Abstract
Globally, over 33.2 million people who mostly live in developing countries with limited access to the appropriate medical care suffer from the human immunodeficiency virus (HIV) infection. We developed an on-chip HIV capture and imaging method using quantum dots (Qdots) from fingerprick volume (10 μl) of unprocessed HIV-infected patient whole blood in anti-gp120 antibody-immobilized microfluidic chip. Two-color Qdots (Qdot525 and Qdot655 streptavidin conjugates) were used to identify the captured HIV by simultaneous labeling the envelope gp120 glycoprotein and its high-mannose glycans. This dual-stain imaging technique using Qdots provides a new and effective tool for accurate identification of HIV particles from patient whole blood without any pre-processing. This on-chip HIV capture and imaging platform creates new avenues for point-of-care diagnostics and monitoring applications of infectious diseases.
Keywords: Human immunodeficiency virus (HIV), Quantum dot (Qdot), Viral capture, Dual-stain imaging, Anti-gp120 antibody, lectin
1. Introduction
Overall, 2.0 million (1.8-2.3 million) HIV (Human Immunodeficiency Virus)-infected patients died due to AIDS (Acquired Immune Deficiency Syndrome) in 2007, compared to an estimated 1.7 million (1.5-2.3 million) in 2001 (Cohen 2007; HIV/AIDS 2008). Moreover, globally, there were an estimated 33.2 million patients living with HIV in 2007 (HIV/AIDS 2008). CD4+ T lymphocyte count give physicians a good discernment how much damage HIV has done to immune system (Cheng et al. 2007a; Cheng et al. 2007b; Hammer et al. 2008; Moon et al. 2009; Ozcan and Demirci 2008). However, there are many factors, other than HIV activity, that affect the amount of CD4+ T lymphocytes present in the blood at any given time. Thus, plasma HIV viral load count, which is the number of HIV per unit volume of blood, is essential to determine the initiation and dosage of antiretroviral therapy (ART) along with the CD4+ T lymphocytes count (Simon and Ho 2003).
HIV has a spherical shape with a diameter of about 120 nm (Zhu et al. 2006). The structure of the HIV consists of a lipid membrane envelope studded with gp41 and gp120 proteins supported by matrix proteins surrounding the two single-strand RNA and reverse transcriptase enclosed by p24 capsid proteins (Fig. S1). The envelop glycoprotein spike, gp120 (~14 spikes per virus particle), directly mediate a recognition of CD4 receptors expressed on the target cells (Wyatt et al. 1998; Zhu et al. 2006). Considering the HIV envelope structure, gp120 which is highly glycosylated with high-mannose glycans has been among the first targets of HIV vaccine development and is the most attractive target molecule to capture and fluorescently image the viruses in our current research. Previously, two groups, Geyer et al. and Mizuochi et al., reported that a series of high-mannose type oligosaccharides (Man5GlcNAc2 to Man9GlcNAc2) is predominant oligosaccharide structures (60-80%) of gp120 glycoproteins produced by human T cells (H9) (Geyer et al. 1988; Mizuochi et al. 1990). The previous results suggest that the high-mannose type oligosaccharide can be a supplemental HIV recognition molecule along with the envelope protein. In addition, importantly, the anti-gp120 antibody (raised in goat) does not express the high-mannose type oligosaccharides (Raju et al. 2000). This means that ConA lectin majorly binds to high-mannose oligosaccharides of the HIV gp120. However, current organic dyes and fluorescent proteins are not well suited for long-term imaging of these tiny viral particles under a high magnification objective which generate high excitation light intensity in the focal plane of the objective (Joo et al. 2008). Moreover, their ambiguous fluorescence signal intensity is not suitable for virus detection and imaging.
Luminescent quantum dots (Qdots), colloidal semiconductor nanocrystals, are promising alternatives to organic fluorophores for fluorescence-based biological imaging applications (Bruchez et al. 1998; Chan and Nie 1998). The available Qdots for biological applications are made of CdSe which is coated with an additional layer of ZnS to improve the optical properties of the materials (Medintz et al. 2005). Because of their unique size-dependent characteristics (Smith et al. 2006) including extraordinary photostability, narrow and more symmetric emission spectra and multicolor light emission, the nanometer-sized semiconductor particles have been widely used in immunolabeling (Dahan et al. 2003), cell trafficking studies (Michalet et al. 2005; Pellegrino et al. 2003), in situ hybridization (Levsky and Singer 2003) and in vivo imaging (Gao et al. 2004; Gao et al. 2005). Especially, HIV (Joo et al. 2008) and envelope protein (Bentzen et al. 2005; Cambi et al. 2007) labeled with Qdots have been used in visualization of dynamic interaction between viruses and target human immune cells. While their excellent optical properties have been verified, there have not been studies of their use for imaging of nanoscale HIV which is captured in microfluidic device. Recently, Akin et al. reported real-time virus trapping and imaging method in microfluidic devices (Akin et al. 2004). They used dielectrophoretic filters to only trap from a flow and image vaccinia virus from a pure populations that does not require selective capture. Moreover, the verification of capture was performed by fluorescently labeling using virus membrane staining dyes and nucleophilic dyes, which often suffer from photobleaching and specific excitation ranges. Therefore, Qdots, which are exceptionally bright due to their high extinction coefficients and quantum yields, are more adaptable for virus imaging and tracking studies requiring long-term analysis under the high magnification objective.
Here, we developed HIV capture and imaging method by using an integrated microfluidic device from microliter quantities (10 μl) of HIV-infected patient whole blood (Fig. 1). It enables on-chip selective HIV capture and rapid imaging within 10 minutes using quantum dot (Qdot) which has remarkable photostability and brightness allowing the continuous imaging of individual viruses (Agrawal et al. 2006; Bentzen et al. 2005; Cambi et al. 2007; Liu et al. 2005). To verify the captured HIV particles onto the chamber surface, two types of fluorescent nanoparticles were used: (i) Qdot525 streptavidin conjugate which is coupled with biotinylated anti-gp120 antibody and (ii) Qdot655 streptavidin conjugate which is coupled with biotinylated Concanavalin A (ConA) lectin (Fig. 1b). The dual-stain labeling of HIV and imaging technique gives reliably countable virus images using a fluorescence microscope. We designed a polymethymetacrylate (PMMA)-on-glass microfluidic device to perform HIV capture and imaging directly from unprocessed patient whole blood (Fig. 1a, Fig. 2b). Our purpose in this paper is to demonstrate the proof-of-concept that HIV can be captured and imaged from unprocessed whole blood using an immuno-based microfluidic chip. We have the vision to leverage this technology to a point-of-care HIV monitoring device by further optimization steps in our future studies. To the best of our knowledge, on-chip HIV capture and imaging from patient whole blood has not been reported elsewhere.
Figure 1.

Integrated microfluidic device for HIV capturing and imaging from whole blood. (a) A top-down image of the HIV patient blood containing device. (b) Schematic illustration of HIV capturing/imaging strategy using the dual-stain imaging technique.
Figure 2.

(a) Surface chemistry to immobilize anti-gp120 antibody onto a glass bottom of a microfluidic device. (b) Disposable microfluidic device fabrication. The laser cuts the PMMA lid (inlet - outlet port), a microfluidic channel, and a double-sided adhesive film (50 μm DSA microfluidic channel were manually aligned and bonded. The oxygen plasma treated cover slide glass and the PMMA-DSA were assembled. Blood sample is injected into the microfluidic channel. (c) Validation of the surface chemistry through the direct capture of FITC-conjugate gp120 according to various concentrations (10, 20 and 40 ng/μl, 0.1 and 0.2 μg/μl) using anti-gp120 antibody-immobilized microfluidic device.
2. Material and Methods
2.1 Chemical reagents
3-Mercaptopropyl trimethoxysilane was purchased from Gelest (Morrisville, PA). Ethanol (200 proof) and glass slides (Gold Seal® Cover glass - 24 mm × 35 mm No.1) were purchased from Fisher Scientific (Fair Lawn, NJ). Dimethyl sulfoxide (DMSO), lyophilized bovine serum albumin (BSA), and a glove-bag to handle the moisture-sensitive silane were obtained from Aldrich Chemical Co. (Milwaukee, WI). N-y-maleimidobutyryloxy succinimide ester (GMBS) and NeutrAvidin™ biotin binding protein were obtained from Pierce Biotechnology (Rockford, IL). Phosphate buffered saline (PBS) 1× was purchased from Gibco (Grand Island, NY). Biotinylated goat anti-HIV1 gp120 antibody was purchased from Abcam Inc. (Cambridge, MA). Biotinylated Concanavalin A (ConA) lectin was purchased from EY Laboratories Inc. (San Mateo, CA). Recombinant HIV1 IIIB gp120 -FITC conjugate was obtained from ImmunoDiagnositcs Inc. (Woburn, MA). Qdot525 streptavidin conjugate and Qdot 655 streptavidin conjugate were purchased from Invitrogen (Carlsbad, CA).
2.2 Solution preparation
We prepared 4% (v/v) solutions of 3-mercaptopropyl trimethoxysilane in ethanol, silanization solution, and a 1 μmol/ml of GMBS stock solution (50 mg of GMBS dissolved in 0.5mL DMSO) in ethanol, GMBS solution, were prepared inside a glove bag containing nitrogen gas. Neutravidin, 1 mg in 1 ml PBS, was restored as recommended by the manufacturer. Then 10 % (v/v) biotinylated anti-gp120 antibody solution in PBS containing 1% (w/v) BSA was prepared. A virus staining solutions, 40 μg/ml biotinylated anti-HIV1 gp120 antibody and 50 μg/ml biotinylated ConA, were prepared by dilution with PBS containing 1% (w/v) BSA. 20 nM Qdot525 streptavidin conjugate and 20 nM Qdot 655 streptavidin conjugate were prepared for recognition of the biotinylated anti-HIV1 gp120 antibody and biotinylated ConA.
2.3 Microfluidic device fabrication
We built a microfluidic channel that can be formed with a PMMA inlet/outlet port and a microfluidic channel using a laser prototyping technique and double-sided adhesive film (DSA) (Moon et al. 2009). The DSA bonding approach is suitable for quick channel assembly with surface chemistry. Fig. 2 shows a schematic of the fabrication process and a fabricated device. The microfluidic chip fabrication takes less than one minute per device on a lab bench without employing expensive lithographic methods. Design changes of fluidic channel can be made by simply updating the CAD software that runs a laser cutter, which is applicable to rapid prototyping of a microfluidic device. For the microfluidic device fabrication, a 3.175 mm thick military specification clear cast acrylic sheet (PMMA, Polymethymetacrylate, 8473K276) and a 50 μm thick optically clear laminating adhesive sheet (DSA, 3M 8142) were purchased from McMaster Carr Supply Co. Inc (Los Angeles, CA) and 3M (St. Paul, MN), respectively. The PMMA and DSA films are machined using the VersaLASER Platform (Universal Laser Systems Inc., Models VLS2.3, Scottsdale, AZ)) with a 30W CO2 laser to cut 0.75mm diameter inlets and outlets and 25 mm long × 4 mm width fluidic channels. To cut the 3.2 mm thick PMMA sheet, the power, scan speed, and pulse repulsion rate of the laser are set to 30 W, 10 mm/s (5% of full speed), and 40 pulses per mm, respectively. 50 μm thick DSA film is cut at 6 W, 40 mm/s, and 40 pulses per mm. The laser machine is capable of ~25 μm planar materials. The cut PMMA and adhesive film are manually aligned (~250 μm tolerance) and bonded to each other after removing the protective film backing. The fabricated assay of PMMA-DSA film is then immediately sealed with a microscope cover glass newly exposed to oxygen plasma (100 mW, 1% O2, 60 seconds).
2.4 Surface modification
Glass slides were treated with oxygen plasma (100 mW, 1% oxygen, and 60 seconds) in a PX-250 plasma chamber (March Instruments, Concord, MA). The slides were then immediately assembled with the assay of PMMA-DSA film to form a microfluidic channel. The microfluidic channels were pre-treated with silanization solution for 30 min at room temperature, followed by incubation with GMBS solution for 15 min, also at room temperature. Afterwards, Neutravidin was reacted onto the GMBS immobilized glass surface by incubation with Neutravidin solution for at least 1 hr in a refrigerator (4°C). Finally, the biotinylated anti-gp120 antibody solution was injected into the channel and allowed to react for 30 min at 4°C. After each surface modification step, the channel surface was cleaned with ethanol or PBS, depending on the solvent used in the previous step. The surface-modified channel maintained the functionality for one month in a refrigerator (4 °C) with parafilm sealing (Moon et al. 2009).
2.5 HIV capture
Unprocessed three discarded HIV-infected patient whole blood samples containing undetermined virus copy numbers and two HIV-negative healthy blood samples were obtained from Brigham Women's Hospital (BWH), Harvard Medical School (HMS). These blood specimens are discarded sample under approved institutional review board (IRB) protocols. To prevent coagulation, the blood is collected in tubes containing EDTA, Citrate of heparin. After BSA blocking (1% (w/v) in PBS, 30 min at room temperature), the sample (10 μl) was directly introduced into the fabricated microfluidic channel that is coated with the anti-gp120 antibody. The whole blood was injected for 30 sec using an electronic auto-pipette (Eppendorf, Hamburg, Germany) with 0.5 μl/sec and incubated for 2 min at room temperature. The sample volume, 10 μl, was determined to fully cover the entire channel floor that has 5 μl of microfluidic chamber volume. In addition, there was no bubble or clotting formation during the blood injection into the microfluidic device. Immediately after sample injection, PBS was flowed through to wash the microfluidic chamber.
2.6 Fluorescence and SEM imaging
For fluorescence imaging, the captured HIVs were initially recognized by biotinylated anti-gp120 antibody (2 min at room temperature) followed by Qdot525 streptavidin conjugate (Qdot525/anti-gp120 antibody, 1 min at room temperature). Afterward, they were reconfirmed using biotinylated ConA lectin (2 min at room temperature) followed by Qdot655 streptavidin conjugate (Qdot655/ConA lectin, 1 min at room temperature). After washing the channel with PBS to remove excess proteins and Qdots solution, captured viruses were imaged using an inverted microscope (10× and 100× objective lens, Axio Observer D1, Carl Zeiss, Germany; 6.45 μm × 6.45 μm/pixel CCD). Each fluorescence image was obtained by using two color filter cubes: GFP (489 nm/509 nm) for Qdot525, and Cy5 (650 nm/670 nm) for Qdot655 to filter a specific excitation and emission wavelength. The green and red quantum dot images which were obtained at the same position were manually merged using microscope image-processing software (AxioVision 4.7.1, Carl Zeiss, Germany).
For SEM imaging, the captured HIVs were recognized by biotinylated anti-gp120 antibody and following Qdot525 streptavidin conjugate (Qdot525/anti-gp120 antibody). After washing the channel with PBS, the chamber surface was rinsed two times with distilled water to remove salts which can interfere with SEM imaging. The PMMA part was detached from glass slide and dehydrated for 15 min at room temperature. The captured HIVs and Qdots were analyzed on an environmental scanning electron microscopy (XL30 ESEM-FEG, FEI/Phillips).
3. Results and Discussion
3.1 HIV capture and imaging
The basic principle of HIV capture and imaging using dual-stain method via colocalized green/red Qdots is illustrated in Fig. 1b. To capture the HIVs from unprocessed whole blood, we used two key HIV-specific proteins for double staining the viruses: (i) anti-gp120 antibody that binds to the gp120 glycoprotein exposed on the outer surface of HIV envelop and (ii) ConA lectin is highly specific to high-mannose residues which are mainly expressed on the gp120 glycoprotein. The lectin was used to avoid false-positive signal by just using HIV-1 gp120 antibody. HIVs captured from whole blood patient samples were recognized and imaged via anti-gp120 antibody and ConA lectin without being impeded by concern of non-specific bindings.
3.2 Validation of the surface chemistry for HIV capture
To initially validate the surface chemistry of microfluidic device for HIV capture, we used recombinant gp120-FITC conjugate into PBS at different concentrations, i.e. 10, 20, 40, 100 and 200 ng/μl (Fig. 2c), and captured the HIV envelope protein using the developed microfluidic device. According to the concentrations, our device can evidently capture the target proteins and also sensitively recognize them below 20 ng/μl which is recommended as the lowest dilution range. In addition, we evaluated the non-specific binding of HIV imaging molecules, i.e. anti-gp120 antibody, ConA lectin and Qdot streptavidin conjugates, on the microfluidic chamber surface without HIV capture (Fig. S2). After HIV-uninfected whole blood injection, the biotinylated anti-gp120 antibody followed by Qdot525 streptavidin conjugate and biotinylated ConA lectin followed by Qdot655 streptavidin conjugate were subsequently introduced to anti-gp120 antibody-coated microfluidic device surface, and then unbound molecules were washed out by using PBS buffer. In this control experiment, the anti-gp120 antibody and ConA lectin followed with Qdot incubation hardly bound on the surface without the target HIV (The average number of nonspecific Qdot signal was 1.1 ± 0.9 on 1 mm × 1mm.) (Fig. S2). We thus concluded that the anti-gp120 antibody-immobilized microfluidic surface sensitively recognizes HIV gp120 glycoprotein.
3.3 HIV capture from HIV-infected whole blood and dual-stain imaging in a microfluidic device
Using our microfluidic chip, we performed HIV capturing from HIV-infected patient whole blood (10 μl) and imaging with Qdots and HIV-specific proteins. The HIVs captured onto anti-gp120 antibody-coated surface were first recognized by Qdot525/anti-gp120 antibody. To confirm the captured HIV viruses, ConA lectin solution was injected and subsequently Qdot655 streptavidin conjugate was introduced into the microfluidic chamber. In the merged (10× and 100×) microscope images, colocalized signals (yellow) were detected by both green and red probes binding to the HIV gp120 glycoprotein and its high-mannose oligosaccharides, respectively (Fig. 3a-d). The selected areas in the wide-field images are magnified to allow direct visualization of colocalized signals (yellow). Because of high brightness of the Qdots, we could image the dual-stained HIV through low magnification (10×) optical imaging system which increases the fluorescence lifetime. Through the captured whole image analysis, the dualstain efficiency of anti-gp120 antibody and ConA lectin at the captured HIV was observed as 82.4% ± 12.7. In our future work, we will further optimize critical factors such as the minimum time for effective HIV binding to the chip and quantitative HIV capturing efficiency comparing to the gold standard, i.e. real-time polymerase chain reaction, to use in a point-of-care clinical HIV monitoring devices.
Figure 3.

Fluorescent images 10× (a-b) and 100× (c-d) objective from the HIVs using dual-stain imaging techniques, Qdot525/anti-gp120 antibody (green) and Qdot655/ConA (red).
To verify the HIV recognition of Qdots, we applied scanning electron microscope (SEM) to visualize microfluidic chip surfaces and image viruses detected by Qdot525/anti-gp120 antibody at high magnification. We observed the captured HIV having a spherical shape with a diameter of ranging between 100 - 200 nm without any non-specific binding of human blood cells (Fig. 4a). Finally, the scanning electron micrographs of HIV recognized by Qdot525/anti-gp120 antibody revealed efficient binding of Qdot525/anti-gp120 antibody to HIV captured onto the surface (Fig. 4b-e). These results indicate that HIVs were captured from unprocessed HIV-infected patient whole blood onto our microfluidic chip surface and also the captured HIV can be directly imaged by using Qdots on the microfluidic device surface.
Figure 4.
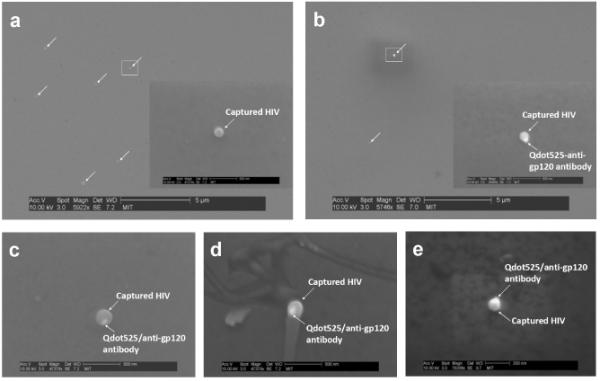
(a) SEM images of the HIV captured onto the chamber surface without Qdot525/anti-gp120 antibody. (b-e) SEM images of the captured HIV with Qdot525/anti-gp120 antibody.
4. Conclusions
In conclusion, we demonstrated an integrated microfluidic platform to rapidly capture and image HIV from a fingerprick volume of unprocessed HIV-infected patient whole blood on a microfluidic chip that achieves sequentially: (i) selective HIV capture using anti-gp120 antibody on a microfluidic chip from whole blood (10 μl) within 10 minutes, (ii) sensitive detection and imaging of the captured HIV particles using colocalized Qdot signals via anti-gp120 antibody and ConA lectin, (iii) reliably countable HIV imaging on a microchip with only standard 10× fluorescence microscope, and (iv) SEM imaging to show directly the specific recognition of Qdots on the captured HIV in a microfluidic chamber. Our ultimate objective is to develop a microfluidic HIV monitoring platform that is rapid (< 10 min), handheld, low-cost and disposable. In the future, we intend to use this device directly with HIV-infected whole blood, and to achieve performance within clinical ±10% error limits compared to the gold standard, i.e. real-time polymerase chain reaction. The presented disposable microfluidic chip potentially paves the way for a new approach to clinical HIV viral load monitoring at the point-of-care.
Supplementary Material
Acknowledgement
The authors acknowledge Center for Integration of Medicine and Innovative Technology (CIMIT), the MIT Deshpande Center, the Wallace H. Coulter Foundation and RO1 (1R01AI081534). We also thank Kimin Jun for his help with SEM imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Zhang C, Byassee T, Tripp RA, Nie S. Anal Chem. 2006;78:1061–1070. doi: 10.1021/ac051801t. [DOI] [PubMed] [Google Scholar]
- Akin M, Li H, Bashir R. Nano Lett. 2004;4:257–259. [Google Scholar]
- Bentzen EL, House F, Utley TJ, Crowe JE, Jr., Wright DW. Nano Lett. 2005;5:591–595. doi: 10.1021/nl048073u. [DOI] [PubMed] [Google Scholar]
- Bruchez M, Jr., Moronne M, Gin P, Weiss S, Alivisatos AP. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- Cambi A, Lidke DS, Arndt-Jovin DJ, Figdor CG, Jovin TM. Nano Lett. 2007;7:970–977. doi: 10.1021/nl0700503. [DOI] [PubMed] [Google Scholar]
- Chan WC, Nie S. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- Cheng X, Irimia D, Dixon M, Sekine K, Demirci U, Zamir L, Tompkins RG, Rodriguez W, Toner M. Lab Chip. 2007a;7:170–178. doi: 10.1039/b612966h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Irimia D, Dixon M, Ziperstein JC, Demirci U, Zamir L, Tompkins RG, Toner M, Rodriguez WR. J Acquir Immune Defic Syndr. 2007b;45:257–261. doi: 10.1097/QAI.0b013e3180500303. [DOI] [PubMed] [Google Scholar]
- Cohen J. Science. 2007;318:1360–1361. doi: 10.1126/science.318.5855.1360. [DOI] [PubMed] [Google Scholar]
- Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- Gao X, Cui Y, Levenson RM, Chung LW, Nie S. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. Curr Opin Biotechnol. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Geyer H, Holschbach C, Hunsmann G, Schneider J. J Biol Chem. 1988;263:11760–11767. [PubMed] [Google Scholar]
- Hammer SM, Eron JJ, Jr., Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- HIV/AIDS, J.U.N.P. 2008 Report on the global AIDS epidemic. 2008 [Google Scholar]
- Joo KI, Lei Y, Lee CL, Lo J, Xie J, Hamm-Alvarez SF, Wang P. ACS Nano. 2008;2:1553–1562. doi: 10.1021/nn8002136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levsky JM, Singer RH. J Cell Sci. 2003;116:2833–2838. doi: 10.1242/jcs.00633. [DOI] [PubMed] [Google Scholar]
- Liu WT, Zhu L, Qin QW, Zhang Q, Feng H, Ang S. Lab Chip. 2005;5:1327–1330. doi: 10.1039/b509086e. [DOI] [PubMed] [Google Scholar]
- Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Nat Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuochi T, Matthews TJ, Kato M, Hamako J, Titani K, Solomon J, Feizi T. J Biol Chem. 1990;265:8519–8524. [PubMed] [Google Scholar]
- Moon S, Keles HO, Ozcan A, Khademhosseini A, Hæggstrom E, Kuritzkes D, Demirci U. Biosens Bioelectron. 2009 doi: 10.1016/j.bios.2009.03.037. In Press, doi:10.1016/j.bios.2009.1003.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan A, Demirci U. Lab Chip. 2008;8:98–106. doi: 10.1039/b713695a. [DOI] [PubMed] [Google Scholar]
- Pellegrino T, Parak WJ, Boudreau R, Le Gros MA, Gerion D, Alivisatos AP, Larabell CA. Differentiation. 2003;71:542–548. doi: 10.1111/j.1432-0436.2003.07109006.x. [DOI] [PubMed] [Google Scholar]
- Raju TS, Briggs JB, Borge SM, Jones AJ. Glycobiology. 2000;10:477–486. doi: 10.1093/glycob/10.5.477. [DOI] [PubMed] [Google Scholar]
- Simon V, Ho DD. Nat Rev Microbiol. 2003;1:181–190. doi: 10.1038/nrmicro772. [DOI] [PubMed] [Google Scholar]
- Smith AM, Ruan G, Rhyner MN, Nie S. Ann Biomed Eng. 2006;34:3–14. doi: 10.1007/s10439-005-9000-9. [DOI] [PubMed] [Google Scholar]
- Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- Zhu P, Liu J, Bess J, Jr., Chertova E, Lifson JD, Grise H, Ofek GA, Taylor KA, Roux KH. Nature. 2006;441:847–852. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


