Introduction
Malaria is one of the most common infectious diseases in over 100 countries in Africa, Southeast Asia, and South America. It kills more people than any other communicable disease except for tuberculosis. Worldwide, malaria affects approximately 300 million patients, leading to more than 2 million death a year.1 The increasing prevalence of multiple drug resistant strains of Plasmodium falciparum in most malaria endemic areas has significantly reduced the efficacy of current anti-malarial drugs for prophylaxis and treatment of this disease. For instance, resistance to the inexpensive anti-malarial mainstays, such as chloroquine, is worldwide. Similarly, resistance to mefloquine, which was proposed as the drug of choice for chloroquine-resistant malaria, has been reported from Africa and Southeast Asia.2, 3 Therefore, medicinal agents based on novel mode of action are required to overcome the emergence of resistance and to control an ever-increasing number of epidemics caused by the malaria parasite.
Febrifugine (1) and isofebrifugine (2) were isolated as the active components against malaria in the Chinese herb Chang Shan (Dichroa febrifuga Lour),4, 5 which has been employed by the local people as medicine against fevers caused by malaria parasites for a long time. Febrifugine acts by impairing haemazoin formation required for maturation of the parasite at the trophozoite stage.6 The use of febrifugine as anti-malarial agent is initially appealing not only because of its rapid effect and no drug resistance, but also because of its availability. Subsequent pre-clinical researches have found that febrifugine possesses adverse side effects. Strong liver toxicity has precluded febrifugine as a clinical drug.7, 8, 9 Major drug toxicities can be grouped into five categories in terms of the underlying toxicity mechanism: (I) on-target, or mechanism-relayed, toxicity; (II) hypersensitivity and related immunological reactions; (III) off-target pharmacology; (IV) biological activation to toxic metabolites; (V) idiosyncratic toxicities. One concept in the study of toxicity is the so-called ‘metabolic activation’ of drugs and other chemicals. One explanation is that drugs can be transformed into reactive electrophiles, which bind covalently with proteins, DNA and low-molecular-mass nucleophiles such as glutathione (GSH).
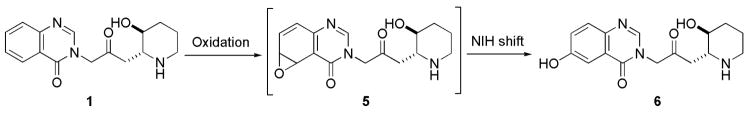
Total synthesis and structural modifications of febrifugine have been made.10, 11, 12, 13, 14, 15, 16 It has been elucidated the essential role played by the 4-quinazolinone ring and 1″-amino group in the appearance of activity. It is also known that febrifugine 1 and isofebrifugine 2 are in equilibrium when they are in solution (Scheme 1). This equilibrium most likely follows a three-step sequence: (i) retro-Michael reaction (1 → 3); (ii) Michael addition (3 → 4); (iii) hemi-ketal cyclization (4 → 2). Intermediate 3, as a Michael acceptor, is highly electrophilic. If half-life of 3 is sufficiently long, then it is very possible that this electrophile will non-selectively alkylate biomolecules such as proteins, peptides, DNA, or RNA inside the host cell, resulting in the observed toxicity.
Figure 2.

Febrifugine could be metabolized to the corresponding arene oxide 5 by cytochrome P-450 enzymes (Scheme 2). When arene oxide 5 escapes deactivation process by certain enzymes such as epoxide hydrase or glutathione S-transferase, toxicity can result because this reactive electrophile will form covalent adducts with DNA, RNA, proteins, or other biomolecules of the host. Such binding can cause mutations and result in cell damage. In a recent metabolic study of febrifugine, Oshima and coworkers isolated metabolite 6.14 This study indicates that arene oxide 5 is most probably a short-lived reactive metabolic intermediate because metabolite 6 would have been derived from intermediate 5 through the rearrangement known as NIH shift.17
Figure 3.
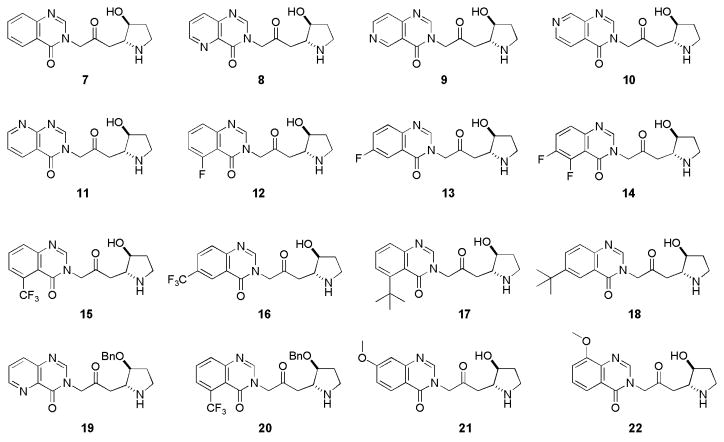
There are two possible ways to make this process unfavorable: (I) Blocking the C-5 or C-6 position of the quinoline ring; and (II) Increasing the oxidation potential of the molecule. As part of an ongoing malaria chemotherapy project in our laboratory, we undertook the synthesis and antimalarial activity evaluation of some febrifugine analogues (compounds 7–22, shown in Figure 1). Lower toxicity of these newly designed compounds will be achieved by reducing or eliminating the tendency to form chemically reactive and toxic intermediates. Formation of five-membered rings are kinetically more favored than that of six-membered rings due to entropy effect. In the case of compounds 7–22 where the original piperidine ring was replaced by a pyrrolidine ring, retro-Michael reaction would less likely occur. Also, the half-life of the corresponding Michael acceptor, once formed, would be much shorter. Thus, the host is less exposed to toxic electrophiles resulted from the retro-Michael reaction of the administered drug. For compounds 8–16, an extra nitrogen atom or a substitution group was also introduced on the aromatic ring to block the C-5 or C-6 position of the quinazolinone ring or to increase the oxidation potential of the molecule. Compounds 17 and 18 each bear a bulky group, on C-5 or C-6 position of the quinazolinone ring. Compounds 19 and 20 each bear a benzyloxy group on C-3″ position of the quinazolinone ring. These compounds are designed to block the aforementioned unwarranted metabolic pathway. Overall, these compounds closely resemble febrifugine itself by possessing a planar aromatic ring, a 1″-amino group and C-2′, C-3″ O-functionality and are therefore expected to possess same or similar mode of action. Meanwhile, they would be much less likely to produce toxic intermediates because of the replacement of the original piperidine ring by a pyrrolidine moiety, the blocking of C-5 and C-6 position or the increase of the oxidation potential of the molecule.
Figure 1.
As a comparative study, compounds 21 and 22 were also designed. These compounds each bear an electron-donating methoxy group on the C-7 or C-8 position of the aromatic ring. These compounds have comparable or even greater tendency to undergo oxidation. Biological data from these compounds should further validate, or nullify, the hypothesis that some oxidized febrifugine metabolites have contributed to the observed toxicity.
Chemistry
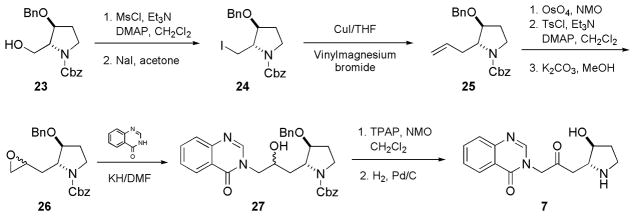
Scheme 3 illustrated the synthesis of compound 7. Other compounds (8–22) were synthesized by similar procedures. The known alcohol 23, available from borane reduction of commercially available trans-4-benzyloxy-N-Cbz-L-proline, was converted to the corresponding alkyl iodide 24. Coupling between 24 and vinyl magnesium bromide, in the presence of copper (I) iodide, furnished alkene 25 in good yield. 18 Although initial oxidation of alkene 25 by mCPBA to give oxirane 26 was not successful, the transformation was smooth and high-yielding via a three-step sequence: (i) dihydroxylation; (ii) tosylation of the primary alcohol; and (iii) epoxide ring formation facilitated by potassium carbonate in methanol. 3H-Quinazolin-4-one condensed with oxirane 26, to furnish corresponding alcohol 27, as a pair of diastereomers, this follows the methods of Ogasawara, et al.10 Compound 27 then underwent TPAP oxidation19 and hydrogenolysis, to afford compound 7. The synthesis of compounds 8–22 follows similar route. The respective aromatic moieties are available from previously published procedures.20, 21, 22
Figure 4.
Results and Discussion
Synthesized compounds (7–22) were tested against Plasmodium falciparum clones W-2, a chloroquine resistant cell line for in vitro efficacy. All compounds were also evaluated for antimalarial efficacy and toxicity in mouse models. The data are summarized in Table 1. Most compounds (7–16, 19–22) have exhibited comparable or superior in vitro and in vivo antimalarial activity, as compared to parent natural product febrifugine. This confirms earlier observation that it is crucial to possess a planar aromatic ring, a 1″-amino group and C-2′, C-3″ O-functionality in order to preserve the excellent antimalarial activity of febrifugine. Compounds 17 and 18, bearing a bulky tert-butyl group at the C-5 or C-6 position of the aromatic ring, are less active. The acute toxicity study of new compounds was conducted and the MTDs (maximum tolerated dose) were determined. Compound 7, bearing a pyrrolidine ring instead of the original piperidine, becomes 4 times less toxic as compared to febrifugine itself. Compounds 8–18, where the aromatic ring has either an extra nitrogen atom, an electron-withdrawing group, or a bulky group, have shown drastically reduced toxicity.
Table 1.
Antimalarial Efficacy and Toxicity Evaluationa
| Test Compounds | In Vitro W2 Strain | In Vivo P. berghei (ED50, mg/kg) | MCD (mg/kg) | MTD (mg/kg) | TIb |
|---|---|---|---|---|---|
| 7 | 0.56 | 1.6 | 8.4 | 144 | 17 |
| 8 | 0.12 | 0.44 | 3.1 | 345 | 111 |
| 9 | 0.14 | 0.49 | 2.9 | 310 | 107 |
| 10 | 0.31 | 1.2 | 6.6 | 338 | 51 |
| 11 | 0.34 | 1.3 | 5.9 | 439 | 74 |
| 12 | 0.29 | 1.0 | 7.1 | 399 | 56 |
| 13 | 0.26 | 1.1 | 6.8 | 425 | 63 |
| 14 | 0.40 | 1.7 | 8.9 | 379 | 43 |
| 15 | 0.23 | 0.74 | 4.7 | 335 | 71 |
| 16 | 0.28 | 1.5 | 7.9 | 440 | 56 |
| 17 | 2.9 | 8.7 | 39 | 429 | 11 |
| 18 | 3.4 | 10 | 42 | 504 | 12 |
| 19 | 0.14 | 0.14 | 0.77 | 102 | 132 |
| 20 | 0.19 | 0.12 | 0.69 | 88 | 128 |
| 21 | 0.62 | 1.7 | 5.6 | 55 | 10 |
| 22 | 0.57 | 1.5 | 4.9 | 51 | 10 |
| Febrifugine | 0.76 | 2.3 | 12 | 35 | 3 |
| Chloroquine | 224 | 4.5 | 40.0 | 240 | 6 |
Antimalarial activities were determined after i.p. administration of the compounds once daily for 4 days to infected mice.
Therapeutic indices were calculated as MTD divided by MCD.
Compounds 19 and 20 each bears a benzyloxy group on C-3″ position of the aromatic ring. They possess excellent in vitro antimalarial activity. The IC50’s are comparable to compounds 8 and 15, which bear a free hydroxyl group on C-3″ position of the aromatic ring. It is interesting to note that the in vivo efficacy of compounds 19 and 20 is superior to that of 8 and 15. The toxicity level is also elevated. Compounds 19 and 20, with a benzyloxy group on C-3″ position of the aromatic ring, are more lipophilic. Consequently, they may possess improved bioavailability.
Although the antimalarial efficacy of compounds 21 and 22 is about the same as that of compound 7, these compounds are considerably more toxic. Compounds 21 and 22 each possess an electron-donating methoxy group attached to the C-7 or C-8 of the aromatic ring and possess even greater tendency to undergo oxidation than that of compound 7. These biological data further validate the hypothesis that some oxidized febrifugine metabolites have contributed to the observed toxicity.
Antimalarial potency was also measured by determination of the MCD (minimum clearance dose), a commonly used efficacy measurement. The therapeutic indices, which were obtained by using the MCDs as the effective parameters, were calculated. Based on the therapeutic-index data, compounds 8–16, 19, and 20 are much less toxic than the natural product febrifugine and existing antimalarial drug chloroquine and are expected to possess wide therapeutic windows. Four compounds (17, 18, 21, and 22) possess narrow therapeutic windows. They are either less active (compounds 17, 18) or quite toxic (compounds 21, 22).
In conclusion, new febrifugine analogues were designed and synthesized. Some of these compounds (8, 9, 19, 20) possess a therapeutic index over ten times superior to that of the commonly used antimalarial drug chloroquine. These compounds, as well as the underlying design rationale, may find usefulness in the discovery and development of new antimalarial drugs.
Experimental
Chemistry
Melting points were determined on a Mettler FP62 melting point apparatus and are uncorrected. Unless otherwise noted, all nonaqueous reactions were performed under an oxygen-free atmosphere of nitrogen with rigid exclusion of moisture from reagents and glassware. Analytical thin layer chromatography (TLC) was performed using EM Reagents 0.25 mm silica gel 60-F plates. Visualization of the developed chromatogram was performed by UV absorbance, aqueous potassium permanganate, or ethanolic anisaldehyde. Liquid chromatography was performed using a force flow (flash chromatography) of the indicated solvent system on EM Reagents Silica Gel 60 (70–230 mesh). Preparative TLC was performed using Whatman Silica Gel C8 TLC plates (PLK5F). 1H NMR spectra were recorded in deuteriochloroform, unless otherwise noted, on a Bruker Avance 600 spectrometer at the frequency of 600.1 MHz. Chemical shifts are reported in parts per million on the δ scale from an internal standard of tetramethylsilane. Data are reported as follows: chemical shifts, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, qn = quintet, m = multiplet, and br = broad), coupling constant in Hertz, integration, and assignment. 13C NMR spectra were recorded in deuteriochloroform, unless otherwise noted, on a Bruker Avance 600 spectrometer at the frequency of 150.9 MHz. Chemical shifts are reported in parts per million on the δ scale from an internal standard of tetramethylsilane. Combustion analyses were performed by Atlantic Microlab, Inc. (Norcross, Georgia). All purified compounds possess a purity of at least 95%. When necessary, solvents and reagents were dried as follows: ether, tetrahydrofuran, benzene, and toluene were stored and distilled from sodium benzophenone ketyl; dichloromethane, triethylamine, pyridine, and hexane were distilled over calcium hydride. Unless otherwise stated, the reagents were purchased from Fisher Scientific, Aldrich Chemical Company, Lancaster, or Fluka, and used as received.
3-Benzyloxy-2-iodomethyl-pyrrolidine-1-carboxylic acid benzyl ester (24)
Mesyl chloride (MsCl, 8.0 mL) was added into a stirred solution of alcohol 23 (25.3 g), triethylamine (Et3N, 30 mL) and 4-dimethylaminopyridine (DMAP, 1.0 g) in 200 mL of methylene chloride (CH2Cl2) in an ice-water bath. The reaction mixture was then warmed to room temperature and stirred for additional 2 h. Solvent was evaporated. The resulting slurry was dissolved in ethyl acetate (180 mL), washed with water (150 mL), then aqueous sodium bicarbonate (80 mL), and brine (70 mL), dried over anhydrous sodium sulfate, and evaporated in a rotary evaporator under reduced pressure to furnish the essentially pure mesylate. This mesylate (29.5 g) and sodium iodide (NaI, 23.0 g) were dissolved in 180 mL of acetone. The resulting solution was then heated at reflux for 12 h. Solvent was evaporated. The resulting slurry was dissolved in ethyl acetate (150 mL), washed with water (130 mL), then aqueous sodium bicarbonate (60 mL), and brine (50 mL), dried over anhydrous sodium sulfate, and evaporated in a rotary evaporator under reduced pressure to furnish the crude product. Silica gel flash chromatography (15% ethyl acetate in hexanes) furnishes 24 as viscous oil. Yield: 85% (2 steps). 1H NMR: 7.31-7.27 (m, 10H), 5.34 (m, 2H), 4.61 (m, 2H), 4.09 (m, 1H), 3.85 (m, 1H), 3.57 (m, 2H), 3.38 (dd, J = 11 and 5.4 Hz, 1H), 3.24 (dd, J = 11 and 4.2 Hz, 1H), 1.94 (m, 2H); 13C NMR: 159.4, 140.9, 137.9, 128.7, 128.5, 127.7, 127.6, 127.4, 127.3, 80.2, 76.2, 70.8, 59.0, 39.1, 28.5, 13.5. HRESIMS m/z 474.0531 [M+Na]+ (calcd for C20H22INNaO3+, 474.0542) (100%). Anal. Calcd for C20H22INO3: C, 53.23; H, 4.91; I, 28.12; N, 3.10. Found: C, 53.29; H, 4.88; I, 28.21; N, 3.04.
2-Allyl-3-benzyloxy-pyrrolidine-1-carboxylic acid benzyl ester (25)
Vinyl magnesium bromide [1.0M in tetrahydrofuran (THF), 53.0 mL] was added into a stirred solution of alkyl iodide 24 (11.0 g) and copper (I) iodide (CuI, 2.5 g) in 100 ML of THF at −78°C under nitrogen atmosphere. The reaction mixture was kept at −78°C for 1 h, then slowly warmed to room temperature over a period of 2 h. The reaction mixture was quenched with aqueous sodium bicarbonate (60 mL). After partition between ethyl acetate (120 mL) and water (120 mL), the separated organic layer was washed with brine (55 mL), dried over anhydrous sodium sulfate, and evaporated in a rotary evaporator under reduced pressure to furnish the crude product. Silica gel flash chromatography (15% ethyl acetate in hexanes) furnished 25 as viscous oil. Yield: 94%. 1H NMR: 7.31-7.27 (m, 10H), 5.73 (m, 1H), 5.34 (m, 2H), 5.07 (m, 1H), 4.99 (m, 1H), 4.61 (m, 2H), 4.09 (m, 1H), 3.85 (m, 1H), 3.57 (m, 2H), 2.38 (m, 2H), 1.94 (m, 2H); 13C NMR: 158.6, 142.1, 140.5, 138.2, 128.6, 128.4, 127.8, 127.7, 127.5, 127.2, 115.5, 79.2, 75.8, 71.2, 57.6, 38.7, 31.4, 27.5. HRESIMS m/z 374.1739 [M+Na]+ (calcd for C22H25NNaO3+, 374.1732) (100%). Anal. Calcd for C22H25NO3: C, 75.19; H, 7.17; N, 3.99. Found: C, 75.24; H, 7.15; N, 3.92.
3-Benzyloxy-2-oxiranylmethyl-pyrrolidine-1-carboxylic acid benzyl ester (26)
Alkene 25 (9.0 g), osmium tetraoxide (OsO4, 4% in water, 1.0 mL), and N-methyl morpholine N-oxide (NMO, 10.5 g) were dissolved in 80 mL of water and 80 mL of tetrahydrofuran (THF). The resulting heterogeneous solution is stirred vigorously for 4 days at room temperature. After partition between ethyl acetate (150 mL) and water (100 mL), the separated organic layer was washed with brine (70 mL), dried over anhydrous sodium sulfate, and evaporated in a rotary evaporator under reduced pressure to furnish the essentially pure diol. Tosyl chloride (TsCl, 4.6 g) was added into a stirred solution of this diol (8.9 g), triethylamine (Et3N, 7.0 mL) and 4-dimethylaminopyridine (DMAP, 300 mg) in 100 mL of methylene chloride (CH2Cl2) in an ice-water bath. The reaction mixture was then warmed to room temperature and stirred for additional 2 h. Solvent was evaporated. The resulting slurry was dissolved in ethyl acetate (100 mL), washed with water (80 mL), then aqueous sodium bicarbonate (80 mL), and brine (50 mL), dried over anhydrous sodium sulfate, and evaporated in a rotary evaporator under reduced pressure to furnish the essentially pure mono-tosylate. Potassium carbonate (K2CO3, 1.0 g) was added into a stirred solution of this mono-tosylate (11.4 g) in 25 mL of methanol (MeOH) at room temperature. After 4 h, reaction mixture was partitioned between ethyl acetate (80 mL) and water (80 mL), the separated organic layer was then washed with brine (40 mL), dried over anhydrous sodium sulfate, and evaporated in a rotary evaporator under reduced pressure to furnish the crude product. Silica gel flash chromatography (25% ethyl acetate in hexanes) furnishes 26 as viscous oil. Yield: 76% (3 steps). 1H NMR: 7.30-7.22 (m, 10H), 5.37 (m, 2H), 4.65 (m, 2H), 3.85 (m, 1H), 3.57 (m, 2H), 3.34 (m, 1H), 3.05 (m, 1H), 2.79 (m, 1H), 2.52 (m, 1H), 1.94 (m, 2H), 1.87-1.82 (m, 2H); 13C NMR: 158.8, 141.7, 139.3, 128.5, 128.2, 127.9, 127.6, 127.4, 126.8, 76.2, 73.8, 71.9, 51.4, 47.5, 43.3, 35.8, 34.8, 28.4. HRESIMS m/z 390.1677 [M+Na]+ (calcd for C22H25NNaO4+, 390.1681) (100%). Anal. Calcd for C22H25NO4: C, 71.91; H, 6.86; N, 3.81. Found: C, 71.84; H, 6.91; N, 3.85.
3-Benzyloxy-2-[2-hydroxy-3-(4-oxo-4H-quinazolin-3-yl)-propyl]-pyrrolidine-1-carboxylic acid benzyl ester (27)
Potassium hydride (KH, 30% in mineral oil, 3.6 g) was suspended in 50 mL of dimethylformamide (DMF). It was cooled in an ice-water bath, and solid 3H-quinazolin-4-one (4.0 g) was added in. After 30 min, a solution of oxirane 26 (2.2 g) in 10 mL of DMF was added in. The reaction mixture was then heated at 80°C for 12 h under nitrogen atmosphere. It was partitioned between ethyl acetate (80 mL) and water (80 mL), separated organic layer was washed with water (3 × 60 mL), then brine (40 mL), dried over anhydrous sodium sulfate, and evaporated in a rotary evaporator under reduced pressure to furnish the crude product. Silica gel flash chromatography (80% ethyl acetate in hexanes) furnished 27 as off white solid. Yield: 77%. MP: 187°C. 1H NMR: 8.28 (d, J = 7.5 Hz, 1H), 8.24 (s, 1H), 7.73 (m, 2H), 7.48 (m, 1H), 7.31-7.27 (m, 10H), 5.45 (m, 2H), 5.19 (br, 1H), 4.81 (m, 2H), 4.24 (m, 1H), 4.16 (m, 1H), 3.85 (m, 2H), 3.62 (m, 2H), 3.23 (m, 1H), 1.89 (m, 2H), 1.57 (m, 2H); 13C NMR: 168.4, 163.1, 158.7, 145.3, 141.2, 138.2, 134.1, 129.3, 128.9, 128.6, 127.8, 127.6, 127.4, 127.1, 127.0, 126.7, 123.8, 80.8, 77.4, 71.3, 66.4, 55.1, 51.9, 39.4, 32.4, 26.6. HRESIMS m/z 536.2170 [M+Na]+ (calcd for C30H31N3NaO5+, 536.2161) (100%). Anal. Calcd for C30H31N3O5.HCl: C, 65.51; H, 5.86; Cl, 6.45; N, 7.64. Found: C, 65.56; H, 5.83; Cl, 6.51; N, 7.62.
(2R, 3S)-3-[3-(3-Hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-3H-quinazolin-4-one (7)
A solution of alcohol 27 (2.1 g) in 5 mL of CH2Cl2 was added into a stirred slurry of tetrapropylammonium perruthenate (TPAP, 70 mg), N-methylmorpholine N-oxide (NMO, 936 mg), and grounded molecular sieve (800 mg) in 10 mL of CH2Cl2 at room temperature. After 1 h, the reaction mixture was loaded directly into a short column of silica gel and eluted with 5% MeOH/EtOAc. Concentration of the eluant under reduced pressure afforded the corresponding ketone. This ketone (1.89 g) was dissolved in 15 mL of 95% EtOH/H2O. 300 mg of 10% Pd on carbon was added in. It was then treated with hydrogen (60 psi) in a Parr apparatus for 12 h. Solid was filtered off and the solution was evaporated under vacuum to dryness. Recrystallization from ethanol-water (with addition of dilute aqueous HCl solution, 4–5 equiv. of HCl) furnishes compound 7 (HCl salt) as off-white crystals. Yield: 901 mg, 76% (two steps). MP: 217–218°C. [α]25D +24.9 (c 0.51, EtOH). 1H NMR (CD3OD): 8.27(dd, J = 7.9, 1.0 Hz, 1H), 7.92 (s, 1H), 7.77 (ddd, J = 8.1, 7.9, 1.0 Hz, 1H), 7.71 (dd, J = 8.1, 0.9 Hz, 1H), 7.50 (dt, J = 7.9, 0.9 Hz, 1H), 5.11 (d, J = 17.1 Hz, 1H), 4.96 (d, J = 17.1 Hz, 1H), 4.41 (m, 1H), 4.14 (m, 1H), 3.49-3.42 (m, 2H), 2.89 (dd, J = 15.9, 4.2 Hz,, 1H), 2.81 (dd, J = 15.9, 6.1 Hz, 1H), 2.24 (m, 1H), 1.90 (m, 1H); 13C NMR (CD3OD): 204.1, 169.2, 165.2, 147.9, 133.2, 128.1, 127.4, 124.3, 121.9, 75.1, 57.9, 56.4, 39.2, 33.4, 31.7. HRESIMS m/z 310.1159 [M+Na]+ (calcd for C15H17N3NaO3+, 310.1168) (100%). Anal. Calcd for C15H17N3O3.HCl: C, 55.64; H, 5.60; Cl, 10.95; N, 12.98. Found: C, 55.60; H, 5.63; Cl, 10.99; N, 12.89.
(2R, 3S)-3-[3-(3-Hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-3H-pyrido[3,2-d]pyrimidin-4-one (8)
Yellow crystal. MP: 261°C. [α]25D +21.8 (c 0.5, EtOH). 1H NMR (CD3OD): 9.71 (d, J = 7.2 Hz, 1H), 9.24 (d, J = 7.2 Hz, 1H), 8.80 (s, 1H), 8.21 (t, J = 7.2 Hz, 1H), 5.08 (d, J = 17.3 Hz, 1H), 4.92 (d, J = 17.3 Hz, 1H), 4.41 (m, 1H), 4.14 (m, 1H), 3.49-3.42 (m, 2H), 2.89 (m, 1H), 2.81 (dd, J = 15.1 and 6.1 Hz, 1H), 2.24 (m, 1H), 1.90 (m, 1H); 13C NMR (CD3OD): 202.1, 163.7, 159.2, 151.5, 149.6, 146.4, 132.9, 131.2, 81.0, 57.9, 54.1, 51.9, 44.7, 36.7. HRESIMS m/z 311.1116 [M+Na]+ (calcd for C14H16N4NaO3+, 311.1120) (100%). Anal. Calcd for C14H16N4O3.HCl: C, 51.78; H, 5.28; Cl, 10.92; N, 17.25. Found: C, 51.82; H, 5.26; Cl, 10.88; N, 17.28.
(2R, 3S)-3-[3-(3-Hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-3H-pyrido[4,3-d]pyrimidin-4-one (9)
Pale- yellow crystal. MP: 251°C. [α]25D −31.4 (c 0.5, EtOH). 1H NMR (CD3OD): 9.54 (s, 1H), 9.19 (d, J = 7.5 Hz, 1H), 8.71 (s, 1H), 8.13 (d, J = 7.5 Hz, 1H), 5.07 (d, J = 17.4 Hz, 1H), 4.90 (d, J = 17.4 Hz, 1H), 4.39 (m, 1H), 4.18 (m, 1H), 3.46-3.41 (m, 2H), 2.94 (m, 1H), 2.85 (dd, J = 15.1 and 6.1 Hz, 1H), 2.23 (m, 1H), 1.87 (m, 1H); 13C NMR (CD3OD): 202.6, 166.6, 163.6, 159.1, 154.5, 149.0, 147.1, 135.2, 80.3, 56.9, 55.3, 50.9, 44.3, 35.6. HRESIMS m/z 311.1128 [M+Na]+ (calcd for C14H16N4NaO3+, 311.1120) (100%). Anal. Calcd for C14H16N4O3.HCl: C, 51.78; H, 5.28; Cl, 10.92; N, 17.25. Found: C, 51.74; H, 5.31; Cl, 10.90; N, 17.22.
(2R, 3S)-3-[3-(3-Hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-3H-pyrido[3,4-d]pyrimidin-4-one (10)
Yellow crystal. MP: 221–222°C. [α]25D +27.4 (c 0.43, EtOH). 1H NMR (CD3OD): 9.24 (s, 1H), 8.74 (d, J = 7.3 Hz, 1H), 8.49 (d, J = 7.3 Hz, 1H), 8.25 (s, 1H), 5.16 (d, J = 17.1 Hz, 1H), 5.03 (d, J = 17.1 Hz, 1H), 4.37 (m, 1H), 4.23 (m, 1H), 3.44-3.38 (m, 2H), 2.91 (m, 1H), 2.89 (dd, J = 15.4 and 6.2 Hz, 1H), 2.27 (m, 1H), 1.92 (m, 1H); 13C NMR (CD3OD): 202.6, 167.8, 164.6, 159.2, 155.2, 154.7, 139.9, 134.8, 79.2, 56.1, 54.9, 51.2, 45.1, 34.8. HRESIMS m/z 311.1114 [M+Na]+ (calcd for C14H16N4NaO3+, 311.1120) (100%). Anal. Calcd for C14H16N4O3.HCl: C, 51.78; H, 5.28; Cl, 10.92; N, 17.25. Found: C, 51.84; H, 5.24; Cl, 10.99; N, 17.31.
(2R, 3S)-3-[3-(3-Hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-3H-pyrido[2,3-d]pyrimidin-4-one (11)
Yellow crystal. MP: 213–214°C. [α]25D −19.6 (c 0.42, EtOH). 1H NMR (CD3OD): 8.88 (d, J = 6.8 Hz, 1H), 8.75 (d, J = 6.8 Hz, 1H), 8.28 (s, 1H), 8.19 (t, J = 6.8 Hz, 1H), 5.03 (d, J = 16.8 Hz, 1H), 4.92 (d, J = 16.8 Hz, 1H), 4.37 (m, 1H), 4.24 (m, 1H), 3.45-3.40 (m, 2H), 2.82 (dd, J = 15.5, 4.6 Hz,, 1H), 2.76 (dd, J = 15.5, 6.2 Hz, 1H), 2.28 (m, 1H), 1.84 (m, 1H); 13C NMR (CD3OD): 204.1, 171.8, 167.3, 162.8, 152.1, 142.6, 133.1, 130.9, 77.4, 58.5, 56.8, 38.7, 33.9, 32.1. HRESIMS m/z 311.1122 [M+Na]+ (calcd for C14H16N4NaO3+, 311.1120) (100%). Anal. Calcd for C14H16N4O3.HCl: C, 51.78; H, 5.28; Cl, 10.92; N, 17.25. Found: C, 51.83; H, 5.25; Cl, 10.81; N, 17.34.
(2R, 3S)-5-Fluoro-3-[3-(3-hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-3H-quinazolin-4-one (12)
Pale- yellow crystals. MP: 242°C. [α]25D −33.8 (c 0.5, EtOH). 1H NMR (CD3OD): 8.32 (s, 1H), 8.20 (d, J = 7.1 Hz, 1H), 7.73 (d, J = 7.2 Hz, 1H), 7.55 (t, J = 7.1 Hz, 1H), 5.10 (d, J = 17.4 Hz, 1H), 4.93 (d, J = 17.4 Hz, 1H), 4.43 (m, 1H), 4.10 (m, 1H), 3.48-3.40 (m, 2H), 2.82 (m, 1H), 2.77 (dd, J = 15.2 and 5.8 Hz, 1H), 2.28 (m, 1H), 1.93 (m, 1H); 13C NMR (CD3OD): 203.1, 164.1, 158.5, 152.7, 149.3, 147.5, 132.8, 132.2, 129.6, 80.2, 58.6, 53.2, 50.9, 43.8, 35.9. HRESIMS m/z 328.1082 [M+Na]+ (calcd for C15H16FN3NaO3+, 328.1073) (100%). Anal. Calcd for C15H16FN3O3.HCl: C, 52.71; H, 5.01; Cl, 10.37; F, 5.56; N, 12.30. Found: C, 52.80; H, 5.00; Cl, 10.26; F, 5.51; N, 12.34.
(2R, 3S)-6-Fluoro-3-[3-(3-hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-3H-quinazolin-4-one (13)
Pale- yellow crystal. MP: 196–197°C. [α]25D +26.3 (c 0.5, EtOH). 1H NMR (CD3OD): 8.24 (s, 1H), 7.84 (d, J = 7.1 Hz, 1H), 7.53 (s, 1H), 7.46 (d, J = 7.1 Hz, 1H), 5.08 (d, J = 17.3 Hz, 1H), 4.95 (d, J = 17.3 Hz, 1H), 4.48 (m, 1H), 4.15 (m, 1H), 3.47-3.39 (m, 2H), 2.86 (m, 1H), 2.79 (dd, J = 15.1 and 5.6 Hz, 1H), 2.32 (m, 1H), 1.89 (m, 1H); 13C NMR (CD3OD): 202.9, 167.9, 163.6, 147.1, 133.2, 129.7, 128.9, 128.0, 127.7, 78.4, 60.4, 54.0, 51.1, 42.5, 34.5. HRESIMS m/z 328.1066 [M+Na]+ (calcd for C15H16FN3NaO3+, 328.1073) (100%). Anal. Calcd for C15H16FN3O3.HCl: C, 52.71; H, 5.01; Cl, 10.37; F, 5.56; N, 12.30. Found: C, 52.64; H, 5.05; Cl, 10.42; F, 5.52; N, 12.24.
(2R, 3S)-5,6-Difluoro-3-[3-(3-hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-3H-quinazolin-4-one (14)
Pale-yellow crystal. MP: 241°C. [α]25D −11.8 (c 0.5, EtOH). 1H NMR (CD3OD): 8.31 (s, 1H), 7.80 (d, J = 6.9 Hz, 1H), 7.74 (d, J = 6.9 Hz, 1H), 5.06 (d, J = 17.4 Hz, 1H), 4.92 (d, J = 17.4 Hz, 1H), 4.39 (m, 1H), 4.12 (m, 1H), 3.48-3.40 (m, 2H), 2.92 (m, 1H), 2.78 (dd, J = 14.9 and 5.8 Hz, 1H), 2.28 (m, 1H), 1.87 (m, 1H); 13C NMR (CD3OD): 202.8, 162.8, 161.6, 158.5, 152.1, 149.4, 147.2, 132.4, 130.5, 80.7, 58.5, 53.8, 51.4, 43.6, 37.2. HRESIMS m/z 346.0984 [M+Na]+ (calcd for C15H15F2N3NaO3+, 346.0979) (100%). Anal. Calcd for C15H15F2N3O3.HCl: C, 50.08; H, 4.48; Cl, 9.85; F, 10.56; N, 11.68. Found: C, 50.14; H, 4.44; Cl, 9.91; F, 10.49; N, 11.75.
(2R, 3S)-3-[3-(3-Hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-5-trifluoromethyl-3H-quinazolin-4-one (15)
Off-white crystals. MP: 262°C. [α]25D +27.5 (c 0.5, EtOH). 1H NMR (CD3OD): 8.28 (s, 1H), 7.87 (d, J = 7.2 Hz, 1H), 7.59 (d, J = 7.2 Hz, 1H), 7.50 (t, J = 7.2 Hz, 1H), 5.05 (d, J = 16.8 Hz, 1H), 4.84 (d, J = 16.8 Hz, 1H), 4.40 (m, 1H), 4.19 (m, 1H), 3.47-3.41 (m, 2H), 2.85 (m, 1H), 2.78 (dd, J = 15.3 and 6.2 Hz, 1H), 2.27 (m, 1H), 1.93 (m, 1H); 13C NMR (CD3OD): 202.8, 162.7, 158.2, 150.5, 148.3, 141.4, 133.3, 132.7, 131.7, 116.8, 80.5, 57.6, 53.6, 50.5, 42.4, 36.5. HRESIMS m/z 378.1050 [M+Na]+ (calcd for C16H16F3N3NaO3+, 378.1041) (100%). Anal. Calcd for C16H16F3N3O3.HCl: C, 49.05; H, 4.37; Cl, 9.05; F, 14.55; N, 10.73. Found: C, 49.12; H, 4.33; Cl, 9.01; F, 14.62; N, 10.82.
(2R, 3S)-3-[3-(3-Hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-6-trifluoromethyl-3H-quinazolin-4-one (16)
Off-white crystal. MP: 206–207°C. [α]25D +16.7 (c 0.5, EtOH). 1H NMR (CD3OD): 8.26 (s, 1H), 7.81 (d, J = 7.2 Hz, 1H), 7.75 (s, 1H), 7.58 (d, J = 7.2 Hz, 1H), 5.09 (d, J = 17.4 Hz, 1H), 4.98 (d, J = 17.4 Hz, 1H), 4.39 (m, 1H), 4.24 (m, 1H), 3.47-3.41 (m, 2H), 2.85 (dd, J = 15.7 4.4 Hz,, 1H), 2.77 (dd, J = 15.7, 5.9 Hz, 1H), 2.20 (m, 1H), 1.92 (m, 1H); 13C NMR (CD3OD): 203.3, 167.8, 162.6, 147.3, 137.1, 133.5, 129.8, 128.1, 127.4, 119.2, 76.9, 58.2, 57.1, 38.8, 33.1, 30.9. HRESIMS m/z 378.1035 [M+Na]+ (calcd for C16H16F3N3NaO3+, 378.1041) (100%). Anal. Calcd for C16H16F3N3O3.HCl: C, 49.05; H, 4.37; Cl, 9.05; F, 14.55; N, 10.73. Found: C, 48.98; H, 4.41; Cl, 9.11; F, 14.49; N, 10.67.
(2R, 3S)-5-tert-Butyl-3-[3-(3-hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-3H-quinazolin-4-one (17)
Off-white crystal. MP: 193–194°C. [α]25D +12.4 (c 0.51, EtOH). 1H NMR (CD3OD): 8.25 (d, J = 7.2 Hz, 1H), 8.17 (s, 1H), 7.74 (d, J = 7.2 Hz, 1H), 7.63 (t, J = 7.2 Hz, 1H), 5.12 (d, J = 16.9 Hz, 1H), 5.00 (d, J = 16.9 Hz, 1H), 4.34 (m, 1H), 4.12 (m, 1H), 3.49-3.42 (m, 2H), 2.91 (dd, J = 15.5 4.5 Hz,, 1H), 2.82 (dd, J = 15.5, 5.8 Hz, 1H), 2.22 (m, 1H), 1.89 (m, 1H), 1.31 (s, 9H); 13C NMR (CD3OD): 202.3, 169.5, 165.1, 152.9, 147.2, 143.1, 133.9, 130.1, 129.9, 77.3, 58.6, 56.4, 39.4, 35.6, 33.6, 31.9, 30.5. HRESIMS m/z 366.1782 [M+Na]+ (calcd for C19H25N3NaO3+, 366.1794) (100%). Anal. Calcd for C19H25N3O3.HCl: C, 60.07; H, 6.90; Cl, 9.33; N, 11.06. Found: C, 60.13; H, 6.87; Cl, 9.29; N, 11.12.
(2R, 3S)-6-tert-Butyl-3-[3-(3-hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-3H-quinazolin-4-one (18)
Off- white crystal. MP: 190–191°C. [α]25D −10.3 (c 0.45, EtOH). 1H NMR (CD3OD): 8.20 (s, 1H), 7.94 (d, J = 7.1 Hz, 1H), 7.69 (d, J = 7.1 Hz, 1H), 7.56 (s, 1H), 5.09 (d, J = 17.1 Hz, 1H), 4.97 (d, J = 17.1 Hz, 1H), 4.30 (m, 1H), 4.18 (m, 1H), 3.48-3.40 (m, 2H), 2.93 (dd, J = 15.9 4.6 Hz,, 1H), 2.84 (dd, J = 15.9, 5.9 Hz, 1H), 2.29 (m, 1H), 1.92 (m, 1H), 1.34 (s, 9H); 13C NMR (CD3OD): 202.9, 167.9, 164.6, 154.1, 143.2, 134.7, 129.9, 128.8, 128.4, 79.2, 59.1, 56.9, 38.8, 36.2, 34.2, 31.7, 30.2. HRESIMS m/z 366.1803 [M+Na]+ (calcd for C19H25N3NaO3+, 366.1794) (100%). Anal. Calcd for C19H25N3O3.HCl: C, 60.07; H, 6.90; Cl, 9.33; N, 11.06. Found: C, 60.01; H, 6.93; Cl, 9.37; N, 10.98.
(2R, 3S)-3-[3-(3-Benzyloxy-pyrrolidin-2-yl)-2-oxo-propyl]-3H-pyrido[3,2-d]pyrimidin-4-one (19)
Yellow crystal. MP: 232–233°C. [α]25D +25.7 (c 0.5, EtOH). 1H NMR (CD3OD): 9.61 (d, J = 7.2 Hz, 1H), 9.19 (d, J = 7.2 Hz, 1H), 8.76 (s, 1H), 8.31 (t, J = 7.2 Hz, 1H), 7.22-7.18 (m, 5H), 5.08 (d, J = 17.2 Hz, 1H), 4.89 (d, J = 17.2 Hz, 1H), 4.69 (m, 2H), 4.40 (m, 1H), 4.12 (m, 1H), 3.48-3.42 (m, 2H), 2.88 (m, 1H), 2.78 (dd, J = 15.1 and 6.0 Hz, 1H), 2.26 (m, 1H), 1.92 (m, 1H); 13C NMR (CD3OD): 204.3, 163.2, 159.9, 151.1, 149.3, 146.1, 137.9, 133.8, 131.6, 128.9, 127.8, 127.5, 81.1, 75.1, 573, 54.8, 51.3, 44.2, 36.2. HRESIMS m/z 401.1586 [M+Na]+ (calcd for C21H22N4NaO3+, 401.1590) (100%). Anal. Calcd for C21H22N4O3.HCl: C, 60.79; H, 5.59; Cl, 8.55; N, 13.50. Found: C, 60.83; H, 5.55; Cl, 8.62; N, 13.44.
(2R, 3S)-3-[3-(3-Benzyloxy-pyrrolidin-2-yl)-2-oxo-propyl]-5-trifluoromethyl-3H-quinazolin-4-one (20)
Pale yellow crystals. MP: 229–230°C. [α]25D −17.9 (c 0.5, EtOH). 1H NMR (CD3OD): 8.22 (s, 1H), 7.84 (d, J = 7.1 Hz, 1H), 7.62 (d, J = 7.1 Hz, 1H), 7.55 (t, J = 7.1 Hz, 1H), 7.23-7.19 (m, 5H), 5.09 (d, J = 16.9 Hz, 1H), 4.82 (d, J = 16.9 Hz, 1H), 4.71 (m, 2H), 4.39 (m, 1H), 4.13 (m, 1H), 3.46-3.40 (m, 2H), 2.89 (m, 1H), 2.79 (dd, J = 15.4 and 6.0 Hz, 1H), 2.29 (m, 1H), 1.91 (m, 1H); 13C NMR (CD3OD): 203.7, 162.1, 158.9, 151.2, 149.1, 140.7, 139.2, 133.5, 132.4, 131.9, 128.9, 127.8, 127.7, 116.8, 80.1, 76.2, 57.9, 52.9, 51.5, 42.1, 33.8. HRESIMS m/z 468.1519 [M+Na]+ (calcd for C23H22F3N3NaO3+, 468.1511) (100%). Anal. Calcd for C23H22F3N3O3.HCl: C, 57.32; H, 4.81; Cl, 7.36; F, 11.83; N, 8.72. Found: C, 57.39; H, 4.76; Cl, 7.42; F, 11.74; N, 8.80.
(2R, 3S)-3-[3-(3-Hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-7-methoxy-3H-quinazolin-4-one (21)
Off- white crystal. MP: 181–182°C. [α]25D +18.3 (c 0.5, EtOH). 1H NMR (CD3OD): 8.13 (s, 1H), 7.72 (s, 1H), 7.43 (d, J = 7.2 Hz, 1H), 7.33 (d, J = 7.2 Hz, 1H), 5.08 (d, J = 17.3 Hz, 1H), 4.92 (d, J = 17.3 Hz, 1H), 4.38 (m, 1H), 4.11 (m, 1H), 3.81 (s, 3H), 3.43-3.36 (m, 2H), 2.91 (dd, J = 15.4, 4.3 Hz,, 1H), 2.79 (dd, J = 15.4, 6.2 Hz, 1H), 2.29 (m, 1H), 1.81 (m, 1H); 13C NMR (CD3OD): 202.5, 168.3, 165.5, 161.3, 188.5, 138.4, 134.5, 130.6, 128.9, 76.4, 58.7, 57.2, 55.9, 38.8, 34.1, 31.4. HRESIMS m/z 340.1285 [M+Na]+ (calcd for C16H19N3NaO4+, 340.1273) (100%). Anal. Calcd for C16H19N3O4.HCl: C, 54.32; H, 5.70; Cl, 10.02; N, 11.88. Found: C, 54.41; H, 5.67; Cl, 10.11; N, 11.79.
(2R, 3S)-3-[3-(3-Hydroxy-pyrrolidin-2-yl)-2-oxo-propyl]-8-methoxy-3H-quinazolin-4-one (22)
Off- white crystal. MP: 184–185°C. [α]25D −15.6 (c 0.5, EtOH). 1H NMR (CD3OD): 8.18 (s, 1H), 7.55 (t, J = 7.3 Hz, 1H), 7.36 (d, J = 7.3 Hz, 1H), 7.19 (d, J = 7.3 Hz, 1H), 5.09 (d, J = 17.5 Hz, 1H), 5.01 (d, J = 17.5 Hz, 1H), 4.43 (m, 1H), 4.08 (m, 1H), 3.77 (s, 3H), 3.51-3.44 (m, 2H), 2.81 (dd, J = 16.1, 4.3 Hz,, 1H), 2.75 (dd, J = 16.1, 6.0 Hz, 1H), 2.27 (m, 1H), 1.92 (m, 1H); 13C NMR (CD3OD): 203.1, 166.2, 162.3, 160.5, 147.3, 137.4, 132.9, 129.9, 128.7, 80.1, 58.9, 57.3, 56.9, 39.7, 34.8, 30.8. HRESIMS m/z 340.1266 [M+Na]+ (calcd for C16H19N3NaO4+, 340.1273) (100%). Anal. Calcd for C16H19N3O4.HCl: C, 54.32; H, 5.70; Cl, 10.02; N, 11.88. Found: C, 54.25; H, 5.74; Cl, 9.98; N, 11.96.
Parasite culture
Chloroquine-resistant W2-strain of Plasmodium falciparum were cultivated in RPMI 1640 media with 6% human erythrocytes supplemented with 10% of human serum. The parasites were cultured in an atmosphere of 5% CO2, 5% O2 and 90% N2 at 37°C.
In vitro drug susceptibility assay.23, 24
Febrifugine and analogs were tested in a cell-based in vitro drug susceptibility assay to determine if they were capable of interrupting Plasmodium metabolism and growth. The semi-automated micro-dilution technique was used to assess the sensitivity of the parasites to the selected compounds. The incorporation of [3H]hypoxanthine into the parasites was measured as a function of compound concentration to determine IC50 values.
In vivo efficacy test.25
In conducting the research described in this report, the investigators adhered to the Guide for the Care and Use of Laboratory Animals by the Institute of Laboratory Animal Resources, National Research Council. Typically, 4–5 weeks old IRC mice were housed in plastic cages with free access to water and food. P. burghei parasite-infected erythrocytes were obtained from donor mice. On experiment day 0, the donor mice were anesthetized and exsanguinated via cardiac puncture. The pooled blood from the donor mice was then diluted with normal mouse serum to a concentration of 1 × 106 P. berghei-infected erythrocytes per inoculum (0.1 ml). The groups of experimental and control mice were inoculated with this parasitized blood on day 0. The tested mice were treated with either candidate antimalarial drugs or with vehicle alone to serve as negative controls from day 3 to day 10 orally (PO) or subcutaneously (SC) once a day for eight days. Each experimental group received a different dose level, with up to 5 different dose groups per compound. Blood films and body weights were taken on the third and sixth days post-infection, then at weekly intervals through day 60. Films were Giemsa stained and examined microscopically to determine parasitemia. All mice were observed twice a day to assess their clinical signs that were recorded. All treated mice with negative smear on day 60 were considered cured.
Acknowledgments
This research was partially supported by a grant from the National Institutes of Health (R43 AI077109 to S.Z.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.WHO. The World Health Report. 2004 [Google Scholar]
- 2.Winstanley PA. Chemotherapy for Falciparum Malaria: The Armoury, the Problems and the Prospects. Parasitology Today. 2000;16:146–153. doi: 10.1016/s0169-4758(99)01622-1. [DOI] [PubMed] [Google Scholar]
- 3.Oduola AM, Milhous WK, Salako LA, Walker O, Desjardins RE. Reduced In Vitro Susceptibility to Mefloquine in West African Isolates of Plasmodium falciparum. Lancet. 1987;2:1304–1305. doi: 10.1016/s0140-6736(87)91195-0. [DOI] [PubMed] [Google Scholar]
- 4.Koepfly JB, Mead JF, Brockman JA., Jr An Alkaloid with High Antimalarial Activity from Dichroa febrifuga. J Am Chem Soc. 1947;69:1837. doi: 10.1021/ja01199a513. [DOI] [PubMed] [Google Scholar]
- 5.Koepfly JB, Mead JF, Brockman JA., Jr Alkaloids of Dichroa febrifuga. I. Isolation and Degradative Studies. J Am Chem Soc. 1949;71:1048–1054. doi: 10.1021/ja01171a080. [DOI] [PubMed] [Google Scholar]
- 6.WHO Report; Meeting on Antimalarial Drug Development; Shanghai, China. 16–17 November 2001. [Google Scholar]
- 7.Ablondi F, Gordon S, Morton J, II, Williams JH. An Antimalarial Alkaloid from Hydrangea. II. Isolation. J Org Chem. 1952;17:14–18. [Google Scholar]
- 8.Hewitt RI, Wallace WS, Gill ER, Williams JH. An Antimalarial Alkaloid from Hydrangea. Am J Trop Med Hyg. 1952;1:768–772. [PubMed] [Google Scholar]
- 9.Kato M, Inaba H, Itahana H, Ohara E, Nakamura K, Uesato S, Inouye H, Fujita T. Studies on Anticoccidial Constituents of Crude Drugs and Related Plants. (I). Isolation and Biological Activities of cis- and trans-Febrifugine from Hydrangea. Macrophylla Shoyakugaku Zasshi. 1990;44:288–292. [Google Scholar]
- 10.Taniguchi T, Ogasawara K. A Diastereocontrolled Synthesis of (+)-Febrifugine: A Potent Antimalarial Piperidine Alkaloid. Org Lett. 2000;2:3193–3195. doi: 10.1021/ol006384f. [DOI] [PubMed] [Google Scholar]
- 11.Takaya Y, Tasaka H, Chiba T, Uwai K, Tanitsu M, Kim HS, Wataya Y, Miura M, Takeshita M, Oshima Y. New Type of Febrifugine Analogues, Bearing a Quinolizidine Moiety, Show Potent Antimalarial Activity against Plasmodium Malaria Parasite. J Med Chem. 1999;42:3163–3166. doi: 10.1021/jm990131e. [DOI] [PubMed] [Google Scholar]
- 12.Fishman M, Cruickshank PA. Febrifugine Antimalarial Agents. I. Pyridine Analogs of Febrifugine. J Med Chem. 1970;13:155–156. doi: 10.1021/jm00295a050. [DOI] [PubMed] [Google Scholar]
- 13.Chien PL, Cheng CC. Structural Modification of Febrifugine. Some Methylenedioxy analogs. J Med Chem. 1970;13:867–870. doi: 10.1021/jm00299a018. [DOI] [PubMed] [Google Scholar]
- 14.Hirai S, Kikuchi H, Kim HS, Begum K, Wataya Y, Tasaka H, Miyazawa Y, Yamamoto K, Oshima Y. Metabolites of Febrifugine and Its Synthetic Analogue by Mouse Liver S9 and Their Antimalarial Activity against Plasmodium Malaria Parasite. J Med Chem. 2003;46:4351–4359. doi: 10.1021/jm0302086. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi Y, Koike M, Azuma K, Nishioka H, Abe H, Kim HS, Wataya Y, Harayama T. Synthesis and Antimalarial Activity of Febrifugine Derivatives. Chem Pharm Bull. 2001;49:721–725. doi: 10.1248/cpb.49.721. [DOI] [PubMed] [Google Scholar]
- 16.Zhu S, Meng L, Zhang Q, Wei L. Synthesis and Evaluation of Febrifugine Analogues As Potential Antimalarial Agents. Bioorg Med Chem Lett. 2006;16:1854–1858. doi: 10.1016/j.bmcl.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guroff G, Daly JW, Jerina DM, Renson J, Witkop B, Udenfriend S. Hydroxylation-Induced Migration: the NIH shift. Science. 1967;157:1524–1530. doi: 10.1126/science.157.3796.1524. [DOI] [PubMed] [Google Scholar]
- 18.It should be noted that alkene 25 was also available from tartaric acid in 17 steps: Yoda H, Egawa T, Takabe K. First Total Synthesis of A New Pyrrolizidine Alkaloid, Amphorogynine A. Tetrahedron Lett. 2003;44:1643–1646.
- 19.Ley SV, Norman J, Griffith WP, Marsden SP. Tetrapropylammonium Perruthenate, Pr4N+RuO4−, TPAP: Catalytic Oxidant For Organic Synthesis. Synthesis. 1994:639–666. [Google Scholar]
- 20.Williams EJ, Kenny PW, Kettle JG, Mwashimba PG. Synthesis of a 5- Alkoxypyrido[4,3-d]pyrimidin-4(3H)-one Derivative via a Regioselective Meisenheimer N-oxide Rearrangement. Tetrahedron Lett. 2004;45:3737–3739. [Google Scholar]
- 21.Alexandre FR, Berecibar A, Besson T. Microwave-assisted Niementowski Reaction. Back to the Roots. Tetrahedron Lett. 2002;43:3911–3913. [Google Scholar]
- 22.Armarego WLF, Smith JIC. Quinazolines. Part IX. Covalent Hydration in the Neutral Species of Substituted Quinazolines. J Chem Soc B. 1967:449–454. [Google Scholar]
- 23.Desjardins RE, Canfield CJ, Haynes DE, Chulay JD. Quantitative Assessment of Antimalarial Activity In Vitro by a Semiautomated Microdilution Technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chulay JD, Haynes JD, Diggs CL. Plasmodium falciparum: Assessment of In Vitro Growth by [3H]hypoxanthine Incorporation. Exp. Parasitol. 1983;55:138–146. doi: 10.1016/0014-4894(83)90007-3. [DOI] [PubMed] [Google Scholar]
- 25.Jiang S, Zeng Q, Gettayacamin M, Tungtaeng A, Wannaying S, Lim A, Hansukjariya P, Okunji CO, Zhu S, Fang D. Antimicrob. Agents. Chemother. 2005;49:1169–1176. doi: 10.1128/AAC.49.3.1169-1176.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]