Abstract
Transcutaneous electrical nerve stimulation (TENS) is a non-pharmacological modality used clinically to relieve pain. Central involvement of serotonin and endogenous opioids are implicated in TENS-induced analgesia. Activation of spinal cholinergic receptors is antinociceptive and these receptors interact with opioid and serotonin receptors. In the current study, the possible involvement of spinal cholinergic receptors in TENS analgesia was investigated in rats. Hyperalgesia was induced by inflaming one knee joint with 3% kaolin—carrageenan and assessed by measuring paw withdrawal latency (PWL) to heat before and 4 h after injection. The non-selective nicotinic antagonist mecamylamine (50 μg), non-selective muscarinic antagonist atropine (30 μg) or one of the muscarinic subtype antagonists: pirenzepine (M1, 10 μg), methoctramine (M2, 10 μg), 4-DAMP (M3, 10 μg), or saline was administered intrathecally just prior to TENS treatment. Low or high frequency TENS was then applied to the inflamed knee and PWL was determined again. Atropine, pirenzepine and 4-DAMP significantly attenuated the antihyperalgesic effects of low and high frequency TENS while mecamylamine and methoctramine had no effects, compared to saline control. The results show that TENS-induced antihyperalgesia is mediated partially by activation of spinal muscarinic receptors but not spinal nicotinic receptors. Further, the results also indicate that spinal M1 and M3 muscarinic receptor subtypes mediate the muscarinic component of TENS antihyperalgesia.
Keywords: Electrical nerve stimulation, Cholinergic, Muscarinic, Inflammation, Pain, Spinal cord
1. Introduction
Transcutaneous electrical nerve stimulation (TENS) is a therapeutic modality, clinically used to relieve acute and chronic pain (Johnson et al., 1992). TENS is easy to use for the patient and is devoid of major side effects. Although the use of TENS is very common, its analgesic mechanism is not fully understood. Two types of TENS are used clinically, low frequency TENS (frequency of stimulation < 10 Hz) and high frequency TENS (frequency > 50 Hz). Different theories have been proposed for the mechanism of action of TENS, the popular one being the gate control theory proposed by Melzack and Wall (1965). According to gate control theory, the nociceptive information from small diameter afferents is overridden by the stimulation of large diameter fibers and the pain stimulus is prevented from reaching supraspinal centers. However, neuropharmacological studies point towards neurotransmitter-related spinal and supraspinal mechanisms in TENS-induced analgesia. Endogenous opioids released in the central nervous system are implicated in the analgesic mechanism of TENS by various investigators (see Sluka and Walsh, 2003).
The antihyperalgesic effect of TENS predominantly involves central (spinal and supraspinal) mechanisms (Sluka et al., 1999; Kalra et al., 2001) rather than peripheral mechanisms (Janko and Trontelj, 1980). Spinally, opioid and serotonin receptors mediate TENS antihyperalgesia (Suka et al., 1999; Radhakrishnan et al., 2003). Further, sensitization of dorsal horn neurons induced by carrageenan inflammation is reversed by high and low frequency TENS (Ma and Sluka, 2001). Descending inhibitory systems from the periaqueductal grey (PAG) and rostral ventromedial medulla (RVM) mediate analgesia through opioid, adrenergic and serotonin receptors spinally (Fields and Basbaum, 1999). In the spinal cord, inhibition also involves cholinergic receptors and they are clearly involved in spinal antinociception (Zhuo and Gebhart, 1991; Fang and Proudfit, 1996; reviewed by Eisenach, 1999; Pinardi et al., 2003). Among the spinal receptors, cholinergic receptors are unique in that they mediate antinociception through interactions with most of the inhibitory receptors, i.e. 5-HT, adrenergic or opioid receptors, in the spinal cord (Chiang and Zhuo, 1989; Gordh et al., 1989; Li et al., 1994; Obata et al., 2002; Chen and Pan, 2001; Honda et al., 2002). Both cholinergic nicotinic (Arimatsu et al., 1981; Ninkovic and Hunt, 1983; see review by Coggeshall and Carlton, 1997) and cholinergic muscarinic (Kayaalp and Neff, 1980; see review by Coggeshall and Carlton, 1997; Eisenach, 1999) receptors are localized to the dorsal horn, in laminae I–IV. Although nicotinic receptors are located in the spinal cord, their role in spinal antinociception is controversial. Thus, spinal cholinergic receptors are important in the inhibition of nociception through activation of opioid and 5-HT receptors.
Since there is compelling evidence for spinal and/or supraspinal opioid and 5-HT receptor mediation in TENS analgesia, we investigated the involvement of spinal cholinergic nicotinic and muscarinic receptors in the antihyperalgesic mechanisms of low and high frequency TENS, using selective antagonists. Selectivity and functional differences of the spinal cholinergic receptor subtypes were studied using selective antagonists against the spinal antinociceptive effects of muscarinic agonists.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats (n = 136, Harlan, St. Louis, Missouri, USA), weighing 225–300 g, kept at 12 h dark—light cycle with free access to standard rat chow and water, were used for the experiments. All experiments were approved by University of Iowa Animal Care and Use Committee and were carried out according to the guidelines of the International Association for the Study of Pain and National Institutes of Health.
2.2. Behavior testing
Animals were brought to the behavioral testing room the day before to acclimatize them to the testing environment. All behavioral testing was done between 9 a.m. and 5 p.m. Animals were kept in Plexiglas® restrainers on an elevated platform with a clear glass top for at least 30 min for acclimatization. A radiant heat source was used as the stimulus. This produces a gradually increasing skin temperature until the animal withdraws from the stimulus. The heat source was positioned on the plantar skin of the hind limb and the beam was switched on, simultaneously starting a built-in timer. When the animal withdrew the paw abruptly to heat stimulus, the heat source and the timer were stopped. The duration in seconds from the start of heat application to the paw withdrawal was taken as the paw withdrawal latency (PWL). PWLs were determined five times bilaterally, with an interval of 5 min between each test, and the mean of five readings was taken as the PWL for each time. Any significant reduction in PWL compared to baseline was considered as hyperalgesia. The intensity of the heat source was set at optimum level with an adjustable voltage power supply to obtain a baseline response time between 12 and 16 s. This voltage, and therefore the intensity of the heat source, was kept constant throughout the study. Cut-off time was set to 30 s to minimize heat damage to the skin.
2.3. Intrathecal catheter placement
A 32G polyethylene catheter was placed intrathecally. Briefly, the animals were anesthetized with 2% halothane and the dorsal surface shaved and cleaned with Betadine® solution. A 2 cm incision was made at the iliac crest. A 32 G polyethylene catheter was introduced into the lumbar space between L4 and L5 with the help of a 23G guide needle and advanced to a length of 3.5–4 cm rostrally. The catheter was fixed in place and the tip connected to a saline filled PE10 tube, which was externalized dorsally between the scapulas. The tip of the catheter was sealed and the animal was allowed to recover for 5–7 days.
2.4. Intra-articular injection
After baseline PWL recordings, animals were injected with 0.1 ml suspension of 3% kaolin and 3% carrageenan (K/C suspension) in normal saline (pH 7.0), in the left knee joint, under light halothane (2–4% v/v in medical oxygen) anesthesia.
2.5. Drugs
The following drugs were used: Carbamylcholine chloride (carbachol, non-selective cholinergic antagonist, 500 ng, intrathecal (i.t.); Smith et al., 1989), 1-Methyl-1,2,5,6-tetrahydro-3-pyridine carboxylic acid propargyl ester hydrobromide (arecaidine, muscarinic agonist slightly selective at M2, 20 μg; Baba et al., 1998, in vitro), α-(Hydroxymethyl)benzeneacetic acid 8-methyl-8-azabicyclo(3.2.1)oct-3-yl ester Tropine tropate (atropine, non-selective muscarinic antagonist, 30 μg; Chen and Pan, 2001), N,2,3,3-Tetramethylbicyclo [2.2.1]heptan-2-amine hydrochloride (mecamylamine, non-selective nicotinic antagonist, 50 μg; Chen and Pan, 2001), 5,11-Dihydro-11-[(4-methyl-1-piperazinyl) acetyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one dihydrochloride (pirenzepine, M1 receptor antagonist, 10 μg, i.t.; Obata et al., 2002), N,N’-bis[6[[(2-methoxyphenyl)methyl]amino]hexyl]-1,8-octanediamine tetrahydrochloride (methoctramine, M2 receptor antagonist, 10 μg; Honda et al., 2002), 4-diphenylacetoxy-N-methylpiperdine methiodide (4-DAMP, M3 receptor antagonist, 10 μg; Hwang et al., 1999), kaolin, lambda carrageenan (type IV). All chemicals were dissolved in normal saline and obtained from Sigma Chemical Co. USA. The doses used, shown against each drug, were adapted or modified from previously published studies that showed pharmacological activation or antagonism of individual receptor subtypes. In some cases, the effective doses were determined by titration.
2.6. Intrathecal drug administration
Drugs or saline were injected into the intrathecal space in a volume of 10 μl, using a Hamilton syringe connected to the i.t. catheter via a PE10 tubing followed by 10 μl saline to flush the catheter. After the experiment, proper placement of the catheter was confirmed by injection of 10 μl of 2% lidocaine i.t. and observing animals for hind limb paralysis. Methylene blue dye solution (10 μl., i.t.) was then injected. Animals were sacrificed, spinal cord dissected and the dye spread was assessed. The data from those animals, which did not show hind limb paralysis with lidocaine and in which the dye was not found in L4—L6 levels of spinal cord, were not included in the analysis.
2.7. TENS application
TENS units and electrodes (Eclipse+, EMPI Inc., Minnesota, USA) were used in this study. Immediately after drug or saline was administered intrathecally, the inflamed hind limb of the rat was shaved and cleaned with alcohol. Circular, pregelled electrodes of 2.5 cm diameter were attached to the medial and lateral aspects of the inflamed knee joint. Either high (100 Hz) or low frequency (4 Hz) TENS, at sensory intensity, was then applied through the electrodes for 20 min under light halothane anesthesia (1–2% in oxygen). Sensory intensity was determined by increasing the intensity until motor contraction was observed and then turning down the intensity to just below the motor contraction level. The pulse width was kept constant at 100 μs in both low and high frequency TENS, the only variable among TENS groups being frequency. There is a complete reversal of kaolin/carrageenan-induced hyperalgesia by both low and high frequency TENS, using this protocol (Sluka et al., 1999).
2.8. Protocol
Five to seven days after intrathecal catheter placement, baseline PWLs to heat for both hind paws were determined and knee joint inflammation was induced by intra-articular injection of kaolin/carrageenan unilaterally. Four hours after injection, PWLs were determined again. Animals were then administered with one of the antagonists intrathecally viz. mecamylamine (no TENS, n = 4; low TENS, n = 4; high TENS, n = 4), atropine (no TENS, n = 8; low TENS, n = 6; high TENS, n = 6), pirenzepine (no TENS, n = 6; low TENS, n = 6; high TENS, n = 8), methoctramine (no TENS, n = 6; low TENS, n = 4; high TENS, n = 6), 4-DAMP (no TENS, n = 6; low TENS, n = 6; high TENS, n = 6) or saline (no TENS, n = 8; low TENS, n = 6; high TENS, n = 6). After 15 min, animals were anesthetized (2–4% halothane) and either high or low frequency TENS or no TENS was applied to the ipsilateral knee for 20 min. PWLs were determined again 30 min after removal of TENS, when the animals were fully awake from halothane anesthesia. Animals in the ‘no TENS’ (control) groups were kept under halothane anesthesia for 20 min (as in TENS group), but TENS was not applied. A previous study from our laboratory shows that the hyperalgesia caused by kaolin—carrageenan is not affected by shaving the knee joint area, placing TENS electrodes or halothane anesthesia (Sluka, 2000).
The selectivity of various agonists against their respective antagonists was studied in separate groups of animals. All drugs were tested in animals with knee joint inflammation at the same 4 h time point that TENS was applied in the previous experiment. Specifically, (1) mecamylamine was tested for its ability to antagonize the effects of carbachol (mecamylamine + carbachol, n = 3; saline + carbachol, n = 6); (2) atropine was tested against carbachol (n = 3); (3) pirenzepine was tested against carbachol (n = 4); (4) methoctramine was tested against carbachol and arecaidine (methoctramine + carbachol, n = 4; methoctramine + arecaidine, n = 3; saline + arecaidine, n = 3); and (5) 4-DAMP was tested against carbachol (n = 4). The antagonist was given 15 min prior to agonist, as done in the TENS experiment. All groups were compared against a control group that received saline (instead of antagonist) 15 min prior to the agonist.
2.9. Statistical analysis
All data are presented as mean ± SEM. Data were compared with a multivariate analysis of variance for treatment (no TENS, high frequency TENS, low frequency TENS), drug (saline and five drugs) and three time points (baseline, hour 4, and postTENS). Since only postTENS showed significant effects for TENS treatment, drug and the interaction of TENS treatment with drug, a post hoc Tukey’s test compared differences between the appropriate saline control group treated with TENS and the drug groups treated with TENS. Paired t-tests compared differences between baseline and 4-h PWLs to confirm hyperalgesia. Multivariate tests followed by Tukey’s post hoc test compared the effects of saline vs. mecamylamine, atropine, pirenzepine, methoctramine and 4-DAMP on carbachol-induced antihyperalgesia. Independent samples t-test was used to compare the effects of methoctramine on the antinociceptive effects of arecaidine. The level of significance was set at p≤0.01 to correct for multiple comparisons. Statistical analysis was performed with SPSS version 10.1.
3. Results
An overall effect for the PWL to heat occurred for treatment (F2,102 = 95.2, p < 0.0001) such that PWLs were significantly higher following treatment with either high (p < 0.0001) or low (p < 0.0001) frequency TENS, when compared to ‘no TENS’. An interaction occurred between treatment and drug (F10,102 = 7.8, p < 0.0001) for PWLs after treatment with or without TENS. The results below are thus outlined by drug treatments.
3.1. Carrageenan/kaolin-induced hyperalgesia and effect of TENS
Four hours after the injection of kaolin and carrageenan into the knee joint of rats, there was a significant reduction in the ipsilateral PWL when compared to baseline (p < 0.0001). This reduction in PWL by carrageenan injection was completely reversed by low (p = 0.002) or high frequency (p = 0.003) TENS in control (i.t. saline) animals (Figs. 1 and 2). PWLs remained unchanged in animals not treated with TENS (Fig. 3). There were no significant changes in PWL on the contralateral side.
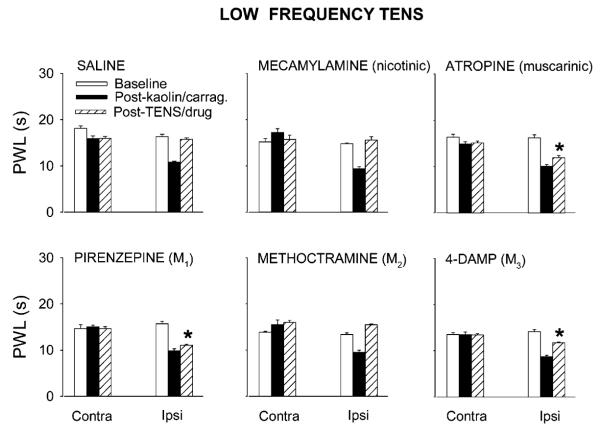
Fig. 1.
Bar graphs showing effects of saline or cholinergic antagonists viz. mecamylamine (50 μg), atropine (30 μg), pirenzepine (10 μg), methoctramine (10 μg) or 4-DAMP (10 μg), on the antihyperalgesia produced by low frequency TENS. Intrathecal treatment with atropine, pirenzepine and 4-DAMP significantly (*, p < 0.01, Tukey’s post hoc test) attenuated the antihyperalgesic effects of low frequency TENS compared to intrathecal treatment with saline. Values are mean ± S.E.M. Contra = contralateral, Ipsi = Ipsilateral.
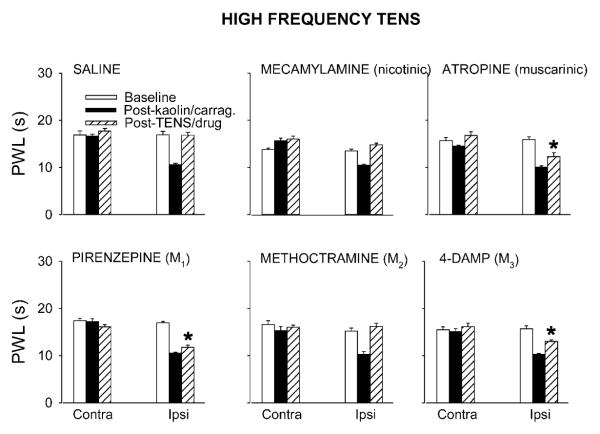
Fig. 2.
Bar graphs showing effects of saline or cholinergic antagonists viz. mecamylamine (50 μg), atropine (30 μg), pirenzepine (10 μg), methoctramine (10 μg) or 4-DAMP (10 μg), on the antihyperalgesia produced by high frequency TENS. Atropine, pirenzepine and 4-DAMP significantly (*, p < 0.01, Tukey’s post hoc test) attenuated the antihyperalgesic effects of high frequency TENS, compared to saline group. Data are mean ± S.E.M. Contra = contralateral, Ipsi = Ipsilateral.
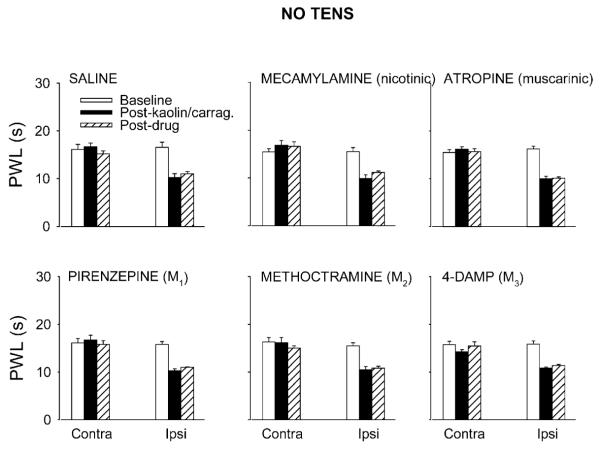
Fig. 3.
Bar graphs showing the effects of intrathecal administration of saline or cholinergic antagonists viz. mecamylamine (50 μg), atropine (30 μg), pirenzepine (10 μg), methoctramine (10 μg) or 4-DAMP (10 μg) on kaolin/carrageenan-induced hyperalgesia, without TENS application. There was no effect on the hyperalgesia by any antagonist or saline. Values are mean ± S.E.M. Contra = contralateral, Ipsi = ipsilateral.
3.2. Effect of nicotinic antagonist
Intrathecal injection of the nicotinic antagonist mecamylamine (50 μg) had no effect on the reversal of ipsilateral PWL produced by low (Fig. 1) or high (Fig. 2) frequency TENS, when compared to saline controls. Mecamylamine alone had no effects on PWL when given intrathecally at the same dose in animals with knee joint inflammation (Fig. 3).
3.3. Effect of non-selective muscarinic antagonist
Atropine (30 μg, i.t.), a non-selective muscarinic antagonist, significantly inhibited the reversal of ipsilateral PWLs produced by both low (Fig. 1) and high (Fig. 2) frequency TENS (p<0.0001 for both) compared to saline controls. A similar dose of intrathecal atropine alone had no effect on PWL in animals with knee joint inflammation (Fig. 3).
3.4. Effects of selective muscarinic antagonists
The M1 subtype receptor antagonist, pirenzepine (10 μg, i.t., p < 0.0001 and p = 0.002 for low and high TENS, respectively) and M3 subtype antagonist 4-DAMP (10 μg, i.t.), significantly attenuated the antihyperalgesia produced by both low (Fig. 1) and high (Fig. 2) frequency TENS, compared to saline control. The same doses of pirenzepine or 4-DAMP had no effect on the reduction in PWL produced by knee joint inflammation (Fig. 3).
The selective antagonist at the M2 receptor subtype, methoctramine (10 μg, i.t.), had no effect on the antihyperalgesia produced by low or high frequency TENS when compared to saline control animals (Figs. 1 and 2). The same dose of methoctramine had no effect on the decreased PWL produced by knee joint inflammation (Fig. 3).
3.5. Selectivity of antagonists
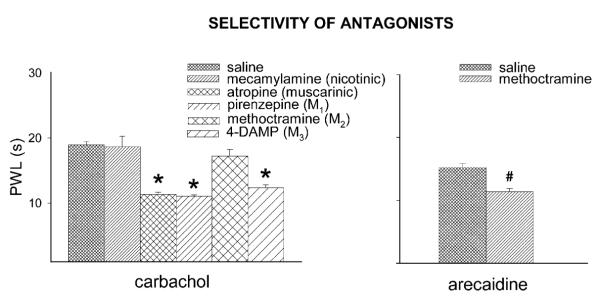
There was an overall effect for PWLs following drug treatment (F5,22 = 21.24, p < 0.0001). The nicotinic receptor antagonist, mecamylamine (i.t.), did not block the spinal antinociceptive effect of carbachol. The muscarinic antagonists, atropine (non-selective) (p < 0.0001), pirenzepine (M1) (p < 0.0001) and 4-DAMP (M3) (p = 0.001), administered i.t., significantly inhibited the spinal antinociceptive effects of carbachol. Spinal methoctramine, an M2 antagonist, did not affect the carbachol antinociception. But arecaidine (M2 agonist)-induced antinociception was significantly attenuated by methoctramine (p = 0.01) (Fig. 4).
Fig. 4.
Effects of muscarinic antagonists viz. atropine (30 μg), pirenzepine (10 μg), methoctramine (10 μg) or 4-DAMP (10 μg) on the antihyperalgesia caused by spinal carbachol (500 ng) (left panel) and effects of methoctramine on the antinociception caused by arecaidine (20 μg) (right panel). Methoctramine did not affect the antihyperalgesia caused by carbachol but reversed the antihyperalgesia caused by arecaidine. *Significantly different from saline effects (p≤0.01, Tukey’s) post hoc test, #Significantly different from saline control (p = 0.01, Independent samples t-test). Data shown are mean ± S.E.M.
4. Discussion
The data from the present study clearly show that the antihyperalgesia produced by low and high frequency TENS is mediated to a large extent by spinal muscarinic receptors since the non-selective muscarinic antagonist atropine markedly attenuated both low and high frequency TENS-induced antihyperalgesia, when administered intrathecally. Further, pirenzepine and 4-DAMP which are antagonists at M1 and M3 muscarinic receptor subtypes, respectively, also attenuated the effects of both low and high frequency TENS, indicating involvement of these receptor subtypes in TENS-induced antihyperalgesia. Methoctramine had no effect on either low or high frequency TENS antihyperalgesia indicating that M2 receptors are not involved. The results also show that antihyperalgesia produced by low or high frequency TENS does not depend on spinal nicotinic receptormediated mechanisms, in this model of knee joint inflammatory pain.
4.1. Descending inhibition and TENS effects
Descending inhibitory systems, originating in the midbrain and terminating in the spinal dorsal horn, consist of two major pathways—noradrenergic and serotoninergic (see Fields and Basbaum, 1999). Descending inhibition is translated into antinociception in the spinal cord mainly by activation of serotoninergic, adrenergic, cholinergic and opioidergic receptors (Li and Zhuo, 2001; see Fields and Basbaum, 1999). Similarly, the antihyperalgesic effects of TENS utilize descending inhibitory systems to reduce hyperalgesia through activation of spinal opioid, serotonergic and cholinergic receptors (Woolf et al., 1980; Men and Matsui, 1994; Sluka et al., 1999; Kalra et al., 2001; Radhakrishnan et al., 2003). However, in contrast to studies on descending inhibition, spinal adrenergic receptors are not involved in TENS antihyperalgesia (Radhakrishnan et al., 2003). Systemic serotonin depletion or spinal serotonin receptor antagonists attenuate the antihyperalgesia produced by TENS (Woolf et al., 1980; Radhakrishnan et al., 2003) and peripheral nerve stimulation increases serotonin metabolites in the spinal cord (Men and Matsui, 1994). Further, repeated application of low or high frequency TENS leads to opioid tolerance and in animals made tolerant to morphine, low frequency TENS is ineffective (Sluka et al., 2000; Chandran and Sluka, 2003). Thus, involvement of central 5-HT and opioid receptors in TENS-induced analgesia is evident. Therefore, both supraspinal as well as spinal neurotransmitters, through descending inhibitory systems, play a pivotal role in the effects of TENS. Opioid and 5-HT receptors interact with cholinergic receptors in the spinal cord (Dirksen and Nijhuis, 1983; Li et al., 1994) and results from our studies show that cholinergic antagonists block TENS effects. Therefore, we suggest that one mechanism for TENS antihyperalgesia is through the activation of descending inhibitory systems, which in turn activate spinal inhibitory receptors including muscarinic receptors. Further, spinal cholinergic receptors are an important interneuronal link in the spinal analgesic effector system.
4.2. Spinal neurotransmitter interactions and TENS effects
Cholinergic agonists and acteylcholinesterase (AChE) inhibitors, administered spinally, cause antinociception (see review by Eisenach, 1999). The antinociceptive effects of cholinergic agonists are mediated primarily through muscarinic receptors (Taylor et al., 1982; Yaksh et al., 1985; Zhuo and Gebhart, 1991; Naguib and Yaksh, 1994, 1997), although some studies suggest a role for cholinergic nicotinic receptors (Chen and Pan, 2001; Abelson and Hoglund, 2002). Although there is strong evidence for local spinal cholinergic interneurons (Borges and Iversen, 1986; Baba et al., 1998), the possibility of a descending cholinergic pathway cannot be ruled out (Bowker et al., 1983; Jones et al., 1986). Muscarinic receptors are located in laminae I–IV, but mostly in the lamina II in spinal dorsal horn, which is a spinal site involved in nociceptive transmission. Nicotinic receptors, however, are located mainly in laminae III and IV (Coggeshall and Carlton, 1997), which receive nonnociceptive inputs.
There is much evidence in the literature regarding the interaction of cholinergic receptors in the spinal cord with other major inhibitory receptors like 5-HT, adrenergic and opioid receptors (Dirksen and Nijhuis, 1983; Chiang and Zhuo, 1989; Gordh et al., 1989; Zhuo and Gebhart, 1991; Li et al., 1994; Obata et al., 2002; Fang and Proudfit, 1996; Chen and Pan, 2001; Honda et al., 2002). For example, spinal morphine (Dirksen and Nijhuis, 1983), clonidine (Gordh et al., 1989), and serotonin (Li et al., 1994) act through muscarinic receptors to produce antinociception. These findings taken together with the results from present study, strongly suggest that TENS stimulation activates spinal muscarinic receptors, possibly through an interaction with opioid and/or serotonergic receptor(s). However, in the current study, the antihyperalgesia produced by either low or high frequency TENS was not affected by spinal pretreatment with mecamylamine, a nicotinic antagonist. Thus, our data indicate that spinal muscarinic, but not nicotinic, receptors play an important role in mediating antinociception produced by low and high frequency TENS, in this model of knee joint inflammatory pain.
4.3. Spinal cholinergic receptor subtypes and TENS effects
Five subtypes of muscarinic receptors have been identified, i.e. M1–5 (Bymaster et al., 2003; Wess et al., 2003). Muscarinic subtypes implicated in the spinal nociceptive processing are mainly M1, M2, and M3 (Gillberg et al., 1989; Zhuo and Gebhart, 1991; Iwamoto and Marion, 1993; Bouaziz et al., 1995; Naguib and Yaksh, 1997; Ma et al., 2001) and probably M4 (Ellis et al., 1999). In the current study, our data show that TENS activates M1 and M3 receptors to reduce hyperalgesia. Our data agree with prior pharmacological studies which show activation of M1 and M3 receptors in the spinal cord produces antinociception, using acute pain tests (Gillberg et al., 1989; Zhuo and Gebhart, 1991; Iwamoto and Marion, 1993; Bouaziz et al., 1995; Naguib and Yaksh, 1997; Hwang et al., 1999; Honda et al., 2002; Obata et al., 2002). In contrast, blockade of M3 receptors causes antinociception in the second phase of the formalin test (Honda et al., 2000). However, in the current study, blockade of M3 receptors with the same antagonist (4-DAMP) has no effect on the hyperalgesia, suggesting different mechanisms are involved in these two conditions.
The role of M2 receptors is unclear. Blockade of spinal M2 receptors attenuates the antinociception in some studies (Gillberg et al., 1989; Iwamoto and Marion, 1993) while others show no involvement (Bouaziz et al., 1995; Naguib and Yaksh, 1997; Honda et al., 2002). Probable reasons for these conflicting findings are the structural homology (Wei et al., 1994), overlapping expression patterns of the M1–5 muscarinic receptor subtypes (Gomeza et al., 1999), and a lack of selectivity of muscarinic subtype ligands (Gomeza et al., 1999; Bymaster et al., 2003).
In the current study, secondary heat hyperalgesia is reversed by the non-selective cholinergic agonist, carbachol, and the selective M2 agonist arecaidine. Surprisingly, methoctramine, an M2 antagonist, has no effect on the antinociception produced by carbachol. In agreement with the current data, Naguib and Yaksh (1997) also show that carbachol antinociception is not prevented by blockade of M2 receptors with methoctramine. Arecaidine, a relatively specific M2 agonist, produced antinociception that was attenuated by methoctramine. Taken together these data suggest that carbachol does not bind to spinal M2 receptors and that TENS does not activate spinal M2 receptors to cause antihyperalgesia.
4.4. Clinical relevance
This study provides direct evidence for the spinal muscarinic receptor activation during low and high frequency TENS. Clinically, muscarinic receptor antagonists such as atropine, scopolamine, tolterodine, ipratropium; and acetylcholinesterase inhibitors (AChE inhibitors) such as neostigmine and physostigmine, are used to treat a variety of conditions. Systemic AChE inhibitors increase concentrations of ACh in various body tissues. Therefore, it is theoretically possible that in patients taking muscarinic antagonists or AChE inhibitors for unrelated conditions, there could be an attenuation or enhancement of analgesic effects of TENS, respectively.
In conclusion, the results from this study, using pharmacologic manipulation, strongly suggests that the spinal muscarinic receptors mediate antihyperalgesia produced by both low and high frequency TENS in rats. Specifically, pharmacologic blockade of spinal M1 and M2, but not M3, muscarinic receptors attenuates the antihyperalgesia produced by low and high frequency TENS. Further, we show that spinal nicotinic receptors are not involved in the antihyperalgesia produced by either low or high frequency TENS in the knee joint inflammatory pain model.
Acknowledgements
The authors are thankful to Ms. Carol Leigh for secretarial assistance. This work was supported by National Institutes of Health Grant K02 AR02201 (KAS) and the Arthritis Foundation.
References
- Abelson KS, Hoglund AU. Intravenously administered lidocaine in therapeutic doses increases the intraspinal release of acetylcholine in rats. Neurosci. Lett. 2002;317:93–96. doi: 10.1016/s0304-3940(01)02440-5. [DOI] [PubMed] [Google Scholar]
- Arimatsu Y, Seto A, Amano T. An atlas of alpha-bungarotoxin binding sites and structures containing acetylcholinesterase in the mouse central nervous system. J. Comp. Neurol. 1981;198:603–631. doi: 10.1002/cne.901980405. [DOI] [PubMed] [Google Scholar]
- Baba H, Kohno T, Okamoto M, Goldstein PA, Shimoji K, Yoshimura M. Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn. J. Physiol. 1998;508:83–93. doi: 10.1111/j.1469-7793.1998.083br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges LF, Iversen SD. Topography of choline acetyltransferase immunoreactive neurons and fibers in the rat spinal cord. Brain Res. 1986;362:140–148. doi: 10.1016/0006-8993(86)91407-1. [DOI] [PubMed] [Google Scholar]
- Bouaziz H, Tong C, Eisenach JC. Postoperative analgesia from intrathecal neostigmine in sheep. Anesth. Analg. 1995;80:1114–1140. doi: 10.1097/00000539-199506000-00012. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Sullivan MC, Wilber JF, Coulter JD. Descending serotonergic, peptidergic and cholinergic pathways from the raphe nuclei: a multiple transmitter complex. Brain Res. 1983;288:33–48. doi: 10.1016/0006-8993(83)90079-3. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, McKinzie DL, Felder CC, Wess J. Use of M1—M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem. Res. 2003;28:437–442. doi: 10.1023/a:1022844517200. [DOI] [PubMed] [Google Scholar]
- Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102:195–201. doi: 10.1016/s0304-3959(02)00381-0. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Spinal endogenous acetylcholine contributes to the analgesic effect of systemic morphine in rats. Anesthesiology. 2001;95:525–530. doi: 10.1097/00000542-200108000-00039. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Zhuo M. Evidence for the involvement of a descending cholinergic pathway in systemic morphine analgesia. Brain Res. 1989;478:293–300. doi: 10.1016/0006-8993(89)91509-6. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Carlton SM. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Res. Rev. 1997;24:28–66. doi: 10.1016/s0165-0173(97)00010-6. [DOI] [PubMed] [Google Scholar]
- Dirksen R, Nijhuis GM. The relevance of cholinergic transmission at the spinal level to opiate effectiveness. Eur. J. Pharmacol. 1983;91:215–221. doi: 10.1016/0014-2999(83)90467-3. [DOI] [PubMed] [Google Scholar]
- Eisenach JC. Muscarinic-mediated analgesia. Life Sci. 1999;64:549–554. doi: 10.1016/s0024-3205(98)00600-6. [DOI] [PubMed] [Google Scholar]
- Ellis JL, Harman D, Gonzalez J, Spera ML, Liu R, Shen TY, Wypij DM, Zuo F. Development of muscarinic analgesics derived from epibatidine: role of the M4 receptor subtype. J. Pharmacol. Exp. Ther. 1999;288:1143–1150. [PubMed] [Google Scholar]
- Fang F, Proudfit HK. Spinal cholinergic and monoamine receptors mediate the antinociceptive effect of morphine microinjected in the periaqueductal gray on the rat tail, but not the feet. Brain Res. 1996;722:95–108. doi: 10.1016/0006-8993(96)00198-9. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. In: Wall PD, Melzack R, editors. Textbook of Pain. Chrchill Livingstone; 1999. pp. 309–338. [DOI] [PubMed] [Google Scholar]
- Gillberg PG, Gordh T, Jr., Hartvig P, Jansson I, Pettersson J, Post C. Characterization of the antinociception induced by intrathecally administered carbachol. Pharmacol. Toxicol. 1989;64:340–343. doi: 10.1111/j.1600-0773.1989.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordh T, Jr., Jansson I, Hartvig P, Gillberg PG, Post C. Interactions between noradrenergic and cholinergic mechanisms involved in spinal nociceptive processing. Acta Anaesthesiol. Scand. 1989;33:39–47. doi: 10.1111/j.1399-6576.1989.tb02857.x. [DOI] [PubMed] [Google Scholar]
- Honda K, Harada A, Takano Y, Kamiya H. Involvement of M3 muscarinic receptors of the spinal cord in formalin-induced nociception in mice. Brain Res. 2000;859:38–44. doi: 10.1016/s0006-8993(99)02456-7. [DOI] [PubMed] [Google Scholar]
- Honda K, Koga K, Moriyama T, Koguchi M, Takano Y, Kamiya HO. Intrathecal alpha2 adrenoceptor agonist clonidine inhibits mechanical transmission in mouse spinal cord via activation of muscarinic M1 receptors. Neurosci. Lett. 2002;12(322):161–164. doi: 10.1016/s0304-3940(02)00073-3. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Hwang KS, Leem JK, Park PH, Han SM, Lee DM. The antiallodynic effects of intrathecal cholinesterase inhibitors in a rat model of neuropathic pain. Anesthesiology. 1999;90:492–499. doi: 10.1097/00000542-199902000-00025. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET, Marion L. Characterization of the anti-nociception produced by intrathecally administered muscarinic agonists in the rats. J. Pharmacol. Exp. Ther. 1993;266:329–338. [PubMed] [Google Scholar]
- Janko M, Trontelj JV. Transcutaneous electrical nerve stimulation: a microneurographic and perceptual study. Pain. 1980;9:219–230. doi: 10.1016/0304-3959(80)90009-3. [DOI] [PubMed] [Google Scholar]
- Johnson MI, Ashton CH, Thompson JW. Long term use of transcutaneous electrical nerve stimulation at Newcastle Pain Relief Clinic. J. Roy. Soc. Med. 1992;85:267–268. doi: 10.1177/014107689208500508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, Pare M, Beaudet A. Retrograde labeling of neurons in the brain stem following injections of [3H]choline into the rat spinal cord. Neuroscience. 1986;18:901–916. doi: 10.1016/0306-4522(86)90108-9. [DOI] [PubMed] [Google Scholar]
- Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J. Pharmacol. Exp. Ther. 2001;298:257–263. [PubMed] [Google Scholar]
- Kayaalp SO, Neff NH. Regional distribution of cholinergic muscarinic receptors in spinal cord. Brain Res. 1980;196:429–436. doi: 10.1016/0006-8993(80)90406-0. [DOI] [PubMed] [Google Scholar]
- Li P, Zhuo M. Cholinergic, noradrenergic, and serotonergic inhibition of fast synaptic transmission in spinal lumbar dorsal horn of rat. Brain Res. Bull. 2001;54:639–647. doi: 10.1016/s0361-9230(01)00470-1. [DOI] [PubMed] [Google Scholar]
- Li YJ, Zhang ZH, Chen JY, Qiao JT. Effects of intrathecal naloxone and atropine on the nociceptive suppression induced by norepinephrine and serotonin at the spinal level in rats. Brain Res. 1994;666:113–116. doi: 10.1016/0006-8993(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Ma HC, Dohi S, Wang YF, Ishizawa Y, Yanagidate F. The antinociceptive and sedative effects of carbachol and oxycodone administered into brainstem pontine reticular formation and spinal subarachnoid space in rats. Anesth. Analg. 2001;92:1307–1315. doi: 10.1097/00000539-200105000-00043. [DOI] [PubMed] [Google Scholar]
- Ma YT, Sluka KA. Reduction in inflammation-induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Exp. Brain Res. 2001;137:94–102. doi: 10.1007/s002210000629. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Men DS, Matsui Y. Peripheral nerve stimulation increases serotonin and dopamine metabolites in rat spinal cord. Brain Res. Bull. 1994;33:625–632. doi: 10.1016/0361-9230(94)90225-9. [DOI] [PubMed] [Google Scholar]
- Naguib M, Yaksh TL. Antinociceptive effects of spinal cholinesterase inhibition and isobolographic analysis of the interaction with mu and alpha 2 receptor systems. Anesthesiology. 1994;80:1338–1348. doi: 10.1097/00000542-199406000-00022. [DOI] [PubMed] [Google Scholar]
- Naguib M, Yaksh TL. Characterization of muscarinic receptor subtypes that mediate antinociception in the rat spinal cord. Anesth. Analg. 1997;85:847–853. doi: 10.1097/00000539-199710000-00025. [DOI] [PubMed] [Google Scholar]
- Ninkovic M, Hunt SP. Alpha-bungarotoxin binding sites on sensory neurons and their axonal transport in sensory afferents. Brain Res. 1983;272:57–69. doi: 10.1016/0006-8993(83)90364-5. [DOI] [PubMed] [Google Scholar]
- Obata H, Saito S, Sasaki M, Goto F. Possible involvement of a muscarinic receptor in the anti-allodynic action of a 5-HT2 receptor agonist in rats with nerve ligation injury. Brain Res. 2002;932:124–128. doi: 10.1016/s0006-8993(02)02288-6. [DOI] [PubMed] [Google Scholar]
- Pinardi G, Sierralta F, Miranda HF. Atropine reverses the antinociception of nonsteroidal anti-inflammatory drugs in the tailflick test of mice. Pharmacol. Biochem. Behav. 2003;74:603–608. doi: 10.1016/s0091-3057(02)01046-8. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, King EW, Dickman JK, Herold CA, Johnston NF, Spurgin ML, Sluka KA. Spinal 5-HT2 and 5-HT3 receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain. 2003 doi: 10.1016/s0304-3959(03)00207-0. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA. Systemic morphine in combination with TENS produces an increased antihyperalgesia in rats with acute inflammation. J. Pain. 2000;1:204–211. doi: 10.1054/jpai.2000.7149. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J. Pain. 2003;4:109–121. doi: 10.1054/jpai.2003.434. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J. Pharmacol. Exp. Ther. 1999;289:840–846. [PubMed] [Google Scholar]
- Sluka KA, Judge MA, McColley MM, Reveiz PM, Taylor BM. Low frequency TENS is less effective than high frequency TENS at reducing inflammation-induced hyperalgesia in morphine-tolerant rats. Eur. J. Pain. 2000;4:185–193. doi: 10.1053/eujp.2000.0172. [DOI] [PubMed] [Google Scholar]
- Smith MD, Yang XH, Nha JY, Buccafusco JJ. Antinociceptive effect of spinal cholinergic stimulation: interaction with substance P. Life Sci. 1989;45:1255–1261. doi: 10.1016/0024-3205(89)90127-6. [DOI] [PubMed] [Google Scholar]
- Taylor JE, Yaksh TL, Richelson E. Agonist regulation of muscarinic acetylcholine receptors in rat spinal cord. J. Neurochem. 1982;39:521–524. doi: 10.1111/j.1471-4159.1982.tb03975.x. [DOI] [PubMed] [Google Scholar]
- Wei J, Walton EA, Milici A, Buccafusco JJ. m1—m5 muscarinic receptor distribution in rat CNS by RT-PCR and HPLC. J. Neurochem. 1994;63:815–821. doi: 10.1046/j.1471-4159.1994.63030815.x. [DOI] [PubMed] [Google Scholar]
- Wess J, Duttaroy A, Gomeza J, Zhang W, Yamada M, Felder CC, Bernardini N, Reeh PW. Muscarinic receptor subtypes mediating central and peripheral antinociception studied with muscarinic receptor knockout mice: a review. Life Sci. 2003;72:2047–2054. doi: 10.1016/s0024-3205(03)00082-1. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Mitchell D, Barrett GD. Antinociceptive effect of peripheral segmental electrical stimulation in the rat. Pain. 1980;8:237–252. doi: 10.1016/0304-3959(88)90011-5. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Dirksen R, Harty GJ. Antinociceptive effects of intrathecally injected cholinomimetic drugs in the rat and cat. Eur. J. Pharmacol. 1985;117:81–88. doi: 10.1016/0014-2999(85)90474-1. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Tonic cholinergic inhibition of spinal mechanical transmission. Pain. 1991;46:211–222. doi: 10.1016/0304-3959(91)90078-C. [DOI] [PubMed] [Google Scholar]