Abstract
Objective
To determine the release pattern of serotonin and noradrenaline in the spinal cord in response to transcutaneous electric nerve stimulation (TENS) delivered at low or high frequency.
Design
Prospective randomized allocation of 3 treatments.
Setting
Research laboratory.
Animals
Male Sprague-Dawley rats (weight range, 250–350g).
Intervention
Knee joints of rats were inflamed with a mixture of 3% carrageenan and 3% kaolin for 24 hours prior to placement of push-pull cannulae into the dorsal horn of the spinal cord. Push-pull samples were collected in 10-minute intervals before, during, and after treatment with low-frequency TENS (4Hz), high-frequency TENS (100Hz), or sham TENS. TENS was applied to the inflamed knee joint for 20 minutes at sensory intensity and 100-μs pulse duration. Push-pull samples were analyzed for serotonin and noradrenaline by high performance liquid chromatography with coulemetric detection.
Main Outcome Measures
Spinal concentrations of serotonin and noradrenaline.
Results
Low-frequency TENS significantly increased serotonin concentrations during and immediately after treatment. There was no change in serotonin with high-frequency TENS, nor was there a change in noradrenaline with low- or high-frequency TENS.
Conclusions
Low-frequency TENS releases serotonin in the spinal cord to produce antihyperalgesia by activation of serotonin receptors.
Keywords: Hyperalgesia, Pain, Rehabilitation, Serotonin, Transcutaneous electric nerve stimulation
TRANSCUTANEOUS ELECTRIC NERVE stimulation (TENS) is a common yet important nonpharmacologic treatment used as an adjunct treatment for a variety of painful conditions by health care practitioners. TENS is the application of surface electrodes to the skin for pain relief. TENS has the advantage of being efficacious, inexpensive, and essentially free of undesirable side effects.
TENS was initially reported as a method for pain relief in 1967 by Wall and Sweet1 in direct response to the gate control theory of pain as a clinical test for implantation of dorsal column stimulators. Several early studies suggested that TENS activates opioid and serotonin receptors to produce analgesia in humans2 and animals.3 These initial animal studies used animals without tissue injury. We extended these studies by examining effects of selective receptor antagonists on low- and high-frequency TENS effects in an animal model of joint inflammation. The antihyperalgesia produced by both high- and low-frequency TENS in animals with arthritis is prevented by blockade of δ- and μ-opioid receptors, respectively, in the spinal cord or supraspinally in the rostral ventromedial medulla (RVM).4,5 Further, antihyperalgesia produced by low-frequency TENS is prevented by blockade of serotonin, but not noradrenergic, receptors in the spinal cord.6 Thus, we hypothesize that there will be an increase in serotonin release in the spinal cord dorsal horn during low-frequency TENS, but not high-frequency TENS. We further hypothesized that noradrenaline would remain unchanged during either high or low-frequency TENS. We tested this hypothesis by measuring release of serotonin and noradrenaline using push-pull perfusion coupled to electrochemical high performance liquid chromatography (HPLC).
METHODS
All experiments were approved by the Animal Care and Use Committee at the University of Iowa. Experiments follow the guidelines for use of animals by the National Institutes of Health and the International Association for the Study of Pain.
Injection of Kaolin and Carrageenan Into the Knee Joint
All experiments were performed in male Sprague-Dawley ratsa (n=18; weight range, 250–350g) with knee joint inflammation. A mixture of 3% kaolin and 3% carrageenan (0.1mL in sterile saline; pH, 7.2–7.4) was injected into the knee joint, just lateral to the patellar tendon, while the rat was anesthetized with halothane, initially with 5% and maintained with 2%.7 The animals were allowed to recover 24 hours before placement of the push-pull cannulae.
Placement of Push-Pull Probes
All push-pull probes were placed in rats weighing 250 to 350g while anesthetized with sodium pentobarbital (50mg/kg given intraperitoneally). Anesthesia was maintained with sodium pentobarbital (1–2mg·kg-1·h-1), given intravenously throughout the experiments. This anesthetic was chosen to maintain adequate depth of anesthetic for a prolonged period of time. Rats remained anesthetized throughout the sample collection. All samples were collected on ice, immediately frozen on dry ice, and analyzed within 12 hours for serotonin and noradrenaline. Push-pull samples were filtered through 0.2μ low-protein binding polyvinylidene fluoride microcentrifuge tube immediately after collection. At the end of the experiment, rats were killed with an overdose of sodium pentobarbital, spinal cord or brain removed, fixed in 10% formalin, and cut for analysis of probe placement.
Push-pull probes were placed according to previously published procedures.8 A 125-μm tungsten wireb was epoxied to a piece of microdialysis fiberc (outside diameter, 200μm). A 1.0-mm section of the fiber was cut out and a stainless steel guide cannula inserted into the fiber. One end of the microprobe was inserted into a piece of PE20 tubingd and a stainless steel guide cannulae superglued to the other end of the microprobe. After insertion of the microprobe and fixation to bone with dental cement, the guide cannula was removed and the free ends of the microprobe connected to PE20 tubing. Four 2.5mL syringes with 26-gauge needles were filled with artificially cerebrospinal fluid and connected to PE20 tubing. The push-pull pump was operated at 10μL/min and artificial cerebrospinal fluid infused throughout the experiments. It is difficult to maintain flow for several hours with lower flow rates.8 We previously showed we can detect baseline concentrations of serotonin and noradrenaline with push-pull, but not for serotonin with microdialysis.9
Application of TENS
Electrodes and TENS units were placed on the inflamed knee joint prior to collection of samples. After 1 hour of baseline testing, TENS was applied to the inflamed knee for 20 minutes while the rat was anesthetized with halothane. The electrodes were placed on the medial and lateral aspects of the joint. Either high-(100Hz) or low-frequency (4Hz) stimulation was used. All other parameters were kept constant as follows: pulse width, 100μs; amplitude and intensity, sensory level (8–15mA, just below motor contraction). This intensity level likely represents a strong sensory intensity in human subjects and is defined as the intensity just below motor contraction. In all rats, a motor twitch is achieved and then intensity is reduced to where the twitch disappears. Although many studies demonstrate differences between high-frequency and low-frequency TENS, the intensity of stimulation is usually different. Typically high-frequency TENS is applied at sensory-level stimulation and low-frequency TENS is applied at motor-level stimulation. From these studies, we are unsure if the effects were a result of frequency (as is claimed) or just intensity of stimulation. Thus, we chose to keep the intensity (amplitude) the same and to change the frequency to either 4 or 100Hz. Previously we demonstrated that reduction of secondary mechanical or heat hyperalgesia was equivalent with high- or low-frequency TENS at sensory intensity.4,10
The TENS units used in these studies are utilized clinically.e The waveform is a balanced asymmetrical biphasic square wave. Amplitude is adjustable from 0 to 60mA; pulse width is adjustable from 30 to 250μs; pulse rate (frequency) is adjustable from 2 to 125Hz. Electrodes are 1.3-cm (0.5-in) diameter round pregelled and used clinically for TENS treatment (of small areas such as the hand and fingers). The size of electrodes used in the proposed experiments compares to the area of tissue that would be covered by electrodes in human subjects receiving TENS to the knee joint.
Analysis of Norepinephrine and Serotonin9
Because of the loss of serotonin and noradrenaline within 24 hours,9 all samples were run immediately after collection. Aliquots of 20μL were injected without pretreatment onto an MD-150 HPLC column (particle diameter, 3μm; outside distance, 3mm; length, 15cm) with a coulometric electrochemical detector. The electrochemical detector guard cell was set at +350mV, analytical cell 1 was set at -150mV, and analytical cell 2 was set at +220mV. The column temperature was maintained at ambient temperature throughout the analysis. The mobile phase for serotonin and noradrenaline analysis consists of 10% acetonitrile, 75mM NaH2PO4, 1.7mM of 1-octanesulfonic acid sodium salt, 00μl/L of triethylamine, and 25μM of disodium ethylenediaminetetraacetic acid, adjusted to a pH of 3.0 with phosphoric acid. The HPLC flow rate was 0.6mL/min. Standards were dispersed through the run so that there were 3 standards at the beginning of the run, 1 standard every 5 samples, and 2 standards at the end of the run. Peak heights were used to determine the concentration for serotonin and noradrenaline from the corresponding standard curve. The limit of detection for noradrenaline is .28fg/mL, the limit of quantification is 0.8fg/mL, and precision is 4%. The limit of detection for serotonin is .06fg/mL, the limit of quantification is .18fg/mL, and precision is 4%.
Experimental Protocol
The animals were randomly assigned to the following treatment groups: high-frequency TENS, sensory intensity (n=6); low-frequency TENS, sensory intensity (n=6); and sham TENS (n=6). Dialysate samples were collected in 10-minute intervals after a 30-minute washout period. There were four 10-minute samples collected that served as baseline; two 10-minute samples during TENS (TENS1, TENS2), and four 10-minute samples collected after TENS (Post1, Post2, Post3, Post4).
Statistical Analysis
Data were converted to a percentage of baseline ([experimental/baseline]×100) so that baseline was set at 100% for each individual animal. Percentage of baseline was analyzed with a repeated-measures analysis for time, group, and time by group. Post hoc testing was performed with a Tukey t test.
RESULTS
Serotonin
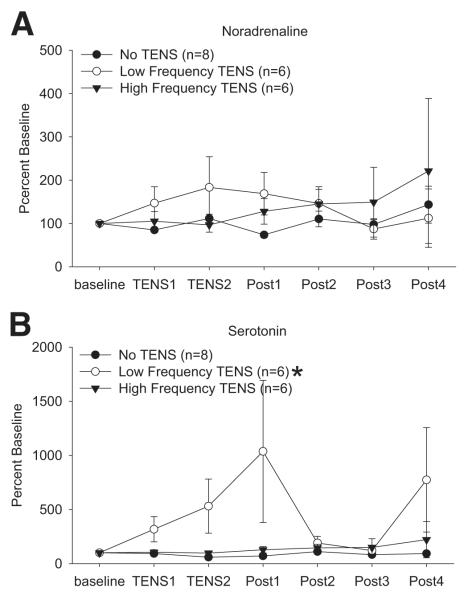
Concentrations of serotonin were similar between groups 24 hours after induction of inflammation (table 1). To compare relative changes during and after TENS data were converted to a percentage of baseline. In the groups treated with TENS there was a significant interaction for time by group (F2,7=5.0, P=.04). The concentrations of serotonin from the group that received low-frequency TENS (n=6) were significantly greater than the group that received no TENS (P=.004) (n=6) or high-frequency TENS (P=.012) (n=6) (fig 1).
Table 1.
Baseline Concentrations of Noradrenaline and Serotonin
| Group | Noradrenaline (ng/mL) | Serotonin (ng/mL) |
|---|---|---|
| No TENS | 1.41±0.10 | 0.57±0.10 |
| Low-frequency TENS | 1.21±0.23 | 0.50±0.09 |
| High-frequency TENS | 0.80±0.15 | 0.55±0.07 |
NOTE. Values are mean ± standard deviation.
Fig 1.
Time course of release of (A) noradrenaline and (B) serotonin in response to treatment with low-frequency, high-frequency, or sham TENS. Significant increases in serotonin occur during and immediately after low-frequency TENS. Dialysate samples were collected in 10-minute intervals. Data are presented as a percentage of baseline, with baseline set at 100%. The four 10-minute baseline samples were averaged to give one baseline number per animal for calculation of percentage of baseline responses. *Significantly different from sham TENS and high-frequency TENS.
Noradrenaline
Baseline concentrations of noradrenaline were similar between groups (see table 1). To compare relative changes during and after TENS data were converted to a percentage of baseline. The concentrations of noradrenaline remained unchanged across the sampling period for all groups.
DISCUSSION
The current study shows that serotonin increases in the spinal dorsal horn during and immediately after low-frequency TENS applied to the inflamed knee joint. There was no change in serotonin in response to high-frequency TENS, and there was no change in noradrenaline in response to either low- or high-frequency TENS. Early studies, in animals without tissue injury, show that spinal blockade of serotonin receptors with methysergide prevents low-frequency (20Hz) TENS analgesia.11 However, systemic depletion of serotonin prevents high-frequency TENS analgesia,12 suggesting a role for serotonin outside the spinal cord. In parallel with the lack of change in noradrenaline in the spinal cord, we demonstrated that blockade of α2-noradrenergic receptors in the spinal cord has no effect on either high- or low-frequency TENS antihyperalgesia in either mice or rats with knee joint inflammation.6,13 Further blockade of serotonin receptors spinally prevents the antihyperalgesia produced by low-, but not high-frequency TENS.6 Thus, low-frequency TENS invokes endogenous mechanisms for the release of serotonin in the spinal cord, which activates 5-hydroxytryptamine (5-HT) receptors reducing hyperalgesia.
Serotonin in the spinal cord comes from the RVM (see Fields and Basbaum14), is released spinally following electric or chemical stimulation of the RVM,15-17 and blockade of spinal serotonin receptors prevents RVM stimulation-induced analgesia.18 Analgesia produced by spinally applied serotonin is prevented by blockade of spinal μ-opioid receptors.19 Low-frequency TENS pharmacology parallels these data in animals with joint inflammation. The antihyperalgesia produced by low-frequency TENS, but not high-frequency TENS, is prevented by blockade of μ-opioid receptors in the spinal cord or the RVM.4,5 Similarly, in human subjects, systemic blockade of opioid receptors with naloxone prevents low-, but not high-, frequency TENS analgesia.2 In animals, higher concentrations of systemic naloxone also prevent the effects of high-frequency TENS analgesia,12,20 and blockade of δ-opioid receptors in the spinal cord or the RVM prevents the antihyperalgesia produced by high-frequency TENS in arthritic rats.4,5 In parallel, opioid peptides are released in the cerebrospinal fluid and plasma in humans in response to either low- or high-frequency TENS in a frequency-dependent manner.21-23 Increases in β-endorphin occur in the cerebrospinal fluid and plasma in response to both low- and high-frequency stimulation.22,23 Han et al21 show increases in the cerebrospinal fluid methionine (met)-enkephalin-Arg-Phe with low-frequency TENS and increases in dynorphin A with high-frequency TENS. Surface electrodes were applied to the hand, leg, and abdomen over acupuncture points and stimulation was applied with pulse widths of 300μs at a motor intensity. This type of TENS is likely distinctly different from application of TENS directly to the site of injury with shorter pulse widths and lower intensity.
Serotonin is released in response to dorsal column stimulation at a frequency of 100Hz and a pulse width of 200μs.24 However, it should be noted that these experiments were done in cats without tissue injury that were previously decerebrated. Several explanations could account for the differences between these studies. Distinct differences occur in the spinal cord and RVM after tissue injury particularly for opioids and γ-aminobutyric acid25-27 and thus different mechanisms of action could exist for TENS between animals with tissue injury and those without. Differences between the decerebrated and the intact animal, or species differences, could also contribute to these observations of a lack of change in serotonin in the inflamed rat treated with 100Hz TENS to the inflamed knee joint. The results of the current study, however, directly parallel prior pharmacology studies that examined the role of spinal serotonin and noradrenergic receptors in TENS-induced antihyperalgesia. Specifically, we show that blockade of 5-HT2 (ketanserin) and 5-HT3 (MDL-72222) receptors in the spinal cord prevents the antihyperalgesic effects of low-frequency TENS, but not that of high-frequency TENS, in rats 4 hours after induction of knee joint inflammation.6 Thus, low-frequency TENS releases serotonin in the spinal cord to activate 5-HT2 and 5-HT3 receptors to reduce hyperalgesia.
Electric stimulation has many adjustable parameters; one of these is pulse width. Spinal cord stimulation typically uses pulse widths of 200μs or longer; while TENS pulse widths vary greatly ranging between 50 and 250μs. Prior studies show that electric stimulation of brain sites involved in pain inhibition (periaqueductal gray, nucleus raphe magnus) with longer pulse widths, regardless of frequency of stimulation, is generally required to produce serotonin release. Electric stimulation of the brainstem periaqueductal gray with pulse widths of 500μs (333Hz or 30Hz) evokes release of serotonin in the spinal cord, while pulse widths of 100μs (67Hz or 30Hz) have no effect.17 Similarly, electric stimulation of the periaqueductal gray with 200μs pulse duration (333Hz) or 500μs (67Hz) increased release of serotonin in the spinal cord that was greater with increased pulse widths.28 For low-frequency stimulation, however, serotonin is released with shorter pulse widths and agrees with our current data. For example, electric stimulation of the nucleus raphe magnus at low frequencies and the same pulse width as utilized in the current study (10Hz and 100μs) increases serotonin release in the spinal cord.16 Thus, it is concluded that pulse widths greater than 100μs are required to evoke serotonin release in the spinal cord for higher frequencies; but a 100μs pulse width is sufficient for low-frequency stimulation (<10Hz). These observations are entirely consistent with results from our studies showing release of serotonin with low-frequency (4Hz) and not with high-frequency (100Hz) TENS with pulse widths of 100μs. Therefore, low-frequency TENS is capable of releasing serotonin in the spinal cord using this inhibitory mechanism to shift the balance of the endogenous pain control system for pain relief.
CONCLUSIONS
Understanding the mechanisms of action of TENS could lead to more appropriate treatment strategies using judicious combinations of pharmaceutical agents and TENS. For example, because low-frequency TENS increases release of serotonin, use of serotonin reuptake inhibitors in combination with low-frequency TENS could enhance and prolong the effectiveness of TENS. Furthermore, clinical use of TENS and future clinical outcome studies should be carefully evaluated with respect to the current medication of the patient. The uncertainties about the mechanisms and effectiveness of TENS for pain control can contribute to the hesitation of clinicians for its use. Understanding the mechanism of action for pain reduction should dramatically influence the utilization of this noninvasive treatment option.
Acknowledgments
Supported by the Arthritis Foundation, National Institutes of Health (grant no. K0202201). The transcutaneous electric nerve stimulators were donated by EMPI.
Footnotes
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
Harlan Industries, PO Box 29176, Indianapolis, IN 46229-0176.
Small Parts Inc, 13980 NW 58th Ct, PO Box 4650, Miami Lakes, FL 33014-0650.
Hospital Filtral AN69; Gambro Inc, 10810 W Collins Ave, Lakewood, CO 80215-4498.
Clay Adams; Becton Dickinson, 1 Becton Dr, Franklin Lakes, NJ 07417.
Eclipse+; EMPI, 599 Cardigan Rd, St. Paul, MN 55126-4099.
References
- 1.Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108–9. doi: 10.1126/science.155.3758.108. [DOI] [PubMed] [Google Scholar]
- 2.Sjolund BH, Eriksson MB. The influence of naloxone on analgesia produced by peripheral conditioning stimulation. Brain Res. 1979;173:295–301. doi: 10.1016/0006-8993(79)90629-2. [DOI] [PubMed] [Google Scholar]
- 3.Woolf DJ, Barrett GD, Mitchell D, Myers RA. Naloxone-reversible peripheral electroanalgesia in intact and spinal rats. Eur J Pharmacol. 1977;45:311–4. doi: 10.1016/0014-2999(77)90016-4. [DOI] [PubMed] [Google Scholar]
- 4.Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289:840–6. [PubMed] [Google Scholar]
- 5.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298:257–63. [PubMed] [Google Scholar]
- 6.Radhakrishnan R, King EW, Dickman JK, et al. Spinal 5-HT(2) and 5-HT(3) receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain. 2003;105:205–13. doi: 10.1016/s0304-3959(03)00207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain. 1993;55:367–77. doi: 10.1016/0304-3959(93)90013-F. [DOI] [PubMed] [Google Scholar]
- 8.Patterson SL, Sluka KA, Arnold MA. A novel transverse push—pull microprobe: in vitro characterization and in vivo demonstration of the enzymatic production of adenosine in the spinal cord dorsal horn. J Neurochem. 2001;76:234–46. doi: 10.1046/j.1471-4159.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- 9.Lisi TL, Westlund KN, Sluka KA. Comparison of microdialysis and push-pull perfusion for retrieval of serotonin and norepinephrine in the spinal cord dorsal horn. J Neurosci Methods. 2003;126:187–94. doi: 10.1016/s0165-0270(03)00093-1. [DOI] [PubMed] [Google Scholar]
- 10.King EW, Sluka KA. The effect of varying frequency and intensity of transcutaneous electrical nerve stimulation on secondary mechanical hyperalgesia in an animal model of inflammation. J Pain. 2001;2:128–33. doi: 10.1054/jpai.2001.19963. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu T, Koja T, Fujisaki T, Fukuda T. Effects of methysergide and naloxone on analgesia induced by the peripheral electric stimulation in mice. Brain Res. 1981;208:463–7. doi: 10.1016/0006-8993(81)90578-3. [DOI] [PubMed] [Google Scholar]
- 12.Woolf CJ, Mitchell D, Barrett GD. Antinociceptive effect of peripheral segmental electrical stimulation in the rat. Pain. 1980;8:237–52. doi: 10.1016/0304-3959(88)90011-5. [DOI] [PubMed] [Google Scholar]
- 13.King EW, Audette K, Athman GA, Nguyen HO, Sluka KA, Fairbanks CA. Transcutaneous electrical nerve stimulation activates peripherally located alpha-2A adrenergic receptors. Pain. 2005;115:364–73. doi: 10.1016/j.pain.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation. In: Wall PD, Melzack R, editors. Textbook of pain. Churchill Livingstone; New York: 1999. pp. 243–57. [Google Scholar]
- 15.Hammond DL, Tyce GM, Yaksh TL. Efflux of 5-hydroxytryptamine and noradrenaline into spinal cord superfusates during stimulation of the rat medulla. J Physiol (London) 1985;359:151–62. doi: 10.1113/jphysiol.1985.sp015579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowker RM, Abhold RH. Evoked changes in 5-hydroxytryptamine and norepinephrine release: in vivo dialysis of the rat dorsal horn. Eur J Pharmacol. 1990;175:101–6. doi: 10.1016/0014-2999(90)90159-4. [DOI] [PubMed] [Google Scholar]
- 17.Sorkin LS, McAdoo DJ, Willis WD. Raphe magnus stimulation induced antinociception in the cat is associated with release of amino acids as well as serotonin in the lumbar dorsal horn. Brain Res. 1993;618:95–108. doi: 10.1016/0006-8993(93)90433-n. [DOI] [PubMed] [Google Scholar]
- 18.Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaquaductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31:123–36. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- 19.Goodchild GS, Guo Z, Freeman J, Gent JP. 5-HT spinal antinociception involves mu opioid receptors: cross tolerance and antagonist studies. Br J Anaesthesia. 1997;78:563–9. doi: 10.1093/bja/78.5.563. [DOI] [PubMed] [Google Scholar]
- 20.Han JS, Xie GX, Ding ZX, Fan SG. High and low frequency electroacupuncture analgesia are mediated by different opioid peptides [abstract] Pain Suppl. 1984;2:543. [Google Scholar]
- 21.Han JS, Chen XH, Sun SL, et al. Effect of low and high frequency TENS on met-enkephalin-arg-phe and dynorphin A immunoreactivity in human lumbar CSF. Pain. 1991;47:295–8. doi: 10.1016/0304-3959(91)90218-M. [DOI] [PubMed] [Google Scholar]
- 22.Salar G, Job I, Mingrino S, Bosio A, Trabucchi M. Effect of transcutaneous electrotherapy on CSF β-endorphin content in patients without pain problems. Pain. 1981;10:169–72. doi: 10.1016/0304-3959(81)90192-5. [DOI] [PubMed] [Google Scholar]
- 23.Hughes GS, Lichstein PR, Whitlock D, Harker Response of plasma beta-endorphins to transcutaneous electrical nerve stimulation in healthy subjects. Phys Ther. 1984;64:1062–6. doi: 10.1093/ptj/64.7.1062. [DOI] [PubMed] [Google Scholar]
- 24.Linderoth B, Gazelius B, Franck J, Brodin E. Dorsal column stimulation induces release of serotonin and substance-P in the cat dorsal horn. Neurosurgery. 1992;31:289–97. doi: 10.1227/00006123-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Dmitrieva N, Rodriguez-Malaver AJ, Perez J, Hernandez L. Differential release of neurotransmitters from superficial and deep layers of the dorsal horn in response to acute noxious stimulation and inflammation of the rat paw. Eur J Pain. 2004;8:245–52. doi: 10.1016/j.ejpain.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Draisci G, Kajander KC, Dubner R, Bennett GJ, Iadarola MJ. Up-regulation of opioid gene expression in spinal cord evoked by experimental nerve injuries and inflammation. Brain Res. 1991;560:186–92. doi: 10.1016/0006-8993(91)91231-o. [DOI] [PubMed] [Google Scholar]
- 27.Stanfa L, Dickenson A. Spinal opioid systems in inflammation. Inflamm Res. 1995;44:231–41. doi: 10.1007/BF01782974. [DOI] [PubMed] [Google Scholar]
- 28.Cui M, Feng Y, McAdoo CJ, Willis WD. Periaqueductal gray stimulation-induced inhibition of nociceptive dorsal horn neurons in rats is associated with the release of norepinephrine, serotonin, and amino acids. J Pharmacol Exp Ther. 1999;289:868–76. [PubMed] [Google Scholar]