Abstract
Muscle fatigue is associated with a number of clinical diseases, including chronic pain conditions. Decreases in extracellular pH activates acid-sensing ion channel 3 (ASIC3), depolarizes muscle, protects against fatigue, and produces pain. We examined whether ASIC3-/- mice were more fatigable than ASIC3+/+ mice in a task-dependent manner. We developed two exercise protocols to measure exercise-induced muscle fatigue: ( fatigue task 1, three 1-h runs; fatigue task 2, three 30-min runs). In fatigue task 1, male ASIC3+/+ mice muscle showed less fatigue than male ASIC3-/- mice and female ASIC3+/+ mice. No differences in fatigue were observed in fatigue task 2. We then tested whether the development of muscle fatigue was dependent on sex and modulated by testosterone. Female ASIC3+/+ mice that were ovariectomized and administered testosterone developed less muscle fatigue than female ASIC3+/+ mice and behaved similarly to male ASIC3+/+ mice. However, testosterone was unable to rescue the muscle fatigue responses in ovariectomized ASIC3-/- mice. Plasma levels of testosterone from male ASIC3-/- mice were significantly lower than in male ASIC3+/+ mice and were similar to female ASIC3+/+ mice. Muscle fiber types, measured by counting ATPase-stained whole muscle sections, were similar in calf muscles from male and female ASIC3+/+ mice. These data suggest that both ASIC3 and testosterone are necessary to protect against muscle fatigue in a task-dependent manner. Also, differences in expression of ASIC3 and the development of exercise-induced fatigue could explain the female predominance in clinical syndromes of pain that include muscle fatigue.
Keywords: proton, exercise, pain, gender, sex, testosterone, estrogen
FATIGUE IS ASSOCIATED WITH a number of clinical diseases, including chronic pain conditions, such as chronic fatigue syndrome and fibromyalgia (4, 13, 36, 37, 39, 64, 67, 70, 71, 73). In these chronic conditions, fatigue is generally described as a whole body feeling of muscle fatigue and loss of energy. Fatigue, experimentally, is defined as a temporary decrease in muscle force in response to exercise (19). Patients with chronic fatigue syndrome or fibromyalgia exhibit an enhanced fatiguing response to exercise (57) as well as muscle weakness and decreased endurance (33, 34, 69), suggesting potential interactions between fatigue and pain. Many peripheral factors affect muscle fatigue including differences in local metabolic changes, sex, and muscle fiber types, and these factors may interact to either enhance or protect against fatigue (19).
Changes in the extracellular environment directly affect muscle fatigability. Factors associated with pain that also affect fatigability of skeletal muscle are increases in extracellular K+, and decreases in pH due to metabolic by-products and lactic acid accumulation (19). Increases in extracellular K+ decreases muscle membrane excitability, resulting in decreased force production (58). Extracellular pH decreases in muscle tissue in response to high-intensity short-duration exercise, in several pain conditions (19, 29, 32, 50, 52, 52, 54) and causes hyperalgesia in both humans and animals (28, 32, 60). Although decreases in extracellular pH have typically been thought to enhance fatigue, recent work suggests that these decreases may protect against fatigue (14). When the extracellular pH is decreased, muscle cells depolarize and prevent the movement of Na+ out of the cell through the Na+/K+ pump, resulting in the enhancement of membrane excitability (23). Interestingly, lactic acid and the consequent decreases in pH restores the reduced amplitude of the muscle action potential (45), and muscle force production is reduced by accumulation of extracellular K+ (14). Furthermore, decreases in pH activate acid-sensing ion channels (ASICs) (68). The activity of ASIC3, which has been found on sensory neurons innervating muscle (41, 61), increases the presence of lactic acid (44). More recently, mRNA for ASIC3 and ASIC1a were detected in whole muscle tissue with the greater expression observed for ASIC3 (23).
Gender, sex hormones, and muscle fiber types can influence the fatigability of muscle. In human subjects, males have greater overall strength, but can fatigue to a greater extent than females in a task-dependent manner (22, 27, 31). When males and females are matched for strength, women fatigued less than men during intermittent contractions but performed similarly during sustained low-intensity contractions as males (27). In contrast, several studies show an increase in muscle strength following testosterone replacement therapy in men with low levels of testosterone (5, 6, 18), and testosterone enhances force and resistance to fatigue in normal males (2, 65). In parallel, androgen receptors are located on muscle fibers (1, 42) and long-term treatment of orchidectomized male mice with testosterone alters the neuromuscular junction to enhance synaptic efficacy (43). The effect of fatigue after testosterone treatment is muscle type-specific being observed in the soleus muscle Type I fibers but not in the extensor digitorum longus muscle Type II fibers (2). This suggests that gender differences in fatigability could be related to effects of testosterone on fiber types. Reports on the existence of differences in muscle fiber types between genders is conflicting with some studies showing no differences (20, 63) and others showing females with a greater portion of Type I fibers than males (59). Due to the discrepancy in the literature, this study examined whether differences in muscle fiber types between male and female mice could account for fatigue differences between genders.
The purpose of this study was to assess the influence of sex and the deficiency of ASIC3 in the development of exercise-induced muscle fatigue. We tested the hypothesis that ASIC3-/- mice would be more fatigable than ASIC3+/+ mice in a sex- and task-dependent manner. This study is the first to suggest that both ASIC3 and testosterone are important in the protection against the development of muscle fatigue.
MATERIALS AND METHODS
Mice
All experiments were approved by the University of Iowa Animal Care and Use Committee and were conducted in accordance with National Institutes of Health guidelines. Congenic ASIC3-/- and ASIC3+/+ mice on a C57BL/6J background (51) were bred at the University of Iowa Animal Care Facility. Both male (n = 27) and female (n = 50) mice, 4 – 8 mo of age, were used in these studies. The female mice were divided into five groups: 1) ASIC3+/+ untreated mice (n = 12), 2) ASIC3+/+ mice that were ovariectomized (n = 10), 3) ASIC3+/+ that were ovariectomized and treated with testosterone (n = 16), 4) ASIC3-/- untreated mice (n = 12), and 5) ASIC3-/- mice that were ovariectomized and treated with testosterone (n = 8). Male mice were divided into two groups: ASIC3+/+ untreated mice (n = 12) and ASIC3-/- untreated mice (n = 15). For the plasma testosterone measurements, blood samples were taken from male ASIC3+/+ (n = 8) and male ASIC3-/- (n = 9) mice at 8 wk of age. For comparison, we also measured testosterone from age-matched female ASIC3+/+ (n = 3) mice.
Behavior Experiments with Rota-Rod and Grip Force
Acclimation training
Mice were trained for 2 days on the Rota-Rod (IITC Life Science, Woodland Hills, CA) and the Grip Strength System (San Diego Instruments, San Diego, CA) for acclimation purposes. The day 1 training regimen consisted of having the mice perform the grip-force test five times each on both the front and back paws. Following grip-force measurement, mice were placed on the Rota-Rod and run at speeds of 13 rpm for 15 min. They were replaced on the Rota-Rod if they fell off. After 30 min of rest, the mice were replaced on the Rota-Rod and run at speeds ramping from 13–20 rpm for 15 min. Mice were then tested five times on both paws on the grip-force gauge. The day 2 training regimen was similar to the day 1 protocol, but the first run speed was from 13–20 rpm for 15 min and the second run was ramping from 13–20 rpm in 180 s for 15 min.
Fatigue task 1
The first muscle fatigue task had mice run three times for 1 h on each run, with a 15-min rest after run 1 and a 10-min rest after run 2. Mice were placed on the Rota-Rod (IITC Life Science) and were run at speeds ramping from 13–20 rpm in 180 s, and maintained at 20 rpm for a total duration of 1-h run time. Mice were allowed to rest for 15 min and then were placed on the Rota-Rod and run at speeds ramping from 13–20 rpm in 180 s and maintained at 20 rpm for a total duration of 1 h. After 10-min rest, mice were placed on the Rota-Rod for the final run at speeds ramping from 13–21 rpm in 180 s and maintained at 21 rpm for a total duration of 1 h. If a mouse fell off four times within 60 s of each fall, the mouse was termed fatigued and the task stopped. The number of times the mouse fell off the Rota-Rod during the task and the time to task failure was recorded for each mouse. The time to task failure was recorded only for the time the animal spent running and thus for fatigue task 1 there was a maximal possible running time of 10,800 s.
Fatigue task 2
The second fatigue task was the same as fatigue task 1 (see above) except that the tests were terminated after 30 min instead of 1 h. For fatigue task 2 there was a maximal possible running time of 5,400 s. Separate groups of animals were used in fatigue task 1 and fatigue task 2 for each condition tested.
Grip-force protocol
In the same animals, fatigue was tested by measuring grip force. Fatigue was confirmed by measurement of grip force immediately after the fatigue task by comparing to the grip force prior to the fatigue task for both the hindlimbs and the forelimbs. Mice were trained 2 days prior to data collection to the grip-force device. Mice were pulled by the tail to read grip force on the forelimb and then pulled again by the tail to read grip force on the hindlimb. Grip force was measured before, immediately, and 1 h and 2 h after the third run. Grip force was analyzed on both the forelimbs and hindlimbs, and an average of five trials were recorded. Each mouse had forelimb and hindlimb grip force measured before moving on to the next mouse. A decrease in grip force after running was interpreted as muscle fatigue.
Surgery
Ovariectomized ASIC3+/+ mice
Female mice were ovariectomized according to previously published procedures (55). Mice were anesthetized with isoflurane (3%), a small incision was made in the abdomen, and the uterine horns were lifted out of the body cavity. The ovaries were snipped off of the uterine horns along with attached connective tissue. The incision was closed by suturing with 5-0 silk. The acclimation training and testing of the mice was performed 1 wk after surgery.
Ovariectomized mice and testosterone
Mice were ovariectomized as described above. The training and testing of the mice were performed 1 wk after surgery. Before testing on the third day, the ovariectomized mice were given testosterone (30 mg/kg sc; Sigma, St. Louis MO) based on previous literature (21). Testing and data collection were done 1 h after the testosterone injection.
Protocols for Fatigue Tasks
ASIC3-/- and ASIC3+/+ mice
The first test examined the difference in fatigue between ASIC3-/- and ASIC3+/+ mice in separate groups of animals as follows: ASIC3+/+ males (n = 6/task), ASIC3+/+ females (n = 6/task), ASIC3-/- males (n = 6 task 1; n = 9 task 2), and ASIC3-/- females (n = 6/task). Grip force was measured before, immediately, and 1 h and 2 h after the fatigue task.
Ovariectomized mice
Since there was a difference in fatigue to fatigue task 1 (1-h fatigue task) between male and female ASIC3+/+ mice, we tested female mice that were ovariectomized to remove the female hormones. One week after ovariectomy, ASIC3+/+ female mice performed either fatigue task 1 (n = 6) or fatigue task 2 (n = 4). Grip force was measured before, immediately, and 1 h and 2 h after the fatigue task.
Ovariectomized ASIC3+/+ mice with testosterone
Since differences in fatigue to fatigue task 1 (1-h fatigue task) still existed between male and female mice after ovariectomy, we tested the effects of testosterone supplementation in ovariectomized female mice. One week after ovariectomy, female ASIC3+/+ mice were given a single dose of testosterone (30 mg/kg sc) 1 h before the task (21). Mice then performed either fatigue task 1 (n = 8) or fatigue task 2 (n = 8). For comparison, ASIC3-/- mice were ovariectomized and given a single dose of testosterone (30 mg/kg sc) 1 h before the fatigue task 1 (n = 8). Grip force was measured before and after each run, and 1 h and 2 h after the fatigue task.
Measurement of Testosterone
Mouse blood was collected into heparinized hematocrit tubes by retroorbital puncture of mice anesthetized with isoflurane (Hospira, Lake Forest, IL). Plasma was separated from blood by centrifugation at 800 g for 15 min at room temperature. Testosterone concentrations of plasma samples, each tested in quadruplicate, were determined with an ELISA kit (Neogen, Lexington, KY). Prior to testing, plasma samples were extracted with ethyl ether and prepared according to the manufacturer’s instructions.
ATPase Staining
The gastrocnemius, soleus, and plantaris muscles were collected from each of three male and three female C57BL/6J mice. Before dissection, each mouse was deeply anesthetized with pentobarbital sodium (150 mg/kg ip), decapitated, and excess blood drained. A thin piece of cork was placed beneath the intact muscle, and pins were placed through the tendons at both ends of the muscle through the cork before excising to keep the muscle from contracting. Optimal cutting temperature embedding media for frozen tissues, (Tissue-Tek, Torrance, CA) was placed between the muscle and the cork. The embedded muscle preparation was placed into a liquid nitrogen/isopentane bath for 30 s and then held in liquid nitrogen until freezing was complete. After being frozen, the muscles were stored at -80°C until cryosectioning within the next 24 to 48 h. The gastrocnemius/soleus/plantaris muscle group was serially sectioned at 16-μm thickness in the transverse plane on a cryostat and placed on slides. This ensured that the muscle could be sampled from the top, middle, and lower portions of the muscle group. Sections were stored at -80°C and stained for ATPase within 1 wk.
Sections of muscle tissue from ASIC3+/+ male and female mice were stained with the 4.3 and 4.6 ATPase staining method according to procedures posted by the Department of Pathology at Washington University (http://www.neuro.wustl.edu/neuromuscular/pathol/histol/atp.htm) with the following adjustments, clarifications, and substitutions. Preincubation solutions were adjusted to pH 4.5 for 4.6 stains, and pH 4.2 for the 4.3 stains with 1 N hydrochloric acid, upon the protocol’s suggestion when staining nonhuman tissue. To avoid calcium precipitation, the incubating solution was always made by adding reagents in the following order 1) barbital sodium, 2) water (allowing room for pH adjustment), 3) ATP powder, and 4) calcium chloride. Preincubation times of 5 min and incubation times of 25 min were strictly adhered to. Muscle fibers that stained darkest were identified as Type I in both the 4.3 and 4.6 staining procedures. Type IIa and Type IIb fibers stain lightly, and the Type IIc stain intermediate with the 4.3 staining procedure. With the 4.6 stain, Type IIa stains light and Type IIb and Type IIc stain intermediate.
Images of the stained muscle sections were taken on an Olympus BX-51 light microscope equipped with a DP70 camera interfaced to DP Controller (version 3.2.1.276) and DP Manager (version 3,1,1,208) digital image capture. For resolution sufficient for counting fibers, images were taken with a ×20 objective lens. Three sections per muscle per stain were digitally imaged. Montages of up to 83 captured images for each muscle section were printed and manually spliced together for counting. For each mouse [males (n = 3), females (n = 3)], the fiber types of three transverse sections were counted for each of the two ATPase stains. The three sections counted from each mouse represented the rostral portion, the middle, and the caudal portion of the muscle group. Each entire section was counted and included between 8194 and 17,848 muscle fibers. The percentages of Type I, Type IIa + Type IIb combined, and Type IIc were determined from the 4.3 ATPase stain. With the 4.6 ATPase stain, the percentages of Type I, Type IIa, and Type IIb + Type IIc combined were found. By pooling the data from the two stains, a percentage of Type IIb fibers were determined by subtracting the Type IIa percentage determined from the 4.6 stain from the combined Type IIa + IIb percentage calculated from the 4.3 stain.
Statistical Analysis
Statistical calculations were performed with SPSS 16.0 software. Repeated-measures ANOVA was used to compare for differences between groups and across time for the fatigue data. The number of falls and time to task failure was analyzed with a univariate ANOVA for fatigue task, genotype, sex, and testosterone administration. Post hoc testing compared differences between individual groups. All of the statistical results were expressed as means ± SE. Differences were considered to be significant when P ≤ 0.05.
RESULTS
Baseline Grip-Force Measurements
Grip force before the fatigue tasks is shown in Table 1. There were no differences between male and female ASIC3+/+ or ASIC3-/- mice for either the hindpaw or the forepaw. Ovariectomizing mice increased grip force for the forepaw but not the hindpaw, while administration of testosterone in ovariectomized ASIC3+/+ and ASIC3-/- mice significantly increased the grip force for both the hindpaw and forepaw compared with untreated ASIC3+/+ and ASIC3-/- mice (P < 0.05).
Table 1.
Baseline grip force measures
| ASIC3+/+ |
ASIC3-/- |
OVX |
OVX + T, ASIC3+/+ |
OVX + T, ASIC3-/- |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Forepaw | Hindpaw | Forepaw | Hindpaw | Forepaw | Hindpaw | Forepaw | Hindpaw | Forepaw | Hindpaw | |
| Male | 64.3±2.5 | 63.0±1.5 | 64.2±0.84 | 62.2±1.3 | NA | NA | NA | NA | NA | NA |
| Female | 63.4±2.4 | 61.0±1.7 | 64.4±2.2 | 60.0±1.7 | 81.8±3.1* | 65.1±1.3 | 74.5±1.4* | 72.6±1.7* | 87.2±0.61* | 68.6±1.1* |
Values are means ± SE in grams. OVX, ovariectomized; OVX + T, OVX + testosterone; NA, not applicable.
P < 0.05, significantly different than acid-sensing ion channel (ASIC) ASIC3 +/+ , or ASIC3-/-.
Fatigue Responses in ASIC3+/+ Mice and ASIC3-/- Mice
The number of times the mouse fell off of the Rota-Rod was significantly different between ASIC3-/- mice and ASIC3+/+ mice (P = 0.003) (Table 2). The ASIC3-/- mice (23.9 ± 1.6 falls) fell a greater number of times than the ASIC3+/+ mice (17.6 ± 1.4 falls). The time to task failure was greater for fatigue task 1 (8,918 ± 176 s; complete duration 10,800 s) compared with fatigue task 2 (4,939 ± 110 s; complete duration 5,400 s) (P = 0.0001) and for ovariectomized mice that received testosterone (8,330 ± 456 s) compared with ovariectomized mice that did not receive testosterone (6,606 ± 256 s) (P = 0.0001) (Table 3). However, there was no difference in performance between male and female mice for either fall times or for time to task failure, suggesting that male and female mice, regardless of genotype or treatment, performed each task similarly.
Table 2.
Performance on fatigue task
| ASIC3+/+ |
ASIC3-/- |
OVX |
OVX + T, ASIC3+/+ |
OVX + T, ASIC3-/- |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Task 1 | Task 2 | Task 1 | Task 2 | Task 1 | Task 2 | Task 1 | Task 2 | Task 1 | Task 2 | |
| Male | 13.3±1.5 | 12.7±1.8 | 26±1.6 | 14±2.1 | NA | NA | NA | NA | NA | NA |
| Female | 22±2.9 | 17.8±3.3 | 30±4.6 | 17.8±2.1 | 16.7±1.3 | 5.5±1.7 | 23.8±6 | 27.8±4.7 | 30±5.3 | NA |
Values are means ± SE of number of falls from the Rota-Rod trainer.
Table 3.
Performance on fatigue task
| ASIC3+/+ |
ASIC3-/- |
OVX |
OVX + T, ASIC +/+ |
OVX + T, ASIC3-/- |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Task 1 | Task 2 | Task 1 | Task 2 | Task 1 | Task 2 | Task 1 | Task 2 | Task 1 | Task 2 | |
| Male | 8423±204 | 4915±222 | 8724±176 | 4379±195 | NA | NA | NA | NA | NA | NA |
| Female | 8211±178 | 5324±398 | 7901±211 | 4742±235 | 9445±622 | 5032±368 | 9789±309 | 5400±0 | 10,800±0 | NA |
Values are means ± SE of time to task failure in seconds. Maximal duration for fatigue task 1 is 10,800 s and for fatigue task 2 is 5400 s.
Fatigue task 1
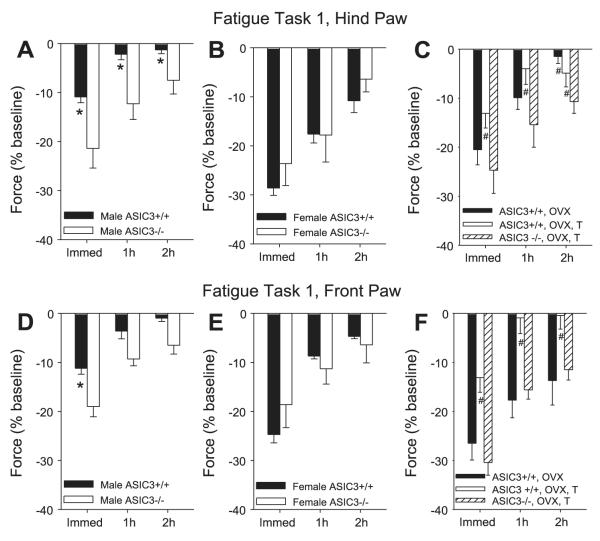
Analysis of the grip-force data shows there was a significant difference for sex (hindpaw, P = 0.003; forepaw, P = 0.006), for genotype (hindpaw, P = 0.01), and an interaction between sex and genotype (hindpaw, P = 0.001; forepaw, P = 0.002). Male ASIC3+/+ mice showed significantly less fatigue than female ASIC3+/+ mice (hindpaw, P = 0.0001; forepaw, P = 0.01), and male ASIC3-/- mice (hindpaw, P = 0.001; forepaw, P = 0.001). However, the grip force immediately after the fatigue task was similar between the female ASIC3-/- and the female ASIC3+/+ mice for the hindpaw and the forepaw. The male ASIC3-/- mice showed similar reductions in grip force as the female ASIC3+/+ and ASIC3-/- mice (Fig. 1). Recovery from fatigue was also significantly slower in the hindpaw in the female ASIC3+/+ compared with the male ASIC3+/+ (1 h, P = 0.003; 2 h, P = 0.02), in the male ASIC3-/- mice compared with the male ASIC3+/+ (1 h, P = 0.008), and in the female ASIC3-/- mice compared with the male ASIC3+/+ mice (P = 0.001). There were no differences in recovery from fatigue for the forepaw.
Fig. 1.
%Change in grip force from baseline (set at 0%) immediately (Immed) and 1 h and 2 h after fatigue task 1, for the hindpaw (A—C) and the forepaw (D—F). A: change in hindpaw grip force in male acid-sensing ion channel (ASIC) ASIC3+/+ and ASIC3-/- mice after the fatigue task. *Significantly different between ASIC3+/+ and ASIC3-/- mice. B: change in hindpaw grip force in female ASIC3+/+ and ASIC3-/- mice after the fatigue task. C: change in hindpaw grip force in ASIC3+/+ and ASIC3-/- mice after various manipulations. Female mice were either ovariectomized (OVX) or ovariectomized and administered testosterone systemically (OVX, T). #Significantly less than female ASIC3+/+ mice. D: change in forepaw grip force in male ASIC3+/+ and ASIC3-/- mice after the fatigue task. *Significantly different between ASIC3+/+ and ASIC3-/- mice. E: change in forepaw grip force in female ASIC3+/+ and ASIC3-/- mice after the fatigue task. F: change in forepaw grip force in ASIC3+/+ and ASIC3-/- mice after various manipulations. Female mice were either ovariectomized or ovariectomized and administered testosterone systemically. #Significantly less than female ASIC3+/+ mice.
Fatigue task 2
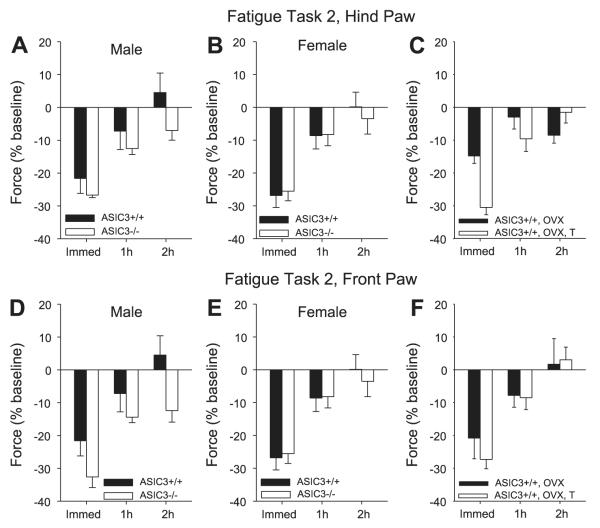
Analysis of the grip-force data for the hindpaw and forepaw (Fig. 2) shows there were no significant differences for sex or for genotype. Immediately after the fatigue task, male ASIC3+/+ mice showed a similar reduction in grip force as the female ASIC3+/+ mice. Both female and male ASIC3-/- showed a similar reduction in force as female and male ASIC3+/+ mice immediately after the fatigue task. Recovery from fatigue was similar between male and female ASIC3+/+ and ASIC3-/-.
Fig. 2.
%Change in grip force from baseline (set at 0%) immediately, 1 h, and 2 h after fatigue task 2 for the hindpaw (A—C) and the forepaw (D—F). A: change in hindpaw grip force in male ASIC3+/+ and ASIC3-/- mice after the fatigue task. B: change in hindpaw grip force in female ASIC3+/+ and ASIC3-/- mice after the fatigue task. C: change in hindpaw grip force in ASIC3+/+ mice after various manipulations. Female mice were either ovariectomized or ovariectomized and administered testosterone systemically. D: change in forepaw grip force in male ASIC3+/+ and ASIC3-/- mice after the fatigue task. E: change in forepaw grip force in female ASIC3+/+ and ASIC3-/- mice after the fatigue task. F: change in forepaw grip force in ASIC3+/+ mice after various manipulations. Female mice were either ovariectomized or ovariectomized and administered testosterone systemically.
Effects of Ovariectomy and Testosterone in ASIC3+/+ Mice
Fatigue task 1
The grip-force reduction was significantly greater in female mice compared with male mice (hindpaw, P = 0.0001; forepaw, P = 0.01) (Fig. 1). Adding testosterone (30 mg/kg ip) to the ovariectomized mice prevented the reduction in grip force to female ASIC3+/+ mice (hindpaw, P = 0.0001; forepaw, P = 0.006) and was similar to the male ASIC3+/+ mice. However, adding testosterone to ovariectomized ASIC3-/- mice had no effect on the fatigue response compared with ovariectomized ASIC3+/+ mice (hindpaw and forepaw, P = 0.001) (Fig. 1). Recovery from fatigue was also significantly slower in the ovariectomized mice (hindpaw, 1 h P = 0.05; 2h P = 0.01; forepaw, 1 h P = 0.003; 2 h P = 0.03), and in the ovariectomized mice with testosterone (hindpaw, 1 h and 2 h P = 0.0001; forepaw, 1 h P = 0.001 and 2 h P = 0.01) (Fig. 1).
Fatigue task 2
Analysis of the grip-force data for the hindpaws and forepaws shows that there were no significant differences between groups, i.e., males, females, ovariectomized, and ovariectomized with testosterone (Fig. 2). All groups showed similar reductions in grip force and recovery.
Plasma Testosterone Concentration in ASIC3+/+ and ASIC3-/- Mice
The concentration of testosterone in the blood from male ASIC3-/- mice was significantly less than that of male ASIC3+/+ mice (P = 0.018). Testosterone in the ASIC3+/+ mice averaged 1.2 ± 0.4 ng/ml and that for ASIC3-/- mice was 0.22 ± 0.1 ng/ml. For comparison, testosterone measured in an additional three female ASIC3+/+ mice averaged 0.12 ± 0.07 ng/ml and was similar to the male ASIC3-/- mice.
Muscle Fiber Type in Male and Female ASIC3+/+ Mice
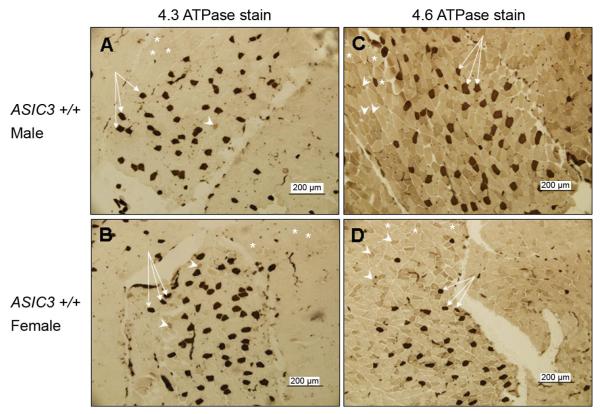
Figure 3 shows an example of a stain from ASIC3+/+ males and females stained with the 4.3 and with the 4.6 ATPase protocol. The distribution of fiber types across a given muscle was not uniform when examining cross sections through the muscle, and thus, we sampled and counted the entire muscle. We counted a total of 10,458 ± 1,360 muscle cells in each female for the 4.6 ATPase stain, 12,413 ± 1,725 in each female for the 4.3 stain, 14,570 ± 900 in each male for the 4.6 ATPase stain, and 14,706 ± 1,396 in each male for the 4.3 ATPase stain. Analysis of the fiber types in both females and males showed no difference in fiber types between males and females. The percentage of fiber types were as followed: Type I male, 3.0% ± 1.2 vs. Type I female, 2.4% ± 0.36; Type IIa male, 86.2% ± 1.6 vs. Type IIa female, 86.5% ± 1.2; Type IIb male, 9.8% ± 1.3 vs. Type IIb female, 9.1% ± 1.2; Type IIc male, 1.5% ± 0.32 vs. Type IIc female, 1.4% ± 0.
Fig. 3.
Fiber type staining in the gastrocnemius of male and female ASIC3+/+ mice. The images represent a portion of a cross section through the gastrocnemius muscle stained with one of two ATPase staining protocols, i.e., 4.3 or 4.6. In the 4.3 ATPase staining, (A and B), Type I fibers stain dark (arrows), Type IIc fibers stain intermediate (arrowheads), and Type IIa and Type IIb fibers stain light (*). In the 4.6 ATPase staining (C and D), Type I fibers stain dark (arrows), Type IIb and Type IIc fibers stain intermediate (arrowheads), and Type IIa fibers stain light (asterisks). Counting of a large sample set of ATPase-stained sections, revealed no significant gender-based differences in the percentages of fiber types.
DISCUSSION
Fatigue Tasks
The current study showed that we could produce exercise-induced fatigue, defined as a decrease in muscle force, with two different fatigue tasks. These tasks resulted in different fatigue levels among male ASIC3+/+ mice with less fatigue for the long-duration task (3 1-h runs) compared with the shorter fatigue duration task (3 30-min runs). Although there were similar amounts of falls between the two tasks, the ASIC3-/- mice fell more often than the ASIC3+/+ mice. As expected, the total time the mice ran was longer for the longer duration task (fatigue task 1), and there were no differences in the time to develop fatigue between the ASIC3-/- mice and the ASIC3+/+ for each of the two tasks. Similar amounts of fatigue were found between female ASIC3+/+ mice in the two fatigue tasks. Interestingly, both male and female mice, regardless of genotype or treatment, performed similarly between the two tasks in terms of number of falls and the time to task failure. It is possible that with increasing fatigue the male mice are unable to perform the task as a result of a loss in motor coordination to a greater extent than the female mice. The Rota-Rod is commonly used to test coordination and motor performance in rodents at a gross level. To test motor performance, mice are run on a Rota-Rod for shorter durations (1–5 min) and at higher maximal speeds (up to 45 rpms) (7, 10, 35) than that used in the current study. Furthermore, in human subjects, fatigue affects coordination resulting in changes in firing patterns of muscles; however, there are no apparent gender differences in abilities to perform the exercise-related tasks (12, 17). Together, our data suggest that there was no difference in the ability to perform the fatigue tasks between male and female mice, regardless of genotype, despite different levels of fatigue. The long-duration fatigue tasks on the Rota-Rod used in the current study are uniquely different from previously published fatigue protocols in animals, which typically involve spontaneous wheel running (72), treadmill running (9, 49), or electrical stimulation of isolated muscles (2, 49).
The current study shows that ASIC3-/- mice fatigue more than ASIC3+/+ mice in males, but not females, in a task-dependent manner. The differences in fatigue in the male ASIC3-/- mice was only evident with the longer duration fatigue task, i.e., fatigue task 1, three 1-h runs. In both tasks the animals were able to complete the first two runs without reaching fatigue, but were generally not able to complete the third run. Thus, the animals in fatigue task 1 ran substantially longer than those in fatigue task 2. There was a greater fatigue observed in females than male mice in the longer duration fatigue task (fatigue task 1), which was surprising given prior data showing fatigability is greater in males than in female human subjects (22, 24, 27, 38, 66). However, in human studies, differences in the development of muscle fatigue between sexes are task dependent (31). Women that are strength matched to men could perform a greater number of intermittent contractions before they fatigued, but they perform similarly when asked to sustain a submaximal voluntary contraction (31).
In our animal study, the lack of differences in the shorter duration fatigue task ( fatigue task 2) suggests that fatigue and differences in fatigue are dependent on the duration of the task itself. Several clinical studies show differences in fatigue based on variations of the task, the intensity of exercise, and the muscle group involved (11, 24, 30). Specifically, a 2-h running task results in fatigue in the knee flexors and extensors but not the hip muscles and shows how different muscle groups respond differently to fatigue (24). Differences in fatigue could also relate to differences in fiber type. For example, Type I fibers are fatigue resistant, Type IIb fibers are fatigable, and Type IIa and Type IIc fibers have an intermediate fatigue response. This data would support that muscles with a large portion of Type I, Type IIa, and Type IIc fibers would fatigue less than those with predominately Type IIb fibers (11). However, the current study measuring grip force involves multiple muscle groups and suggests that the effect is not related to muscle fiber type or specific muscle groups. Furthermore, in the current study, examination of muscle fiber types in the calf muscles show there were no differences between male and female mice in the distribution of Type I, IIa, IIb, or IIc fibers.
Interactions Between ASIC3 and Testosterone
Our data show that plasma testosterone is reduced in male ASIC3-/- mice compared with male ASIC3+/+ mice and that exogenous administration of testosterone protects females from fatigue in ASIC3+/+ mice but not ASIC3-/- mice. This data therefore support the hypothesis that both ASIC3 and testosterone are required to protect from fatigue in the longer duration exercise regimen. It further suggests that testosterone production, and/or secretion, is influenced by ASIC3, most likely in the testes. The lack of effect of testosterone in ASIC3-/- mice suggests an interaction between the androgen receptor and ASIC3 in the muscle, either directly or indirectly. ASIC3 has been found in the testes (3), and testosterone regulates expression of other ion channels (i.e., calcium channels) in skeletal, cardiac, and smooth muscle (8, 25, 40, 47).
Fatigue is reduced in female mice that were ovariectomized and administered testosterone subcutaneously and is consistent with studies in male mice that show increased levels of testosterone increased muscle force and in some cases reduced muscle fatigue (2, 65). Furthermore, testosterone increases intracellular calcium in myocytes producing increases in phosphorylation and transcription factors through a G protein-linked receptor (16). In agreement, testosterone administration to myocytes decreases the action potential threshold and increases synaptic facilitation resulting in an enhancement of synaptic efficiency in the flexor carpi radialis muscle of the frog (43). Thus, testosterone can have relatively fast effects on the function of the myocyte that would affect the efficacy of synaptic transmission through phosphorylation of ion channels. Increases in transcription factors could enhance the production of ion channels or androgen receptors, which could also increase membrane excitability. Exercise alters androgen receptor expression in mice depending on muscle fiber type by increasing expression in Type II and decreasing expression in Type I fibers (15). We hypothesize that testosterone, through intracellular signaling pathways, increases efficacy of ASIC3 and/or increases production of ASIC3 to reduce fatigue, allowing myocytes to contract with a lower motor neuron output.
There are many mechanisms of muscle fatigue, both peripheral and central. Peripherally, extracellular changes in the microenvironment of ions (K+,H+,Na+) can enhance fatigue, or, in some cases, protect against fatigue (14, 19, 53). For example, increases in extracellular K+ that occur during exercise, would activate and depolarize muscle cells resulting in an inactivation of sodium channels and a reduction in force (53). We suggest that H+ ions play a critical role in fatigue and recovery from fatigue through activation of ASIC3 on muscle fibers. Excitability of muscle fibers is modified by the Na+-K+ cotransporter and depends on extracellular K+ concentration (46). Depolarization could inactivate voltage-sensitive sodium channels and produce fatigue (56, 58). Thus, exercise that increases extracellular K+ may depolarize muscle cells resulting in inactivation of sodium channels and decreased excitability of muscle fibers. The Na+/K+ gradient across the membrane is critical for generation of action potential amplitude (46). By mimicking extracellular sodium decreases and potassium increases in muscle, thus reducing the gradients for Na+ and K+, there is a reduction in the M-wave similar to that observed with fatiguing contractions (48). Restoring the sodium gradient through activation of ASICs could potentially improve the Na+/K+ gradient to reduce fatigue. Prior studies show that amiloride and benzamil, nonselective antagonists of ASIC channels, enhanced the development of fatigue (74). These studies concluded that the Na+-H+ cotransporter played a role in this response. However, since amiloride and benzamil also inhibit ASICs, it could be interpreted that ASICs were involved in the changes in pH and the development of muscle fatigue. Prior studies also show that increases in lactic acid and H+ have protective effects on muscle excitability and restores the K+-induced decrease in force (14, 45). These data are consistent with the present study, which shows enhanced muscle fatigue and slower recovery from fatigue in the male ASIC3-/- mice in the longer-duration fatigue task.
Clinical Implications and Perspective
Fatigue in clinical conditions is generally measured subjectively and is a whole body feeling of muscle fatigue and loss of energy. This type of fatigue is common in several chronic pain conditions, including fibromyalgia, osteoarthritis, and rheumatoid arthritis (4, 13, 36, 37, 39, 64, 67, 70, 71, 73). As many as 76% of people with chronic widespread musculoskeletal pain report fatigue, and as many as 94% of people with chronic fatigue syndromes report muscle pain (39, 71, 73). These conditions are predominantly expressed in women and thus suggest a difference between males and females in the development of fatigue. During exercise, there is increased pain in people with chronic fatigue syndrome and in people with fibromyalgia (36, 64, 67, 70). In an animal model of pain, induced by intramuscular injections of acidic saline, exercise increases nociceptive responses in mice during a long-duration fatigue task (72). These data suggest an overlap between fatigue and pain such that fatigue plays a significant role in the development of pain, and that pain is enhanced in response to fatigue. Furthermore, the nociceptive response to intramuscular injections of acidic saline or to muscle inflammation is reduced in ASIC3-/- mice, both males and females (61, 62). Since both fatigue and muscle pain involve ASIC3, a common pathway may occur in the periphery to generate the pain and fatigue associated with chronic muscle pain conditions. It should be noted, however, that most studies in clinical syndromes measure fatigue as a whole body feeling of a loss of energy using subjective scales. However, the current study measured fatigue as a temporary reduction in force as a result of exercise. Muscle fatigability has been measured in people with fibromyalgia and chronic fatigue syndrome in response to high-intensity exercise. Interestingly, in these studies there is no difference in fatigability compared with controls for fibromyalgia patients, but there is for chronic fatigue syndrome (4, 13, 26, 37). This may be related to the task performed, and longer duration tasks may be necessary to observe differences in the clinical population. Subjects do show a decrease in muscle strength and this reduction in strength may contribute to the overall feeling of fatigue (4, 13, 33, 34, 37, 69). Future studies should assess endurance and low intensity exercise tests in clinical populations that have pain and fatigue.
Acknowledgments
GRANTS These studies were supported by the National Institutes of Arthritis and Muscloskelectal and Skin Diseases Grants AR-052316 and AR-053509.
REFERENCES
- 1.Altuwaijri S, Lee DK, Chuang KH, Ting HJ, Yang Z, Xu Q, Tsai MY, Yeh S, Hanchett LA, Chang HC, Chang C. Androgen receptor regulates expression of skeletal muscle-specific proteins and muscle cell types. Endocrine. 2004;25:27–32. doi: 10.1385/endo:25:1:27. [DOI] [PubMed] [Google Scholar]
- 2.Axell AM, MacLean HE, Plant DR, Harcourt LJ, Davis JA, Jimenez M, Handelsman DJ, Lynch GS, Zajac JD. Continuous testosterone administration prevents skeletal muscle atrophy and enhances resistance to fatigue in orchidectomized male mice. Am J Physiol Endocrinol Metab. 2006;291:E506–E516. doi: 10.1152/ajpendo.00058.2006. [DOI] [PubMed] [Google Scholar]
- 3.Babinski K, Catarsi S, Biagini G, Seguela P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+ J Biol Chem. 2000;275:28519–28525. doi: 10.1074/jbc.M004114200. [DOI] [PubMed] [Google Scholar]
- 4.Bazelmans E, Bleijenberg G, Voeten MJ, van der Meer JW, Folgering H. Impact of a maximal exercise test on symptoms and activity in chronic fatigue syndrome. J Psychosom Res. 2005;59:201–208. doi: 10.1016/j.jpsychores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supra-physiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 6.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82:407–413. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 7.Blundell J, Hoang CV, Potts B, Gold SJ, Powell CM. Motor coordination deficits in mice lacking RGS9. Brain Res. 2008;1190:78–85. doi: 10.1016/j.brainres.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowles DK, Maddali KK, Ganjam VK, Rubin LJ, Tharp DL, Turk JR, Heaps CL. Endogenous testosterone increases L-type Ca2+ channel expression in porcine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2091–H2098. doi: 10.1152/ajpheart.00258.2004. [DOI] [PubMed] [Google Scholar]
- 9.Carter GT, Kikuchi N, Abresch RT, Walsh SA, Horasek SJ, Fowler WM., Jr Effects of exhaustive concentric and eccentric exercise on murine skeletal muscle. Arch Phys Med Rehabil. 1994;75:555–559. [PubMed] [Google Scholar]
- 10.Colebrooke RE, Humby T, Lynch PJ, McGowan DP, Xia J, Emson PC. Age-related decline in striatal dopamine content and motor performance occurs in the absence of nigral cell loss in a genetic mouse model of Parkinson’s disease. Eur J Neurosci. 2006;24:2622–2630. doi: 10.1111/j.1460-9568.2006.05143.x. [DOI] [PubMed] [Google Scholar]
- 11.Coyle EF. Physiological determinants of endurance exercise performance. J Sci Med Sport. 1999;2:181–189. doi: 10.1016/s1440-2440(99)80172-8. [DOI] [PubMed] [Google Scholar]
- 12.Danion F, Latash ML, Li ZM, Zatsiorsky VM. The effect of a fatiguing exercise by the index finger on single- and multi-finger force production tasks. Exp Brain Res. 2001;138:322–329. doi: 10.1007/s002210100698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Becker P, Roeykens J, Reynders M, McGregor N, De Meirleir K. Exercise capacity in chronic fatigue syndrome. Arch Intern Med. 2000;160:3270–3277. doi: 10.1001/archinte.160.21.3270. [DOI] [PubMed] [Google Scholar]
- 14.de Paoli FV, Overgaard K, Pedersen TH, Nielsen OB. Additive protective effects of the addition of lactic acid and adrenaline on excitability and force in isolated rat skeletal muscle depressed by elevated extracellular K+ J Physiol. 2007;581:829–839. doi: 10.1113/jphysiol.2007.129049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deschenes MR, Maresh CM, Armstrong LE, Covault J, Kraemer WJ, Crivello JF. Endurance and resistance exercise induce muscle fiber type specific responses in androgen binding capacity. J Steroid Biochem Mol Biol. 1994;50:175–179. doi: 10.1016/0960-0760(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 16.Estrada M, Espinosa A, Muller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- 17.Fagenbaum R, Darling WG. Jump landing strategies in male and female college athletes and the implications of such strategies for anterior cruciate ligament injury. Am J Sports Med. 2003;31:233–240. doi: 10.1177/03635465030310021301. [DOI] [PubMed] [Google Scholar]
- 18.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–E607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 19.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 20.Fox J, Garber P, Hoffman M, Johnson D, Schaefer P, Vien J, Zeaton C, Thompson LV. Morphological characteristics of skeletal muscles in relation to gender. Aging Clin Exp Res. 2003;15:264–269. doi: 10.1007/BF03324508. [DOI] [PubMed] [Google Scholar]
- 21.Fregoso-Aguilar TA, Zamudio SR. Differential effect of testosterone and repetitive induction on cataleptic and dorsal immobility in mice. Horm Behav. 2006;50:27–32. doi: 10.1016/j.yhbeh.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Fulco CS, Rock PB, Muza SR, Lammi E, Cymerman A, Butterfield G, Moore LG, Braun B, Lewis SF. Slower fatigue and faster recovery of the adductor pollicis muscle in women matched for strength with men. Acta Physiol Scand. 1999;167:233–239. doi: 10.1046/j.1365-201x.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- 23.Gitterman DP, Wilson J, Randall AD. Functional properties and pharmacological inhibition of ASIC channels in the human SJ-RH30 skeletal muscle cell line. J Physiol. 2005;562:759–769. doi: 10.1113/jphysiol.2004.075069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glace BW, McHugh MP, Gleim GW. Effects of a 2-hour run on metabolic economy and lower extremity strength in men and women. J Orthop Sports Phys Ther. 1998;27:189–196. doi: 10.2519/jospt.1998.27.3.189. [DOI] [PubMed] [Google Scholar]
- 25.Golden KL, Marsh JD, Jiang Y. Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Horm Metab Res. 2004;36:197–202. doi: 10.1055/s-2004-814445. [DOI] [PubMed] [Google Scholar]
- 26.Hakkinen A, Hakkinen K, Hannonen P, Alen M. Force production capacity and acute neuromuscular responses to fatiguing loading in women with fibromyalgia are not different from those of healthy women. J Rheumatol. 2000;27:1277–1282. [PubMed] [Google Scholar]
- 27.Hakkinen K. Neuromuscular fatigue and recovery in male and female athletes during heavy resistance exercise. Int J Sports Med. 1993;14:53–59. doi: 10.1055/s-2007-1021146. [DOI] [PubMed] [Google Scholar]
- 28.Hamamoto DT, Ortiz-Gonzalez XR, Honda JM, Kajander KC. Intraplantar injection of hyaluronic acid at low pH into the rat hindpaw produces tissue acidosis and enhances withdrawal responses to mechanical stimuli. Pain. 1998;74:225–234. doi: 10.1016/s0304-3959(97)00185-1. [DOI] [PubMed] [Google Scholar]
- 29.Hood VL, Chubert C, Keller U, Muller S. Effect of systemic pH on pHi and lactic acid generation in exhaustive forearm exercise. Am J Physiol Renal Fluid Electrolyte Physiol. 1988;255:F479–F485. doi: 10.1152/ajprenal.1988.255.3.F479. [DOI] [PubMed] [Google Scholar]
- 30.Howlett RA, Hogan MC. Effect of hypoxia on fatigue development in rat muscle composed of different fiber types. Exp Physiol. 2007;92:887–894. doi: 10.1113/expphysiol.2007.037291. [DOI] [PubMed] [Google Scholar]
- 31.Hunter SK, Critchlow A, Shin IS, Enoka RM. Men are more fatigable than strength-matched women when performing intermittent submaximal contractions. J Appl Physiol. 2004;96:2125–2132. doi: 10.1152/japplphysiol.01342.2003. [DOI] [PubMed] [Google Scholar]
- 32.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett. 1996;208:191–194. doi: 10.1016/0304-3940(96)12576-3. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen S, Danneskiold-Samsoe B. Isometric and isokinetic muscle strength in patients with fibrositis syndrome. New characteristics for a difficult definable category of patients. Scand J Rheumatol. 1987;16:61–65. [PubMed] [Google Scholar]
- 34.Jacobsen S, Danneskiold-Samsoe B. Dynamic muscular endurance in primary fibromyalgia compared with chronic myofascial pain syndrome. Arch Phys Med Rehabil. 1992;73:170–173. [PubMed] [Google Scholar]
- 35.Jyotika J, McCutcheon J, Laroche J, Blaustein JD, Forger NG. Deletion of the Bax gene disrupts sexual behavior and modestly impairs motor function in mice. Dev Neurobiol. 2007;67:1511–1519. doi: 10.1002/dneu.20525. [DOI] [PubMed] [Google Scholar]
- 36.Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain. 2007;11:39–47. doi: 10.1016/j.ejpain.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Maquet D, Croisier JL, Renard C, Crielaard JM. Muscle performance in patients with fibromyalgia. Joint Bone Spine. 2002;69:293–299. doi: 10.1016/s1297-319x(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 38.Martin V, Millet GY, Martin A, Deley G, Lattier G. Assessment of low-frequency fatigue with two methods of electrical stimulation. J Appl Physiol. 2004;97:1923–1929. doi: 10.1152/japplphysiol.00376.2004. [DOI] [PubMed] [Google Scholar]
- 39.Meeus M, Nijs J, Meirleir KD. Chronic musculoskeletal pain in patients with the chronic fatigue syndrome: a systematic review. Eur J Pain. 2006;11:377–386. doi: 10.1016/j.ejpain.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Michels G, Er F, Eicks M, Herzig S, Hoppe UC. Long-term and immediate effect of testosterone on single T-type calcium channel in neonatal rat cardiomyocytes. Endocrinology. 2006;147:5160–5169. doi: 10.1210/en.2006-0186. [DOI] [PubMed] [Google Scholar]
- 41.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monks DA, O’Bryant EL, Jordan CL. Androgen receptor immunore-activity in skeletal muscle: enrichment at the neuromuscular junction. J Comp Neurol. 2004;473:59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]
- 43.Nagaya N, Herrera AA. Effects of testosterone on synaptic efficacy at neuromuscular junctions in a sexually dimorphic muscle of male frogs. J Physiol. 1995;483:141–153. doi: 10.1113/jphysiol.1995.sp020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naves LA, McCleskey EW. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res. 2005;38:1561–1569. doi: 10.1590/s0100-879x2005001100001. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen OB, de Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol. 2001;536:161–166. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nielsen OB, Overgaard K. Ion gradients and contractility in skeletal muscle: the role of active Na+, K+ transport. Acta Physiol Scand. 1996;156:247–256. doi: 10.1046/j.1365-201X.1996.204000.x. [DOI] [PubMed] [Google Scholar]
- 47.Nudler SI, Pagani MR, Urbano FJ, McEnery MW. Uchitel OD. Testosterone modulates Ca(v2.2) calcium channels’ functional expression at rat levator ani neuromuscular junction. Neuroscience. 2005;134:817–826. doi: 10.1016/j.neuroscience.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 48.Overgaard K, Nielsen OB, Flatman JA, Clausen T. Relations between excitability and contractility in rat soleus muscle: role of the Na+-K+ pump and Na+/K+ gradients. J Physiol. 1999;518:215–225. doi: 10.1111/j.1469-7793.1999.0215r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pagala M, Namba T, Grob D. Mechanisms of fatigue in normal young and old mice. Ann NY Acad Sci. 1998;841:712–715. doi: 10.1111/j.1749-6632.1998.tb11006.x. [DOI] [PubMed] [Google Scholar]
- 50.Pan JW, Hamm JR, Rotham DL, Shulman RG. Intracellular pH in human skeletal muscle by 1H NMR. Proc Natl Acad Sci USA. 1988;85:7836–7839. doi: 10.1073/pnas.85.21.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 52.Reeh PW, Steen KH. Tissue acidosis in nociception and pain. Prog Brain Res. 1996;113:143–151. doi: 10.1016/s0079-6123(08)61085-7. [DOI] [PubMed] [Google Scholar]
- 53.Renaud JM. Modulation of force development by Na+, K+, Na+ K+ pump and KATP channel during muscular activity. Can J Appl Physiol. 2002;27:296–315. doi: 10.1139/h02-017. [DOI] [PubMed] [Google Scholar]
- 54.Revici E, Stoopen E, Frenk E, Ravich RA. The painful focus. II. The relation of pain to local physiochemical changes. Bull Inst Appl Biol. 1949;1:21–38. [Google Scholar]
- 55.Riffo-Vasquez Y, de Oliveira AP Ligeiro, Page CP, Spina D. Tavaresde-Lima W. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy. 2007;37:459–470. doi: 10.1111/j.1365-2222.2007.02670.x. [DOI] [PubMed] [Google Scholar]
- 56.Ruff RL, Simoncini L, Stuhmer W. Comparison between slow sodium channel inactivation in rat slow- and fast-twitch muscle. J Physiol. 1987;383:339–348. doi: 10.1113/jphysiol.1987.sp016412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schillings ML, Kalkman JS, van der Werf SP, van Engelen BG, Bleijenberg G, Zwarts MJ. Diminished central activation during maximal voluntary contraction in chronic fatigue syndrome. Clin Neurophysiol. 2004;115:2518–2524. doi: 10.1016/j.clinph.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Sejersted OM, Sjogaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- 59.Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol Endocrinol Metab. 1989;257:E567–E572. doi: 10.1152/ajpendo.1989.257.4.E567. [DOI] [PubMed] [Google Scholar]
- 60.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 61.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 62.Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48:623–629. doi: 10.1177/002215540004800506. [DOI] [PubMed] [Google Scholar]
- 64.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, Casaburi R, Bhasin S. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478–1485. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- 66.Torisu T, Wang K, Svensson P, De Laat A, Fujii H, Arendt-Nielsen L. Effects of muscle fatigue induced by low-level clenching on experimental muscle pain and resting jaw muscle activity: gender differences. Exp Brain Res. 2006;174:566–574. doi: 10.1007/s00221-006-0497-4. [DOI] [PubMed] [Google Scholar]
- 67.Vierck CJ, Jr, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 68.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 69.Wallman KE, Sacco P. Sense of effort during a fatiguing exercise protocol in chronic fatigue syndrome. Res Sports Med. 2007;15:47–59. doi: 10.1080/15438620601184331. [DOI] [PubMed] [Google Scholar]
- 70.Whiteside A, Hansen S, Chaudhuri A. Exercise lowers pain threshold in chronic fatigue syndrome. Pain. 2004;109:497–499. doi: 10.1016/j.pain.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 71.Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407–1417. [PubMed] [Google Scholar]
- 72.Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle fatigue increases the probability of developing hyperalgesia in mice. J Pain. 2007;8:692–699. doi: 10.1016/j.jpain.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zautra AJ, Fasman R, Parish BP, Davis MC. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2007;128:128–135. doi: 10.1016/j.pain.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Zavecz JH, Anderson WM, Adams B. Effect of amiloride on diaphragmatic contractility: evidence of a role for Na+-Ca2+ exchange. J Appl Physiol. 1991;70:1309–1314. doi: 10.1152/jappl.1991.70.3.1309. [DOI] [PubMed] [Google Scholar]