Abstract
The question whether stem cells age remains an enigma. Traditionally, aging was thought to change the properties of hematopoietic stem cells (HSC). We discuss here a new model of stem cell aging that challenges this view. It is now well-established that the HSC compartment is heterogeneous, consisting of epigenetically fixed subpopulations of HSC that differ in self-renewal and differentiation capacity. New data show that the representation of these HSC subsets changes during aging. HSC that generate lymphocyte-rich progeny are depleted, while myeloid-biased HSC are enriched in the aged HSC compartment. Myeloid-biased HSC, even when isolated from young donors, have most of the characteristics that had been attributed to aged HSC. Thus, the distinct behavior of the HSC isolated from aged hosts is due to the accumulation of myeloid-biased HSC. By extension this means that the properties of individual HSC are not substantially changed during the lifespan of the organism and that aged hosts do not contain many aged HSC. Myeloid-biased HSC give rise to mature cells slowly but contribute for a long time to peripheral hematopoiesis. We propose that such slow, “lazy” HSC are less likely to be transformed and therefore may safely sustain hematopoiesis for a long time.
Keywords: hematopoietic stem cells, aging, lifespan, epigenetic, lymphocytes, myeloid cells
Introduction
Stem cells are characterized by the ability to differentiate into multiple lineages of mature cells and by self-renewal capacity. Self-renewal of the stem cells pool assures a constant supply of stem cells, and consequently of mature cells, throughout the lifespan of an organism. This is particularly important when the mature progeny is short-lived and large numbers of mature cells are required for the survival of the organism. Hematopoietic stem cells (HSC) are well-studied examples of stem cells that generate progeny with short life spans.
The concept of self-renewal implies that HSC generate daughter cells with essentially unaltered properties1. In most mature cells, eroding telomeres act as an internal clock that limits the number of divisions that a cell can perform. However, HSC express telomerase which attenuates telomere loss.2,3 HSC also express drug efflux pumps, 4 which protect cells from environmental insults. Thus, HSC should be well protected from aging. Indeed, the number of HSC is not limiting in either aged humans or mice. However, there is evidence that HSC and stem cells from other tissues obtained from aged organisms behave differently from those isolated from young donors. This raises the question: Do stem cells age and if so, what might be the reason for and the consequence of stem cell aging? We will focus here on recent developments from the field of HSC research that shed new light on stem cell aging. We propose that the aged HSC compartment accumulates a type of HSC that has properties of “aged” stem cells—even when isolated from young donors. While each HSC has a limited lifespan, their differentiation potential is maintained throughout their life. Thus, the function of HSC, and perhaps other types of stem cells, is not limited by age-related pathologies.
What are the Signs of Stem Cell Aging?
On an organismal level, aging manifests as a decrease of organ functions.5,6 This makes it difficult for the organism to respond adequately to environmental stimuli and stresses. For example, slow wound healing and inadequate immune responses contribute to morbidity in the aged. In the lympho-hematopoietic system, diminished immune responses lead to an increased susceptibility to infectious diseases and an impaired immune surveillance, a crucial defense against cancer (reviewed in refs. 7 and 8). An accumulation of myeloid cells leads to increased levels of inflammatory cytokines such as Interleukin (IL)-6, IL-1 and transforming-growth-factors. High levels of proinflammatory cytokines negatively affect many aspects of the organism, including bone and vascular health.9–11 Indeed, an inflammatory environment is considered a hallmark of aging. Thus, a major thrust of aging research is directed towards rejuvenating the immune system and the hematopoietic stem cells (HSC), which generate all the cells of the immune system.
On a cellular level, aging is characterized by an accumulation of genetic mutations and metabolic byproducts.12–15 The response of cells to external signals is sluggish, mirroring the overall organ function.7 Tissue regeneration after stress, such as chemotherapy or heavy bleeding, is attenuated in the aged. Therefore, the cells responsible for renewing organ function, adult tissue stem cells, are thought to lose regenerative function as a result of aging. In the hematopoietic system, where stem cell aging has been studied extensively, many age-related changes have been documented. HSC isolated from aged donors may have a reduced ability to home to the bone marrow,16,17 an altered cell surface phenotype,16,18 and changes in metabolic activity,19,20 compared to HSC from young donors. HSC isolated from aged donors exhausted earlier in serial transplantation than their younger counterparts. However, the effects were rather modest and manifested only after several rounds of transplantation.21 HSC from aged donors do not repopulate the lymphoid lineages as effectively as HSC from young donors and aged HSC are believed to overproduce myeloid cells.18,20–24 The latter is likely a misinterpretation, since the underproduction of lymphoid cells can mimic an increase in myeloid cell production.25
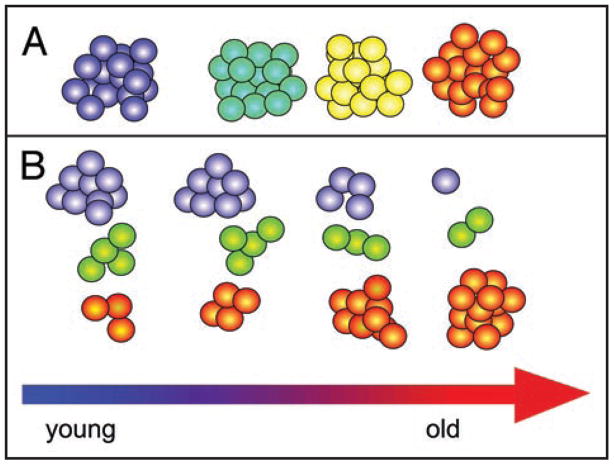
All these changes attributed to aged HSC have led to the idea that age modulates the function of a uniform population of HSC (Fig. 1A). In that model, age-associated pathologies would limit the ability of HSC to give rise to lymphocytes and would restrict the self-renewal ability of the aged HSC. Accumulation of DNA mutations and diminished DNA repair are thought to contribute to the transmogrification of young into aged HSC.12,13 However, it has become increasingly clear that the HSC compartment is heterogeneous, and we showed that the HSC compartment consists of epigenetically fixed classes of HSC that differ in self-renewal and differentiation capacity.25–27 This led to the new hypothesis that the age-related changes in the HSC compartment derive from a changing composition of the different classes of HSC (Fig. 1B). This implies that individual HSC do not change during aging. Rather, the HSC compartment changes because it contains different proportions of the different classes of HSC in the young and the aged.
Figure 1.
Models of HSC aging. (A) The classical model assumes a homogenous population of HSC. All of these HSC progressively change their behavior as a result of aging (blue corresponds to young; orange to old). (B) The new model of HSC aging builds on the insight that HSC are heterogeneous and that the representation of the different classes of HSC shifts during aging.24 The young HSC compartment is dominated by Ly-bi HSC (Blue), while the aged HSC accumulates My-bi HSC (orange). Bala HSC (green) have an intermediate lifespan.25 The changing combination of the different types of HSC (B) generates the averaged behavior of the HSC compartment (A) during the life of the organism.
Heterogeneity in the HSC Compartment
During development, totipotent stem cells differentiate into the various tissue-specific stem cells. Epigenetic mechanisms assure that the tissue specificity of these stem cells is stably maintained through many rounds of self-renewal divisions. Interestingly, heterogeneity can be documented even within the tissue specific stem cell compartments. Differences in self-renewal capacity, phenotype, cell cycling and lineage potential has been documented within populations of embryonic,28,29 sperm,30 neuronal stem cells,31 mesenchymal-stromal cells32,33 and HSC (reviewed in refs. 34 and 35).
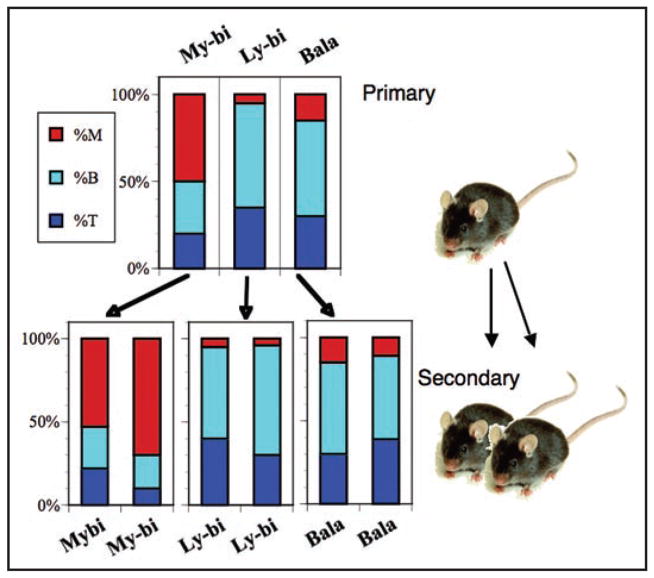
Within the HSC compartment, three classes of HSC can be distinguished based on their epigenetically fixed differentiation potential. These three classes of HSC, called Balanced (Bala), Lymphoid-biased (Ly-bi) and Myeloid-biased (My-bi) have distinct differentiation and self-renewal capacities. Bala HSC differentiate to give rise to about 10% myeloid and 90% lymphoid donor type cells in blood (Fig. 2). Ly-bi HSC generate few myeloid cells and therefore the blood of animals repopulated by a Ly-bi HSC is lymphocyte rich. In contrast, My-bi HSC have an attenuated ability to give rise to lymphocytes but make levels of myeloid cells similar to those generated by Bala HSC. Our data were subsequently confirmed by others.36 Together, these three classes of HSC make up the complete HSC compartment in young mice.
Figure 2.
The differentiation potential of HSC is stable through serial transplantation. The cartoon shows a representative example each of a My-bi, Ly-bi and Bala HSC. The top panel shows the percent of myeloid cells (red), B lymphocyte (light blue) and T lymphocyte (dark blue) in the blood of hosts reconstituted with a single HSC 7 months earlier. Bone marrow cells from these mice were then transplanted into pairs of secondary mice. The lower panel shows the percent of myeloid and lymphoid cells seven months after transplantation in the secondary hosts. For more examples see the supplemental Figures in Cho et al.24
Each of these types of HSC represents true stem cells with pluripotent differentiation capacity and self-renewal. A hallmark of all types of HSC is that their differentiation capacity is stably inherited through many rounds of self-renewal (Fig. 2). When a Ly-bi HSC self-renews it will always give rise to more Ly-bi HSC. It will never generate My-bi or Bala HSC. Similarly, Bala HSC produce Bala daughter HSC, and My-bi HSC generate My-bi daughter HSC. There is no precursor-progeny relationship between these types of HSC and the original heterogeneity is not recreated. Thus, the differentiation capacity of each of these classes of HSC is epigenetically fixed and predetermined.
The Aged HSC Compartment: Enrichment for My-bi HSC
The healthy hematopoietic system is polyclonal: many different HSC act together to generate mature cells and to maintain the HSC compartment.37–40 The behavior of the HSC compartment reflects the combined activities of all of these different HSC clones. To understand how individual HSC contribute to the overall activity it is necessary to examine individual HSC. A clonal analysis of individual HSC showed that the young HSC compartment is dominated by Ly-bi HSC, making up 60% of the HSC compartment of young C57BL/6 (B6) mice and about 40% of that of DBA/2 (D2) mice.24 At the same time, roughly equal numbers of Bala and My-bi HSC are found in both strains of mice. The combined output of these HSC leads to the typical young hematopoietic system, with a lymphocyte rich periphery and fast responses to hematopoietic stress. These two strains of mice were tested because they differ in the number of HSC, and HSC in these strains are affected differently in aging.41–43 The genetics of HSC aging has been reviewed in detail.44,45
When individual HSC from young donors were examined, My-bi HSC showed surprising similarities to HSC isolated from aged donors.24,25 The generation of both T cell and B cell precursors from young My-bi HSC is markedly reduced compared to that of Ly-bi or Bala HSC. At least in part, this is due to an attenuated ability of the lymphoid progeny of My-bi HSC to respond to the lymphopoietin IL-7. Attenuated production of lymphocytes and a blunted response to IL-7 are also found in aged mice.46–55 When My-bi HSC from young donors are transplanted it takes several months to see mature donor type cells in blood.25 The slow onset of repopulation is reminiscent of the sluggish response of the aged hematopoietic system to stress. These resemblances between young My-bi and HSC from aged donors prompted the idea that the aged HSC compartment is enriched for My-bi HSC (Fig. 1B). An extensive clonal analysis of the aged HSC compartment in the strains DBA/2 (D2) and C57BL/6 (B6) proved that the idea was correct.24 The aged HSC compartment is depleted of Ly-bi HSC and enriched for My-bi HSC. Indeed, in aged D2 mice, My-bi HSC make up almost 80% of all HSC.
Remarkably, the hematopoietic system of an aged D2 behaves very much like that of a host repopulated by a My-bi HSC isolated from young mice (of either strains of mice). The peripheral blood cells of aged D2 mice are strongly skewed towards the myeloid lineages, the response of B cell precursors to IL-7 is vanishingly low, and bone marrow from aged D2 is depleted of T cell precursors that home to the thymus.24 As an aside, aged B6 mice show a less pronounced enrichment of My-bi HSC than D2 of the same age. In aged B6 mice manifestation of myeloid-bias is variable in blood. T and B cell precursor levels are only slightly reduced. This suggests that aged B6 but not D2 mice have sufficient numbers of Bala and Ly-bi HSC to compensate for and mask the sluggish response of My-bi HSC. Thus, while the HSC compartments in both strains of mice was shifted from Ly-bi to My-bi HSC (Fig. 2), the extent of that shift is controlled by genetic mechanisms.
Next, we asked whether My-bi HSC from aged and young donors behave differently. An extensive analysis of the self-renewal and differentiation potential of young and aged My-bi HSC did not reveal significant differences.24 Thus, by all criteria examined, My-bi HSC from young and aged donors are functionally equivalent. This raises the intriguing possibility that HSC from aged animals are not changed by aging. Rather, the behavior of populations of HSC isolated from aged hosts is just a reflection of the enrichment of My-bi HSC. A skewing in the clonal composition of the HSC cell populations in aged humans has also been reported,56 suggesting that selection for long-lived HSC is a common feature in aged HSC compartments. This, of course, brings us back to the original conundrum: do HSC age at all?
HSC have a Limited Lifespan
HSC can outlive the original organism in which they were generated. While laboratory strains of mice live 2 to 3 years at best, populations of HSC can generate mature progeny for 6 years when followed in serial transplantation into consecutive young hosts.57 This was taken as evidence that HSC can self-renew limitlessly and that HSC do not age. However, clonal analyses showed that the lifespan of a population of HSC cells reflects the cumulative function of many different HSC clones. The healthy hematopoietic system is polyclonal, that is many different HSC clones work together to generate mature cells. At the same time, new HSC are regularly activated to start to generate mature cells while other HSC extinguish.37,38,58,59 The sequentially activation of HSC contributes to the long lifespan of populations of HSC. The clonal analysis also revealed subsets of HSC that could be distinguished by how long they generated mature cells. Short-term repopulating HSC give rise to mature progeny for only a few weeks after transplantation into an ablated host, while long-term HSC contribute for many months.37,38,58,59 To explain these different types of HSC, the generation-age hypothesis60,61 postulates that all HSC initially have a long lifespan. However, for every self-renewal division, each daughter HSC would have less repopulation and self-renewal capacity. This would shorten the lifespan of the HSC clone until the HSC ceases to generate mature cells. In this context, it is important to realize that clonal analyses are initiated with a single cell, but the overall lifespan of the clone derives from the combined output of all HSC generated through self-renewal by that original HSC. Thus, the lifespan of a HSC clone reflects its self-renewal and differentiation capacity.
We have followed many individual HSC through serial transplantation until they stopped contributing mature cells to the periphery (reviewed in ref. 25 and unpublished). We measured the overall output of mature cells, which is a function of the combined differentiation and proliferation of each individual HSC. All experiments were initiated with a single HSC, which generated a clone of daughter, granddaughter, great-granddaughter, etc., HSC. In all cases, we have found that the overall repopulation kinetics approximate a skewed bell shaped curve (Fig. 3), with an initial increase, followed by an apex, finishing with a decline in the output of mature cells. This was true if individual HSC were followed in serial transplantation or were allowed to remain in the same primary hosts for many months (unpublished). Thus, it is unlikely that the stress of repeated transplantation causes the decline in HSC function as has been surmised previously.62,63
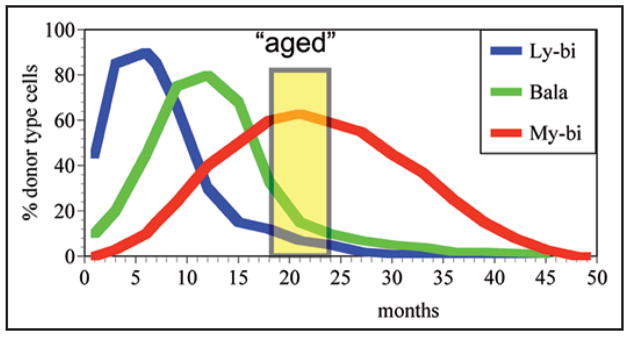
Figure 3.
The different lifespans of HSC affect their representation in the aged host. Lifespans are measured as the time that a HSC contributes to mature cells (Percent donor type cells). Donor type cells were measured in blood every other month as described in detail previously.25 Most Ly-bi HSC (blue) repopulate rapidly after transplantation but have short lifespans. Bala HSC (green) have a medium lifespan and initiate repopulation slower than Ly-bi HSC. My-bi HSC show a very slow onset of repopulation, but these HSC have a very long lifespan. The curves shown here are rough approximations and extrapolations of the average lifespans of these classes of HSC. For original data on the lifespans see Muller-Sieburg et al.25 A mouse is considered old after 18 months of age and most aging experiments are performed with mice aged 18 to 24 months (yellow box). This marks a time in most strains when mortality is still limited, but age-related effects are clearly present.68 Note that all types of HSC will eventually cease to generate mature cells, which is defined as the end of their lifespan. However, as populations, all types of HSC outlive the original donor albeit to different extents. The differences in lifespan can account for the enrichment of My-bi HSC in the aged host.
Extensive heterogeneity of the lifespan of HSC has been detected in all clonal analyses.25,26,37,38,64–67 Yet, every single HSC that we tested had a limited lifespan—the output of mature cells decreased and then ceased at some point. Thus, HSC age in the sense that each HSC clone exhausts at some point. Self-renewal does not reset the clock. This view is supported by our data that all daughter-HSC (derived from a single HSC) follow the same kinetics of repopulation and, in turn, give rise to granddaughters that again are self-similar.27 Remarkably, when granddaughter HSC were tested in many different hosts in parallel, they all stopped contributing mature cells at the same time.27 This indicates that HSC have an intrinsic counter that limits their life span at a predictable and therefore predetermined rate. In that sense, the generation-age hypothesis is correct. However, we found that My-bi HSC, on average, have a much longer lifespan than Ly-bi HSC.25 Yet, My-bi HSC do not generate Ly-bi HSC. This makes it unlikely that all HSC are born with the same lifespan. Rather, this points to a scenario where different types of HSC are endowed with distinct lifespans.
Ly-bi, Bala and My-bi HSC on average have different lifespans (see Fig. 3). Ly-bi HSC, on average, repopulate rapidly, but have a short lifespan. Serial transplants showed that Ly-bi HSC have limited self-renewal capacity. In contrast, it takes My-bi HSC several months to generate mature cells. At the same time, My-bi HSC have the longest lifespan of all HSC, reflecting their extensive self-renewal capacity.25 Thus, the lifespan is coupled with the differentiation capacity of HSC, indicating that both behaviors are epigenetically fixed.
The different lifespans of HSC would predict that many short-lived Ly-bi HSC should exhaust early in the life of an organism and that My-bi HSC accumulate in the aged host because of their long lifespan. While not known, it is tempting to speculate that HSC of the Ly-bi class are activated in shorter intervals than HSC in the My-bi class. Although the classes of HSC can be distinguished based on their average lifespans, there is some variability within each class. This, together with the sequential activation of HSC in all classes would explain why a few Ly-bi HSC are still found in aged hosts.
Aged Mice do not Contain many Old HSC
Another prediction that derives from the analysis of lifespans is that an aged mouse does not contain many old HSC! A mouse is considered aged after 18 months and most experiments in the aging field are preformed with 18 to 24 months old mice (Fig. 3). By 25 months of age, approximately 60% of D2 and 45% of B6 mice have died of old age.68 The lifespan of My-bi, and also of some Bala HSC is sufficiently long, so that they can readily outlast the host by many months even if the HSC is activated early in life. We have seen individual HSC clones last for more than 50 months. Thus, the lifespan of each HSC clone and not the age of the host determines whether a HSC is old. One could consider the first part of the lifespan curve (Fig. 3) with its increasing output of mature cells as the youth of a HSC clone. The right side of the curve with declining output of mature cells would then be the old age or “end-of-life” of the HSC clone. To characterize an old HSC, the behavior of the HSC at the beginning and the end of the repopulation curve should be compared. “End-of-life” HSC, of course, have no, or very limited, self-renewal capacity.26 However, we found that the differentiation potential of end-of-life HSC is unchanged. While graft failure (end-of-life) is associated with an increased level of T cells in blood, this reflects the long lifespan of T lymphocytes rather than a change in the generative potential of HSC. End-of life My-bi HSC retained their myeloid-biased differentiation program and so did Bala and Ly-bi HSC.27 Progeny from end-of-life Ly-bi HSC readily responded to IL-7 with similar kinetics as progeny derived from earlier forms of Ly-bi (unpublished). Thus, besides a diminished proliferative capacity, old HSC do not show the signs attributed to HSC isolated from aged host.
The view that the aged host does not contain many old HSC is supported by data demonstrating that the effects of organismal aging on the self-renewal capacity of HSC are surprisingly mild. For example, a difference in repopulation capacity between HSC from young and aged donors could only be demonstrated after four sequential rounds of transplants.21 Even in models of accelerated aging, detrimental effects could only be demonstrated after severe, prolonged stress to the hematopoietic system.12–14 Thus, the onset of an age-related decline in HSC function is generally observable only long after the normal lifespan of the organism from which the HSC were derived. This agrees with data that the number of HSC is not limiting in either aged humans or mice40,56 (reviewed in ref. 69).
Survival of the Laziest?
Why would the body invest into maintaining several classes of HSC, each with distinct differentiation and self-renewal capacity? One highly speculative, but testable, model is that the different classes of HSC are custom tailored to perform different roles in development and aging.
As all types of stem cells, HSC must maintain proliferative capacity to assure a life-long ability to compensate for cell loss in the adult tissues. During fetal and neonatal life, there is a strong pressure on HSC to self-renew to fill up the expanding tissue compartments to adult levels. At the same time, there is a strong demand to differentiate, since the growing body requires large numbers of mature cells. In the hematopoietic system, demand for lymphocytes is particularly high perinataly, when the immature immune system struggles to keep up with the challenges of the new environment. Mice and humans are born with immature immune systems. In this setting, a rapidly responding HSC that produces copious numbers of lymphocytes, such as the Ly-bi HSC, is required. It is noteworthy that early in life humans have lymphocyte rich blood comparable to mouse blood.70,71 This suggests that Ly-bi HSC dominate early life both in mice and humans. A small number of rapidly repopulating HSC would be maintained throughout the life of the organism to facilitate the response to acute stress and to allow the organism to survive accidents. The importance of rapidly generating lymphocytes, a feature of Ly-bi HSC, is highlighted by data that early recovery of lymphocytes predicts survival after autologous clinical stem cell transplants.72
On the other hand, the system must guard against too much or uncontrolled proliferation to avoid early death due to cancers. An active, rapidly responding HSC would certainly be more vulnerable to environmental insults than a slow HSC, such as the My-bi HSC. Yet, too much protection against transformation can backfire. Overexpression of the tumor suppressor p53 in HSC caused a slow and incomplete response to hematopoietic stress. HSC were depleted prematurely leading to accelerated aging.73,74 Thus, rapidly responding HSC, such as Ly-bi HSC, are needed and maintained throughout the life of the animal. Their short lifespan limits the danger that their susceptibility to insults might cause. On the other hand, populations of slow, “lazy” HSC with long lifespans seem ideally suited for maintaining a healthy hematopoietic system once the demands of the growing body have been fulfilled. The concept that HSC require long periods of rest to perform house keeping tasks including DNA repair has been presaged by Lajtha.75
In some respects the behavior of slow and long-lived My-bi HSC are reminiscent of organisms maintained on caloric restriction. Caloric restriction is the only proven way to significantly extend the lifespan of many different organisms.6,76 Starvation causes metabolic slowdown and increased stress resistance by regulating mitrochondrial activity, glucose homeostasis, oxidative damage, insulin sensitivity, and the production of mitogenic cytokines.5,6,77 Mice maintained on a restricted diet did not show the usual age-related changes in the HSC compartment.78 D2 mice, which are short-lived, have a higher metabolic rate than long-lived B6 mice.79 This agrees with our finding that the shift towards a My-bi HSC dominated HSC compartment is faster and more complete in D2 than in B6 mice. Cells cultured in serum from Rhesus monkeys maintained on caloric restriction proliferated slower than in serum from normally fed animals,80 suggesting that starvation can affect the growth rate of individual cells. It is tempting to speculate that certain cells (such as My-bi HSC) have a naturally attenuated metabolic rate. A slow metabolism would protect the HSC from aging and, at the same time, preserve the regenerative potential of HSC. Such lazy HSC would provide an ideal compromise between the need to tame the proliferative capacity of HSC and the necessity of maintaining an active HSC pool for the lifespan of the organism.
It should also be kept in mind that mice are short-lived and maintain lymphocyte rich blood for much of their life. However, the blood of humans (and other longer-lived species) loses the youthful lymphocyte-rich composition and turns increasingly myeloid biased. By the age of 21 there are only about half of the lymphocytes in blood found in a one year old child.70,71,81 It remains to be established whether the myeloid-biased blood of young large animals derives from an accumulation of My-bi HSC. If proven to be correct, this would mean that My-bi HSC are the principal HSC during much of the lifespan of large animals. New assays will have to be developed to define on the single cell level if humans have similar subsets of HSC.
Outlook
The implications of the aging model as outlined in Figure 1B are rather provocative. Instead of facing a population of HSC sickened by age (Fig. 1A), we are looking at perfectly healthy HSC. Youthful Ly-bi and balanced HSC are present albeit in small numbers. It might be possible to find ways to selectively expand the remaining Ly-bi and Bala HSC to rejuvenate the HSC compartment. Perhaps, ways can be found to extend the lifespan of the populations of Ly-bi and Bala HSC. It may also be possible to re-educate My-bi HSC into more rapidly responding types of HSC. The epigenetic imprinting of adult HSC is remarkably stable in the adult hematopoietic environment over the lifespan of the organism. However, it is not clear whether these cells can be instructed to change their epigenetic settings. When single HSC sorted as RhoLo were injected either directly or after culture in cytokines, a difference in the frequency of My-bi and Bala HSC was noted.36 Whether this change was caused by changing individual HSC or by selecting the subsets of HSC awaits clarification. To critically test this issue, it will be necessary to prospectively isolate the different types of HSC. Mice in which Green Fluorescent Protein (GFP) was knocked in to the Lysozyme locus may be helpful for that purpose. Graf82 noted that GFP+Lin-Sca-1+cKit+ HSC isolated from these mice are enriched for My-bi HSC while the GFP-negative fraction of the Lin-Sca-1+cKit+ population contains Ly-bi HSC. Lastly, if conditions can be found to restore the aged HSC compartment to its youthful level of Ly-bi HSC, it is likely that responses to hematopoietic stresses will be improved. However, the possibility that a reeducation of My-bi into Ly-bi HSC ultimately leads to a depletion of the HSC reserve and hematopoietic failure must be carefully considered.
Acknowledgments
Work cited from this laboratory was supported by grants from the National Institute for Health AG023197 and DK48015. We thank Drs. Gaelle Rondeau, Elisabeth Virtz, Phylis Linton (Sidney Kimmel Cancer Center, San Diego) and Dr. Becky Adkins (University of Miami, Miami) for critical discussion of this manuscript.
Abbreviations
- B6
C57BL/6 mice
- D2
DBA/2 mice
- HSC
hematopoietic stem cells
- Ly-bi
lymphoid biased
- My-bi
myeloid-biased
- Bala
balanced
- IL
interleukin
- GFP
green fluorescent protein
Footnotes
Previously published online as a Cell Cycle E-publication:
References
- 1.Deryugina E, Muller-Sieburg C. The stromal cells’ guide to the stem cell universe. Stem Cells. 1995;13:477–86. doi: 10.1002/stem.5530130505. [DOI] [PubMed] [Google Scholar]
- 2.Yui J, Chiu CP, Lansdorp PM. Telomerase activity in candidate stem cells from fetal liver and adult bone marrow. Blood. 1998;91:3255–62. [PubMed] [Google Scholar]
- 3.Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102:517–20. doi: 10.1182/blood-2002-07-2334. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood TB, Austad SN. Why do we age? Nature. 2000;408:233–8. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- 6.Blagosklonny MV. Paradoxes of aging. Cell Cycle. 2007;6:2997–3003. doi: 10.4161/cc.6.24.5124. [DOI] [PubMed] [Google Scholar]
- 7.Rothstein G. Hematopoiesis in the aged: a model of hematopoietic dysregulation? Blood. 1993;82:2601–4. [PubMed] [Google Scholar]
- 8.Michel J-P. Aging and the Immune System. In: Jones TV, editor. Merck Manual of Geriatrics. 3. Merck & Co., Inc; 2006. pp. 1351–9. Chapter 1313, online edition, http://www.merck.com/mkgr/mmg/sec1316/ch1131/ch1131a.jsp. [Google Scholar]
- 9.Ginaldi L, Di Benedetto M, De Martinis M. Osteoporosis, inflammation and ageing. Immunity & Ageing. 2005;2:14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53:20–6. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- 11.Krasniak A, Drozdz M, Pasowicz M, et al. Factors involved in vascular calcification and atherosclerosis in maintenance haemodialysis patients. Nephrol Dial Transplant. 2007;22:515–21. doi: 10.1093/ndt/gfl564. [DOI] [PubMed] [Google Scholar]
- 12.Nijnik A, Woodbine L, Marchetti C, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–90. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 13.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–9. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 14.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10:1923–40. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Q, Yasumoto H, Tsai RY. Nucleostemin delays cellular senescence and negatively regulates TRF1 protein stability. Mol Cell Biol. 2006;26:9279–90. doi: 10.1128/MCB.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–6. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–87. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–80. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida N, Dykstra B, Lyons K, Leung F, Kristiansen M, Eaves C. ABC Transporter Activities of Murine Hematopoietic Stem Cells Vary According to Their Developmental and Activation Status. Blood. 2004:2003–11. doi: 10.1182/blood-2003-11-3989. [DOI] [PubMed] [Google Scholar]
- 20.Kim M, Moon HB, Spangrude GJ. Major age-related changes of mouse hematopoietic stem/progenitor cells. Ann N Y Acad Sci. 2003;996:195–208. doi: 10.1111/j.1749-6632.2003.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 21.Kamminga LM, van Os R, Ausema A, et al. Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells. 2005;23:82–92. doi: 10.1634/stemcells.2004-0066. [DOI] [PubMed] [Google Scholar]
- 22.Guerrettaz LM, Johnson SA, Cambier JC. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proc Natl Acad Sci USA. 2008;105:11898–902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–61. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller-Sieburg CE, Cho RH, Karlsson L, Huang J-F, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–8. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- 26.Sieburg HB, Cho RH, Dykstra B, Uchida N, Eaves CJ, Muller-Sieburg CE. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood. 2006;107:2311–6. doi: 10.1182/blood-2005-07-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller-Sieburg CE, Cho RH, Thoman M, Adkins B, Sieburg HB. Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood. 2002;100:1302–9. [PubMed] [Google Scholar]
- 28.Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–18. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- 29.Shovlin TC, Durcova-Hills G, Surani A, McLaren A. Heterogeneity in imprinted methylation patterns of pluripotent embryonic germ cells derived from pre-migratory mouse germ cells. Dev Biol. 2008;313:674–81. doi: 10.1016/j.ydbio.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Izadyar F, Pau F, Marh J, et al. Generation of multipotent cell lines from a distinct population of male germ line stem cells. Reproduction. 2008;135:771–84. doi: 10.1530/REP-07-0479. [DOI] [PubMed] [Google Scholar]
- 31.Brazel CY, Limke TL, Osborne JK, et al. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell. 2005;4:197–207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 32.Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10:320–30. doi: 10.1080/14653240802217011. [DOI] [PubMed] [Google Scholar]
- 33.Wineman J, Moore K, Lemischka I, Muller-Sieburg C. Functional heterogeneity of the hematopoietic microenvironment: Rare stromal elements maintain long-term repopulating stem cells. Blood. 1996;87:4082–90. [PubMed] [Google Scholar]
- 34.Muller-Sieburg CE, Sieburg HB. Clonal diversity of the stem cell compartment. Curr Opin Hematol. 2006;13:243–8. doi: 10.1097/01.moh.0000231421.00407.65. [DOI] [PubMed] [Google Scholar]
- 35.Muller-Sieburg CE, Sieburg HB. The GOD of hematopoietic stem cells: a clonal diversity model of the stem cell compartment. Cell Cycle. 2006;5:394–8. doi: 10.4161/cc.5.4.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dykstra B, Kent D, Bowie M, et al. Long-Term Propagation of Distinct Hematopoietic Differentiation Programs In Vivo. Cell Stem Cell. 2007;1:218–29. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Keller G, Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990;171:1407–18. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordan CT, Lemischka IR. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990;4:220–32. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- 39.Harrison DE, Lerner C, Hoppe PC, Carlson GA, Alling D. Large numbers of primitive stem cells are active simultaneously in aggregated embryo chimeric mice. Blood. 1987;69:773–7. [PubMed] [Google Scholar]
- 40.Swierczek SI, Agarwal N, Nussenzveig RH, et al. Hematopoiesis is not clonal in healthy elderly women. Blood. 2008 doi: 10.1182/blood-2008-03-143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller-Sieburg CE, Riblet R. Genetic control of the frequency of hematopoietic stem cells in mice: mapping of a candidate locus to chromosome 1. J Exp Med. 1996;183:1141–50. doi: 10.1084/jem.183.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang Y, Jansen M, Aronow B, Geiger H, Van Zant G. The quantitative trait gene latexin influences the size of the hematopoietic stem cell population in mice. Nat Genet. 2007;39:178–88. doi: 10.1038/ng1938. [DOI] [PubMed] [Google Scholar]
- 43.de Haan GNW, Van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 1997;89:1543–50. [PubMed] [Google Scholar]
- 44.Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3:329–33. doi: 10.1038/ni0402-329. [DOI] [PubMed] [Google Scholar]
- 45.Liang Y, Van Zant G. Aging stem cells, latexin and longevity. Exp Cell Res. 2008;314:1962–72. doi: 10.1016/j.yexcr.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherwood EM, Xu W, Riley RL. B cell precursors in senescent mice exhibit decreased recruitment into proliferative compartments and altered expression of Bcl-2 family members. Mech Ageing Dev. 2003;124:147–53. doi: 10.1016/s0047-6374(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 47.Miller JP, Allman D. The Decline in B Lymphopoiesis in Aged Mice Reflects Loss of Very Early B-Lineage Precursors. J Immunol. 2003;171:2326–30. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Hsu HC, Stockard CR, et al. IL-12 inhibits thymic involution by enhancing IL-7- and IL-2-induced thymocyte proliferation. J Immunol. 2004;172:2909–16. doi: 10.4049/jimmunol.172.5.2909. [DOI] [PubMed] [Google Scholar]
- 49.Rolink A, Haasner D, Nishikawa S, Melchers F. Changes in frequencies of clonable pre B cells during life in different lymphoid organs of mice. Blood. 1993;81:2290–300. [PubMed] [Google Scholar]
- 50.Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol. 1997;158:1598–609. [PubMed] [Google Scholar]
- 51.Thoman ML. Early steps in T cell development are affected by aging. Cell Immunol. 1997;178:117–23. doi: 10.1006/cimm.1997.1133. [DOI] [PubMed] [Google Scholar]
- 52.Mackall CL, Punt JA, Morgan P, Farr AG, Gress RE. Thymic function in young/old chimeras: substantial thymic T cell regenerative capacity despite irreversible age-associated thymic involution. Eur J Immunol. 1998;28:1886–93. doi: 10.1002/(SICI)1521-4141(199806)28:06<1886::AID-IMMU1886>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 53.Hirokawa K, Kubo S, Utsuyama M, Kurashima C, Sado T. Age-related change in the potential of bone marrow cells to repopulate the thymus and splenic T cells in mice. Cell Immunol. 1986;100:443–51. doi: 10.1016/0008-8749(86)90043-2. [DOI] [PubMed] [Google Scholar]
- 54.Sharp A, Kukulansky T, Globerson A. In vitro analysis of age-related changes in the developmental potential of bone marrow thymocyte progenitors. Eur J Immunol. 1990;20:2541–6. doi: 10.1002/eji.1830201203. [DOI] [PubMed] [Google Scholar]
- 55.Eren R, Globerson A, Abel L, Zharhary D. Quantitative analysis of bone marrow thymic progenitors in young and aged mice. Cell Immunol. 1990;127:238–46. doi: 10.1016/0008-8749(90)90129-f. [DOI] [PubMed] [Google Scholar]
- 56.Abkowitz JL, Taboada M, Shelton GH, Catlin SN, Guttorp P, Kiklevich JV. An X chromosome gene regulates hematopoietic stem cell kinetics. Proc Natl Acad Sci USA. 1998;95:3862–6. doi: 10.1073/pnas.95.7.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harrison DE. Normal production of erythrocytes by mouse marrow continuous for 73 months. Proc Natl Acad Sci USA. 1973;70:3184–8. doi: 10.1073/pnas.70.11.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller G. Clonal analysis of hematopoietic stem cell development in vivo. Curr Top Microbiol Immunol. 1992;177:41–57. doi: 10.1007/978-3-642-76912-2_4. [DOI] [PubMed] [Google Scholar]
- 59.Lemischka IR, Raulet DH, Mulligan RC. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986;45:917–27. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- 60.Rosendaal M, Hodgson GS, Bradley TR. Organization of haemopoietic stem cells: the generation-age hypothesis. Cell Tissue Kinet. 1979;12:17–29. doi: 10.1111/j.1365-2184.1979.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 61.Rosendaal M, Hodgson GS, Bradley TR. Haemopoietic stem cells are organised for use on the basis of their generation-age. Nature. 1976;264:68–9. doi: 10.1038/264068a0. [DOI] [PubMed] [Google Scholar]
- 62.Harrison DE, Stone M, Astle CM. Effects of transplantation on the primitive immunohematopoietic stem cell. J Exp Med. 1990;172:431–7. doi: 10.1084/jem.172.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iscove NN, Nawa K. Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Curr Biol. 1997;7:805–8. doi: 10.1016/s0960-9822(06)00341-1. [DOI] [PubMed] [Google Scholar]
- 64.Abkowitz JL, Golinelli D, Harrison DE, Guttorp P. In vivo kinetics of murine hemopoietic stem cells. Blood. 2000;96:3399–405. [PubMed] [Google Scholar]
- 65.Mazurier F, Gan OI, McKenzie JL, Doedens M, Dick JE. Lentivector-mediated clonal tracking reveals intrinsic heterogeneity in the human hematopoietic stem cell compartment and culture-induced stem cell impairment. Blood. 2004;103:545–52. doi: 10.1182/blood-2003-05-1558. [DOI] [PubMed] [Google Scholar]
- 66.Glimm H, Eisterer W, Lee K, et al. Previously undetected human hematopoietic cell populations with short-term repopulating activity selectively engraft NOD/SCID-{beta}2 microglobulin-null mice. J Clin Invest. 2001;107:199–206. doi: 10.1172/JCI11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guenechea G, Gan OI, Dorrell C, Dick JE. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol. 2001;2:75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- 68.Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. Faseb J. 2003;17:690–2. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warren LA, Rossi DJ. Stem cells and aging in the hematopoietic system. Mech Ageing Dev. 2008 doi: 10.1016/j.mad.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ryan DE. Examination of the blood. 6. New York: McGrawHill Book Co; 2001. [Google Scholar]
- 71.Lichtman MA, Williams WJ. Hamatology in the aged. 6. New York: McGrawHill Book Co; 2001. [Google Scholar]
- 72.Porrata LF, Gertz MA, Inwards DJ, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–85. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 73.Tyner SD, Venkatachalam S, Choi J, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 74.Dumble M, Moore L, Chambers SM, et al. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109:1736–42. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lajtha LG. Stem cell concepts. Differentiation. 1979;14:23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 76.Partridge L, Pletcher SD, Mair W. Dietary restriction, mortality trajectories, risk and damage. Mech Ageing Dev. 2005;126:35–41. doi: 10.1016/j.mad.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 77.Ferguson M, Sohal BH, Forster MJ, Sohal RS. Effect of long-term caloric restriction on oxygen consumption and body temperature in two different strains of mice. Mech Ageing Dev. 2007;128:539–45. doi: 10.1016/j.mad.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Astle CM, Harrison DE. Hematopoietic senescence is postponed and hematopoietic stem cell function is enhanced by dietary restriction. Exp Hematol. 2003;31:1097–103. doi: 10.1016/s0301-472x(03)00238-8. [DOI] [PubMed] [Google Scholar]
- 79.Ferguson M, Rebrin I, Forster MJ, Sohal RS. Comparison of metabolic rate and oxidative stress between two different strains of mice with varying response to caloric restriction. Exp Gerontol. 2008;43:757–63. doi: 10.1016/j.exger.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Cabo R, Furer-Galban S, Anson RM, Gilman C, Gorospe M, Lane MA. An in vitro model of caloric restriction. Exp Gerontol. 2003;38:631–9. doi: 10.1016/s0531-5565(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 81.Sandmaier B, Bethge W, Wilbur D, et al. Bismuth 213-labeled anti-CD45 radioimmuno-conjugate to condition dogs for nonmyeloablative allogeneic marrow grafts. Blood. 2002;100:318–26. doi: 10.1182/blood-2001-12-0322. [DOI] [PubMed] [Google Scholar]
- 82.Graf T. Two Lineages of Hematopoietic Stem Cells Identified by Differences in Lineage Priming. Experimental Hematology. 2007;35:1. [Google Scholar]