Abstract
Adipocytes provide an organism with fuel in times of caloric deficit, and are an important type of endocrine cell in the maintenance of metabolic homeostasis. In addition, as a lipid-sink, adipocytes serve an equally important role in the protection of organs from the damaging effects of ectopic lipid deposition. For the organism, it is of vital importance to maintain adipocyte viability, yet the fat depot is a demanding extracellular environment with high levels of interstitial free fatty acids and associated lipotoxic effects. These surroundings are less than beneficial for the overall health of any resident cell, adipocyte and preadipocyte alike. In this review, we discuss the process of adipogenesis and the potential involvement of the p53 tumor-suppressor protein in alleviating some of the cellular stress experienced by these cells. In particular, we discuss p53-mediated mechanisms that prevent damage caused by reactive oxygen species and the effects of lipotoxicity. We also suggest the potential for two p53 target genes, START domain-containing protein 4 (StARD4) and oxysterol-binding protein (OSBP), with the concomitant synthesis of the signaling molecule oxysterol, to participate in adipogenesis.
Keywords: adipogenesis, diabetes, lipotoxicity, oxysterol, p53
The adipocyte is a truly remarkable cell in many aspects. In recent years several studies have identified adipose tissue as an important endocrine organ involved in regulating whole-body energy homeostasis through the actions of a range of adipocyte-specific secreted cytokines, commonly referred to as ‘adipokines’ [1–3]. The tight connection between adipocytes and several types of cancer further highlights the significance of this endocrine role [4–6]. In addition, the adipocyte stores energy in the form of triglycerides to supply the organism with free fatty acids as fuel in times of fasting. In the evolution of animals, the advent of the adipocyte must have been a life-transforming event, liberating animals to roam unconstrained by the need for a direct and continuous supply of energy [7,8]. As an added bonus, with the development of the adipocyte as a lipid-sink, these cells were further enabled to protect other organs from the deleterious effects of excessive levels of cholesterol, triglycerides and free fatty acids. These effects (commonly referred to as ‘lipotoxicity’) include the induction of endoplasmic reticular (ER)-stress by excessive cholesterol loads and general oxidative damage due to increases in reactive oxygen species (ROS) [9–13]. Failure of the fat depot to store lipids and cholesterol properly results in lipotoxicity that contributes to several serious morbidities associated with insulin-resistance, such as cardiovascular disease, kidney disease and diabetes [14].
The importance of the adipocyte in ensuring proper lipid homeostasis is further highlighted by the onset of devastating dyslipidemia and insulin resistance in both pharmacologically induced and genetically afflicted lipodystrophic patients [15,16]. A milder example is provided in the elderly, where loss of adipogenic differentiation potential with aging is associated with ectopic fat storage [17]. Conversely, the flip side also holds true. Adipocytes from the adipocyte-specific insulin-receptor knockout mouse can still maintain lipid homeostasis, but are no longer able to take up and store glucose in response to insulin; yet, blood glucose levels in these mice are normal, the mice are protected from age- and diet-induced obesity and, interestingly, they live significantly longer [18,19]. The protection against diet-induced obesity could be explained by lack of the permissive effect of insulin on triglyceride storage in fat or by lack of the antilipolytic effects of insulin in adipocytes, causing the organism to burn fat rather than store it. These data elegantly illustrate the deleterious effects fat has on longevity and health [18,19].
Building a fat cell
The differentiation of a committed preadipocyte into an adipocyte is mediated by several profound morphological changes in the cell, such as the formation of caveolae in the plasma membrane, the formation of a unilocular lipid droplet, and a vesicular system regulating the uptake of glucose in response to insulin. Caveolae are omega-shaped membrane invaginations of 50–150 nm in diameter under structural control of the caveolin proteins [20,21]. These lipid-raft-derived structures occupy 30% of the surface of the adipocyte plasma membrane and play an important role in the regulation of lipid and cholesterol uptake by the adipocyte. The concomitant induction of lipogenic and lipid-handling genes, such as the adipocyte-specific fatty acid transporter aP2 and lipoprotein lipase (LPL), provides the adipocyte with its ability to handle and store huge quantities of lipids in its lipid droplet [22]. This defining trait of the adipocyte, the large central lipid droplet, is derived from the endoplasmic reticulum and covered by the adipocyte-specific perilipins [23]. The lipid droplet should not be perceived as a static storage site for fat; rather, a recent proteomic analysis of the lipid droplet suggests that it forms an important platform for signaling events [24]. Moreover, even in a chronically overfed state, the lipid droplet is in a permanent flux between lipogenesis and lipolysis [25]. The change in lipid droplet size induces a fluctuation in cell size throughout life that can be extreme, ranging from roughly 20 μm in diameter in the depleted state to 200 μm in a fully lipid-laden adipocyte. A final hallmark of a fully differentiated adipocyte is the presence of an insulin-responsive vesicular storage system harboring the GLUT4 glucose-transporter proteins [26]. Stimulation of the adipocyte with insulin leads to a rapid redistribution of the GLUT4 transporters, and concomitant initiation of fusion between these storage vesicles and the plasma membrane, thereby conferring insulin responsiveness on the process of glucose uptake by these cells [27].
Adipogenesis: a tale of three kings
At the transcription level, these profound morphological changes associated with the maturation of the preadipocyte are a finely controlled affair involving the interplay of several families of transcription factors (Figure 1). These include PPAR-γ, CCAAT/enhancer-binding proteins (C/EBP)-α, -β and -δ, and sterol regulatory element-binding protein 1/adipocyte determination and differentiation factor 1 (SREBP-1/ADD1) [28]. The nuclear hormone receptor PPAR-γ is a transcription factor functioning as an obligate heterodimer with the retinoid X receptor [29]. Expression of PPAR-γ in fibroblasts is sufficient to induce adipogenic differentiation [30], and adipose-specific or hypomorphic PPAR-γ-knockout mice are severely lipodystrophic [31,32]. Therefore, PPAR-γ is widely considered to be the master adipogenic transcription factor. Indeed, ligands of PPAR-γ, such as the antidiabeteic thiazolidinediones (TZDs) troglitazone and rosiglitazone, and the naturally occurring agonist 15-deoxy-Δ12,14-prostaglandin J2, all induce adipogenesis in cells expressing PPAR-γ [33–36]. Interestingly, the metabolic cofactor of PPAR-γ, PPAR-γ coactivator (PGC)-1α is itself a transcriptional target of p53 [37]. Conversely, the aforementioned PPAR-γ agonists have also been shown to induce p53-dependent apoptosis in tumor cells, thereby providing an early example of the interconnection between metabolic and p53-signaling pathways [38–41].
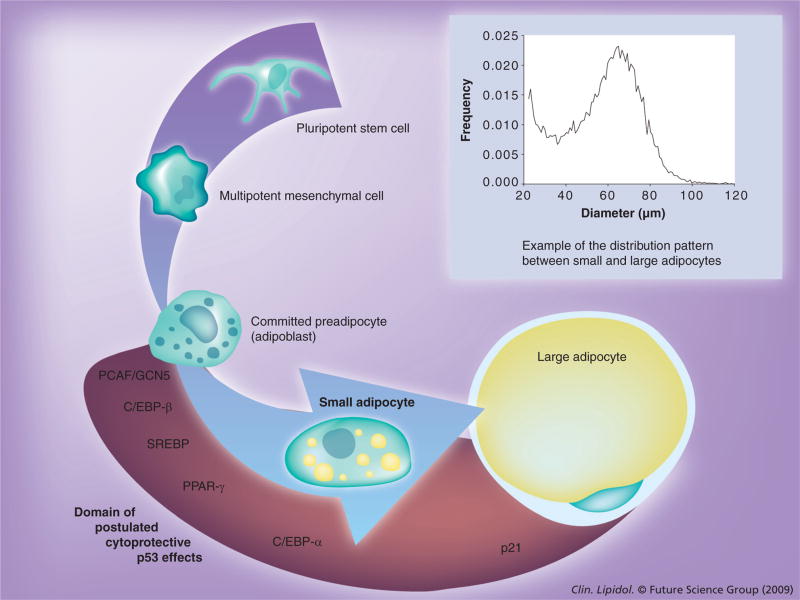
Figure 1. Stages in the adipogenic conversion process.
The insert depicts a typical example of the distribution between the small and large adipocytes we observe in an organism. Initial pluripotent stem cells are committed to the mesenchymal lineage. Subsequent differentiation steps lead to loss of potency and the ability to divide in the formation of a fully committed preadipocyte cell. Upon initiation of adipogenesis these cells accumulate small lipid droplets in the perinuclear region and undergo marked alterations in cellular morphology and transcriptional activity. Ultimately, these small lipid droplets engorge themselves with lipids and become enlarged, fully lipid-laden adipocytes. The cytoplasm and nucleus of these rounded cells are squeezed by the enormous lipid droplet into a narrow area just underneath the plasma membrane. The relative position of several key transcription factors involved in adipogenic conversion (PCAF/GCN5, C/EBP-β, SREBP, PPAR-γ and C/EBP-α) is depicted at the relative position of their action during adipogenesis. The red shaded area and arrow indicate the time wherein we postulate p53 to be active in the manner described in the text. It is of interest to note that in the initiation phase of adipogenesis in the 3T3-L1 cell line, after the contact-inhibited cell-cycle arrest and clonal expansion phase, a large amount of cells are lost owing to apoptosis. Indicative of an involvement of p53-signaling pathways as this may be, it is at present unclear if these stages in 3T3-L1 adipogenesis (a semi-transformed cell-line) accurately reflect the process of adipogenesis in vivo. C/EBP: CCAAT/enhancer-binding protein; GCN5: General control nonderepressible; PCAF: p300/CREB-binding protein associated factor; SREBP: Sterol regulatory element-binding protein.
Meanwhile, the C/EBP-family, albeit not unique for the adipocyte, also appears to play an important role in adipogenesis. C/EBP-β (possibly in synergy with C/EBP-δ) induces the transcription of PPAR-γ in the preadipocyte [42,43], thereby triggering full-blown adipocyte differentiation. In the context of this review, it is worthwhile to note that p300/CREB-binding protein-associated factor/general control nonderepressible 5 (PCAF/GCN5) is a direct target of p53 activity [44]. PCAF-dependent acetylation of C/EBP-β is in turn involved in unlocking the adipogenic potential of this transcription factor [45]. Conversely, PCAF is itself also a regulator of p53-transcriptional activity, locking the two in a positive-feedback loop [44,46]. Another C/EBP family member, C/EBP-α, synergizes with PPAR-γ in a positive feedback loop in the regulation of the later stages of adipogenesis, such as the induction of the GLUT4 glucose transporter and adiponectin, and the maintenance of the adipocyte phenotype through a sustained elevation in levels of PPAR-γ [47,48]. With the onset of C/EBP-α, the adipocyte matures visibly, as can be seen by the formation of lipid droplets in the perinuclear region [49]. Interestingly, expression of C/EBP-α is also regulated by a transcriptional target of p53 activity, the transcription factor hematopoietic zinc-finger (Hzf) [50,51]. Combined, the regulation of both these adipogenic C/EBP transcription factors by p53 target genes provides an interesting mechanism for the regulation of the metabolic settings of the organism by the p53 master transcription factor.
A final important adipogenic transcription factor to be considered here is SREBP1: a transcription factor predominantly involved in regulating cholesterol homeostasis [52]. When cholesterol is abundant, this transcription factor is anchored to the endoplasmic reticulum through the ER retention protein Insig (insulin-induced gene) [53] and the escort-protein SREBP cleavage-activating protein (SCAP) [54]. A drop in cholesterol levels allows the release of the SREBP1/SCAP-complex from Insig followed by translocation of this complex to the Golgi complex. Within the Golgi, proteolytic processing events release the basic helix–loop–helix dimerization and DNA-binding domain of SREBP1. This soluble, transcriptionally active cleavage product, nSREBP1, translocates to the nucleus where it initiates transcription of SREBP1-responsive genes, such as PPAR-γ, leading to lipogenesis [55] and also to the production of an endogenous ligand for PPAR-γ activation [56]. In this way, SREBP1-activation potently enhances PPAR-γ activity, locking the two in a positive feed-forward loop. An alternative, hormonal pathway mediating SREBP1-transcriptional activation involves PKB-mediated, rapamycin-sensitive, mammalian target of rapamycin complex (mTORC) activation, which is responsible for the insulin-induced increases in lipogenesis [57]. In concert, these transcription factors induce the collection of genes leading to maturation of the adipocyte-precursor to its fully differentiated lipid-laden form.
Living in the fat lane
As much as this specialized system has evolved to deal with free fatty acids and store fat, it seems reasonable to assume that a price must be paid for the protection offered. More than any other cell type in the body, cells in the fat depot are confronted with extreme levels of free fatty acids, cholesterol and the effects of lipotoxicity [58]. To give an inkling of the challenges faced in the fat depot, in blood, the levels of free fatty acids range in the area from 0.2 to 0.4 mM and even a small increase in these levels is associated with a reduction in insulin release by the β-cell and a marked loss of insulin sensitivity in muscle [59–61]. In comparison to blood it is difficult, if not technically impossible, to obtain a reliable measurement of free fatty acids in the interstitial space between adipocytes within the fat depot. However, that it will be higher than 0.4 mM, even staggeringly high, seems quite a safe bet. The deleterious effects of prolonged exposure to these conditions in the fat depot are readily apparent as committed preadipocytes from older animals demonstrate changes in their ability to undergo adipogenic differentiation and lipogenesis [62–64].
Remarkably, even under such adverse conditions, adipocytes and preadipocytes turn out to be fairly long-lived cells. Recently, Arner and coworkers made clever use of 14C derived from nuclear bomb tests. Levels of this radionucleotide in the atmosphere markedly increased during the Cold War and have dropped exponentially since the Test-Ban Treaty in 1963. As DNA is synthesized at the moment of cell division and is stable afterwards, comparing the levels of 14C incorporated into the DNA during its synthesis to known atmospheric 14C levels can mark the date a cell was born. Their data suggest that the number of adipocytes in an adult is set during childhood and adolescence [65]. Furthermore, they found that roughly half of all adipocytes are replaced every 8.3 years irrespective of the level of obesity (and associated increases in levels of free fatty acids). Interestingly, this implies a theoretical possibility for some adipocytes (~0.5%) to not turn over at all in the span of a human life.
In an adult, it must now be concluded that it is mainly hypertrophy of differentiated, committed preadipocytes as opposed to hyperplasia from stem cells and subsequent de novo adipogenesis that is responsible for the expansion of the fat depot under high caloric intake in an adult [65]. In essence, after the initial waves of cellular hyperplasia from pluripotent and multipotent cells, and commitment to the adipocyte lineage during youth and early adolescence, a large proportion of the cells lie dormant in a semi-senescent state in the fat depot until cued for enlargement (Figure 1). Kirkland et al. suggest that these preadipocytes account for 15–50% of the total cell population in the fat depot [66,67]. Importantly, it suggests that these preadipocytes must maintain viability and the ability to differentiate into a lipid-laden adipocyte from early adolescence onwards and throughout life. As a consequence, a certain amount of innate tolerance to the cellular and oxidative damage caused by high levels of free fatty acids can be expected as a trait of all cells that have residence in the fat depot.
Studies performed by our collaborators and at our laboratories uncovered a further important principle when we compared obese individuals that succumb to insulin-resistance, and potentially diabetes, with obese individuals that maintain insulin sensitivity. We found that the insulin-resistant group are characterized by the larger presence of ‘small’ differentiated adipocytes, suggesting the final stages of the adipogenic conversion process are hampered [68–70]. As shown in the light blue graph inset of Figure 1, these ‘small’ adipocytes form a major component of any fat depot and can perhaps best be seen as immature adipocytes with little lipid and very small droplets. These cells do float when suspended, thereby clearly distinguishing them from other cells in the fat depot, such as fibroblasts, committed preadipocytes and macrophages. It seems intuitive and plausible that these cells form a transitional stage between the more fibroblastic preadipocyte and a fully engorged, lipid-laden adipocyte. In that light, our hypothesis is that these cells fail to make the final adipogenic step in insulin-resistant individuals. By failing to engorge themselves on lipids, the problem persists, prompting more cells to undergo terminal differentiation, and at the same time exposing the organism to an excessive lipid load with many deleterious side-effects to bear. However, it could be argued that in theory these small cells could also originate from a large adipocyte cell after undergoing massive lipolysis, or they could form a completely separate end point, independent from the large adipocytes and without any movement between these cellular populations whatsoever. Future research will be needed to tease apart these different possibilities.
With that caveat in mind let us for now resume our train of thought following the assumption made in Figure 1. The beneficial protective effects these cells have in permitting a happy, healthy ‘Burgundian Life’ [71] to the obese individual should then be readily apparent. For these individuals have a reservoir of cells at their disposal that maintain viability and the ability to differentiate to a fully enlarged lipid-engorged state. As a consequence, they can mobilize these cells to deal with the additional lipid and cholesterol load to protect these individuals from the lipotoxic and inflammatory effects of free fatty acids, ectopic lipid accumulation and the ER-stress caused by cholesterol. Failure to do so results in the obese individual succumbing to diabetes and its associated morbidities. It is of interest to note in this discourse the observation made by Blüher et al., of an even more profound heterogeneity between small and large adipose cells in the adipose-specific insulin receptor-knockout mice, although the molecular mechanisms remain enigmatic [72]. In continued close collaboration with Reaven, Smith and coworkers at the Universities of Stanford, USA, and Gothenburg, Sweden, we took this material from our previous studies [68–70,73] and cross-referenced it against all other publicly available arrays using gene-set enrichment analyses. This approach gives a very direct, unbiased readout of which signaling pathways are involved in a biological phenomenon as the data from the studies are matched against all possible array-information in the public domain, without being influenced by the scientist. As an example, in this case, by comparing expression profiles between groups of equally obese people separated into groups of insulin-resistant and insulin-sensitive subjects, one can assume that differences in gene expression between these individuals would match up with signaling pathways characterized in other array studies, such as a study into immortalization of cancer cells, thereby shedding some light, even if only correlative, at the molecular mechanisms involved in turning the small adipocytes into fully lipid-laden cells. Much to our surprise, alongside the expected matches with studies on adipogenesis or studies on the insulin-sensitizing effects of TZDs, our analyses also matched profiles of studies describing several well-known canonical p53-transcriptional targets, such as p21waf1 [74–76], as well as lesser-known targets, such as oxysterol-binding protein (OSBP) [44], suggesting an involvement of the p53 cell cycle-control pathway in the regulation of this adipogenic conversion process. Unfortunately, data in the literature are sparse, and only a few mouse models have been studied in any detail with respect to this question. For one, it has been demonstrated that p21waf1 is crucial for protecting the hypertrophied adipocytes in diet-induced obese mice from apoptosis [77]. Another important study by the same group from the University of Tsukuba, Japan, identified p53 as a component in a negative-feedback loop preventing excessive fat accumulation in the adipocytes of obese, leptin-deficient mice [78]. Unfortunately, however, no measurement was made on the size distribution of the adipocytes in this latter study. It is to be hoped that these studies will be revisited in the near future and complemented with an analysis of cell-size distribution, which will provide valuable additional information. Perhaps the most intriguing study in this respect involves Donehower’s hypermorphic p53 mice: these mice exhibit altered fat depots and dyslipidemia reminiscent of accelerated aging [79,80]. However, it remains to be determined to what extent this mouse model is a true hypermorph of p53 activity and not a p53-mutant inducing transcription of some, but not all transcriptional targets of p53 [81]. Another caveat is that all these studies are performed on whole-body mouse knockout models, thus making it very difficult to attribute alterations in the metabolic settings of these mice directly to the fat depots. Irrespective of these considerations, these studies do seem to corroborate a role for p53 in the regulation of adipogenesis throughout adult life and, thereby, its profound influence on the metabolic setting of the organism.
The p53 transcription factor is predominantly known as a critical regulator of cell cycle arrest and apoptosis in cells when confronted with a genotoxic insult such as UV-damage or ionizing radiation [82,83]. Indeed, it has been estimated that aberrant p53 signaling is involved in at least 50% of human cancers [84,85]. It is this role of p53 in preventing neoplasias that gave rise to its nickname ‘guardian of the genome’ [86]. Notwithstanding the importance of the p53 tumor suppressor in safeguarding the organism against cancer, what could possibly be its role in the protection against age- or obesity-induced Type 2 diabetes?
A recent major shift in paradigm in the field of p53-signaling provides an important clue to the resolution of this theoretical conundrum. Currently, it is suggested that aside from its key apoptotic role in the face of acute and extreme genotoxic insults, low levels of p53 expression, induced by the rigors and strains of daily life, make an important contribution to the maintenance of the organism [81,87]. Far from promptly inducing apoptosis, a low but continuous level of p53 activity actually induces a cytoprotective set of genes involved in oxygen radical scavenging and cellular maintenance [88,89]. Both free fatty acid-derived ROS and cholesterol-derived ER stress are exactly the ‘rigors and strains of daily life’ that activate p53 in this manner. In the remainder of this review, we will introduce some of the basic tenets of p53 signaling and discuss the potential for several of its transcriptional targets to either protect the preadipocytes from oxidative stress or to provide the immature, small adipocyte with an enhanced lipogenic capacity, thereby greatly improving the ability of the cell to act as a lipid sink. Please see Figure 2 for a depiction of the following hypothesis.
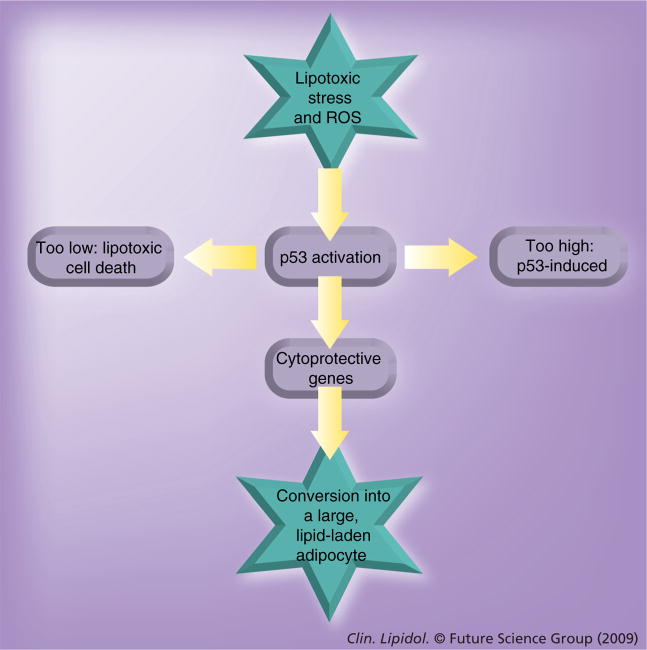
Figure 2. The underlying hypothesis on the role of p53 in adipogenesis.
Exposure to free fatty acids in the interstitial fluid will activate p53 through lipotoxic stress and ROS-induced cellular damage. Is the level of p53-activation too high and persistent? The tumor-suppressor p53 will eventually induce apoptosis, leading to loss of the cell. Is the level of p53 activation too low? There will not have been an effect of this ‘guardian’ at all and the cell will ultimately succumb to the damage induced by reactive oxygen radicals and lipotoxic effects. Only when the level of p53 activation is ‘just right’, will it confer protection to the small adipocytes by the induction of reactive oxygen-scavenging genes and possibly even by enhancing the lipogenic capacity of these cells. Thereby, p53 activation, when properly controlled, will ensure the clearance of lipotoxic stress, protecting not just the cell, but once again the organism as a whole. However, it is worthy to note for consideration, that in our present-day society ‘just right’ may have shifted considerably from our original evolutionary settings.
ROS: Reactive oxygen species.
The guardian of corpulence
Control over the p53-signal transduction pathway is achieved by two major p53-binding and regulating proteins: Mdm2 and Mdm4 [90]. Mdm2 was identified in the early 1990s as a gene present on the double-minute amplicon of a spontaneously transformed mouse cell-line [91]. Several studies identified Mdm2 as a major regulator of p53 through its ability to bind p53 and shuttle it out of the nucleus, thereby preventing its ability to activate transcription [92–94]. Furthermore, Mdm2 also acts as an E3-ubiquitin ligase and thereby destabilizes the p53 protein through ubiquitin-mediated proteasomal degradation [95,96]. However, a recent paper describing a knock-in mouse expressing mutant Mdm2 (which cannot degrade p53) demonstrates that simple binding is not sufficient for inhibition of p53-activity [97]. This manuscript highlights the prime importance of this ubiquitin-ligase function of Mdm2. As the Mdm2 gene itself is a transcriptional target of p53, the two are locked in a negative-feedback loop ensuring p53 levels are maintained at very low levels (Figure 3). The vital importance of this activity is demonstrated in the mdm2 homozygous null mice, which die in utero owing to p53-induced apoptosis [98,99].
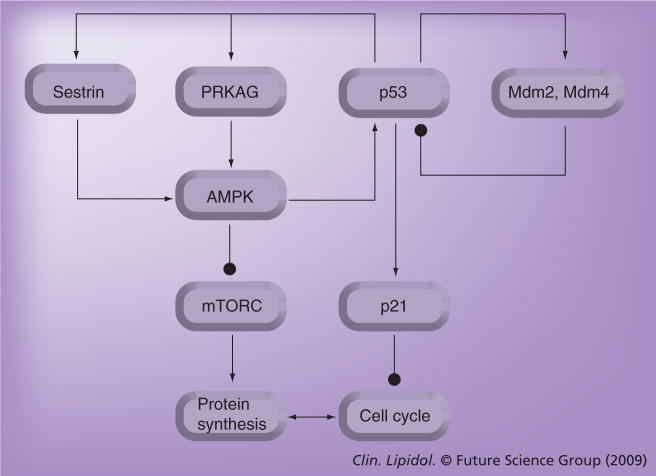
Figure 3. Negative- and positive-feedback loops involved in p53 signaling.
Negative- (closed arrow –•) and positive- (open arrow →) feedback loops involved in p53 signaling are highlighted and the manner in which they intertwine to synchronize regulation of the cell cycle with the regulation of protein synthesis. Indicated are classical components of the p53-mediated cell-cycle arrest pathway: Mdm2, Mdm4 and the p53-effector p21waf1; essential components of the metabolic fuel-sensing pathway: AMPK and mTORC; and the recently positioned sestrins, as discussed in the text.
mTORC: Mammalian target of rapamycin complex.
A homologous protein, Mdm4 (originally termed MdmX), was identified in the mid-1990s in a screen for p53-binding proteins [100,101]. In contrast to Mdm2, Mdm4 does not cycle in and out of the nucleus on its own and does not act as a ubiquitin ligase [102]. Surprisingly, however, Mdm4-knockout mice are embryonically lethal owing to massive cell cycle arrest, and akin to the Mdm2-knockout mice, this lethality can also be prevented in a p53-homozygous null background [103]. Combined, these studies identified the Mdm2 and Mdm4 proteins as crucial non-redundant regulators of p53 [104]. Upon disruption of the complex between p53 and the Mdm proteins, levels of nuclear p53 rapidly increase and p53-mediated transcription is initiated.
Transcriptional activity of p53 is further regulated through deacetylation by Sirt1. These NAD+-dependent protein deacetylases appear to mimic the cell-survival and life-prolonging effects of caloric restriction by attenuating p53-activation, promoting fat mobilization and preventing adipogenesis [105–107]. Vital to the effects of p53 is its activity as a transcription factor initiating the synthesis of a wide range of proteins. One of the best known targets of p53-transcriptional activity is p21waf1 (wild-type-p53-activated factor), which acts as a cell cycle inhibitor and protects cells from undergoing apoptosis [74–76].
Another important point of cross-talk between p53 and metabolic signaling pathways is AMPK. Several reviews by Hardie and coworkers present a detailed description of this major metabolic regulator of cellular energy homeostasis [108–110]. In the context of this review, it is important to note that AMPK can stabilize and activate p53 by phosphorylation of Ser15 [111,112]. Through this mechanism, a checkpoint is emplaced ensuring arrest of the cell cycle in periods of glucose deprivation. Indeed, as such, AMPK-mediated activation of p53 may be a far more common event in the life of a cell than ionizing radiation-mediated p53-stabilization and apoptosis. Remarkably, data by Karin et al. illustrate that the reverse holds true as well: active p53 induces the transcription of sestrins [113], which subsequently mediate the autophosphorylation and activation of AMPK and concomitant inactivation of mTORC, the rapamycin-sensitive metabolic regulator of protein synthesis. In essence, this pathway is thought to ensure arrest of protein synthesis when further cellular division is halted by p53. Furthermore, PRKAG2, the regulatory subunit of AMPK, is itself a transcriptional target of p53 activity [44], suggesting the two pathways are intimately interconnected in positive-feedback mechanisms (Figure 3).
All about stress
The previously mentioned sestrins are members of an ancient, evolutionarily conserved family of proteins involved in the regeneration of the thiol-containing peroxidase-scavenging proteins. As such, their original role appears to be in forming an important line of defense against ROS [114]. In addition, this cytoprotective effect against ROS may well be of significant functional importance in protecting the preadipocyte against the oxidative damage associated with lipotoxicity. Another very interesting p53 target gene with regard to protection against oxidative stress is Tp53-induced glycolysis and apoptosis regulator (TIGAR) [115]. TIGAR shows significant homology to the bisphosphatase domain of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (Fru-2,6-P2) and indeed, overexpression of TIGAR causes a decline in intracellular levels of Fru-2,6-P2. Fru-2,6-P2 is both a positive allosteric effector of 6-phosphofructo-1-kinase and also an inhibitor of fructose-1,6-bisphosphatase; thereby the net effect of TIGAR-mediated reductions in levels of Fru-2,6-P2 is a reduction of glycolysis and redirection of the accumulated glucose-6-P into the pentose phosphate shunt. The generation of NADPH and increases in reduced glutathione as a resultant of the pentose phosphate shunt also serve to protect the cell from oxidative damage [87,115]. Furthermore, the contribution of p53-induced target genes, such as glutathione peroxidase (GPXI) [116] and aldehyde dehydrogenase (ALDH4A1) [117], in protecting the cell against oxidative damage [89] is well established. In essence, this combined transcriptional profile of p53 could serve the cells residing in the fat depot well in conferring protection to one of the major mediators of lipotoxicity: ROS.
The StARD of the story: a thought on oxysterol
Aside from providing cytoprotection from ROS, some recent observations suggest p53 could, in theory, provide the adipocytes with a more direct handle on free fatty acids and cholesterol by enhancing the lipogenic capacity of these cells. Thereby, lipotoxicity-induced p53 activation would aid these cells in an attempt to attenuate the sources of stress in the fat depot directly (see Figure 4 for a depiction of this thought). Wei and coworkers identified two p53-target genes, StARD4 and OSBP, in a genome-wide map [44]. StARD4 was originally identified as a mediator of intracellular lipid metabolism under the control of SREBP1 transcription factors [118,119] and belongs to a family of steroidogenic acute regulatory proteins that are involved in transporting the water-insoluble cholesterol across the cytosol to the mitochondrion where it can be converted into the potent signaling molecule oxysterol [120–122]. How oxysterol interacts with metabolic settings in the body is currently not understood in detail. However, this more soluble derivative of cholesterol acts in part through its cognate receptor liver X receptor (LXR) that, in turn, has been demonstrated to induce PPAR-γ upon its activation [123–125].
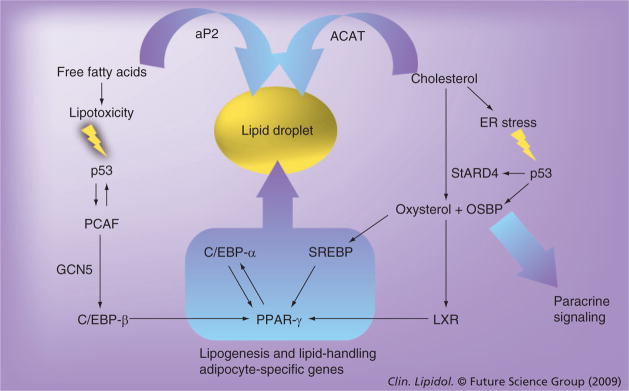
Figure 4. Potential involvement of p53 activity in adipogenesis, as hypothesized in the text.
Free fatty acid-mediated lipotoxity and associated oxidative stress and cholesterol-overload with associated ER stress can both induce p53 activation. Several recently identified transcriptional targets of p53, such as PCAF, StARD4 and OSBP, impinge on known components of the transcriptional machinery governing adipogenic conversion. In particular, StARD4 is of interest as it contributes to the synthesis of oxysterol. This powerful signaling molecule can mediate paracrine signaling and acts on a known, but enigmatic component of adipogenesis, the LXR receptors. The net increase in lipogenic activity and the genesis of the lipid droplet in these cells will subsequently provide a sink for the excessive amounts of free fatty acids and cholesterol (represented by curved arrows) governed by the aP2 fatty acid transporter and the ACAT mediator of cholesterol esterification. This ultimately leads to removal of the stresses that initiated p53-activation and termination of the p53-signal (prior to the initiation of apoptotic processes). It is noteworthy that the respective position of StARD4, OSBP and PCAF is only due to diagrammatic constraints: either lipotoxicity or ER-stress can induce these transcriptional targets of p53 through the activation of p53-mediated transcription. A detailed description of this model and its respective components can be found in the text along with accompanying references. ACAT: Acyl-coenzyme A:cholesterol acyl-transferase; C/EBP: CCAAT/enhancer-binding protein; ER: Endoplasmic reticulum; LXR: Liver X receptor; OSBP: Oxysterol-binding protein; PCAF: p300/CREB-binding protein associated factor; SREBP: Sterol regulatory element-binding protein.
In light of the observations made on our human subjects [61–63], the involvement of this pathway starts making some sense: for, even though the LXR receptors are not required for the initiation of adipogenesis per se, these receptors are involved in initiating age- and diet-induced adipocyte hypertrophy. LXR-knockout mice demonstrate a reduced adipocyte size and do not gain weight when fed a high-fat diet [125,126]. Furthermore, and importantly, oxysterol levels in the serum of subjects markedly associates with obesity, oxidative stress and components of the metabolic syndrome in adolescents [127]. Furthermore, it is of interest to note that it has recently been shown that a potent proadipogenic enzyme, 11β-hydroxysteroid dehydrogenase (11-HSD), catalyzes the reduction of 7-ketocholesterol leading to the accumulation of the oxysterol 7β-hydroxycholesterol in the adipocyte [128–131]. Furthermore, oxysterol allosterically activates an ER-resident enzyme, acyl-coenzyme a:cholesterol acyl-transferase (ACAT) [132,133]. This enzyme converts cholesterol into cholesterol esters, which are stored in the neutral core of the lipid droplet [134,135]. Combined with the initiation of LXR-mediated lipogenesis and enhanced lipid droplet biogenesis this creates a cholesterol-sink, thereby liberating the SREBP-transcription complex from the negative feedback imposed by cholesterol and a further enhancement of the lipogenic capacity of the cell. These indirect boosts of LXR- and PPAR-γ-activity downstream of p53 may enhance the adipogenic conversion of preadipocyte or prompt a small adipocyte to engorge itself with lipids (Figure 4). Based on the observations mentioned previously, oxysterol certainly merits further study as an attractive theoretical mediator of these effects.
Another p53-target with potential close association to this oxysterol-signaling pathway is OSBP. OSBP was originally identified as a putative oxysterol receptor involved in maintaining cholesterol and lipid homeostasis [136]. However, these roles are actually mediated through the previously mentioned LXR and SREBP1 [137]. OSBP is the founding member of a large family of 16 predicted proteins [138,139]. These family members have several different membrane– and protein–protein interaction domains in conjunction with their OBD, but their function remains enigmatic. It has been demonstrated that OSBP potentiates SREBP1 signaling and is crucial for the insulin-induced induction of SREBP1 and lipogenesis [137]. Furthermore, it has also been demonstrated that LXR-transcriptional activity is unaffected by OSBP [137]. Thereby, the combined effect of OSBP on both SREBP1 and LXR-PPAR-γ enhances these lipogenic transcription factors even further. It seems these novel p53 targets are capable of orchestrating a finely tuned sequence of events within the adipogenic process, initiating the genesis of the lipid droplet and strongly potentiating lipogenesis. Thereby, in contrast to the destruction inherent to an apoptotic process, these cells could rely on the p53 transcription factor to build themselves into a lipid-sink with a large capacity to deal with free fatty acids in the protection of the organism.
Future perspective
Current research in our laboratories and studies by our colleagues are aimed at verifying and validating key components of this hypothesis. In particular, we aim to elucidate the effects of alterations in p53 signaling on animal metabolism in collaboration with Dr Lozano (Houston, TX, USA), by employing available mouse models such as the floxed mdm2 and mdm4 mice. Naturally, we are also very interested in studying the physiological role of oxysterol in the maturation of adipocytes and the regulation of adipocyte cell size, and would welcome anyone interested in collaborating with us on this topic. The suggested fundamental link between p53, cytoprotection and adipogenesis provides an interesting new way of thinking about signaling pathways involved in age-related afflictions such as diabetes and cancer. In the coming years, if proven correct, a deeper understanding of these pathways can provide attractive new avenues for pharmacological intervention influencing adipocyte mobilization during times of increased requirement for lipid storage, the prevention of the ectopic deposition of fat with advancing age, and the onset of Type 2 diabetes.
Executive summary
Adipocytes are of vital importance in protecting the organism against the deleterious effects of ectopic lipid deposition.
Preadipocytes must survive the lipotoxic conditions in the fat depot throughout adult life and maintain the ability to differentiate.
Data obtained by our studies suggest an involvement of the p53-transcription factor in protecting obese individuals from diabetes.
The antioxidant function of the p53-transcription factor may confer protection from the oxidative damage associated with lipotoxicity.
Furthermore, transcriptional targets of p53 could induce lipogenesis and enhance the lipid-storage capacity of adipocytes.
Combined, p53 may aid the cells residing in the fat pad in dealing with the effects of lipid and cholesterol overload, thereby protecting the organism from ectopic lipid deposition and lipotoxicity.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol. 2008;22(5):1023–1031. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arner P. Insulin resistance in Type 2 diabetes – role of the adipokines. Curr Mol Med. 2005;5(3):333–339. doi: 10.2174/1566524053766022. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Cushman SW, Pannell LK, Hess S. Quantitative proteomic analysis of the secretory proteins from rat adipose cells using a 2D liquid chromatography-MS/MS approach. J Proteome Res. 2005;4(2):570–577. doi: 10.1021/pr049772a. [DOI] [PubMed] [Google Scholar]
- 4.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, Type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8(5):395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 5.Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296(5570):1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMillan DC, Sattar N, McArdle CS. ABC of obesity. Obesity and cancer. BMJ. 2006;333(7578):1109–1111. doi: 10.1136/bmj.39042.565035.BE1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002:53319–53336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 8.Neel JV. The ‘thrifty genotype’ in 1998. Nutr Rev. 1999;57(5 Pt 2):S2–S9. doi: 10.1111/j.1753-4887.1999.tb01782.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Hirose H, Ohneda M, et al. β-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-β-cell relationships. Proc Natl Acad Sci USA. 1994;91(23):10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittra S, Bansal VS, Bhatnagar PK. From a glucocentric to a lipocentric approach towards metabolic syndrome. Drug Discov Today. 2008;13(5–6):211–218. doi: 10.1016/j.drudis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Robertson RP, Harmon J, Tran PO, Poitout V. β-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in Type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119–S124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 12.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29(1):42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 13.Banhegyi G, Baumeister P, Benedetti A, et al. Endoplasmic reticulum stress. Ann NY Acad Sci. 2007;111:358–71. [Google Scholar]
- 14.Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121–131. doi: 10.1146/annurev.me.44.020193.001005. [DOI] [PubMed] [Google Scholar]
- 15.Capeau J, Magre J, Lascols O, et al. Diseases of adipose tissue: genetic and acquired lipodystrophies. Biochem Soc Trans. 2005;33(Pt 5):1073–1077. doi: 10.1042/BST0331073. [DOI] [PubMed] [Google Scholar]
- 16.Villarroya F, Domingo P, Giralt M. Lipodystrophy associated with highly active anti-retroviral therapy for HIV infection: the adipocyte as a target of anti-retroviral-induced mitochondrial toxicity. Trends Pharmacol Sci. 2005;26(2):88–93. doi: 10.1016/j.tips.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 17▪.Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp Gerontol. 2007;42(6):463–471. doi: 10.1016/j.exger.2007.03.003. Summarizes over a decade of outstanding work on the effect of age on cellularity and adipogenesis in the fat depot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299(5606):572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 19.Bluher M, Michael MD, Peroni OD, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3(1):25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 20.Cohen AW, Razani B, Wang XB, et al. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol. 2003;285(1):C222–C235. doi: 10.1152/ajpcell.00006.2003. [DOI] [PubMed] [Google Scholar]
- 21.Bickel PE. Lipid rafts and insulin signaling. Am J Physiol Endocrinol Metab. 2002;282(1):E1–E10. doi: 10.1152/ajpendo.2002.282.1.E1. [DOI] [PubMed] [Google Scholar]
- 22.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Londos C, Brasaemle DL, Gruia-Gray J, et al. Perilipin: unique proteins associated with intracellular neutral lipid droplets in adipocytes and steroidogenic cells. Biochem Soc Trans. 1995;23(3):611–615. doi: 10.1042/bst0230611. [DOI] [PubMed] [Google Scholar]
- 24.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279(45):46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 25.Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149(3):942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 26.Holman GD, Cushman SW. Subcellular localization and trafficking of the GLUT4 glucose transporter isoform in insulin-responsive cells. Bioessays. 1994;16(10):753–759. doi: 10.1002/bies.950161010. [DOI] [PubMed] [Google Scholar]
- 27.Lizunov VA, Matsumoto H, Zimmerberg J, Cushman SW, Frolov VA. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J Cell Biol. 2005;169(3):481–489. doi: 10.1083/jcb.200412069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000:16145–16171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 29.Tontonoz P, Graves RA, Budavari AI, et al. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR-γ and RXR α. Nucleic Acids Res. 1994;22(25):5628–5634. doi: 10.1093/nar/22.25.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR-γ 2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 31.He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA. 2003;100(26):15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koutnikova H, Cock TA, Watanabe M, et al. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR-γ hypomorphic mice. Proc Natl Acad Sci USA. 2003;100(24):14457–14462. doi: 10.1073/pnas.2336090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amri EZ, Bonino F, Ailhaud G, Abumrad NA, Grimaldi PA. Cloning of a protein that mediates transcriptional effects of fatty acids in preadipocytes. Homology to peroxisome proliferator-activated receptors. J Biol Chem. 1995;270(5):2367–2371. doi: 10.1074/jbc.270.5.2367. [DOI] [PubMed] [Google Scholar]
- 34.Forman BM, Tontonoz P, Chen J, et al. 15-deoxy-δ 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR-γ. Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 35.Kliewer SA, Lenhard JM, Willson TM, et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83(5):813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 36.Negrel R, Gaillard D, Ailhaud G. Prostacyclin as a potent effector of adipose-cell differentiation. Biochem J. 1989;257(2):399–405. doi: 10.1042/bj2570399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saleem A, Adhihetty PJ, Hood DA. Role of p53 in mitochondrial biogenesis and apoptosis in skeletal muscle. Physiol Genomics. 2008 doi: 10.1152/physiolgenomics.90346.2008. [DOI] [PubMed] [Google Scholar]
- 38.Su RY, Chi KH, Huang DY, Tai MH, Lin WW. 15-deoxy-δ12,14-prostaglandin J2 up-regulates death receptor 5 gene expression in HCT116 cells: involvement of reactive oxygen species and C/EBP homologous transcription factor gene transcription. Mol Cancer Ther. 2008;7(10):3429–3440. doi: 10.1158/1535-7163.MCT-08-0498. [DOI] [PubMed] [Google Scholar]
- 39.Mrowka P, Glodkowska E, Mlynarczuk-Bialy I, et al. Pioglitazone, a PPAR-γ ligand, exerts cytostatic/cytotoxic effects against cancer cells, that do not result from inhibition of proteasome. Acta Biochim Pol. 2008;55(1):75–84. [PubMed] [Google Scholar]
- 40.Gaetano C, Colussi C, Capogrossi MC. PEDF, PPAR-γ, p53: deadly circuits arise when worlds collide. Cardiovasc Res. 2007;76(2):195–196. doi: 10.1016/j.cardiores.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Ho TC, Chen SL, Yang YC, et al. PEDF induces p53-mediated apoptosis through PPAR-γ signaling in human umbilical vein endothelial cells. Cardiovasc Res. 2007;76(2):213–223. doi: 10.1016/j.cardiores.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 42.Wu Z, Xie Y, Bucher NL, Farmer SR. Conditional ectopic expression of C/EBPβ in NIH-3T3 cells induces PPAR-γ and stimulates adipogenesis. Genes Dev. 1995;9(19):2350–2363. doi: 10.1101/gad.9.19.2350. [DOI] [PubMed] [Google Scholar]
- 43.Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol Cell Biol. 1996;16(8):4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44▪.Wei CL, Wu Q, Vega VB, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124(1):207–219. doi: 10.1016/j.cell.2005.10.043. Genome-wide analysis of p53-responsive genes demonstrating p53’s involvement in several unexpected pathways, such as oxysterol signaling. [DOI] [PubMed] [Google Scholar]
- 45.Wiper-Bergeron N, Salem HA, Tomlinson JJ, Wu D, Hache RJ. Glucocorticoid-stimulated preadipocyte differentiation is mediated through acetylation of C/EBPβ by GCN5. Proc Natl Acad Sci USA. 2007;104(8):2703–2708. doi: 10.1073/pnas.0607378104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Scolnick DM, Trievel RC, et al. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol. 1999;19(2):1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christy RJ, Yang VW, Ntambi JM, et al. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989;3(9):1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- 48.Wang ND, Finegold MJ, Bradley A, et al. Impaired energy homeostasis in C/EBPα knockout mice. Science. 1995;269(5227):1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 49.Freytag SO, Paielli DL, Gilbert JD. Ectopic expression of the CCAAT/enhancer-binding protein α promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994;8(14):1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- 50.Kawagishi H, Wakoh T, Uno H, et al. Hzf regulates adipogenesis through translational control of C/EBPα. EMBO J. 2008;27(10):1481–1490. doi: 10.1038/emboj.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugimoto M, Gromley A, Sherr CJ. Hzf, a p53-responsive gene, regulates maintenance of the G2 phase checkpoint induced by DNA damage. Mol Cell Biol. 2006;26(2):502–512. doi: 10.1128/MCB.26.2.502-512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10(9):1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Takaishi K, Cook W, McCorkle SK, Unger RH. Insig-1 ‘brakes’ lipogenesis in adipocytes and inhibits differentiation of preadipocytes. Proc Natl Acad Sci USA. 2003;100(16):9476–9481. doi: 10.1073/pnas.1133426100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McPherson R, Gauthier A. Molecular regulation of SREBP function: the Insig-SCAP connection and isoform-specific modulation of lipid synthesis. Biochem Cell Biol. 2004;82(1):201–211. doi: 10.1139/o03-090. [DOI] [PubMed] [Google Scholar]
- 55.Fajas L, Schoonjans K, Gelman L, et al. Regulation of peroxisome proliferator-activated receptor γ expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol. 1999;19(8):5495–5503. doi: 10.1128/mcb.19.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPAR-γ through the production of endogenous ligand. Proc Natl Acad Sci USA. 1998;95(8):4333–4337. doi: 10.1073/pnas.95.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Porstmann T, Santos CR, Griffiths B, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8(3):224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirkland JL, Hollenberg CH, Kindler S, Roncari DA. Long-chain fatty acids decrease lipoprotein lipase activity of cultured rat adipocyte precursors. Metabolism. 1994;43(2):144–151. doi: 10.1016/0026-0495(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 59.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277(52):50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 60.Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86(1):205–243. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- 61.Poitout V, Robertson RP. Minireview: Secondary β-cell failure in Type 2 diabetes – a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143(2):339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 62.Djian P, Roncari DA, Hollenberg CH. Adipocyte precursor clones vary in capacity for differentiation. Metabolism. 1985;34(9):880–883. doi: 10.1016/0026-0495(85)90114-3. [DOI] [PubMed] [Google Scholar]
- 63.Gregerman RI. Aging and hormone-sensitive lipolysis: reconciling the literature. J Gerontol. 1994;49(4):B135–B139. doi: 10.1093/geronj/49.4.b135. [DOI] [PubMed] [Google Scholar]
- 64.Carraro R, Li ZH, Gregerman RI. Catecholamine-sensitive lipolysis in the rat: different loci for effect of age on the lipolytic cascade in epididymal vs perirenal fat cells. J Gerontol. 1994;49(4):B140–B143. doi: 10.1093/geronj/49.4.b140. [DOI] [PubMed] [Google Scholar]
- 65▪▪.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. These authors cunningly use known atmospheric levels of 14C to retrospectively birth-date adipocytes allowing them to show that the number of adipocytes in adult life is set in early adolescence. [DOI] [PubMed] [Google Scholar]
- 66.Kirkland JL, Hollenberg CH, Kindler S, Gillon WS. Effects of age and anatomic site on preadipocyte number in rat fat depots. J Gerontol. 1994;49(1):B31–B35. doi: 10.1093/geronj/49.1.b31. [DOI] [PubMed] [Google Scholar]
- 67.Kirkland JL, Dobson DE. Preadipocyte function and aging: links between age-related changes in cell dynamics and altered fat tissue function. J Am Geriatr Soc. 1997;45(8):959–967. doi: 10.1111/j.1532-5415.1997.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 68.McLaughlin T, Deng A, Gonzales O, et al. Insulin resistance is associated with a modest increase in inflammation in subcutaneous adipose tissue of moderately obese women. Diabetologia. 2008;51(12):2303–2308. doi: 10.1007/s00125-008-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McLaughlin T, Sherman A, Tsao P, et al. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50(8):1707–1715. doi: 10.1007/s00125-007-0708-y. [DOI] [PubMed] [Google Scholar]
- 70▪.Yang X, Jansson PA, Nagaev I et al. Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun. 2004;317(4):1045–1051. doi: 10.1016/j.bbrc.2004.03.152. This proverb refers to the late 14th Century in the Netherlands and Flanders when the Dukes of Burgundy ruled the area. Their courts were such a spectacle of luxury, wealth and culture that a ‘Burgundian lifestyle’ still refers to the carefree enjoyment of good food, fine wine and a laid-back life. [DOI] [PubMed] [Google Scholar]
- 71.Bluher M, Patti ME, Gesta S, Kahn BB, Kahn CR. Intrinsic heterogeneity in adipose tissue of fat-specific insulin receptor knock-out mice is associated with differences in patterns of gene expression. J Biol Chem. 2004;279(30):31891–31901. doi: 10.1074/jbc.M404569200. [DOI] [PubMed] [Google Scholar]
- 72.Cam MC, Stiles D, Lee JC, et al. Reduced oxidative phosphorylation in adipocytes from human insulin resistant subjects. 2009 (Epub ahead of print) [Google Scholar]
- 73.Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 2003;13(2):65–70. doi: 10.1016/s0962-8924(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 74.Ju Z, Choudhury AR, Rudolph KL. A dual role of p21 in stem cell aging. Ann NY Acad Sci. 2007;1100:333–344. doi: 10.1196/annals.1395.036. [DOI] [PubMed] [Google Scholar]
- 75.Child ES, Mann DJ. The intricacies of p21 phosphorylation: protein/protein interactions, subcellular localization and stability. Cell Cycle. 2006;5(12):1313–1319. doi: 10.4161/cc.5.12.2863. [DOI] [PubMed] [Google Scholar]
- 76.Inoue N, Yahagi N, Yamamoto T, et al. Cyclin-dependent kinase inhibitor, p21WAF1/CIP1, is involved in adipocyte differentiation and hypertrophy, linking to obesity, and insulin resistance. J Biol Chem. 2008;283(30):21220–21229. doi: 10.1074/jbc.M801824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yahagi N, Shimano H, Matsuzaka T, et al. p53 Activation in adipocytes of obese mice. J Biol Chem. 2003;278(28):25395–25400. doi: 10.1074/jbc.M302364200. [DOI] [PubMed] [Google Scholar]
- 78.Dumble M, Gatza C, Tyner S, Venkatachalam S, Donehower LA. Insights into aging obtained from p53 mutant mouse models. Ann NY Acad Sci. 2004;1019:171–177. doi: 10.1196/annals.1297.027. [DOI] [PubMed] [Google Scholar]
- 79.Tyner SD, Venkatachalam S, Choi J, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415(6867):45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 80▪.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8(4):275–283. doi: 10.1038/nrm2147. Provides an important and comprehensive synthesis of the novel insights in the role of p53 as a cytoprotective agent beyond its functions in oncogenesis. [DOI] [PubMed] [Google Scholar]
- 81.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 82.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 83.Soussi T, Lozano G. p53 mutation heterogeneity in cancer. Biochem Biophys Res Commun. 2005;331(3):834–842. doi: 10.1016/j.bbrc.2005.03.190. [DOI] [PubMed] [Google Scholar]
- 84.Elledge RM, Lee WH. Life and death by p53. Bioessays. 1995;17(11):923–930. doi: 10.1002/bies.950171105. [DOI] [PubMed] [Google Scholar]
- 85.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 86.Bensaad K, Vousden KH. p53: new roles in metabolism. Trends Cell Biol. 2007;17(6):286–291. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 87.Vousden KH. Outcomes of p53 activation – spoilt for choice. J Cell Sci. 2006;119(Pt 24):5015–5020. doi: 10.1242/jcs.03293. [DOI] [PubMed] [Google Scholar]
- 88▪▪.Sablina AA, Budanov AV, Ilyinskaya GV, et al. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11(12):1306–1313. doi: 10.1038/nm1320. Breakthrough study that demonstrated how low levels of p53 activity are involved in protecting the cell against reactive oxygen species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marine JC, Francoz S, Maetens M, et al. Keeping p53 in check: essential and synergistic functions of Mdm2 and Mdm4. Cell Death Differ. 2006;13(6):927–934. doi: 10.1038/sj.cdd.4401912. [DOI] [PubMed] [Google Scholar]
- 90.Fakharzadeh SS, Trusko SP, George DL. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 1991;10(6):1565–1569. doi: 10.1002/j.1460-2075.1991.tb07676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 92.Roth J, Dobbelstein M, Freedman DA, Shenk T, Levine AJ. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 1998;17(2):554–564. doi: 10.1093/emboj/17.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freedman DA, Levine AJ. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol. 1998;18(12):7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2(3):169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- 95.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420(1):25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 96.Itahana K, Mao H, Jin A, et al. Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell. 2007;12(4):355–366. doi: 10.1016/j.ccr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 97.Montes de Oca LR, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378(6553):203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 98.Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378(6553):206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 99.Shvarts A, Bazuine M, Dekker P, et al. Isolation and identification of the human homolog of a new p53-binding protein, Mdmx. Genomics. 1997;43(1):34–42. doi: 10.1006/geno.1997.4775. [DOI] [PubMed] [Google Scholar]
- 100.Shvarts A, Steegenga WT, Riteco N, et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 1996;15(19):5349–5357. [PMC free article] [PubMed] [Google Scholar]
- 101.Marine JC, Jochemsen AG. Mdmx as an essential regulator of p53 activity. Biochem Biophys Res Commun. 2005;331(3):750–760. doi: 10.1016/j.bbrc.2005.03.151. [DOI] [PubMed] [Google Scholar]
- 102.Parant J, Chavez-Reyes A, Little NA, et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat Genet. 2001;29(1):92–95. doi: 10.1038/ng714. [DOI] [PubMed] [Google Scholar]
- 103.Marine JC, Jochemsen AG. Mdmx and Mdm2: brothers in arms? Cell Cycle. 2004;3(7):900–904. [PubMed] [Google Scholar]
- 104.Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429(6993):771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vaziri H, Dessain SK, Ng EE, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 106.Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107(2):137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 107.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8(10):774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 108.Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008;582(1):81–89. doi: 10.1016/j.febslet.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 109.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 110.Jones RG, Plas DR, Kubek S, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18(3):283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 111.Thoreen CC, Sabatini DM. AMPK and p53 help cells through lean times. Cell Metab. 2005;1(5):287–288. doi: 10.1016/j.cmet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 112.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134(3):451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304(5670):596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 114▪.Bensaad K, Tsuruta A, Selak MA, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–120. doi: 10.1016/j.cell.2006.05.036. This study, identifying Tp53-induced glycolysis and apoptosis regulator, links p53-activity with protection against reactive oxygen species, mitochondrial respiration and glycolysis. [DOI] [PubMed] [Google Scholar]
- 115.Tan M, Li S, Swaroop M, et al. Transcriptional activation of the human glutathione peroxidase promoter by p53. J Biol Chem. 1999;274(17):12061–12066. doi: 10.1074/jbc.274.17.12061. [DOI] [PubMed] [Google Scholar]
- 116.Yoon KA, Nakamura Y, Arakawa H. Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. J Hum Genet. 2004;49(3):134–140. doi: 10.1007/s10038-003-0122-3. [DOI] [PubMed] [Google Scholar]
- 117.Shea-Eaton WK, Trinidad MJ, Lopez D, Nackley A, McLean MP. Sterol regulatory element binding protein-1a regulation of the steroidogenic acute regulatory protein gene. Endocrinology. 2001;142(4):1525–1533. doi: 10.1210/endo.142.4.8075. [DOI] [PubMed] [Google Scholar]
- 118.Christenson LK, Osborne TF, McAllister JM, Strauss JF., III Conditional response of the human steroidogenic acute regulatory protein gene promoter to sterol regulatory element binding protein-1a. Endocrinology. 2001;142(1):28–36. doi: 10.1210/endo.142.1.7867. [DOI] [PubMed] [Google Scholar]
- 119.Soccio RE, Adams RM, Romanowski MJ, et al. The cholesterol-regulated StARD4 gene encodes a StAR-related lipid transfer protein with two closely related homologues, StarD5 and StarD6. Proc Natl Acad Sci USA. 2002;99(10):6943–6948. doi: 10.1073/pnas.052143799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Russell DW. Oxysterol biosynthetic enzymes. Biochim Biophys Acta. 2000;1529(1–3):126–135. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 121.Bjorkhem I, Diczfalusy U. Oxysterols: friends, foes, or just fellow passengers? Arterioscler Thromb Vasc Biol. 2002;22(5):734–742. doi: 10.1161/01.atv.0000013312.32196.49. [DOI] [PubMed] [Google Scholar]
- 122.Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80(1):361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- 123.Edwards PA, Kennedy MA, Mak PA. LXRs; oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. Vascul Pharmacol. 2002;38(4):249–256. doi: 10.1016/s1537-1891(02)00175-1. [DOI] [PubMed] [Google Scholar]
- 124▪▪.Seo JB, Moon HM, Kim WS, et al. Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor γ expression. Mol Cell Biol. 2004;24(8):3430–3444. doi: 10.1128/MCB.24.8.3430-3444.2004. Demonstrates the involvement of liver X receptor receptors in the induction of PPAR-γ and the stimulation of adipogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gerin I, Dolinsky VW, Shackman JG, et al. LXRβ is required for adipocyte growth, glucose homeostasis, and β cell function. J Biol Chem. 2005;280(24):23024–23031. doi: 10.1074/jbc.M412564200. [DOI] [PubMed] [Google Scholar]
- 126.Kalaany NY, Gauthier KC, Zavacki AM, et al. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 2005;1(4):231–244. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 127.Alkazemi D, Egeland G, Vaya J, Meltzer S, Kubow S. Oxysterol as a marker of atherogenic dyslipidemia in adolescence. J Clin Endocrinol Metab. 2008;93(11):4282–4289. doi: 10.1210/jc.2008-0586. [DOI] [PubMed] [Google Scholar]
- 128.Wamil M, Andrew R, Chapman KE, et al. 7-oxysterols modulate glucocorticoid activity in adipocytes through competition for 11β-hydroxysteroid dehydrogenase type. Endocrinology. 2008;149(12):5909–5918. doi: 10.1210/en.2008-0420. [DOI] [PubMed] [Google Scholar]
- 129.Hult M, Elleby B, Shafqat N, et al. Human and rodent type 1 11β-hydroxysteroid dehydrogenases are 7β-hydroxycholesterol dehydrogenases involved in oxysterol metabolism. Cell Mol Life Sci. 2004;61(7–8):992–999. doi: 10.1007/s00018-003-3476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996;20(4):291–302. [PubMed] [Google Scholar]
- 131.Mattsson C, Olsson T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr Med Chem. 2007;14(27):2918–2924. doi: 10.2174/092986707782359972. [DOI] [PubMed] [Google Scholar]
- 132.Cheng D, Chang CC, Qu X, Chang TY. Activation of acyl-coenzyme A:cholesterol acyltransferase by cholesterol or by oxysterol in a cell-free system. J Biol Chem. 1995;270(2):685–695. doi: 10.1074/jbc.270.2.685. [DOI] [PubMed] [Google Scholar]
- 133.Chang TY, Chang CC, Cheng D. Acyl-coenzyme A:cholesterol acyltransferase. Annu Rev Biochem. 1997;66:613–638. doi: 10.1146/annurev.biochem.66.1.613. [DOI] [PubMed] [Google Scholar]
- 134.Thiele C, Spandl J. Cell biology of lipid droplets. Curr Opin Cell Biol. 2008;20(4):378–385. doi: 10.1016/j.ceb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 135.Olofsson SO, Bostrom P, Andersson L, et al. Triglyceride containing lipid droplets and lipid droplet-associated proteins. Curr Opin Lipidol. 2008;19(5):441–447. doi: 10.1097/MOL.0b013e32830dd09b. [DOI] [PubMed] [Google Scholar]
- 136.Levanon D, Hsieh CL, Francke U, et al. cDNA cloning of human oxysterol-binding protein and localization of the gene to human chromosome 11 and mouse chromosome 19. Genomics. 1990;7(1):65–74. doi: 10.1016/0888-7543(90)90519-z. [DOI] [PubMed] [Google Scholar]
- 137▪▪.Yan D, Lehto M, Rasilainen L, et al. Oxysterol binding protein induces upregulation of SREBP-1c and enhances hepatic lipogenesis. Arterioscler Thromb Vasc Biol. 2007;27(5):1108–1114. doi: 10.1161/ATVBAHA.106.138545. Makes the crucial and counterintuitive observation that oxysterol-binding protein potentiates sterol regulatory element-binding protein and regulates insulin-induced lipogenesis. [DOI] [PubMed] [Google Scholar]
- 138.Olkkonen VM, Johansson M, Suchanek M, et al. The OSBP-related proteins (ORPs): global sterol sensors for co-ordination of cellular lipid metabolism, membrane trafficking and signalling processes? Biochem Soc Trans. 2006;34(Pt 3):389–391. doi: 10.1042/BST0340389. [DOI] [PubMed] [Google Scholar]
- 139.Fairn GD, McMaster CR. Emerging roles of the oxysterol-binding protein family in metabolism, transport, and signaling. Cell Mol Life Sci. 2008;65(2):228–236. doi: 10.1007/s00018-007-7325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]