Abstract
Varenicline, a partial α4β2 and full α7 nicotinic receptor agonist, has been shown to inhibit nicotine self-administration and nicotine-induced increases in extracellular dopamine in the nucleus accumbens. In the present study, we investigated whether varenicline inhibits nicotine-enhanced electrical brain-stimulation reward (BSR), and if so, which receptor subtypes are involved. Systemic administration of nicotine (0.25–1.0 mg/kg, i.p.) or varenicline (0.03–3 mg/kg, i.p.) produced biphasic effects, with low doses producing enhancement (e.g., decreased BSR threshold), and high doses inhibiting BSR. Pretreatment with low dose (0.03–1.0 mg/kg) varenicline dose-dependently attenuated nicotine (0.25 or 0.5 mg/kg)-enhanced BSR. The BSR-enhancing effect produced by varenicline was blocked by mecamylamine (a high affinity nicotinic receptor antagonist) or dihydro-β-erythroidine (a relatively selective nicotinic α4-containing receptor antagonist), but not methyllycaconitine (a selective α7 receptor antagonist), suggesting an effect mediated by activation of α4β2 receptors. This suggestion is supported by findings that the α4β2 receptor agonist SIB-1765F produced a dose-dependent enhancement of BSR, while pretreatment with SIB-1765F attenuated nicotine (0.5 mg/kg)-enhanced BSR. In contrast, the selective α7 receptor agonist ARR-17779, altered neither BSR itself nor nicotine-enhanced BSR, at any dose tested. These findings suggest that: 1) varenicline inhibits nicotine-enhanced BSR, supporting its use as a smoking cessation aid; and 2) varenicline-enhanced BSR by itself and varenicline's anti-nicotine effects are mediated by activation of α4β2, but not α7, receptors.
Keywords: Nicotine, Varenicline, Dopamine, α4β2 receptors, Reward, Addiction
1. Introduction
Cigarette smoking is the primary cause of many preventable diseases, and much attention has focused on various therapies for smoking cessation. Currently, smoking cessation therapies include nicotine replacement products and the monoamine reuptake inhibitor bupropion. In addition, the U.S. Food and Drug Administration (FDA) has recently approved the nicotine receptor agonist varenicline and nicotine vaccines as newer medications for smoking cessation (Rollema et al., 2007b; Tutka, 2008; Xi et al., in press).
Nicotine is considered the major reinforcing component of cigarette tobacco for both humans and experimental animals (Goldberg et al., 1981; Watkins et al., 2000). Neuronal nicotinic acetylcholine (nAChR) receptors are either homomeric or heteromeric pentameric ion channels, consisting of different combinations of α2–α10 and β2–β4 subunits (Cooper et al., 1991; Le Novère et al., 2002). The non-neuronal subunits – α1, β1, γ, δ and ε – form peripheral nicotinic acetylcholine receptors at the neuromuscular junction (Le Novère et al., 2002). The predominant nAChRs are the α4β2-containing heteromers or α7-containing homomers (Couturier et al., 1990; Zoli et al., 1998), which modulate release of neurotransmitters such as dopamine (DA), glutamate or GABA (Zhu and Chiappinelli, 2002; Lambe et al., 2003; Cao et al., 2005).
Recent research has indicated that α4β2 receptors play a major role in mediating nicotine's action in the brain (Markou, 2008; Xi et al., in press). Repeated exposure to nicotine significantly increases nicotine binding to α4β2 receptors (Marks et al., 1992), and blockade of α4β2 nAChRs by dihydro-β-erythroidine (DHβE) inhibits nicotine self-administration (Watkins et al., 1999). Deletion of the β2 subunits significantly attenuates or abolishes nicotine self-administration, nicotine-induced taste aversion, nicotine discrimination, and nicotine-enhanced DA in the nucleus accumbens (NAc) (Picciotto et al., 1998; Grady et al., 2001; Shoaib et al., 2002). In contrast, animals lacking α7 subunits show normal acquisition of stimulus discrimination for nicotine (Stolerman et al., 2004).
Varenicline is a partial agonist at α4β2 and a full agonist at α7 nicotinic receptors (Foulds, 2006; Mihalak et al., 2006). Clinical trials indicate that varenicline is effective in decreasing relapse to smoking in humans (Zierler-Brown and Kyle, 2007; Tutka, 2008). Preclinical studies demonstrate that varenicline inhibits nicotine self-administration and nicotine-enhanced NAc DA (Rollema et al., 2007a). Varenicline partially substitutes for nicotine in self-administration testing in animals and partially generalizes to nicotine in the drug discrimination preclinical animal paradigm (Rollema et al., 2007a; Smith et al., 2007). Varenicline has also been reported to reduce ethanol, but not sucrose, self-administration, and decrease voluntary ethanol, but not water, consumption in rats (Steensland et al., 2007).
In addition to the self-administration and drug discrimination paradigms, electrical brain-stimulation reward (BSR) is another commonly used animal model to assess nicotine's action in the brain (Wise, 1996; Pak et al., 2006). In the present study, we first examined the effects of both varenicline and nicotine on BSR, and the effect of varenicline pretreatment on nicotine-enhanced BSR. To study the underlying receptor mechanisms, we further observed the effects of various high affinity nAChR agonists or antagonists with relative selectivity for α4β2 or α7 receptors on BSR and on varenicline- or nicotine-enhanced BSR.
2. Materials and methods
2.1. Animals
Male Long–Evans rats (Charles River Laboratories, Raleigh, NC, USA), 300–325 g at time of surgery, were used. They were housed individually in a climate-controlled environment with food and water freely available with the exception of the time spent each day in the test chambers. All experiments were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the U.S. National Institutes of Health, and were carried out in compliance with applicable United States federal and Maryland state laws and regulations.
2.2. Surgery and general BSR procedures
Surgery and general procedures for electrical BSR were as we have reported previously (Pak et al., 2006; Spiller et al., 2008). Briefly, the surgery was performed under sodium pentobarbital anesthesia (65 mg/kg i.p.) with standard aseptic surgical and stereotaxic technique. A unilateral monopolar stainless-steel stimulating electrode (Plastics One, Roanoke, VA, USA) was placed into the medial forebrain bundle at the anterior–posterior level of the lateral hypothalamus (AP −2.5, ML ±1.9, and DV −8.6) according to the rat brain atlas of Paxinos and Watson (1998). After 7 days of recovery from surgery, rats were allowed to self-train (autoshape) to lever press for rewarding BSR. Following establishment of lever-pressing for BSR, animals were presented with a series of 16 different pulse frequencies, ranging from 141 to 25 Hz in descending order. At each pulse frequency, animals responded for two 30-s time periods (“bins”), after which the pulse frequency was decreased by 0.05 log units. The response rate for each frequency was defined as the mean number of lever responses during the two 30-s bins. Following each 30-s bin, the lever retracted for 5 s. Throughout the experiment, animals were run for 3 sessions a day. Since lever-pressing behavior was variable during the first session, but was stable during the second and third sessions, the data from the first session were discarded, and the data from the second and third sessions were designated as the baseline session data and test session data, respectively. The BSR threshold (θ0) was defined as the minimum frequency at which the animal responded for rewarding stimulation. The Ymax was defined as the maximal rate of response for rewarding brain stimulation. Both the θ0 and Ymax values were mathematically derived for each ‘baseline’ run and each ‘drug’ run by analyzing each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies using ‘best-fit’ mathematical algorithms (Spiller et al., 2008).
2.3. Testing the effects of varenicline, nicotine, mecamylamine, ARR-17779, SIB-1765F, methyllycaconitine or DHβE on BSR
Once a baseline value was achieved (<10% variation in θ0 over 5 continuous days), animals were divided into 7 groups to observe the effects of the following compounds on BSR: nicotine (0, 0.25, 0.5, 1.0 mg/kg), varenicline (0, 0.03, 0.1, 0.3, 1, 3 mg/kg), mecamylamine (0, 3, 6 mg/kg), ARR-17779 (0, 0.1, 1, 10 mg/kg), SIB-1765F (0, 3, 1, 10 mg/kg), methyllycaconitine (MLA) (0, 3, 6 mg/kg) and DHβE (0, 0.3, 1 mg/kg). All animals received, between the baseline and test BSR session, an intraperitoneal (i.p.) injection of saline (vehicle) or one dose of test compound. At 30 min after injection, the BSR test began. After each test, animals received an additional 5–7 days of BSR re-stabilization until a new baseline θ0 was established. The order of testing for different drug doses was counterbalanced according to a Latin square design.
2.4. Testing the effects of mecamylamine, DHβE, or MLA on varenicline-enhanced BSR
Three additional groups of animals were used to evaluate the effects of pretreatment with mecamylamine (3.0 or 6.0 mg/kg), DHβE (1 mg/kg), or MLA (3 or 6 mg/kg) on varenicline (0.1 mg/kg)-enhanced BSR. The drug injection and BSR protocols were the same as described above, with the addition that the pretreatments were administered 30 min prior to varenicline. At 30 min after varenicline injection, the BSR test session began. Drug-induced alterations in θ0 were measured.
2.5. Testing the effects of varenicline, SIB-1765F, or ARR-17779 on nicotine-enhanced BSR
Six additional groups of animals were used to evaluate the effects of pretreatment with varenicline (0.3 or 1.0 mg/kg), SIB-1765F (3 mg/kg), and ARR-17779 (1 mg/kg) on nicotine (0.25, 0.5 mg/kg)-enhanced BSR. The drug injection protocol was the same as described above, with the addition that pretreatments were administered 30 min prior to nicotine. Drug-induced alterations in θ0 were measured.
2.6. Drugs and chemicals
Nicotine (as nicotine bitartrate) was obtained from the National Institute on Drug Abuse (Baltimore, MD 21224, USA). Varenicline, mecamylamine, SIB-1765F [(+/−)-5-ethynyl-3-(1-methyl-2-pyrrolidinyl)pyridine fumarate], and ARR-17779 [(2)-spiro[1-azabicyclo(2.2.2)octane-3,59-oxazolidin]-29-one)] were provided by Memory Pharmaceuticals (Montvale, NJ, USA). Dihydro-β-erythroidine (DHβE) was purchased from Tocris Bioscience (Ellisville, MO, USA). Methyllycaconitine (MLA) was purchased from Sigma Life Science (St. Louis, MO, USA). All compounds were dissolved in sterile physiological saline.
2.7. Statistical analysis
One-way analysis of variance (ANOVA) was used to analyze the effects of each test compound on BSR. Two-way analysis of variance (ANOVA) was used to analyze the effects of each test compound on varenicline- or nicotine-enhanced BSR. Post-hoc individual group comparisons were carried out using the Student–Newman–Keuls procedure. The minimally acceptable statistical significance was set at a probability level of p < 0.05 for all tests.
3. Results
3.1. Biphasic effects of varenicline and nicotine on BSR
Fig. 1A shows representative rate-frequency function curves for BSR, indicating the BSR threshold (θ0, Hz) and the maximal work amount (Ymax, lever presses per 30 s) produced by a representative animal for BSR after either varenicline or nicotine treatment. Both varenicline (0.1 mg/kg, i.p.) and nicotine (0.5 mg/kg, i.p.) produced a significant enhancement of BSR, as indicated by the leftward shifts in the rate-frequency function curves, reflecting lowered θ0 values without changes in Ymax. Fig.1B shows that systemic administration of nicotine produced dose-dependent biphasic effects on BSR; that is, the lower doses of nicotine (0.25, 0.5 mg/kg) produced a significant enhancement of BSR (i.e., reduction in BSR θ0 threshold), while the highest dose (1 mg/kg, i.p.) of nicotine produced an inhibition of BSR (i.e., increased BSR θ0 threshold). One-way ANOVA for the data shown in Fig. 1B revealed a statistically significant treatment (i.e., nicotine dose) main effect (F3,43 = 22.89, p < 0.001). Individual group comparisons revealed a statistically significant enhancement of BSR after 0.25 mg/kg (q = 5.89, p < 0.001) and 0.5 mg/kg (q = 7.17, p < 0.001) nicotine, and a significant inhibition of BSR after 1.0 mg/kg (q = 3.94, p < 0.01) nicotine administration. Nicotine failed to alter the Ymax levels at any dose tested (F3,43 = 3.60, p = NS) (data not shown).
Fig. 1.
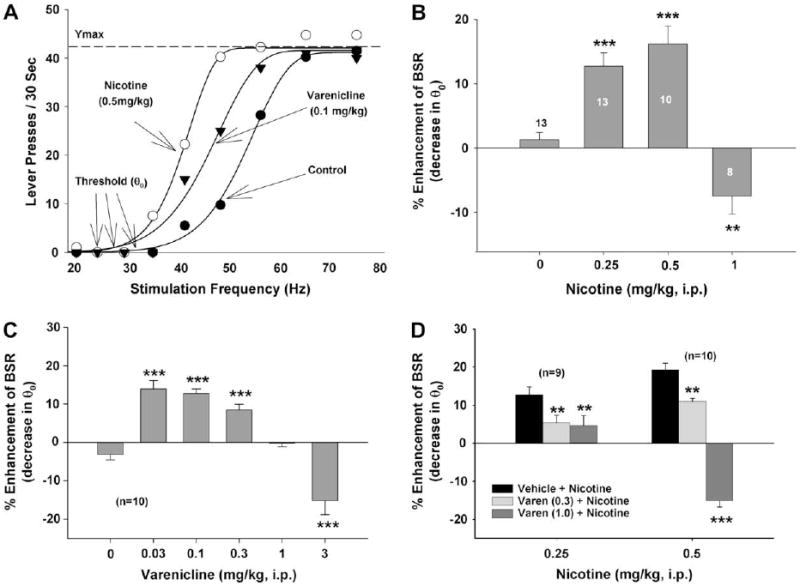
Effects of nicotine and/or varenicline on electrical BSR in rats. Panel A shows typical effects of varenicline and nicotine on the rate-frequency BSR curve, and also indicates the definitions of BSR threshold (θ0) and Ymax. Both varenicline (0.1 mg/kg, i.p.) and nicotine (0.5 mg/kg, i.p.) produced significant leftward shifts in the rate-frequency function, lowering BSR θ0 thresholds without altering Ymax. Panel B shows the effects of nicotine on BSR. Nicotine, at low doses (0.25, 0.5 mg/kg), enhanced, while, at a high dose (1 mg/kg, i.p.), inhibited BSR. Panel C shows the effects of varenicline on BSR. Varenicline, at low doses (0.03, 0.1, 0.3 mg/kg), enhanced BSR, while the highest dose (3 mg/kg i.p.) inhibited BSR. Panel D shows that varenicline (0.3 or 1.0 mg/kg) pretreatment dose-dependently attenuated the BSR-enhancing effects produced by either 0.25 mg/kg or 0.5 mg/kg nicotine. *p < 0.05, **p < 0.01, ***p < 0.001, compared to the vehicle treatment group.
Fig. 1C shows that systemic administration of varenicline also produced a dose-dependent biphasic effect. That is, lower doses (0.03, 0.1, 0.3 mg/kg i.p.) of varenicline produced a significant BSR-enhancing effect, a modestly high dose (1 mg/kg) had no effect, and the highest dose (3 mg/kg) produced a BSR-inhibiting effect. Oneway ANOVA for repeated measurements revealed a statistically significant treatment (varenicline dose) main effect (F5,57 = 28.80, p < 0.001). Individual group comparisons revealed a statistically significant enhancement of BSR after 0.03 mg/kg (q = 7.72, p < 0.001), 0.1 mg/kg (q = 7.89, p < 0.001), and 0.3 mg/kg (q = 5.79, p < 0.001) varenicline, and a significant inhibition of BSR after 3.0 mg/kg (q = 5.95, p < 0.001) varenicline administration. Varenicline had no effect on Ymax levels at any dose tested (F5,57 = 2.19, p = NS) (data not shown).
3.2. Varenicline pretreatment attenuates nicotine-enhanced BSR
Fig. 1D shows that pretreatment with varenicline (0.3, 1.0 mg/kg, i.p., 30 min prior to nicotine) significantly attenuated the BSR-enhancing effects produced by either 0.25 mg/kg or 0.5 mg/kg nicotine. Two-way ANOVA for repeated measurements over varenicline dose for the data shown in Fig. 1D revealed a significant varenicline treatment main effect (F2,32 = 56.05, p < 0.001). Individual group comparisons revealed a statistically significant reduction in 0.25 mg/kg nicotine-enhanced BSR after 0.3 mg/kg (q = 4.20, p < 0.01) or 1.0 mg/kg varenicline (q = 4.74, p < 0.01), and in 0.5 mg/kg nicotine-enhanced BSR after 0.3 mg/kg (q = 4.36, p < 0.01) or 1.0 mg/kg (q = 16.31, p < 0.001) varenicline.
3.3. Mecamylamine blocks varenicline-enhanced BSR
Fig. 2 shows that pretreatment with mecamylamine, a high affinity non-competitive nAChR antagonist (3, 6 mg/kg, i.p., 30 min prior to varenicline), dose-dependently blocked varenicline-enhanced BSR. Two-way ANOVA with repeated measures over mecamylamine dose revealed a significant mecamylamine treatment main effect (F2,30 = 5.60, p < 0.01) and varenicline treatment main effect (F1,15 = 13.44, p < 0.01). Individual group comparisons showed significant augmentation of BSR after 0.1 mg/kg varenicline (q = 5.19, p < 0.001) and significant inhibition of varenicline-enhanced BSR after 3 mg/kg (q = 3.38, p < 0.05) or 6 mg/kg (q = 6.00, p < 0.001) mecamylamine. Mecamylamine alone had no effect on BSR.
Fig. 2.
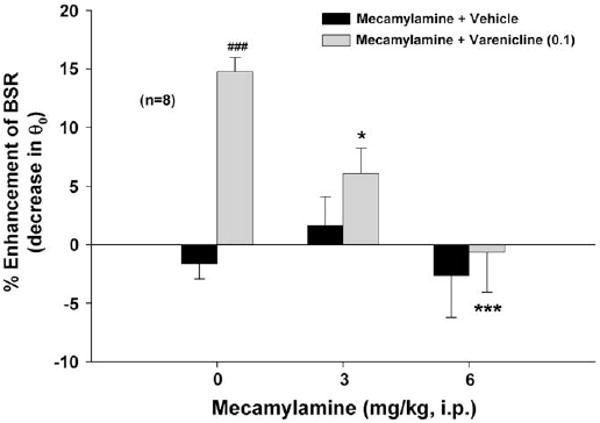
Effects of mecamylamine on varenicline-enhanced BSR. Mecamylamine (3 or 6 mg/kg) alone failed to alter BSR, while pretreatment with mecamylamine dose-dependently attenuated varenicline-enhanced BSR. *p < 0.05, ***p < 0.001, compared to the mecamylamine (0 mg/kg) + varenicline (0.1 mg/kg) baseline group. ###p < 0.001, compared to the mecamylamine (0 mg/kg) + vehicle control group.
3.4. DHβE, but not MLA, attenuates varenicline-enhanced BSR
Fig. 3A shows that pretreatment with DHβE, a relatively selective nicotinic receptor antagonist with high affinity for the α4 receptor subunit (0.3 or 1 mg/kg), dose-dependently attenuated varenicline-enhanced BSR. Two-way ANOVA with repeated measures over DHβE dose revealed a statistically significant DHβE treatment main effect (F2,31 = 4.64, p < 0.05) and varenicline treatment main effect (F1,17 = 36.36, p < 0.001). Individual group comparisons showed significant enhancement of BSR after varenicline (q = 8.65, p < 0.001), and a significant inhibition of varenicline-enhanced BSR after 0.3 mg/kg DHβE (q = 4.02, p < 0.01) and 1 mg/kg DHβE (q = 5.64, p < 0.001), when compared with the varenicline control group. Fig. 3B shows that pretreatment with MLA, a selective α7 receptor antagonist (3 or 6 mg/kg), failed to attenuate varenicline-enhanced BSR. MLA alone had no effect on BSR at any dose tested.
Fig. 3.
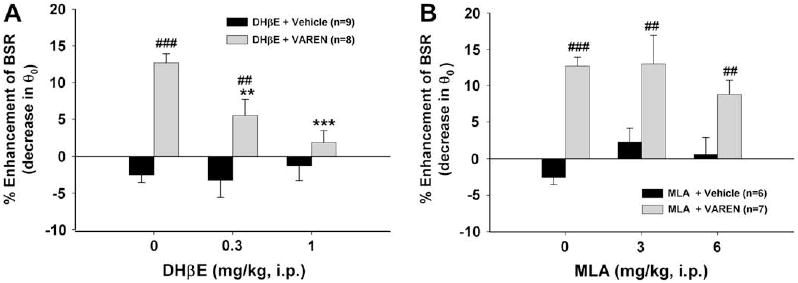
Effects of DHβE (an α4-containing receptor antagonist) or MLA (a selective α7 receptor antagonist) on varenicline-enhanced BSR. Panel A shows that DHβE (0.3 or 1 mg/kg, i.p.) itself has no effect on BSR, but its pretreatment attenuated varenicline-enhanced BSR. Panel B shows that MLA (3 or 6 mg/kg, i.p.) had no significant effect on either BSR itself or on varenicline-enhanced BSR. **p < 0.01, ***p < 0.001, compared to the DHβE (0 mg/kg) + varenicline baseline group. ##p < 0.01, ###p < 0.001, compared to the DHβE + vehicle (Panel A) or the MLA + vehicle (Panel B) control group at each dose of DHβE or MLA.
3.5. Effects of SIB-1765F on BSR and nicotine-enhanced BSR
Fig. 4A shows that the α4β2 receptor agonist SIB-1765F produced dose-dependent enhancement of BSR. One-way ANOVA for repeated measurements for the data shown in Fig. 4A revealed a statistically significant treatment main effect (F3,36 = 20.00, p < 0.001). Individual group comparisons revealed a statistically significant enhancement of BSR after 3 mg/kg (q = 7.76, p < 0.001) or 10 mg/kg SIB-1765F (q = 8.63, p < 0.001). SIB-1765F did not affect Ymax at any dose tested (F3,36 = 0.67, p = NS) (data not shown). Fig. 4B shows that pretreatment with SIB-1765F (3 mg/kg, i.p., 30 min prior to nicotine) significantly attenuated the BSR-enhancing effect produced by 0.5 mg/kg, but not 0.25 mg/kg, nicotine. Two-way ANOVA over SIB-1765F dose revealed a statistically significant SIB-1765F treatment main effect (F1,40 = 9.32, p < 0.01). Individual group comparisons indicated a significant reduction in 0.5 mg/kg nicotine-enhanced BSR (q = 6.30, p < 0.001), but not in 0.25 mg/kg nicotine-enhanced BSR (q = 0.14, p = NS), after 3 mg/kg SIB-1765F.
Fig. 4.
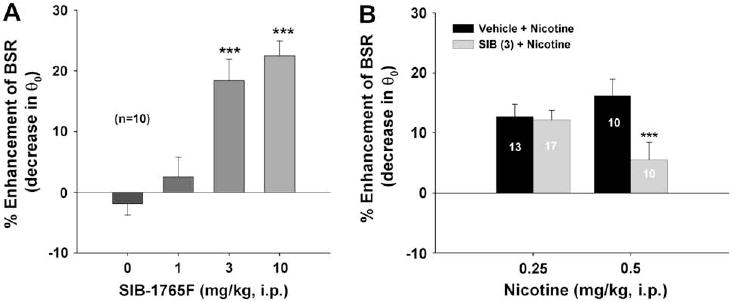
Effects of SIB-1765F (a relatively selective α4β2 receptor agonist) on BSR itself or on nicotine-enhanced BSR. Panel A shows that SIB-1765 (1, 3, 10 mg/kg i.p.) alone dose-dependently enhanced BSR. Panel B shows that SIB-1765 (3 mg/kg, i.p.) pretreatment attenuated the BSR-enhancing effect produced by 0.5 mg/kg, but not 0.25 mg/kg nicotine. ***p < 0.001, compared to the vehicle control group (Panel A) or the 0.5 mg/kg nicotine group (Panel B).
3.6. ARR-17779 alters neither BSR itself nor nicotine-enhanced BSR
Fig. 5A shows that the selective α7 receptor agonist ARR-17779 had no significant effect on BSR (F3,39 = 1.50, p = NS). Fig. 5B shows that pretreatment with ARR-17779 (1 mg/kg) also failed to alter the BSR-enhancing effect produced by either 0.25 mg/kg or 0.5 mg/kg nicotine.
Fig. 5.
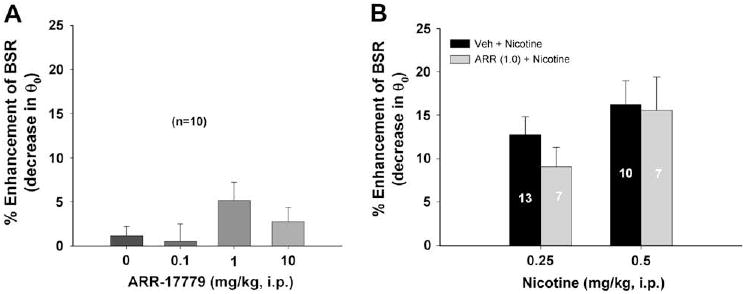
Effects of ARR-17779 (a selective α7 receptor agonist) on BSR or nicotine-enhanced BSR. Panel A shows that ARR-17779 (0.1–10 mg/kg, i.p.) failed to alter BSR. Panel B shows that pretreatment with ARR-17779 (ARR) also failed to alter nicotine (0.25 or 0.5 mg/kg)-enhanced BSR.
4. Discussion
The major findings of the present study are: 1) both nicotine and varenicline produced a similar biphasic effect on brain reward function; that is, low doses enhanced, while high doses inhibited BSR; 2) pretreatment with lower doses of varenicline dose-dependently attenuated nicotine-enhanced BSR; 3) the BSR-enhancing effect produced by varenicline was blocked by either mecamylamine (a high affinity nAChR antagonist) or DHβE (a relatively selective α4-containing receptor antagonist), but not by MLA (a selective α7 receptor antagonist); 4) the α4β2 receptor agonist SIB-1765F dose-dependently enhanced BSR, while pretreatment with SIB-1765F significantly “attenuated” 0.5 mg/kg nicotine-, but not 0.25 mg/kg nicotine-, enhanced BSR. In contrast, the selective α7 receptor agonist ARR-17779 neither altered BSR itself, nor altered nicotine-enhanced BSR at any dose tested. These data suggest that nicotine- or varenicline-enhanced BSR and the pharmacotherapeutic anti-nicotine effects of varenicline are mediated by activation of α4β2, rather than α7, nicotinic receptors.
As noted above, BSR is commonly used for assessing brain reward function and the rewarding effects of addictive drugs (Wise, 1996). Addictive drugs produce highly characteristic reductions in BSR thresholds, indicating summation between the electrical stimulation reward and the drug-induced reward. Both nicotine and varenicline produced a dose-dependent biphasic effect on BSR in the present study, an effect that is similar to the dual motivational effects that lower doses to nicotine produce rewarding, while higher doses produce aversive effects as assessed by conditioned place preference or aversion to nicotine (Fudala et al., 1985; Fudala and Iwamoto, 1986; Picciotto, 2003; Laviolette and van der Kooy, 2003). These data suggest that both nicotine and varenicline may act at the same nicotinic receptors, producing similar biphasic motivational effects. It is well documented that the rewarding effects of nicotine appear to be mediated by activation of α4β2 nAChRs (Gotti et al., 2006; Tutka, 2008; Xi et al., in press). This is supported by the findings that a mutation in the α4 subunit of nicotinic receptors causes sensitization of nAChR to nicotine (Tapper et al., 2004), and deletion of either α4 or β2 subunits attenuates the behavioral effects of nicotine (Picciotto et al., 1998; Marubio et al., 2003). Further, re-expression of the β2-subunit in the VTA and its axonal projections in β2-knockout mice restored nicotine self-administration (Maskos et al., 2005).
To further determine which receptor subtype underlies varenicline-enhanced BSR, we observed the effects of relatively selective α4β2 or α7 receptor antagonists on varenicline-induced increases in BSR. Mecamylamine is a nAChR subtype-nonselective and non-competitive nAChR antagonist that is commonly used to study the CNS effects of nicotine. DHβE is a competitive nAChR antagonist with high affinity to the α4β2, α4β4, α3β2, α2β2, and α2β4 nAChR subtypes (Harvey and Maddox, 1996; Williams and Robinson, 1984). DHβE is often used as an α4β2 nAChR antagonist because the α4β2 nAChR is the most prominent high affinity nAChR subtype in the brain (Picciotto et al., 2001; Khiroug et al., 2004). In contrast, MLA is a selective α7 receptor antagonist (Romanelli and Gualtieri, 2003), although it also binds to α6-containing nAChRs in vitro (Klink et al., 2001; Salminen et al., 2004). In the present study, mecamylamine or DHβE, but not MLA, significantly attenuated varenicline-enhanced BSR, suggesting that varenicline-enhanced BSR is mediated by activation of α4β2, but not α7, nicotinic receptors. This is consistent with previous studies demonstrating that varenicline is a highly potent and selective α4β2 receptor partial agonist (Coe et al., 2005; Mihalak et al., 2006). Consistent with partial agonist activity, varenicline moderately elevates NAc DA but not beyond 70% of the maximal DA response to nicotine (Rollema et al., 2007a), and partially (∼60%) generalizes to nicotine-like discriminative stimulus effects (Rollema et al., 2007a; Smith et al., 2007). In addition, varenicline also partially substitutes for nicotine in self-administration but with lower progressive-ratio break-point levels (Rollema et al., 2007a). These data suggest that the rewarding and discriminative stimulus effects produced by varenicline or nicotine are mediated predominantly by α4β2 nicotinic receptors (Rollema et al., 2007b; Tutka, 2008).
In addition to binding to α4β2 receptors, varenicline also acts as a partial agonist at α3β4, α3β2 and α6, and a full agonist at α7 nicotinic receptors (Rollema et al., 2007a, 2007b; Mihalak et al., 2006). However, it has much lower affinity and functional activity at these non-α4β2 receptors, with reported EC50 values ranging from 1 to 55 μM. Although α7 receptors are also found to be expressed on midbrain DA neurons (Wu et al., 2004), neither deletion of α7 receptors nor pharmacological blockade of α7 receptors alter the nicotine-produced discriminative stimulus effect (Brioni et al., 1996; Gommans et al., 2000; Stolerman et al., 2004). In the present study, we found that the selective α7 receptor agonist ARR-17779 failed to alter BSR, and the selective α7 receptor antagonist MLA failed to block varenicline-enhanced BSR. Together, these data suggest that activation of α7 nicotinic receptors produces neither reward nor aversion, and that α7 receptors are not involved in the reward produced by varenicline or nicotine.
Another present finding is that pretreatment with varenicline significantly attenuated nicotine-enhanced BSR. This is consistent with previous findings that varenicline pretreatment inhibits nicotine self-administration (Rollema et al., 2007a) and nicotine-augmented extracellular NAc DA (Coe et al., 2005; Rollema et al., 2007a). This finding is also consistent with the reports that bupropion, another U.S. FDA approved smoking cessation aid (Xi et al., in press), attenuates nicotine-enhanced BSR (Cryan et al., 2003; Paterson et al., 2008). Thus, blockade of the BSR-enhancing effects of nicotine may contribute to the beneficial effects of varenicline or bupropion in smoking cessation. Varenicline's α4β2 partial agonist properties presumably underlie this anti-nicotine effect. Having lower intrinsic agonist activity than the full agonist nicotine, varenicline produces smaller maximal rewarding or BSR-enhancing effects than nicotine. It is this partial agonist property that is thought to be helpful in relieving nicotine craving and withdrawal. In addition, varenicline's high affinity for α4β2 nAChRs may also prevent nicotine binding to α4β2 nAChRs, therefore, adding to the anti-nicotine effect.
To further determine whether the same α4β2 receptors underlie varenicline's anti-nicotine effects, we studied the effects of other relatively selective α4β2 or α7 receptor agonists on both BSR itself and nicotine-enhanced BSR. SIB-1765F is a relatively selective α4β2 agonist (Sacaan et al., 1997; Menzaghi et al., 1997), and ARR-17779 is reported to be the most selective and potent α7 receptor agonist available (Gordon et al., 1998; Gurley et al., 1998; Van Kampen et al., 2004). In the present study, SIB-1765F produced a robust, dose-dependent BSR-enhancing effect. Pretreatment with SIB-1765F attenuated the BSR-enhancing effect produced by 0.5 mg/kg, but not 0.25 mg/kg, nicotine. We believe that the most parsimonious explanation for this finding relates to summation effects between similarly acting drugs. At low to moderate doses, nicotine enhances BSR (Huston-Lyons and Kornetsky, 1992; Bozarth et al., 1998). However, at high doses, nicotine inhibits BSR (Fig. 1B). This is congruent with reports that at low to moderate doses nicotine produces a dose-orderly increase in conditioned place preference, followed by a dose-orderly increase in conditioned place aversion at high doses (Fudala et al., 1985; Fudala and Iwamoto, 1986), and congruent with the finding that nicotine-enhanced BSR is lost at doses above 1.0 mg/kg (Bozarth et al., 1998). Thus, nicotine's effect on brain reward mechanisms may be viewed as lying along an inverted U-shaped curve – with low doses dose-dependently enhancing brain reward and high doses inhibiting brain reward (Fudala and Iwamoto, 1986). Therefore, if SIB-1765F and nicotine share similar pharmacological actions, their effects should be additive. We find that SIB-1765F alone enhances BSR in a strikingly nicotine-like manner (Fig. 4A). It would not be unexpected, then, for high-dose SIB-1765F to summate with nicotine and push the combined effect on BSR rightward on the inverted U-shaped curve – into the range of brain reward inhibition (Fig. 4B, right panel). Whatever the mechanisms, the present findings suggest that: 1) activation of α4β2 receptors by nicotine, varenicline or SIB-1765F produces rewarding effects; and 2) SIB-1765F is a full α4β2 receptor agonist. In contrast, the selective α7 receptor agonist ARR-17779 neither altered BSR itself nor attenuated nicotine-enhanced BSR. All these data suggest that the attenuation of nicotine-enhanced BSR produced by varenicline is most likely mediated by activation of the α4β2, rather than the α7 receptor subtype.
In conclusion, the present study demonstrates that low doses of varenicline produce reward by themselves, while pretreatment with varenicline significantly attenuates nicotine-enhanced BSR. These effects are mimicked by the selective α4β2 receptor agonist SIB-1765F, but not the selective α7 receptor agonist ARR-17779, and are blocked by the selective α4β2 receptor antagonist DHβE, but not the selective α7 receptor antagonist MLA, suggesting an important role of α4β2 receptors in mediating varenicline's pharmacotherapeutic action.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), U.S.A.
Footnotes
5. Disclosure/conflict of interest
Co-authors Spiller, Xi, Li, and Gardner declare that, except for income received from their primary employers, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. Co-author Ashby declares that, in addition to income received from his primary employer, he served as an expert consultant to Memory Pharmaceuticals in Montvale, NJ, USA. Co-authors Callahan and Tehim declare that their primary employer is Memory Pharmaceuticals in Montvale, NJ, USA.
References
- Bozarth MA, Pudiak CM, KuoLee R. Effect of chronic nicotine on brain stimulation reward. I. Effect of daily injections. Behav Brain Res. 1998;96:185–188. doi: 10.1016/s0166-4328(98)00050-3. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Kim DJB, O'Neill AB. Nicotine cue: lack of effect of the alpha7 nicotinic receptor antagonist methyllycaconitine. Eur J Pharmacol. 1996;301:1–5. doi: 10.1016/0014-2999(96)00010-6. [DOI] [PubMed] [Google Scholar]
- Cao YJ, Surowy CS, Puttfarcken PS. Different nicotinic acetylcholine receptor subtypes mediating striatal and prefrontal cortical [3H]dopamine release. Neuropharmacology. 2005;48:72–79. doi: 10.1016/j.neuropharm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, et al. Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Cooper E, Couturier S, Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal acetylcholine receptor. Nature. 1991;350:235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology. 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Foulds J. The neurobiological basis for partial agonist treatment of nicotine dependence: varenicline. Int J Clin Pract. 2006;60:571–576. doi: 10.1111/j.1368-5031.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Further studies on nicotine-induced conditioned place preference in the rat. Pharmacol Biochem Behav. 1986;25:1041–1049. doi: 10.1016/0091-3057(86)90083-3. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22:237–241. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Gommans J, Stolerman IP, Shoaib M. Antagonism of the discriminative and aversive stimulus properties of nicotine in C57BL/6J mice. Neuropharmacology. 2000;39:2840–2847. doi: 10.1016/s0028-3908(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Gordon J, Gurley DA, Tran O, Machulskis A, Zongrone J, et al. AR-R 17779: the first high affinity, subtype-selective full agonist at the rodent α7 nicotinic acetylcholine receptor. Soc Neurosci Abstr. 1998;24:331.9. [Google Scholar]
- Gotti C, Riganti L, Vailati S, Clementi F. Brain neuronal nicotinic receptors as new targets for drug discovery. Curr Pharm Des. 2006;12:407–428. doi: 10.2174/138161206775474486. [DOI] [PubMed] [Google Scholar]
- Grady SR, Meinerz NM, Cao J, Reynolds AM, Picciotto MR, Changeux JP, McIntosh JM, Marks MJ, Collins AC. Nicotinic agonists stimulate acetylcholine release from mouse interpeduncular nucleus: a function mediated by a different nAChR than dopamine release from striatum. J Neurochem. 2001;76:258–268. doi: 10.1046/j.1471-4159.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- Gurley DA, Lanthorn T, Ryan T, Luhowskyj S, Gordon J. Comparison of nicotine and the α7-selective agonist AR-R 17779 on nAchR α7 expressed in Xenopus oocytes. Soc Neurosci Abstr. 1998;24:331.10. [Google Scholar]
- Harvey SC, Maddox FN. Multiple determinants of dihydro-β-erythroidine sensitivity on rat neuronal nicotinic receptor α subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol Biochem Behav. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Khiroug SS, Khiroug L, Yakel JL. Rat nicotinic acetylcholine receptor α2β2 channels: comparison of functional properties with α4β2 channels in Xenopus oocytes. Neuroscience. 2004;124:817–822. doi: 10.1016/j.neuroscience.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Klink R, de kerchove d'Exacrde A, Zoli M, Changeux J. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The motivational valence of nicotine in the rat ventral tegmental area is switched from rewarding to aversive following blockade of the α7-subunit-containing nicotinic acetylcholine receptor. Psychopharmacology. 2003;166:306–313. doi: 10.1007/s00213-002-1317-6. [DOI] [PubMed] [Google Scholar]
- Le Novère N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53:447–456. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- Markou A. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, McIntosh JM, Rossi F, Champtiaux N, Zoli M, Changeux JP. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Whelan KT, Risbrough VB, Rao TS, Lloyd GK. Effects of a novel cholinergic ion channel agonist SIB1765F on locomotor activity in rats. J Pharmacol Exp Ther. 1997;280:384–392. [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Pak AC, Ashby CR, Jr, Heidbreder CA, Pilla M, Gilbert J, Xi ZX, Gardner EL. The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol. 2006;9:585–602. doi: 10.1017/S1461145706006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob Res. 2008;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. fourth. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Picciotto MR. Nicotine as a modulator of behavior: beyond the inverted U. Trends Pharmacol Sci. 2003;23:494–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lèna C, Marubio LM, Merlo Pich E, Fuxe K, Changeux JP. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007a;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4-beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007b;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Romanelli MN, Gualtieri F. Cholinergic nicotinic receptors: competitive ligands, allosteric modulators, and their potential applications. Med Res Rev. 2003;23:393–426. doi: 10.1002/med.10037. [DOI] [PubMed] [Google Scholar]
- Sacaan I, Reid RT, Santori EM, Addmas P, Correa LD, et al. Pharmacological characterisation of SIB-1765F: a novel cholinergic ion channel agonist. J Pharmacol Exp Ther. 1997;280:373–383. [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh M, Drago J, Marks MJ, Collins AC, Grady SR. Submit composition and pharamacology of two classes of striatal presynaptic nicotinic acetycholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Smith J, Mogg AM, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M. Ligands selective for α4β2 but not α3β4 or α7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology. 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Peng XQ, Newman A, Ashby C, Heidbreder C, Gaál J, Gardner EL. The selective dopamine D3 receptor antagonists SB-277011A and NGB 2904 and the putative partial D3 receptor agonist BP-897 attenuate methamphetamine-enhanced brain stimulation reward in rats. Psychopharmacology. 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Chamberlain S, Bizarro L, Fernandes C, Schalkwyk L. The role of nicotinic receptor α7 subunits in nicotine discrimination. Neuropharmacology. 2004;46:363–371. doi: 10.1016/j.neuropharm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tutka P. Nicotine receptor partial agonists as novel compounds for the treatment of smoking cessation. Expert Opin Investig Drugs. 2008;17:1473–1485. doi: 10.1517/13543784.17.10.1473. [DOI] [PubMed] [Google Scholar]
- Van Kampen M, Selbach K, Schneider R, Schiegel E, Boess F, Schreiber R. AR-R 17779 improves social recognition in rats by activation of nicotinic alpha7 receptors. Psychopharmacology. 2004;172:375–383. doi: 10.1007/s00213-003-1668-7. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Williams M, Robinson JL. Binding of the nicotinic cholinergic antagonist, dihydro-β-erythroidine, to rat brain tissue. J Neurosci. 1984;4:2906–2911. doi: 10.1523/JNEUROSCI.04-12-02906.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wu J, George AA, Schroeder KM, Xu L, Marxer-Miller S, Lucero L, Lukas RJ. Electrophysiological, pharmacological and molecular evidence for α7-nicotinic acetylcholine receptors in rat midbrain dopamine neurons. J Pharmacol Exp Ther. 2004;311:80–91. doi: 10.1124/jpet.104.070417. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Spiller K, Gardner EL. Mechanism-based medication development for the treatment of nicotine dependence. Acta Pharmacol Sinica. doi: 10.1038/aps.2009.46. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Chiappinelli VA. Nicotinic receptors mediate increased GABA release in brain through a tetrodotoxin-insensitive mechanism during prolonged exposure to nicotine. Neuroscience. 2002;115:137–144. doi: 10.1016/s0306-4522(02)00371-8. [DOI] [PubMed] [Google Scholar]
- Zierler-Brown SL, Kyle JA. Oral varenicline for smoking cessation. Ann Pharmacother. 2007;41:95–99. doi: 10.1345/aph.1H310. [DOI] [PubMed] [Google Scholar]
- Zoli M, Lèna C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using β2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


