Abstract
Background
Children who receive high dose radiation therapy to the hypothalamic-pituitary axis may be at risk for both early and late puberty. Data on risk of altered timing of menarche after higher dose RT, as used in the treatment of CNS tumors, are limited.
Patients & Methods
We evaluated 235 female survivors of CNS tumors, diagnosed between 1970-1986, and >1,000 sibling controls who were participants in the Childhood Cancer Survivor Study, and provided self-reported data on age at menarche.
Results
Survivors of CNS tumors were more likely to have onset of menarche before age ten compared to their siblings (11.9% vs. 1.0%) (Odds Ratio [OR] =14.1, 95% confidence interval [95% CI] 7.0-30.9). Of the 138 survivors who received RT to the HP axis, 20 (14.5%) had onset of menarche before age 10, compared to 4.3% of those who did not receive RT (OR=3.8, 95% CI=1.2-16.5). Age ≤4 years at diagnosis was associated with an increased risk (OR=4.0, 95% CI=1.7-10.0) of early menarche. Additionally, survivors of CNS tumors were more likely than siblings to have onset of menarche after age 16 (10.6% vs. 1.9%) (OR=6.6, 95% CI=3.4-11.4). Doses of RT to the H-P axis >50 Gy (OR=9.0, 95% CI 2.3-59.5) and spinal RT conferred an increased risk of late menarche, as did older age (>10 years) at the time of diagnosis (OR = 3.0, 95% CI 1.3-7.0).
Conclusion
Survivors of CNS tumors are at significant risk of both early and late menarche associated with RT exposure and age at treatment.
Keywords: menarche, puberty, late-effects, brain tumor, pediatric
INTRODUCTION
The onset of puberty and menarche is a complex process, which is under the regulation of the hypothalamic-pituitary (H-P) axis. Damage to the H-P axis can alter the timing of both puberty and menarche in addition to the other hormonal systems regulated by the H-P axis. Children with central nervous system (CNS) tumors in proximity to the H-P axis, and/or children who have a surgical resection or radiation therapy (RT) in this region are at particular risk for injury. Growth hormone deficiency is the most common outcome after hypothalamic injury, however central hypothyroidism is common as well.
The strongest evidence that cranial radiation causes early sexual development and early menarche comes from extensive acute lymphoblastic leukemia (ALL) literature in which cranial radiation in doses between 18-24 Gy were used for CNS prophylaxis of disease.1-8 However, few studies have addressed the effect of radiation on the timing of puberty or menarche in female survivors of CNS tumors. It appears that while lower doses of cranial radiation (18-24 Gy), such as those used in treatment of childhood ALL, increase the incidence of early puberty and menarche, it is unknown whether higher doses of radiation, like those used to treat children with CNS tumors, cause increased rates of early pubertal development.9-12 Additionally, high dose cranial radiation may cause central hypogonadism, while patients who receive craniospinal radiation are at risk for primary hypogonadism, both of which increase the risk for delayed or absent menarche.8, 13 Therefore, we conducted an analysis of the effect of radiotherapy on timing of menarche in survivors of pediatric CNS malignancies within the Childhood Cancer Survivor Study (CCSS) population to determine the incidence of both early and delayed menarche following CNS directed RT among survivors of childhood CNS tumors.
METHODS
The CCSS Cohort
The CCSS is a retrospective cohort of children and adolescents treated for childhood cancer at 26 collaborating institutions in the United States and Canada. Eligibility criteria for the cohort included: diagnosis of childhood cancer with initial treatment at one of the collaborating CCSS institutions between January 1, 1970 and December 31, 1986; diagnosis of cancer before age 21; and survival for at least five years from diagnosis of leukemia, CNS cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, renal tumor, neuroblastoma, soft tissue sarcoma, or bone tumor.
Beginning in 1994 all participants (or parents of participants < 18 years of age) completed a self-administered baseline questionnaire or telephone interview to collect: demographic data, medication usage, physician-diagnosed medical conditions, and health-related behaviors, in addition to recurrence of the primary malignancy or occurrence of a second malignant neoplasm. Since that time, a follow-up questionnaire was sent to all participants asking detailed information about menstrual history including: ever had a menstrual period (yes/no/unsure), age at menarche, and ascertainment of medications required to induce menses. Medical records were abstracted for treatment information including: surgical procedures, chemotherapy, and radiotherapy exposure. Details of this cohort and the study design have been previously described.14 The institutional review board at each participating center reviewed and approved the CCSS protocol and documents sent to participants.
Of the 20,691 participants eligible for the cohort, 2,887 (14.0%) were survivors of CNS tumors. Of these 491 (17.0%) were unable to be located and thus lost to follow-up, 511 (17.7%) refused to participate, and 9 (0.3%) were unable to participate because of language difficulties, resulting in 1,876 participants with CNS tumors. Participants, non-participants, and those lost to follow-up were similar with regard to: sex, cancer diagnosis, age at diagnosis, age when cohort assembled, and type of cancer treatment.14, 15
A random sample of siblings of all CCSS participating cases was invited to participate to provide a comparison population neither diagnosed nor treated with cancer. In the setting where a case had multiple siblings, the sibling of closest age to the case was recruited. Of 6,098 selected siblings, 3,899 (63.9%) (of both sexes) participated in CCSS.
Ascertainment of Cases and Controls
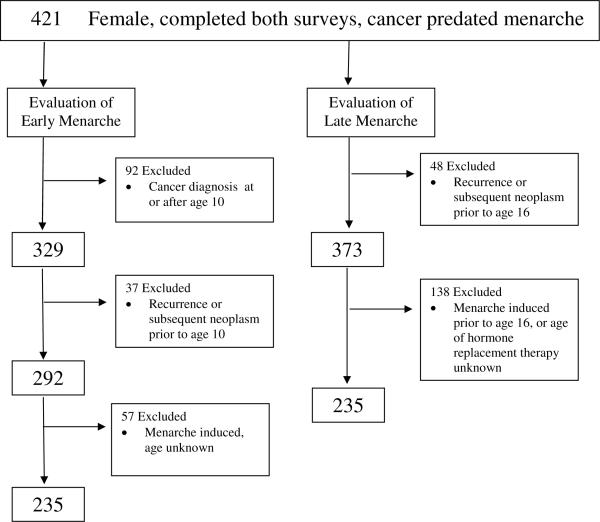
Of the 1,876 participants identified to have primary CNS tumors, 843 (44.9%) were female. Of these females 557 (66.1%) completed both baseline and follow-up surveys, and had complete menarche and radiotherapy exposure data. Of these, 136 experienced menarche prior to cancer diagnosis resulting in 421 females available for evaluation of early and late onset of menarche (Figure 1).
Figure 1.
Female Survivors of CNS Tumors Evaluable for Early and Late Menarche
Evaluation for Early Menarche
Early menarche was defined as menarche before 10 years of age. Of the 421 females available for evaluation, 92 participants were excluded because their cancer was diagnosed at or after age 10; 37 females were excluded due to recurrence//progression of primary malignancy or diagnosis of second malignancy before age 10 or at unknown age; and 57 were excluded because medical therapy to induce menarche was used at an unknown age resulting in 235 participants eligible for evaluation of early menarche
Evaluation for Late Menarche
Late menarche was defined as onset of menarche at 16 years of age or greater. Of the 421 females available for evaluation, 48 were excluded because recurrence/progression of primary malignancy or diagnosis of second malignancy occurred before age 16 and predated onset of menarche, or occurred at unknown age. An additional 138 were excluded because medical therapy to induce menarche was used prior to the age of 16 or at unknown age resulting in 235 participants eligible for evaluation of late menarche. Participants with amenorrhea, who received medical therapy routinely used to induce menarche after the age of 16, were not excluded. Thus, the populations evaluable for both early and late menarche contained 235 participants, 158 (67%) of which were mutually eligible for both evaluations.
The comparison group for the early-menarche analysis comprised 1129 female siblings who reported their age at menarche and did not have medical therapy to induce menarche before age at menarche, or at unknown age. One thousand and fifty-one female siblings were used for comparison in the late-menarche analysis that reported age at menarche, and had no menarche-inducing therapy before age 16.
Survivor treatment exposures were categorized into: surgery alone, surgery with cranial radiotherapy (RT), surgery with cranial RT and chemotherapy, surgery with cranial and spinal RT, and all modalities combined. Absorbed radiation doses to the hypothalamic-pituitary axis were calculated for each participant who received CNS RT based on measurements in a tissue-equivalent phantom and a three-dimensional computer model of the patient. To quantify radiation exposure, the brain was partitioned into four anatomic segments (frontal cortex, temporal lobes including H-P axis, posterior fossa, parietal and occipital cortex) and maximum radiation doses were estimated for each region. For this study, it was assumed that the H-P axis received the full-beam dose if at least half of the total temporal lobe/H-P axis region was included in the beam, otherwise this segment was considered to have received scatter dose. Treatment diagrams and photographs taken in the treatment position were reviewed to make the determination of which brain segments were irradiated. If diagrams were not available, a written description of the medical record was used to estimate the regions included and the dose administered. Further details of the dosimetry method have been previously reported.16, 17
Statistical analysis
Logistic regression was used to investigate the univariate and multivariate associations between early or late menarche and potential risk factors. Models were first fit to compare menarche outcomes between survivors and siblings using generalized estimating equations with robust variance estimates to adjust for case-sibling pairings within the same family.18 Additional sets of models were fit within the survivor population to evaluate diagnosis and treatment related risk factors. Covariates were selected for inclusion in final multivariate models by examining combinations of risk factors and determining the factors which best fit the data using the Akaike Information Criterion.19
To further explore the dose response relationship of CNS radiotherapy with both early and late menarche, we combined the current population of CNS tumor survivors at risk for early menarche (n = 235) and late menarche (n = 235) with a previously published cohort of 949 patients treated for acute lymphoblastic leukemia and evaluated by the CCSS. Eight hundred and seventy-four met the eligibility criteria outlined above for evaluation of early menarche and 678 for late menarche.20 Combining these cohorts provided a wider dose distribution with increased power to detect a dose-response relationship between radiotherapy and risk of early or late menarche. Logistic regression was used to investigate the multivariate associations in this combined analysis of CNS tumor and leukemia survivors.
RESULTS
Subject Characteristics
The mean age at the time of survey completion was 26 years (Standard Deviation [SD] 5.0 years) for the early menarche cohort, 29 years (SD 5.1 years) for the late menarche cohort, and 32 years of age (SD 8.2 years for the early-menarche cohort, 8.1 years for the late-menarche cohort) for each for the sibling comparison groups. Survivors and their siblings shared similar distributions in ethnicity (Table 1). Among the survivors of childhood CNS malignancies, the mean age at diagnosis was 5 years (SD 2.6 years) for the early menarche cohort and 7 years (SD 3.6 years) for the late menarche cohort; however, a significant proportion (43% early menarche, 23% late menarche) were diagnosed and treated by four years of age. Gliomas were the most common histological subtype among both early and late cohorts of five-year survivors. While a significant proportion of this population received surgery alone, most participants received some form of radiotherapy, resulting in 44.9% of the population (46.5% in late menarche cohort) receiving more than 40 Gy to the H-P axis.
Table 1.
Characteristics of Female Survivors of CNS Tumors and Siblings
| Characteristic | Survivors: Early Menarche (n=235) | Siblings: Early Menarche (n=1129) | Survivors: Late Menarche (n=235) | Siblings: Late Menarche (n=1051) |
|---|---|---|---|---|
| Age at cancer diagnosis, years | ||||
| >2 | 25 (10.8) | 14 (6.0) | ||
| 2-4 | 75 (31.9) | 41 (17.4) | ||
| 5-9 | 135 (51.4) | 103 (43.8) | ||
| >10 | 0 | 77 (32.8) | ||
| Histological Subtype | ||||
| Glioma | 152 (64.7) | 147 (62.6) | ||
| Low Grade Glioma | 26 | 20 | ||
| High Grade Glioma | 37 | 30 | ||
| Astrocytoma NOS | 89 | 97 | ||
| Medulloblastoma/PNET | 52 (22.1) | 65 (27.7) | ||
| Other | 31 (13.2) | 23 (9.8) | ||
| Ethnicity | ||||
| Caucasian, non Hispanic | 206 (87.7) | 993 (88.0) | 207 (88.1) | 924 (87.9) |
| Black, non-Hispanic | 10 (4.3) | 27 (2.4) | 9 (3.8) | 25 (2.4) |
| Hispanic/Latino | 8 (3.4) | 39 (3.5) | 7 (3.0) | 37 (3.5) |
| Asian/Native American/Pac. Islander | 1 (0.4) | 12 (1.1) | 1 (0.4) | 12 (1.1) |
| Other | 10 (4.3) | 18 (1.6) | 11 (4.7) | 11 (4.7) |
| Unknown | 0 | 40 (3.5) | 0 | 35 (3.3) |
| Treatment Exposure, % | ||||
| Surgery only | 66 (28.1) | 61 (26.0) | ||
| Surgery + Brain RT | 66 (28.1) | 67 (26.5) | ||
| Surgery + Brain RT + spine RT | 29 (12.3) | 35 (14.9) | ||
| Surgery +Brain RT + chemo | 21 (8.9) | 15 (6.4) | ||
| All 4 treatment modalities | 21 (8.9) | 25 (10.6) | ||
| Chemo only | 2 (0.9) | 0 | ||
| Unknown | 30 (12.8) | 32 (13.6) | ||
| Dose to H-P Axis a | ||||
| None | 82 (41.8) | 72 (38.5) | ||
| 1 - 20 Gy | 0 | 0 | ||
| 20.01 - 30 Gy | 3 (1.5) | 4 (2.1) | ||
| 30.01 - 40 Gy | 23 (11.7) | 24 (12.8) | ||
| 40.01 - 50 Gy | 47 (24.0) | 30 (16.0) | ||
| >50 Gy | 41 (20.1) | 57 (30.5) | ||
| Timing of Menarche | ||||
| Early (<10 years) | 28 (11.9) | 10 (1.0) | ||
| Late (>16 years) | 25 (10.6) | 20 (1.9) |
Percentages exclude survivors (n = 39) and siblings (n = 48) in whom dose was unknown
RT = Radiation Therapy, H-P Axis = Hypothalamic-Pituitary Axis, Gy = Grey
Early menarche risk profile
The mean age at onset of menarche among survivors was 11.9 years (SD 2.1 years), whereas siblings experienced menarche at a mean of 12.7 years (SD 1.5 years) (p<.0001). Survivors of CNS tumors were more likely to have onset of menarche before age ten (11.9%) than their siblings (1%) (OR = 14.1, 95% confidence interval [95% CI] 6.9-30.9).
The risk of early menarche was strongly associated with radiation exposure to the H-P axis. Among the 138 patients with radiation exposure to this location, 14.5% (n = 20) had onset of menarche before age 10, compared to only 4.3% of those who did not receive radiotherapy to the H-P axis (OR = 3.8, 95% CI 1.2-16.5). Notably, 17% of participants receiving >50 Gy to the H-P axis experienced menarche before age 10 (OR vs. no H-P axis RT = 4.6, 95% CI 1.2-22.4). Additionally among all eligible patients, those diagnosed and treated under five years of age had four-fold odds (OR = 4.0, 95% CI 1.7-10.0) of early menarche. There were no observed associations between histological diagnosis, use of chemotherapy, or ethnicity with early age at onset of menarche. Multivariate analysis demonstrated RT dose >50 Gy (OR = 3.7, 95% CI 1.1-13.7) and young age (≤4 years) (OR = 3.9, 95% CI 1.5-11.9) as significant independent risk factors for early menarche (Table 2). RT strata <50 Gy (1-40 Gy: OR = 2.58, 95% CI 0.48-11.96, and 40.01-50 Gy: OR = 1.86, 95% CI 0.48-7.17) demonstrated increased odds of early menarche but did not reach statistical significance.
Table 2.
Risk of Abnormal Timing of Menarche in Survivors of CNS Tumors : Multivariate Model
| Odds Ratio | 95% CI | ||
|---|---|---|---|
| Early Menarche | |||
| RT Dose H-P Axis † | |||
| 1 - 40 Gy | 2.58 | 0.48-11.96 | |
| 40.01 - 50 Gy | 1.86 | 0.48-7.17 | |
| >50 Gy | 3.69 * | 1.07-13.74 | |
| No RT (referent) | 1.00 | - | |
| Age at Diagnosis | |||
| ≤4 years | 3.96 * | 1.48-11.92 | |
| 5-9 years (referent) | 1.00 | - | |
| Late Menarche | |||
| RT to H-P Axis +/- Spine RT | |||
| H-P axis ≤ 50 Gy, no spine RT | 1.84 | 0.21-16.07 | |
| H-P axis ≤ 50 Gy, plus spine RT | 4.73 | 0.86-36.0 | |
| H-P axis > 50 Gy, no spine RT | 6.90 * | 1.46-49.57 | |
| H-P axis >50 Gy, plus spine RT | 12.40 * | 2.66-89.64 | |
| No RT (referent) | 1.00 | - | |
| Age at Diagnosis | |||
| 10-20 years | 2.39 | 0.90-6.45 | |
| 0-9 years (referent) | 1.00 |
= value is statistically significant (p<.05)
RT = Radiation Therapy, H-P Axis = Hypothalamic-Pituitary Axis, Gy = Grey, CI = Confidence Interval
Late Menarche Risk Profile
The mean age of onset of menarche among survivors was 12.4 years (SD 2.3 years) as compared to 12.7 years (SD 1.5 years) in the sibling cohort. The survivor cohort eligible for evaluation of late menarche was more likely to have onset of menarche after age 16 (10.6%) than their siblings (1.9%) (OR = 6.1, 95% CI 3.4-11.4). The risk of late menarche was associated with radiation exposure to both the H-P axis (OR = 4.7, 95% CI 1.3-22.9) and to the spine (OR = 3.0, 95% CI 1.2-7.5). Greatest risk for late menarche was associated with >50 Gy RT to the H-P axis (OR vs. no H-P axis RT = 9.0, 95% CI 2.3-59.5). Additionally, children diagnosed with cancer at an older age (age ≥10 years) were at increased risk for late menarche (OR = 3.0, 95% CI 1.3-7.0) compared to those age less than 10 years from diagnosis. Risk for late menarche was also greater for those diagnosed with medulloblastoma (OR = 3.3, 95% CI 1.4-7.8) than for other diagnostic groups, presumably due to the use of both cranial and spinal RT in this population. There was no association with race, ethnicity, or use of alkylating chemotherapy, though notably, few participants (n = 33) received alkylating agents during this era.
Multivariate analysis was used to further assess the independent effects of RT to both the H-P axis and the spine with the development of late menarche (Table 2). Increasing risk for late menarche was observed across these combinations of RT exposure with participants receiving >50 Gy RT to the H-P axis, without spine RT (OR = 6.9, 95% CI 1.5-49.6) and participants receiving >50 Gy RT to the H-P axis with additional spine RT (OR = 12.4, 95% CI 2.7-89.6) having the greatest risk for late onset of menarche.
Evaluation of Timing of Menarche Across a Broad Range of Radiation Doses by Combining CNS Tumor and Leukemia Survivors
We performed additional analysis combining this CNS tumor population with a population treated for acute lymphoblastic leukemia with CNS-directed RT and, thus, at risk for abnormal timing of menarche in order to identify if a dose-response relationship between RT and timing of menarche existed. Multivariate analysis (Table 3), controlling for diagnosis (CNS tumor vs. leukemia), H-P axis exposure to RT at any dose was significantly associated with an increased risk for early menarche with H-P axis dose >50 Gy conferring the highest risk (OR = 5.7, 95% CI 1.6-22.1). Age ≤4 years at the time of diagnosis remained a significant independent risk for early menarche (OR = 4.2, 95% CI, 2.1-8.6) after controlling for RT exposure. A test for trend across RT dose to the H-P axis was statistically significant (p <0.001).
Table 3.
Risk of Early Menarche in Survivors of Childhood CNS Tumors and Acute Lymphoblastic Leukemia: Multivariate Model
| Odds Ratio | 95% CI | |
|---|---|---|
| RT Dose to H-P axis † | ||
| 1-19.99 Gy | 4.25 * | 1.88-12.28 |
| 20-29.99 Gy | 3.55 * | 1.42-9.70 |
| 30-49.99 Gy | 4.01 * | 1.30-13.27 |
| >50 Gy | 5.68 * | 1.57-22.10 |
| No RT (referent) | 1.00 | - |
| Age at Diagnosis | ||
| 0-4 | 4.06 * | 2.09-8.58 |
| 5-9 (referent) | 1.00 | |
| Diagnosis | ||
| Leukemia | 0.34 | .11-1.11 |
| CNS Tumors (referent) | 1.00 |
= value is statistically significant (p<.05)
RT = Radiation Therapy, H-P Axis = Hypothalamic-Pituitary Axis, Gy = Grey, CI = Confidence Interval
For assessment of dose response for development of late menarche (Table 4), controlling for diagnosis and spinal radiation exposure, only RT exposure >50 Gy (OR 13.24, 95% CI 1.81-142.7) was significantly associated with late menarche, however, evidence for a dose-response relationship with RT exposure to the H-P axis may exist (p<0.008, test for trend) Older age at the time of RT exposure (OR 3.14, 95% CI 1.31-7.66) retains significance in this model.
Table 4.
Risk of Late Menarche in Survivors of Childhood CNS Tumors and Acute Lymphoblastic Leukemia: Multivariate Model✶
| Odds Ratio | 95% CI | |
|---|---|---|
| Age at Diagnosis | ||
| 5-9 | 0.67 | 0.25-1.73 |
| 10-20 | 3.14 ✶ | 1.31-7.66 |
| 0-4 | 1.00 | |
| RT Dose to H-P axis † | ||
| <=20 Gy | 1.00 | 0.18-5.58 |
| >20 - <=30 Gy | 3.17 | 0.96-14.39 |
| >30 - <=50 Gy | 6.34 | 0.81-60.51 |
| >50 Gy | 13.24 ✶ | 1.81-142.17 |
Controling for primary cancer diagnosis and spinal RT exposure
* = value is statistically significant (p<.05)
RT = Radiation Therapy, H-P Axis = Hypothalamic-Pituitary Axis, Gy = Grey, CI = Confidence Interval
DISCUSSION
The onset of puberty and menarche marks a time of rapid linear growth, sexual development, and transition from childhood to maturity. As a result, children experience the appearance of secondary sexual characteristics, the adolescent growth spurt, and the establishment of fertility. This occurs as a consequence of CNS maturation and release of pituitary gonadotropins resulting in stimulation of gonadal end organs (testis/ovaries).21 The diagnosis and treatment of a childhood CNS malignancy prior to the onset of puberty has the potential to profoundly impact the timing and the tempo of both puberty and menarche. The clinical impact is particularly important as patients with early onset of puberty are at increased risk for premature epiphyseal fusion and shortened final height. The risk of short stature is compounded by the increased incidence of growth hormone deficiency in this population.22, 23 Large epidemiologic studies have identified small increases in risk for breast cancer among girls with early onset of puberty24. Additionally, girls with true precocious puberty are at increased risk for a number of behavioral problems, increased social withdrawal, and potential sexual abuse.24, 25 Patients with delayed puberty and menarche are at increased risk for low bone mineral density and long-term risk for osteoporosis, in addition to incomplete sexual development and reduced fertility.26
Early identification of the association between CNS directed radiotherapy and early onset of puberty occurred in populations treated for childhood ALL. However, far less research has occurred describing alterations of pubertal and menarchal timing in patients with CNS tumors, who typically receive higher doses of RT than children treated for ALL. Investigations to date have been limited to case series or small retrospective studies with limited ability to identify risk factors for early maturity.9, 27-29
Brauner et. al., identified the risk for precocious puberty in patients with CNS tumors following cranial radiation doses of 24-45 Gy delivered to the hypothalamic-pituitary region in a case series of 6 patients.27 Five of these six children, in addition, had growth hormone deficiency, and a blunted growth spurt resulting in extremely short stature. Ogilvy-Stuart et. al., followed by reporting on a small retrospective cohort of 46 CNS tumor survivors suggesting that onset of puberty occurred at an earlier age in both sexes compared to population norms.9 Similarly, Oberfield, et. al., has reported, in a retrospective cohort of 36 patients, that females, but not males, experienced early onset of puberty after treatment for CNS tumors. Thus, the current study represents the largest population of CNS tumor survivors evaluated for early onset of menarche to date and includes a large sibling cohort for comparison. Our data demonstrate that female survivors of CNS tumor have a greater than fourteen-fold risk of developing menarche before the age of 10 years, compared to siblings.
Previously, both Ogilvy-Stuart et al and Oberfield et al, suggested that younger age at diagnosis was a significant risk factor for premature puberty. Our data further corroborate this increased risk for early puberty in younger children with CNS tumors. More difficult to determine in these smaller studies, however, is whether there is an effect of increasing doses of radiotherapy (dose-response effect) on the risk of early menarche. Our combined analysis of both ALL and CNS tumor populations provides data on over 1,000 patients who received a broad spectrum of radiation doses for analysis. It is clear from these data that a wide range of doses of RT to the H-P axis is associated with an increased risk of early menarche; the threshold for this effect lies somewhere below 20 Gy. However, while the test for trend suggests a statistically significant dose-response relationship exists, the small increase in risk across these dose ranges may be clinically insignificant.
Whole brain and/or focal radiation to the H-P axis places patients at risk not only for early puberty, but also for the gradual onset of hypothalamic-pituitary failure which may result in gonadotropin deficiency and subsequent pubertal delay.30 A previous evaluation of 251 patients, suggested a greater incidence of gonadotropin deficiency in patients who received 35-45 Gy compared to those who received only 20 Gy.31 Additionally, females who receive spinal radiation may have added risk of delayed puberty or menarche due to direct gonadal damage20. In addition to quantifying this risk for delayed menarche among survivors of CNS tumors at greater than six-fold, our findings document that cranial RT doses >50 Gy confer the greatest risk for delayed menarche. The addition of spinal radiation augments this risk, such that patients who received both >50 Gy to the H-P axis and spinal RT have a twelve-fold increase in their risk of delayed menarche compared to patients with CNS tumors who did not receive RT. While exposure to alkylating agents is known to increase the risk of gonadal damage, no such association was noted in this study, likely due to the small number of participants who received alkylators for treatment of CNS tumors in this era32.
The self-report nature of data collection may be a limitation of this study, although previous work suggests women's recall of age at menarche is generally quite accurate.33 Additionally, recall of menarche is better than that of other pubertal milestones.34 However, lack of data on other pubertal milestones prevented assessment of tempo of female puberty. Also, 17% of the eligible population was lost to follow-up and an additional 18% refused participation creating the potential for bias within this analysis. However, previous analysis has determined few differences between participants in the CCSS cohort, non-participants, and those lost to follow-up reducing the potential for participation bias14. An additional limitation was our inability to define the precise location of tumor in relation to the H-P axis, and the extent of surgical intervention in this cohort. Thus, it is unclear to what extent tumor location and surgical resection may have independently affected timing of menarche. Finally, this analysis is unable to determine the contribution of chemotherapy, most significantly alkylating agents, to altered timing of menarche, due to the relatively few number of survivors who received chemotherapy during this treatment era (1970-1986). We are currently expanding the CCSS cohort to include patients diagnosed between 1987-1999, a period in which chemotherapy was used increasingly for treatment of CNS tumors. Future investigations of this cohort will be needed to determine independent risk for altered timing of menarche associated with these agents.
In summary, radiation used for treatment of CNS tumors confers a significant risk for both early and delayed timing of menarche. Thus, it is imperative that clinicians following these patients anticipate this increased propensity for altered pubertal timing in those at greatest risk. Advances in the delivery of RT to the CNS over the last decade, with the use of techniques such as conformal RT and proton beam RT, may limit the dose exposure to the H-P axis and reduce the subsequent risk for some of these abnormalities. Future investigation of large cohorts of survivors of childhood CNS tumors will be necessary to confirm these expectations.
ACKNOWLEDGEMENTS
CCSS is supported by a grant from the National Cancer Institute (U24 CA55727, L.L. Robison, Principal Investigator) and the American Lebanese Syrian Associated Charities (ALSAC - St. Jude Children's Research Hospital).
Abbreviations
- (H-P)
Hypothalamic-pituitary
- (CNS)
Central Nervous System
- (RT)
Radiation Therapy
- (ALL)
Acute Lymphoblastic Leukemia
- (CCSS)
Childhood Cancer Survivor Study
- (OR)
Odds Ratio
- (SD)
Standard Deviation
Footnotes
Conflicts of Interest: None of the authors report a conflict of interest or report a financial or personal relationship that could inappropriately bias their work.
REFERENCES
- 1.Leiper AD, Stanhope R, Kitching P, Chessells JM. Precocious and premature puberty associated with treatment of acute lymphoblastic leukaemia. Arch Dis Child. 1987;62(11):1107–12. doi: 10.1136/adc.62.11.1107. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=3479948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasqualini T, Escobar ME, Domene H, Muriel FS, Pavlovsky S, Rivarola MA. Evaluation of gonadal function following long-term treatment for acute lymphoblastic leukemia in girls. Am J Pediatr Hematol Oncol. 1987;9(1):15–22. doi: 10.1097/00043426-198721000-00004. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=3109271. [DOI] [PubMed] [Google Scholar]
- 3.Moell C, Garwicz S, Westgren U, Wiebe T. Disturbed pubertal growth in girls treated for acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1987;4(1):1–5. doi: 10.3109/08880018709141243. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=3152908. [DOI] [PubMed] [Google Scholar]
- 4.Quigley C, Cowell C, Jimenez M, Burger H, Kirk J, Bergin M, et al. Normal or early development of puberty despite gonadal damage in children treated for acute lymphoblastic leukemia. N Engl J Med. 1989;321(3):143–51. doi: 10.1056/NEJM198907203210303. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=2501681. [DOI] [PubMed] [Google Scholar]
- 5.Maneschi F, Fugardi MG, Corsello G, LoCurto M. Pubertal maturation in girls treated for childhood acute leukaemia. Eur J Pediatr. 1991;150(9):630–3. doi: 10.1007/BF02072622. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=1915514. [DOI] [PubMed] [Google Scholar]
- 6.Hokken-Koelega AC, van Doorn JW, Hahlen K, Stijnen T, de Muinck Keizer-Schrama SM, Drop SL. Long-term effects of treatment for acute lymphoblastic leukemia with and without cranial irradiation on growth and puberty: a comparative study. Pediatr Res. 1993;33(6):577–82. doi: 10.1203/00006450-199306000-00008. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=8378115. [DOI] [PubMed] [Google Scholar]
- 7.Melin AE, Adan L, Leverger G, Souberbielle JC, Schaison G, Brauner R. Growth hormone secretion, puberty and adult height after cranial irradiation with 18 Gy for leukaemia. Eur J Pediatr. 1998;157(9):703–7. doi: 10.1007/s004310050918. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=9776525. [DOI] [PubMed] [Google Scholar]
- 8.Mills JL, Fears TR, Robison LL, Nicholson HS, Sklar CA, Byrne J. Menarche in a cohort of 188 long-term survivors of acute lymphoblastic leukemia. J Pediatr. 1997;131(4):598–602. doi: 10.1016/s0022-3476(97)70069-6. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=9386666. [DOI] [PubMed] [Google Scholar]
- 9.Ogilvy-Stuart AL, Clayton PE, Shalet SM. Cranial irradiation and early puberty. J Clin Endocrinol Metab. 1994;78(6):1282–6. doi: 10.1210/jcem.78.6.8200926. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=8200926. [DOI] [PubMed] [Google Scholar]
- 10.Rappaport R, Brauner R, Czernichow P, Thibaud E, Renier D, Zucker JM, et al. Effect of hypothalamic and pituitary irradiation on pubertal development in children with cranial tumors. J Clin Endocrinol Metab. 1982;54(6):1164–8. doi: 10.1210/jcem-54-6-1164. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=6804477. [DOI] [PubMed] [Google Scholar]
- 11.Roth C, Lakomek M, Schmidberger H, Jarry H. Cranial irradiation induces premature activation of the gonadotropin-releasing-hormone. Klin Padiatr. 2001;213(4):239–43. doi: 10.1055/s-2001-16854. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=11528557. [DOI] [PubMed] [Google Scholar]
- 12.Roth C, Schmidberger H, Schaper O, Leonhardt S, Lakomek M, Wuttke W, et al. Cranial irradiation of female rats causes dose-dependent and age-dependent activation or inhibition of pubertal development. Pediatr Res. 2000;47(5):586–91. doi: 10.1203/00006450-200005000-00005. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=10813581. [DOI] [PubMed] [Google Scholar]
- 13.Noorda EM, Somers R, van Leeuwen FE, Vulsma T, Behrendt H. Adult height and age at menarche in childhood cancer survivors. Eur J Cancer. 2001;37(5):605–12. doi: 10.1016/s0959-8049(00)00438-x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=11290436. [DOI] [PubMed] [Google Scholar]
- 14.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–39. doi: 10.1002/mpo.1316. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=11920786. [DOI] [PubMed] [Google Scholar]
- 15.Mertens AC, Walls RS, Taylor L, Mitby PA, Whitton J, Inskip PD, et al. Characteristics of childhood cancer survivors predicted their successful tracing. J Clin Epidemiol. 2004;57(9):933–44. doi: 10.1016/j.jclinepi.2004.01.005. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=15504636. [DOI] [PubMed] [Google Scholar]
- 16.Packer RJ, Gurney JG, Punyko JA, Donaldson SS, Inskip PD, Stovall M, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21(17):3255–61. doi: 10.1200/JCO.2003.01.202. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=12947060. [DOI] [PubMed] [Google Scholar]
- 17.Stovall M, Weathers R, Kasper C, Smith SA, Travis L, Ron E, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166(1 Pt 2):141–57. doi: 10.1667/RR3525.1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=16808603. [DOI] [PubMed] [Google Scholar]
- 18.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–60. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=3233245. [PubMed] [Google Scholar]
- 19.Akaike H. A new look at the statistical identification model. IEEE Transactions on Automatic Control. 1974;19:716–23. [Google Scholar]
- 20.Chow EJ, Friedman DL, Yasui Y, Whitton JA, Stovall M, Robison LL, et al. Timing of menarche among survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2007 doi: 10.1002/pbc.21316. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=17729247. [DOI] [PubMed] [Google Scholar]
- 21.Styne D, Grumbach MM. Puberty: Ontogeny, Neuroendocrinology, Physiology, and Disorders. In: Kronenberg H, editor. Williams Textbook of Endocrinology. 11th edition Saunders El Sevier; Phhiladelphia: 2008. pp. 969–1166. [Google Scholar]
- 22.Stubberfield TG, Byrne GC, Jones TW. Growth and growth hormone secretion after treatment for acute lymphoblastic leukemia in childhood. 18-Gy versus 24-Gy cranial irradiation. J Pediatr Hematol Oncol. 1995;17(2):167–71. doi: 10.1097/00043426-199505000-00012. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=7749768. [DOI] [PubMed] [Google Scholar]
- 23.Birkebaek NH, Fisker S, Clausen N, Tuovinen V, Sindet-Pedersen S, Christiansen JS. Growth and endocrinological disorders up to 21 years after treatment for acute lymphoblastic leukemia in childhood. Med Pediatr Oncol. 1998;30(6):351–6. doi: 10.1002/(sici)1096-911x(199806)30:6<351::aid-mpo9>3.0.co;2-d. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=9589084. [DOI] [PubMed] [Google Scholar]
- 24.Golub MS, Collman GW, Foster PM, Kimmel CA, Rajpert-De Meyts E, Reiter EO, et al. Public health implications of altered puberty timing. Pediatrics. 2008;121(Suppl 3):S218–30. doi: 10.1542/peds.2007-1813G. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=18245514. [DOI] [PubMed] [Google Scholar]
- 25.Sonis WA, Comite F, Blue J, Pescovitz OH, Rahn CW, Hench KD, et al. Behavior problems and social competence in girls with true precocious puberty. J Pediatr. 1985;106(1):156–60. doi: 10.1016/s0022-3476(85)80489-3. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=3965676. [DOI] [PubMed] [Google Scholar]
- 26.Ho AY, Kung AW. Determinants of peak bone mineral density and bone area in young women. J Bone Miner Metab. 2005;23(6):470–5. doi: 10.1007/s00774-005-0630-7. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=16261454. [DOI] [PubMed] [Google Scholar]
- 27.Brauner R, Rappaport R. Precocious puberty secondary to cranial irradiation for tumors distant from the hypothalamo-pituitary area. Horm Res. 1985;22(1-2):78–82. doi: 10.1159/000180076. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=4029883. [DOI] [PubMed] [Google Scholar]
- 28.Constine LS, Woolf PD, Cann D, Mick G, McCormick K, Raubertas RF, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328(2):87–94. doi: 10.1056/NEJM199301143280203. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=8416438. [DOI] [PubMed] [Google Scholar]
- 29.Oberfield SE, Soranno D, Nirenberg A, Heller G, Allen JC, David R, et al. Age at onset of puberty following high-dose central nervous system radiation therapy. Arch Pediatr Adolesc Med. 1996;150(6):589–92. doi: 10.1001/archpedi.1996.02170310023003. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=8646307. [DOI] [PubMed] [Google Scholar]
- 30.Toogood AA. Endocrine consequences of brain irradiation. Growth Horm IGF Res. 2004;14(Suppl A):S118–24. doi: 10.1016/j.ghir.2004.03.038. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=15135792. [DOI] [PubMed] [Google Scholar]
- 31.Littley MD, Shalet SM, Beardwell CG, Robinson EL, Sutton ML. Radiation-induced hypopituitarism is dose-dependent. Clin Endocrinol (Oxf) 1989;31(3):363–73. doi: 10.1111/j.1365-2265.1989.tb01260.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=2559824. [DOI] [PubMed] [Google Scholar]
- 32.Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91(5):1723–8. doi: 10.1210/jc.2006-0020. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=16492690. [DOI] [PubMed] [Google Scholar]
- 33.Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155(7):672–9. doi: 10.1093/aje/155.7.672. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=11914195. [DOI] [PubMed] [Google Scholar]
- 34.Casey VA, Dwyer JT, Coleman KA, Krall EA, Gardner J, Valadian I. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Ann Hum Biol. 1991;18(2):155–66. doi: 10.1080/03014469100001492. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citati on&list_uids=2024949. [DOI] [PubMed] [Google Scholar]