Abstract
Guiding non-neural, retinal pigment epithelium (RPE) to produce retinal neurons may offer a source of developing neurons for cell-replacement. Sox2 plays important roles in maintaining neural progenitor/stem cell properties and in converting fibroblasts into pluripotent stem cells. This study tests the possibility of using Sox2 to reprogram RPE to differentiate towards retinal neurons in vivo and in vitro. Expression of Sox2 in the chick retina was detected in progenitor cells, in cells at a discrete location in the layers of amacrine and ganglion cells, and in Műller glia. Overexpression of Sox2 in the developing eye resulted in hypo-pigmentation of the RPE. In the affected regions, expression of retinal ganglion cell markers became apparent in the RPE layer. In RPE cell culture, Sox2 promoted the expression of retinal ganglion and amacrine markers and suppressed the expression of genes associate with RPE properties. Mechanistic investigation using the developing retina revealed a co-expression of Sox2 and bFGF, a growth factor commonly used in stem cell culture and capable of inducing RPE-to-retina transdifferentiation (or reprogramming) during early development. Similar patterns of changes in Sox2 expression and in bFGF expression were observed in atrophic retina and in injured retina. In RPE cell culture, Sox2 and bFGF mutually enhanced one another's expression. Up-regulation of bFGF expression by Sox2 also occurred in the retina. These results suggest that Sox2 can initiate a reprogramming of RPE cells to differentiate towards retinal neurons and may engage bFGF during the process.
Keywords: differentiation, fibroblast growth factor, regeneration, retinal ganglion cells, transcription factor
Introduction
In the vertebrate eye, the multilayered neural retina lies anatomically beneath a homogenous-looking monolayer of non-neural, darkly pigmented cells, the retinal pigment epithelium (RPE). Developmentally, the non-neural RPE and the neural retina share a common developmental origin – the optic vesicle, which originates from the lateral aspects of the forebrain. Invagination of the optic vesicle forms a double-layered optic cup. Cells in the outer layer of the optic cup are destined to become the RPE, and cells in the inner layer will constitute the neural retina. The RPE maintains its simple, monolayer structure throughout life. The neural retina, on the other hand, consists of highly ordered structure with several major neuron types, including ganglion, amacrine, bipolar, horizontal and photoreceptor cells, and Műller glia. The diverse cell types in the retina are generated from a pool of multipotent progenitors through states of developmental competence by coordinated cell cycle exit and cell fate specification [1-4].
Degeneration of retinal neurons results in vision loss, because retinal neurons are unable to regenerate. RPE cells, on the other hand, can re-enter the cell cycle to proliferate. Further, progeny cells from RPE proliferation can differentiate into other cell types [5]. Classic studies demonstrated that embryonic chick RPE can be induced by basic fibroblast growth factor (bFGF), either administered exogenously or released from the retina, or by aFGF, to transdifferentiate into a neural retina [6-10]. This capability of bFGF-induced RPE-to-retina transdifferentiation/reprogramming is lost in chick embryos older than embryonic day 4.5 [E4.5; 6, 8]. Additionally, under in vitro conditions, the transdifferentiation/reprogramming occurs only when RPE is cultured as an explant or sheet, and does not occur when RPE is cultured as dissociated cells [8]. In the mature eye, RPE cells normally remain quiescent. However, when stimulated, such as by physical triggers that occur during surgery, RPE cells can proliferate. This proliferative response is presently regarded as an undesirable side effect, because the amplified RPE cells often differentiate into cells that generate tractional force that causes retinal detachment and leads to visual impairment. On the other hand, the proliferative response raises an intriguing possibility of exploring the RPE as a convenient source of retinal neurons for replacement in situ, provided that the RPE progeny cells can be guided to differentiate into retinal neurons.
Sox2 is one of four genes used to convert adult fibroblasts into induced pluripotent stem cells in mouse and in human [11-14]. Its protein belongs to the SoxB1 subfamily of transcription factors characterized by a high mobility group (HMG) DNA-binding domain [15, 16]. Gene targeting studies show a cell-autonomous requirement for Sox2 in both epiblast and extraembryonic ectoderm, and mice homozygous for Sox2 mutants die shortly after implantation [17]. During embryonic neurogenesis, Sox2 is expressed in the neural plate and in neural stem cells and progenitors. Expression of Sox2 in neural stem cells and progenitors also occurs in neurogenesis of adulthood [17-20]. Constitutive expression of Sox2 inhibits neuronal differentiation. Conversely, inhibition of Sox2 signaling results in the loss of progenitor markers, premature cell cycle exit, and initiation of neuronal differentiation [19, 21]. Sox2 could therefore play an important role in maintaining neural progenitor/stem cell properties. In mouse retina, the lack of Sox2 expression causes retinal progenitor cells to lose competence to both proliferation and differentiation, and there is a dose-dependent regulatory effect of Sox2 on retinal neurogenesis [22]. In human, Sox2 mutations cause anophthalmia with variable extraocular defects [18]. In the chick and the mouse retina, Sox2 is expressed in proliferating cells and a subpopulation of amacrine cells [22-24].
To investigate whether Sox2 can reprogram RPE cells to differentiate towards retinal neurons, we used the Replication Competent Avian Splice (RCAS) retrovirus [25] to ectopically express Sox2 in RPE cells in the developing chick eye and in cultured chick RPE cells. We then analyzed the expression of retinal neuronal markers in the otherwise RPE cells. We found that Sox2 induced the expression of genes active in retinal neurons, inhibited the expression of genes associated with RPE properties, and caused hypo-pigmentation of the RPE cells. Furthermore, we found a positive relationship between Sox2 and bFGF expression and a negative relationship between Sox2 and the expression of pigment epithelium-derived factor (PEDF).
Materials and Methods
Chick embryos
Fertilized, pathogen-free White Leghorn chicken eggs were purchased from Spafas and incubated in a Petersime incubator. All use of animals adhered to the procedures and policies set by the Institutional Animal Use and Care Committee at the University of Alabama at Birmingham.
Construction of retroviruses expressing Sox2, Sox2ΔB, Sox2ΔC, and En-Sox2
To generate RCAS expressing Sox2, the coding region of chick Sox2 was amplified by RT-PCR based on the published sequence [26] and cloned into pGEMT (Promega, Madison, WI). The cloned sequence was verified. To generate deletion constructs, the DNA-binding domain was removed by restriction digestion with Tth111 I and Psi I, followed by Klenow filling-in and inframe, self-ligation to create Sox2ΔB, and the transactivation domain [27] was removed by restriction digestion with Psi I and Spe I (in the multiple cloning site) followed by self-ligation to create Sox2ΔC. To generate an active repression construct, the repressor domain of Drosophila Engrailed [28-30] was fused (in frame) to the N-terminus of Sox2, creating En-Sox2. After sequence verification, the DNA sequences were subcloned into shuttle vector Cla12Nco and then inserted into RCAS [25]. Recombinant viral particles were produced as previously described [31]. The titers of the virus stocks ranged from 5 × 107 to 2 × 108 pfu/ml. Different batches of independently produced viruses were used when repeating experiments to rule out the possibility that experimental observations were attributable to retroviral recombination.
Infection of embryonic chick eye with RCAS-Sox2, RCAS-NSCL2, and RCAS-GFP
Retroviruses expressing Sox2, NSCL2 [32], a neural-tissue specific basic helix-loop-helix gene belonging to the stem cell leukemia (SCL) family, or GFP [31] as a control, were microinjected into the subretinal space between E2.5 and E3, as previously described [33]. The eyes were enucleated between E5 to E18 and fixed with ice-cold 4% paraformaldehyde. The fixed samples were cryoprotected with OCT: 20% sucrose (2:1), frozen with liquid nitrogen, and kept at −80°C. Cross sections of 10 μm were used in immunocytochemistry and in situ hybridization.
RPE cell culture
Chick E6 RPE was dissected free from the neural retina as described [31]. Pooled RPE tissues were incubated with typsin-EDTA, and the dissociated cells were cultured with knock-out DMEM plus 20% serum replacement (Invitrogen, San Diego, CA). For bFGF treatment, RPE cells were cultured in the presence of 10 μg/ml bFGF. When the culture became ∼50% confluent, 10-20 μl of concentrated retrovirus expressing Sox2, Sox2ΔB, Sox2ΔC, En-Sox2 or GFP was added to a 35 mm dish. Cultures were maintained for an additional 8-15 days, and cells in the culture were then harvested for reverse transcription-polymerase chain reaction (RT-PCR) or fixed for immunocytochemistry.
Glial cell culture
E18 chick eyes were enucleated. The anterior segment along the middle between limbus and equator was surgically removed. After an incubation of the eye cup in 2% dispase at 37°C for 10-20 minutes, the retina was peeled off the RPE. Retinal cells were dissociated with typsin-EDTA and seeded in 35 mm dishes with Medium 199 supplemented with 10% fetal calf serum for 4 hours. At this point, cells not attached to the dish were removed, and new medium was added. When the culture reached about 40-50% confluency, RCAS-Sox2 or RCAS-GFP (30 μl) was added to the dishes. Cells in the culture were harvested for RT-PCR 3 days after the administration of the virus.
Immunocytochemistry
Monoclonal antibody RA4 (1:1000 dilution) was a gift from Dr. Steven McLoon (University of Minnesota, Minneapolis, MN). Monoclonal antibody (HM-2; 1:200) against microtubule-associated proteins (MAP2) was purchased from Sigma. Monoclonal antibody against Brn3a (1:100) was purchased from Chemicon (Temecula, CA). The following monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA): anti-bromodeoxyuridine (anti-BrdU, G3G4, 1:100; developed by Dr. Stephen J. Kaufman), 3A10 (1:100; developed by Dr. Thomas Jessell), anti-islet-1 (39.4D5, 1:100; developed by Dr. Thomas Jessell), anti-Ap2a (3B5, 1:200, developed by Dr. Trevor Williams), anti-vimentin (H5, developed by Dr. Joshua Sanes), and anti-visinin (7G4, 1:500; developed by Dr. Constance Cepko). Standard immunocytochemistry was performed with horse radish peroxidase (HRP)-conjugated, alkaline phosphatase-conjugated (Vector Laboratories, Burlingame, CA), or fluorophore-conjugated (Molecular Probes, Eugene, OR) secondary antibodies.
In situ hybridization and double labeling
Digoxigenin (Dig)-labeled antisense RNA probes against the coding sequences of Sox2 (555 nucleotides) and bFGF (476 nucleotides) [34] were synthesized using the Genius kit (Roche Molecular Biochemicals, Indianapolis, IN) following the manufacturer's instructions. FITC-labeled antisense RNA probes against bFGF mRNA was also synthesized for double-in situ hybridization along with dig-labeled antisense RNA probes against Sox2 mRNA, following procedures previously described [35]. In situ hybridization was performed with 8-10 μm frozen sections on glass slides with final staining with alkaline phosphatase substrate NBT [35] or with fluorescein-tyramide or rhodamine-tyramide [35]. For double labeling, in situ hybridization was performed first followed by immunocytochemistry.
Double labeling for BrdU incorporation and Sox2 expression
Chick embryos at E8, E10, or E14 infected with RCAS-Sox2, or RCAS-GFP as a control, were pulsed with BrdU (50 μg in 50 μl of HBSS) dropped through an opening window in the shell through the vitelline membrane. The embryos were incubated for 4 hours before the eyes were harvested and fixed with 4% paraformaldehyde. Frozen sections on glass slides were first subjected to in situ hybridization with dig-labeled anti-Sox2 RNA probes and then to BrdU detection using a specific antibody as previously described [36]. E15 embryos infected with RCAS-Sox2 or RCAS-GFP were subjected to anti-BrdU immunostaining only.
Chemically damaging the retina
N-methyl-D-aspartate (NMDA, 2-3 μl of 34 mM solution in 0.9% saline) was injected into the vitreous of E12 chick eyes to induce retinal injuries [37-39]. Injection of saline solution alone served as the control. At E15, retinas from 3 experimental eyes or control eyes were collected for analysis.
RT-PCR
Total RNAs were isolated from the RPE and the retina dissected from chick embryos and from cells in RPE and glial cell cultures, using the RNeasy protect mini kit (QIAGEN). First-strand cDNA was synthesized with a cDNA synthesis kit (Ambion) using oligo-dT as the primer. After a 5-10 fold dilution, 1 μl of the first-strand cDNA was used as the template in each 30 μl PCR reaction. The small ribosomal protein 17 (S17) was used as an internal control to normalize the amount of cDNA in each sample [40] with 20 cycles of amplification using primers gtgatcatcgagaag and agcaacataacgagc annealed at 44°C. Mitf, mp115, Otx2, Npy-1, Pax6, and PEDF were amplified by 30 cycles and bFGF was amplified by 25 cycles, at an annealing temperature of 56°C derived empirically using temperature gradients. The specific primer set for each of the genes spans intron of 689 to 7396 bp: Mitf, ccttggcttgatggaccca and gcatgatcagtgtcctccat (GenBank accession # D88363); Mmp115, cacagcagtggagggaagc and ggctgtgggcagcagcg (GenBank accession # NM_205112); Otx2, gcatgatgtcttatcttaagcaa and ggaacttccatgaggatgtc (GenBank accession # AY600957); Npy-1, cctctcccagagccgctcc and ggaatgcacaaataatttatttaaat (GenBank accession # M87294); Pax6, caacactcccagccacatc and ccaacacagatcaaacatcc (GenBank accession # NM_205066); bFGF, atggcggcgggggcgg and tcagcttttagcagacattgg (GenBank accession # NM_205433); and PEDF, gaagatgtgatttctcgtgc and gaggctttcctcgatcag (NCBI's Annotation Process, XM_001234864). For quantificational and statistical analyses, the integrated optical density (IOD) of the PCR product on agarose gels was measured using LabWorks™ (version 4.0, UVP Inc.). The means and SDs of IODS from 3 independent RT-PCR reactions were calculated using Origin 7.0 (OriginLab Corp.) One-way ANOVA of Origin 7.0 was used for statistical significance analysis at 0.05 (*) and 0.01 (**) levels.
Results
The spatial and temporal expression pattern of Sox2 in the chick retina
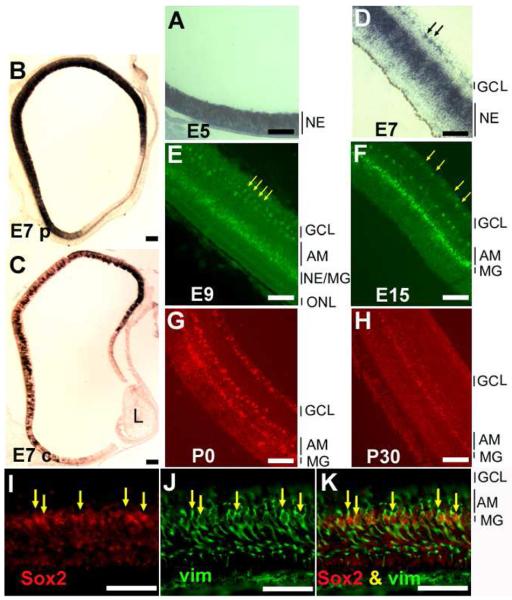
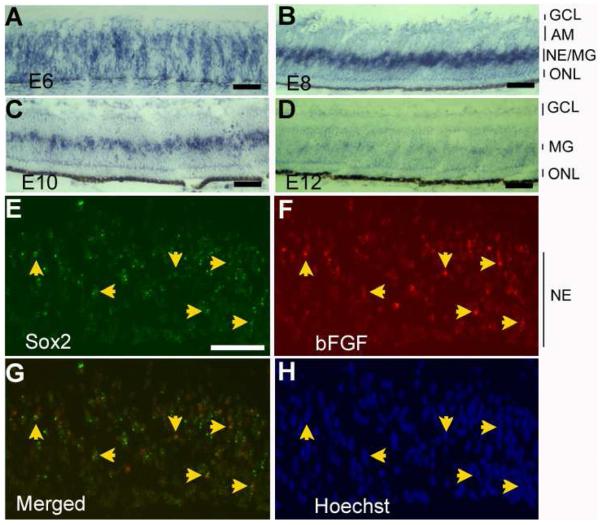
To facilitate an understanding of how Sox2 participates in retinal neurogenesis, we examined its expression pattern at different developmental stages, from E5 to post-hatching day 30 (P30). In situ hybridization showed heavy staining of Sox2 expression between E5 and E7 (Fig. 1), the time when retinal cell proliferation is most active [41, 42]. During this period, cells expressing Sox2 localized across the entire thickness of the neuroepithelium, as shown in E5 central retina (Fig. 1A) or in E7 peripheral retina (Fig. 1B). In E7 central retina, where development is more advanced than at the periphery, cell proliferation activity subsides, and postmitotic cells accumulate on the vitreous side as differentiating ganglion cells and amacrine cells and on the RPE side as developing photoreceptor cells. At this stage, fewer cells remained positive for Sox2 mRNA (Fig. 1C), and most of the positive cells were confined to the zone of progenitor cells (Fig. 1D). Sox2 expression continued to decrease From E7 through E15, with the majority of the expression confined to a narrowing zone of cells in the middle of the developing retina (Fig. 1E, F). This expression pattern parallels that of retinal neurogenesis in general. The lens, the RPE, and the surrounding mesenchymal cells appeared to lack Sox2 expression (Fig. 1A-D)
Fig. 1.
Spatial and temporal pattern of Sox2 expression in chick retina. The in situ hybridization signals were visualized with nitroblue tetrazolium (A-D), fluorescein-tyramide (E,F), or rhodamine-tyramide (G,H). A: E5 retina. B: E7 peripheral retina. C: E7 central retina. D: E7 central retina at higher magnification. E: E9 retina; F: E15 retina. G: P0 retina. H: P30 retina. I-K: Double-labeling for Sox2 expression and for Muller glia protein vimentin (recognized by Mab H5) in E15 retina. I: In situ hybridization detection of Sox2 mRNA with rhodamine-tyramide. J: Immunostaining for vimentin with fluorescein. K: Simultaneous view of both stainings. Arrows in D: positive cells in the inner plexiform layer. Arrows in E and F: positive cells that appear spatially paired. Arrows in I-K point to double-labeled cells. AM: amacrine cells. GCL: ganglion cell layer. L: lens. MG: Muller glia. NE: neuroepithelium. ONL: outer nuclear layer. Scale bars: 50 μm.
Starting from E7, Sox2 expression was also apparent in a small number of cells spatially separated from those of the proliferating zone (arrows, Fig. 1D). These cells localized to the inner plexiform layers at E9 (Fig. 1E), to the amacrine layer at E9 and thereafter (Fig. 1E-H), and to the ganglion cell layer in E15 and thereafter (Fig. 1F-H).
In E15 retina, where there is little cell proliferation activity [41, 42], Sox2 expression persisted in cells at the middle of the inner nuclear layer (INL; Fig. 1F). Similar expression was detected in retinas of P0 (Fig. 1G) and P30 (Fig. 1H). Double-labeling showed that these cells were positive for vimentin (arrows, Fig. 1I-K), indicating Sox2 expression in Műller glia.
Changes in RPE appearance induced by Sox2
The expression of Sox2 in the retinal neuroepithelium and its known role in maintaining neural stem cell properties prompted us to ask whether Sox2 could induce de novo retinal neurogenesis in the context of RPE cells. RCAS, a replication-competent retrovirus, was used to drive Sox2 overexpression (or misexpression) in the developing chick eye by microinjecting RCAS-Sox2 into the space between the two layers of the optic cup between E2.5 and E3. Before E8, the eye and the retina from embryos infected with RCAS-Sox2 were indistinguishable from those of the control. BrdU incorporation analysis showed no significant changes in retinal cell proliferation activities between retinas overexpressing Sox2 and the controls at E8 and E10 (data not shown). Anatomically, the majority of the retina infected with RCAS-Sox2 appeared normal, with the exception of discreet places between the central and peripheral retina, as described below.
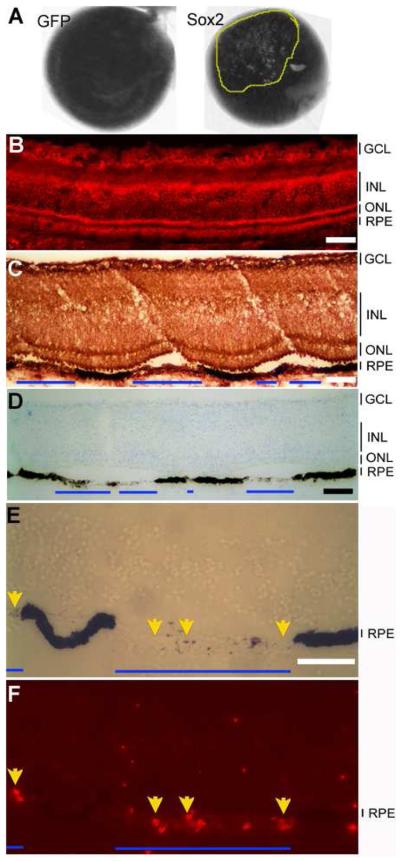
From E8 and thereon, the experimental eyes appeared “spotty,” with regions markedly lacking the dark pigmentation (Fig. 2A). Cross sections showed that at the “spotty” places, the RPE cells partially or completely lacked dark pigments (Fig. 2A). At these affected places, the retina often detached from the de-pigmented RPE (Fig. 2C). The de-pigmentation of the RPE and the retinal detachment suggest that Sox2 might have interfered with RPE development and function. Neither de-pigmentation nor retinal detachment was observed with control eyes infected with RCAS-GFP (Fig. 2A, B).
Fig.2.
RPE de-pigmentation from Sox2 overexpression. A: E15 eyes from an embryo infected with RCAS-Sox2 (left) or RCAS-GFP (right). The “patchy” area is encircled by a yellow line. B: Immunostaining with anti-viral protein P27 to detect viral infection in E15 retina infected with RCAS-GFP. C: Immuno-detection of viral infection (anti-viral protein P27 staining) in E15 retina infected with RCAS-Sox2. D: Immuno-detection of viral infection (with anti-viral protein P27) in E15 retina infected with RCAS-GFP. Blue lines mark regions with de-pigmentation of the RPE and retinal detachment. E-F: BrdU incorporation in de-pigmented regions of E15 retina infected with RCAS-Sox2. E: A bright-field views. F: BrdU immunostaining for BrdU. The arrows show BrdU+ cells in the RPE layer. GCL: ganglion cell layer. INL: inner nuclear layer. ONL: outer nuclear layer. Scale bars: 50 μm.
To examine whether cells in the de-pigmented regions could re-enter the cell cycle, we subjected the embryos to BrdU at E14, when cell proliferation has long ceased in the RPE, and analyzed BrdU incorporation at E15. While absent in the control (data not shown), BrdU+ cells were detected in the de-pigmented regions of the RPE infected with RCAS-Sox2 (Fig. 2E, F).
Induction of neuron markers in the RPE by Sox2
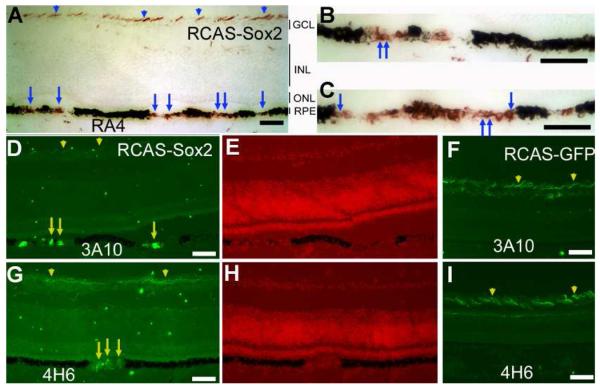
To investigate whether Sox2 had initiated the development of neural properties in the context of RPE, we examined the eyes infected with RCAS-Sox2 for the expression of genes associated with retinal neurons. One of the earliest neural markers in the developing chick retina is RA4 immunoreactivity in newborn ganglion cells [43]. In regions with significant de-pigmentation, a large portion of the cells (estimated to be >50%) in the RPE were RA4+ (Fig. 3A-C). Some of the RA4+ cells exhibited neural morphologies (arrows in Fig. 3A-C). Cells in the de-pigmented regions were also immunopositive for a neurofilament-associated protein recognized by monoclonal antibody 3A10 (Fig. 3D), and a 160-kDa neurofilament recognized by monoclonal antibody 4H6 (Fig. 3G). Neither protein was detected in the RPE cell layer of the control retina infected with RCAS-GFP (Fig. 3E-I). The RPE de-pigmentation and the ectopic expression of RA4, 3A10, and 4H6 were detected from E8 through E18, the last developmental stage analyzed. Notably, cells in the de-pigmented region of the RPE infected with RCAS-Sox2 did not express other ganglion markers, such as Brn3a or Islet-1, and they remained negative for general neural maker Map2, photoreceptor marker visinin, and amacrine markers Pax6 and AP2α (data not shown).
Fig. 3.
Ectopic expression of retinal ganglion cell markers in the RPE of E18 embryos infected with RCAS-Sox2. A: Immunostaining (in red) with RA4. B: Higher magnification of the left side of A. C: Higher magnification of the right side of A. D: Immunostaining (FITC) with 3A10 of retina infected with RCAS-Sox2. E: Nuclear staining of C. F: Immunostaining (FITC) with 3A10 of retina infected with RCAS-GFP. G: Immunostaining (FITC) with 4H6 of retina infected with RCAS-Sox2. H: Nuclear staining of C. I: Immunostaining (FITC) with 4H6 of retina infected with RCAS-GFP. Arrows point to cells with neuron-like morphologies. Arrowheads point to immunostaining of the neural fibers of retinal ganglion cells. GCL: ganglion cell layer. INL: inner nuclear layer. ONL: outer nuclear layer. Scale bars: 50 μm.
Reprogramming cultured RPE cells by Sox2
The possibility of Sox2 reprogramming RPE to differentiate towards retinal neurons was also investigated in cultures of dissociated RPE cells. When E6 chick RPE cells are dissociated and seeded at low density in culture dishes, they enter the cell cycle and proliferate. During this proliferating phase of culture, RPE cells lose their pigment granules [44]. Later, as the culture becomes confluent, the cells become re-pigmented. When infected with RCAS-Sox2, however, cells in the culture failed to become re-pigmented after confluence and a week thereafter, in contrast to those in the control culture infected with RCAS-GFP (Fig. 4B). Thus, as in the eye, Sox2 inhibited the pigmentation of RPE progeny cells in culture.
Fig. 4.
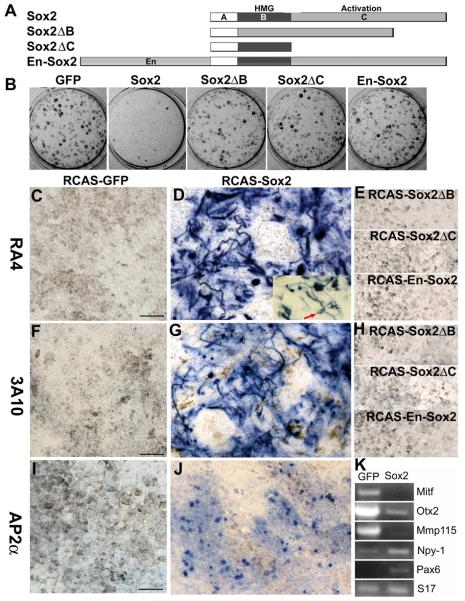
Suppression of RPE properties and induction of neural genes by Sox2 in RPE cell cultures. A: A schematic diagram showing the structural regions included in each recombinant DNA construct. B: Appearance of RPE cell cultures, showing hypopigmentation of the infected with RCAS-Sox2 as compared with the control infected with RCAS-GFP. Also shown are parallel cultures infected with RCAS expressing Sox2ΔB, Sox2ΔC, or En-Sox2. C-J: Expression of retinal neural markers in RPE cell cultures infected with RCAS-Sox2. C-E: Immunocytochemistry with RA4 of cultures infected with RCAS-GFP, RCAS-Sox2, and RCAS expressing 3 mutations constructs of Sox2: Sox2ΔB, Sox2ΔC, and En-Sox2. F-H: Immunocytochemistry with 3A10. I, J: Immunocytochemistry for AP2α. The arrow in the inset of D: a cell displaying morphology typical of a retinal ganglion cell. K: RT-PCR analysis of the expression of RPE genes (Mitf, Otx2, and Mmp115) and of neural genes (Npy-1 and Pax6) in RPE cell cultures infected with RCAS-Sox2 or RCAS-GFP. Small ribosomal protein 17 (S17) was used as an internal control for the amount of first-strand cDNA present in the samples. Scale bars: 50 μm.
Immunocytochemistry analysis showed a large number of RA4+ cells (accounting for >50% of the total cells) in RPE cultures infected with RCAS-Sox2 (Fig. 4D) and 3A10+ (accounting for >30% of the total cells; Fig. 4G). Expression of AP2α was also detected in RPE cell cultures infected with RCAS-Sox2 (Fig. 4J). Thus, unlike in vivo in the eye, Sox2 was able to induce AP2α expression in vitro in RPE cell culture. The control cultures lacked the expression of these markers (Fig. 4C, F, I). Morphologically, some of the RA4+ or 3A10+ cells exhibited long processes, which markedly deviated from the hexagonal or fibroblast-like morphologies of RPE cells in culture (Fig. 4D, F).
For additional controls and to determine whether transcription activation by Sox2 is required for the observed changes, we generated RCAS expressing mutation constructs of Sox2 (Fig. 4A), including omission of the DNA-binding domain (Sox2ΔB), omission of the activation domain (Sox2ΔC), and the addition of the repressor domain of Drosophila Engrailed (En-Sox2). Drosophila Engrailed actively and specifically represses activated transcription [28, 28]. Its repressor domain can confer repression activity to heterologous DNA-binding domains when linked to them [28, 29]. RPE cell cultures infected with RCAS viruses expressing these mutation constructs showed no differences from that of the GFP control in re-pigmentation (Fig. 4B), in lacking of RA4+ cells (Fig. 4E), and in lacking of 3A10+ cells (Fig. 4H).
RT-PCR was used to examine whether Sox2 suppressed the expression of genes key to RPE development and specification and/or promoted the expression of genes associated with neural properties. Genes important for RPE development include Mitf [45, 46], Otx2 [47-50] and melanosomal matrix protein Mmp115 [51]. Mutation of Mitf caused RPE-to-retina transformation [52, 53]. In cultures infected with RCAS-Sox2, we found reduced expression of Mitf, Otx2, and Mmp115 (Fig. 4K), genes which are important in development and maintaining of RPE properties. On the other hand, Sox2 increased the expression of Pax6, a homeodomain gene expressed in retinal progenitor cells, amacrine cells, and ganglion cells [54] and regulating neural identity and differentiation [55]. Sox2 also increased the expression of Npy-1 (Fig. 4K), a gene that encodes neuropeptide Y [56] and is expressed in a discrete set of amacrine cells [57]. The sizes of the RT-PCR products were as expected from the corresponding mRNA, not the genomic sequence with intron sequence.
Co-expression of Sox2 with bFGF in the developing retina
The observed changes in RPE properties induced by Sox2 share similarities with bFGF-induced RPE transdifferentiation/reprogramming [5, 7, 8, 58]. In reprogramming embryonic chick RPE at early developmental stages to develop into a neural retina, aFGF (or bFGF) is thought to potentiate the fate of the first born neurons, the retinal ganglion cells, thereby setting the stage for subsequent retinal neurogenesis. Population analysis of dissociated cells several days after exposing RPE tissue to aFGF (or bFGF) indicated that as much as 80% of the cells in the transdifferentiated RPE cells express antigens normally present in retinal ganglion cells [9]. In RPE cell culture, bFGF, like Sox2, prevents the cells from becoming re-pigmented and induces the expression of early, but not later markers of retinal ganglion cells [59]. These similarities prompted us to examine the inductive relationship between Sox2 and bFGF in our experimental systems.
In situ hybridization was used to examine the bFGF expression pattern in the developing chick retina. At E6, bFGF mRNA was present in cells across the neuroepithelium, while the developing ganglion cells accumulating on the vitreal side lacked obvious expression (Fig. 5A). By E8, cells expressing bFGF were concentrated into a zone at the middle of the INL where residual proliferating cells and/or developing Műller glia reside (Fig. 5B). Expression of bFGF in cells localized to where Műller glia reside became apparent in retinas of E10 (Fig. 5C), E12 (Fig. 5D), and E16 (data not shown), albeit the levels of expression were barely detectable with our in situ hybridization method in E12 and older retinas.
Fig.5.
The spatial pattern of bFGF expression in the developing chick retina and its co-expression with Sox2. A-D: In situ hybridization for bFGF expression in E6 (A), E8 (B), E10 (C), and E12 (D) chick retina. E-F: Double in situ hybridization for Sox2 mRNA (E, fluorescein-tyramide, in green) and bFGF mRNA (F, rhodamine-tyramide, in red) in E8 chick retina. G: A merged view of E and F. H: Hoechst nuclear staining. Arrows point to double-labeled cells in the neuroepithelial layer (NE). AM: amacrine cells. GCL: ganglion cell layer. MG: Muller glia. NE: neuroepithelium. ONL: outer nuclear layer. Scale bars: 50 um.
The similarity in the spatial patterns of bFGF expression and Sox2 expression prompted an inquiry into whether the two were co-expressed. Double-in situ hybridization was carried out using thin (8 μm) E8 retinal sections and with prolonged proteinase K treatment, two measures incorporated to reduce cell density to minimize ambiguity in identifying double-labeled cells. This method detected double-labeled cells in the middle region of the neuroepithelial layer of the E8 retina (Fig. 5E-H).
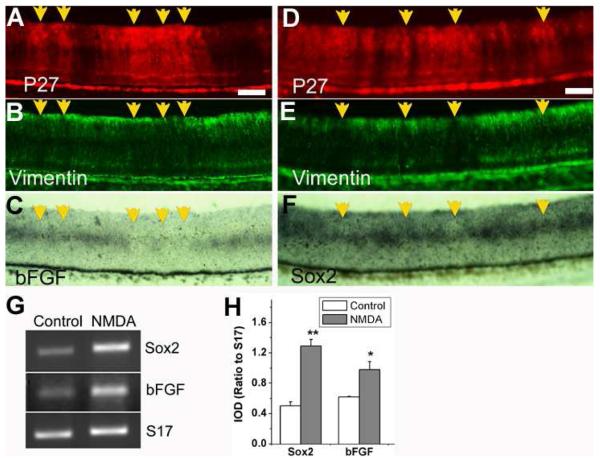
We then examined atrophic retinas and injured retinas to address the question of whether Sox2 and bFGF were co-regulated in the retina. For atrophic retina, we used those partially infected with RCAS-NSCL2. Previous study has shown than retinas infected with RCAS-NSCL2 undergo severe Műller glial atrophy [32]. We found that in the retina partially infected with RCAS-NSCL2, the infected regions (Fig. 6A) lacked vimentin+ (Műller) cells (Fig. 6B). These regions also lacked bFGF-expressing cells, which were present in the adjacent, uninfected regions (Fig. 6C). Similarly, in infected regions (Fig. 6D) with fewer vimentin+ cells (Fig. 6E), fewer Sox2-expressing cells were present than in the adjacent, uninfected regions (Fig. 6F). Thus, in Műller glia-deficient atrophic retina, the expression of Sox2 and bFGF were down-regulated.
Fig. 6.
Similar patterns of changes in expression of Sox2 and bFGF in Muller glia atrophic retina and after chemically induced retinal injury. A-F: E10 retinas partially infected with RCAS-NSCL2, which causes Muller glia atrophy [ref]. A, D: Immunostaining for antiviral protein p27 to identify regions infected by RCAS-NSCL2. B, E: Immunostaining for Muller glial protein vimentin. C, F: In situ hybridization for bFGF expression (C) and Sox2 expression (F). Arrows point to RCAS-NSCL2 infected regions with fewer vimentin+ cells and fewer bFGF+ cells or with fewer Sox2+ cells than the adjacent, uninfected regions. G: RT-PCR analysis of Sox2 and bFGF expression in control and in NMDA-damaged retina. H: A plot of IOD ratios to S17 of the DNA band intensities of Sox2 and bFGF PCR products. Shown are the means and S.D.s from 3 independent RT-PCR reactions. “*” indicates statistically significant at p<0.05 level, and “**” at p<0.01 level. Scale bars: 50 um.
Vitreal injection of NMDA is known to cause degeneration of retinal neurons, particularly amacrine and bipolar cells [37-39] and to induce glial activation [60]. One of the reactive responses of Műller glia to NMDA-induced retinal injury is to re-enter the cell cycle, producing progeny cells that migrate into all layers of the neural retina, leading to the hypothesis that Műller glia may serve as “stem cells” of the retina [60-62]. RT-PCR was used to analyze the effect of NMDA damage on the expression of Sox2 and bFGF, with primers spanning intron sequence to verify amplification from mRNA/cDNA and not from genomic DNA contamination. In retinas subjected to NMDA damage, the level of expression of Sox2 was increased, and the level of bFGF expression was also increased (Fig. 6G). Computer assisted calculation of the integrated optical density (IOD) ratio to “housekeeping” gene S17 showed a more than 2-fold increase (p<0.01) in Sox2 expression, from 0.50 ± 0.05 in the control to 1.29 ± 0.09 in the injured retina. The expression of bFGF showed a 58% increase (p<0.01), from 0.62 ± 0.01 in the control to 0.98 ± 0.11 in the experimental retina (Fig. 6H). Thus, in NMDA-treated retina, the expression of Sox2 and bFGF was up-regulated.
Inductive relationship between Sox2 and bFGF
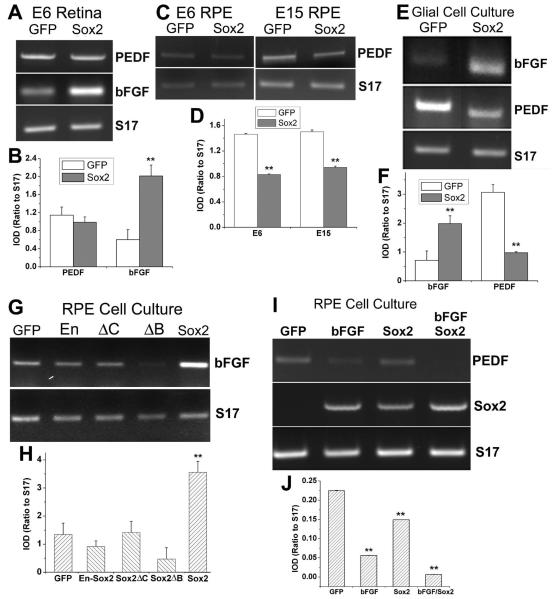
The coincidental expression and the similar patterns of changes in expression between Sox2 and bFGF prompted an examination of the inductive relationship between Sox2 and bFGF. Semi-quantitative RT-PCR was carried out to examine the effect of Sox2 overexpression in retinas infected with RCAS-Sox2 on bFGF expression. In these retinas, the level of bFGF mRNA was increased in comparison to the control infected with RCAS-GFP (Fig. 7A). The IOD ratio of bFGF to “housekeeping” gene S17 was increased over 3-fold (p<0.01), from 0.60 ± 0.22 in the control to 2.02 ± 0.24 in the experimental retina (Fig. 7B). No significant change was observed in the mRNA level of PEDF (p>0.05; Fig. 7A, B), a growth factor produced by Műller glia and the RPE [63-65]. The RPE from these embryos was also analyzed for alterations in bFGF expression, but no changes were found (data not shown). However, the level of PEDF expression in the RPE was significantly decreased by at least 30% (p<0.01) in embryos infected with RCAS-Sox2 (Fig. 7C, D). Similar results were obtained with RPE isolated from E15 embryos (Fig. 7C, D).
Fig. 7.
Increasing bFGF expression and decreasing PEDF expression by Sox2. A: RT-PCR analysis of bFGF and PEDF expression in E6 retina infected with RCAS-GFP (GFP) or RCAS-Sox2 (Sox2). Housekeeping gene S17 was used as an internal control of the relative amount of cDNA present in each sample. B: A plot of IOD ratios (to S17) of the DNA band intensities of PEDF and bFGF PCR products. C, D: RT-PCR and a plot of IOD ratios (to S17) of PEDF expression in E6 and E15 RPE infected RCAS-GFP (GFP) or RCAS-Sox2 (Sox2). E, F: RT-PCR and a plot of IOD ratios (to S17) of bFGF expression and PEDF expression in glial cell culture infected with RCAS-GFP (GFP) or RCAS-Sox2. G, H: RT-PCR and a plot of IOD ratios (to S17) of bFGF expression E6 RPE cell culture infected with RCAS-GFP (GFP), RCAS-Sox2ΔB, RCAS-Sox2ΔC, RCAS-En-Sox2, or RCAS-Sox2. I, J: RT-PCR and a plot of IOD ratios (to S17) of the expression of PEDF and Sox2 in RPE cell cultures infected with RCAS-GFP (GFP), treated with bFGF (bFGF), infected with RCAS-Sox2 (Sox2), or treated with bFGF as well as infected with RCAS-Sox2 (bFGF Sox2). Bars shown in all plots are the means and S.D.s from 3 independent RT-PCR reactions. “*” indicates statistically significant at p<0.05 level, and “**” at p<0.01 level.
To confirm the induction of bFGF in the retina and the suppression of PEDF in the RPE by Sox2, we analyzed cultures of Műller glial cells and dissociated RPE cells. In Műller glial cell culture, the level of bFGF mRNA was increased (Fig. 7E), with its IOD ratio to S17 increased 2-fold (p<0.01), from 0.71 ± 0.33 in the control culture infected with RCAS-GFP to 1.98 ± 0.28 in the culture infected with RCAS-Sox2 (Fig. 7F). The level of PEDF expression (Fig. 7E), on the other hand, showed a 3 fold decrease (p<0.01), from 3.07 ± 0.27 in the control to 0.98 ± 0.04 in the experimental culture (Fig. 7F). In dissociated RPE cell culture, levels of bFGF expression was more than doubled (p<0.01) in a culture infected with RCAS-Sox2, in comparison with the control infected with RCAS-GFP (Fig. G, H). In RPE cell cultures infected with RCAS expressing Sox2ΔB, Sox2ΔC, or En-Sox2, the levels of bFGF expression was similar (p>0.05) to that of the GFP control (Fig. 7G, H). In RPE cell cultures infected with RCAS-Sox2, PEDF expression was decreased (Fig. 7I, J).
We cultured dissociated RPE cells in the presence of bFGF, either alone or in combination with RCAS-Sox2, in order to examine bFGF's effect on the expression of PEDF and Sox. We found that bFGF, like Sox2, significantly (p<0.01) reduced PEDF expression (Fig. 7I, J). A further reduction was observed with a combination of bFGF and Sox2 (from 0.22 to 0.01) than with either bFGF (to 0.05) or Sox2 (to 0.15) alone (Fig. 7I, J). We also found that addition of bFGF to RPE cell culture induced Sox2 expression (Fig. 7I).
Discussion
In situ hybridization detected Sox2 mRNA in different retinal cell populations. The most prominent expression of Sox2 was detected in cells of the neuroepithelium, suggesting a primary expression of Sox2 in retinal progenitor cells. Although the number of cells expressing Sox2 significantly decreased starting at E7 in the central retina, as cell proliferation diminished, Műller cells and a small number of cells in the amacrine and ganglion cell layers maintained detectable levels of Sox2 mRNA. These results are in good agreement with previous reports of Sox2 expression in the proliferating cells and a subpopulation of amacrine cells in the chick [24] and mouse retina [22]. The peak of Sox2 expression during active cell proliferation in the chick retina is consistent with its established role in maintaining neural stem cell properties. Expression of Sox2 in Műller glia could be related to its role in maintaining stem cell properties, as Műller glia have been shown to exhibit some properties of retinal stem cells [60-62]. The significance of Sox2 expression in a small number of cells in the amacrine and ganglion cell layers is unclear, and it remains to be examined whether they were quiescent progenitor cells, reminiscent of rod progenitors in the inner nuclear layer of the goldfish retina [66]. In the mouse retina, this particular subgroup of Sox2-expressing cells coexpress neural markers Islet-1 and calretinin [22].
A large number of studies show Sox2's importance in maintaining neural progenitor/stem cell properties, and recent studies have established its role in reprogramming adult fibroblasts into pluripotent stem cells. Yet, our overexpression experiments showed no increase in the size of the retina or the eye, suggesting that Sox2 alone is insufficient to prolong cell proliferation activity in retinal progenitor cells. It remains to be investigated whether prolonging retinal cell proliferation activity by Sox2 will require the other 3 genes in the group of 4 used to reprogram fibroblasts into pluripotent stem cells [11-14]. The most profound effect of overexpression of Sox2 in the developing chick eyes was to change certain RPE cell properties. In the eye, Sox2 induced de-pigmentation and the expression of ganglion cell markers recognized by RA4, 3A10, and 4H6 in cells within the RPE layer. This suggests that Sox2 is able to redirect the differentiation of RPE cells towards that of retinal neurons. Reprogramming by Sox2 also occurred in RPE cell culture, in which genes key to RPE properties were suppressed, and genes associated with neurons were induced. These suggest that a reprogramming of RPE cells toward retinal neurons was initiated by Sox2 both in vivo and in vitro. Phenotypically, Sox2-induced reprogramming of RPE towards retinal neuron bears certain similarities to bFGF-induced RPE reprogramming.
The importance of Sox2 and bFGF signaling in maintaining the undifferentiated state of stem cells is widely known; the underlying mechanisms, however, remain unclear. In osteoblasts, activating bFGF signaling induces Sox2 expression [67]. During neural induction in Xenopus embryos, FGF signaling is not required for the induction of Sox2 expression, but is required for the maintenance of Sox2 expression [68]. Results from the current study suggest an inductive relationship between Sox2 and bFGF. Their spatial patterns of expression overlapped in the developing retina, and their co-expression was detected in some retinal cells. Expression of both Sox2 and bFGF was reduced with Műller glia atrophy and was enhanced by NMDA damage. Furthermore, Sox2 was induced in RPE cell cultures treated with bFGF, while bFGF expression was enhanced by Sox2 in the retina and in Műller glia culture.
Conclusion
The importance of Sox2 and bFGF for stem cells is widely accepted. Our results imply a close relationship between Sox2 expression and bFGF expression. Sox2 can reprogram RPE cells towards retinal neurons both in vivo and in vitro. In future studies, Sox2 may be used in combination with neural differentiation factors to reprogram RPE cells to produce retinal neurons.
Acknowledgements
We thank Dr. Steven McLoon for monoclonal antibody RA4 and Dr. Stephen Hughes for RCAS (B/P) and Cla12Nco. This study is supported by EyeSight Foundation grant 2005-06-21, NIH/NEI grants R01 EY011640 and P30 EY03039, and Research to Prevent Blindness.
Grant support: This study is supported by EyeSight Foundation research grant 2005-06-21, NIH/NEI grants R01 EY011640 and P30 EY03039, and Research to Prevent Blindness.
Footnotes
Commercial relationships: None.
References
- 1.Adler R. A model of retinal cell differentiation in the chick embryo. Prog Retinal Eye Res. 2000;19:529–557. doi: 10.1016/s1350-9462(00)00008-2. [DOI] [PubMed] [Google Scholar]
- 2.Dyer MA, Cepko CL. Regulating proliferation during retinal development. Nat Rev Neurosci. 2001;2:333–342. doi: 10.1038/35072555. [DOI] [PubMed] [Google Scholar]
- 3.Pearson BJ, Doe CQ. Specification of temporal identity in the developing nervous system. Annu Rev Cell Dev Biol. 2004;20:619–647. doi: 10.1146/annurev.cellbio.19.111301.115142. [DOI] [PubMed] [Google Scholar]
- 4.Zaghloul NA, Yan B, Moody SA. Step-wise specification of retinal stem cells during normal embryogenesis. Biol Cell. 2005;97:321–337. doi: 10.1042/BC20040521. [DOI] [PubMed] [Google Scholar]
- 5.Zhao S, Rizzolo LJ, Barnstable CJ. Differentiation and transdifferentiation of the retina pigment epithelium. Int Rev Cytol. 1997;171:225–265. doi: 10.1016/s0074-7696(08)62589-9. [DOI] [PubMed] [Google Scholar]
- 6.Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev Biol. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- 7.Park CM, Hollenberg MJ. Basic fibroblast growth factor induces retinal regeneration in vivo. Div Biol. 1989;134:201–205. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- 8.Pittack C, Jones M, Reh TA. Basic fibroblast growth factor induces retinal pigment epithelium to generate neural retina in vitro. Development. 1991;113:577–588. doi: 10.1242/dev.113.2.577. [DOI] [PubMed] [Google Scholar]
- 9.Guillemot F, Cepko CL. Retinal fate and ganglion cell differentiation are potentiated by acidic FGF in an in vitro assay of early retinal development. Development. 1992;114:743–754. doi: 10.1242/dev.114.3.743. [DOI] [PubMed] [Google Scholar]
- 10.Opas M, Dziak E. bFGF-induced transdifferentiation of RPE to neuronal progenitors is regulated by the mechanical properties of the substratum. Dev Biol. 1994;161:440–454. doi: 10.1006/dbio.1994.1043. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:86–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 14.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 15.Pevny LH, Lovell-Badge R. Sox genes find their feet. Curr Opin Genet Dev. 1997;7:338–344. doi: 10.1016/s0959-437x(97)80147-5. [DOI] [PubMed] [Google Scholar]
- 16.Wegner M. From head to toes: The multiple facets of Sox proteins. Nucleic Acids Res. 1999;7:409–420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avilion AA, Nicolis SK, Pevny LH, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zappone MV, Galli R, Catena, R, et al. Sox2 regulatory sequences direct expression of a (beta)-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development. 2000;127:2367–2382. doi: 10.1242/dev.127.11.2367. [DOI] [PubMed] [Google Scholar]
- 19.Graham V, Khudyakov J, Ellis P, et al. SOX2functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 20.Ferri AL, Cavallaro M, Braida D, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- 21.Bylund M, Andersson E, Novitch BG, et al. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 22.Taranova OV, Magness ST, Fagan MB, et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes & Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagstrom SA, Pauer GJ, Reid J, et al. SOX2 mutation causes anophthalmia, hearing loss, and brain anomalies. Am J Med Genet A. 2005;138:95–98. doi: 10.1002/ajmg.a.30803. [DOI] [PubMed] [Google Scholar]
- 24.Le RD, Rayner K, Rex M, et al. The transcription factor cSox2 and Neuropeptide Y define a novel subgroup of amacrine cells in the retina. J Anat. 2002;200:51–56. doi: 10.1046/j.0021-8782.2001.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes SH, Greenhouse JJ, Petropoulos CJ, et al. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uwanogho D, Rex M, Cartwright EJ, et al. Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev. 1995;49:23–36. doi: 10.1016/0925-4773(94)00299-3. [DOI] [PubMed] [Google Scholar]
- 27.Nowling TK, Johnson LR, Wiebe MS, Rizzino A. Identification of the transactivation domain of the transcription factor Sox-2 and an associated co-activator. J Biol Chem. 2000;275:3810–3818. doi: 10.1074/jbc.275.6.3810. [DOI] [PubMed] [Google Scholar]
- 28.Jaynes JB, O'Farrell PH. Active repression of transcription by the engrailed homeodomain protein. EMBO J. 1991;10:427–433. doi: 10.1002/j.1460-2075.1991.tb07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han K, Manley JL. Functional domains of the Drosophila Engrailed protein. EMBO J. 1993;12:2723–2733. doi: 10.1002/j.1460-2075.1993.tb05934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li CM, Yan RT, Wang SZ. Chick homeobox gene cbx and its role in retinal development. Mech Dev. 2002;116(12):85–94. doi: 10.1016/s0925-4773(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 31.Yan R-T, Wang S-Z. NeuroD induces photoreceptor cell overproduction in vivo and de novo generation in vitro. J Neurobiol. 1998;36:485–496. [PMC free article] [PubMed] [Google Scholar]
- 32.Li C-M, Yan R-T, Wang S-Z. Overexpression of chick NSCL2 causes atrophy of Müller glia and photoreceptor cells. Invest Ophthalmol Vis Sci. 2001;42:3103–3109. [PMC free article] [PubMed] [Google Scholar]
- 33.Mao W, Yan R-T, Wang S-Z. Proneural gene ash1 promotes amacrine cell production in the chick retina. Dev Neurobiol. 2009;69:88–104. doi: 10.1002/dneu.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitrani E, Gruenbaum Y, Shohat H, et al. Fibroblast growth factor during mesoderm induction in the early chick embryo. Development. 1990;109:387–393. doi: 10.1242/dev.109.2.387. [DOI] [PubMed] [Google Scholar]
- 35.Ma W, Yan R-T, Xie W, et al. A role of ath5 in inducing neuroD and the photoreceptor pathway. J Neurosci. 2004;24:7150–7158. doi: 10.1523/JNEUROSCI.2266-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C-M, Yan R-T, Wang S-Z. Misexpression of a bHLH gene, cNSCL1, results in abnormal brain development. Dev Dyn. 1999;215:238–247. doi: 10.1002/(SICI)1097-0177(199907)215:3<238::AID-AJA6>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrington MJ, Sattaysai J, Zappia J, Barrington M. Excitatory amino acids interfere with normal eye growth in posthatch chicks. Curr Eye Res. 1989;8:781–792. doi: 10.3109/02713688909000868. [DOI] [PubMed] [Google Scholar]
- 38.Tung NN, Morgan IG, Ehrlich D. A quantitative analysis of the effects of excitatory neurotoxins on retinal ganglion cells in the chick. Vis Neurosci. 1990;4:217–223. doi: 10.1017/s0952523800003369. [DOI] [PubMed] [Google Scholar]
- 39.Fischer AJ, Morgan IG, Stell WK. Colchicine induces excessive ocular growth and myopia in chicks. Vis Res. 1999;39:685–697. doi: 10.1016/s0042-6989(98)00178-3. [DOI] [PubMed] [Google Scholar]
- 40.Wang S-Z, Adler R. A developmentally regulated basic-leucine zipper-like gene and its expression in embryonic retina and lens. Proc Nat Acad Sci USA. 1994;91:1351–1355. doi: 10.1073/pnas.91.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coulombre AJ. Correlations of structural and biochemical changes in the developing retina of the chick. Am J Anat. 1955;96:153–189. doi: 10.1002/aja.1000960106. [DOI] [PubMed] [Google Scholar]
- 42.Spence SG, Robson JA. An autoradiographic analysis of neurogenesis in the chick retina in vitro and in vivo. Neuroscience. 1989;32:801–812. doi: 10.1016/0306-4522(89)90300-x. [DOI] [PubMed] [Google Scholar]
- 43.Waid DK, McLoon SC. Immediate differentiation of ganglion cells following mitosis in the developing retina. Neuron. 1995;4:117–124. doi: 10.1016/0896-6273(95)90245-7. [DOI] [PubMed] [Google Scholar]
- 44.Liang L, Ma W, Yan R-T, et al. Exploring RPE as a source of photoreceptors: differentiation and integration of transdifferentiating cells grafted into embryonic chick eyes. Invest Ophthalmol Vis Sci. 2006;47:5066–5074. doi: 10.1167/iovs.06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mochii M, Mazak Y, Mizuno N, et al. Role of mitf in differentiation and transdifferentiation of chicken pigmented epithelial cell. Dev Biol. 1998;193:47–62. doi: 10.1006/dbio.1997.8800. [DOI] [PubMed] [Google Scholar]
- 46.Amae Shintaro, Fuse Nobuo, Yasumoto Ken-ichi, et al. Identification of a novel isoform of microphthalmia-associated transcription factor that is enriched in retinal pigment epithelium. Biochem Biophys Res Commun. 1998;247:710–715. doi: 10.1006/bbrc.1998.8838. [DOI] [PubMed] [Google Scholar]
- 47.Martínez-Morales JR, Dolez V, Rodrigo I, et al. OTX2 Activates the Molecular Network Underlying Retina Pigment Epithelium Differentiation. J Biol Chem. 2003;278:21721–21731. doi: 10.1074/jbc.M301708200. [DOI] [PubMed] [Google Scholar]
- 48.Plouhinec JL, Leconte L, Sauka-Spengler T, et al. Comparative analysis of gnathostome Otx gene expression patterns in the developing eye: implications for the functional evolution of the multigene family. Dev Biol. 2005;278:560–575. doi: 10.1016/j.ydbio.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Bovolenta P, Mallamaci A, Briata P, et al. Implication of OTX2 in pigment epithelium determination and neural retina differentiation. J Neurosci. 1997;17:4243–4252. doi: 10.1523/JNEUROSCI.17-11-04243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez-Morales JR, Signore M, Acampora D, et al. Otx genes are required for tissue specification in the developing eye. Development. 2001;128:2019–2030. doi: 10.1242/dev.128.11.2019. [DOI] [PubMed] [Google Scholar]
- 51.Fuhrmann Sabine, Levine Edward M, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127:4599–4609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- 52.Bumsted KM, Barnstable CJ. Dorsal retinal pigment epithelium differentiates as neural retina in the microphthalmia (mi/mi) mouse. Invest Ophthalmol Vis Sci. 2000;41:903–908. [PubMed] [Google Scholar]
- 53.Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–3591. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- 54.Belecky-Adams T, Tomarev S, Li H-S, et al. Pax-6, Prox1, and Chx10 homeobox gene expression correlates with phenotyppic fate of retinal precursor cells. Invest Ophthalmol Vis Sci. 1997;38:1293–1303. [PubMed] [Google Scholar]
- 55.Zaghloul NA, Moody SA. Alterations of rx1 and pax6 expression levels at neural plate stages differentially affect the production of retinal cell types and maintenance of retinal stem cell qualities. Dev Biol. 2007;306:222–240. doi: 10.1016/j.ydbio.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Blomqvist AG, Söderberg C, Lundell I, et al. Strong evolutionary conservation of neuropeptide Y: sequences of chicken, goldfish, and Torpedo marmorata DNA clones. Proc Natl Acad Sci USA. 1992;89:2350–2354. doi: 10.1073/pnas.89.6.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moody SA, Chow I, Huang S. Intrinsic bias and lineage restriction in the phenotype determination of dopamine and neuropeptide Y amacrine cells. J Neurosci. 2000;20:3244–3253. doi: 10.1523/JNEUROSCI.20-09-03244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan R-T, Ma W, Wang S-Z. neurogenin2 elicits the genesis of retinal neurons from cultures of non-neural cells. Proc Natl Acad Sci USA. 2001;98:15014–15019. doi: 10.1073/pnas.261455698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan Run-Tao, Wang Shu-Zhen. Differential induction of gene expression by basic fibroblastgrowth factor and neuroD in cultured retinal pigment epithelial cells. Vis Neurosci. 2000;17:57–164. doi: 10.1017/s0952523800171172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fischer AJ, Reh TA. Müller glia are a potential source of neuronal regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- 61.Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 62.Bernardos RL, Barthel LK, Meyers JR, et al. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tombran-Tink J, Shivaram SM, Chader GJ, et al. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci. 1995;15:4992–5003. doi: 10.1523/JNEUROSCI.15-07-04992.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ortego J, Escribano J, Becerra SP, et al. Gene expression of the neurotrophic pigment epithelium-derived factor in the human ciliary epithelium. synthesis and secretion into the aqueous humor. Invest Ophthalmol Vis Sci. 1996;37:2759–2767. [PubMed] [Google Scholar]
- 65.Ogata N, Wada M, Otsuji T, et al. Expression of pigment epithelium-derived factor in normal adult rat eye and experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;431:1168–1175. [PubMed] [Google Scholar]
- 66.Otteson DC, D'Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- 67.Mansukhani A, Ambrosetti D, Holmes G, et al. Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. J Cell Biol. 2005;168:1065–1076. doi: 10.1083/jcb.200409182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogers CD, Archer TC, Cunningham DD, et al. Sox3 expression is maintained by FGF signaling and restricted to the neural plate by Vent proteins in the Xenopus embryo. Dev Biol. 2008;313:307–319. doi: 10.1016/j.ydbio.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]