Abstract
Transcutaneous electrical nerve stimulation (TENS) is a form of non-pharmacological treatment for pain. Involvement of descending inhibitory systems is implicated in TENS-induced analgesia. In the present study, the roles of spinal 5-HT and α2-adrenoceptors in TENS analgesia were investigated in rats. Hyperalgesia was induced by inflaming the knee joint with 3% kaolin—carrageenan mixture and assessed by measuring paw withdrawal latency (PWL) to heat before and 4 h after injection. The (1) α2-adrenergic antagonist yohimbine (30 μg), (2) 5-HT antagonist methysergide (5-HT1 and 5-HT2,30 μg), one of the 5-HT receptor subtype antagonists, (3) NAN-190 (5-HT1A, 15 μg), (4) ketanserin (5-HT2A, 30 μg), (5) MDL-72222 (5-HT3, 12 μg), or (6) vehicle was administered intrathecally prior to TENS treatment. Low (4 Hz) or high (100 Hz) frequency TENS at sensory intensity was then applied to the inflamed knee for 20 min and PWL was determined. Selectivity of the antagonists used was confirmed using respective agonists administered intrathecally. Yohimbine had no effect on the antihyperalgesia produced by low or high frequency TENS. Methysergide and MDL-72222 prevented the antihyperalgesia produced by low, but not high, frequency TENS. Ketanserin attenuated the antihyperalgesic effects of low frequency TENS whereas NAN-190 had no effect. The results from the present study show that spinal 5-HT receptors mediate low, but not high, frequency TENS-induced antihyperalgesia through activation of 5-HT2A and 5-HT3 receptors in rats. Furthermore, spinal noradrenergic receptors are not involved in either low or high frequency TENS antihyperalgesia.
Keywords: Inflammation, Serotonin, Adrenergic, Pain, Carrageenan, Spinal cord
1. Introduction
Transcutaneous electrical nerve stimulation (TENS) is a form of non-pharmacological therapy, clinically used to relieve acute and chronic pain (Johnson et al., 1992; Chesterton et al., 2002). This treatment modality is easy to use and devoid of major side effects. Although the use of TENS is very common, its analgesic mechanism is not fully understood. Conclusions regarding the efficacy of TENS from systematic reviews have been contradictory (for review, see Sluka and Walsh, 2003). Cochrane database reviews conclude that TENS has significant analgesic effects above placebo in people with osteoarthritis (Osiri et al., 2000) but not for people with low back pain (Milne et al., 2001). Carroll et al. (2001) state that the results of a systematic review on TENS efficacy are inconclusive for people with chronic pain. Lack of randomized, controlled, blinded clinical trials are seen as the major reason for contradictory and inconclusive findings. Two types of TENS are used clinically, low frequency TENS (frequency of stimulation < 10 Hz) and high frequency TENS (frequency > 50 Hz). Different theories have been proposed for the mechanism of action of TENS, the popular one being the gate control theory proposed by Melzack and Wall (1965). According to the gate control theory, nociceptive information from small diameter afferents is overridden by stimulation of large diameter fibres and the pain stimulus is prevented from reaching supraspinal centers. Endogenous opioids released in the central nervous system are also implicated in the analgesic mechanism of TENS by various investigators (Woolf et al., 1977; Sjölund et al., 1977; Shimizu et al., 1981; Hughes et al., 1984; Sluka et al., 1999; Kalra et al., 2001). Data from our laboratory show that low frequency TENS produces antihyperalgeisa by activating central μ-opioid receptors while high frequency TENS activates δ-opioid receptors both spinally and supraspinally in the rostral ventromedial medulla (RVM) (Sluka et al., 1999; Kalra et al., 2001).
The antihyperalgesic effect of TENS predominantly involves central (spinal and supraspinal) mechanisms (Sluka et al., 1999; Kalra et al., 2001) rather than peripheral mechanisms (Janko and Trontelj, 1980). Descending inhibition from the periaqueductal gray (PAG) and RVM is mediated by serotoninergic and noradrenergic receptors spinally (Aimone et al., 1987; Fields and Basbaum, 1999) and the effector neurotransmitters are serotonin and norepinephrine. Norepinephrine is antinociceptive in the spinal dorsal horn and produces analgesic effects mainly through activating α2-adrenoceptors (Reddy et al., 1980; for review, see Yaksh, 1985). Although α1-adrenergic receptors are found in the spinal cord, they may not be involved in pain inhibition (Miller and Proudfit, 1990). Serotonin also has antinociceptive effects spinally, depending on the receptor type activated and dose used (Yaksh and Wilson, 1979; Schmauss et al., 1983). Seven types of serotonin receptors have been identified (5-HT1–7) with a total of 14 subtypes. Serotonin receptor types commonly implicated in spinal pain processing are 5-HT1, 5-HT2 or 5-HT3 (Danzebrink and Gebhart, 1991a; Giordano, 1991; Zhuo and Gebhart, 1991; Eide and Hole, 1993). Spinal blockade of serotonin receptors with methysergide reduces low frequency (20 Hz) peripheral electrical stimulation (PES) analgesia and systemic depletion of serotonin with p-chlorophenylalanine (PCPA) reduces high frequency PES analgesia (Woolf et al., 1980; Chen, 1992; Scherder and Bouma, 1993; Liss and Liss, 1996).
Since serotonin and norepinephrine receptors in spinal cord mediate descending inhibition of pain from supraspinal structures, we investigated the roles of spinal serotoninergic and noradrenergic receptors in the antihyperalgesic mechanism of TENS. Preliminary results from this study were presented in abstract form (Radhakrishnan et al., 2002).
2. Materials and methods
2.1. Animals
Male Sprague—Dawley rats (n = 171, 225–300 g, Harlan, St Louis, MO, USA) were housed in a 12 h dark—light cycle with free access to food and water. All experiments were approved by University of Iowa Animal Care and Use Committee and were carried out according to the guidelines of the International Association for the Study of Pain and National Institute of Health.
2.2. Behavior testing
Animals were acclimated overnight in the behavioral testing room and all behavioral tests were done between 9 a.m. and 5 p.m. Prior to testing, rats were placed in plexi-glass restrainers on an elevated glass platform for at least 30 min for acclimatization. A radiant heat source, connected to a built-in timer, was shone on the plantar skin of the hind limb. When the animal withdrew the paw abruptly to heat stimulus, the heat source and timer were stopped. The duration in seconds from the start of heat application to the paw withdrawal was taken as the paw withdrawal latency (PWL). PWLs were determined five times bilaterally, with an interval of 5 min between each test, and the mean of five readings was taken as the PWL for each time. The intensity of the heat source was set at optimum level with an adjustable voltage power supply, to obtain a baseline response time between 12 and 16 s. Cut-off time was set to 30 s to minimize heat damage to the skin.
2.3. Intrathecal catheter placement
A 32 G polyethylene catheter was placed intrathecally as described before (Pogatzki et al., 2000). Briefly, animals were anesthetized with 2% halothane and the dorsal surface shaved and cleaned with Betadine® solution. A 2 cm incision was made at the iliac crest. A 32 G polyethylene catheter was introduced into the lumbar space between L4 and L5 with the help of a 23 G guide needle and advanced to a length of 3.5–4 cm rostrally. The catheter was fixed in place and the tip connected to a saline filled PE10 tube, which was externalized dorsally between the scapulas. The tip of the catheter was sealed and the animal was allowed to recover for 5–7 days.
2.4. Intra-articular injection
After baseline PWL measurements, the left knee joint was injected with 0.1 ml suspension of 3% kaolin and 3% carrageenan in normal saline (pH 7.0) while the rat was anesthetized with halothane (2–4% in oxygen).
2.5. Drugs
Clonidine hydrochloride (α2-adrenoceptor agonist, 5 μg, Danzebrink and Gebhart, 1991b), serotonin hydrochloride (30 μg, Bardin et al., 1997), (±)-8-hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT, 5-HT1 agonist, 50 μg, Obata et al., 2001), (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI, 5-HT2 agonist, 30 μg, Sasaki et al., 2001), yohimbine hydrochloride (α2-adrenoceptor antagonist, 30 μg; Solomon et al., 1989), methysergide maleate (5-HT1 and 5-HT2 receptor antagonist, 30 μg; Hammond et al., 1998) and ketanserin tartrate (5-HT2A receptor antagonist, 30 μg; Sasaki et al., 2001) were dissolved in normal saline (pH = 7.0). 2-Methyl-serotonin maleate (5-HT agonist, 50 μg, Giordano, 1991), 1-(2-methoxyphenyl)-4-(4-[2-phthalimido]butyl)piperazine hydrobromide (NAN-190, 5-HT1A receptor antagonist, 15 μg; Mjellem et al., 1993) and 3-tropanyl-3,5-dichlorobenzoate (MDL-72222, 5-HT3 receptor antagonist, 12 μg; Sasaki et al., 2001) were dissolved in 20% dimethyl sulfoxide (DMSO). 8-OH-DPAT was obtained from Tocris (Ellisville, MO, USA) and others from Sigma Chemical Co. (St Louis, MO, USA). The doses of antagonists utilized were selected based on previously published studies that show selectivity for the individual receptor types/subtypes and confirmed in the current study against their agonists.
2.6. Intrathecal drug administration
Drugs or vehicles were injected into the intrathecal (i.t.) space in a volume of 5 or 10 μl, using a Hamilton syringe connected to the i.t. catheter via a PE10 tubing followed by 10 μl vehicle to flush the catheter. After the experiment, proper placement of the catheter was confirmed by injection of 10 μl of 2% lidocaine i.t. and observing animals for hind limb paralysis. Methylene blue dye solution (10 μl., i.t.) was then injected. Animals were sacrificed, spinal cord dissected and the dye spread assessed. The data from those animals, which did not show hind limb paralysis with lidocaine and in which the dye was not found in L4—L6 levels of spinal cord, were not included in the analysis.
2.7. TENS application
Commercially available TENS units and electrodes (EMPI Inc., Minnesota, USA) were used in this study. After drug or vehicle was administered intrathecally, the inflamed hind limb of the rat was shaved and cleaned with alcohol. Two circular, pregelled electrodes of 2.5 cm diameter were placed, one to the medial and the other to the lateral aspect, on the inflamed knee joint. This electrode placement is similar to placement of larger electrodes on the knee joint of human subjects. Either high frequency (100 Hz) or low frequency (4 Hz) TENS, at sensory intensity, was then applied through the electrodes for 20 min under light halothane anesthesia (1–2% in oxygen). Sensory intensity was defined as just below threshold for motor contraction. The pulse duration was kept constant at 100 μs. A complete reversal of kaolin—carrageenan-induced hyperalgesia occurs with this protocol by both low and high frequency TENS (Sluka et al., 1999). In this study, the effects of various antagonists were studied only on different frequencies of TENS and not different intensities or pulse durations, since a previous study shows that TENS effects depend on modulation of frequency, but not on intensity or pulse duration (Gopalkrishnan and Sluka, 2000).
2.8. Protocol
Five to 7 days after intrathecal catheter placement, baseline PWLs to heat for both hind paws were determined and knee joint inflammation was induced by intra-articular injection of kaolin—carrageenan unilaterally. Four hours after injection, PWLs were determined again. Animals were then intrathecally injected with the one of the following drugs: yohimbine (30 μg; no TENS, n = 5; low frequency TENS, n = 5; high frequency TENS, n = 5), methysergide (30 μg, no TENS, n = 6; low frequency = TENS, n = 11; high frequency TENS, n = 12), NAN-190 (15 μg, no TENS, n = 7; low frequency TENS, n = 8), ketanserin (30 μg, no TENS, n = 7; low frequency TENS, n = 8), MDL-72222 (12 μg; no TENS, n = 8; low frequency TENS, n = 8) or the vehicles, normal saline (no TENS, n = 8; low frequency TENS, n = 8; high frequency TENS, n = 8) or 20% DMSO (no TENS, n = 7; low frequency TENS, n = 8). After 15 min, either high or low frequency TENS was applied to the ipsilateral knee. PWLs were determined again, 30 min after TENS/no TENS treatment was completed, when the animals were fully awake from halothane anesthesia. Another group of animals did not receive TENS, ‘no TENS’ (control) group, were also anesthetized with halothane. Prior experiments show that shaving and placement of electrodes to the knee joint does not reduce hyperalgesia (Sluka, 2000). Since methysergide, a non-selective antagonist at 5-HT receptors, had effects only on low frequency, but not on high frequency TENS antihyperalgesia, selective 5-HT antagonists were studied for effects on low frequency TENS antihyperalgesia. Since methysergide does not block 5-HT3 receptors, MDL-72222 was tested in an additional group (n = 4) that received high frequency TENS.
The selectivity of various agonists against their respective antagonists was studied in separate groups of animals. All drugs were tested in animals with knee joint inflammation at the same 4 h time point that TENS was applied in the previous experiment. Specifically (1) yohimbine was tested for its ability to antagonize the effects of clonidine (yohimbine + clonidine, n = 4; vehicle + clonidine, n = 3); (2) methysergide was tested against serotonin (methysergide + serotonin, n = 4; vehicle + serotonin, n = 3); (3) NAN-190 was tested against 8-OH-DPAT (NAN-190 + 8-OH-DPAT, n = 4; vehicle + 8-OH-DPAT, n = 4); (4) ketanserin was tested against DOI (ketanserin + DOI, n = 4; vehicle + DOI, n = 4); and (5) MDL-72222 was tested against 2-methyl-5-HT (MDL-72222 + 2-methyl-5-HT, n = 4; vehicle + 2-methyl-5-HT, n = 4). The antagonist was given 15 min prior to agonist, as done in the TENS experiment. All groups were compared against a control group that received saline or 20% DMSO (instead of antagonist) 15 min prior to the agonist.
2.9. Statistical analysis
All data are presented as mean ± SEM. Data were compared with a multivariate analysis of variance for TENS treatment (no TENS, high frequency TENS, low frequency TENS) and drug treatment (two vehicles, five drugs) for time (baseline, hour 4, and post TENS). Since only post TENS showed significant effects for TENS treatment, drug and the interaction of TENS treatment with drug, Tukey’s post hoc test compared differences between the appropriate vehicle control group treated with TENS and the drug group treated with TENS. Paired t-tests compared differences between baseline and hour 4 to establish hyperalgesia. The level of significance was set at p < 0.05. Statistical analysis was performed with SPSS versions 10.1.
3. Results
3.1. Carrageenan—kaolin-induced hyperalgesia and effect of TENS
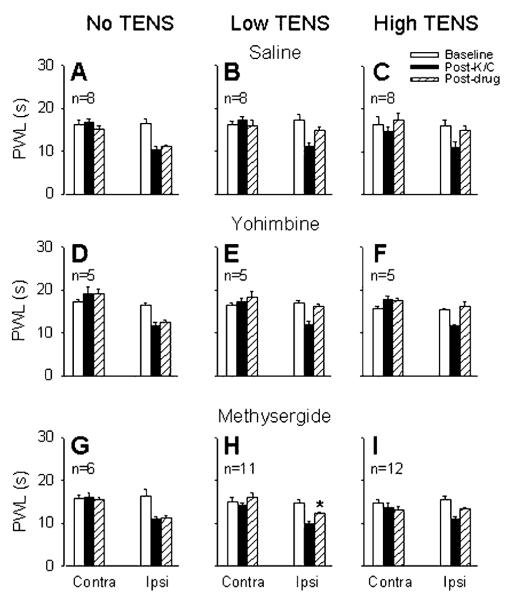
Four hours after injection of kaolin and carrageenan into the knee joint of rats, there was a significant reduction in the ipsilateral PWL when compared to baseline (p = 0.0001). There was an overall significant effect for PWL following TENS treatment (F1,128 = 53.6, p = 0.0001) such that both high (p = 0.0001) or low frequency (p = 0.0001) TENS significantly reverse their hyperalgesia (Fig. 1). There were no significant differences between groups for the PWL ipsilaterally before or after induction of inflammation, or for the contralateral side. In saline controls, the ipsilateral mean PWLs (s) were: baseline = 17.2 ± 1.3, 4 h after carrageenan = 11.1 ± 0.78, after low frequency TENS = 14.8 ± 0.87, after high frequency TENS = 14.98 ± 0.96; and contralateral mean PWLs (s) were: baseline = 16.24 ± 0.75, 4 h after carrageenan = 17.3 ± 0.91, after low frequency TENS = 15.96 ± 1.41, after high frequency TENS = 17.3 ± 1.48.
Fig. 1.
Bar graphs show the PWLs before (open bars), after joint inflammation (solid bars) and after treatment with drug and TENS/no TENS (hatched bars) for the ipsilateral and contralateral paws. Effects of intrathecal yohimbine and methysergide on carrageenan-induced hyper-algesia compared to saline control are shown in the graphs on the left panel (no TENS; graphs A, D, G). Effects of intrathecal saline, yohimbine or methysergide on low frequency TENS antihyperalgesia are shown in the middle panel (low TENS; graphs B, E, H), and effects of saline, yohimbine (30 μg) or methysergide (30 μg) on high frequency TENS are shown in the right panel (high TENS; graphs C, F, I). *, Significantly different from vehicle control group and also from its own baseline. K/C, kaolin and carrageenan; Contra, contralateral; Ipsi, ipsilateral.
There was a significant effect for drug (F6,128 = 19.1, p = 0.001) such that saline was significantly different from methysergide (p = 0.001) and ketanserin (p = 0.006), and DMSO was significantly different from MDL-72222 (p = 0.0001). Importantly, there was an interaction between drug and TENS treatment (F8,128 = 5:4, p = 0.0001). Post hoc results are listed below in appropriate sections.
3.2. Effect of α2-adrenoceptor antagonist
Intrathecal (i.t.) injection of the α2-adrenoceptor antagonist yohimbine (30 μg) had no effect on the reversal of the ipsilateral PWL produced by low or high frequency TENS when compared to vehicle controls (i.t. saline). Yohimbine alone had no effects on PWL when given intrathecally at the same dose in animals with knee joint inflammation (Fig. 1).
3.3. Effect of non-selective 5-HT antagonist
Methysergide (30 μg, i.t.), a non-selective antagonist at 5-HT receptors acting on 5-HT1 and 5-HT2, significantly inhibited the reversal of the ipsilateral PWL produced by low frequency TENS (p = 0.04), but not that produced by high frequency TENS, when compared to vehicle controls (i.t. saline, Fig. 1). The same dose of intrathecal methysergide alone had no effect on PWL in animals with knee joint inflammation (Fig. 1). Since methysergide blocked only low frequency TENS-induced antihyperalgesia, the effects of selective 5-HT receptor antagonists were tested on the antihyperalgesia produced by low frequency TENS.
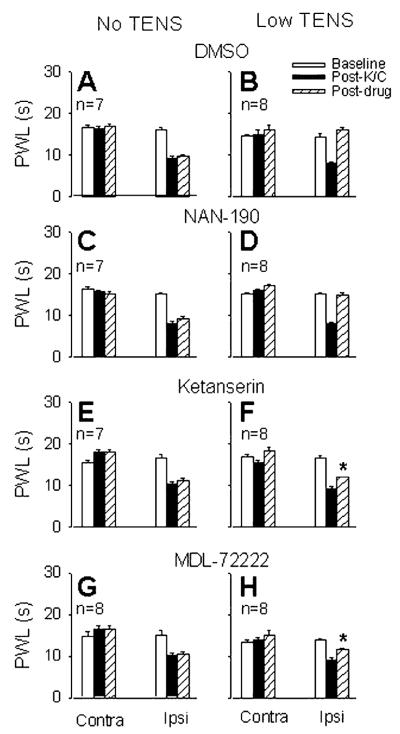
3.4. Effects of selective 5-HT antagonists
The selective antagonist of the 5-HT1A receptor subtype, NAN-190 (15 μg, i.t.), had no effect on the antihyperalgesia produced by low frequency TENS when compared to vehicle control animals (i.t. 20% DMSO, Fig. 2). The same dose of NAN-190 had no effect on the decreased PWL produced by knee joint inflammation (Fig. 2).
Fig. 2.
Bar graphs show the PWLs before (open bars), after joint inflammation (solid bars) and after treatment with drug and TENS/no TENS (hatched bars) for the ipsilateral and contralateral paws. Effects of intrathecal 20% DMSO, NAN-190 (15 μg, 5-HT1A antagonist), ketanserin (30 μg, 5-HT2 antagonist) and MDL-72222 (12 μg, 5-HT3 antagonist) on the carrageenan-induced decrease in PWL are shown in the left panels (no TENS; graphs A, C, E, G). Effects of intrathecal 20% DMSO, NAN-190, ketanserin and MDL-72222, on low frequency TENS antihyperalgesia are shown in the right panels (low TENS; graphs B, D, F, H). *, Significantly different from vehicle control group and also from their own baseline. K/C, kaolin and carrageenan; Contra, contralateral; Ipsi, ipsilateral.
The 5-HT2A subtype selective antagonist, ketanserin (30 μg, i.t.), significantly attenuated the antihyperalgesia produced by low frequency TENS, compared to vehicle control (i.t. saline, p = 0.03). The same dose of ketanserin had no effect on the reduction in PWL produced by knee joint inflammation (Fig. 2).
The 5-HT3 receptor selective antagonist, MDL-72222 (12 μg, i.t.), significantly attenuated the antihyperalgesia produced by low frequency TENS, compared to vehicle control (i.t. 20% DMSO, p = 0.0001). The same dose of MDL-72222 had no effect on the decreased PWL produced by knee joint inflammation (Fig. 2). MDL-72222 had no effect on antihyperalgesia produced by high frequency TENS (16.32 ± 0.96 s) when compared to vehicle controls (15.85 ± 0.83 s).
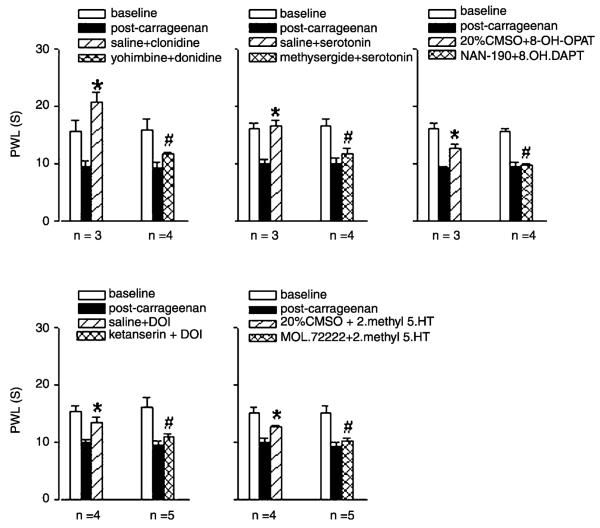
3.5. Selectivity of antagonists
Intrathecal administration of all adrenergic and 5-HT agonists studied, significantly reversed the decreased PWL to heat produced kaolin—carrageenan injection. The reversal was blocked by intrathecal pre-administration of selective antagonists used in this study. Pre-treatment with (1) the α2-antagonist yohimbine (30 μg, p = 0.0008), or (2) the non-selective serotonin antagonist methysergide (30 μg, p = 0.004), significantly prevented the antihyperalgesia produced by clonidine (p = 0.02) or serotonin (p = 0.03), respectively (Fig. 3). Similarly, in kaolin—carrageenan inflamed rats, antihyperalgesia produced by intrathecal administration of selective serotonin receptor agonists to 5-HT1A (8-OH-DPAT, 50 μg, p = 0.008), 5-HT2A (DOI, 30 μg, p = 0.03) and 5-HT3 (2-methyl 5-HT, 50 μg, p = 0.02), administered intrathecally, were blocked by their selective antagonists, NAN-190 (15 μg, p = 0.002), ketanserin (30 μg, p = 0.02), and MDL-72222 (12 μg, p = 0.0002), respectively (Fig. 3).
Fig. 3.
The left cluster of bars in each graph shows ipsilateral PWLs before (open bar), 4 h after injection of kaolin—carrageenan injection into the knee joint (solid bar), and after intrathecal vehicle + agonists (hatched bar). Right cluster shows ipsilateral PWLs before (open), 4 h after kaolin—carrageenan injection (solid), and after intrathecal antagonist + agonist (hatched). *, PWLs were significantly increased compared to PWLs after kaolin—carrageenan. #, PWLs for antagonist + agonist were significantly less than PWLs from vehicle + agonist.
4. Discussion
The data from the present study show that spinal serotonin (5-HT) receptors mediate, at least in part, the antihyperalgesia produced by low frequency TENS in rats, since the non-selective 5-HT antagonist methysergide attenuated the low frequency TENS-induced hyper-algesia when administered intrathecally. Further, ketanserin and MDL-72222, antagonists at 5-HT2A and 5-HT3 receptor subtypes, respectively, also attenuated the effects of low frequency TENS, indicating involvement of these receptor subtypes in low frequency TENS antihyperalgesia. NAN-190 had no effect on low frequency TENS antihyperalgesia indicating that 5-HT1A receptors are not involved. The results also show that antihyperalgesia produced by high frequency TENS does not depend on 5-HT mediated mechanisms in the spinal cord. Another major finding from this study is that the spinal α2-adrenergic receptors are not involved in the antihyperalgesia produced by either low or high frequency TENS.
4.1. Descending inhibition and TENS effects
Descending inhibitory systems, originating in the midbrain and terminating in the spinal dorsal horn, consists of two major pathways—noradrenergic and serotoninergic. Electrical or chemical stimulation of PAG induces spinal nociception by the activation of descending adrenergic and serotonergic pathways that arise from pontine noradrenergic nuclei and RVM, respectively (Odeh and Antal, 2001). Opioid, glutamate (Jensen and Yaksh, 1989; Fields and Basbaum, 1999) and possibly serotonin (Fields and Basbaum, 1999; Kishimoto et al., 2001) receptors in PAG or RVM are believed to initiate descending inhibition in the midbrain or brainstem. Both norepinephrine (Reddy et al., 1980; for review, see Yaksh, 1985; Aran and Proudfit, 1990; Fairbanks et al., 2002) and serotonin (Yaksh and Wilson, 1979; Schmauss et al., 1983; Peng et al., 1996; Paul et al., 2001) are antinociceptive in the spinal cord. Descending serotoninergic pathways are implicated in the analgesia produced by peripheral stimulation in the current study and in previous investigations (Chen-yu et al., 1979; Woolf et al., 1980; Shimizu et al., 1981; Chen, 1992; Scherder and Bouma, 1993). Shimizu et al. (1981) showed that PES increased the turnover rate of 5-HT in the cortex and brainstem, systemic administration of a 5-HT precursor, L-5-hydroxytryptophan, enhanced the antinociception produced by peripheral stimulation and spinal methysergide abolished peripheral stimulation-induced antinociception. Serotonin concentrations increase in brain homogenate after peripheral stimulation (Wang et al., 1985) and depletion of serotonin systemically prevents peripheral stimulation analgesia (Woolf et al., 1980). Furthermore, μ-opioid antagonists injected into RVM block the effects of TENS in rats (Kalra et al., 2001). Results from the current study extend previous studies showing that spinal 5-HT2A and 5-HT3 receptor subtypes are involved in the low, but not high, frequency TENS-induced antihyperalgesia. Taken together, these findings strongly suggest a pivotal role for central serotonergic system in mediating antinociception produced by low frequency PES.
4.2. Lack of effect of yohimbine
Spinal antinociceptive effect of noradrenaline is mainly mediated through α2-adrenoceptors. Selective α2-adrenoceptor agonists are antinociceptive when administered intrathecally and selective antagonists of α2-adrenergic receptors block these antinociceptive effects (Howe et al., 1983; Fairbanks and Wilcox, 1999). In the current study, the α2-adrenoceptor agonist clonidine reversed the hyperalgesia produced by knee joint inflammation. This reversal was prevented by prior treatment with 30 μg yohimbine showing that this dose blocks antihyperalgesia produced by activation of α2-adrenoceptors. Since intrathecal yohimbine did not affect the antihyperalgesic effects of low or high frequency TENS in the current study, we conclude that the analgesic mechanism of TENS does not involve spinal α2-adrenoceptor activation.
4.3. Spinal serotonin receptor subtypes
In the present study, methysergide attenuated the effects of low frequency TENS but did not affect high frequency TENS antihyperalgesia. Methysergide is a non-selective antagonist at 5-HT receptors acting on 5-HT1 and 5-HT2 families. Since low frequency TENS antihyperalgesia was attenuated by methysergide, we conclude that serotonin released in response to low frequency TENS in the spinal cord acts on one or more 5-HT receptor subtypes to reduce hyperalgesia. Serotonin has seven types of receptors identified so far (5-HT1–7) and a total of 14 subtypes. All types, except 5-HT3, are G-protein coupled and 5-HT3 is ligand-gated ion channel receptor (Hoyer et al., 1994). 5-HT1, 5-HT2 and 5-HT3 serotonin receptor types are located in the spinal dorsal horn (Hamon et al., 1989; Marlier et al., 1991). The role of spinal serotonin receptor subtypes, especially those in 5-HT1 family, in pain processing is controversial (Obata et al., 2001). 5-HT1 receptors have been shown to be antinociceptive (Eide et al., 1990; Oyama et al., 1996), nociceptive (Solomon and Gebhart, 1988; Alhaider and Wilcox, 1993) or have no role (Bardin et al., 2000; Sasaki et al., 2001) in spinal pain modulation. In our study, although the 5-HT1A antagonist NAN-190 had no effect on low frequency TENS antihyperalgesia, the 5-HT1A agonist 8-OH-DPAT (50 μg) significantly reduced the hyperalgesia induced by injection of kaolin—carrageenan into the knee joint of rats. These data suggest that spinal 5-HT1A receptors are not activated by the physiological levels of serotonin produced by low frequency TENS stimulation, but can be activated by an exogenous agonist at a pharmacological dose.
Our results clearly show the involvement of 5-HT2 and 5-HT3 receptors in low frequency TENS-induced anti-hyperalgesia in rats and are in agreement with other investigators that show spinal antinociception produced by 5-HT agonists is mediated by 5-HT2 (Solomon and Gebhart, 1988; Danzebrink and Gebhart, 1991a; Sasaki et al., 2001; Obata et al., 2001) and 5-HT3 (Danzebrink and Gebhart, 1991a; Bardin et al., 2000; Paul et al., 2001; Peng et al., 1996; Sasaki et al., 2001) receptors. In contrast, a recent study by Zeitz et al. (2002) shows that 5-HT3 receptor knockout and spinal blockade of spinal 5-HT3 receptors reduces nocifensive behaviors associated with the second phase of the formalin test, but not hyperalgesia associated with CFA or partial sciatic nerve lesion. The difference in observation could be due to the different antagonists, different testing methods, different animal models and species used.
4.4. TENS, opioids and serotonin
Endogenous opioids are involved in both low and high frequency TENS analgesia. Low frequency TENS activates μ-opioid receptors and high frequency TENS activates δ-opioid receptors in the spinal cord and rostral ventral medulla (Sluka et al., 1999; Kalra et al., 2001). Similarly, human studies show increased endogenous opioid concentrations in cerebrospinal fluid (CSF) following PES (Hughes et al., 1984; Almay et al., 1985). Further, in rats, repeated application of low or high frequency TENS can lead to development of tolerance of spinal μ- and δ-opioid receptors, respectively (Chandran and Sluka, 2003). Also, in rats which were made tolerant to morphine, low frequency TENS was ineffective (Sluka et al., 2000). Thus, the involvement of endogenous opioids in TENS-induced analgesia is clear. Activation of μ-opioid receptors in the supraspinal structures such as PAG and RVM exerts antinociceptive effects through either serotonin or norepinephrine receptors spinally (Aimone et al., 1987; Fields and Basbaum, 1999). Further, spinal antinociceptive effects of 5-HT are blocked by naloxone, an opioid receptor antagonist (Yang et al., 1994; Goodchild et al., 1997). Conversely, spinal morphine analgesia is blocked by serotonin receptor subtype antagonists (Crisp et al., 1991). In either case, an interaction between opioid receptors and serotonin receptors in the spinal cord plays a significant role in antinociception. Lastly, supraspinal administration of morphine produces increased release of GABA spinally that is blocked by 5-HT3 receptor antagonists (Kawamata et al., 2002) suggesting that activation of supraspinal opioid receptors produces inhibition through spinal serotonin release. Since low frequency TENS-induced analgesia is mediated by both opioid and serotonin receptors the interaction spinally and/or supraspinally between these receptors could be important in TENS analgesia.
4.5. Clinical relevance
This study provides direct evidence for spinal serotonin receptor mediation in low frequency TENS analgesia in rats. As such, there exists a possibility that serotonin agonists, serotonin selective reuptake inhibitors (SSRIs) or monoamine oxidase (MAO) inhibitors could enhance the analgesic effects of low frequency TENS clinically. Also, analgesic effects of low frequency TENS could be attenuated in patients who are under treatment with serotonin antagonists like methysergide, cyproheptadine or granisetron for unrelated conditions.
Acknowledgements
The authors are thankful to Mr Chuck Cibula and Ms Tammy Lisi for their technical assistance and Ms Carol Leigh for secretarial assistance. This work was supported by grants from The Arthritis Foundation and the National Institutes of Health K02 AR02201. TENS units were donated by EMPI, Inc. (Minneapolis, MN).
References
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31:123–36. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Alhaider AA, Wilcox GL. Differential roles of 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptor subtypes in modulating spinal nociceptive transmission in mice. J Pharmacol Exp Ther. 1993;265:378–85. [PubMed] [Google Scholar]
- Almay BG, Johansson F, von Knorring L, Sakurada T, Terenius L. Long-term high frequency transcutaneous electrical nerve stimulation (hi-TNS) in chronic pain. Clinical response and effects on CSF-endorphins, monoamine metabolites, substance P-like immunoreactivity (SPLI) and pain measures. J Psychosom Res. 1985;29:247–57. doi: 10.1016/0022-3999(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Aran S, Proudfit HK. Antinociception produced by interactions between intrathecally administered adenosine agonists and norepinephrine. Brain Res. 1990;513:255–63. doi: 10.1016/0006-8993(90)90464-m. [DOI] [PubMed] [Google Scholar]
- Bardin L, Bardin M, Lavarenne J, Eschalier A. Effect of intrathecal serotonin on nociception in rats: influence of the pain test used. Exp Brain Res. 1997;113:81–7. doi: 10.1007/BF02454144. [DOI] [PubMed] [Google Scholar]
- Bardin L, Lavarenne J, Eschalier A. Serotonin receptor subtypes involved in the spinal antinociceptive effect of 5-HT in rats. Pain. 2000;86:11–18. doi: 10.1016/s0304-3959(99)00307-3. [DOI] [PubMed] [Google Scholar]
- Carroll D, Moore RA, McQuay HJ, Fairman F, Tramer M, Leijon G. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database Syst Rev. 2001;(3):CD003222. doi: 10.1002/14651858.CD003222. [DOI] [PubMed] [Google Scholar]
- Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102:195–201. doi: 10.1016/s0304-3959(02)00381-0. [DOI] [PubMed] [Google Scholar]
- Chen A. An introduction to sequential electric acupuncture (SEA) in the treatment of stress related physical and mental disorders. Acupunct Electrother Res. 1992;17:273–83. doi: 10.3727/036012992816357675. [DOI] [PubMed] [Google Scholar]
- Chen-yu C, Huan-chi T, Yen-fang C, Yao-hui P, Hsi-ken K, Jui-kang C, Hong-ying S, Fu-yao Y. Effects of electrolytic lesions or intracerebral injections of 5,6-dihydroxytryptamine in raphe nuclei on acupuncture analgesia in rats. Chin Med J (Engl) 1979;92:129–36. [PubMed] [Google Scholar]
- Chesterton LS, Barlas P, Foster NE, Lundeberg T, Wright CC, Baxter GD. Sensory stimulation (TENS): effects of parameter manipulation on mechanical pain thresholds in healthy human subjects. Pain. 2002;99:253–62. doi: 10.1016/s0304-3959(02)00118-5. [DOI] [PubMed] [Google Scholar]
- Crisp T, Stafinsky JL, Uram M, Perni VC, Weaver MF, Spanos LJ. Serotonin contributes to the spinal antinociceptive effects of morphine. Pharmacol Biochem Behav. 1991;39:591–5. doi: 10.1016/0091-3057(91)90133-m. [DOI] [PubMed] [Google Scholar]
- Danzebrink RM, Gebhart GF. Evidence that spinal 5-HT1, 5-HT2 and 5-HT3 receptor subtypes modulate responses to noxious colorectal distension in the rat. Brain Res. 1991a;538:64–75. doi: 10.1016/0006-8993(91)90377-8. [DOI] [PubMed] [Google Scholar]
- Danzebrink RM, Gebhart GF. Intrathecal coadministration of clonidine with serotonin receptor agonists produces supra-additive visceral antinociception in the rat. Brain Res. 1991b;555:35–42. doi: 10.1016/0006-8993(91)90856-q. [DOI] [PubMed] [Google Scholar]
- Eide PK, Hole K. The role of 5-hydroxytryptamine (5-HT) receptor subtypes and plasticity in the 5-HT systems in the regulation of nociceptive sensitivity. Cephalalgia. 1993;13:75–85. doi: 10.1046/j.1468-2982.1993.1302075.x. [DOI] [PubMed] [Google Scholar]
- Eide PK, Joly NM, Hole K. The role of spinal cord 5-HT1A and 5-HT1B receptors in the modulation of a spinal nociceptive reflex. Brain Res. 1990;536:195–200. doi: 10.1016/0006-8993(90)90025-7. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Stone LS, Kitto KF, Nguyen HO, Posthumus IJ, Wilcox GL. Alpha(2C)-adrenergic receptors mediate spinal analgesia and adrenergic—opioid synergy. J Pharmacol Exp Ther. 2002;300:282–90. doi: 10.1124/jpet.300.1.282. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Wilcox GL. Moxonidine, a selective alpha2-adrenergic and imidazoline receptor agonist, produces spinal antinociception in mice. J Pharmacol Exp Ther. 1999;290:403–12. [PubMed] [Google Scholar]
- Fields HL, Basbaum AI. Endogenous pain control systems: brainstem and spinal pathways and endorphin circuitry. In: Melzack R, Wall PD, editors. Textbook of pain. Churchill Livingstone; London: 1999. pp. 308–38. [Google Scholar]
- Giordano J. Analgesic profile of centrally administered 2-methylserotonin against acute pain in rats. Eur J Pharmacol. 1991;199:233–6. doi: 10.1016/0014-2999(91)90462-y. [DOI] [PubMed] [Google Scholar]
- Goodchild CS, Guo Z, Freeman J, Gent JP. 5-HT spinal antinociception involves mu opioid receptors: cross tolerance and antagonist studies. Br J Anaesth. 1997;78:563–9. doi: 10.1093/bja/78.5.563. [DOI] [PubMed] [Google Scholar]
- Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil. 2000;81:984–90. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Nelson V, Thomas DA. Intrathecal methysergide antagonizes the antinociception, but not the hyperalgesia produced by microinjection of baclofen in the ventromedial medulla of the rat. Neurosci Lett. 1998;244:93–6. doi: 10.1016/s0304-3940(98)00142-6. [DOI] [PubMed] [Google Scholar]
- Hamon M, Gallissot MC, Menard F, Gozlan H, Bourgoin S, Verge D. 5-HT3 receptor binding sites are on capsaicin-sensitive fibres in the rat spinal cord. Eur J Pharmacol. 1989;164:315–22. doi: 10.1016/0014-2999(89)90472-x. [DOI] [PubMed] [Google Scholar]
- Howe JR, Wang JY, Yaksh TL. Selective antagonism of the antinociceptive effect of intrathecally applied alpha adrenergic agonists by intrathecal prazosin and intrathecal yohimbine. J Pharmacol Exp Ther. 1983;224:552–8. [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hughes GS, Jr, Lichstein PR, Whitlock D, Harker C. Response of plasma beta-endorphins to transcutaneous electrical nerve stimulation in healthy subjects. Phys Ther. 1984;164:1062–6. doi: 10.1093/ptj/64.7.1062. [DOI] [PubMed] [Google Scholar]
- Janko M, Trontelj JV. Transcutaneous electrical nerve stimulation: a microneurographic and perceptual study. Pain. 1980;9:219–30. doi: 10.1016/0304-3959(80)90009-3. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of the antinociceptive effect of morphine and glutamate at coincidental sites in the periaqueductal gray and medial medulla in rats. Brain Res. 1989;476:1–9. doi: 10.1016/0006-8993(89)91529-1. [DOI] [PubMed] [Google Scholar]
- Johnson MI, Ashton CH, Thompson JW. Long term use of transcutaneous electrical nerve stimulation at Newcastle Pain Relief Clinic. J R Soc Med. 1992;85:267–8. doi: 10.1177/014107689208500508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298:257–63. [PubMed] [Google Scholar]
- Kawamata T, Omote K, Toriyabe M, Kawamata M, Namiki A. Intracerebroventricular morphine produces antinociception by evoking gamma-aminobutyric acid release through activation of 5-hydroxytryptamine 3 receptors in the spinal cord. Anesthesiology. 2002;96:1175–82. doi: 10.1097/00000542-200205000-00022. [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Koyama S, Akaike N. Synergistic mu-opioid and 5-HT1A presynaptic inhibition of GABA release in rat periaqueductal gray neurons. Neuropharmacology. 2001;41:529–38. doi: 10.1016/s0028-3908(01)00100-9. [DOI] [PubMed] [Google Scholar]
- Liss S, Liss B. Physiological and therapeutic effects of high frequency electrical pulses. Integr Physiol Behav Sci. 1996;31:88–95. doi: 10.1007/BF02699781. [DOI] [PubMed] [Google Scholar]
- Marlier L, Teilhac JR, Cerruti C, Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–9. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Miller JF, Proudfit HK. Antagonism of stimulation-produced antinociception from ventrolateral pontine sites by intrathecal administration of alpha-adrenergic antagonists and naloxone. Brain Res. 1990;530:20–34. doi: 10.1016/0006-8993(90)90653-s. [DOI] [PubMed] [Google Scholar]
- Milne S, Welch V, Brosseau L, Saginur M, Shea B, Tugwell P, Wells G. Transcutaneous electrical nerve stimulation (TENS) for chronic low back pain. Cochrane Database Syst Rev. 2001;(2):CD003008. doi: 10.1002/14651858.CD003008. [DOI] [PubMed] [Google Scholar]
- Mjellem N, Lund A, Hole K. Different functions of spinal 5-HT1A and 5-HT2 receptor subtypes in modulating behavior induced by excitatory amino acid receptor agonists in mice. Brain Res. 1993;626:78–82. doi: 10.1016/0006-8993(93)90565-5. [DOI] [PubMed] [Google Scholar]
- Obata H, Saito S, Sasaki M, Ishizaki K, Goto F. Antiallodynic effect of intrathecally administered 5-HT(2) agonists in rats with nerve ligation. Pain. 2001;90:173–9. doi: 10.1016/s0304-3959(00)00401-2. [DOI] [PubMed] [Google Scholar]
- Odeh F, Antal M. The projections of the midbrain periaqueductal grey to the pons and medulla oblongata in rats. Eur J Neurosci. 2001;14:1275–86. doi: 10.1046/j.0953-816x.2001.01760.x. [DOI] [PubMed] [Google Scholar]
- Osiri M, Welch V, Brosseau L, Shea B, McGowan J, Tugwell P, Wells G. Transcutaneous electrical nerve stimulation for knee osteoarthritis. Cochrane Database Syst Rev. 2000;(4):CD002823. doi: 10.1002/14651858.CD002823. [DOI] [PubMed] [Google Scholar]
- Oyama T, Ueda M, Kuraishi Y, Akaike A, Satoh M. Dual effect of serotonin on formalin-induced nociception in the rat spinal cord. Neurosci Res. 1996;25:129–35. doi: 10.1016/0168-0102(96)01034-6. [DOI] [PubMed] [Google Scholar]
- Paul D, Yao D, Zhu P, Minor LD, Garcia MM. 5-Hydroxytryptamine3 (5-HT3) receptors mediate spinal 5-HT antinociception: an antisense approach. J Pharmacol Exp Ther. 2001;298:674–8. [PubMed] [Google Scholar]
- Peng YB, Lin Q, Willis WD. The role of 5-HT3 receptors in periaqueductal gray-induced inhibition of nociceptive dorsal horn neurons in rats. J Pharmacol Exp Ther. 1996;276:116–24. [PubMed] [Google Scholar]
- Pogatzki EM, Zahn PK, Brennan TJ. Lumbar catheterization of the subarachnoid space with a 32-gauge polyurethane catheter in the rat. Eur J Pain. 2000;4:111–3. doi: 10.1053/eujp.1999.0157. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Dickman JK, Richtsmeier C, Schardt N, Spurgin M, Sluka KA. Involvement of 5-HT receptor subtypes in TENS-induced analgesia. Soc Neurosci Abstr. 2002:C-17. [Google Scholar]
- Reddy SV, Maderdrut JL, Yaksh TL. Spinal cord pharmacology of adrenergic agonist-mediated antinociception. J Pharmacol Exp Ther. 1980;213:525–33. [PubMed] [Google Scholar]
- Sasaki M, Ishizaki K, Obata H, Goto F. Effects of 5-HT2 and 5-HT3 receptors on the modulation of nociceptive transmission in rat spinal cord according to the formalin test. Eur J Pharmacol. 2001;424:45–52. doi: 10.1016/s0014-2999(01)01117-7. [DOI] [PubMed] [Google Scholar]
- Scherder EJ, Bouma A. Possible role of the nucleus raphe dorsalis in analgesia by peripheral stimulation: theoretical considerations. Acupunct Electrother Res. 1993;18:195–205. doi: 10.3727/036012993816357485. [DOI] [PubMed] [Google Scholar]
- Schmauss C, Hammond DL, Ochi JW, Yaksh TL. Pharmacological antagonism of the antinociceptive effects of serotonin in the rat spinal cord. Eur J Pharmacol. 1983;90:349–57. doi: 10.1016/0014-2999(83)90556-3. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Koja T, Fujisaki T, Fukuda T. Effects of methysergide and naloxone on analgesia induced by the peripheral electric stimulation in mice. Brain Res. 1981;208:463–7. doi: 10.1016/0006-8993(81)90578-3. [DOI] [PubMed] [Google Scholar]
- Sluka KA. Systemic morphine in combination with TENS produces an increased antihyperalgesia in rats with acute inflammation. J Pain. 2000;1:204–11. doi: 10.1054/jpai.2000.7149. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289:840–6. [PubMed] [Google Scholar]
- Sluka KA, Judge MA, McColley MM, Reveiz PM, Taylor BM. Low frequency TENS is less effective than high frequency TENS at reducing inflammation-induced hyperalgesia in morphine-tolerant rats. Eur J Pain. 2000;4:185–93. doi: 10.1053/eujp.2000.0172. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Walsh D. TENS: basic science mechanisms and clinical effectiveness. J Pain. 2003;4:109–21. doi: 10.1054/jpai.2003.434. [DOI] [PubMed] [Google Scholar]
- Sjölund B, Terenius L, Eriksson M. Increased cerebrospinal fluid levels of endorphins after electro-acupuncture. Acta Physiol Scand. 1977;100:382–4. doi: 10.1111/j.1748-1716.1977.tb05964.x. [DOI] [PubMed] [Google Scholar]
- Solomon RE, Brody MJ, Gebhart GF. Pharmacological characterization of alpha adrenoceptors involved in the antinociceptive and cardiovascular effects of intrathecally administered clonidine. J Pharmacol Exp Ther. 1989;251:27–38. [PubMed] [Google Scholar]
- Solomon RE, Gebhart GF. Mechanisms of effects of intrathecal serotonin on nociception and blood pressure in rats. J Pharmacol Exp Ther. 1988;245:905–12. [PubMed] [Google Scholar]
- Wang YJ, Wu J, Wang SK. The role of acetylcholine in the rat brain and its effect on 5-hydroxytryptamine metabolism during electroacupuncture analgesia. J Tradit Chin Med. 1985;5:297–300. [PubMed] [Google Scholar]
- Woolf CJ, Barrett GD, Mitchell D, Myers RA. Naloxone-reversible peripheral electroanalgesia in intact and spinal rats. Eur J Pharmacol. 1977;45:311–4. doi: 10.1016/0014-2999(77)90016-4. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Mitchell D, Barrett GD. Antinociceptive effect of peripheral segmental electrical stimulation in the rat. Pain. 1980;8:237–52. doi: 10.1016/0304-3959(88)90011-5. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav. 1985;22:845–58. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Wilson PR. Spinal serotonin terminal system mediates antinociception. J Pharmacol Exp Ther. 1979;208:446–53. [PubMed] [Google Scholar]
- Yang SW, Zhang ZH, Wang R, Xie YF, Qiao JT, Dafny N. Norepinephrine and serotonin-induced antinociception are blocked by naloxone with different dosages. Brain Res Bull. 1994;35:113–7. doi: 10.1016/0361-9230(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–9. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Spinal serotonin receptors mediate descending facilitation of a nociceptive reflex from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Brain Res. 1991;550:35–48. doi: 10.1016/0006-8993(91)90402-h. [DOI] [PubMed] [Google Scholar]