Abstract
Adiponectin is an adipocyte-derived cytokine with beneficial effects on insulin sensitivity and the development of atherosclerosis. Id3 is a helix-loop-helix (HLH) factor that binds to E-proteins such as E47 and inhibits their binding to DNA. While the bHLH factor SREBP-1c is a known activator of adiponectin transcription, this study provides the first evidence of a role for Id3 and E47 in adiponectin expression. Decreased Id3 in differentiating adipocytes correlates with increased adiponectin expression and forced expression of Id3 inhibits adiponectin expression. Moreover, Id3 null mice have increased adiponectin expression in visceral fat tissue and in serum. We demonstrate that E47 potentiates SREBP-1c-mediated adiponectin promoter activation and that Id3 can dose-dependently inhibit this action via interaction with E47. Mutation of a consensus E47 binding site results in nearly complete loss of promoter activation. Further, we demonstrate E47 binding to the endogenous adiponectin promoter both in vitro and in vivo by ChIP analysis. Binding is not detected in undifferentiated cells which express Id3 but peaks during differentiation in parallel with Id3 decline. This promoter binding can be completely abolished by the overexpression of Id3 and is enhanced in adipose tissue null for Id3. These data establish Id3 and E47 as novel regulators of SREBP-1c-mediated adiponectin expression in differentiating adipocytes and provide evidence that Id3 regulates adiponectin expression in vivo.
Keywords: basic helix-loop-helix proteins, differentiation, gene regulation, adiponectin, adipocytes
Introduction
Adiponectin is produced by adipocytes and is secreted into the circulation1,2. Adiponectin levels are reduced in obese, insulin-resistant and diabetic rodents3, monkeys4 and humans5. Administration of adiponectin in rodent models increases insulin sensitivity and lowers plasma glucose6,7. Low adiponectin levels are associated with the development of coronary artery disease8,9 and ApoE-/- mice overexpressing adiponectin demonstrate significantly less atherosclerosis in response to high fat feeding10,11. While these studies implicate adiponectin as an important modulator of insulin sensitivity and the development of atherosclerosis, the transcriptional mechanisms that regulate adiponectin expression are incompletely understood.
Both the human and mouse adiponectin promoters contain binding sites for transcription factors including sterol regulatory elements (SREs), PPAR-response elements, C/EBP sites and E-boxes12. Accordingly, many factors influence adiponectin expression either directly or indirectly, including SREBP-1c12, PPARγ12,13, and C/EBP12,14. To date, no study has addressed the role of the three putative E-boxes present in the adiponectin promoter12.
Sterol regulatory element binding protein -1c (SREBP-1c) is a member of the basic HLH-leucine zipper (bHLH-LZ) family of proteins that binds to SREs15. Originally implicated in cholesterol-regulated gene expression16, SREBP-1c is also a major regulator of adipogenic genes, including fatty acid synthase and adiponectin12,17. Also known as adipocyte determination- and differentiation-dependent factor-1 (ADD1), SREBP-1c is expressed preferentially in adipose tissue and is upregulated as adipocyte differentiation progresses12,15.
The inhibitor of differentiation (Id) family of proteins contains four members, Id1-4, which have both redundant and unique functions18. Ids are HLH proteins that function as dominant-negative transcription factors that are incapable of binding to DNA. Instead, the Ids bind to a subset of bHLH factors known as E-proteins, including E12, E47, ITF2 and HEB, thereby preventing their dimerization and DNA binding. Although the Ids bind preferentially to E-proteins, one report has suggested that Id3 binds to and inhibits SREBP-1c directly19.
Previous studies have proposed a role for Id proteins in adipocytes. The mRNAs for Ids 1-3 are expressed in 3T3-L1 pre-adipocytes and decrease to undetectable levels as they differentiate into adipocytes19-21. Id3 has been shown to negatively regulate fatty acid synthase in vitro19; however, no other adipocyte target genes have been identified.
In the current study, we examine the role of Id3 and E47 in the control of adiponectin transcription. We demonstrate that Id3 inhibits adiponectin expression in vitro and in vivo. In addition, we show that E47 and SREBP-1c bind the endogenous adiponectin promoter in vitro and in vivo, and synergistically activate the adiponectin promoter. We refute previous suggestions that Id3 directly binds to SREBP-1c, and propose instead that Id3 regulates SREBP-1c activity indirectly by interacting with E47, thereby preventing E47 binding to the adiponectin promoter and inhibiting adiponectin expression.
Materials and Methods
Methods are described in detail in the online data supplement. Transfections, adenoviral transductions, promoter-reporter assays, Western blotting and two-hybrid analysis were performed as previously described22,23. OP9 and 3T3-L1 cell culture and differentiation were carried out as described by Wolins et al24. Chromatin immunoprecipitation methods were based on Francis et al and are described in supplemental materials25.
Results
Increased levels of adiponectin protein and mRNA in Id3 knockout mice
To address the potential in vivo regulation of adiponectin by Id3 in an atherogenic model, total adiponectin levels in ApoE-/- and Id3-/-ApoE-/- mice were determined. Sera were collected from eight week-old, weight-matched ApoE-/- and Id3-/-ApoE-/- mice, and adiponectin levels were measured by radioimmunoassay. Loss of Id3 resulted in a significant increase in serum adiponectin levels (Figure 1A). To determine whether this was due to an effect on adiponectin production, we analyzed visceral adipose tissue, the major site of adiponectin production in rodents1,2. Epididymal and mesenteric fat depots from ApoE-/- and Id3-/-ApoE-/- mice were harvested from eight week-old, weight-matched animals. Relative amounts of adiponectin were determined by Western blotting. When compared to ApoE-/- mice, Id3-/-ApoE-/- mice had increased adiponectin in both the epididymal (3.7-fold) and mesenteric (2.8-fold) fat pads (Figures 1B and 1C). To determine whether Id3 affects adiponectin expression at the mRNA level, total RNA was isolated from fat pads, and adiponectin transcripts were quantified by real-time PCR. As compared to ApoE-/- mice, Id3-/-ApoE-/- animals expressed 2.7 and 3.1-fold more adiponectin mRNA in the epididymal and mesenteric fat pads, respectively (Figure 1D).
Figure 1. Increased adiponectin levels in serum and adipose tissue of Id3 knockout mice.
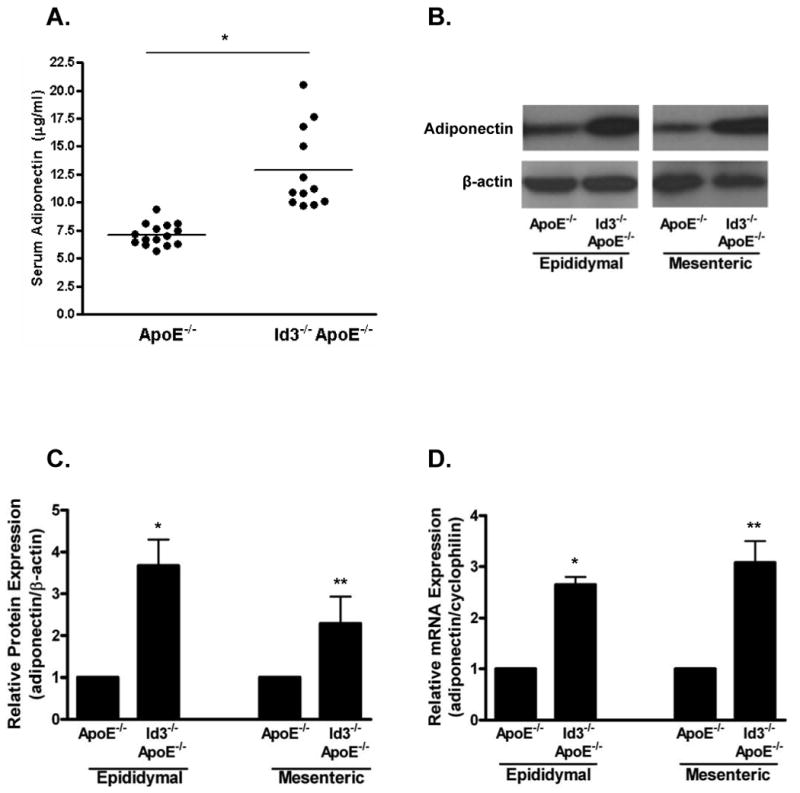
Blood, epididymal and mesenteric adipose tissues were harvested from eight week-old ApoE-/- and Id3-/-ApoE-/- mice. A, Serum levels of adiponectin were determined by RIA. Points represent serum measurements from individual animals, bar represents the average. *=p<0.005. B, Total protein was extracted from adipose tissue, separated by SDS-PAGE and analyzed by Western blotting. Representative blot from one animal is shown. C, Densitometric analysis of the experiment described in B. Adiponectin levels were normalized to β-actin. Results are average values obtained from four animals per group presented relative to the value in the ApoE-/- group. *=p<0.01, **=p<0.05. D, Total RNA was harvested from adipose tissue and analyzed by real-time PCR. Adiponectin was normalized to the corresponding cyclophilin signal. Three samples from each fat pad were analyzed, each in triplicate PCR measurements. Average adiponectin values from three animals per group are presented relative to adiponectin value in the ApoE-/- group. *=p<0.005, **=p<0.01.
Forced expression of Id3 inhibits adiponectin expression
Throughout our study, we have confirmed many of our results in two adipocyte cell lines: 3T3-L1, a widely used adipocyte line, and OP9, a new alternative model24. Wolins et al have demonstrated that OP9 cells express the same adipocyte lineage markers as 3T3-L1 cells, but differentiate more rapidly (three to seven days versus two weeks26 for complete differentiation after plating)24. We have confirmed that OP9 cells can be transiently transfected with high efficiency and are efficiently transduced with an adenovirus (95% transduction (data not shown) versus 50% or less with 3T3-L1 cells27), enabling us to assay changes in expression of endogenous proteins in the total cell population. In addition, the shorter differentiation time allows OP9 cells to maintain expression of transfected genes over the course of differentiation.
We evaluated the expression of Id3 and adiponectin in both 3T3-L1 and OP9 cells. Undifferentiated (pre-adipocytes) or fully differentiated (adipocytes) cells were analyzed by Western blotting, revealing that Id3 is detected in undifferentiated but not in differentiated 3T3-L1 or OP9 cells. Conversely, adiponectin is present in differentiated but not undifferentiated cells (Figure 2A).
Figure 2. Id3 expression decreases adiponectin.
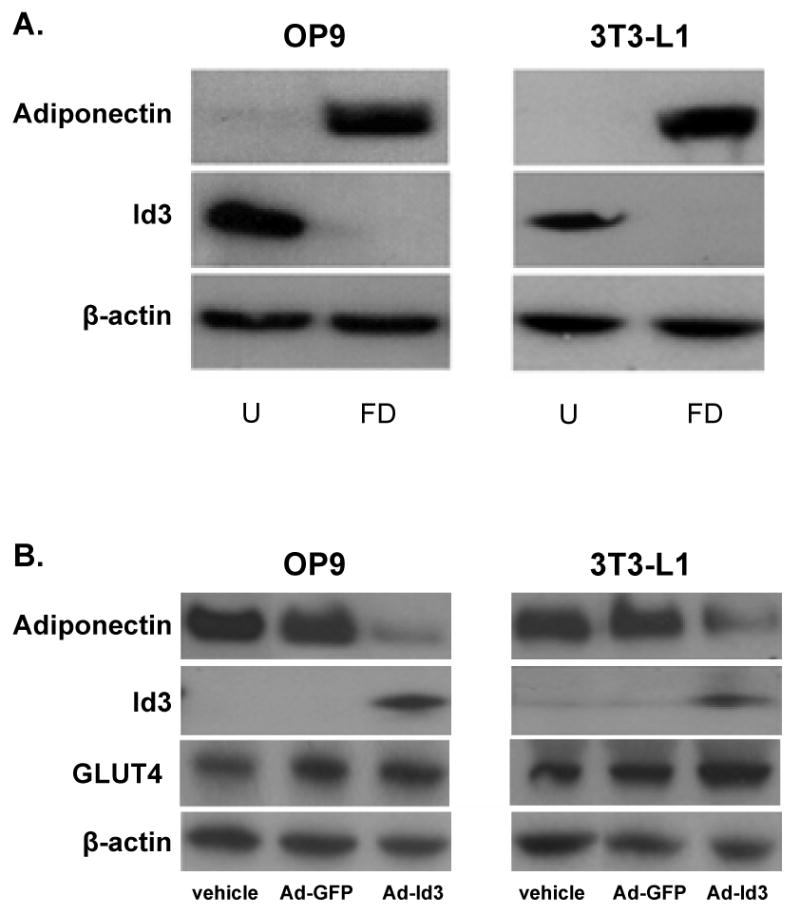
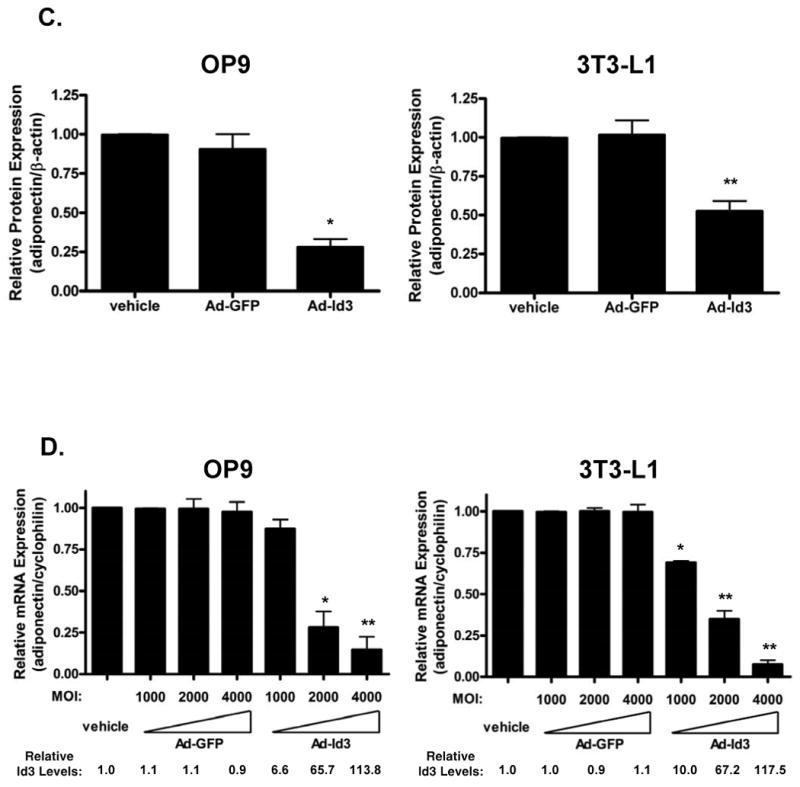
A, OP9 and 3T3-L1 cells were harvested 24 hours after plating as undifferentiated (“U”) cells and after six days (OP9) or ten days (3T3-L1) of incubation in adipogenic cocktail as fully differentiated (“FD”) cells. Total lysates were analyzed by Western blotting. B, Undifferentiated OP9 and 3T3-L1 cells were transduced with adenovirus encoding GFP (Ad-GFP), Id3 (Ad-Id3) or were treated with vehicle for 24 hours. Cells were then differentiated as in A. Total lysates were analyzed by Western blotting. C, Adiponectin protein levels in samples from the experiment described in B were quantified as in Fig. 1C. Results are relative to the adiponectin signal in the vehicle group. *=p<0.01, **=p<0.05; Ad-GFP versus Ad-Id3. D, OP9 cells were infected with Ad-GFP or Ad-Id3 with an MOI of 1000, 2000 or 4000 and differentiated as in A. Total mRNA was isolated and analyzed by real-time PCR. Results are relative to the vehicle treated group. Relative Id3 mRNA levels are indicated below the graph. *=p<0.01, **=p<0.005; Ad-GFP versus Ad-Id3.
To determine whether Id3 modulates adiponectin expression, undifferentiated OP9 or 3T3-L1 cells were transduced with an adenovirus expressing either Id3 (Ad-Id3) or GFP (Ad-GFP) and then differentiated for three (OP9) or five (3T3-L1) days. Expression of adiponectin, Id3 or GLUT4 (a marker of differentiated adipocytes28) was analyzed by Western blotting. Exogenous Id3 expression significantly decreased adiponectin protein levels (Figure 2B) by approximately three-fold in OP9 cells and two-fold in 3T3-L1 cells (Figure 2C). GLUT4 expression in the Ad-Id3 and Ad-GFP groups was similar, indicating that the effect of Id3 on adiponectin expression is not due to inhibition of differentiation. To ascertain whether Id3 inhibits adiponectin expression independent of differentiation state, differentiated OP9 cells were transduced with Ad-GFP or Ad-Id3 and analyzed 72 hours later. Differentiated OP9 adipocytes transduced with Id3 showed a two-fold decrease in adiponectin expression as compared to vehicle or Ad-GFP-treated cells (Online Figure I).
To assess whether forced Id3 expression inhibits adiponectin at the transcriptional level, we also compared adiponectin mRNA levels in cells transduced with increasing amounts of Ad-GFP or Ad-Id3. Dose-dependent increases in Id3 mRNA expression in Ad-Id3 treated cells were confirmed by real-time PCR. While increasing doses of GFP did not alter adiponectin expression, expression of Id3 in either OP9 or 3T3-L1 cells reduced adiponectin mRNA levels in a dose-dependent manner (Figure 2D).
Id3 inhibits adiponectin promoter activation
Since the level of Id3 expression affected adiponectin mRNA levels, regulation of the adiponectin promoter by Id3 was tested next. NIH3T3 fibroblasts were transfected with a 0.41kb adiponectin promoter/luciferase reporter construct in combination with SREBP-1c (a potent activator of the adiponectin promoter12) and/or Id3 (Figure 3). NIH3T3 cells were used to eliminate the potentially confounding effect of adipocyte differentiation state and to avoid background activity from the endogenous expression of SREBP-1c. Id3 alone had no effect on basal luciferase activity, while SREBP-1c expression resulted in a twelve-fold activation of the promoter. When SREBP-1c and Id3 were co-transfected, a dose-dependent reduction in SREBP-1c-mediated activation of the adiponectin promoter was observed. As previously shown, basal and SREBP-1c-induced adiponectin promoter activity were similar with either the proximal 0.41 or 0.98 kb promoter fragments (data not shown)12.
Figure 3. Id3 inhibits SREBP-1c mediated adiponectin promoter activation.
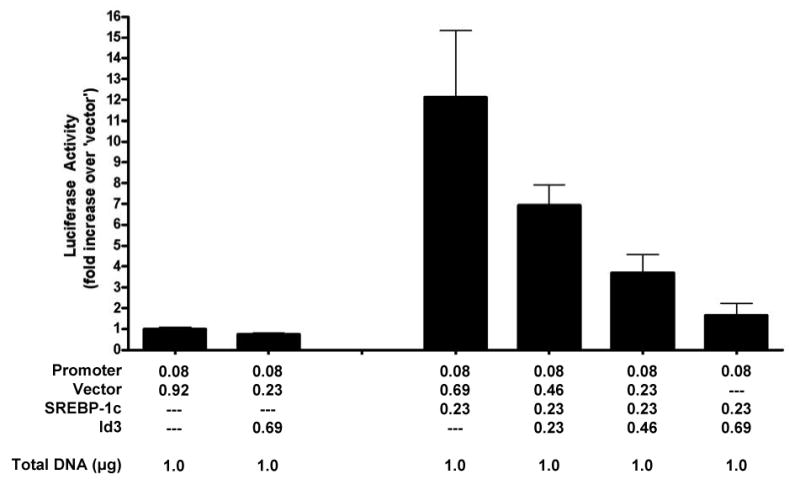
NIH3T3 cells were transfected in triplicate with a 0.41kb adiponectin promoter-reporter and expression vectors as indicated. Seventy-two hours after transfection, cells lysates were assayed for luciferase activity. Luciferase activity was normalized to protein concentration and is presented as fold activation relative to the first group (promoter plus vector only).
SREBP-1c partners with E47 but not Id3 in adipocytes
The established paradigm of Id function involves dimerization with E-proteins such as E47 and not a direct interaction with bHLH-LZ proteins such as SREBP-1c21,29. A previous study demonstrated an Id3:SREBP-1c interaction using in vitro translated proteins outside of a cellular context19. To determine whether SREBP-1c interacts with either Id3 or E47 in adipocytes, mammalian two-hybrid analysis in differentiated OP9 cells was employed (see methods). Large T antigen and p53, which are known to interact, were used as a positive control, and partners producing equal or greater signal than these were considered positive (Figure 4A). SREBP-1c bound to E47 but not to Id3, which is in contrast with the aforementioned study, but consistent with previous results demonstrating E47:SREBP-1c interaction in other tissues30. To extend the two-hybrid findings, co-immunoprecipitation of Id3 with E47 or SREBP-1c was tested. COS7 cells were transfected with HA-Id3 and either FLAG-SREBP-1c or FLAG-E47 before immunoprecipitation with anti-FLAG beads. HA-Id3 co-immunoprecipitated with FLAG-E47 but not with FLAG-SREBP-1c (Figure 4B), confirming the results of the two-hybrid experiment. These findings provide evidence that, in adipocytes, SREBP-1c binds to E47 and not to Id3.
Figure 4. SREBP-1c interacts with E47 but not Id3.
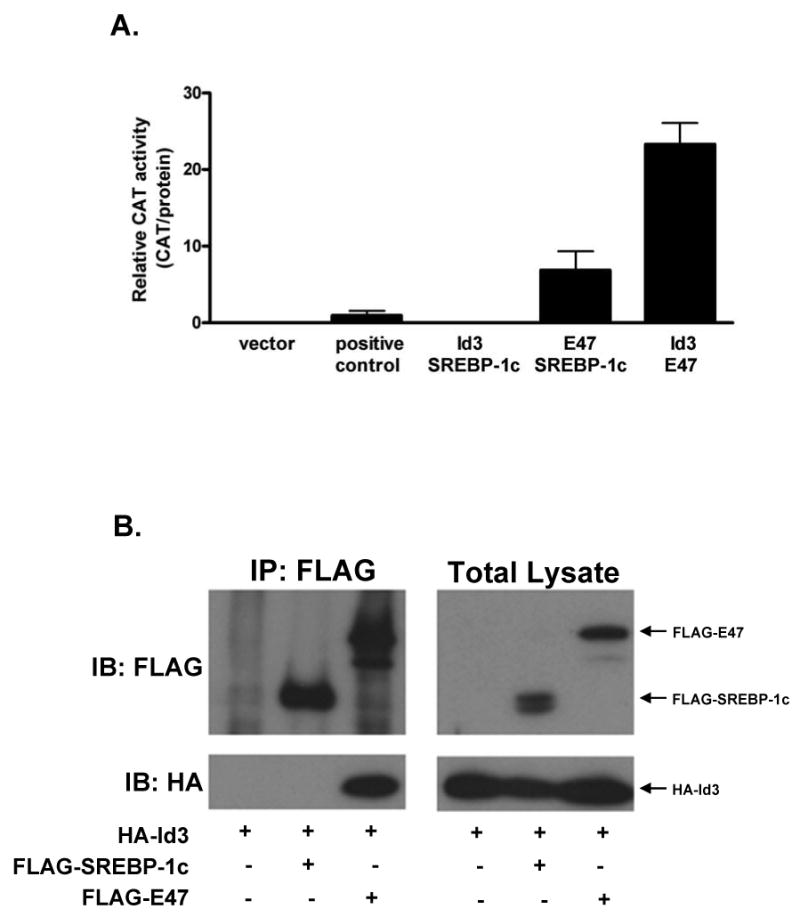
A, OP9 cells were transiently transfected with expression vectors encoding the indicated factors expressed as fusion proteins with either the GAL4 binding domain or GAL4 activation domain and with a CAT promoter-reporter plasmid in order to perform two-hybrid analysis (see methods). B, COS cells were transiently transfected with HA-Id3 and either FLAG-SREBP-1c or FLAG-E47. Forty-eight hours after transfection, total lysates were harvested and precipitated using anti-FLAG beads. Immunoprecipitates and total lysates were separated by SDS-PAGE and immunoblotted with FLAG or HA antibodies. Results are representative of two independent experiments.
E47 potentiates SREBP-1c mediated adiponectin promoter activation
To determine if E47:SREBP-1c interaction influences adiponectin promoter-reporter activation, NIH3T3 cells were transfected with the 0.41kb adiponectin promoter-reporter construct together with SREBP1-c and varying amounts of E47. E47 alone did not activate the promoter, but co-expression of E47 with SREBP-1c augmented adiponectin promoter activation six-fold more than the same dose of SREBP-1c alone (Figure 5).
Figure 5. E47 potentiates adiponectin promoter activation by SREBP-1c.
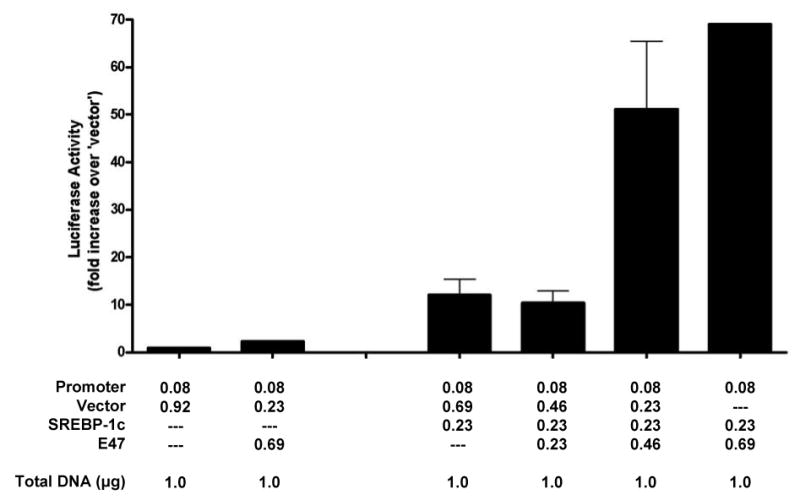
NIH3T3 cells were transfected as described in Figure 3. Seventy-two hours after transfection, cells lysates were assayed for luciferase activity. Values are relative to protein levels and are presented relative to promoter plus vector.
To identify possible sites at which E47 acts, the two E-boxes within the proximal 400 base pairs of the adiponectin promoter were mutated. The most distal E-box was not tested because, as mentioned above, the 0.41kb promoter-reporter construct, in which this E-box is deleted, responds to E47 and Id3 in the same manner as the 0.98 kb construct. Approximate positions of the E-boxes are shown in the schematic (Figure 6A). Mutations within E-box #1 but not E-box #2 inhibited SREBP-1c-mediated adiponectin promoter activity (Figure 6B). Moreover, the mutation of E-box #1, but not E-box #2, led to the loss of promoter activation by SREBP-1c and E47 (Figure 6C).
Figure 6. Mutagenesis of E-box #1 inhibits adiponectin promoter activation by SREBP-1c and E47.
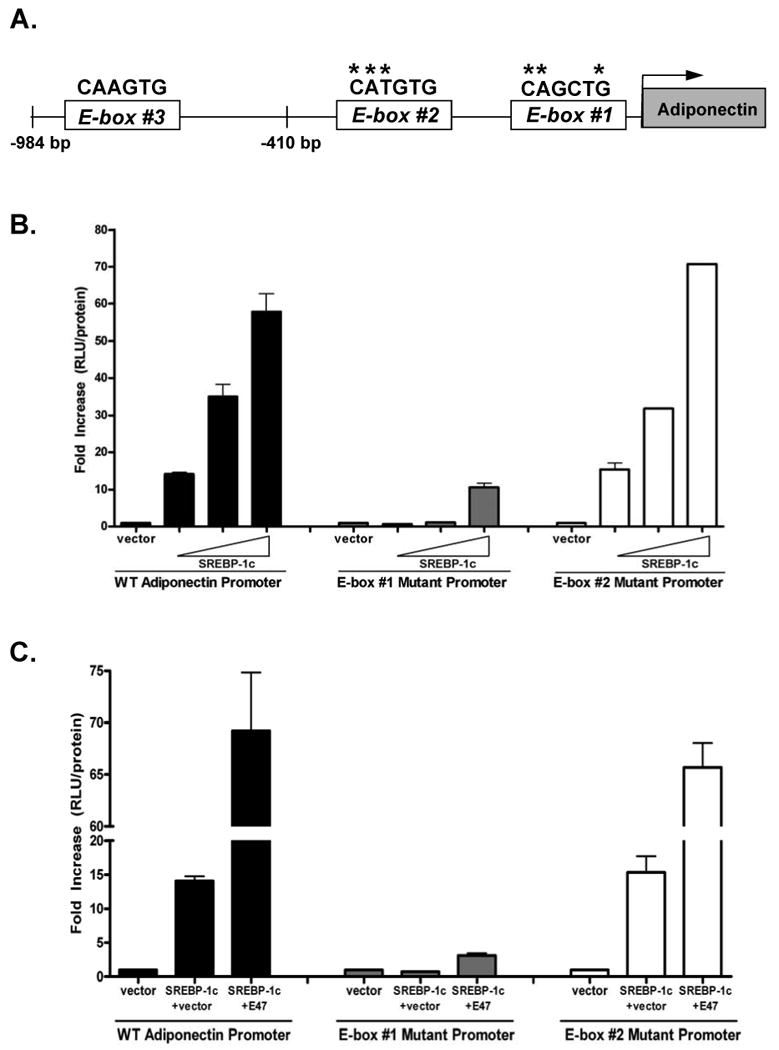
A, Schematic representation of the proximal 984 base pairs of the adiponectin promoter depicting the relative locations of three E-boxes. E-box #1 begins at -137bp, E-box #2 begins at -396bp and E-box #3 begins at -679bp. The nucleotide sequence appears above each E-box with asterisks marking the base pairs which were mutated. Specific mutations are described in the methods section. B, NIH3T3 cells were transiently transfected with wild type or mutant adiponectin promoters and an expression vector for SREBP-1c. Seventy-two hours after transfection, cells were harvested and assayed for luciferase activity. Luciferase values are relative to protein levels and are presented relative to promoter plus vector. C, NIH3T3 cells were transiently transfected with wild type or mutant adiponectin promoters as well as with expression vectors for SREBP-1c, Id3 and E47. The experiment was performed as in B.
E47 binds to the adiponectin promoter during adipocyte differentiation
To determine whether E47 binds the endogenous adiponectin promoter, chromatin immunoprecipitation was performed. OP9 and 3T3-L1 cells were harvested during differentiation, at day three and day five respectively, and precipitated with anti-E47 antibody. As SREBP-1c is known to bind the adiponectin promoter, it was used as a positive control. Adiponectin promoter recovery was determined by real-time PCR using primers spanning E-box #1 (Figure 7A). In OP9 cells, precipitation with E47 antibody led to a four-fold enrichment of the adiponectin promoter compared to isotype control and in 3T3-L1 cells, five-fold enrichment was obtained (Figure 7B). Precipitation with an antibody for E12, an alternate splice product of the E2A gene that also produces E47, did not lead to any detectable recovery of the adiponectin promoter in either cell line. Similar results were obtained using a second set of adiponectin promoter primers, while a negative set of primers gave no specific signal (Online Figure IIA and IIB). To investigate whether Id3 expression can interrupt binding of E47 to the promoter, OP9 cells were transduced with Ad-Id3 during differentiation (day three), when endogenous Id3 had dropped to undetectable levels. OP9 cells transduced with Ad-GFP and precipitated with E47 antibody showed a 4.8-fold increase in adiponectin promoter recovery in the E47 immunoprecipitate compared to isotype control whereas cells transduced with Ad-Id3 had no detectable promoter binding of E47 (Figure 7C). Immunoblotting of of lysates transduced with either GFP or Id3 revealed that levels of E47 and SREBP-1c were similar between the two groups (Figure 7C), confirming that the observed decrease in binding was not due to a reduction in E47 or SREBP-1c expression. Transduction with Ad-Id3 also inhibited SREBP-1c binding to the adiponectin promoter. Results using a second set of primers were similar and no specific signal was obtained using negative control primers (Online Figure IIC).
Figure 7. E47 binds the endogenous adiponectin promoter in a differentiation-state dependent manner.
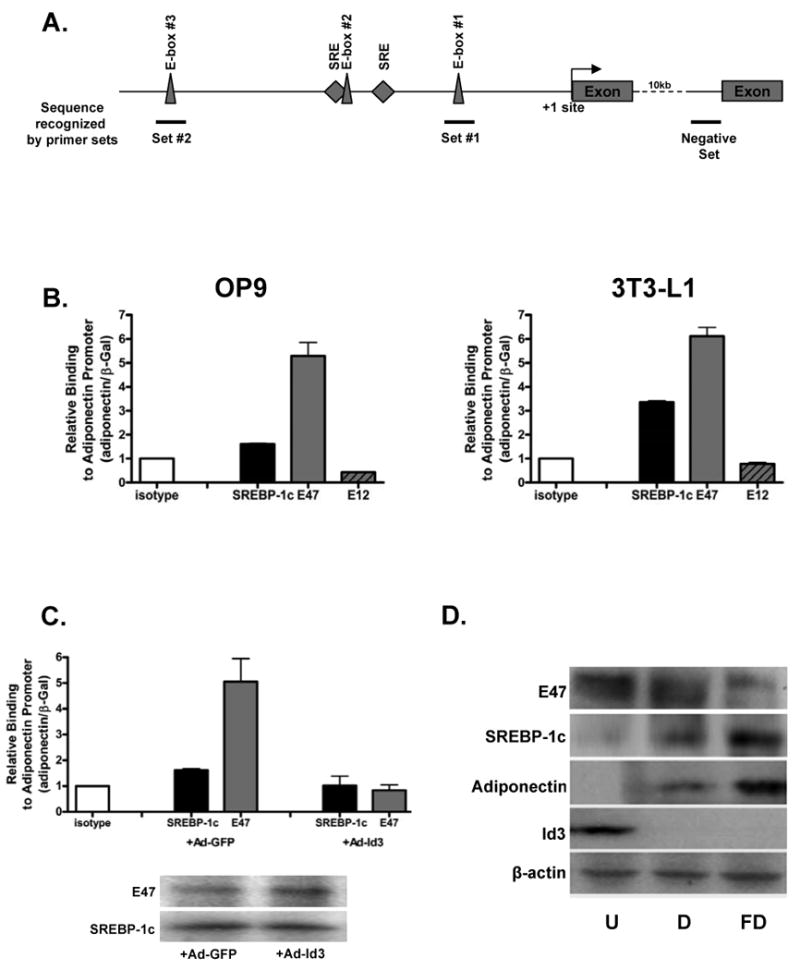
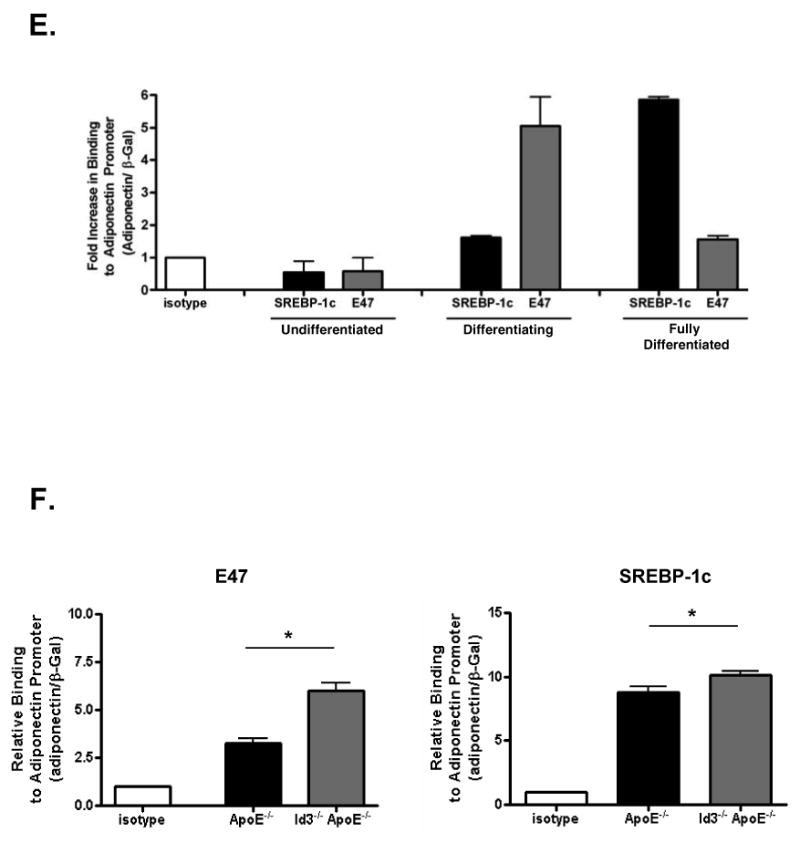
A, Schematic depicting relative locations of the primer sets used for ChIP analysis. Lines beneath the schematic represent the promoter area amplified by the indicated primer sets. B, OP9 and 3T3-L1 cells were differentiated for three and five days respectively, then cross-linked and precipitated with the indicated antibodies. Immunoprecipitated fragments were quantified by real-time PCR and normalized to an internal β-Gal control for recovery. Results are presented relative to IP with isotype control and are the average of triplicate PCR measurements from three independent experiments. C, OP9 cells were transduced with adenovirus encoding GFP (Ad-GFP) or Id3 (Ad-Id3) and then differentiated for three days. Cells were harvested for ChIP as in B. In parallel, transduced lysates were separated by SDS-PAGE and membranes were immunoblotted with the indicated antibodies. D, OP9 cells were harvested at day zero (undifferentiated, “U”), day three (differentiating, “D”) or day six (fully differentiated, “FD”). Total lysates were immunoblotted as in C. E, Undifferentiated, differentiating and fully differentiated OP9 cells were harvested in parallel to cells used for Western blotting as described in D and ChIP was carried out as described in B. F, Adipose tissue was harvested from eight week-old ApoE-/- or Id3-/-ApoE-/- mice, snap frozen and homogenized. Homogenates were cross-linked and precipitated for ChIP analysis as in B. Left panel: Precipitation with E47 antibody. *=p<0.05; as determined by paired t-test. Right panel: Precipitation with SREBP-1c antibody. *=p<0.05; as determined by paired t-test.
In order to determine the temporal pattern of Id3, E47, SREBP-1c and adiponectin expression in differentiating adipocytes, OP9 cells were harvested at day zero (undifferentiated), day three (differentiating) and day six (fully differentiated). Immunoblotting revealed that E47 is expressed in undifferentiated pre-adipocytes and during differentation while SREBP-1c is expressed in differentiating and fully differentiated cells (Figure 7D). To test whether endogenous E47 and SREBP-1c binding to the adiponectin promoter is consistent with the temporal pattern of protein expression during differentiation, we performed ChIP analysis of samples harvested in parallel to those used for Western blotting (Figure 7E). Consistent with our data that Id3 levels are high in undifferentiated OP9 and 3T3-L1 cells, E47 did not bind the adiponectin promoter. At that stage, SREBP-1c is not expressed in undifferentiated cells and no detectable adiponectin promoter binding was observed. In OP9 cells undergoing differentiation, Id3 levels are declining, E47 levels remain high and SREBP-1c is expressed. During this phase, both SREBP-1c and E47 bound the promoter with a 1.8 and 5.0-fold increase in binding compared to isotype respectively. In mature adipocytes, Id3 is undetectable, E47 is reduced and SREBP-1c is abundantly expressed. At this stage, both E47 and SREBP-1c bound the promoter (1.5 and 5.9-fold increased binding respectively). Consistent with Western blot data in Figure 7D, E47 binding peaks during differentiation when E47 levels are high, and SREBP-1c binding peaks later in differentiation when SREPB-1c levels are maximal (Figure 7E). To confirm that E47 and SREBP-1c bind the adiponectin promoter in vivo and that Id3 regulates this, in vivo ChIP was performed. Epididymal adipose tissue from ApoE-/- and Id3-/-ApoE-/- mice was harvested from eight week-old animals and ChIP analysis was performed as above. Precipitation with an E47 antibody led to a 3.4-fold enrichment of the adiponectin promoter in ApoE-/- mice while in Id3-/-ApoE-/- mice, the enrichment was 6.0-fold compared to isotype control. Precipitation with an SREBP-1c antibody resulted in an 8.8-fold enrichment of adiponectin promoter from ApoE-/- adipose tissue and 10.1-fold enrichment from Id3-/-ApoE-/- adipose tissue. Taken together, these data provide evidence that E47 promotes and Id3 inhibits the expression of adiponectin in adipocytes in vitro and in vivo.
Discussion
A strong link has been established between adiponectin levels and clinical atherosclerosis and Type 2 diabetes; however, the molecular regulation of adiponectin is incompletely understood. The present study provides the first evidence that the HLH proteins Id3 and E47 regulate adiponectin transcription during adipocyte differentiation.
Here, we have shown that Id3 can negatively regulate adiponectin expression. Id proteins are unable to bind DNA, therefore they regulate transcription by dimerizing with other HLH proteins, known as E-proteins, in order to prevent the DNA binding of these factors. We sought to identify an E-protein that may play a role in the regulation of adiponectin. A previous study demonstrated expression of E-proteins in 3T3-L1 cells at the mRNA level31. The current study is the first to attribute a function to any E-protein in adipocyte biology. We show that E47 is expressed in pre-adipocytes and differentiating adipocytes and binds the adiponectin promoter. Further, our data suggest that E47 interacts with SREBP-1c, a known positive regulator of adiponectin, to enhance SREBP-1c-mediated promoter activation. Consistent with our findings in adipocytes, a recent study reported that E47 partners with SREBP-1c in pancreatic islet cells to activate the insulin promoter30.
Like Id3 and E47, SREBP-1c is a member of the HLH family of transcription factors. In contrast to the Ids and the E-proteins, SREBP-1c also possesses a leucine zipper (HLH-LZ)15. Based on structural and empirical data, the HLH-LZ factors are not capable of partnering with Id proteins29,32. Contrary to this, one previous report suggested that Id3 and SREBP-1c bind directly to one another, proposing this as a possible mechanism for Id3 antagonism of SREBP-1c activation of the fatty acid synthase promoter. Using in vitro translated versions of Id3 and SREBP-1c, the authors showed that Id3 altered SREBP-1c binding to the promoter in a gel-shift mobility assay and that Id3 and SREBP-1c co-immunoprecipitated in a cell free system19. We were unable to reproduce these results in a cellular context using either mammalian-two hybrid or immunoprecipitation techniques. Instead, both Id3:E47 and E47:SREBP-1c were shown to interact. This suggests that the action of Id3 on SREBP-1c is via inhibition of the SREBP-1c partner, E47. Accordingly, E47 binding to the promoter was dramatically reduced by the overexpression of Id3 in vitro and was increased in Id3-/-ApoE-/- adipose tissue. While we demonstrate that Id3 inhibits SREBP-1c activity in NIH3T3 cells (Figure 3), we believe that this effect is not direct, but instead due to the interaction of Id3 with endogenous E47 present in NIH3T3 cells33.
Id3 has been proposed to regulate the differentiation of adipocytes since constitutive expression of Id3 in 3T3-F442A cells inhibits the expression of adipsin and adipocyte lipid binding protein mRNAs20. Our data show that acute overexpression of Id3 inhibits adiponectin, but does not change the levels of GLUT4, another marker of differentiation. This is true whether Id3 is expressed before or after the cells undergo differentiation. This suggests that the effect of Id3 on adiponectin is specific rather than via a global blockade of differentiation.
The current study shows that E47 is highly expressed in undifferentiated cells and during differentiation, while expression is significantly decreased in differentiated cells. Correspondingly, E47 binding to the adiponectin promoter occurs in a differentiation state specific manner. No binding is observed in undifferentiated cells when Id3 expression is high. Maximal binding occurs during differentiation when Id3 levels have fallen and a small amount of binding persists in fully differentiated cells that have reduced E47 levels. SREBP-1c expression and promoter binding increase throughout differentiation and are maximal in fully-differentiated adipocytes. The expression of E47 and SREBP-1c overlap during differentiation when Id3 is undetectable which may allow E47:SREBP-1c interaction and activatation of the adiponectin promoter.
SREBP-1c is known to act at two distinct sterol response elements (SREs) within the mouse adiponectin promoter12. This same study reported the existence of three putative E-boxes within the promoter, which we investigated here. These E-boxes are relatively conserved in the human adiponectin promoter, which shares 56% similarity to the mouse promoter12. This similarity includes several E-boxes in the same region as mouse E-box #1, raising the possibility that at least one of these elements could function similarly in the human promoter. In our study, the elimination of E-box #3 (the 0.41kb promoter construct) and the mutation of E-box #2 (Figure 5A) did not significantly change promoter activation by SREBP-1c and E47. While these sequences can be identified as E-boxes based on their similarity to the “canntg” DNA sequence, neither of them represent the type of E-box that is considered to be the classical binding site (cagctg or cacctg) for E-proteins such as E4734. In addition, E-box #2 overlaps with a functional SRE site, making it less likely to be the location of E-protein binding12. E-box #1 represents a classical E-box for E-protein binding and mutational analysis shows that this E-box is necessary for the activation observed with E47 and SREBP-1c (Figure 5). Interestingly, mutation of E-box #1 also decreases the effect seen with SREBP-1c alone. While it is plausible that SREBP-1c binds to E-box #1 itself as SREBP-1c proteins can bind to both SRE and E-box elements35, this latter scenario is less likely given that SREBP-1c binding sites within the adiponectin promoter have been mapped by DNase footprinting, and SREBP-1c binding does not protect the region of the promoter containing E-box #112. Id3 overexpression during differentiation inhibits not only E47 but also SREBP-1c binding to the adiponectin promoter (Figure 7C). Id3 does not appear to bind to SREBP-1c (Figure 6), indicating that SREBP-1c binding at this stage may depend on E47 binding.
Other protein partners for E47 and SREBP-1c are likely to exist, particularly in the case of SREBP-1c, which continues to be expressed and to bind the adiponectin promoter later in differentiation even after E47 expression has been downregulated. Taken together, these data suggest that during differentiation, efficient adiponectin promoter activation by SREBP-1c is dependent on interaction with E47 and E47 binding to E-box #1. Little E47 and prominent SREBP-1c binding to the adiponectin promoter in differentiated cells suggest the existence of E47-independent mechanisms to activate adiponectin expression by SREBP-1c in mature adipocytes. Given the role for Id3 in regulating adiponectin expression both in vitro and in vivo, it will be important to determine what controls Id3 expression in adipocytes. Id3 is known to be an early response gene whose expression is stimulated in response to mitogenic stimuli21 and decreased when cells are growth arrested or induced to differentiate36. To date, no specific factor has been demonstrated to directly control Id3 expression in adipocytes. Further studies to identify such factors in these cells seem warranted given the significant role Id3 plays in the control of adiponectin expression.
In summary, we have shown that Id3 regulates the expression of adiponectin in differentiating adipocytes in culture and in adipose tissue in vivo. During differentiation, E47 is capable of partnering with SREBP-1c, a previously identified positive regulator of adiponectin expression, to enhance SREBP-1c-mediated adiponectin transcription. It will be important to identify other HLH factors that may regulate adiponectin as well as other adipocyte markers in order to more fully understand the role of HLH factors in adipocyte biology.
Figure 8. Schematic representation of adiponectin regulation by SREBP-1c and HLH factors.
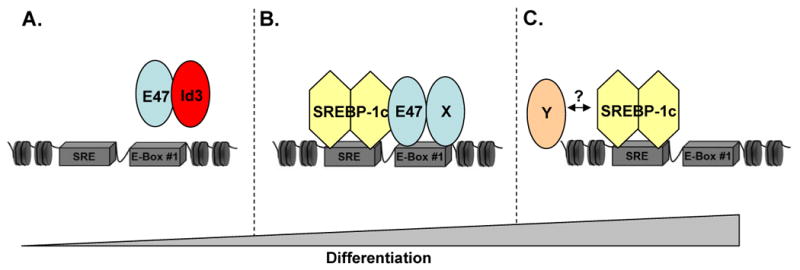
A, In undifferentiated cells, Id3 and E47 are expressed, and no binding to the promoter is observed. SREBP-1c expression is undetectable. B, When endogenous Id3 expression is low during differentiation, E47 is free to bind to the adiponectin promoter. X is either a tissue-specific bHLH protein or an E-protein. SREBP-1c is also expressed and binds the promoter. The synergism observed between E47 and SREBP-1c may be due to E47 enhancement of SREBP-1c binding or recruitment of coactivators. C, In fully differentiated adipocytes, E47 expression is reduced but SREBP-1c expression is maximal. At this stage, SREBP-1c may activate the adiponectin promoter alone or in conjunction with an unidentified partner (Y).
Acknowledgments
The authors would like to thank Toni Barbera and the Diabetes Center Animal Characterization Core at the University of Virginia (NIH DK063609) for their assistance.
Sources of Funding: This work was supported by NIH P01 HL55798 (C.A.M.), NIH Training Grant 5-T32 HL007355-29 (A.C. and H.D.) and an American Heart Association Pre-Doctoral Fellowship (A.C.D.).
Footnotes
Disclosures: None.
References
- 1.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 2.Trujillo ME, Scherer PE. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 3.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. The Journal of biological chemistry. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 4.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 5.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 6.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 7.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 9.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. Jama. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. The Journal of biological chemistry. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 12.Seo JB, Moon HM, Noh MJ, Lee YS, Jeong HW, Yoo EJ, Kim WS, Park J, Youn BS, Kim JW, Park SD, Kim JB. Adipocyte determination- and differentiation-dependent factor 1/sterol regulatory element-binding protein 1c regulates mouse adiponectin expression. The Journal of biological chemistry. 2004;279:22108–22117. doi: 10.1074/jbc.M400238200. [DOI] [PubMed] [Google Scholar]
- 13.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 14.Park SK, Oh SY, Lee MY, Yoon S, Kim KS, Kim JW. CCAAT/enhancer binding protein and nuclear factor-Y regulate adiponectin gene expression in adipose tissue. Diabetes. 2004;53:2757–2766. doi: 10.2337/diabetes.53.11.2757. [DOI] [PubMed] [Google Scholar]
- 15.Tontonoz P, Kim JB, Graves RA, Spiegelman BM. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol. 1993;13:4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 17.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 18.Barone MV, Pepperkok R, Peverali FA, Philipson L. Id proteins control growth induction in mammalian cells. Proc Natl Acad Sci U S A. 1994;91:4985–4988. doi: 10.1073/pnas.91.11.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moldes M, Boizard M, Liepvre XL, Feve B, Dugail I, Pairault J. Functional antagonism between inhibitor of DNA binding (Id) and adipocyte determination and differentiation factor 1/sterol regulatory element-binding protein-1c (ADD1/SREBP-1c) trans-factors for the regulation of fatty acid synthase promoter in adipocytes. Biochem J. 1999;344(Pt 3):873–880. [PMC free article] [PubMed] [Google Scholar]
- 20.Moldes M, Lasnier F, Feve B, Pairault J, Djian P. Id3 prevents differentiation of preadipose cells. Mol Cell Biol. 1997;17:1796–1804. doi: 10.1128/mcb.17.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norton JD, Deed RW, Craggs G, Sablitzky F. Id helix-loop-helix proteins in cell growth and differentiation. Trends Cell Biol. 1998;8:58–65. [PubMed] [Google Scholar]
- 22.Forrest ST, Barringhaus KG, Perlegas D, Hammarskjold ML, McNamara CA. Intron retention generates a novel Id3 isoform that inhibits vascular lesion formation. The Journal of biological chemistry. 2004;279:32897–32903. doi: 10.1074/jbc.M404882200. [DOI] [PubMed] [Google Scholar]
- 23.Forrest ST, Taylor AM, Sarembock IJ, Perlegas D, McNamara CA. Phosphorylation regulates Id3 function in vascular smooth muscle cells. Circ Res. 2004;95:557–559. doi: 10.1161/01.RES.0000142735.67542.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K, Bickel PE. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res. 2006;47:450–460. doi: 10.1194/jlr.D500037-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Francis J, Chakrabarti SK, Garmey JC, Mirmira RG. Pdx-1 links histone H3-Lys-4 methylation to RNA polymerase II elongation during activation of insulin transcription. The Journal of biological chemistry. 2005;280:36244–36253. doi: 10.1074/jbc.M505741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. The Journal of biological chemistry. 1980;255:4745–4750. [PubMed] [Google Scholar]
- 27.Orlicky DJ, Schaack J. Adenovirus transduction of 3T3-L1 cells. J Lipid Res. 2001;42:460–466. [PubMed] [Google Scholar]
- 28.Charron MJ, Katz EB, Olson AL. GLUT4 gene regulation and manipulation. The Journal of biological chemistry. 1999;274:3253–3256. doi: 10.1074/jbc.274.6.3253. [DOI] [PubMed] [Google Scholar]
- 29.Sun XH, Copeland NG, Jenkins NA, Baltimore D. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol. 1991;11:5603–5611. doi: 10.1128/mcb.11.11.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amemiya-Kudo M, Oka J, Ide T, Matsuzaka T, Sone H, Yoshikawa T, Yahagi N, Ishibashi S, Osuga J, Yamada N, Murase T, Shimano H. Sterol regulatory element-binding proteins activate insulin gene promoter directly and indirectly through synergy with BETA2/E47. The Journal of biological chemistry. 2005;280:34577–34589. doi: 10.1074/jbc.M506718200. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Weng YC, Schatteman GC, Sanders L, Christy RJ, Christy BA. Expression of the dominant-negative regulator Id4 is induced during adipocyte differentiation. Biochem Biophys Res Commun. 1999;256:614–619. doi: 10.1006/bbrc.1999.0386. [DOI] [PubMed] [Google Scholar]
- 32.Beckmann H, Kadesch T. The leucine zipper of TFE3 dictates helix-loop-helix dimerization specificity. Genes Dev. 1991;5:1057–1066. doi: 10.1101/gad.5.6.1057. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, Cano A. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. The Journal of biological chemistry. 2001;276:27424–27431. doi: 10.1074/jbc.M100827200. [DOI] [PubMed] [Google Scholar]
- 34.Jones S. An overview of the basic helix-loop-helix proteins. Genome Biol. 2004;5:226. doi: 10.1186/gb-2004-5-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JB, Spotts GD, Halvorsen YD, Shih HM, Ellenberger T, Towle HC, Spiegelman BM. Dual DNA binding specificity of ADD1/SREBP1 controlled by a single amino acid in the basic helix-loop-helix domain. Mol Cell Biol. 1995;15:2582–2588. doi: 10.1128/mcb.15.5.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zilberfarb V, Siquier K, Strosberg AD, Issad T. Effect of dexamethasone on adipocyte differentiation markers and tumour necrosis factor-alpha expression in human PAZ6 cells. Diabetologia. 2001;44:377–386. doi: 10.1007/s001250051630. [DOI] [PubMed] [Google Scholar]


