Abstract
A broad range of nanomedicines is being developed to improve drug delivery for CNS disorders. The structure of the blood–brain barrier (BBB), the presence of efflux pumps and the expression of metabolic enzymes pose hurdles for drug-brain entry. Nanoformulations can circumvent the BBB to improve CNS-directed drug delivery by affecting such pumps and enzymes. Alternatively, they can be optimized to affect their size, shape, and protein and lipid coatings to facilitate drug uptake, release and ingress across the barrier. This is important as the brain is a sanctuary for a broad range of pathogens including HIV-1. Improved drug delivery to the CNS would affect pharmacokinetic and drug biodistribution properties. This article focuses on how nanotechnology can serve to improve the delivery of antiretroviral medicines, termed nanoART, across the BBB and affect the biodistribution and clinical benefit for HIV-1 disease.
Keywords: BBB, CNS, drug delivery, HIV, nanoparticles, nanotechnology
To maximize treatment of CNS diseases, drugs must cross the blood–brain barrier (BBB) and show limited systemic toxicities. This task is by no means simple. Owing to its structural and functional complexities, the BBB represents one of the greatest challenges impeding drug penetration for combating nervous system diseases [1]. For example, drugs, nucleic acids, proteins, imaging agents and other low-molecular-weight compounds and macromolecules are restricted in brain entry. Thus, the means to improve delivery of compounds across the BBB in an efficient, safe and site-specific manner remain a primary goal to achieve optimal therapeutics in the battle against neuroinflammatory and neurodegenerative diseases [2–5].
The major bottleneck in the development of both diagnostic tools and therapies aimed at combating CNS disorders is in modulating BBB structure, function and biophysiology. With regards to structure, the ability of the barrier to effectively limit transport is attributed, in part, to brain microvessel endothelial cells (BMVECs) that form brain capillaries. Without question, BMVECs are a principal means for limiting transport and solute passage from blood to the CNS [1,6–9]. The function and biophysiology of the barrier is also linked to its tight intercellular junctions, low pinocytic potentials and the presence of high levels of drug efflux transporters and metabolizing enzymes.
In our quest to improve drug delivery to the CNS and specifically for neuroAIDS we have, over the past quarter of a century, researched the pathobiology of neuroAIDS and BBB function [10–27]. Our most recent pursuits in this area involve nanomedicine [28–34]. We have used this review to highlight our own progress and those of others from studies of CNS neuropathogenesis to the development of nanomedicine approaches designed to improve BBB penetration of drugs to attenuate disease.
The review is divided into four major parts. The first provides a background on the BBB towards affecting drug penetration into the CNS; the second focuses on nanoformulated antiretroviral therapy (ART) currently being developed; the third brings ART into the context of viral pathobiology; and the fourth addresses nanomedicine approaches being developed or now available for neuroAIDS and specifically what is being developed in our own laboratories. It is our intent to bring a basic understanding of microbial pathogenesis into schemes for nanoformulated drug development that will have a broader impact in combating adverse disease outcomes.
Barriers to CNS drug delivery
BBB
The BBB evolved to protect the brain from toxic substances but in doing so, it hinders the delivery of diagnostic and therapeutic agents [6]. Understanding the physiological features of the barrier is required in order to discover ways to effectively deliver compounds to diseased brain regions. The BBB consists of walls formed by capillaries that separate the brain from circulating blood. The human brain contains approximately 100 billion capillaries that have a net surface area of approximately 20 m2. Despite this extremely large surface area, permeability of many drugs to the BBB is low [7,8]. The lack of permeability can be attributed to its anatomical structure, expression of efflux transporters and level of enzyme activity. The BBB is composed of multiple cells and cell components, which together form the neurovascular unit. These cells include BMVECs and perivascular elements such as astrocyte endfeet, pericytes, leptomeningeal cells, basement membrane and parenchymal coverings [35]. Astrocytes and pericytes secrete a variety of soluble factors that are necessary for normal differentiation, homeostasis and barrier function [36,37]. Unlike the rest of the body, capillaries within the CNS lack intercellular clefts and fenestrae. This is because the BMVECs form tight junctions by the interaction of various transmembrane proteins, which effectively prevent the paracellular movement of drug agents. This essentially divides the cell into two distinct halves: luminal (blood) and abluminal (brain). On the abluminal side of the BMVECs are pericytes, which, along with BMVECs, are ensheathed by basal lamina and surrounded by astrocyte endfeet. In addition to these physical barriers, BMVECs have an increased amount of mitochondria and low pinocytotic activity, which decrease the passage of antiretroviral molecules even further [1,38]. Therefore, the CNS is essentially anatomically sealed from the rest of the body and any transport of drugs to the brain from the periphery must occur by a transcellular route across the BMVECs.
Some lipophilic and low-molecular-weight substances can cross the BMVECs by passive diffusion. However, a large number of lipophilic compounds are rapidly discharged from BMVEC cytoplasm by efflux systems. The BBB possesses a number of active and passive efflux mechanisms. Active transporters can reduce the amount of measured penetration to levels lower than predicted when considering the physiochemical characteristics of drugs [39,40]. Multidrug transporters such as multiple drug resistance protein, P-glycoprotein (Pgp), breast cancer resistance protein and multiple organic anion transporter all belong to the ATP-binding cassette family and can affect drug entry [39–42]. Pgp is a product of the MDR gene, is highly expressed by BMVECs and has a broad range of specificity to a structurally diverse group of lipophilic and amphiphilic molecules, including many antiretroviral compounds [43,44]. In addition to the efflux transporters, BMVECs also possess an enzymatic barrier to drug transport. The activity and expression of enzymes that participate in metabolism and activation of endogenous compounds is elevated within BMVECs and include, but are not limited to, g-glutamyl transpeptidase, alkaline phosphatase and aromatic acid decarboxylase [45].
The BBB, however, does not completely prevent all molecules from passing into the brain [46–48]. The BBB must allow the passage of necessary components to maintain proper and normal brain function. In addition to isolating the brain from unwanted molecules, the BBB also provides selective transport of small molecules, polypeptides and even cells to the CNS. Nutrients and endogenous compounds required by the CNS such as amino acids, glucose, essential fatty acids, vitamins, minerals and electrolytes are effectively carried to the brain by numerous saturatable transport systems expressed at the BBB [46]. By first restricting the entry of unwanted molecules and then selectively transporting desired substances from circulation, the BBB acts as a regulatory membrane that largely defines the brain's homeostatic environment [49,50]. This includes maintaining the interstitial and cerebral spinal fluid (CSF) within very narrow limits. Neurons are especially sensitive to their environmental milieu and even small fluctuations within it can result in dysfunction, degeneration and death. Thus, proper maintenance of the fluid compartments of the brain is necessary for neurons to be able to carry out their complex functions. Another important function of the BBB is neuroprotection. In addition to being sensitive to their surroundings, neurons are vulnerable to a wide range of toxins. This includes metabolites, chemicals or xenobiotics that may be present in the blood [51].
During times of infection and tissue death, immune cells must be able to enter the brain and remove necrotic tissue and microbial infections [52]. Immunocytes have the same barriers to cross if they are to enter the CNS. As a consequence, the process of leukocyte penetration into the brain involves several steps. The first is penetration of the endothelial monolayer through integrin β1-mediated processes, and the second is penetration through the BBB basement membrane and glia limitans by matrix metalloproteinases [53].
Blood–CSF barrier
The BMVECs discussed previously make up 95% of brain vasculature; the remaining 5% is composed of the circumventricular regions of the CNS. These regions contain microvessels that are analogous to those of the periphery. Although these capillaries are more permeable to solutes, choroid plexus epithelial cells and regions of tanycytes adjacent to ventricles also form tight junctions that help to inhibit drug transport. The choroid plexus is the major site of CSF production. Blood is filtered through ependymal cells to form CSF. The blood–CSF barrier (BCB) is found within the epithelial layer of the choroid plexus. The BCB is more ‘leaky’ than the BBB, and the fact that its electric resistance is 10-times less than that of the BBB reflects this. Despite the fact that the BCB surface area is much smaller than that of the BBB, its leakiness could account for the small amount of albumin normally found in CSF and may have functional consequences. However, penetration across the BCB does not guarantee transport into the brain due to the diffusion distance between CSF and brain interstitial fluids.
Virus detection & disease
Reliable detection of HIV is of great importance for resource-limited areas that have a high incidence of infection. In these regions there is a great need for detection assays that are both sensitive and specific, in addition to being cost effective, portable and easy to operate. By diagnosing patients earlier in the course of disease, antiviral drugs could be administered sooner, improving clinical outcomes. Nanotechnology-based techniques are being evaluated for their use in medical testing and could potentially provide a new generation of diagnostic assays. These technologies have high degrees of sensitivity and specificity, multiplexing capabilities and can operate without enzymes. One example is the development of the bio-barcode assay for the detection of protein and nucleic acid. Two types of nanoparticles (NPs) are used in this assay: magnetic or gold NPs with a recognition agent that forms a sandwich around the target and hundreds of thiolated single-strand oligonucleotide barcodes. After particle–target interaction, a magnetic field is used to localize and collect the structures and then the barcode strands are released and removed. The barcode strands can be identified on a microarray via scanometric detection or in situ [54]. As these methods do not require enzymatic amplification, they have the potential to be translated into a powerful and useful detection assay. This method has been modified for the detection of HIV-1 p24 and Gag proteins. Each method was far more sensitive than the traditional ELISA method, by approximately 150-times in the case of p24 detection and exhibited or neared 100% sensitivity and specificity [55,56].
During HIV infection, enumeration of CD4+ T lymphocytes is required in order to establish the level at which the immune system has been compromised. The gold standard method used in developed countries is based on flow cytometry, which is precluded from widespread use in resource-scarce settings owing to its high expense and technical requirements. In an effort to address the need for a simpler and more cost-effective measure of this necessary diagnostic criteria, the integration of quantum dots (semiconductor nanocrystals) into a portable microfluidic-based lymphocyte capture and detection system has been developed. This integrated system is capable of isolating and counting CD3+CD4+ lymphocyte sub-populations from whole blood. Combining the unique optical properties of quantum dots with microfluidic systems has reduced the optical requirements signifIcantly relative to molecular fluorophores and showed good correlation with flow cytometry measurements [57].
Another method uses a gold electrode surface with thiolated single-walled carbon nanotubes/gold NPs modified with ferrocene–pepstatin to detect HIV-1 protease. The interaction between the ferrocene–pepstatin-modified NPs and HIV-1 protease showed enhanced electrochemical responses as the protease level increased. When modified with thiolated nanotubes and pepstatin NPs, the sensing electrode showed remarkable detection sensitivity with an estimated detection limit of 0.8 pM [58].
Nanomedicine & HIV disease
Nanomedicines are being developed for HIV therapies. Nanotechnology and the properties of nanomaterials allow for unique ways to inhibit viral mechanisms such as infection, growth and spread. Metal NPs, such as gold and silver, have been extensively researched for their use in imaging, bioassays and therapy [59–61], and are now being studied for their possible use in HIV therapy. Gold has proven to be a good material for nanomedical approaches since it is biocompatible, its NPs can easily be constructed and molecules are readily conjugated to its surface. Gold NPs were conjugated to SDC-1721, a fragment of the potent HIV inhibitor TAK-779, which acts by allosteric inhibition of the CCR5 receptor. Free SDC-1721 has no inhibitory effect on HIV infection; however, SDC-1721-gold NPs displayed activity comparable to that of TAK-779. This suggests that presentation of small molecules on the surface of gold NPs can convert inactive drugs into potent viral inhibitors [62]. The interaction of metal NPs with proteins such as amyloid-β in Alzheimer's disease and α-synuclein in Parkinson's disease were investigated. However, within this field, an area that has been largely unexplored is the interaction of metal NPs with viruses. Silver NPs can interact with HIV and inhibit infection in vitro. The silver NPs interacted with HIV in a size-dependent manner, as only NPs within the 1–10 nm size range attached to the virus. The suggested mechanism of this interaction occurs through preferential binding to HIV-1 gp120 glycoprotein knobs because of the regular spatial relationships of attached NPs, the center-to-center distance between them and the fact that the exposed sulfur-bearing residues of the glycoprotein knobs of HIV-1 gp120 would be attractive sites for interaction. This is an interesting drug-free method of viral inhibition and more work is needed to investigate the interactions between metal NP and virus [63].
Residual virus is present in the lymphoid tissues of patients on ART [64,65]. Since viruses isolated from these tissues are also susceptible to antiretroviral medications, it was suggested that insufficient drug exposure is a major cause of viral persistence [66]. In order to address this issue, work was carried out with NPs that target lymphoid tissues. Indinavir (IDV)-NPs composed of disteroyl phosphatidylcholine and methyl polyethylene glycol-disteroyl phosphatidylethanolamine were designed. At physiologic pH, IDV binds to the lipid. When administered subcutaneously, the NPs provided IDV concentrations up to 2000% higher in the lymph nodes when compared with the plasma of HIV-infected macaques. However, the plasma concentration achieved was only equivalent to the trough concentration of an oral dose [67]. While it is important to deliver drugs to virus-specific tissue regions, ensuring therapeutic drug levels in the plasma is also required. Therefore, it would be necessary to pair a therapy such as this with one that maintains high drug levels in the plasma.
Since many antiretroviral drugs are rapidly metabolized and eliminated from circulation, one issue that has long plagued HIV therapy has been the poor pharmacokinetics and biodistribution of ART. As such, long-acting parenteral formulations of ART could facilitate treatment maintenance during HIV infection. Rilpivirine, a poorly water-soluble non-nucleoside reverse transcriptase inhibitor (NNRTI), NPs have been manufactured by wet milling in an aqueous carrier and coated with either poloxamer 338 or d-α-tocopheryl polyethylene glycol 1000 succinate surfactants. When compared with intramuscular injections, a single subcutaneous administration resulted in the most stable plasma levels, which were constant at 25 ng/ml for 20 days, followed by a slow subsequent decline to 1–3 ng/ml at 3 months. NPs with a diameter of 200 nm achieved higher and less variable plasma concentrations than 400 or 800 nm NPs. This study provides evidence that subcutaneous injections of antiretrovial NPs (nanoART) may serve as a long-acting therapy [68].
The pharmacokinetics and biodistribution produced with the use of the two particles described earlier are promising. To be able to achieve stable, long-term drug levels in the plasma, or penetration into poor drug penetrating tissue regions would greatly improve ART. However, the major concern with this method is the route of administration. It is not uncommon to have reactions at the injection site in addition to other untoward side effects when concentrated doses of drugs are injected subcutaneously. This has been an issue for drugs formulated for this type of delivery [69,70]. Issues such as this would first have to be addressed before these types of formulation could be used in humans.
HIV-1-associated neurocognitive disorders
HIV-1-associated neurocognitive disorders (HAND) is a neurodegenerative disorder that occurs as a consequence of HIV infection. Nearly half of all people infected with HIV display some form of neurological dysfunction, most likely due to entry of HIV-1 into the CNS [71,72]. HAND, worldwide, is one of the top causes of dementia and is reflected by varying degrees of cognitive and motor impairments [2,45,73–75]. The virus enters the brain within CD4+ T lymphocytes and/or mononuclear phagocytes (MPs: dendritic cells, monocytes and macrophages) [76]. The pathophysiology of HAND revolves around the formation of multinucleated giant cells of MP lineage. These formations serve as the main reservoirs for virus within the CNS [77].
In HAND, reactive MP neurotoxic responses dominate at the site of neuronal injury and are amplified through paracrine and autocrine signaling [14]. Secretion of neurotoxins from MPs can induce neuronal injury, adding to neurocognitive and motor impairments. It is likely that microglial responses also play a major role in the loss of neurons [20,71,78–82]. Activated microglia can induce neuronal injury via secretion of glutamate, proinflammatory factors, reactive oxygen species and quinolinic acid. By secreting various signaling molecules, activated microglia can also mobilize adaptive immune responses and stimulate cell chemotaxis. This leads to transendothelial immune cell migration across the BBB and perpetuation of neural damage [83–86]. Clear connections between inflammation and neurodegeneration have been established [87,88]. As the disease progresses, inflammatory secretions from activated microglia and immune cells engage nearby astrocytes and endothelial cells, perpetuating a vicious positive feedback cycle of inflammation, which results in significant tissue injury [89]. Successful delivery of antiretroviral compounds to the brain and elimination of this viral sanctuary could effectively eliminate this potentially treatable form of dementia.
ART, CNS drug penetration & the BBB
The CNS is a site that ART often has difficulty reaching. Clinically, the penetration of ART into the CNS shows limitations [90,91]. Owing to its physical structure, the presence of efflux pumps and its high expression of metabolizing enzymes, the BBB is an effective barrier against many antiretrovirals [90,92]. In addition, ART are commonly protein bound within the plasma, further limiting their access to the CNS [90,93]. Since HIV can easily enter the CNS but antiretroviral drugs cannot, the brain essentially becomes a sanctuary for HIV [94,95], acting not only as a reservoir, making it difficult to completely eradicate the virus, but also increasing the chance of a viral mutation leading to drug resistance [96]. Even though the BCB is easier to penetrate than the BBB, it has not been considered an option for delivery of HIV therapeutic agents owing to its small surface area and diffusion limitations. Cells of the MP lineage have the dual properties of both being major targets of HIV infection and having the ability to cross the BBB. As such, entry of HIV-infected MPs into the CNS is of particular importance to the spread of HIV into the brain and persistence of infection in this immunoprivileged site [94]. Owing to the importance of getting antiretroviral drugs into the brain, a number of nanotechnologybased methods for crossing the BBB are being specifically developed for this class of compounds. These methods include disruption of the BBB, development of NPs with increased BBB permeability, uptake by BMVECs via adsorptive-mediated transcytosis and cell-mediated delivery.
Modulation of the BBB
An alternative approach to CNS antiretroviral drug delivery is to disrupt the BBB. By altering the structure or function of the BBB, molecules could be allowed passage where otherwise they would not. A drug can be administered systemically in conjunction with transient disruption of the BBB, theoretically increasing the rate of extravasation of the drug into the brain parenchyma. Several experimental and clinical strategies have been employed to disrupt the BBB including chemical, osmotic, biochemical and MRI-guided ultrasound disruption. However, BBB disruption can be dangerous because it may allow unwanted blood components into the CNS, which can lead to serious complications. These methods have been met with less than great success and are generally reserved for extreme situations.
One novel method for increasing the passage of antiretroviral-containing NPs across the BMVECs is to disrupt the BBB with electromagnetic interference (EMI). Application of EMI, in the form of amplitude modulated (AM) and frequency modulated (FM) waves, has been shown to increase the permeability of a number of tissues, including the BBB [97,98]. This led to the possibility of increasing the permeability of the BBB to antiretroviral-containing NPs with the use of AM and FM waves being tested. Using human BMVECs as a BBB, it was demonstrated that both saquinavir containing polymeric NPs and solid lipid NPs (SLNs) had increased permeability across the cell monolayer with the application of EMI [99]. Not all EMI had the same effect on BMVECs. While all waves (sine, square and triangle) and both AM and FM signals did increase permeability, square AM waves had the strongest effect by increasing permeability to NPs by more than 14-times, while having almost no effect on the permeability of the free drug. This would be a unique and noninvasive way of increasing the passage of antiretroviral NPs into the CNS.
The use of nanomaterials to modulate the function of the BBB has also been explored to increase drug penetration into the brain. As discussed earlier, Pgp is an ATP-driven drug efflux transporter that is a critical element of the BBB. Since Pgp is highly expressed, present on the luminal membrane and multispecific, it is a selective gatekeeper of the BBB and thus a major obstacle to HIV drug delivery into the brain [100]. Modulating Pgp could therefore improve CNS HIV drug delivery [101,102]. Pluronic® P85 (P85) is an amphiphilic block copolymer with two chains of hydrophilic polyethylene oxide groups flanking a hydrophobic polypropylene oxide group. P85 has been shown to inhibit the action of Pgp by energy depletion (via depletion of the available pool of ATP) and by inhibition of Pgp ATPase activity (via membrane fluidization) [103]. In vitro and in vivo experiments have shown how P85 can improve the penetration of HIV drugs into the brain by inhibiting drug efflux by Pgp. In one study, it was demonstrated that the presence of P85 increased the accumulation of saquinavir and nelfinavir across a cell monolayer with possible inhibition of multiple drug resistance proteins in addition to Pgp [104]. The in vivo efficacy of P85 has been demonstrated in a severe combined immunodeficiency mouse model of HIV-1 encephalitis inoculated with HIV-1-infected monocyte-derived macrophages into the brain. In addition to enhancing the efficacy of zidovudine, lamivudine and nelfinavir, P85 also demonstrated potent antiretroviral effects when used alone [33]. By having the dual properties of increasing BBB penetration of HIV drugs and having direct antiretroviral effects, P85 and other polymers will probably play a future role in HIV therapy.
NanoART uptake by receptor-mediated adsorptive endocytosis in BMVECs
One method for increasing delivery of antiretroviral compounds to the brain would be to specifically increase the uptake of ART-containing NPs by BMVECs. Adsorptive-mediated transcytosis provides a means for delivering medicines across the BBB. BMVECs are readily equipped for this process. These cells can bind and uptake molecules at the luminal surface and then release them by exocytosis at the abluminal surface. This transcytotic pathway provides a means for transporting molecules through the endothelial cytoplasm [105]. This concept could be applied to increase delivery of antiretrovirals to the brain by attaching a molecule to the NP surface that would induce adsorptive-mediated transcytosis by BMVECs. One such molecule, which is known to induce this pathway in BMVECs, is trans-activating transcriptor (Tat). The main function of HIV Tat is to increase transcription of viral genes by binding to cellular factors and affecting phosphorylation [106]. In addition, HIV Tat can act as a director of toxin to noninfected cells and induce apoptosis [107]. Tat peptide conjugated to the surface of NPs can increase the transport of encapsulated ritonavir into BMVECs, bypass the efflux action of Pgp and increase transport into the CNS. The drug level within the brain was 800-fold higher for Tat-NPs than free drug 2 weeks after administration, with clearance seen at 4 weeks. Tat-conjugated NPs can enhance the CNS bioavailability of encapsulated ritonavir and maintain therapeutic drug levels in the brain for a sustained period [108]. Unfortunately, this strategy suffers from several limitations such as neurotoxicity and immunogenicity [105]. The toxicity of Tat to neurons has been well characterized [109]. Uptake by this mechanism, the interactions between neurons and Tat-NPs, and the subsequent effect on the cells must be better understood.
Solid lipid & polymeric NPs
Solid lipid NPs are spherical and dispersed in water or an aqueous surfactant solution. Generally, these NPs consist of a solid hydrophobic core coated with a phospholipid monolayer. Lipophilic drugs can be dissolved within the hydrophobic core and transported by the particle throughout the body or targeted to particular regions. Kaur et al. thoroughly discussed the use of SLNs for drug delivery to the CNS and the advantages these NPs have over others [110]. To summarize, these advantages include, but are not limited to, SLNs less than 200 nm not being readily taken up by the reticuloendothelial system, thereby avoiding uptake by the liver, controlled drug release for up to several weeks, the production of high drug payloads and increased biocompatibility of biodegradeable carrier lipids.
Polymeric NPs have also been researched for their potential use in drug delivery to the brain. Chen et al. thoroughly discussed polymeric NPs and their suitability as drug-delivery systems to the CNS [111]. In this article, the various mechanisms for NP uptake by the brain are outlined. To summarize, these include enhanced retention in brain capillaries, leading to adsorption into the capillary wall and formation of a high concentration gradient, opening of tight junctions and adsorptive-mediated transcytosis. In addition, using polysorbates to coat the particles has been reported to increase penetration of NPs across the BBB [112,113]. Polysorbate-80 has been used to increase the uptake of nanoART into BMVECs [114–117]. Some of the proposed mechanisms by which this might work include: increased BMVEC membrane fluidization, facilitated endocytosis and inhibition of the Pgp efflux system.
Both types of particles are being developed to increase delivery of antiretroviral drugs to the CNS, and their abilities to do this are being compared. Studies have investigated how composition, size, surface characteristics and the type of drug carried affect the ability of the NP to cross the BBB. Two polymeric carriers, which have been well researched for this purpose, are polybutylcyanoacrylate (PBCA) and methylmethacrylate–sulfopropylmethacrylate. PBCA is better than methylmethacrylate–sulfopropylmethacrylate, in some instances by tenfold, at transporting the HIV antiretrovirals zidovudine, lamivudine, delavridine and saquinavir across the BMVECs. In addition, PBCA is also superior to SLNs at transporting delavirdine and saquinavir. Therefore, PBCA is generally more efficient at transporting antiretroviral drugs than the other two carriers mentioned. These results indicate that the properties of PBCA may make it a preferred material for increasing the delivery of ART across the BBB [114–116]. SLNs have also been developed for atazanavir transport [118].
Cationic-SLNs (CSLNs) are similar to SLNs except that their shells are modified with a positively charged lipid. Positively charged NP carriers can be beneficial to drug loading and improve cellular uptake via electrostatic interactions [114,115]. In addition, a NP with a complex structure can improve the pharmacokinetics of drug entrapment and release from the NP itself. One example is a CSLN composed of a nonionic core consisting of Compritol 888 ATO and cacao butter surrounded by cationic lipids stearylamine and dioctadecyldimethyl ammonium bromide. One issue with nanoART is that there is a large initial burst of drug released from the NP, leading to less drug release over time. If the initial amount of drug that is released could be reduced, then more drug would be present to be released over a potentially longer time span. Work with this particle indicated that a lipid core made up of complicated components could extend the time of ART release by limiting the initial burst and decelerating the rate of release. This was further demonstrated by the fact that a core consisting of both nonionic lipids had a longer period of drug release than a NP made up of just one of the lipids [117]. A suggested reason for why a core of multiple nonionic lipids is superior is that a simple core may form a regular crystal lattice structure that leaves less space for drug molecules, while a complex core may result in a variety of formations and leave multiple available spatial vacancies. This has beneficial effects upon both drug loading of the NP and release and may prove to be an important future concept for nanoART.
The studies discussed in this section demonstrate the importance of the relationship between the carrier compound and the drug being transported. Generally, there is an optimum ratio of different lipids and surfactants that provides the best loading efficiency, entrapment efficiency, stability, uptake, transport and release for each individual drug based upon its structure, size, charge and degree of lipophilicity. Therefore, it is necessary to discover which lipid and surfactant composition and ratio best facilitates delivery of each drug. It would be extremely useful if a computer program could be developed that was capable of modeling the interactions between a drug and its potential carrying compound, in order to provide information about the physical characteristics, and thus the possible biologic behavior of a potential ART–NP complex. Unfortunately, we are currently limited to seeing what combinations have succeeded or failed in the past for each individual drug and continuing to narrow down the optimal carrier by trial and error. While we can make predictions in this area, we will continue to be very dependent on the successes and failures of previous studies to guide future experiments.
While it is encouraging to see evidence that BMVECs are capable of taking up polymer-NPs, SLNs, CSLNs and other similar particles, there are many obstacles to overcome before this could be a viable therapy. These cells must be able to take up the particles before they are removed from circulation and made unavailable. This is one issue that we have been attempting to address in our laboratory. Many examples have shown that it is possible to deliver nanoART to BMVECs, however, delivering those compounds to those same cells in vivo is another challenge. To bypass this problem, we have been using monocytes and macrophages as vehicles for antiretroviral-NP delivery.
Cell-mediated nanoART
Nanocarriers for CNS drugs offer many benefits that can aid increased delivery to the brain. As drugs circulate throughout the body, they encounter blood proteins and enzymes that may result in degradation. Protective carriers could prevent interaction between the drug and these degrading elements. In addition, a drug carrier can be targeted to the brain by linking a specific moiety that would aid in receptor-mediated transport across the BBB. There are nanocarriers that have very high drug-to-carrier ratios and offer high ‘payload’ potential by allowing many drug molecules to be transported by a single carrier. This could improve the efficacy of delivery by minimizing involvement of the number of targeted receptors while maximizing the amount of drug delivered per interaction. Generally, studying the pharmacokinetic handling of nanomaterials by cells is achieved by exposing phagocytic cells to nanoART. The source of the cells can be from cell lines, cells harvested from animals or obtained from human donors. Cells are then exposed to the nanomaterials within culture media and uptake within the cells is often measured by reverse-phase high performance liquid chromatography or liquid chromatography mass spectrometry. Subsequent release of the NPs can be studied by exchanging treatment media with fresh, compound-free media and measuring particle content of the cells and the media. Interaction of the cells with the nanomaterials can be studied by fluorescence microscopy, fluorometry, flow cytometry and scintillation counting methods.
For cell-mediated delivery, MPs can incorporate micro- and nanosized drug carriers (such as liposomes, dendrimers, SLNs, CSLNs and polymeric-gelatin NPs) and act as Trojan horses by crossing the BBB and delivering drugs to affected brain areas. A broad range of neurodegenerative diseases have an inflammatory component [14,20,119]. MPs have the unique ability to migrate to sites of inflammation through diapedisis and chemotaxis [120]. The cells also have the ability to phagocytize, produce cytotoxic compounds and subsequently release these compounds by exocytosis. Therefore, these cells make great candidates for cell-mediated drug delivery to the CNS. Indeed, MPs are capable of endocytosing colloidal nanomaterials and subsequently releasing them into surrounding media, as discussed [9,121]. These cells seem to prefer charged compounds as they preferentially uptake charged rather than relatively neutral NPs [122,123].
Using MPs as vehicles for drug carriage ensures that drugs with intracellular action that are incapable of crossing cell membranes, such as antiretroviral compounds, can be assisted in reaching their target cells or tissue. Cellular uptake mechanisms vary according to cell type and the physical/chemical properties of the compound [30,124–126]. Intracellular targeting is feasible through the use of ligands that trigger receptor-mediated endocytosis or by modification of the NP surface, such as coating the surface of the particle in IgG to induce or enhance cell uptake (Figure 1). Some NPs have been shown to be transported into primary endosomes and sorted to either recycling endosomes or secondary endosomes [127]. Within the acidic environment of the secondary endosome, the NP surface changes from anionic to cationic, leading to the escape of the drug into the cytoplasm. In a study where pentamidine-laden NPs were administered to Leishmania-infected mice, ultrastructural studies showed NP trafficking inside Leishmania-infected Kupffer cells. In these cells, the NPs were located within vacuoles and primary lysosomes, which mature into secondary lysosomes. Secondary lysosomes containing NPs and parasitophorous vacuoles were also observed [128]. During HIV infection, these same MPs are infected and transport the virus. Thus, similar trafficking of antiretroviral-NPs within MPs could help not only to target drug delivery to the cells that are infected, but to the very compartments where the virus is developing.
Figure 1. Surface modification of nanoparticles greatly affects cellular utilization.
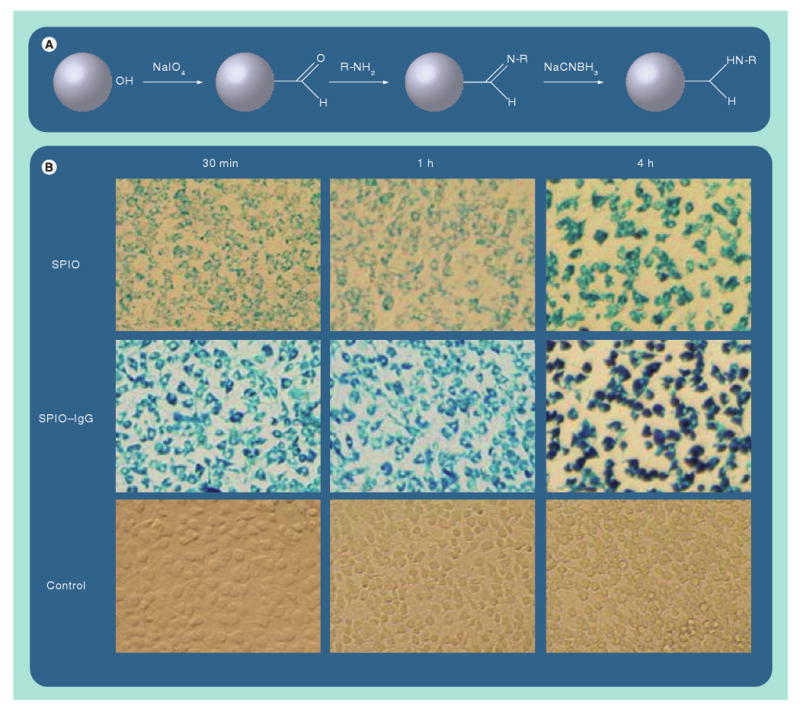
(A) Schematic example of one method for modifying the surface of dextran-coated SPIO-nanoparticles (NPs) by enabling attachment to group -R: immunoglobulins, receptor ligands or other proteins. (B) Demonstration of how conjugation of IgG to SPIO-NPs greatly enhances their uptake by mononuclear phagocytes when compared with uncoated SPIO-NPs.
SPIO: Superparamagnetic iron oxide.
A variety of NPs encapsulating different antiretroviral drugs have been designed for uptake by macrophages including: dendimers, gelatin NPs, polymer blend NPs, lipid-drug liposomes and nanocrystals. A model has been developed to help predict uptake of NPs and release of drug from MPs [129]. Fifth-generation poly(propyleneimine) dendrimers conjugated to mannose or tuftsin (which is a peptide fragment produced by enzymatic cleavage of the Fc-domain of the heavy chain of IgG and can bind to receptors on the cells to stimulate phagocytosis) were used in order to study the effects of surface modification on pharmacokinetics and cellular handling in vitro. These molecules were chosen because they have specific membrane receptors that are expressed by macrophages. While mannose significantly increased uptake, tuftsin caused the greatest increase in drug uptake by MPs [130,131]. Mannosylated gelatin NPs are another example of using mannose to modify the surface of NPs in order to increase uptake. Coupling of didanosine-containing gelatin NP with mannose significantly enhanced the lung, liver and lymph node uptake of the drug 1 h after intravenous administration (Figure 2) [132,133]. Polymeric blends, such as poly(lactide acid)–poly(ethylene glycol) for zidovudine and poly(ethylene oxide)–poly(ε-caprolactone) for saquinivir, have also been used to target antiretroviral-NPs to phagocytes and study NP pharmacokinetics in vitro [126,134]. These examples demonstrate how using different blends of polymers and surface modifiers can influence the rate and extent of uptake by phagocytes, as well as aid in targeting or avoiding these cells as necessary.
Figure 2. Tissue distribution of drug nanoparticles after uptake by circulating monocytes.
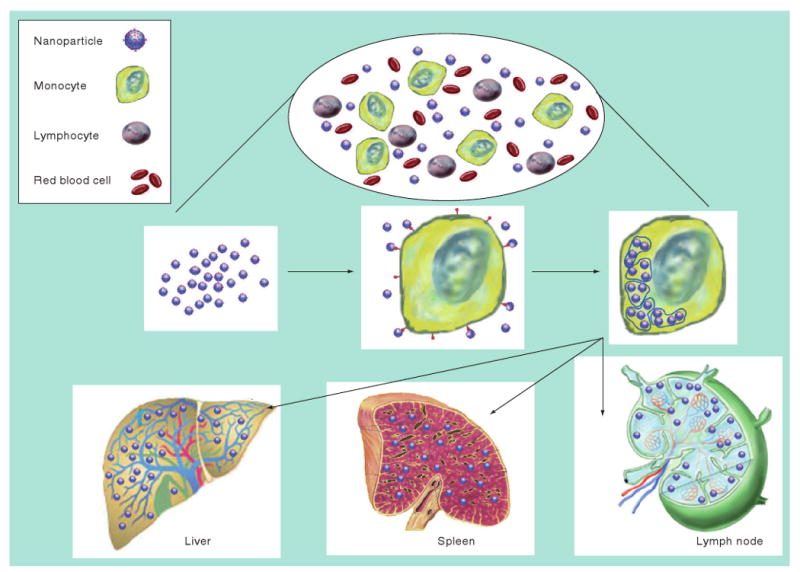
While in the blood (top), circulating immunocytes will specifically engulf nanoparticles by receptor-mediated endocytosis and store them within cytoplasmic vesicles (middle). The drug-laden cells will then respond to secreted chemokine messages and migrate from the circulation by diapedesis through vascular epithelial cells into target organs or travel through the reticuloendothelial system (bottom). The drugs are then released slowly ensuring that therapeutic concentrations are maintained for extended time periods.
Dendrimers, gelatin and polymeric NPs designed for uptake by phagocytes usually contain a single antiretroviral compound; however, attempts are being made to combine multiple drugs into a single NP. An interesting study investigated the ability of lipid–IDV-NPs to encapsulate the hydrophilic antiretroviral drug phosphonylmethoxypropyl-adenine (PMPA). The composition of this particle consisted of a lipid–IDV stable bilayer capable of enclosing PMPA in the aqueous compartment. At neutral pH, IDV was incorporated into the lipid bilayer and PMPA was maintained within. However, at an acidic pH, IDV was dissociated and PMPA was released [135]. This pH-dependent release of drug-containing NPs could be very useful for cell-mediated delivery. NPs that are stable at physiologic pH could be injected intravenously and would remain intact while traveling through the blood. No drug would be released until the particle encountered an environment with a low pH, such as a secondary phagolysosome within a macrophage.
In order for nanoART to be transported by MPs to specific diseased regions of the brain, potential hurdles need be overcome (Figure 3). Studies carried out in our laboratory using IDV nanosuspensions demonstrate the development process and highlight the importance of cell-based drug delivery for the treatment of HIV infection [29,31,32,34]. IDV can be fabricated into nanosuspensions of nanosized crystals coated in a monolayer of lipid or polymer, which are stable in an alkaline environment. Laboratory experiments revealed internalization of NPs into macrophage endosomes. When ingested into lysosomes, the acidic environment can cause the NPs to dissolve and allow release of the drug. The extracellular release was dependent on steady-state drug equilibrium within and outside the macrophages. Results of these experiments also revealed that nanoART-laden bone marrow-derived macrophages (BMMs) have the ability to carry and release the drug in tissue. After intravenous injection of nanoART-laden BMMs, tissue and sera IDV levels were greater than or equal to 50 mM for 2 weeks. Similar cells administered to HIV-1-challenged humanized mice revealed reduced numbers of virus-infected cells as well as CD4+ T-cell protection. In addition to IDV, similar nanosuspensions containing ritonavir and efavirenz were recently developed in our laboratories. We have begun to package all three drugs for carriage by MPs. Parallel efforts in our laboratory are using the same system for delivery of growth factors and anti-inflammatory medicines for treatment of other neurodegenerative diseases.
Figure 3. Interdisciplinary development of nanomedicines for HIV-1 encephalitis and other human diseases.
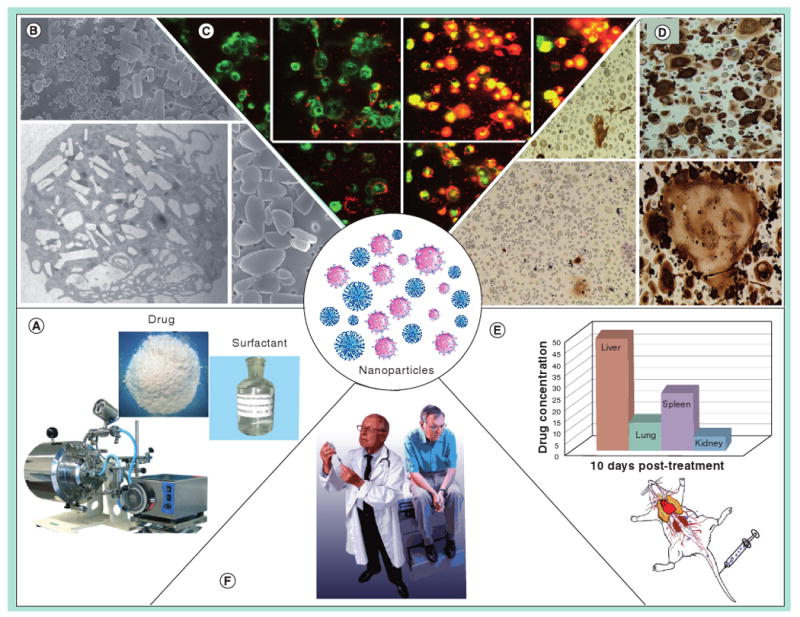
Modalities that are site specific hold considerable promise for human use and their development is illustrated herein. (A) Neuroprotective, anti-inflammatory, anti-apoptotic or antimicrobial agents are packaged into nanoparticles (NPs) with surfactant and protein coats that target circulating immunocytes or diseased regions within the CNS. (B) The NPs are developed in size, shape, charge and coating to facilitate their uptake into circulating monocytes. (C) The cells circulating in the body will scavenge the particles into cytoplasmic vesicles as a delivery vehicle. Laboratory tests can measure uptake and release of fluorescently tagged drug-laden NPs from monocytes and monocyte-derived macrophages (D). In the case of HIV-1 infections, for example, the drug-laden NPs not housed within monocyte-derived macrophages would release antiretroviral drugs leading to inhibition of viral replication, measured by the absence of multinucleated giant cells and HIV-1 p24 protein expression. (E) Such formulations can then be tested in animals demonstrating sustained drug levels for an extended period. (F) Final therapeutic use in humans will be dependent upon toxicity measures, pharmacokinetic responses and untoward side effects.
Several critical questions regarding cell-based delivery of nanoART need addressing. The first is whether MPs are capable of crossing the BBB once they are loaded with nanoART? We have shown that MP-carrying nanoART have no decrease in functional or migratory capabilities as measured by cytokine section in an in vitro model of the BBB. The second is whether these cells can reach disease-affected brain regions? It has been demonstrated that after adoptive transfer the majority of macrophages accumulate in the lungs, liver and spleen rather than in the targeted diseased tissue [29]. However, a significant portion of cells do in fact reach the brain, and once localized there, can persist and release the drug to the extracellular space for a long period of time. For example, BMM loaded with IDV-NPs adoptively transferred into an HIV-1 encephalitis mouse model can migrate across the BBB specifically to the diseased regions of the brain and release the drug for 14 days [34].
As discussed previously, various antiretroviral compounds can be entrapped by a variety of carriers to make stable nanoART that can be taken up by MPs and delivered to the brain. However, administration of these NPs is still an issue. To date, the only successful way to achieve macrophage delivery of nanoART to diseased brain regions is by exposing the cells to the compounds ex vivo and then adoptively transferring them into another animal. This is not a viable method for clinical therapy. We know that once the cells have taken in the NPs they will be able to cross the BBB and deliver the drug. In order to make this method of drug delivery useful in humans, the nanoformulations of antiretroviral drugs must be able to specifically target monocytes and macrophages after systemic injection prior to removal from circulation.
Conclusion
The introduction of ART in the mid-1990s provided a treatment that could effectively inhibit viral replication and cause a significant decrease in morbidity and mortality. Despite this achievement, problems with HIV therapy were plentiful due to the limited pharmacokinetics and biodistribution of these drugs. Since complex dosing regimes were necessary to maintain effective drug concentrations and many unpleasant side effects were associated with their long-term use, treatment failure was not uncommon. In addition, in a significant number of people infected, HIV would eventually find its way into the brain and these patients would develop HAND. After more than 20 years of research exploring the mechanisms behind HIV spread to the brain, viral perpetuation and neuronal degeneration have provided many targets for therapy. However, this condition remains particularly difficult to treat since the BBB is very effective at keeping antiretroviral drugs out of the brain. Getting therapeutic compounds across the BBB and into the brain has also been a major goal for the treatment of a variety of other diseases of the CNS, including neoplastic, degenerative and ischemic conditions.
The structure and function of the BBB have been studied for nearly 100 years, and yet we are still elucidating its workings and trying to develop methods for transporting needed drugs.With the advent of nanotechnology, new tools have been made available for accomplishing this goal. Nanocarriers with unique physical and chemical properties allow for loading, entrapment, protection and transport of drugs. This is especially important for ART prone to rapid degradation and elimination from circulation. As technology advances, it is now becoming possible to target nanoART to specific cells, such as BMVECs or MPs, or to avoid the reticuloendothelial system altogether. This allows us to either vastly extend the circulation time of these drugs or pinpoint them to specific regions. A variety of carriers such as dendrimers, polymer-NPs, SLNs, CSLNs and nanocrystals are being used to transport antiretroviral drugs. Table 1 summarizes information for the nanoART discussed herein. Two main avenues for increasing ART drug delivery to the CNS include targeting BMVECs to increase drug delivery directly and using MPs for cell-mediated transport and delivery. Both methods are promising. Our laboratories have focused on nanoART manufacturing and characterization, cell NP uptake and drug release, pharmacokinetics and effective viral suppression in animal models of HIV/AIDS. While hurdles remain that need to be overcome, we are confident that this method of antiretroviral drug delivery to the brain will be realized as a treatment modality in humans.
Table 1.
NanoART developed to treat HIV-1 infection and increase antiretroviral drug delivery to the brain.
| Drug delivery mechanism | Antiretroviral | Particle composition | Manufacture method | Finding | Ref. |
|---|---|---|---|---|---|
| BBB disruption | Saquinavir | PBCA and MMA-SPM | Emulsion polymerization and free-radical polymerization | Application of AM waves greatly increases permeability of BMVECs to HIV-drug NPs | [99] |
| Nelfinavir, zidovudine and lamivudine | Pluronic® 85 | Nanomaterial used as is | Pluronic 85 greatly increases the permeability of the BBB in vitro/in vivo to ART | [33,104] | |
| Adsorptive endocytosis by BMVEC | Saqunavir | Core: Compritol 888 ATO, cacao butter Shell: stearylamine, dioctadecyldimethyl ammonium bromide Stabilizer: polysorbate 80 | Emulsion polymerization and free-radical polymerization | Decreased initial burst of drug release and extended overall time of drug release | [117] |
| Zidovudine | PBCA and MMA-SPM | Emulsion polymerization and free-radical polymerization | PBCA as a carrier; alcohol increases permeability of BMVECs to NPs | [114] | |
| Cell-mediated delivery | Phosphonylmethoxypropyl-adenine and indinavir | Egg phosphatidylcholine and cholesterol | Sonication | NPs stable at physiologic pH and disassociate at low pH | [135] |
| Indinavir | Drug nanocrystals and lipoid E80 | High-pressure homogenization | Avid uptake and long-term release by MDMs | [31,32] | |
| Indinavir | Drug nanocrystals and mPEG-DSPE | High-pressure homogenization | Adoptive transfer of NP-loaded MDMs can significantly improve outcome in a mouse model of HIVE | [34] |
AM: Amplitude modulation; ART: Antiretroviral therapy; BBB: Blood-brain barrier; BMVEC: Brain microvessel endothelial cell; HIVE: HIV-1 encephalitis; MDM: Monocyte-derived macrophage; MMA-SPM: Methylmethacrylate-sulfopropylmethacrylate; mPEG-DPSE: Methyl polyethylene glycol-disteroyl phosphatidylethanolamine; NP: Nanoparticle; PBCA: Polybutylcyanoacrylate.
Future perspective
Nanotechnology has and continues to rapidly advance the field of drug delivery, especially in HIV therapy. The use of NPs is helping to improve the pharmacokinetics of old drugs and is finding new ways to inhibit viral replication. The interesting result that unmodified silver NPs are capable of binding and inhibiting HIV is an exciting discovery that opens up a whole new field of direct NP–virus interactions. Widespread research on the use of polymers, surfactants and lipids as carrying agents is helping us to narrow down the compounds that provide optimal entrapment efficiency and stability for each antiretroviral drug.
Work with various surface-modifying molecules, such as mannose and tuftsin, is improving the accuracy at which we can specifically target nanoART-affected cells. The success that is being achieved at targeting nanoART to BMVECs and MPs does not make it unreasonable to expect that within the next 5 years an injectable formulation of nanoART that can easily reach these cells and drastically improve HIV therapy and drug delivery to the brain will be realized. Within the next 10 years, it may even be possible to completely eliminate the virus from the brain and eradicate this potentially treatable form of dementia. In addition to the brain, other sites where the virus persists will be reached, thus making no place in the body safe for HIV.
Executive summary
Barriers to CNS drug delivery
The CNS is separated from the body by the blood–brain barrier (BBB). The BBB is subdivided into its physical and chemical constituents. This barrier is effective at keeping foreign molecules out while allowing in vital nutrients and cells.
The physical BBB is the neurovascular unit, which is comprised of brain microvessel endothelial cells (BMVECs), pericytes, astrocytes, basement membrane and perivascular neurons. Unlike peripheral capillaries, there are no fenestrae between BMVECs due to tight junctions between cells. This serves to eliminate the passage of molecules by paracellular routes. Antiretroviral therapy (ART) must cross transcellularly to enter the brain.
The chemical barrier consists of efflux pumps and enzymes, both highly expressed by BMVECs. Antiretroviral drugs are substrates for both types of proteins. Thus, even if the ART can enter the BMVECs, many can be ejected into blood or metabolized before entering the brain.
The blood–cerebrospinal fluid barrier is more ‘leaky’ than the rest of the BBB. However, owing to its relatively small surface area and diffusion limitations, the blood–cerebrospinal fluid barrier has not been considered as a target for antiretroviral drug delivery to the CNS.
The BBB allows selective passage of immune cells. Passage of HIV-1-infected monocytes and monocyte-derived macrophages into the brain is an important mechanism of viral spread and persistence.
Advancements in nanomedicine for HIV disease
Gold and silver nanoparticles (NPs), which are used for therapy and imaging in many other diseases, are also being explored for their use in HIV therapies. Conjugation of SDC-1721, an inactive fragment of the HIV inhibitor TAK-779, to gold NPs resulted in the complex NPs having potent antiretroviral activities. Silver NP, without modification, inhibits HIV.
NanoART designed for subcutaneous injection greatly increased the amount of drug in lymph nodes and extended the presence of detectable drug within the plasma for up to 3 months.
Virus detection & disease monitoring
By using a bio-barcode assay modified for HIV-1 p24 interactions, detection is increased to 150-times that of traditional ELISA assays, with nearly 100% sensitivity and specificity.
Enumeration of the CD4+ T lymphocytes is required to establish how much of the immune system has been compromised during HIV-1 infection. The gold standard for this is flow cytometry, which is not accessible in many parts of the world. A detection system consisting of semiconductor nanocrystals in a portable microfluid was nearly able to match the detection capabilities of flow cytometry.
HIV-1-associated neurocognitive disorders
The advent of ART significantly reduced morbidity and mortality during HIV infection. However, owing to the limited pharmacokinetics and biodistribution of HIV antiretroviral drugs, sanctuary sites develop where the virus can persist. This not only makes eradication of the virus very difficult but also increases the risk of viral mutation, leading to drug resistance and treatment failure. One of these sites is the CNS.
HIV infection of the brain can lead to HIV-1-associated neurocognitive disorders. A major avenue by which HIV enters the brain is through infected mononuclear phagocytes (MPs). The pathophysiology revolves around the formation of multinucleated giant cells within the brain. These serve as viral reservoirs and perpetuate an inflammatory signaling cascade, which can result in neuronal death.
HIV within the CNS is still susceptible to antiretroviral medications. Therefore, if HIV therapies can be delivered to the brain, this potentially treatable form of dementia could be eliminated. New methods for increased HIV antiretroviral drug delivery to the brain are being developed with the help of nanotechnology.
ART & BBB disruption
ART delivery to the brain may be enhanced by disruption of the BBB. Several methods were developed to modify BBB permeability including chemical, osmotic, biochemical, electromagnetic and physical disruptions.
Electromagnetic energy in the form of amplitude modulated/frequency modulated waves can alter the permeability of BMVECs. Application of these waves causes a 14-fold increase in BMVEC permeability for nanoART.
Pluronic® P85, a triblock polymeric copolymer, has the ability to inhibit the action of P-glycoprotein. By using it in conjunction with antiretroviral medications, it was able to increase drug delivery both in vitro and in vivo. In addition, it was shown that Pluronic P85 alone, without HIV drugs, had antiretroviral properties.
Solid lipid & polymeric NPs
Solid lipid nanoparticles are spherical and dispersed in water or an aqueous surfactant solution. They consist of a solid hydrophobic core coated with a phospholipid monolayer. Lipophilic drugs can be dissolved within the hydrophobic core and transported by the particle throughout the body. Several characteristics make these particles useful for antiretroviral drug delivery to the CNS including: small size (<200 nm), controlled drug release, high drug payloads and biocompatible constituents.
Polymeric NPs are made from polymer surfactants. Characteristics that make these particles useful for antiretroviral delivery to the CNS include: enhanced retention by BMVECs, opening of tight junctions and adsorption-mediated endocytosis.
Work with multiple HIV therapies and a variety of polymers, lipids and surfactants has demonstrated the ability of these types of NPs to enter BMVECs and greatly increase the delivery of antiretroviral drugs in vitro. In addition, these studies continue to show the strong effect the choice of carrier and drug combination has upon loading efficiency, entrapment efficiency and transport.
One major obstacle that still needs to be overcome is successful in vivo delivery of these particles to BMVECs after intravenous injection. These cells must take up the particles before they are removed from circulation and made unavailable. A potential way to avoid this problem would be by utilizing cell-mediated drug delivery.
Cell-mediated CNS delivery for nanoART
MPs, such as monocytes and macrophages, have the ability to both uptake NPs and cross the BBB by diapedesis. Thus, these cells can be used to uptake, transport and deliver NPs containing antiretroviral drugs to the brain. In addition, these cells are infected by HIV, which makes them ideal targets for HIV therapies.
Characteristics such as size, shape, charge and surface modification affect NP uptake by MPs. As such, NPs can be targeted for uptake by these cells. Within the cell, NPs are trafficked to secondary lysosomes. This is useful for antiretroviral drug delivery because NPs that are stable at physiologic pH will be able to reach cells intact, be transported and then release the drug once they encounter the low pH environment inside a secondary lysosome.
A variety of NP-encapsulating multiple antiretroviral drugs have been designed for uptake by macrophages including: dendrimers, gelatin NPs, polymer blend NPs, lipid-drug liposomes and nanocrystals. HIV drug NP surfaces are being modified with molecules, such as mannose and tuftsin, to bind with specific receptors expressed on macrophage membranes to increase receptor-mediated uptake.
Work with HIV drug nanocrystals has shown that this NP is ideally suited for cell-mediated drug delivery. Antiretroviral nanocrystals are avidly taken up by MPs and then slowly released for up to 15 days. They demonstrate strong antiretroviral drug effects both in vitro and in vivo. Monocyte-derived macrophages loaded with HIV drug nanocrystals ex vivo and then adoptively transferred maintained tissue and sera drug levels greater than 50 mM for 2 weeks, in addition to potent viral inhibition. These cells were also capable of crossing the BBB, delivering drug and reducing viral loads in a mouse model of HIV-1 encephalitis.
Acknowledgments
The authors thank H Dou and J McMillan for helpful discussions and for technical assistance and R Taylor for critical reading of the manuscript and outstanding graphic and literary support.
This work was supported by the Frances and Louie Blumkin Foundation, the Community Neuroscience Pride of Nebraska Research Initiative, the Alan Baer Charitable Trust, and NIH grants 5P01NS31492, 2R37 NS36126, 2R01 NS034239, P20RR15635, U54NS43011, P01MH64570 and P01 NS43985.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
For reprint orders, please contact: reprints@futuremedicine.com
Bibliography
- 1.Pardridge WM. The blood–brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pathan SA, Iqbal Z, Zaidi SM, et al. CNS drug delivery systems: novel approaches. Recent Pat Drug Deliv Formul. 2009;3(1):71–89. doi: 10.2174/187221109787158355. [DOI] [PubMed] [Google Scholar]
- 3.Silva GA. Nanotechnology approaches to crossing the blood–brain barrier and drug delivery to the CNS. BMC Neurosci. 2008;9(Suppl 3):S4. doi: 10.1186/1471-2202-9-S3-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanson LR, Frey WH., 2nd Intranasal delivery bypasses the blood–brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9(Suppl 3):S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denora N, Trapani A, Laquintana V, Lopedota A, Trapani G. Recent advances in medicinal chemistry and pharmaceutical technology – strategies for drug delivery to the brain. Curr Top Med Chem. 2009;9(2):182–196. doi: 10.2174/156802609787521571. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood–brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res. 2007;24(9):1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Chen C, Smith BJ. Progress in brain penetration evaluation in drug discovery and development. J Pharmacol Exp Ther. 2008;325(2):349–356. doi: 10.1124/jpet.107.130294. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Chen C. Strategies to optimize brain penetration in drug discovery. Curr Opin Drug Discov Devel. 2005;8(4):505–512. [PubMed] [Google Scholar]
- 9.Kabanov AV, Gendelman HE. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog Polym Sci. 2007;32:1054–1082. doi: 10.1016/j.progpolymsci.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buescher JL, Gross S, Gendelman HE, Ikezu T. The neuropathogenesis of HIV-1 infection. Handb Clin Neurol. 2007;85:45–67. doi: 10.1016/S0072-9752(07)85004-4. [DOI] [PubMed] [Google Scholar]
- 11.Gendelman HE, Ding S, Gong N, et al. Monocyte chemotactic protein-1 regulates voltage-gated K+ channels and macrophage transmigration. J Neuroimmune Pharmacol. 2009;4(1):47–59. doi: 10.1007/s11481-008-9135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorantla S, Liu J, Wang T, et al. Modulation of innate immunity by copolymer-1 leads to neuroprotection in murine HIV-1 encephalitis. Glia. 2008;56(2):223–232. doi: 10.1002/glia.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X, Reynolds AD, Mosley RL, Gendelman HE. CD4+ T cells for the pathobiology of neurodegenerative disorders. J Neuroimmunol. 2009 doi: 10.1016/j.jneuroim.2009.04.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadiu I, Glanzer JG, Kipnis J, Gendelman HE, Thomas MP. Mononuclear phagocytes in the pathogenesis of neurodegenerative diseases. Neurotox Res. 2005;8(1–2):25–50. doi: 10.1007/BF03033818. [DOI] [PubMed] [Google Scholar]
- 15.Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. HIV-1 gp120 compromises blood–brain barrier integrity and enhances monocyte migration across blood–brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27(1):123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keblesh JP, Dou H, Gendelman HE, Xiong H. 4-aminopyridine improves spatial memory in a murine model of HIV-1 encephalitis. J Neuroimmune Pharmacol. 2009 doi: 10.1007/s11481-009-9161-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kingsley JD, Dou H, Morehead J, Rabinow B, Gendelman HE, Destache CJ. Nanotechnology: a focus on nanoparticles as a drug delivery system. J Neuroimmune Pharmacol. 2006;1(3):340–350. doi: 10.1007/s11481-006-9032-4. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Gong N, Huang X, Reynolds AD, Mosley RL, Gendelman HE. Neuromodulatory activities of CD4+CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration. J Immunol. 2009;182(6):3855–3865. doi: 10.4049/jimmunol.0803330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Uberti MG, Dou H, et al. Ingress of blood-borne macrophages across the blood– brain barrier in murine HIV-1 encephalitis. J Neuroimmunol. 2008;200(1–2):41–52. doi: 10.1016/j.jneuroim.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds A, Laurie C, Mosley RL, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- 21.Ricardo-Dukelow M, Kadiu I, Rozek W, et al. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood–brain barrier dysfunction for HIV-1-associated dementia. J Neuroimmunol. 2007;185(1–2):37–46. doi: 10.1016/j.jneuroim.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rozek W, Ricardo-Dukelow M, Holloway S, et al. Cerebrospinal fluid proteomic profiling of HIV-1-infected patients with cognitive impairment. J Proteome Res. 2007;6(11):4189–4199. doi: 10.1021/pr070220c. [DOI] [PubMed] [Google Scholar]
- 23.Schiftto G, Zhong J, Gill D, et al. Lithium therapy for human immunodeficiency virus type 1-associated neurocognitive impairment. J Neurovirol. 2009;15(2):176–186. doi: 10.1080/13550280902758973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, Gong N, Liu J, et al. Proteomic modeling for HIV-1 infected microglia-astrocyte crosstalk. PLoS ONE. 2008;3(6):e2507. doi: 10.1371/journal.pone.0002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gendelman HE, Orenstein JM, Martin MA, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persidsky Y, Gendelman H. Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia. J Leukoc Biol. 1997;62(1):100–106. doi: 10.1002/jlb.62.1.100. [DOI] [PubMed] [Google Scholar]
- 27.Nottet HS, Persidsky Y, Sasseville VG, et al. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156(3):1284–1295. [PubMed] [Google Scholar]
- 28.Batrakova EV, Li S, Reynolds AD, et al. A macrophage–nanozyme delivery system for Parkinson's disease. Bioconjug Chem. 2007;18(5):1498–1506. doi: 10.1021/bc700184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorantla S, Dou H, Boska M, et al. Quantitative magnetic resonance and SPECT imaging for macrophage tissue migration and nanoformulated drug delivery. J Leukoc Biol. 2006;80(5):1165–1174. doi: 10.1189/jlb.0206110. [DOI] [PubMed] [Google Scholar]
- 30.Beduneau A, Ma Z, Grotepas C, et al. Facilitated monocyte-macrophage uptake and tissue distribution of superparmagnetic iron-oxide nanoparticles. PLoS ONE. 2009;4(2):e4343. doi: 10.1371/journal.pone.0004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dou H, Destache CJ, Morehead JR, et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108(8):2827–2835. doi: 10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dou H, Morehead J, Destache CJ, et al. Laboratory investigations for the morphologic, pharmacokinetic, and anti-retroviral properties of indinavir nanoparticles in human monocyte-derived macrophages. Virology. 2007;358(1):148–158. doi: 10.1016/j.virol.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Spitzenberger TJ, Heilman D, Diekmann C, et al. Novel delivery system enhances efficacy of antiretroviral therapy in animal model for HIV-1 encephalitis. J Cereb Blood Flow Metab. 2007;27(5):1033–1042. doi: 10.1038/sj.jcbfm.9600414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dou H, McMillan JM, Destache CJ, et al. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol. 2009 doi: 10.4049/jimmunol.0900274. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss N, Miller F, Cazaubon S, Couraud PO. Part I: Biology of the blood–brain barrier. Rev Neurol (Paris) 2009 doi: 10.1016/j.neurol.2009.03.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Newton HB. Advances in strategies to improve drug delivery to brain tumors. Expert Rev Neurother. 2006;6(10):1495–1509. doi: 10.1586/14737175.6.10.1495. [DOI] [PubMed] [Google Scholar]
- 37.Abbott NJ, Ronnback L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 38.Smith MW, Gumbleton M. Endocytosis at the blood–brain barrier: from basic understanding to drug delivery strategies. J Drug Target. 2006;14(4):191–214. doi: 10.1080/10611860600650086. [DOI] [PubMed] [Google Scholar]
- 39.Girardin F. Membrane transporter proteins: a challenge for CNS drug development. Dialogues Clin Neurosci. 2006;8(3):311–321. doi: 10.31887/DCNS.2006.8.3/fgirardin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76(1):22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Begley DJ. ABC transporters and the blood–brain barrier. Curr Pharm Des. 2004;19:1295–1312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- 42.Liang XJ, Aszalos A. Multidrug transporters as drug targets. Curr Drug Targets. 2006;7(8):911–921. doi: 10.2174/138945006778019264. [DOI] [PubMed] [Google Scholar]
- 43.Pastor-Anglada M, Cano-Soldado P, Molina-Arcas M, et al. Cell entry and export of nucleoside analogues. Virus Res. 2005;107(2):151–164. doi: 10.1016/j.virusres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Bousquet L, Roucairol C, Hembury A, et al. Comparison of ABC transporter modulation by atazanavir in lymphocytes and human brain endothelial cells: ABC transporters are involved in the atazanavir-limited passage across an in vitro human model of the blood– brain barrier. AIDS Res Hum Retroviruses. 2008;24(9):1147–1154. doi: 10.1089/aid.2007.0022. [DOI] [PubMed] [Google Scholar]
- 45.Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood–CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82(2):A99–A109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27(11):1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziylan YZ, Baltaci AK, Mogulkoc R. Leptin transport in the central nervous system. Cell Biochem Funct. 2009;27(2):63–70. doi: 10.1002/cbf.1538. [DOI] [PubMed] [Google Scholar]
- 48.Pan W, Kastin AJ. Urocortin and the brain. Prog Neurobiol. 2008;84(2):148–156. doi: 10.1016/j.pneurobio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banks WA. Delivery of peptides to the brain: emphasis on therapeutic development. Biopolymers. 2008;90(5):589–594. doi: 10.1002/bip.20980. [DOI] [PubMed] [Google Scholar]
- 50.Kastin AJ, Pan W. Blood–brain barrier and feeding: regulatory roles of saturable transport systems for ingestive peptides. Curr Pharm Des. 2008;14(16):1615–1619. doi: 10.2174/138161208784705423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kostrzewa RM. Evolution of neurotoxins: from research modalities to clinical realities. Curr Protoc Neurosci. 2009;Chapter 1(Unit 118) doi: 10.1002/0471142301.ns0118s46. [DOI] [PubMed] [Google Scholar]
- 52.Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108(6):1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agrawal S, Anderson P, Durbeej M, et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203(4):1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hill HD, Mirkin CA. The bio-barcode assay for the detection of protein and nucleic acid targets using DTT-induced ligand exchange. Nat Protoc. 2006;1(1):324–336. doi: 10.1038/nprot.2006.51. [DOI] [PubMed] [Google Scholar]
- 55.Tang S, Zhao J, Storhoff JJ, et al. Nanoparticle-based biobarcode amplification assay (BCA) for sensitive and early detection of human immunodeficiency type 1 capsid (p24) antigen. J Acquir Immune Defic Syndr. 2007;46(2):231–237. doi: 10.1097/QAI.0b013e31814a554b. [DOI] [PubMed] [Google Scholar]
- 56.Kim EY, Stanton J, Korber BT, et al. Detection of HIV-1 p24 Gag in plasma by a nanoparticle-based bio-barcode-amplification method. Nanomed. 2008;3(3):293–303. doi: 10.2217/17435889.3.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jokerst JV, Floriano PN, Christodoulides N, Simmons GW, McDevitt JT. Integration of semiconductor quantum dots into nano-biochip systems for enumeration of CD4+ T cell counts at the point-of-need. Lab Chip. 2008;8(12):2079–2090. doi: 10.1039/b817116e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahmoud KA, Hrapovic S, Luong JH. Picomolar detection of protease using peptide/single walled carbon nanotube/gold nanoparticle-modified electrode. ACS Nano. 2008;2(5):1051–1057. doi: 10.1021/nn8000774. [DOI] [PubMed] [Google Scholar]
- 59.Gasparyan VK. Gold and silver nanoparticles in bioassay, cell visualization and therapy. Curr Clin Pharmacol. 2009;4(2):159–163. doi: 10.2174/157488409788184954. [DOI] [PubMed] [Google Scholar]
- 60.Bhattacharya R, Mukherjee P. Biological properties of “naked” metal nanoparticles. Adv Drug Deliv Rev. 2008;60(11):1289–1306. doi: 10.1016/j.addr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Jennings T, Strouse G. Past, present, and future of gold nanoparticles. Adv Exp Med Biol. 2007;620:34–47. doi: 10.1007/978-0-387-76713-0_3. [DOI] [PubMed] [Google Scholar]
- 62.Bowman MC, Ballard TE, Ackerson CJ, Feldheim DL, Margolis DM, Melander C. Inhibition of HIV fusion with multivalent gold nanoparticles. J Am Chem Soc. 2008;130(22):6896–6897. doi: 10.1021/ja710321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elechiguerra JL, Burt JL, Morones JR, et al. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hockett RD, Kilby JM, Derdeyn CA, et al. Constant mean viral copy number per infected cell in tissues regardless of high, low, or undetectable plasma HIV RNA. J Exp Med. 1999;189(10):1545–1554. doi: 10.1084/jem.189.10.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brodie SJ, Patterson BK, Lewinsohn DA, et al. HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death. J Clin Invest. 2000;105(10):1407–1417. doi: 10.1172/JCI8707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Ruiz L, van Lunzen J, Arno A, et al. Protease inhibitor-containing regimens compared with nucleoside analogues alone in the suppression of persistent HIV-1 replication in lymphoid tissue. AIDS. 1999;13(1):F1–F8. doi: 10.1097/00002030-199901140-00001. [DOI] [PubMed] [Google Scholar]
- 67.Kinman L, Bui T, Larsen K, et al. Optimization of lipid–indinavir complexes for localization in lymphoid tissues of HIV-infected macaques. J Acquir Immune Defic Syndr. 2006;42(2):155–161. doi: 10.1097/01.qai.0000214822.33905.87. [DOI] [PubMed] [Google Scholar]
- 68.Baert L, van't Klooster G, Dries W, et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur J Pharm Biopharm. 2009 doi: 10.1016/j.ejpb.2009.03.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 69.Inafuku H, Kasem Khan MA, Nagata T, Nonaka S. Cutaneous ulcerations following subcutaneous interferon-β injection to a patient with multiple sclerosis. J Dermatol. 2004;31(8):671–677. doi: 10.1111/j.1346-8138.2004.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 70.Shiota M, Tokuda N, Kanou T, Yamasaki H. Injection-site granulomas resulting from the administration of both leuprorelin acetate and goserelin acetate for the treatment of prostatic cancer. J Nippon Med Sch. 2007;74(4):306–308. doi: 10.1272/jnms.74.306. [DOI] [PubMed] [Google Scholar]
- 71.McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157(1–2):3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 72.Valcour V, Paul R. HIV infection and dementia in older adults. Clin Infect Dis. 2006;42(10):1449–1454. doi: 10.1086/503565. [DOI] [PubMed] [Google Scholar]
- 73.Best BM, Letendre SL, Brigid E, et al. Low atazanavir concentrations in cerebrospinal fluid. AIDS. 2009;23(1):83–87. doi: 10.1097/QAD.0b013e328317a702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murri R, Lepri AC, Cicconi P, et al. Is moderate HIV viremia associated with a higher risk of clinical progression in HIV-infected people treated with highly active antiretroviral therapy: evidence from the Italian cohort of antiretroviral-naive patients study. J Acquir Immune Defic Syndr. 2006;41(1):23–30. doi: 10.1097/01.qai.0000188337.76164.7a. [DOI] [PubMed] [Google Scholar]
- 75.Alos L, Navarrete P, Morente V, et al. Immunoarchitecture of lymphoid tissue in HIV-infection during antiretroviral therapy correlates with viral persistence. Mod Pathol. 2005;18(1):127–136. doi: 10.1038/modpathol.3800267. [DOI] [PubMed] [Google Scholar]
- 76.Meltzer MS, Gendelman HE. Mononuclear phagocytes as targets, tissue reservoirs, and immunoregulatory cells in human immunodeficiency virus disease. Curr Top Microbiol Immunol. 1992;181:239–263. doi: 10.1007/978-3-642-77377-8_9. [DOI] [PubMed] [Google Scholar]
- 77.Luo X, Carlson KA, Wojna V, et al. Macrophage proteomic fingerprinting predicts HIV-1-associated cognitive impairment. Neurology. 2003;60(12):1931–1937. doi: 10.1212/01.wnl.0000064396.54554.26. [DOI] [PubMed] [Google Scholar]
- 78.Anderson E, Zink W, Xiong H, Gendelman HE. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S43–S54. doi: 10.1097/00126334-200210012-00004. [DOI] [PubMed] [Google Scholar]
- 79.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 80.Wang T, Gong N, Liu J, et al. HIV-1-infected astrocytes and the microglial proteome. J Neuroimmune Pharmacol. 2008;3(3):173–186. doi: 10.1007/s11481-008-9110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghorpade A, Persidsky Y, Swindells S, et al. Neuroinflammatory responses from microglia recovered from HIV-1-infected and seronegative subjects. J Neuroimmunol. 2005;163(1–2):145–156. doi: 10.1016/j.jneuroim.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 82.Borgmann K, Gendelman HE, Ghorpade A. Isolation and HIV-1 infection of primary human microglia from fetal and adult tissue. Methods Mol Biol. 2005;304:49–70. doi: 10.1385/1-59259-907-9:049. [DOI] [PubMed] [Google Scholar]
- 83.Gendelman HE, Orenstein JM, Baca LM, et al. The macrophage in the persistence and pathogenesis of HIV infection. AIDS. 1989;3(8):475–495. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Ghorpade A, Persidskaia R, Suryadevara R, et al. Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1-associated dementia. J Virol. 2001;75(14):6572–6583. doi: 10.1128/JVI.75.14.6572-6583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang ZG, Piggee C, Heyes MP, et al. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J Neuroimmunol. 2001;117(1–2):97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- 86.Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332(14):934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 87.Cotter RL, Burke WJ, Thomas VS, Potter JF, Zheng J, Gendelman HE. Insights into the neurodegenerative process of Alzheimer's disease: a role for mononuclear phagocyte-associated inflammation and neurotoxicity. J Leukoc Biol. 1999;65(4):416–427. doi: 10.1002/jlb.65.4.416. [DOI] [PubMed] [Google Scholar]
- 88.Gendelman HE, Persidsky Y, Ghorpade A, et al. The neuropathogenesis of the AIDS dementia complex. AIDS. 1997;11(Suppl A):S35–S45. [PubMed] [Google Scholar]
- 89.Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52(4):344–353. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- 90.McGee B, Smith N, Aweeka F. HIV pharmacology: barriers to the eradication of HIV from the CNS. HIV Clin Trials. 2006;7(3):142–153. doi: 10.1310/AW2H-TP5C-NP43-K6BY. [DOI] [PubMed] [Google Scholar]
- 91.Thomas SA. Anti-HIV drug distribution to the central nervous system. Curr Pharm Des. 2004;10(12):1313–1324. doi: 10.2174/1381612043384835. [DOI] [PubMed] [Google Scholar]
- 92.Ronaldson PT, Persidsky Y, Bendayan R. Regulation of ABC membrane transporters in glial cells: relevance to the pharmacotherapy of brain HIV-1 infection. Glia. 2008;56(16):1711–1735. doi: 10.1002/glia.20725. [DOI] [PubMed] [Google Scholar]
- 93.Boffito M, Back DJ, Blaschke TF, et al. Protein binding in antiretroviral therapies. AIDS Res Hum Retroviruses. 2003;19(9):825–835. doi: 10.1089/088922203769232629. [DOI] [PubMed] [Google Scholar]
- 94.Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6(5):388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Banks WA, Robinson SM, Nath A. Permeability of the blood–brain barrier to HIV-1 Tat. Exp Neurol. 2005;193(1):218–227. doi: 10.1016/j.expneurol.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 96.Bagasra O. A unified concept of HIV latency. Expert Opin Biol Ther. 2006;6(11):1135–1149. doi: 10.1517/14712598.6.11.1135. [DOI] [PubMed] [Google Scholar]
- 97.Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol. 1996;41(11):2251–2269. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- 98.Salford LG, Brun A, Sturesson K, Eberhardt JL, Persson BR. Permeability of the blood–brain barrier induced by 915 MHz electromagnetic radiation, continuous wave and modulated at 8, 16, 50, and 200 Hz. Microsc Res Tech. 1994;27(6):535–542. doi: 10.1002/jemt.1070270608. [DOI] [PubMed] [Google Scholar]
- 99.Kuo YC, Kuo CY. Electromagnetic interference in the permeability of saquinavir across the blood–brain barrier using nanoparticulate carriers. Int J Pharm. 2008;351(1–2):271–281. doi: 10.1016/j.ijpharm.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 100.Owen A, Chandler B, Back DJ. The implications of P-glycoprotein in HIV: friend or foe? Fundam Clin Pharmacol. 2005;19(3):283–296. doi: 10.1111/j.1472-8206.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 101.Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood–brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60(2):196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bauer B, Hartz AM, Fricker G, Miller DS. Modulation of p-glycoprotein transport function at the blood–brain barrier. Exp Biol Med (Maywood) 2005;230(2):118–127. doi: 10.1177/153537020523000206. [DOI] [PubMed] [Google Scholar]
- 103.Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood–brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299(2):483–493. [PubMed] [Google Scholar]
- 104.Shaik N, Pan G, Elmquist WF. Interactions of pluronic block copolymers on P-gp efflux activity: experience with HIV-1 protease inhibitors. J Pharm Sci. 2008;97(12):5421–5433. doi: 10.1002/jps.21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herve F, Ghinea N, Scherrmann JM. CNS delivery via adsorptive transcytosis. AAPS J. 2008;10(3):455–472. doi: 10.1208/s12248-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marcello A, Zoppe M, Giacca M. Multiple modes of transcriptional regulation by the HIV-1 Tat transactivator. IUBMB Life. 2001;51(3):175–181. doi: 10.1080/152165401753544241. [DOI] [PubMed] [Google Scholar]
- 107.Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- 108.Rao KS, Reddy MK, Horning JL, Labhasetwar V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29(33):4429–4438. doi: 10.1016/j.biomaterials.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res. 2005;8(1–2):119–134. doi: 10.1007/BF03033824. [DOI] [PubMed] [Google Scholar]
- 110.Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127(2):97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 111.Chen Y, Dalwadi G, Benson HA. Drug delivery across the blood–brain barrier. Curr Drug Deliv. 2004;1(4):361–376. doi: 10.2174/1567201043334542. [DOI] [PubMed] [Google Scholar]
- 112.Wilson B, Samanta MK, Santhi K, Kumar KP, Paramakrishnan N, Suresh B. Targeted delivery of tacrine into the brain with polysorbate 80-coated poly(n-butylcyanoacrylate) nanoparticles. Eur J Pharm Biopharm. 2008;70(1):75–84. doi: 10.1016/j.ejpb.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 113.Wang CX, Huang LS, Hou LB, et al. Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res. 2009 doi: 10.1016/j.brainres.2009.01.011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 114.Kuo YC, Chen HH. Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate on the permeability of zidovudine and lamivudine across the in vitro blood–brain barrier. Int J Pharm. 2006;327(1–2):160–169. doi: 10.1016/j.ijpharm.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 115.Kuo YC. Loading efficiency of stavudine on polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate copolymer nanoparticles. Int J Pharm. 2005;290(1–2):161–172. doi: 10.1016/j.ijpharm.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 116.Kuo YC, Su FL. Transport of stavudine, delavirdine, and saquinavir across the blood–brain barrier by polybutylcyanoacrylate, methylmethacrylate-sulfopropylmethacrylate, and solid lipid nanoparticles. Int J Pharm. 2007;340(1–2):143–152. doi: 10.1016/j.ijpharm.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 117.Kuo YC, Chen HH. Entrapment and release of saquinavir using novel cationic solid lipid nanoparticles. Int J Pharm. 2009;365(1–2):206–213. doi: 10.1016/j.ijpharm.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 118.Chattopadhyay N, Zastre J, Wong HL, Wu XY, Bendayan R. Solid lipid nanoparticles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharm Res. 2008;25(10):2262–2271. doi: 10.1007/s11095-008-9615-2. [DOI] [PubMed] [Google Scholar]
- 119.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8(4):382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 120.Vestweber D. Molecular mechanisms that control leukocyte extravasation through endothelial cell contacts. Ernst Schering Found Symp Proc. 2007;(3):151–167. doi: 10.1007/2789_2007_063. [DOI] [PubMed] [Google Scholar]
- 121.Gilmore JL, Yi X, Quan L, Kabanov AV. Novel nanomaterials for clinical neuroscience. J Neuroimmune Pharmacol. 2008;3(2):83–94. doi: 10.1007/s11481-007-9099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chung TH, Wu SH, Yao M, et al. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials. 2007;28(19):2959–2966. doi: 10.1016/j.biomaterials.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 123.Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol) Langmuir. 2006;22(19):8178–8185. doi: 10.1021/la060951b. [DOI] [PubMed] [Google Scholar]
- 124.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25(8):1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mainardes RM, Gremiao MP, Brunetti IL, da Fonseca LM, Khalil NM. Zidovudine-loaded PLA and PLA–PEG blend nanoparticles: influence of polymer type on phagocytic uptake by polymorphonuclear cells. J Pharm Sci. 2009;98(1):257–267. doi: 10.1002/jps.21406. [DOI] [PubMed] [Google Scholar]
- 127.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(dl-lactide-co-glycolide) nanoparticles implications for drug and gene delivery. FASEB J. 2002;16(10):1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 128.Gibbs WJ, Drew RH, Perfect JR. Liposomal amphotericin B: clinical experience and perspectives. Expert Rev Anti Infect Ther. 2005;3(2):167–181. doi: 10.1586/14787210.3.2.167. [DOI] [PubMed] [Google Scholar]
- 129.Ece Gamsiz D, Shah LK, Devalapally H, Amiji MM, Carrier RL. A model predicting delivery of saquinavir in nanoparticles to human monocyte/macrophage (Mo/Mac) cells. Biotechnol Bioeng. 2008;101(5):1072–1082. doi: 10.1002/bit.21958. [DOI] [PubMed] [Google Scholar]
- 130.Dutta T, Agashe HB, Garg M, Balakrishnan P, Kabra M, Jain NK. Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro. J Drug Target. 2007;15(1):89–98. doi: 10.1080/10611860600965914. [DOI] [PubMed] [Google Scholar]
- 131.Dutta T, Garg M, Jain NK. Targeting of efavirenz loaded tuftsin conjugated poly(propyleneimine) dendrimers to HIV infected macrophages in vitro. Eur J Pharm Sci. 2008;34(2–3):181–189. doi: 10.1016/j.ejps.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 132.Jain SK, Gupta Y, Jain A, Saxena AR, Khare P. Mannosylated gelatin nanoparticles bearing an anti-HIV drug didanosine for site-specific delivery. Nanomedicine. 2008;4(1):41–48. doi: 10.1016/j.nano.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 133.Kaur A, Jain S, Tiwary AK. Mannan-coated gelatin nanoparticles for sustained and targeted delivery of didanosine: in vitro and in vivo evaluation. Acta Pharm. 2008;58(1):61–74. doi: 10.2478/v10007-007-0045-1. [DOI] [PubMed] [Google Scholar]
- 134.Shah LK, Amiji MM. Intracellular delivery of saquinavir in biodegradable polymeric nanoparticles for HIV/AIDS. Pharm Res. 2006;23(11):2638–2645. doi: 10.1007/s11095-006-9101-7. [DOI] [PubMed] [Google Scholar]
- 135.Choi SU, Bui T, Ho RJ. pH-dependent interactions of indinavir and lipids in nanoparticles and their ability to entrap a solute. J Pharm Sci. 2008;97(2):931–943. doi: 10.1002/jps.21020. [DOI] [PubMed] [Google Scholar]


