Abstract
Background
The molecular mechanisms of stress-induced depressive behaviors have been characterized extensively in male rodents; however, much less is known about female subjects, despite the fact that human depression is far more prevalent in women.
Methods
To gain insight into these mechanisms, we performed microarray analysis in nucleus accumbens (NAc), a key brain reward region implicated in depression, in ovariectomized (OVX) and gonadally intact female mice after chronic unpredictable stress and measured stress-induced depression-like behavior in the forced swim test (FST). Male mice were studied in the FST for comparison.
Results
We find that stress regulation of genes in NAc of gonadally intact female mice is blunted in OVX mice. This pattern of gene regulation is consistent with behavioral findings on the FST: the pro-depression-like effect of stress in intact female mice is absent in OVX female and gonadally intact male mice. We identified, among many genes regulated by stress, several nuclear factor κB(NFκB) subunits—a pro-survival transcription factor involved in cellular responses to stress—as being highly upregulated in NAc of OVX mice. Given the role of NFκB during stress, we hypothesized that upregulation of NFκB by OVX decreases susceptibility to stress. Indeed, we show that inhibition of NFκB in NAc of OVX animals increases susceptibility to stress-induced depressive behaviors, whereas activation of NFκB in NAc of intact female subjects blocks susceptibility.
Conclusions
These results suggest a hormonal mechanism of NFκB regulation that contributes to stress-induced depressive behaviors in female subjects and might represent a mechanism for gender differences in prevalence rates of these disorders in humans.
Keywords: Depression, estrogen, gender, gene expression, nucleus accumbens, nuclear factor κB, stress, progesterone, sex differences
Stress is a common factor in the onset of many depressive disorders in humans. Among the >10% of the US population suffering from major depression (1), a disproportionately high number are women (2), leading some to hypothesize that the presence of ovarian hormones during a stressful life event might be a risk factor for the development of depressive symptoms (3). The majority of previously published data in rodents suggest that male subjects are generally more resistant than female subjects to the effects of chronic stress on certain aspects of depressive-like behavior (4 – 6), a finding that might be related to gender differences in the reorganization of neural circuitry in response to stress (7). However, the effects of chronic stress on neural and behavioral plasticity are far less characterized in female rodents compared with male rodents, despite the predominance of the human syndrome in women.
Although the literature is conflicting, it is generally thought that ovarian hormones contribute to the enhanced stress sensitivity in women and might be a predisposing factor in several psychiatric disorders, including depression (6) and addiction (8,9). There are many reports indicating that administration of estrogen (E) or progesterone (P) to ovariectomized (OVX) female rats can alleviate depressive-like behaviors or facilitate antidepressant action (10–15); however, these studies did not measure the relative contribution of endogenous hormones to the stress response in female subjects. In fact, there is evidence that endogenous gonadal hormones in female subjects might be involved in regulating certain aspects of stress-induced depressive behavior. For example, gonadal hormones influence stress-induced release of corticosterone (16–18) and stress-induced cognitive function (19–24), both of which are affected in depression.
In the present study, we examined the role of endogenous ovarian hormones in stress-induced depression-like behavior. We also analyzed hormone effects on stress regulation of gene expression in the nucleus accumbens (NAc), a key brain reward region implicated in depression (25–27). We find that chronic unpredictable stress (CUS) in female subjects produces a dramatic pro-depressant effect in the forced swim test (FST) that is mediated, at least in part, through ovarian hormones. We show further that stress-induced gene expression changes in the NAc are generally blunted in OVX mice, consistent with the behavioral findings. Finally, our data suggest a novel nuclear factor κB (NFκB)-dependent mechanism for ovarian hormone regulation of depression-like behaviors.
Methods and Materials
Animals
Eight- to ten-week-old OVX c57bl/6 female or male mice from Charles River (Wilmington, Massachusetts) weighing 20–30 g were used for these studies. All animals were habituated to the animal facility for at least 1 week before experimental manipulation and maintained at 23°–25°C on a 12-hour light/dark cycle in which lights were on between 7:00 am and 7:00 pm. All animals were provided with food and water ad libitum. Experiments were conducted in accordance with guidelines of the Society for Neuroscience and the institutional animal care and use committees at Mount Sinai School of Medicine and The University of Texas Southwestern Medical Center. Separate cohorts of mice naïve to any behavioral testing were used for all biochemical and molecular assays.
Ovariectomy Surgery
In some experiments, NFκB-LacZ transgenic reporter mice (28) were ovariectomized at approximately 8–10 weeks of age. Mice were deeply anesthetized with ketamine (100 mg/kg) and xylazine (100 mg/kg), and a small incision (.3 cm) was made bilaterally to access both ovaries. A suture knot (3.0 silk) was tied around the blood supply to the ovary; the ovary was excised and discarded. The muscle layer was then closed with a suture and the skin was closed with a wound clip. After surgery, mice were allowed 7–10 days' recovery. Wound clips were then removed and, after an additional week of recovery, animals were exposed to the CUS paradigm described in the following text.
Viral-Mediated Gene Transfer
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and positioned in a small-animal stereotaxic instrument (Kopf Instruments, Tujunga, California), and the skull surface was exposed. With a 33-gauge syringe needle (Hamilton, Reno, Nevada), .5 μL of a herpes simplex virus (HSV) vector expressing Inhibitor of kappa b kinase (IKKβ) mutants was infused bilaterally into the NAc at a rate of .1 mL/min (29).
Coding sequences for the β form of either a dominant negative mutant (IKKdn; Lysine 44 was substituted with Methionine) (30) or a constitutively active mutant (IKKca; Serine 177 and 181 were mutated to glutamate) (31) were subcloned into the bi-cistronic p1005+ Herpes simplex virus (HSV) plasmid (32).
CUS
For CUS, we used a stress protocol that does not induce depressive behavior in male subjects but is consistent with Willner's model to prevent stress habituation (33). Mice were exposed to 1 hour of unpredictable stress each day for 6 days, consisting of footshock, tail suspension, or restraint on different days. On days 1 and 4, animals received a mild footshock. Mice were placed inside fear conditioning boxes (Med Associates, St. Albans, Vermont) and received 100 random shocks of .45 mA (3 sec each) over a period of 1 hour. On days 2 and 5, animals were subjected to tail suspension stress. All mice were fixed to a bar and left to hang in an inverted position for a period of 1 hour. On days 3 and 6, animals were administered a restraint stress, in which they were placed inside a 50 mL falcon tube to prevent movement for 1 hour. After each stress, animals were returned to their home cage for the duration of the CUS protocol. All control animals were handled daily and group housed throughout the experiment.
RNA Isolation and Microarrays
Twenty-four hours after the last stress event, animals were rapidly decapitated, and brains were removed and placed on ice. Dissections of NAc were taken with a 14-gauge needle punch and quickly frozen on dry ice until RNA was extracted. Bilateral punches were pooled from four animals, totaling 3 independent tissue samples/group (total 12 mice/group). The RNA isolation, microarray processing, and data analysis were performed as previously described (34). Briefly, RNA was isolated with TriZol reagent (Invitrogen, Carlsbad, California), further purified with the RNAeasy micro kit from Qiagen (Hilden, Germany), and checked for quality with Agilent's Bioanalyzer (Santa Clara, California). Reverse transcription, amplification, labeling, and hybridization to Illumina (San Diego, California) MouseWG-6 V2.0 arrays were performed with standard procedures by University of Texas Southwestern's microarray core. Raw data were background subtracted and quantile normalized with Beadstudio software (Illumina). Normalized data were then analyzed with GeneSpring software (Agilent), and genelists were generated with significance criteria of 1.3 fold change cutoff coupled with a nonstringent p value cutoff of p < .05.
To ensure validity of our microarray experiments, all animals were handled, treated, and killed at the same time, under the same conditions. As well, all RNA and array processing were performed at the same time. Additionally, all microarray data reported here were from triplicate arrays, in which we pooled multiple animals/array sample, thereby minimizing differences due to individual variability and increasing statistical power (35). Next, we performed a pilot microarray experiment under the same experimental conditions but with a smaller number of arrays (n = 1 or 2 depending on group) and found the same global patterns of stress-induced gene regulation. Finally, the data analysis criteria used for our study are recommended by the MicroArray Quality Control (MAQC) project, because these criteria have been validated to have the highest intersite reproducibility and interplatform and intraplatform reproducibility (36,37).
Real-Time Quantitative Polymerase Chain Reaction
Real-time polymerase chain reaction (PCR) was performed for validation of stress and OVX regulated genes as previously described (38). Briefly, PCR was carried out with an Applied Biosystems 7500 RT PCR system (Foster City, California) with the following cycle parameters: 10 min at 95°C; 40 cycles of 95°C for 1 min, 60°C for 33 sec, 72°C for 33 sec; graded heating to 95°C to generate dissociation curves for confirmation of single PCR products. Data were analyzed by comparing C(t) values of the treatment condition (stress or OVX) with the control condition with the 2∧-ΔΔC(t) method (39). Briefly, these values were averaged for all of the control samples, and this value (average ΔC(t)) was subtracted from the ΔC(t) generated for each sample in the preceding text. This gives the ΔΔC(t) value for each sample. The value 2 was raised to the negative power of each ΔΔC(t), giving the fold change induced by stress or OVX in regulated genes. Samples were normalized such that control samples showed no fold change.
Immunohistochemistry and Stereology
Mice were sequentially perfused with cold 1× phosphate buffered saline (PBS) followed by 4% paraformaldehyde in 1× PBS at a pH of 7.4, and the intact brains were removed, cryoprotected in PBS containing 30% sucrose, and later sectioned and processed for immunohistochemistry according to previously published protocols (40). Briefly, sections were incubated in blocking buffer (containing rabbit serum) and then with anti-goat β-galactosidase (β-gal) (Biogenesis, Kingston, New Hampshire) primary antibody. Sections were then incubated with a biotinylated rabbit anti-goat secondary antibody (Dako, Carpinteria, California) and avidin-biotin complexes followed by peroxidase labeling with a DAB substrate kit (Vector, Burlingame, California). Neurons containing β-gal were analyzed under brightfield microscopy at 100× magnification with a Zeiss Axioimager (Thornwood, New York). For stereological quantification we used methods previously described (41) with Stereoinvestigator software from MicroBrightField (Williston, Vermont).
FST
The FST was performed according to previously published protocols (29,42). Mice were tested in a 4-L Pyrex glass beaker containing 3 L of water at 24 ± 1°C for 6 min. Two trained observers, blinded to the experimental condition, manually scored video from each 6-min session. Total immobility was measured as the time spent without noticeable movement except for single limb paddling to maintain flotation.
Elevated Plus Maze
The elevated plus maze (EPM) was performed as previously described (43). Mice were placed in the center of an elevated plus maze (arms are 33 cm–5 cm, with 25-cm-tall walls on the closed arms) under dim lighting and their behavior was videotaped for 5 min. The time spent on the closed and open arms as well as the number of explorations of open and closed arms, frequency of rearing, and mean velocities were determined by video tracking software (Ethovision 3.0 Noldus, Leesburg, Virginia). Time spent on the open arm and the numbers of entries to the open arm are both negatively correlated with anxiety-like behavior and increased by anxiolytic medications.
Statistical Analysis
All data are expressed as the mean ± SEM. Mean differences between groups were determined with a Student t test at p < .05 or analyses of variance (ANOVAs) and Newman Keuls post hoc analysis when the main effect or interaction was significant. Statistical analyses were conducted with software from GraphPad Prism 4.0 (San Diego, California).
Results
OVX Blunts CUS-Induced Responses in the FST
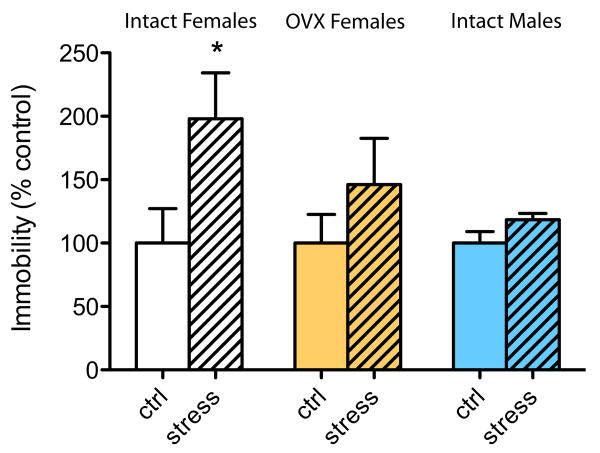
Intact and OVX female mice were subjected to CUS for 6 consecutive days and then tested for depressive- and anxiety-like behaviors in the FST and EPM tests. Intact male mice were studied for comparison. Two-way ANOVAs revealed a main effect of stress and an interaction between Stress and OVX [ANOVA F(1,53) = 9.535, p < .05, Main effect of STRESS and F(2,53) = 2.931, p = 0.06, Interaction STRESS × OVX]. As shown in Figure 1, stress causes a significant increase in the duration of immobility in normal gonadally intact female subjects (p < .05), an effect that was not observed in OVX female subjects (p > .05) or in intact male subjects (p > .05). These findings suggest that endogenous gonadal hormones in female subjects enhance the animals' sensitivity to stress. We also did not observe any significant effects of stress or OVX on the time spent in the open arm of an EPM, between the groups (p > .05) (Supplement 1), indicating that, unlike the case for the FST, these manipulations did not affect behavior in a model of anxiety.
Figure 1.
Ovarian hormones mediate chronic unpredictable stress (CUS)-induced depressive-like behavior in the forced swim test (FST). Intact and ovariectomized (OVX) female mice (n = 9 –10) were subjected to CUS and tested for depressive behavior in the FST. A significant increase in the duration of immobility (ranging from 50 to 180 sec immobile), which reflects a pro-depressant phenotype, was observed in intact stressed female subjects but not in OVX female subjects or intact male subjects, compared with normal-handled control subjects (ctrl). Data are expressed as % of baseline (nonstressed) immobility (which did not differ among the treatment groups) to normalize data across several experiments. *indicates statistically significant differences at p < .05.
Genome-Wide Expression Analysis Reveals Role of OVX in Attenuating Stress-Induced Gene Expression Changes in the NAc
Our behavioral findings indicate that CUS selectively causes a pro-depressant-like phenotype in gonadally intact female mice. We therefore conducted a microarray experiment on NAc tissue to shed light on the molecular mechanisms that might contribute to the stress susceptibility seen in these mice as well as the stress resiliency observed in OVX mice. We find, in accordance with our behavioral data, several lines of evidence that support a greater transcriptional effect of stress in intact female mice compared with OVX mice. First, a much larger number of genes—almost fourfold more—were significantly regulated by stress in the NAc of gonadally intact female mice compared with OVX mice: 619 and 170 genes, respectively. Second, analysis of the 619 genes significantly regulated by stress in intact animals demonstrates attenuated regulation in stressed OVX mice (Figure 2A). Surprisingly, analysis of the 170 genes regulated in the NAc of stressed OVX mice reveals an opposite pattern of stress-induced regulation in gonadally intact female mice (Figure 2B). Overall, 73% of genes significantly regulated by stress in OVX mice are regulated in the opposite direction in intact female mice. This suggests that, in the face of stress, ovariectomy might serve an active role that in some cases reverses stress-induced changes in gene expression (Figure 2B) and in other cases dampens such changes (Figure 2A). Indeed, we find that ovariectomy itself robustly regulates gene expression in the NAc. Approximately 1325 genes are significantly regulated by ovariectomy compared with intact control mice. Among the genes that were significantly upregulated by ovariectomy, we identified three NFκB genes, p105 (NFκB1), IKKγ (NEMO), and IKKβ, that are involved in protecting cells from damage during periods of cellular stress (see Supplement 2 for a complete list of regulated genes).
Figure 2.
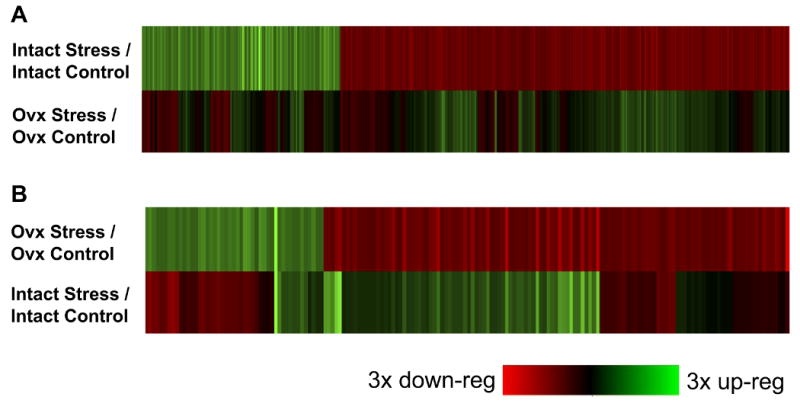
The CUS-induced changes in gene expression in the nucleus accumbens (NAc) are blunted in OVX female subjects. Microarray analysis was performed on the NAc of intact and OVX female mice in control and CUS conditions 24 hours after the last stress (n = 3). (A) Heatmap of 619 genes significantly regulated by stress in intact mice and how these genes are regulated by stress in OVX mice. Stressed-induced gene expression is blunted or reversed by OVX. (B) Heatmap of 170 genes significantly regulated by stress in OVX mice and how these genes are regulated by stress in intact female mice. Stress-induced changes in gene expression are strikingly reversed in OVX animals. See Supplement 2 for complete gene lists. down-reg, downregulated; up-reg, upregulated; other abbreviations as in Figure 1.
OVX Enhances NFκB Signaling and Gene Expression in the NAc
The striking finding of three NFκB subunit genes among those regulated by ovariectomy led us to directly study this transcription factor. To measure the effects of endogenous ovarian hormones on NFκB signaling, we quantified mean values of β-gal expression in the NAc of three to four individual NFκB-LacZ reporter mice/group. We find that OVX increases levels of NFκB-dependent β-gal expression in the NAc [Figure 3A; t (11) = 2.941, p < .05]. The increased expression of β-gal is consistent with the increased levels of IKKβ IKKγ, and p105 we observed on gene expression arrays under these conditions. We next analyzed the messenger RNA expression levels of several NFκB genes by real-time quantitative PCR at 3 and 6 weeks after OVX, as shown in Figures 3B–3E, and observed a similar induction of the NFκB subunits p105 [gene expression at 3 weeks, Figure 3B: t (14) = 2.008, p < .05, and at 6 weeks, Figure 3C: t (16) = 2.418, < .05] and p65 [gene expression at 6 weeks, Figure 3E: t (16) = 2.081, p < .05]. Regulation of both subunits was more pronounced 6 weeks after OVX.
Figure 3.
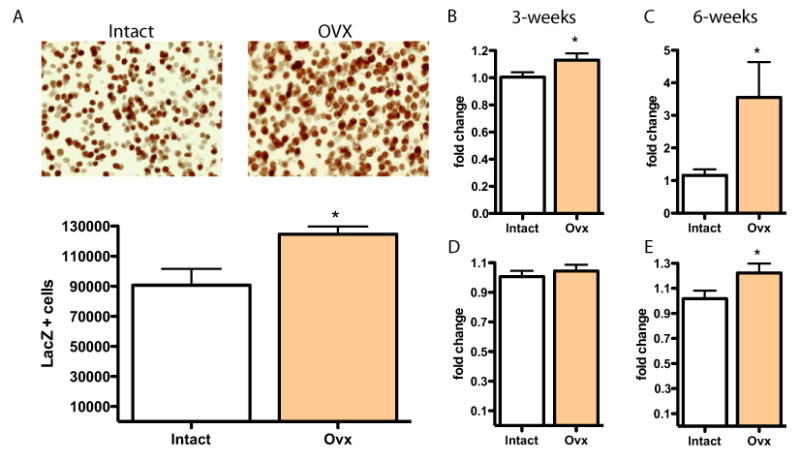
The OVX upregulates nuclear factor κ B (NFκB) subunit expression and transcriptional activity in the NAc. (A) The NFκB activity was greatly increased in the NAc of OVX NFκB-LacZ reporter mice (n =4). (B–D) Likewise we validated OVX regulation of NFκB subunits, as seen on gene expression arrays, and find that these changes are long-lived. Real time quantitative polymerase chain reaction data of NFκB genes shows increased expression of the NFκB subunits p105 (B, C) and p65 (D, E) at 3 and 6 weeks after OVX (n = 6 –7). *indicates statistically significant differences at p < .05. Abbreviations as in Figure 1.
Altered NFκB Signaling in the NAc Influences Stress-Induced Depressive-Like Behavior
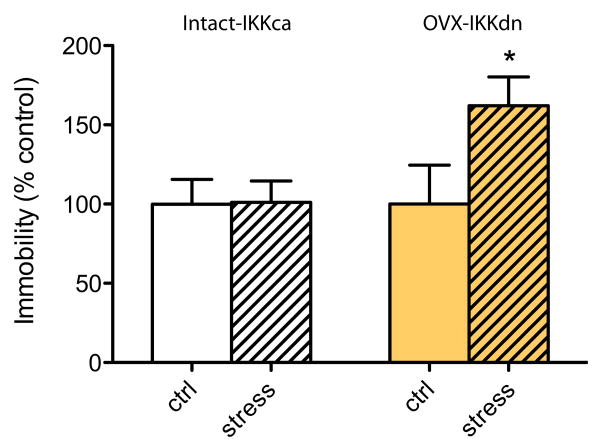
Given the dramatic induction of NFκB activity in the NAc resulting from OVX, we hypothesized that this signaling pathway might be important in mediating the susceptibility of gonadally intact versus OVX female mice to CUS. To directly test this possibility, we injected HSV-IKKdn in the NAc of OVX female subjects; overexpression of this dominant negative NFκB antagonist would reduce the overactivation of NFκB produced by OVX. Conversely, we injected HSV-IKKca, which expresses a constitutively active mutant of this NFκB subunit, to activate NFκB in the NAc of intact female subjects and thereby mimic this aspect of ovariectomy. In both experiments, control mice were unstressed and received injection with the same viral vector. Under these conditions, inhibition of NFκB was sufficient in OVX mice to increase susceptibility for CUS-induced depressive behavior as measured by the FST [Figure 4; t (18) = 2.260, p < .05]. Likewise, activation of NFκB in intact animals was sufficient to protect them from CUS-induced depressive behavior (p > .05). Together, these findings suggest that NFκB signaling in the NAc is highly important in the expression of depression-like behavior in female subjects.
Figure 4.
The NFκB signaling in the NAc regulates CUS-induced depressive behaviors in female subjects. Intra-NAc injections of herpes simplex virus (HSV) dominant negative inhibitor of kappa b kinase (IKKdn), to inhibit NFκB activity, increases susceptibility to CUS-induced depressive behavior in OVX animals compared with normal-handled control subjects (ctrl) as measured by increased immobility in the FST (n = 9 –10). Conversely, intra-NAc infusion of HSV-constitutively active inhibitor of kappa b kinase (IKKca), to increase NFκB activity, was sufficient to block stress-induced depressive behavior in the absence of any peripheral hormone manipulations in normally cycling intact female subjects (time immobile ranging from 100 to 150 sec). Data are expressed as % of control animals, which received intra-NAc HSV-green fluorescent protein injections, to normalize data across several experiments. *indicates statistically significant differences at p < .05. Abbreviations as in Figures 1 and 3.
Discussion
Gonadal hormones have been shown to be powerful regulators of mood and anxiety and are thought to contribute to gender-related differences in the prevalence of mood disorders in humans (3,44). In this study, we examined the contribution of endogenous ovarian hormones in mice to the development of stress-induced depressive- and anxiety-like behavior and gene expression in the NAc, a brain area known to modulate mood (25,27). We find that CUS produces a dramatic pro-depressantlike effect in intact female subjects as measured by the FST, an effect that was completely absent in OVX female subjects and gonadally intact male subjects. These effects were in the absence of any motor deficits or changes in anxiety-like behaviors that were measured in the EPM. Although the previously published literature is conflicting with regard to gender differences in stress effects on FST behavior, much of these studies in rat are confounded by other factors. For example, unlike rats, mice do not show gender differences in baseline FST (45); nor are there any baseline gender differences in neurogenesis (46), which has been implicated in depressive behavior in some studies. Our data support findings in humans, which show that the increased prevalence of depressive disorders in female subjects is linked, at least in part, to ovarian hormone-mediated hypersensitivity to stress (3).
The results from these studies suggest that E and P might predispose woman to depression during periods of stress. Previous reports, however, show that under baseline conditions E or P administration to OVX female subjects can produce what seems to be an antidepressant phenotype in the FST, which is in opposition to our data (11–15). Key methodological differences might explain these paradoxical findings. We compared OVX animals with those that are gonadally intact and normal cycling as well as with intact male subjects, thereby assessing the relative contribution of endogenous ovarian hormones in FST responses. Furthermore, OVX mice in this study did not show altered baseline motor activity consistent with previously published data (47), and therefore our results, unlike those published in rat studies (48), are not confounded by increased immobility due to OVX-induced hypoactivity. Additionally, the previously published work discussed in the preceding text examined the role of E in baseline depressive phenotypes. Here we examined the role of ovarian hormones in mediating depressive behaviors induced by chronic stress. Our data suggest that ovarian hormones do predispose female subjects to enhanced sensitivity to stress and might point to a mechanism for higher prevalence rates of depression and other stress-related disorders in woman.
Microarray analysis of stress- and OVX-regulated genes in the NAc identified numerous interesting candidates for the ovarian hormone-mediated enhanced stress responses in intact female subjects. Of particular interest, we found that several NFκB genes were dramatically increased in the NAc by OVX. Results from quantitative real-time PCR substantiated these array findings, and studies of NFκB reporter mice demonstrated that increased subunit expression is associated with OVX induction of NFκB transcriptional activity in this brain region. This is consistent with previous data in nonhuman primates, which showed similar NFκB induction after OVX that is blocked by E administration (49).
In our studies, we provided direct, causal evidence that the induction of NFκB in the NAc after OVX decreases susceptibility to stress-induced depressive behavior. To accomplish this, we injected HSV vectors expressing IKK mutants directly into the NAc to either increase or decrease NFκB activity selectively in this brain region. As predicted, our results show that the enhanced NFκB signaling induced in the NAc of OVX animals is sufficient to protect animals against stress-induced depressive behavior: inhibition of NFκB activity in OVX mice increases depression-like behavior in the FST, whereas stimulation of NFκB activity in intact mice reduces depressive-like behavior. Although further studies are needed, our data suggest that fluctuations of ovarian hormones and the resulting changes in NFκB signaling in the NAc during the female reproductive cycle might play a crucial role in susceptibility to depressive disorders. Further studies are also needed to examine the influence of the many other genes in the NAc identified by our microarray analysis on depressive-like behavior as well as to investigate many other brain areas also implicated in stress responses.
For example, it would be interesting in future studies to determine how NFκB signaling is affected by stress in other brain regions involved in mood regulation and the behavioral phenotypes that result from such regulation. Gender differences in stress sensitivity have been observed in many neural circuits, and it is likely that ovarian hormones regulate distinct aspects of female stress sensitivity depending on the target brain region. It is well known that gonadally intact female subjects are more sensitive to stress effects on cognitive function, which are generally blunted or absent in OVX female subjects (23). Whereas the brain regions that mediate the effects of OVX on cognitive function remain unknown, the influence of ovarian hormones on cognition is in line with our current findings, which show that the presence of ovarian hormones mediates hypersensitivity to stress on the FST. By use of NFκB-LacZ reporter mice and viral gene transfer, it will be possible in future investigations to determine the specific role of NFκB in regulating numerous aspects of stress-induced behaviors.
In addition, it will be important to understand, at a molecular level, how these targeted manipulations of NFκB activity modulate complex behavior, with perhaps different mechanisms involved in distinct brain regions. For example, it is well known that induction of brain-derived neurotrophic factor (BDNF) or activation of the transcription factor cyclic AMP response element binding protein (CREB) in the hippocampus produce an antidepressant-like phenotype (50,51). In fact, the influence of BDNF is gender-specific: loss of BDNF from hippocampus and other forebrain regions induces depressive symptoms in gonadally intact female mice, an effect not seen in male mice (45). In striking contrast, BDNF and CREB induction in the NAc (and its dopaminergic input from the ventral tegmental area) results in a pro-depressant phenotype (40,52–55). Given that BDNF signaling and CREB both interact with NFκB, future studies will address whether the NFκB pathway, acting in the hippocampus versus NAc, differentially modulates FST behavior.
Our results show that normal, gonadally intact female subjects are more susceptible to stress-induced depressive-like behaviors compared with OVX female mice and with intact male mice. Gene expression arrays revealed that the NFκB signaling pathway, a pathway that promotes cell survival in many systems, is increased in the NAc after OVX and that this induction serves a protective role in stress-induced depressive behavior. It is of general clinical interest that, in the absence of any hormonal manipulations, which can themselves cause psychiatric side-effects, a spatially localized activation of NFκB in the NAc is sufficient to block stress-induced depressive behaviors. These findings provide fundamentally new insight into the mechanisms mediating stress-induced depressive behaviors and raise the novel possibility that pharmacological agents that target central NFκB signaling might be effective treatments for depression in female subjects.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health and National Alliance for Research on Schizophrenia and Depression.
Footnotes
Authors QL and SC contributed equally to this work.
None of the authors reported any biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Robins LN. Psychiatric epidemiology—a historic review. Soc Psychiatry Psychiatr Epidemiol. 1990;25:16–26. doi: 10.1007/BF00789066. [DOI] [PubMed] [Google Scholar]
- 2.Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28:335–41. doi: 10.1016/s0896-6273(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 3.Deecher D, Andree TH, Sloan D, Schechter LE. From menarche to menopause: Exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology. 2008;33:3–17. doi: 10.1016/j.psyneuen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, et al. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav. 2008;93:595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Pare WP, Redei E. Sex differences and stress response of WKY rats. Physiol Behav. 1993;54:1179–1185. doi: 10.1016/0031-9384(93)90345-g. [DOI] [PubMed] [Google Scholar]
- 6.Becker JB, Monteggia LM, Perrot-Sinal TS, Romeo RD, Taylor JR, Yehuda R, Bale TL. Stress and disease: Is being female a predisposing factor? J Neurosci. 2007;27:11851–11855. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quiñones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 9.Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- 10.Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007;86:407–14. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci U S A. 1998;95:13941–13946. doi: 10.1073/pnas.95.23.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckley EH, Finn DA. Inhibition of progesterone metabolism mimics the effect of progesterone withdrawal on forced swim test immobility. Pharmacol Biochem Behav. 2007;87:412–419. doi: 10.1016/j.pbb.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estrada-Camarena E, Contreras CM, Saavedra M, Luna-Baltazar I, López-Rubalcava C. Participation of the lateral septal nuclei (LSN) in the antidepressant-like actions of progesterone in the forced swimming test (FST) Behav Brain Res. 2002;134:175–183. doi: 10.1016/s0166-4328(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 14.Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- 15.Molina-Hernandez M, Tellez-Alcantara NP. Antidepressant-like actions of pregnancy, and progesterone in Wistar rats forced to swim. Psychoneuroendocrinology. 2001;26:479–491. doi: 10.1016/s0306-4530(01)00007-5. [DOI] [PubMed] [Google Scholar]
- 16.Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 17.Panagiotaropoulos T, Papaioannou A, Pondiki S, Prokopiou A, Stylianopoulou F, Gerozissis K. Effect of neonatal handling and sex on basal and chronic stress-induced corticosterone and leptin secretion. Neuroendocrinology. 2004;79:109–118. doi: 10.1159/000076633. [DOI] [PubMed] [Google Scholar]
- 18.Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- 19.Koshibu K, Levitt P. Gene x environment effects: Stress and memory dysfunctions caused by stress and gonadal factor irregularities during puberty in control and TGF-alpha hypomorphic mice. Neuropsychopharmacology. 2008;33:557–565. doi: 10.1038/sj.npp.1301436. [DOI] [PubMed] [Google Scholar]
- 20.Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- 21.Bowman RE, Maclusky NJ, Diaz SE, Zrull MC, Luine VN. Aged rats: Sex differences and responses to chronic stress. Brain Res. 2006;1126:156–166. doi: 10.1016/j.brainres.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 22.Shively CA, Bethea CL. Cognition, mood disorders, and sex hormones. ILAR J. 2004;45:189–199. doi: 10.1093/ilar.45.2.189. [DOI] [PubMed] [Google Scholar]
- 23.Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: Accounting for sex and age. J Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 24.Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 25.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 27.Zacharko RM, Anisman H. Stressor-induced anhedonia in the mesocorticolimbic system. Neurosci Biobehav Rev. 1991;15:391–405. doi: 10.1016/s0149-7634(05)80032-6. [DOI] [PubMed] [Google Scholar]
- 28.Bhakar AL, Tannis LL, Zeindler C, Russo MP, Jobin C, Park DS, et al. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J Neurosci. 2002;22:8466–8475. doi: 10.1523/JNEUROSCI.22-19-08466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, et al. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 31.Mercurio F, Murray BW, Shevchenko A, Bennett BL, Young DB, Li JW, et al. IkappaB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol Cell Biol. 1999;19:1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 34.Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, et al. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng X, Wood CL, Blalock EM, Chen KC, Landfield PW, Stromberg AJ. Statistical implications of pooling RNA samples for microarray experiments. BMC Bioinformatics. 2003;4:26. doi: 10.1186/1471-2105-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi L, Reid LH, Jones WD, Shippy R, Warrington JA, Baker SC, et al. MAQC Consortium. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol. 2006;24:1151–1161. doi: 10.1038/nbt1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo L, Lobenhofer EK, Wang C, Shippy R, Harris SC, Zhang L, et al. Rat toxicogenomic study reveals analytical consistency across microarray platforms. Nat Biotechnol. 2006;24:1162–1169. doi: 10.1038/nbt1238. [DOI] [PubMed] [Google Scholar]
- 38.Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28:3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- 47.Bekku N, Yoshimura H, Araki H. Factors producing a menopausal depressive-like state in mice following ovariectomy. Psychopharmacology (Berl) 2006;187:170–180. doi: 10.1007/s00213-006-0395-2. [DOI] [PubMed] [Google Scholar]
- 48.Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: Disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25:118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 49.Bethea CL, Reddy AP, Smith LJ. Nuclear factor kappa B in the dorsal raphe of macaques: An anatomical link for steroids, cytokines and serotonin. J Psychiatry Neurosci. 2006;31:105–114. doi: 10.1016/j.yfrne.2006.03.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry. 2001;49:753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- 52.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 53.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: A role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.