Abstract
Transcutaneous electrical nerve stimulation (TENS) is a nonpharmacologic treatment for pain relief. TENS has been used to treat a variety of painful conditions. This review updates the basic and clinical science regarding the use of TENS that has been published in the past 3 years (ie, 2005−2008). Basic science studies using animal models of inflammation show changes in the peripheral nervous system, as well as in the spinal cord and descending inhibitory pathways, in response to TENS. Translational studies show mechanisms to prevent analgesic tolerance to repeated application of TENS. This review also highlights data from recent randomized, placebo-controlled trials and current systematic reviews. Clinical trials suggest that adequate dosing, particularly intensity, is critical to obtaining pain relief with TENS. Thus, evidence continues to emerge from both basic science and clinical trials supporting the use of TENS for the treatment of a variety of painful conditions while identifying strategies to increase TENS effectiveness.
Introduction
Transcutaneous electrical nerve stimulation (TENS) is a commonly used nonpharmacologic and noninvasive treatment for pain. Although a number of clinical studies show the effectiveness of TENS for pain, there is still much controversy over which conditions to treat with TENS and the adequate parameters to use. Prior reports show that TENS reduces pain through both peripheral and central mechanisms. Centrally, sites in the spinal cord and brainstem that utilize opioid, serotonin, and muscarinic receptors are activated by TENS. Peripherally, at the site of TENS application, opioid and α-2 noradrenergic receptors are involved in TENS-induced analgesia [1•]. The purpose of this review is to update the reader on the latest literature concerning TENS: basic science, experimental pain, clinical trials, and systematic reviews.
TENS is the application of electrical current through electrodes placed on the skin for pain control. It can be applied with varying frequencies, from low (< 10 Hz) to high (> 50 Hz). Intensity may also be varied from sensory to motor intensities. Sensory intensity is when the patient feels a strong but comfortable sensation without motor contraction. High intensity usually involves a motor contraction but is not painful. In general, higher-frequency stimulation is delivered at sensory intensity, and low-frequency stimulation is delivered at motor intensity. Prior literature from our laboratory shows that, regardless of intensity, different frequencies activate central mechanisms to produce analgesia. Specifically, we show that low-frequency TENS activates μ-opioid receptors in the spinal cord and the brainstem, whereas high-frequency TENS activates δ-opioid receptors in the spinal cord and the brainstem [2–4]. Subsequent studies have investigated the role of serotoninergic, noradrenergic, muscarinic, and γ-aminobutyric acid (GABA)-ergic systems on the analgesia produced by both low-frequency and high-frequency TENS.
The terms “hyperalgesia” and “allodynia” are widely used in the following text. Hyperalgesia is an increased pain sensitivity to a peripherally applied stimulus [5]. Primary hyperalgesia is an increased pain sensitivity at the site of injury, which is thought to mirror changes in the peripheral nervous system. Secondary hyperalgesia occurs outside the site of injury, and it is thought to be mediated by changes in the central nervous system. We and others have tested the effectiveness of TENS on a variety of measures of both primary and secondary hyperalgesia. Allodynia is defined as pain in response to a normally innocuous (nonpainful) stimuli or activities that are thought to be mediated by changes in the central nervous system, where activation of a peripherally located nonnociceptor is perceived as painful.
Basic Science Mechanisms
General studies
Many previous studies in rats have shown that TENS reduces secondary mechanical hyperalgesia of the paw induced by knee joint inflammation [6•,7,8•,9,10]. More recently, Vance et al. [11•] showed that primary mechanical hyperalgesia induced by joint inflammation was reduced in response to both high- and low-frequency TENS. Compression withdrawal threshold was decreased at 24 hours and 2 weeks following the induction of inflammation, but not when applied at 4 hours after the induction of inflammation. Thus, it can be suggested that because inflammation has already completely developed, TENS inhibits primary hyperalgesia associated with inflammation in a time-dependent manner [9].
The site of electrode application of TENS is typically at the site of injury. However, as central mechanisms are activated by TENS, it is possible that application outside the site may also be effective. Recently, two studies confirmed this hypothesis by showing that application of TENS to the contralateral hind limb reduces hyperalgesia of the inflamed limb [10,11•]. Furthermore, when hyperalgesia developed bilaterally after a unilateral injury, application of either high- or low-frequency TENS to the inflamed or the contralateral side reduced the hyperalgesia bilaterally [11•]. In a different pain model, Somers and Clemente [12] investigated the sites of electrode placement that would best prevent the development of allodynia in a chronic constriction injury (CCI) to the rat sciatic nerve. Repeated daily high-frequency TENS for 12 days with electrodes positioned on the skin covering either ipsilateral or contralateral paraspinal muscles reduced the development of mechanical hyperalgesia in CCI rats. Low-frequency TENS applied to acupuncture points in the ipsilateral or contralateral hind limbs decreased the development of thermal hyperalgesia, but only when TENS was delivered on the contralateral side [12]. Thus, once hyperalgesia develops, application of TENS to either the ipsilateral or the contralateral hind limb is effective.
It is generally thought that TENS produces analgesia by activation of cutaneous afferent fibers at the site of application. However, by differentially blocking primary afferents with local anesthetics, Radhakrishnan and Sluka [8•] showed the importance of deep tissue afferents in the analgesia produced by TENS. Specifically, blockade of cutaneous afferents with an anesthetic cream (eutectic mixture of lidocaine and prilocaine) during TENS application had no effect on the analgesia produced by both high- and low-frequency TENS. However, when a local anesthetic was applied to the inflamed knee joint during TENS application, there was a complete blockade of the analgesic effects of TENS [8•].
It is also generally thought that large-diameter fibers are activated by high-frequency TENS and that low-frequency TENS at motor intensity activates Aδ afferent fibers. Recordings of spinal cord dorsum potentials show that only large-diameter primary afferent fibers from deep tissue are activated by both high- and low-frequency TENS at sensory intensities up to and including motor thresholds. Increasing the intensity of stimulation to twice the motor threshold recruits Aδ afferent fibers [8•]. Thus, high intensities of stimulation well above motor threshold are needed to activate nociceptive afferents by TENS, suggesting that the analgesia produced by TENS is mediated through activation of large-diameter afferent fibers.
TENS units have been commercialized with many different characteristics. However, little is known about whether different waveforms could influence TENS-mediated anal-gesia in a different manner [11•] compared with the effects of high-frequency TENS with different waveforms (such as an asymmetrical or symmetrical biphasic square wave) on inflammatory hyperalgesia. Differences in waveform characteristics do not alter the analgesia produced by TENS, as hyperalgesia is similarly reduced when either an asymmetrical or symmetrical waveform is used. Therefore, different waveforms can be used to improve comfort for the patients but not to increase analgesic efficacy.
Peripheral mechanisms
The effect of both high- and low-frequency TENS was tested in mutant mice lacking a functional α2A-adrenergic receptor (AR) against their respective wild-type counterparts. TENS-induced analgesia, at both high and low frequencies of stimulation, was reduced in α2A mutant mice compared with controls. Furthermore, when an α2 AR-selective antagonist (SK&F 86466) was administered intraarticularly, TENS-induced analgesia was reversed, but analgesia was not reversed when it was delivered intrathecally or intracerebroventricularly. Thus, it seems that peripheral α2 ARs partially contribute to TENS-mediated analgesia [7]. The α2A and α2C AR subtypes mediate antinociception when activated by the endogenous ligand norepinephrine. These receptors also produce antinociceptive synergy when activated concurrently with opioid receptors [7].
Peripheral opioid receptors also appear to play a role in the analgesia produced by low-frequency TENS. Sabino et al. [10] showed that blockade of μ-opioid receptors at the site of application prevents the reduction in hyperalgesia produced by low-frequency TENS but not high-frequency TENS.
Spinal mechanisms
Prior reports show the importance of the opioid pathways in pain inhibition at the spinal level. A recent study showed that the inhibitory neurotransmitter GABA, but not glycine, is also involved in this analgesia at the spinal level. High-frequency, but not low-frequency, TENS applied to the inflamed knee joint increases extracellular GABA concentrations in the spinal cord dorsal horn in animals with and without joint inflammation. Interestingly, blockade of GABAA receptors with bicuculline prevented the reduction in hyperalgesia produced by both high- and low-frequency TENS [13,14•]. In another study, spinal serotonin concentrations were increased during and immediately after treatment with low-frequency, but not high-frequency, TENS. Neither low- nor high-frequency TENS changed spinal noradrenaline concentrations [6•].
Sluka et al. [15•] also showed that high-frequency, but not low-frequency, TENS reduces glutamate and aspartate concentrations in spinal cord dorsal horn in animals with joint inflammation when compared with levels in those without joint inflammation. Moreover, spinal administration of δ-opioid receptor antagonists (naltrindole) prevented the reduced release of glutamate and aspartate by high-frequency TENS [15•]. Thus, it appears that TENS reduces the release of glutamate and aspartate in animals with joint inflammation by activation of opioid receptors.
Supraspinal mechanisms
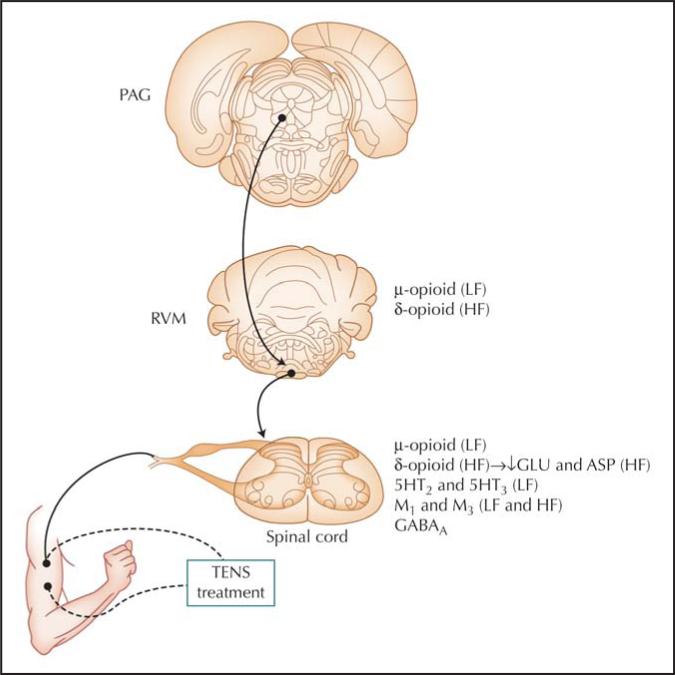
Prior studies show involvement of the rostroventral medial medulla (RVM) in the hyperalgesia produced by TENS. Recently, DeSantana and Sluka [16] showed that the periaqueductal gray (PAG) also contributes to this analgesic effect. The reduction in both the primary and secondary hyperalgesia by high- and low-frequency TENS is prevented by blockade of the ventrolateral PAG with cobalt chloride. Thus, the ventrolateral PAG likely sends projections through the RVM to the spinal cord to produce analgesia. Figure 1 summarizes the main spinal and supraspinal mechanisms and receptors involved in the analgesia produced by high- and low-frequency TENS.
Figure 1.
Schematic diagram showing potential pathways activated by low-frequency (LF) or high-frequency (HF) transcutaneous electrical nerve stimulation (TENS). Projections from the ventrolateral periaqueductal gray (PAG) send input to the rostroventral medial medulla (RVM), which in turn projects to the spinal cord to produce analgesia. Receptors known to be involved in the analgesia produced by TENS are listed for each site. 5HT—serotonin; ASP—aspartate; GABA—γ-aminobutyric acid; GLU—glutamate; M—muscarinic receptor.
Tolerance to TENS
Repeated daily administration of TENS causes analgesic tolerance at spinal opioid receptors on the fourth day [17]. In the past few years, studies have investigated different strategies to improve the efficacy of TENS by preventing or delaying the development of tolerance. Hingne and Sluka [18•] showed that blockade of N-methyl-D-aspartate (NMDA) receptors during application of TENS prevents the onset of tolerance. The NMDA receptor is an ionotropic receptor for glutamate. Calcium flux through NMDA receptors is thought to play a critical role in synaptic plasticity, particularly central sensitization, and development of opioid tolerance. Specifically, TENS reversed the mechanical withdrawal thresholds on day 4 for those rats treated with MK-801 (noncompetitive NMDA receptor antagonist). Moreover, cross-tolerance at spinal opioid receptors developed in animals treated with saline but not in those treated with MK-801 (competitive antagonist to NMDA receptors) [18•]. Thus, blockade of NMDA receptors during TENS prevents the development of analgesic tolerance to TENS by preventing tolerance at spinal opioid receptors. Cholecystokinin (CCK) receptors are a group of G-protein–coupled receptors that bind the peptide hormone CCK. Blockade of CCK receptors (CCK-A or CCK-B) prevents the development of opioid tolerance. Similarly to NMDA receptor antagonists, systemic and intrathecal administration of CCK receptor antagonists prevent the development of tolerance to both high- and low-frequency TENS on day 4 when administered before TENS application in the first 3 days of treatment, and also prevent the cross-tolerance to spinal opioids [19]. Interestingly, modulating the frequency of TENS between high and low frequencies delays the development of analgesic tolerance to TENS [20•].
Basic science studies show that simultaneous activation of μ-opioid and δ-opioid receptors prevents the development of tolerance. Thus, providing low- and high-frequency TENS simultaneously, to activate μ-opioid and δ-opioid receptors, should similarly prevent tolerance to TENS. Either mixed-frequency (high- and low-frequency TENS applied at the same session, cycled every 3 seconds) or alternating-frequency (high- and low-frequency TENS applied separately in alternating days) TENS reversed mechanical hyperalgesia for 9 days. However, by day 10, repeated daily administration of either mixed- or alternating-frequency TENS became ineffective [20•].
In summary, blockade of NMDA and CCK receptors prevents analgesic tolerance to repeated daily TENS by preventing tolerance at spinal opioid receptors [18•,19]. Furthermore, although repeated daily administration of modulating-frequency TENS leads to a development of opioid tolerance, this tolerance effect is delayed by approximately 5 days as compared with treatment with high- or low-frequency TENS [20•].
Experimental Pain in Humans
Experimental pain models have been used to retrieve useful information on electrophysical therapies prior to conducting full-scale, randomized clinical trials. However, their limitations must also be recognized in that they are unable to replicate the complex interactions between the physiologic and psychological processes of clinical pain. Five studies have been recently published on the effect of TENS on upper limb pressure, heat, and ischemic experimental pain models. Brown et al. [21•] failed to demonstrate any significant differences in pain relief in a crossover study using experimental ischemic pain when high-frequency TENS (100 Hz) was applied for 5 minutes at the painful forearm site compared with a remote nonpainful site. This study used a relatively short stimulation period compared with the other experimental pain studies.
Aarskog et al. [22] used pressure pain threshold (PPT) to compare two intensity levels of high-frequency TENS (100 Hz) applied simultaneously for 20 minutes to the hand/forearm on both sides. The intensity levels were either the lowest intensity at which the participant first perceived the electrical stimulation on the skin (sensory threshold) or at a level that the participant described as strong but comfortable. There was a statistically significant increase in PPT on the strong-but-comfortable intensity side but not on the sensory-threshold intensity side. The relevance of stimulus intensity was also highlighted in a study by Claydon et al. [23•]. These authors compared high- and low-frequency (4 Hz and 110 Hz), high- and low-intensity, and segmental versus extrasegmental site of stimulation on PPT recorded from the hand. Their analysis found that TENS applied to segmental and extrasegmental sites, at a high intensity (to tolerance) using different frequencies at each site, produced the greatest hypoalgesia. These results indicate that the high-intensity currents (irrespective of the applied frequency) are the key parameter in TENS applications. In a crossover study, Buonocore and Camuzzini [24] showed that high-frequency TENS (100 Hz) significantly increased heat pain threshold in the area of stimulation of the superficial radial nerve when compared with thresholds recorded during a no-treatment control session. The increase in threshold was observed during the 10 minutes of TENS and up to 60 minutes after stimulation. Tong et al. [25•] compared high-frequency (100 Hz), low-frequency (2 Hz), and alternating-frequency (2/100 Hz) TENS applied to the forearm for 30 minutes to a control group. The alternating-frequency group produced a significant increase in PPT and heat pain threshold, whereas the high-frequency group produced a significant increase in PPT only. The observed changes in thresholds peaked at the end of the stimulation period (30 minutes). The superior hypoalgesic effect of mixed frequencies supports recent work conducted in rats with experimentally induced joint inflammation. As mentioned previously, DeSantana et al. [20•] demonstrated that simultaneous administration of low- and high-frequency TENS in the same session, or alternating administration of low- and high-frequency TENS on subsequent sessions, significantly delays the development of tolerance to TENS. This observation suggests an area for further exploration in humans. The key message from these studies is that high stimulus intensity and an alternating high/low frequency of TENS produced the maximal hypoalgesia using experimental pain models.
Randomized placebo-controlled trials
The ongoing questions of group classification strategies, target outcome measures, and parameter selection when using TENS in humans have been addressed by several randomized placebo controlled trials. Oosterhof et al. [26•] conducted a prospective trial comparing high-frequency (80 Hz, 50 μsec) TENS to placebo TENS to determine if there are predictive characteristics related to TENS effectiveness. One hundred and sixty-three chronic pain patients were classified into three groups: osteoarthritis-related disorders; peripheral neuropathic pain; and soft tissue, bone, and visceral-related pain disorders. Pain intensity and patient satisfaction were chosen as outcome measures. The investigators concluded that patient satisfaction was related to origin of pain, and patients in the soft tissue and bone-disorders group experienced the best results. Pain intensity was not influenced by TENS or placebo TENS.
Narrowing the patient population appears to assist in obtaining answers to questions of TENS effectiveness, as evidenced in three studies. Warke et al. [27•] investigated the hypoalgesic effects of TENS in patients diagnosed with multiple sclerosis with a concurrent diagnosis of chronic low back pain. Ninety patients were randomly assigned to low-frequency TENS (4 Hz, 200 μsec), high-frequency TENS (110Hz, 200 μsec), or placebo TENS. Self-applied TENS treatments of 45 minutes twice daily (minimum) for 6 weeks yielded a significant effect between groups over time for average low back pain. These clinically important results suggest that high-frequency TENS had its greatest effects on pain reduction during the 6 weeks of treatment. Low-frequency TENS demonstrated positive long-term results at 32 weeks. In addition, low-frequency TENS improved functional measures over the treatment period.
TENS was reported to have hypoalgesic effects in two reports of “out of hospital rescue” by emergency responders [28•,29•]. Mora et al. [28•] compared the effects of high-frequency, low-intensity TENS (100 Hz, 200 μsec, 2 mA, for 30 minutes) with sham TENS in patients requiring medical transport determined to have acute renal colic as identified by paramedic evaluation. A second paramedic applied the TENS unit according to a randomized computer-generated code sealed in sequential envelopes. The active TENS group demonstrated a significant reduction in pain, anxiety, and nausea scores, as well as a lower heart rate response. No significant effects were noted with sham TENS [28•]. Similar findings were reported in 63 patients requiring emergency transport for posttraumatic hip pain [25•]. The same study design as in Mora et al. [28•] was used for 30 active TENS and 33 sham TENS treatments. Visual analogue scale pain scores, anxiety scores, and heart rate response were significantly reduced in the active TENS group as compared with sham TENS. Together, these two reports suggest that TENS can be used as a fast-acting pain treatment for victims of painful illnesses requiring transport for medical attention. TENS may also be beneficial in decreasing autonomic responses to acute pain [29•].
The issue of adjusting the stimulation intensity during the treatment session was addressed by Defrin et al. [30•] using interferential current to treat patients with knee osteoarthritis. Their finding of segmental, high-intensity stimulation providing a greater treatment effect than low-intensity stimulation concurs with other investigations. In addition, the finding that fading of the stimulation intensity during the treatment session does not impede the hypoalgesic effects suggests that despite A-fiber adaptation, peripheral nerves remain sufficiently activated to induce a hypoalgesic effect or that only the initial stimulation period is required to produce the treatment effect. Further investigation for minimal effective dose for the parameter of treatment time is warranted.
Recent evidence from five prospective, randomized controlled studies continues to support the benefits of TENS for postoperative pain. DeSantana et al. [31•] tested the hypoalgesic effect of high-frequency TENS (100 Hz) applied using a strong but comfortable sensory intensity used for 30 minutes 2 and 4 hours after unilateral inguinal herniorrhaphy. TENS significantly decreased analgesic requirements and incisional pain intensity for 24 hours postoperatively when compared with placebo TENS. In another study, DeSantana et al. [32•] investigated the effect of both high- and low-frequency TENS (100 Hz and 4 Hz, respectively) immediately after laparoscopic surgery for tubal ligation through placement of Yoon rings. They showed that both high- and low-frequency TENS were able to significantly reduce postoperative pain. Cipriano et al. [33•] tested a 4-hour high-frequency TENS treatment (80 Hz) with a strong but comfortable sensory intensity on 3 postoperative days following cardiac surgery and found significant decreases in incisional pain both at rest and with cough when compared with placebo TENS. This decrease in pain intensity was related to positive effects on pulmonary function, with significant increases in tidal volume, vital capacity, and electrical activity of the trapezius and pectoralis major muscles in patients receiving active TENS but not sham TENS.
Similar results were found when TENS was used for the more severe pain associated with thoracotomy. Solak et al. [34•] tested low-frequency TENS (3 Hz, 100 ms, 12 mA) used for 30 minutes once a day for 10 days postoperatively and found incisional pain intensity and analgesic requirements to be significantly lower compared with placebo TENS. Interestingly, the effect of TENS did not become significant until the fourth postoperative day, and this effect lasted for at least 2 months postoperatively. Pulmonary function measures performed every 2 weeks for 2 months after surgery were not significantly different between groups. This is in contrast to findings reported by Erdogan et al. [35•], who found that high-frequency TENS (100 Hz, 100 μs) used at a sensation to “not disturb the patient”) used continuously for 48 hours postoperatively and then for 20 minutes at 3-hour intervals for 3 days increased forced expiratory volume at 1 second, forced vital capacity, and blood gas results significantly more than placebo TENS at 6 to 48 hours after thoracotomy. Subjective pain levels, both at rest and during coughing, and the need for additional analgesic medication were also significantly reduced in patients receiving TENS compared with placebo TENS. It appears that an adequate dose of TENS is needed to affect the severe pain associated with thoracotomy and to improve related functional performance postoperatively.
In summary, recent randomized, placebo-controlled clinical trials suggest that TENS is effective for several acute and chronic pain conditions associated with emergent and postoperative conditions. TENS is consistently more effective than placebo TENS for pain intensity, anxiety, and heart rate in these populations, and decreases in postoperative pain with cough are associated with improvements in pulmonary function when the TENS dose is adequate. Use of high-intensity stimulation and high-frequency TENS appears to be the most effective, particularly in the short term.
Meta-Analyses, Systematic Reviews, and Cochrane Reviews
Two systematic reviews and one meta-analysis have been published in the past 3 years on the effects of TENS on experimental pain models, chronic musculoskeletal pain, and chronic low back pain. Only two randomized controlled trials of TENS for chronic low back pain [36,37] met the criteria for Khadilkar et al.'s Cochrane systematic review [38•]. Due to heterogeneity among the study populations, the authors were unable to pool the data and concluded that there was inconsistent evidence for the application of TENS for this condition.
The meta-analysis by Johnson and Martinson [39•] of electrical nerve stimulation for chronic musculoskeletal pain pooled data from 32 studies on TENS (high frequency, low frequency, variable frequency, and acupuncture-like) and 6 on percutaneous electrical nerve stimulation. There were 1227 patients with a range of conditions included in the analysis. These conditions were rheumatoid arthritis, low back pain, osteoarthritis, ankylosing spondylitis, and myofascial pain. Results from this diverse population indicated a significant decrease in pain with electrical nerve stimulation compared with placebo. The authors highlighted that lack of statistical power was the main reason for disparity in their findings versus other studies and meta-analyses in this area.
Chen et al. [40] reviewed studies of TENS on experimental pain with the objective of establishing the hypoalgesic effect of pulse frequency. Thirteen studies were included in their review, and only three of these studies reported a significant difference for pulse frequency. The authors concluded that their review did not support the belief that pulse frequency is a key determinant of outcome when pulse intensity is standardized at a strong but comfortable level close to the painful site.
Conclusions
Basic scientific evidence suggests that there are peripheral and central nervous system mechanisms underlying the analgesic action of TENS. Studies also show that tolerance to repeated application of TENS can be prevented by multiple strategies, both pharmacologic and nonpharmacologic. Experimental pain studies and clinical trials are beginning to refine parameters of stimulation to obtain the best pain relief. It seems that stimulation intensity is a critical factor for the effectiveness of TENS. One meta-analysis was able to show the positive treatment effects of electrical stimulation for relief of chronic musculoskeletal pain, and randomized controlled trials consistently demonstrate the effectiveness of TENS for acute, emergent, and postoperative pain conditions. However, the effectiveness of TENS on individual pain conditions, such as low back pain, is still controversial, likely because of poor study designs and small sample size. Thus, continued research of TENS mechanisms and stimulation parameters in adequately characterized patient populations is critical.
Acknowledgments
Deirdre Walsh can be contacted at the Health and Rehabilitation Sciences Research Institute, University of Ulster, Northern Ireland, United Kingdom. Carol Vance, Barbara Rakel, and Kathleen Sluka can be contacted at the University of Iowa in Iowa City, IA.
Footnotes
Disclosures
Dr. Sluka and Dr. DeSantana have received a grant from EMPI. Dr. Sluka has served as a consultant for EMPI, and EMPI has provided TENS units for ongoing studies. The other authors report no potential conflicts of interest for this article.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1•.Sluka KA. The Neurobiology of pain and foundations for electrical stimulation. In: Robinson AJ, Snyder-Mackler L, editors. Clinical Electrophysiology. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 107–149. [• Of importanceA recent review of all the basic science mechanisms underlying TENS] [Google Scholar]
- 2.Sluka KA, Deacon M, Stibal A, et al. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289:840–846. [PubMed] [Google Scholar]
- 3.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). J Pharmacol Exp Ther. 2001;298:257–263. [PubMed] [Google Scholar]
- 4.Sluka KA, Chandran P. Enhanced reduction in hyperalgesia by combined administration of clonidine and TENS. Pain. 2002;100:183–190. doi: 10.1016/s0304-3959(02)00294-4. [DOI] [PubMed] [Google Scholar]
- 5.Loeser JD, Treede RD. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008 doi: 10.1016/j.pain.2008.04.025. (in press) [DOI] [PubMed] [Google Scholar]
- 6•.Sluka KA, Lisi TL, Westlund KN. Increased release of serotonin in the spinal cord during low, but not high, frequency transcutaneous electric nerve stimulation in rats with joint inflammation. Arch Phys Med Rehabil. 2006;87:1137–1140. doi: 10.1016/j.apmr.2006.04.023. [• Of importanceThis article shows that there is an increase in serotonin in the spinal cord during low-frequency, but not high-frequency, TENS using microdialysis and high-performance liquid chromatography analysis in an animal model of joint inflammation] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King EW, Audette K, Athman GA, et al. Transcutaneous electrical nerve stimulation activates peripherally located alpha-2A adrenergic receptors. Pain. 2005;115:364–373. doi: 10.1016/j.pain.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 8•.Radhakrishnan R, Sluka KA. Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation-Induced antihyperalgesia. J Pain. 2005;6:673–680. doi: 10.1016/j.jpain.2005.06.001. [• Of importanceThis article demonstrates the importance of afferents from deep tissues in producing the analgesia produced by TENS by differential anesthetic blockade of the joint or the skin during the TENS stimulation] [DOI] [PubMed] [Google Scholar]
- 9.Vance CG, Radhakrishnan R, Skyba DA, et al. Transcutaneous electrical nerve stimulation at both high and low frequencies reduces primary hyperalgesia in rats with joint inflammation in a time-dependent manner. Phys Ther. 2007;87:44–51. doi: 10.2522/ptj.20060032. [DOI] [PubMed] [Google Scholar]
- 10.Sabino GS, Santos CM, Francischi JN, et al. Release of endogenous opioids following transcutaneous electric nerve stimulation in an experimental model of acute inflammatory pain. J Pain. 2008;9:157–163. doi: 10.1016/j.jpain.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 11•.Ainsworth L, Budelier K, Clinesmith M, et al. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain. 2006;120:182–187. doi: 10.1016/j.pain.2005.10.030. [• Of importanceApplication of TENS over an inflamed muscle in rats reverses the hyperalgesia bilaterally. Furthermore, application of TENS to the contralateral “mirror side” also reverses the hyperalgesia bilaterally] [DOI] [PubMed] [Google Scholar]
- 12.Somers DL, Clemente FR. Transcutaneous electrical nerve stimulation for the management of neuropathic pain: the effects of frequency and electrode position on prevention of allodynia in a rat model of complex regional pain syndrome type II. Phys Ther. 2006;86:698–709. [PubMed] [Google Scholar]
- 13.Hingne PM, Sluka KA. Differences in waveform characteristics have no effect on the anti-hyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) in rats with joint inflammation. J Pain. 2007;8:251–255. doi: 10.1016/j.jpain.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 14•.Maeda Y, Lisi TL, Vance CG, et al. Release of GABA and activation of GABA(A) in the spinal cord mediates the effects of TENS in rats. Brain Res. 2007;1136:43–50. doi: 10.1016/j.brainres.2006.11.061. [• Of importanceGABA is released and activates spinal GABA receptors to produce the analgesia produced by TENS] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Sluka KA, Vance CG, Lisi TL. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. J Neurochem. 2005;95:1794–1801. doi: 10.1111/j.1471-4159.2005.03511.x. [• Of importanceHigh-frequency TENS reduces the release of excitatory neurotransmitters in the spinal cord through activation of δ-opioid receptors] [DOI] [PubMed] [Google Scholar]
- 16.DeSantana JM, Sluka KA. Antinociceptive effect of transcutaneous electric nerve stimulation (TENS) is mediated by ventrolateral periaqueductal grey (vlPAG).. Presented at the XII World Congress in Pain.; Glasgow, Scotland. August 17−22, 2008. [Google Scholar]
- 17.Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102:195–201. doi: 10.1016/s0304-3959(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 18•.Hingne PM, Sluka KA. Blockade of NMDA receptors prevents analgesic tolerance to repeated transcutaneous electrical nerve stimulation (TENS) in rats. J Pain. 2008;9:217–225. doi: 10.1016/j.jpain.2007.10.010. [• Of importanceThis article shows that tolerance to repeated application of TENS can be prevented by blockade of NMDA glutamate receptors during the TENS application when the drug is administered systemically. Systemic blockade of NMDA glutamate receptors also prevents cross-tolerance in the spinal cord] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSantana JM, Sluka KA. Blockade of cholecystokinin receptors prevents the development of analgesic tolerance to transcutaneous electric nerve stimulation (TENS).. Presented at the XII World Congress in Pain.; Glasgow, Scotland. August 17−22, 2008. [Google Scholar]
- 20•.DeSantana JM, Santana-Filho VJ, Sluka KA. Modulation between high- and low-frequency transcutaneous electric nerve stimulation delays the development of analgesic tolerance in arthritic rats. Arch Phys Med Rehabil. 2008;89:754–760. doi: 10.1016/j.apmr.2007.11.027. [• Of importanceModulating high- and low-frequency TENS delays the development of tolerance effect] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Brown L, Tabasam G, Bjordal JM, et al. An investigation into the effect of electrode placement of transcutaneous electrical nerve stimulation (TENS) on experimentally induced ischemic pain in healthy human participants. Clin J Pain. 2007;23:735–743. doi: 10.1097/AJP.0b013e31814b86a9. [• Of importanceThis article showed no significant differences between two TENS eclectrode placement sites using experimental pain] [DOI] [PubMed] [Google Scholar]
- 22.Aarskog R, Johnson MI, Demmink JH, et al. Is mechanical pain threshold after transcutaneous electrical nerve stimulation (TENS) increased locally and unilaterally? A randomized placebo-controlled trial in healthy subjects. Physiother Res Int. 2007;12:251–263. doi: 10.1002/pri.384. [DOI] [PubMed] [Google Scholar]
- 23•.Claydon LS, Chesterton LS, Barlas P, Sim J. Effects of simultaneous dual-site TENS stimulation on experimental pain. Eur J Pain. 2008;12:696–704. doi: 10.1016/j.ejpain.2007.10.014. [• Of importanceHigh-intensity (irrespective of the applied frequency) is a key parameter in TENS applications] [DOI] [PubMed] [Google Scholar]
- 24.Buonocore M, Camuzzini N. Increase of the heat pain threshold during and after high-frequency transcutaneous peripheral nerve stimulation in a group of normal subjects. Europa Medicophysica. 2007;43:155–160. [PubMed] [Google Scholar]
- 25•.Tong KC, Lo SK, Cheing GL. Alternating frequencies of transcutaneous electric nerve stimulation: does it produce greater analgesic effects on mechanical and thermal pain thresholds? Arch Phys Med Rehabil. 2007;88:1344–1349. doi: 10.1016/j.apmr.2007.07.017. [• Of importanceThis article demonstrates the superior hypoalgesic effect of mixed TENS frequencies] [DOI] [PubMed] [Google Scholar]
- 26•.Oosterhof J, Samwel HJ, de Boo TM, et al. Predicting outcome of TENS in chronic pain: A prospective, randomized, placebo controlled trial. Pain. 2008;136:11–20. doi: 10.1016/j.pain.2007.06.009. [• Of importancePredicting the effect of high-frequency TENS in chronic pain depends on the choice of outcome measure, and source of pain influences patients’ satisfaction with treatment result] [DOI] [PubMed] [Google Scholar]
- 27•.Warke K, Al-Smadi J, Baxter D, et al. Efficacy of transcutaneous electrical nerve stimulation (TENS) for chronic low-back pain in a multiple sclerosis population: a randomized, placebo-controlled clinical trial. Clin J Pain. 2006;22:812–819. doi: 10.1097/01.ajp.0000210935.73686.79. [• Of importanceThis article demonstrates the effect of TENS for chronic low-back pain in multiple sclerosis patients] [DOI] [PubMed] [Google Scholar]
- 28•.Mora B, Giorni E, Dobrovits M, et al. Transcutaneous electrical nerve stimulation: an effective treatment for pain caused by renal colic in emergency care. J Urol. 2006;175:1737–1741. doi: 10.1016/S0022-5347(05)00980-8. [• Of importanceThis article demonstrates the hypoalgesic effect of local TENS for renal colic pain] [DOI] [PubMed] [Google Scholar]
- 29•.Lang T, Barker R, Steinlechner B, et al. TENS relieves acute posttraumatic hip pain during emergency transport. J Trauma. 2007;62:184–188. doi: 10.1097/01.ta.0000197176.75598.fc. [• Of importanceThis article demonstrates the hypoalgesic effect of TENS for emergency pain conditions during transportation to the hospital] [DOI] [PubMed] [Google Scholar]
- 30•.Defrin R, Ariel E, Peretz C. Segmental noxious versus innocuous electrical stimulation for chronic pain relief and the effect of fading sensation during treatment. Pain. 2005;115:152–160. doi: 10.1016/j.pain.2005.02.018. [• Of importanceThis article demonstrates the hypoalgesic effects of interferential current for chronic OA knee pain, where segmental noxious stimulation produces a stronger analgesic effect than segmental innocuous stimulation] [DOI] [PubMed] [Google Scholar]
- 31•.DeSantana JM, Santana-Filho VJ, Guerra DR, et al. Hypoalgesic effect of the transcutaneous electrical nerve stimulation following inguinal herniorrhaphy: a randomized, controlled trial. J Pain. 2008;9:623–629. doi: 10.1016/j.jpain.2008.01.337. [• Of importanceThis article demonstrates that TENS significantly decreases analgesic requirements and incisional pain intensity after inquinal herniorrhaphy when compared with placebo] [DOI] [PubMed] [Google Scholar]
- 32•.DeSantana JM, Sluka KA, Lauretti GR. High and low frequency TENS reduce postoperative pain intensity after laparoscopic sterilization for tubal ligation: a randomized controlled trial. Clin J Pain. 2008 doi: 10.1097/AJP.0b013e31817d1070. (in press) [• Of importanceBoth high- and low-frequency TENS significantly reduce pain after laparoscopic tubal ligation] [DOI] [PubMed] [Google Scholar]
- 33•.Cipriano G, Carvalho AC, Bernardelli GF, et al. Short-term transcutaneous electrical nerve stimulation after cardiac surgery: effect on pain, pulmonary function, and electrical muscle activity. Interact CardioVasc Thorac Surg. 2008;7:539–543. doi: 10.1510/icvts.2007.168542. [• Of importanceTENS significantly decreases incisional pain and improves pulmonary function when compared with placebo after cardiac surgery] [DOI] [PubMed] [Google Scholar]
- 34•.Solak O, Turna A, Pekcolaklar A, et al. Transcutaneous electric nerve stimulation for the treatment of postthoracotomy pain: a randomized prospective study. Thorac Cardiov Surg. 2007;55:182–185. doi: 10.1055/s-2006-924631. [• Of importanceTENS, used 30 minutes daily, significantly decreases incisional pain and analgesic requirements but not pulmonary function compared with placebo after thoracotomy] [DOI] [PubMed] [Google Scholar]
- 35•.Erdogan M, Erdogan AE, Erbil N, et al. Prospective, randomized, placebo-controlled study of the effect of TENS on postthoractomy pain and pulmonary function. World J Surg. 2005;29:1563–1570. doi: 10.1007/s00268-005-7934-6. [• Of importanceHigher TENS dosing significantly decreases pain and analgesic requirements and significantly increases pulmonary function compared with placebo after thoracotomy] [DOI] [PubMed] [Google Scholar]
- 36.Cheing GL, Hui-Chan CW. Transcutaneous electrical nerve stimulation: nonparallel antinociceptive effects on chronic clinical pain and acute experimental pain. Arch Phys Med Rehabil. 1999;80:305–312. doi: 10.1016/s0003-9993(99)90142-9. [DOI] [PubMed] [Google Scholar]
- 37.Deyo RA, Walsh NE, Martin DC, et al. A controlled trial of transcutaneous electrical stimulation (TENS) and exercise for chronic low back pain. N Engl J Med. 1990;322:1627–1634. doi: 10.1056/NEJM199006073222303. [DOI] [PubMed] [Google Scholar]
- 38•.Khadilkar A, Milne S, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) for chronic low-back pain. Cochrane Database System Rev. 2005;(3) doi: 10.1002/14651858.CD003008.pub2. [• Of importanceSystematic review of clinical trials on TENS for chronic low back pain] [DOI] [PubMed] [Google Scholar]
- 39•.Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: a meta-analysis of randomized controlled trials. Pain. 2007;130:157–165. doi: 10.1016/j.pain.2007.02.007. [• Of importanceA Meta-analysis that shows that electrical stimulation is superior to placebo for chronic musculoskeletal pain] [DOI] [PubMed] [Google Scholar]
- 40.Chen C, Tabasam G, Johnson MI. Does the pulse frequency of transcutaneous electrical nerve stimulation (TENS) influence hypoalgesia? A systematic review of studies using experimental pain and healthy human participants. Physiotherapy. 2008;94:11–20. [Google Scholar]