Abstract
The following review examines the role of calcium in promoting the in vitro and in vivo activation of transglutaminases in neurodegenerative disorders. Diseases such as Alzheimer's disease, Parkinson's disease and Huntington's disease exhibit increased transglutaminase activity and rises in intracellular calcium concentrations, which may be related. The aberrant activation of transglutaminase by calcium is thought to give rise to a variety of pathological moieties in these diseases, and the inhibition has been shown to have therapeutic benefit in animal and cellular models of neurodegeneration. Given the potential clinical relevance of transglutaminase inhibitors, we have also reviewed the recent development of such compounds.
Keywords: Alzheimer's disease, calcium, Huntington's disease, neurodegeneration, Parkinson's disease, transglutaminase
Transglutaminases (TGs) catalyze various modifications of glutaminyl (Q) residues including covalent intra- and inter-molecular crosslinking with lysyl (K) residues. The activity of these enzymes and the products thereof are markedly increased in a variety of neurodegenerative disorders. Moreover, proteins thought to be pathogenic in these diseases are often TG substrates. Despite these findings, there is ambiguity concerning the role that TGs play in disorders, such as Alzheimer's disease (AD), Parkinson's disease (PD) or Huntington's disease (HD), and of the therapeutic benefit of inhibiting TG activity in these disorders. This ambiguity is partly due to questions concerning the activation of these enzymes in vivo. Depending on the TG, these enzymes are activated by a combination of proteolysis and Ca2+ or Ca2+ alone, and inhibited by GTP. Although little is known regarding the levels of cerebral GTP in neurodegenerative disorders, increases in intracellular Ca2+ (Ca2+i) are recognized to be an important, if not essential, factor in the etiology of neurological diseases. Given the propensity of disease-specific increases in Ca2+i to stimulate in vivo TG activity, we have sought to review the activation of TGs by this cation in both in vitro and neuropathological contexts to better understand the involvement of these enzymes in pathologies such as AD, PD and HD. Since TGs are likely to contribute to these diseases we have also reviewed recent developments in the search for selective TG inhibitors.
Cerebral TGs & γ-glutamylamine formation
The brain expresses at least four of the eight active TGs produced by mammals, namely TGs 1−3 [1-3] and 6 [4]. TGs catalyze a variety of modifications of the carboxamide moiety [-C(O)NH2] of Q residues, including transamidation [5], deamidation [6,7] and esterification [8], in which the carboxamide moiety is modified to [-C(O)NHR], [-CO2−] and [-C(O)OR], respectively. Even so, the only reaction attributed to cerebral TGs thus far is transamidation. This reaction converts the carboxamide moiety of a Q residue to a substituted carboxamide using amine-bearing compounds such as monoamines, diamines, polyamines and K residues as attacking nucleophiles [5]. Of the possible reaction products, the γ-glutamyl-ε-lysine [Nε-(γ-l-glutamyl)-l-lysine] isopeptide linkage formed between the Q and K residues, is the most commonly studied. This bond can be formed both within and between polypeptide chains. The placement of γ-glutamyl-ε-lysine linkages between polypeptides may result in protein aggregation. Since the deposition of multimeric structures is a common feature of neurodegenerative disorders, the formation of γ-glutamyl-ε-lysine bonds and the inhibition thereof has attracted much interest. TGs can also crosslink polypeptides with bis-γ-glutamylpolyamine bridges [9,10]. These bridges are formed by two successive transamidations, the first of which involves the attack of a polyamine on a Q residue to generate a γ-glutamylpolyamine residue. This moiety has a free terminal amine allowing it to participate in a second transamidation and thereby produce a bis-γ-glutamylpolyamine linkage. γ-Glutamylamines are excised unaltered during proteolysis and the amount of these freed molecules reflects in situ TG activity [11-13]. Based on such measurements, bis-γ-glutamylputrescine crosslinks appear to be formed at least as frequently as γ-glutamyl-ε-lysine isopeptide linkages [11]. Of the free γ-glutamylamines thus far identified in human cerebrospinal fluid (CSF; i.e., γ-glutamylspermidine, bis-γ-glutamylputrescine, γ-glutamyl-ε-lysine and bis-γ-glutamylputrescine), γ-glutamylspermidine at a concentration of 670 nM, is the most prevalent [11]. The concentration of bis-γ-glutamylputrescine in CSF exceeds that of γ-glutamylputrescine by approximately sixfold (i.e., 242 vs 39 nM). If the concentration of bis-γ-glutamylspermidine similarly exceeds that of γ-glutamylspermidine then bis-γ-glutamylpolyamine bonds would represent the predominant crosslink formed by TGs in the brain.
Recent studies suggest that some proteins crosslinked by γ-glutamyl-ε-lysine bridges may be relatively soluble [14,15]. In one study, a thioredoxin fusion protein containing a Q65 domain spontaneously formed noncovalent insoluble aggregates [14]. In the presence of TG 2, soluble high-molecular-weight polymers were obtained. However, inhibition of γ-glutamyl-ε-lysine crosslink formation by putrescine or by 5-biotin amidopentylamine antagonized the ability of TG 2 to form soluble high-molecular-weight polymers [14]. The antagonism by putrescine is presumed to be the result of covalent formation of γ-glutamylpolyamine linkages. These studies suggest that endogenous polyamines may modulate the formation of TG-catalyzed soluble aggregates. Despite these observations, little is known regarding the levels of either γ-glutamylpolyamines or bis-γ-glutamylpolyamines in the brain and much less is known regarding the proteins in which these crosslinked species are present.
The ability to infer observations about in situ TG activity from the levels of free γ-glutamylamines derives from a novel aspect of the TG reaction. γ-Glutamylamine bonds are resistant to the action of proteases. Consequently, γ-glutamylamines are excised intact when proteins containing these residues are degraded by proteolysis. The amide bond in free γ-glutamylamines can be cleaved by γ-glutamylamine cyclotransferase to produce free amine and 5-oxoproline. However, the activity of this enzyme in brain is low [16] and we have suggested that levels of free γ-glutamylamines are a good surrogate for in vivo TG activity in brain [11-13]. The resistance of the γ-glutamylpolyamine bonds to proteolysis suggests a novel permanent alteration of proteins. Polyamination increases the activity of a number of enzymes [17-19], notably phospholipase A2. In the case of phospholipase A2, the covalent attachment of polyamines causes a threefold increase in specific activity [17]. If this change persists for the life of this enzyme then it would represent a profound change in overall lipase activity. This is in stark contrast to the transient nature of some other post-translation modifications that affect enzymatic activity (e.g., phosphorylation), which typically last from seconds to minutes depending on the substrates. Thus, polyamination has the potential to bring about global and persistent changes in cellular metabolism. Such changes may herald crucial progressions in disease etiology. For example, the polyamination of phospholipase A2 would significantly increase the production of leukotrienes and prostaglandins and by this mechanism could contribute to the inflammation associated with many neurodegenerative disorders.
Ca2+ & TG activation
Ca2+ is absolutely required for the activation of the well-studied TGs [20]. Moreover, the full activation of these enzymes in most in vitro assays requires millimolar concentrations of Ca2+. Mammalian blood and interstitial fluid contain millimolar amounts of Ca2+ capable of activating extracellular TG 2 and factor XIII, and do so as indicated by the presence of γ-glutamylamine linkages in extracellular matrices, cell envelopes and blood clots [21,22]. Blood clots in the horseshoe crab also contain crosslinked proteins [23]. With the exception of those in the outer epidermis, cells typically maintain basal concentrations of cytosolic Ca2+ between 20 and 100 nM and allow increases of up to 500 nM following cellular activation [24]. This range of permittable Ca2+i concentrations raises the question of how the intracellular TGs are activated. TGs are clearly activated within cells as evidenced by the presence of γ-glutamylamine bonds in cellular proteins [10] and the demonstration of polyamination in cell-based models [25,26]. The tertiary structures of TGs 1−3 have been resolved and indicate the presence of three conserved Ca2+-binding sites on these enzymes [27-32]. Unfortunately, these studies have not suggested a mechanism for the full activation of TGs at cytosolic Ca2+ concentrations. Nonetheless, the known locations of the three TG Ca2+-binding sites have provided valuable insights into the catalysis of transamidation.
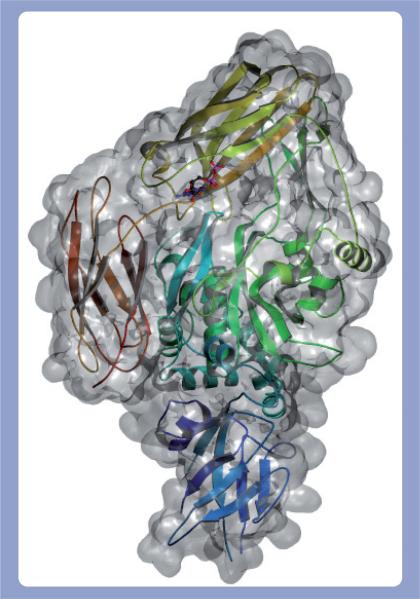
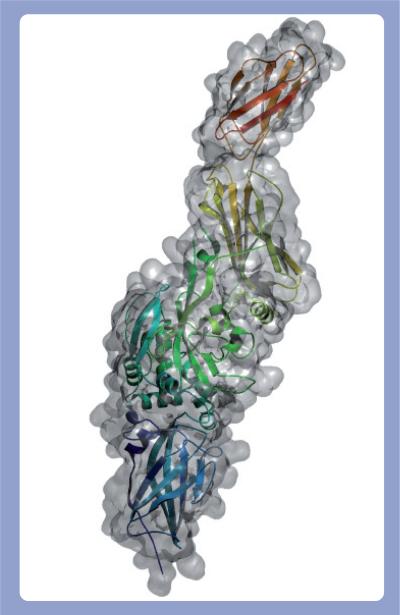
Despite limited sequence homology (e.g., TG 2 and 3 share 40% identity) [27,28], TGs conform to a common architecture of four contiguous ellipsoidal domains: an N-terminal β sandwich followed by a catalytic core, and then two C-terminal β barrels. A hinge region (Leu312–Arg317 in TG 2) connects the catalytic core domain to the first β barrel and facilitates the occupation of at least two conformational states: closed and open [32]. In the closed state, the hinge region resembles a β strand and allows the β barrels to drape across the active site and prevent substrate access (Figure 1). Enzyme activation causes the hinge region to conform to an α helix, which in turn forces the domains into an open conformation exposing the active site (Figure 2). This movement displaces the β barrels by as much as 120 Å and represents a substantial conformational change. The demonstration of these conformational states has been of particular importance, as it has revealed for the first time how two protein TG substrates might interact simultaneously with the catalytic core [32].
Figure 1. Closed state of TG 2.
The secondary structure of the closed form of TG 2 (PDB 1KV3) is rendered as a ribbon inside the molecular surface of the protein with an associated GDP depicted in a ball-and-stick configuration.
Figure 2. Active state of TG 2 with exposed active site.
The secondary structure of the unfolded active form of TG 2 (PDB 2Q3Z) is rendered as a ribbon inside the molecular surface of the protein.
An interesting hypothesis to account for the conversion of the hinge region from a β strand to an α helix invokes a prolyl cis–trans isomerization to power the change. Transition between the cis and trans conformations require a 180° change in the orientation of the prolyl bond and as such can produce profound effects in protein secondary structure [33,34]. TGs 1−3 contain a cis prolyl bond in the hinge region (Gly472–Pro473 in TG 3) and, therefore, isomerization of this bond may be sufficient to induce the hinge β-strand to assume an α-helical conformation [28,31]. This type of mechanism has successfully accounted for the conformational changes of other multidomain proteins [35]. Investigations of prolyl cis–trans isomerizations in proteins usually require nuclear magnetic resonance (NMR)-based techniques [36]. However, the structures of TGs 1−3 are too large to be resolved by current NMR methods. Other tests for the involvement of prolyl isomerization could involve mutation of the Pro473 residue and NMR-based conformational studies of the TG catalytic core domain bound only to the nearest β barrel via the hinge region. Ahvazi and coworkers [31] have pointed out that prolyl isomerization may occur at too slow a rate to account for the kinetics of TG-catalyzed reactions (the activation energy of prolyl isomerization is ∼20 kcal/mol) [33]. Indeed, prolyl isomerization may account for the lag period commonly observed in assays of TG kinetic activity. Prolyl isomerases significantly increase the rate of isomerization [34] and may act in concert with TGs to catalyze transamidation in vivo. This hypothesis could be readily tested by an investigation of the effect of prolyl isomerases on the kinetics of TG reactions.
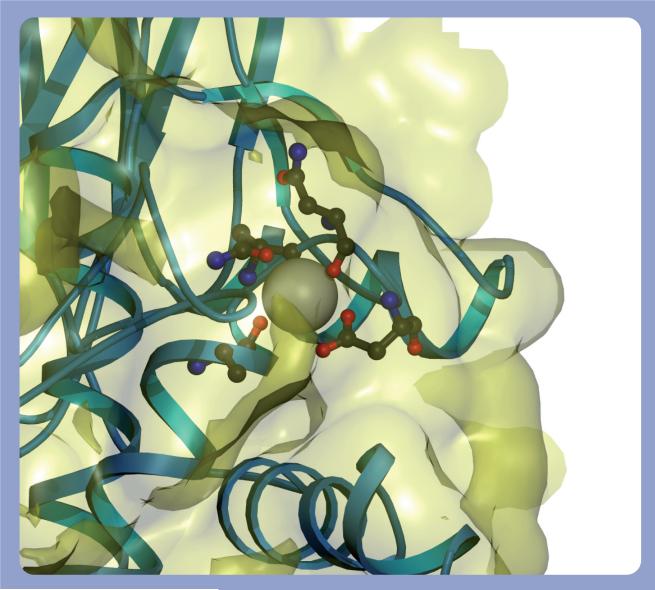
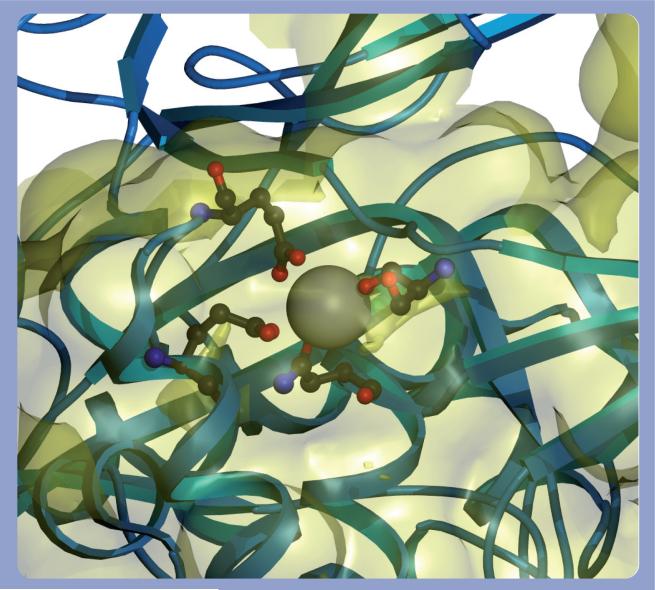
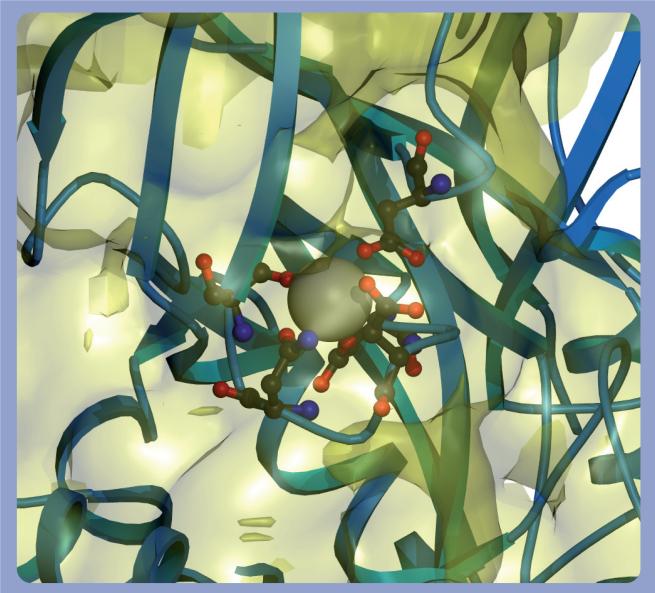
Cis peptide bonds are metastable and are kept in place by extensive hydrogen bonding and hydrophobic side chain interactions. Disruption of these stabilizing forces favors prolyl cis–trans isomerization. However, the TG Ca2+-binding sites are not sufficiently close to the hinge cis prolyl bond to directly impinge on its chemical environment [30]. Nonetheless, Ca2+-induced conformational alterations in the neighboring catalytic core may affect the cis–trans isomerization of the Gly472–Pro473 peptide bond. Ca2+ binds at three highly conserved sites within the catalytic core domain of TGs 1−3 [30,31,37]. Site 1 in TG 3 is defined by Ala221, Asn224, Asn226 and Asp228 (Figure 3) and binds Ca2+ with an affinity of 0.3 μM. This tight binding suggests that the Ca2+ at site 1 is essentially permanently bound to the enzyme and acts to stabilize the local structure [30]. Ca2+ binds to sites 2 (Asn393, Ser415, Glu443 and Glu448; Figure 4) and 3 (Asp301, Asp303, Asn305, Ser307 and Asp324; Figure 5) with a lower average affinity of 3 μM and can dissociate from the enzyme. The binding of Ca2+ to sites 2 and 3 causes large conformational changes in TG [28,30]. Sites 2 and 3 are also in close proximity to cis peptide bonds between Pro372–Tyr373 and Gly472–Lys488 and, therefore, Ca2+ binding may induce the cis–trans isomerization of these bonds. Pro372–Tyr373 and Gly472–Lys488 peptide bond isomerization may, in turn, alter the conformation of the hinge Gly472–Pro473 peptide bond and displace the β barrels from the catalytic core.
Figure 3. Calcium-binding site 1 in TG 3.
The coordinating residues for the calcium bound to site 1 of TG 3 (PDB 1L9N) are rendered as a ribbon, and residues Ala221, Asn224, Asn226 and Asp228 are shown in a ball-and-stick configuration around the calcium. The molecular surface of the protein is shown in yellow. It should be noted that the calcium at this site is almost fully enveloped inside the surface of the protein.
Figure 4. Calcium-binding site 2 in TG 3.
The coordinating residues for the calcium bound to site 2 of TG 3 in both the calcium bound (PDB 1L9N) and calcium free (PDB 1L9M) are shown. The backbone of the bound state is rendered in blue and the free state is rendered in green, as are the atoms with the calcium rendered as in CPK. The residues involved are Asn393, Ser415, Glu443 and Glu448. It should be noted that there is significant motion of Asn393 and Glu443 upon calcium binding.
Figure 5. Calcium-binding site 3 in TG 3.
The coordinating residues for the calcium bound to site 3 of TG 3 in both the calcium-bound (PDB 1L9N) and calcium-free state (PDB 1L9M) are shown. The backbone of the bound state is rendered in blue and the free state is rendered in green, as are the atoms with the calcium rendered as in CPK. The residues involved are Asp301, Asp303, Asn305, Ser307 and Asp324. Of note, there is significant motion of Asp324 in this binding site, which in the free state is significantly displaced from its position in the bound state.
The binding of two Ca2+ ions (given that one Ca2+ is permanently associated with site 1) to the catalytic core domain induces TG 2 to adopt an open conformation exposing the substrate binding sites and the catalytic residues [32]. Q-bearing substrates typically containing sequences QxPϕD(P), QxPϕ or QxxϕDP (where ϕ represents hydrophobic amino acids) [38], bind to a hydrophobic pocket consisting of Ala304, Leu 312, Ile313, Phe316, Ile 331 and Leu420 in the TG 2 catalytic core [32]. The conformational changes that allow substrate binding also juxtapose Cys277 to Trp241, which then form a thiolate–imidazolium ion pair oriented by Asp358. These changes allow a nucleophilic attack by the thiolate ion on the electron-deficient carbonyl of the γ carboxyamine group to generate an oxyanion intermediate [39]. The charge on the oxyanion is stabilized by hydrogen bonding with the backbone nitrogen of Cys277 and Nε1 nitrogen of Try241. Subsequent acylation results in the release of NH3 and the formation of an acyl-enzyme intermediate. The amine-bearing substrate then approaches the acylated Cys277 via a tunnel roofed by Typ241 and Trp332 and guided by Try360 [32,39]. Nucleophilic attack by the amine group leads to the formation of a second oxyanion, again stabilized by hydrogen bonding with Cys277 and Try241. Deacylation then completes the reaction.
The key residues and structural motifs cited above are conserved among TGs 1−3 and make it likely that the aforementioned reaction scheme is shared by these enzymes. Thus, ‘dormant’ TG exists in a closed conformational state in which the β barrels obscure the catalytic core. This self- or auto-inhibition is relieved by Ca2+ binding to two sites in the catalytic core, causing the β-barrels to be displaced in such a manner as to expose the catalytic quartet of Asp358, His335, Cys277 and Trp241 (in TG 2) and allow the binding of up to two protein substrates. The substrate Q residue undergoes a nucleophilic attack by the activated thiolate of Cys277 to generate an acyl intermediate, which in turn undergoes a nucleophilic attack by an amine-bearing substrate. Subsequent deacylation releases the substrate bearing an amide-substituted Q residue.
Equilibrium dialysis studies have established that Ca2+ binds TG 3 at micromolar concentrations [28,30], so why are millimolar amounts of Ca2+ required to activate these enzymes in vitro? This is an important question, since in the absence of an adequate explanation it has been suggested that TGs are rarely activated in some in vivo situations. These suggestions ignore both the presence of these enzymes within cells and the presence of γ-glutamyl linkages in the body. The explanation probably resides with the choice of Q-bearing substrates chosen for in vitro TG assays. Casein (or N,N-dimethylcasein) is commonly used to assay TG activity. This protein is typically extracted in a phosphate buffer and consequently has a high content of covalently bound phosphate [40]. As noted earlier, the binding site for Q-bearing substrates in TG 1−3 is a hydrophobic pocket. The charge imparted by phosphate could interfere with the binding of casein to TG. Ca2+ readily binds and neutralizes the charge on phosphate and this neutralization may account for the millimolar Ca2+ requirement in TG assays. Indeed, the Km of TG 2 for Ca2+ becomes 3−4 μM when dephosphorylated, rather than phosphorylated, casein is used as substrate in in vitro assays [41]. These observations and those demonstrating the formation of γ-glutamylamine linkages in situ indicate that TGs can be, and are, activated by physiological levels of Ca2+i concentrations.
The activation of TG 2 by Ca2+ is attenuated by GTP [25,29]. TG 1 also contains a guanosine-binding site but its ability to catalyze transamidation is not inhibited by GTP [31]. While Ca2+ has little effect on the binding of GTP to TG 2, it does lower the rate of GTP hydrolysis by this enzyme [31]. GTP induces conformational changes in TG 2, but how these changes might affect Ca2+ binding to sites 1, 2 or 3 is unknown. Unfortunately, very little is known regarding guanosine nucleotide concentrations in the brain, making it difficult to evaluate the role of GTP binding and hydrolysis in the regulation of cerebral TGs.
Overview of Ca2+i homeostasis in the brain
An approximately 10,000-fold differential exists between the extra- (∼1−2 mM) and intra- (50−100 nM) cellular pools of Ca2+. Since this gradient is so great the plasma membrane channel responsible for Ca2+ entry into cells only allows small amounts of this ion to access the cytosol at any time. This Ca2+ signal is subsequently amplified by Ca2+ release from the endoplasmic reticulum (ER) via two channels: the inositoltrisphosphate (IP3)-responsive channel and the ryanodine receptor (RyR). IP3 is often cleaved from phosphatidylinositol 4,5-bisphosphate (PIP2) in conjunction with the opening of plasmalemmal Ca2+ channels. The release of Ca2+ from the ER via IP3-channels causes an increase in Ca2+i, which in turn, signals additional Ca2+ release from the ER via the RyR. These elevations in Ca2+ concentrations are countered by ATP-dependent Ca2+ pumps, which operate to either expel Ca2+ from the cell or to sequester it within mitochondria or the ER. Schematically, these processes can be represented as:
Plasma membrane channels (+) → IP3-responsive channels (++) → RyR (++++) → ATP-dependent Ca2+ pumps (−).
The positive and negative signs (shown in parentheses) represent the net effect of these channels and pumps on Ca2+i concentrations.
AD: features & hypotheses
The most common form of dementia affecting humans is AD, which manifests as progressive memory loss and cognitive decline. In most cases, these manifestations of AD appear after 65 years of age and are a harbinger of death. AD primarily affects the pyramidal neurons of the hippocampus and is distinguished by an excessive deposition of amyloid plaques and neurofibrillary tangles in the cerebral cortex [42]. Plaques are external to neurons and are comprised of insoluble aggregates of amyloid β (Aβ) and other proteins, whereas tangles are intracellular aggregates of hyperphosphorylated tau and other cytoskeletal elements. When AD was first described in 1906, a relatively small percentage of adults survived beyond 60 years of age or more, and consequently, the incidence of this disease was thought to be sporadic. With the marked increase in life expectancy over the last 100 years, AD has been revealed to be a frequent cause of death among the elderly. The risk factors for AD include advanced age, poor diet, diabetes, hypertension, head trauma and inheritance of the ε4 allele of the apolipoprotein E [42]. Specific mutations in the genes for Aβ precursor protein (APP), presenilin 1 or presenilin 2, result in a rare and early-onset form of the disease known as familial AD [43].
Two leading hypotheses have been put forward to account for the development of AD. The first of these – the amyloid hypothesis – posits that Aβ deposits initiate the disease [44]. This hypothesis is supported by several observations. The APP gene resides on chromosome 21. Consequently, trisomy 21 (Down syndrome) sufferers have three copies of this gene and invariably develop AD by 40 years of age [45]. Moreover, inheritance of the apolipoprotein E ε4 allele leads to an excessive accumulation of cerebral Aβ-containing plaques, the deposition of which precedes the behavioral changes associated with this disease. However, a number of observations concerning Aβ-containing plaques are not consistent with the amyloid hypothesis. For example, plaque distribution is not coincident with the histological pattern of damage in AD, and ridding the brain of plaques with antibodies does not resolve the disease in humans [46]. Moreover, in a mouse model of AD there is no close correlation between neuronal loss and plaque formation [47]. The other major hypothesis to account for the development of AD – the tau hypothesis – contends that neurofibrillary tangles primarily cause this disease [48].
TG expression & activity in AD
Transglutaminase activity is significantly increased in the affected regions of AD brain [1,49] and TG 1, TG 2 and γ-glutamyl-ε-lysine are present in pathological lesions in AD brain [3,50]. An increase in the amount of γ-glutamyl-ε-lysine crosslinked proteins in insoluble protein in AD brain was shown previously [1]. TG 1 and TG 2 protein expression is increased in AD brain [1], which presumably accounts at least in part for the increased TG activity in AD brain. Many studies have focused on the expression of TG 1 in skin, where the enzyme is regulated via AP1 and Sp1-binding sites [51], but the factors regulating the expression of TG 1 in brain have not been identified. The TG 2 promoter contains an NF-κβ-binding site through which glutamate and inflammatory mediators have been shown to act [52-56], and both glutamate excitotoxicity and inflammation are thought to contribute to AD. An alternatively spliced truncated variant of the TG 2 message, termed ‘short TG 2’, is also generated in brains of AD patients [57]. Short TG 2 expression may be particularly important in the later stages of AD. Truncated TG 2 lacks the ability to bind GTP and promotes apoptosis in vivo independently of the transamidation reaction [58].
γ-Glutamyl-ε-lysine immunoreactivity co-localizes with Aβ in senile plaques in AD brain [3]. For TG 2 to catalyze γ-glutamyl-ε-lysine crosslinks in these plaques, the enzyme would require release from neurons. However, to the best of our knowledge, the export of TGs from neurons has not been demonstrated. Nevertheless, TG 2 is present in CSF and in a greater amount in AD[59] and PD patients [60]. The most likely source for this activity is the brain. However, it should be noted that the activity could result either from cellular export (as shown, for example, for endothelial and epithelial cells of noncerebral origin [61-63]) or from cell lysis. Siegel et al. [64] have reported that extracellular TG 2 is catalytically inactive, but is transiently activated upon tissue injury. However, other studies suggest that TG exported to the extracellular environment is a ctivated by Ca2+ [61-63].
Amyloid β [65-67] and Aβ bearing the Dutch mutation (Q22 → E22) [66] are in vitro substrates of TG2. The resulting polymers resemble the aggregates seen in AD brain, and it has been suggested that TGs contribute to AD by initiating Aβ oligomerization and aggregation at physiological levels [67]. Adballa et al. [68,69] demonstrated that the formation of Aβ leads to oxidation of tyrosine residues of neighboring Ang II receptors (AT2) causing these proteins to dimerize. The dimerized proteins are then substrates for further oligomerization by TGs. Ang II AT2 oligomers sequester the Gαq/11 G protein, inhibiting its activity, and cause a phenotype reminiscent of AD. The notion that TG-catalyzed aggregation causes pathology by removing critical proteins has been proposed earlier. For example, glyceraldehyde 3-phosphate dehydrogenase, α-ketoglutarate dehydrogenase complex and histones [70-72] are in vitro TG substrates whose loss or covalent modification might compromise normal cell functioning. Crosslinking of ubiquitin, HSP27, parkin and α-synuclein by γ-glutamyl-ε-lysine bonds in Alzheimer's neurofibrillary tangles has been detected in AD brain [73]. Crosslinking of tau was not detected in these studies (however, see below).
Transglutaminases may play a significant role in the stabilization of neurofibrillary tangles. γ-Glutamyl-ε-lysine bonds have been identified within tangles [2,3, 50,65] and the proteins that comprise these structures are in vitro TG 2 substrates. These proteins include tau [26, 50, 74-79] and α-synuclein [65,80-82]. The actions of TG on α-synuclein will be discussed below.
Mature TG 1 is variably myristoylated and palmitoylated, which causes this enzyme to associate with the plasma membrane [83]. The binding of this enzyme to membranes profoundly affects its substrate preference, especially with respect to which Q and K residues are modified. Moreover, when bound to membranes, TG 1 can catalyze the addition of ceramides to Q residues [8], which may contribute to the incorporation of fatty materials into tangles. The membrane-binding portion of TG 1 can be removed by proteolysis to generate a cytosolic form of the enzyme [83]. TG 2 and deacylated TG 1 share many of the same substrates [84,85] and, therefore, cytosolic TG 1 may act on a number of the a forementioned TG 2 substrates (e.g., AT2).
Increases in neuronal Ca2+i in AD
The extracellular milieu is sufficiently rich in Ca2+ (1−2 mM) to maximally stimulate proteolytically-activated factor XIII and any active TG 2 exported from cells [61-63], and to contribute to the stabilization of Aβ plaques. Under these circumstances the major limits to activity are likely to be clearance via the cerebral circulation, incorporation into biological matrices (noncovalent associations and covalent attachment where one TG molecule acts as a substrate for another) and substrate availability. With respect to the last point, blood and interstitial fluid are poor sources of polyamines and, consequently, extracellular TG 2 would be expected to produce a greater number of γ-glutamyl-ε-lysine linkages than either bis-γ-glutamylpolyamine or γ-glutamylpolyamine linkages an expectation that has been substantiated by in vitro studies [10,21].
Intracellular TGs may be activated by etiological factors associated with sporadic AD. Kuchibhotla et al. have demonstrated that the pathological deposition of Aβ-containing plaques resulted in a rise in Ca2+i from approximately 80 to approximately 500 nM in 20% of the neurons in the vicinity of the plaques [24]. Similarly, Busche et al. reported an increased frequency of spontaneous Ca2+ transients in approximately 20% of the neurons in neighboring plaques [86]. Thus, Aβ plaques may facilitate the generation of tangles by TGs by elevating Ca2+i concentrations. How plaques allow Ca2+ into neurons has not been established. Busche et al. have argued that plaques may interfere with the glutamatergic inhibition of synaptic activity [86], whereas Kuchibhotla et al. suggest that these structures form Ca2+-conducting pores, modulate voltage-gated Ca2+ channels or generate an oxidative stress that releases calcium from intracellular stores [24]. The observations of these two groups were made using murine models of AD and the differences reported (i.e., increased Ca2+i vs spontaneous Ca2+ transients) are most likely due to differences in methodologies and the genetic background of the mice. Regardless of their differences, these studies demonstrate that elevations in Ca2+i concentrations are an important and consistent feature of sporadic AD etiology.
Some of the mutations that underlie familial AD also increase the concentration of Ca2+i in the affected neurons. The ER is the major reservoir of Ca2+ within cells, and its Ca2+ stores are replenished by ATP-dependent Ca2+ pumps. However, passive Ca2+ efflux leak channels act in opposition to these pumps to ensure that the ER does not become overfilled with Ca2+. One consequence of overfilling the ER is an exaggerated efflux of Ca2+ in response to either IP3 or RyR activation. Presenilins 1 and 2 encode for passive ER Ca2+ leak channels and mutations of these molecules account for approximately 40% of familial AD cases [87]. A significant number of these mutations ablate the leak activity of presenilins, causing both an overfilling of Ca2+ by the ER and an excessive Ca2+ efflux from this organelle upon stimulation [88,89]. Stutzmann and colleagues have demonstrated that mice bearing one of these presenilin 1 mutations (i.e., presenilin 1 M146V) show a threefold greater increase of Ca2+i in response to IP3 relative to control animals [90]. Stutzmann and coworkers subsequently demon strated that this response was primarily due to the passage of Ca2+ through the RyR [91]. The number of RyRs increases with age and is associated with a greater rise in IP3-evoked Ca2+i with age in presenilin 1 M146V-knockin mice [91]. Together, these observations suggest that increases in Ca2+i levels, capable of inducing intracellular TG activity, are a consistent feature in AD.
Increases in neuronal Ca2+i during aging
Aging is one of the major risk factors for AD and other neurodegenerative disorders. As in the case of animal AD models, the activation of RyRs is also thought to contribute to the elevations in Ca2+i, observed in the hippocampal neurons of aging rodents. These elevations are primarily caused by the opening of voltage-dependent Ca2+ channels and contribute to a number of electrophysiological phenomena, including the slowing of the after-hyperpolarization phase of action potentials with age, as well as increases in Ca2+ spikes, currents and transients [92]. Application of the RyR antagonist ryanodine to hippocampal slices lessens the effects of age on the after-hyperpolarization and synaptically activated Ca2+ transients. Based on this observation, Thibault et al. hypothesized that Ca2+ efflux from the ER, as mediated by the RyR, is an important component of the age-dependent increase in neuronal Ca2+i [92]. This hypothesis is further supported by studies that suggest the L-type voltage-dependent Ca2+ channels are situated close enough to RyRs to activate this ER channel [93].
It is important to note that the pathology of the aging rodent brain may be different from that of humans. Aging rodents do not normally succumb to either familial or sporadic AD. Indeed, the transfection of three separate mutated genes is required to produce a facsimile of human AD in mice replete with the behavioral manifestations [94]. Even so, fibroblasts isolated from AD patients do exhibit some of the aspects of altered Ca2+ homeostasis seen in AD mice [95]. However, the relevance of murine aging to AD remains to be established. It will also be important to determine the role of apoptosis in murine and human aging. Apoptosis commonly occurs in rodent models of neuro degeneration. Since apoptosis is usually restricted to dividing cells this is a remarkable observation (nondividing cells typically die by macroautophagy), and suggests that apoptosis is either unique to rodents or diseases such as AD per se. If the latter is true then strategies that limit apoptosis should have therapeutic benefit in human neurodegenerative diseases.
HD: features & TG hypothesis
Huntington's disease is primarily a movement disorder resulting from the death of mediumsized spiny neurons in the striatum, and patients exhibit varying degrees of mental decline as the disease progresses. In addition to the affected sites, HD differs from most cases of AD in that the disease is due to mutations in a single gene. Despite its rarity – a frequency of 0.007% worldwide – the availability of pertinent animal models, and the notion that many fundamental processes underlying neurodegeneration are similar regardless of initiating factors or site of injury, has stimulated significant research interest into HD.
Huntingdon's disease is due to an expansion of CAG repeats in the huntingtin (htt) gene and these mutations are inherited in an autosomal dominant manner. The CAG expansions encode stretches of polyglutamines (poly Q) in the N terminus of the htt protein, and mutations giving rise to 39 or more contiguous Q residues in this protein invariably precipitate the disease [96]; the longer the repeat the earlier the onset of the disease and the greater its rate of progression [97,98]. DNA replication tends to expand mutant CAG repeat sequences, and consequently, the number of mutant poly Q residues in htt tends to increase with each succeeding generation, particularly in the case of paternal transmission. This phenomenon of ‘anticipation’ results in successively earlier disease onsets.
As in the case of AD, polymeric aggregates are deposited in affected brain regions of HD patients [99]. The aggregates include mutant htt, which has a marked propensity to self-aggregate in vitro [100]. Green suggested that mutant htt might be an excellent substrate of TGs and that TG activity toward mutant htt might contribute to the etiology of HD [101]. TGs were hypothesized to promote the aggregation of mutant htt in a manner that correlated with the poly Q length. This hypothesis is supported by in vitro studies showing that contiguous Q residues in model peptides/proteins [102-105] and mutant htt itself [106] are excellent TG substrates. Moreover, γ-glutamyl-ε-lysine linkages are present in htt-containing aggregates in intact HD brain [107].
TG expression & activity in HD
The greater amount of protein-bound and free γ-glutamylamines in HD brains and CSF, as compared with controls, attests to the increased activation of cerebral TGs in this disease [11-13,107]. TG activity is also greater in postmortem samples of HD versus control brain [104,108] and relates to elevations in the message and amounts of TG 2 proteins [106,108]. HD is accompanied by inflammation and mediators of this process, such as TNF-α, may serve to increase the expression of TG 2. The TG 2 promoter contains an NF-κB- binding region and TNF-α stimulates both the translocation and DNA binding of NF-κB, as well as the expression of TG 2 in microglia and astrocytes [55,109]. As noted earlier, the activation of TG 2 (and possibly other TGs) may promote inflammation through the sustained activity of polyaminated phospholipase A2.
Increases in neuronal Ca2+i during HD
The activation of TGs may be an early and significant consequence of HD since mutant htt promotes Ca2+i elevations capable of stimulating the activity of these enzymes. Wild-type htt binds to IP3 receptors and facilitates the release of Ca2+ through this combination channel-receptor [110]. However, mutant htt preferentially enhances the release of Ca2+ via the IP3 receptor following glutamate-dependent hydrolysis of PIP2 [111]. Glutamate excitoxicity in HD is thought to be promoted mainly via the NMDA receptor and the activity of this receptor is markedly increased in mutant htt-expressing mice [112,113]. Dopamine acts synergistically to increase glutamate-dependent Ca2+i, presumably through the protein kinase A-catalyzed phosphorylation of NMDA and AMPA receptors and through the dopamine type 2 receptor-mediated hydrolysis of PIP2 [114]. Together, these processes would be expected to significantly increase Ca2+i, and this has been confirmed in numerous studies with mice bearing disease-causing mutations in htt [115,116].
Mutant htt also appears to increase the sensitivity of the permeability transition pore to Ca2+, leading to dissipation of the mitochondrial membrane potential, and consequently, to a diminished capacity of this organelle to store Ca2+ [117-120]. How mutant htt causes the above effects is not known, but may involve binding partners that attach to mutant poly Q sequences. These binding partners may also regulate TG activity.
Polyglutamine-expanded htt, calmodulin & TG in HD
The Ca2+-binding protein calmodulin associates with poly Q-expanded htt and through this association regulates TG activity. Calmodulin was initially shown to regulate TG activity in platelets, chicken gizzard [121] and the human erythrocyte cytoskeleton [122]. It was subsequently demonstrated that inhibiting calmodulin or disrupting the association of calmodulin with poly Q-expanded htt with a peptide derived from calmodulin (amino acids 76−127), reduces the ability of poly Q-expanded htt to act as a TG substrate [123-125]. In addition to preventing the TG from utilizing poly Q-expanded htt as a substrate, expression of this peptide reduced the cytotoxicity and Ca2+i increase associated with expression of mutant htt [124,125]. These changes were selective, as neither total TG activity nor calmodulin-dependent kinase II activities were inhibited by the calmodulin peptide. In addition, the association of calmodulin with another calmodulin-dependent enzyme, calcineurin, was not disrupted by the calmodulin peptide [125]. Expression of the calmodulin peptide fragment in the striatum of a transgenic mouse model of HD lessened the weight loss and motor dysfunction typically seen in these animals [Muma N, Unpublished Data].
Toxic mechanisms for TGs in HD
Mice with HD (R6/1 transgenic mice expressing exon 1 of human htt that contains a greatly expanded polyglutamine domain [116 Q repeats]) with a TG 2−/− genotype live longer than littermates expressing TG 2+/+ and the mutant htt fragment [126]. In the R6/1:TG2−/− mice, the TG 2 knockout encompassed the whole body. Although one cannot rule out the possibility that the increased life expectancy in the R6/1:TG 2−/− mice is due to some beneficial effect related to ablation of whole body TG2, it seems likely that the beneficial effect is due to local loss of brain TG 2. Thus, the percentage of abnormal brain cells in the various groups of mice at 18 and 25 weeks was found to be in the order R61>R6/1:TG 2+/−>R6/1:TG 2−/− [126]. Moreover, the isopeptide levels in the detergent-insoluble fraction of brain at 25 weeks of age was also of the order R61>R6/1:TG 2+/−>R6/1:TG 2−/− [126]. These gradation effects argue for a local beneficial effect of TG 2 ablation. Interestingly, the number of intranuclear inclusions was in the reverse order (i.e., R61<R6/1:TG 2+/−<R6/1:TG 2−/−), despite the fact that the brains of the R6/1:TG 2−/− mice exhibited tenfold less γ-glutamyl-ε-lysine linkages than their R6/1 littermates [126]. Presumably, the residual γ-glutamyl-ε-lysine bonds in the brains of R6/1, TG 2−/− mice are due to brain TGs other than TG 2. The seemingly paradoxical finding of increased aggregates in the brains of the R6/1:TG 2−/− mice compared with those in brains of the R6/1 mice may be explained by the findings of Lai et al. alluded to above [14]. Thus, in the absence of TG 2, the poly Q domains will noncovalently form insoluble aggregates. In the presence of TG 2, γ-glutamyl-ε-lysine crosslinking will retard the formation of these insoluble noncovalent aggregates. However, TG 2-catalyzed polyamination will a ntagonize this effect. At the present time it is not clear whether the soluble protein aggregates containing htt, the insoluble aggregates, or both, are toxic [14]. However, if the soluble form was more toxic this would explain the findings of Mastroberardino et al. [126] and might suggest that the level of polyamine in the brain is crucial to the contribution of TGs to HD. The levels of polyamines in the brain decrease with age [127]. Thus, the ability of the HD brain to prevent formation of soluble aggregates may be lessened with age. However, this possibility has not yet been investigated.
In addition to HD, there are a number of other CAG/polyglutamine expansion diseases in which TGs are hypothesized to possibly contribute to the disease process. These diseases have been previously reviewed [105,128,129].
Parkinson disease
Parkinson disease is the most common neurodegenerative disorder after AD. This disease results from the selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNc). In most cases, what causes the loss of these neurons is unknown. PD presents as tremor, bradykinesia, rigidity and postural instability. The affected brain regions contain intracytoplasmic aggregates known as Lewy bodies consisting of dense eosinophilic cores surrounded by pale halos [130]. These structures have also been identified in some cases of AD [131]. The major constituent of Lewy bodies is a 140-amino acid protein known as α-synuclein [132]. A fragment of α-synuclein (the nonamyloid component [NAC]) is also found in the Lewy bodies of PD patients [133] and in the neuritic plaques of AD patients [134]. Several lines of evidence suggest a central role of aggregated α-synuclein in the etiology of PD. α-Synuclein fibrils are neurotoxic when added to cultured cells [135,136], and the expression of human α-synuclein in mice results in progressive accumulation of α-synuclein inclusions and a concomitant loss of dopamine-containing neurons in the basal ganglia [137]. Finally, missense point mutations in α-synuclein are associated with an early-onset, inherited form of PD [138,139].
TGs, Ca2+ & Parkinson's disease
As in the case of AD [59], greater amounts of TG 2 have been observed in the CSF of PD patients compared with control CSF [60]. α-Synuclein spontaneously forms aggregates [140]. Interestingly, dopaminergic neurons of the PD SNc exhibit greater staining for TG 2 and γ-glutamyl-ε-lysine than those of aged-matched controls [141]. Immunoprecipitation studies using antibodies that recognize either α-synuclein or γ-glutamyl-ε-lysine have demonstrated the presence of γ-glutamyl-ε-lysine crosslinked mono- and oligomeric α-synuclein in PD nigral neurons [141]. The formation of intramolecular γ-glutamyl-ε-lysine crosslinks in α-synuclein results in a monomeric and more compact form of this protein that is unable to polymerize into α-synuclein fibrils [80,81]. Based on these observations, it has been suggested that TGs in neurodegenerative diseases act, to some extent, to curb the formation of insoluble, toxic oligomers of α-synuclein, htt and other disease-relevant proteins. Notwithstanding the appeal of this idea, it has yet to be demonstrated that soluble α-synuclein monomers or htt oligomers are innocuous. Indeed, the amount of intramolecularly crosslinked α-synuclein in nigral dopaminergic neurons was found to correlate with the progression of PD [141]. γ-Glutamyl-ε-lysine-mediated crosslinking has the potential to modify the ubiquitination of proteins since ubiquitin is bound to lysine residue by a reaction remarkably similar to TG-catalyzed transamidation. Thus, γ-glutamyl-ε-lysine crosslinked proteins may be inappropriately ubiquitinated and congest proteasomes. Components of the ubiquitin proteasome system have been identified in aggregates in two polyglutamine expansion diseases [142-144]. In toto, these observations suggest that the TG substrate pools in the brain change during aging or as neuro degenerative diseases progress, and that these changes may lead to the production of neurotoxic species. These changes range from decrements in substrate pools, as in the case of polyamines in the aging brain [127], to post-translational modifications of the substrates including proteolysis [65], and phosphorylation of α-synuclein [136]. TGs may also contribute to formation of toxic aggregates by polymerizing with molecules, such α-synuclein and poly Q-expanded htt, and TG 2 has been identified within the deposits of these proteins [107].
Unlike other excitable cells in the brain, SNc neurons fire autonomously at a frequency of 2−4 Hz, very much like cardiac pacemaker cells [145], although in PD the neurons exhibit bursts of cyclic firing rather than a continuous train of action potentials [146]. Similar to heart cells, SNc neurons also use Ca2+ rather than Na+ to carry the current. Ca2+ enters SNc cells by way of L-type currents, which open frequently to accommodate the pacemaker functions of these cells. Glutamate-evoked opening of L-type channels causes Ca2+i peak increases of 100 μM [147], which is more than enough to stimulate TG activity. Surmeier has suggested that the mitochondria in diseased SNc neurons leak Ca2+ into the cytosol since these organelles are often damaged in PD [145]. This suggestion is supported by the observation that the expression of calbindin, which binds Ca2+, confers protection against mitochondrial toxins in cultured pheochromacytoma 12 (PC12) cells [148]. Thus, under physiological and pathological conditions, TGs are either continuously activated in SNc neurons or regulated in a different manner than in other cells. The latter possibility is most likely; however, at present, little is known regarding the regulation of TG in either SNc or PD.
Progressive supranuclear palsy
Progressive supranuclear palsy (PSP) is a parkinsonian-like movement disorder characterized clinically by early postural instability, supranuclear vertical gaze palsy, and behavioral and cognitive disturbances [149]. This disease is characterized by neuronal loss, gliosis, neurofibrillary tangles, neuropil thread and tau immuno-reactive astrocytes. Immunoprecipitated proteins containing γ-glutamyl-ε-lysine crosslinks from the affected brain regions in PSP exhibit labeling for paired helical filaments tau, indicative of the actions of TG in this disease [150]. Increased TG activity and increased expression of TG 1 and 2 at the protein and mRNA level has also been demonstrated for the selectively vulnerable brain regions in PSP [151]. Ca2+ homeostasis is thought to be perturbed during PSP and may act to activate TGs in this disease [152].
Depression & TGs
Depression is a common symptom of HD and other neurodegenerative diseases such as AD. Signaling via the serotonergic pathway is an important component of depression, and depression is often successfully treated with drugs that act to modulate this signaling. TGs appear to influence serotonergic signaling by binding serotonin to the Rac1 G protein as indicated by coimmunoprecipitation of Rac 1 and serotonin [153,154]. Moreover, this binding is blocked with two strategies that attenuate TG activity, namely, treatment of cells with either small interfering RNA against TG 2 or cystamine. The identity of the TG-catalyzed bond(s) between serotonin and Rac 1 has as yet not been determined and the significance of its formation in neurodegenerative diseases awaits further research.
TG inhibitors
A major emphasis in this review has been the role of Ca2+ in activating TGs in neurodegenerative diseases. Intracellular Ca2+ concentrations are increased in AD and HD, as is TG activity. However, the mechanisms by which Ca2+ increases in these diseases varies considerably and argues against the use of calcium-channel blockers as possible therapeutics. By contrast, the activation of TGs in these diseases is a common and potentially damaging consequence of the rise in Ca2+i. Thus, TG inhibitors are theoretically good candidates as therapeutics in neurodegenerative disorders.
Numerous small amine-bearing compounds, including polyamines, cysteamine, cystamine, hydroxylamine, methylamine and histamine act as competitive inhibitors of transamidation catalyzed by TGs [5,155,156]. A primary amine bound to an aliphatic unbranched carbon chain of 4 or 5 saturated carbon atoms is a common characteristic of this class of inhibitors, although shorter amines, such as hydroxylamine, methylamine and cysteamine are also active, as is cystamine. These compounds act as alternative TG substrates, and thereby divert the activity of TGs. Of this group of compounds, cystamine (H2NCH2CH2SSCH2CH2SH; which also has the possibility of reacting directly with the cysteine at the active site by sulfide–disulfide interchange) has been the most extensively studied as a potential therapeutic and has been shown to be beneficial in animal models of HD [13, 157, 158] and a neurotoxin model of PD [159]. However, cystamine elicits several changes that may contribute to neuronal survival, making it difficult to assess the role of TG inhibition in cystamine-induced neuroprotection. For example, administration of cystamine to mice raises the level of brain cysteine [160,161]. Both cystamine and cysteamine raise the level of brain-derived neurotropic factor [162] in animal models of HD. The compounds also inhibit macroautophagy [163] and apoptosis [164] in cell models. The inhibition of apoptosis in HD cell models by cystamine is thought to be caused by inhibition of caspase 3 activity [164]. Moreover, cystamine extends the lifespan to a similar extent in a mouse model of HD in which TG 2 is ablated (R6/2:TG2−/− mice) to that observed in control mice (R6/2:TG2+/+ littermates), indicating that the compound may not be directly working through inhibition of TG2 [165]. However, chronic administration of cystamine to mice results in decreased total TG activity in the brain [13,157]. This decrease may be due to an inhibition of the transcription of TGs by cystamine-derived cysteamine. In support of this idea, cysteamine attenuates the DNA binding of two factors – activator protein 1 and NF-κB [166] – that bind to the TG 1 and TG 2 promoters [53,56,167].
Many TG inhibitors have been designed or discovered by screening of compound libraries. Irreversible TG inhibitors include sulfonamides [168], iodoacetamide [169], alkyl isocyanates [170], phenylthioureas [171,172], 3-halo-4,5-dihydroisoxazoles [173-175], tyrosinamidomethyl dihydrohaloisoxazoles [176], sulfonium methyl ketones [177], 2-[(2-oxoproyl)thio]imidazolium derivatives [178], and αβ-unsaturated amides, epoxides and 1,2,4,-thiadiazoles built around the well known acyl substrate Cbz-L-Gln-Gly of guinea pig TG 2 [179-181]. Many compounds in this last category are irreversible inhibitors in the micromolar or submicromolar range.
Comparison of the structures and activities of these compounds revealed the importance of the length and the electronic nature of the side chains of the inhibitor analogues of Cbz-Gln-Gly [180]. Keillor et al. suggest that x-ray crystallographic analysis of TG 2, with or without the inhibitor bound at the active site, might prove useful in the design of a more potent second generation of thiadiazole TG 2 inhibitors [179-181]. Recently, Stein and colleagues used a high-throughput procedure to screen a library of 110,000 drug-like molecules to search for inhibitors of TG 2 [182,183]. The acyl donor and acyl acceptor substrates used were N,N-dimethylcasein and N-boc-lys-NH-CH2-CH2-NH-dansyl, respectively. The screen produced 104 candidates including competitive, mixed-site and GTP-binding site inhibitors [182,183]. Particularly effective inhibitors were thieno[2,3-d]pyrimidin-4-one acylhydrazide and derivatives [184]. Lai et al. have also used a high-throughput screening procedure to screen for inhibitors of human TG 2 [185]. The authors identified several inhibitors, the most potent of which were ZM39923, a Janus kinase inhibitor, and its metabolite ZM49829, with IC50 values of 10 nM and 5 nM, respectively. Tyrphostin and vitamin K3 were found to have IC50 values in the micromolar range. The agents appeared to act in part by interfering with the reduction of the vicinal disulfide needed for activation of TG. Interestingly, several of the agents uncovered by the screening were able to improve survival times in a Drosophila melanogaster model of a polyglutamine expansion disease (Machado-Joseph disease) [185].
A number of groups have investigated the potential of peptide-based TG inhibitors. These peptides are typically derived from TG substrates or binding partners and consequently, have the potential to very precisely modulate the activity of these enzymes. For example, Kim and colleagues, noting that uteroglobin and lipocortin-1 contain common sequences that antagonize the interaction of TG 2 and phospholipase A2, synthesized variants of these sequences that prevented both the polyamination of phospholipase A2 and experimentally induced allergic conjunctivitis [186]. Similarly, a calmodulin-derived peptide that blocks the interaction of calmodulin with expanded poly Q attenuated the action of TG on mutant htt [124,125] and had therapeutic benefit in a transgenic murine HD model [Muma N et al., Unpublished Data].
While the aforementioned peptides presumably act in a competitive manner to inhibit TG activity, other peptides that act irreversibly have been generated using diazo-oxonorleucine (DON) in place of Q residues. The diazo moiety of DON reacts irreversibly with the TG 2 active site catalytic cysteine residue. Khosla and coworkers synthesized Ac-P(DON)LPF-NH2, based on a common sequence in gluten proteins (PQLPY) that is acted upon by TG 2, and employed it to investigate the conformation states of TG 2 [32]. This pentapeptide inhibits TG 2 with a Ki of 60 nM and k1 of 0.5 min−1, but as of yet, has not been tested in models of neurodegeneration. The diazo grouping is moderately reactive and the possibility existed that DON-containing peptides might be cytotoxic. However, Ratan and coworkers have shown that a DON-containing peptide [Z-DON-Gln-Ile-Val-OMe] is especially useful in studying the biological effects of TG inhibition in normal and HD cells in culture and in a Drosophila melanogaster model of HD. Thus far, the results have only been presented at national meetings [187,188]; however, a more comprehensive report has been submitted for publication. The findings may be summarized as follows: TG 2 appears to be a transcriptional regulator, not only of selected metabolic genes (cytochrome c and Pgc1a), but also of a large number of genes that are dysregulated in neural cells expressing a mutant form of htt. Inhibition of TG 2 restores downregulated metabolic genes (cytochrome c and Pgc1a) in mutant htt-expressing cells, and derepression of this pathway increases downstream indicators of metabolic function, increases survival in a cellular model of HD and decreases the incidence of defective ommatidia in a fly model of HD [188]. Dysregulation of metabolism in HD is well documented. For example, Gines et al. demonstrated a deficit in cAMP in HD-knockin mice and in striatal cell models of HD [189]. The findings suggested that the reduction of cAMP leads to a mitochondrial energy deficit [189]. Whether TGs cause a change in cAMP-responsive element-mediated gene transcription of proteins generally involved in metabolic regulation in HD remains to be determined.
While the work from Ratan's group is preliminary, it does strongly implicate a role for TGs in the progression of HD. The next stage is to determine other possible promoters with which TG 2 may interact and identify transcriptional proteins that are possibly covalently modified by TGs. If Z-DON-Gln-Ile-Val-OMe (or analogues) is to prove useful in rodent models of neurodegenerative diseases, further work is needed to determine whether the compound passes the BBB and inhibits endogenous brain TGs.
Yuan et al. have demonsrated that small molecule TG 2 inhibitors, such as the reversible inhibitor monodansylcadaverine and the irreversible dihydroisoxazole inhibitors, KCA075 or KCC009, promote apoptosis in glioma cells and sensitize the tumors to the chemotherapeutic agent N,N′-bis(2-chloroethyl)-N-nitrosourea [190,191]. It was suggested that TG2 is a potential target to enhance cell death and chemosensitivity in glioblastomas [190,191]. The TG inhibitors developed by Yuan et al. [190,191] might also be useful for investigating the role of TGs in neurodegenerative diseases.
Executive summary.
■ Elevation of intracellular Ca2+ (Ca2+i) is a consistent feature of Alzheimer's disease and Huntington's disease.
■ One important consequence of this increase of Ca2+i is likely to be the activation of transglutaminases (TGs), which, increasing evidence suggests, contributes to neurodegenerative processes.
■ The mechanisms by which TG-modified products induce cytotoxicity in neural tissues have not been clearly identified, but studies investigating the toxicity of soluble and insoluble TG-generated crosslinked aggregates suggest some very interesting possibilities.
■ One intriguing possibility is that changes in the ratio of γ-glutamylpolyamine/bis-γ-glutamylpolyamine to γ-glutamyl-ε-lysine linkages may determine the solubility, and by extension, the toxicity of TG-catalyzed aggregates. Other possibilities include alteration of transcription factor machinery.
■ Recent studies with rationally designed TG inhibitors and with inhibitors uncovered by high-throughput screening show that these compounds have beneficial effects in animal/cellular models of polyglutamine-expansion neurodegenerative diseases. TG inhibitors have also been shown to be effective in mice harboring glioblastomas.
■ It is predicted that this promising start with TG inhibitors will be extended to animal models of more common neurodegenerative diseases and will eventually lead to clinical trials in humans with devastating neurological diseases.
Financial & competing interests disclosure
Part of the work described herein from the authors’ laboratories was funded in part by NIH grant 2P01 AG14930 (to Arthur JL Cooper), R01MH068612 (to Nancy A Muma) and a grant from the High Q Foundation (to Nancy A Muma). Part of the work described herein was conducted when Thomas M Jeitner and Arthur JL Cooper were at the Burke Medical Research Institute, White Plains, and when Nancy A Muma was at Loyola University Chicago. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Thomas M Jeitner, Applied Bench Core, Winthrop University Hospital, 222 Station Plaza North, Suite 502, Mineola, NY 11501, USA Tel.: +1 516 663 3455 Fax: +1 516 663 3456 tjeitner@winthrop.org.
Nancy A Muma, Department of Pharmacology & Toxicology, School of Pharmacy, University of Kansas, 1251 Wescoe Hall Drive, 5064 Malott Hall, Lawrence, KS 66045, USA Tel.: +1 785 864 4002 Fax: +1 785 864 5219 nmuma@ku.edu.
Kevin P Battaile, IMCA-CAT, University of Chicago, 9700 S. Cass Ave, Bldg 435A, Argonne, IL 60439, USA Tel.: +1 630 252 0529 Fax: +1 630 252 0521 battaile@anl.gov.
Arthur JL Cooper, Department of Biochemistry & Molecular Biology, New York Medical College, Valhalla, NY 10595, USA Tel.: +1 914 594 3330 Fax: +1 914 594 4058 arthur_cooper@nymc.edu.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1■■.Kim SY, Grant P, Lee JH, et al. Differential expression of multiple transglutaminases in human brain. Increased expression and cross-linking by transglutaminases 1 and 2 in Alzheimer's disease. J. Biol. Chem. 1999;274:30715–33021. doi: 10.1074/jbc.274.43.30715. [■■ of considerable interestFirst study to demonstrate that at least three transglutaminases (TGs) are present in human brain and that Alzheimer's disease (AD) brain has greatly increased levels of protein crosslinks.] [DOI] [PubMed] [Google Scholar]
- 2.Citron BA, SantaCruz KS, Davies PJA, et al. Intron–exon swapping of transglutaminase mRNA and neuronal Tau aggregation in Alzheimer's disease. J. Biol. Chem. 2001;276:3295–3301. doi: 10.1074/jbc.M004776200. [DOI] [PubMed] [Google Scholar]
- 3■.Wilhelmus MM, Grunberg SC, Bol JG, et al. Transglutaminases and transglutaminase-catalyzed cross-links colocalize with the pathological lesions in Alzheimer's disease brain. Brain Pathol. 2008 doi: 10.1111/j.1750-3639.2008.00197.x. DOI: 10.1111/j.1750−3639.2008.00197.x. (Epub ahead of print) [■ of interestImportant demonstration of TGs and pathological lesions in AD brain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4■.Hadjivassiliou M, Aeschlimann P, Strigun A, et al. Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann. Neurol. 2008;64:332–343. doi: 10.1002/ana.21450. [■ of interestDemonstration of TG 6 in human brain.] [DOI] [PubMed] [Google Scholar]
- 5.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 6.Molberg Ø, MacAdam SN, Korner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 1998;4:713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 7.Van de Wal Y, Kooy Y, van Veelen P, et al. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 1998;161:1585–1588. [PubMed] [Google Scholar]
- 8.Nemes Z, Marekov LN, Fésüs L, et al. A novel function for transglutaminase 1: attachment of long-chain Ω-hydroxyceramides to involucrin by ester bond formation. Proc. Natl Acad. Sci. USA. 1999;96:8402–8407. doi: 10.1073/pnas.96.15.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folk JE, Park MH, Chung SI, et al. Polyamines as physiological substrates for transglutaminases. J. Biol. Chem. 1980;255:3695–3700. [PubMed] [Google Scholar]
- 10.Piacentini M, Martinet N, Beninati S, et al. Free and protein-conjugated polyamines in mouse epidermal cells. Effect of high calcium and retinoic acid. J. Biol. Chem. 1988;263:3790–3794. [PubMed] [Google Scholar]
- 11■.Jeitner TM, Matson WR, Folk JE, et al. Increased levels of γ-glutamylamines in Huntington disease CSF. J. Neurochem. 2008;106:37–44. doi: 10.1111/j.1471-4159.2008.05350.x. [■ of interestFirst demonstration of increased γ-glutamylpolyamines in Huntington's disease (HD) brain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeitner TM, Bogdanov MB, Matson WR, et al. Nε-(γ-l-glutamyl)-l-lysine (GGEL) is increased in cerebrospinal fluid of patients with Huntington's disease. J. Neurochem. 2001;79:1109–1112. doi: 10.1046/j.1471-4159.2001.00673.x. [DOI] [PubMed] [Google Scholar]
- 13.Dedeoglu A, Kubilus JK, Jeitner TM, et al. Therapeutic effects of cystamine in a murine model of Huntington's disease. J. Neurosci. 2002;22:8942–8950. doi: 10.1523/JNEUROSCI.22-20-08942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai TS, Tucker T, Burke JR, et al. Effect of tissue transglutaminase on the solubility of proteins containing expanded polyglutamine repeats. J. Neurochem. 2004;88:1253–1260. doi: 10.1046/j.1471-4159.2003.02249.x. [DOI] [PubMed] [Google Scholar]
- 15.Konno T, Morii T, Shimizu H, et al. Paradoxical inhibition of protein aggregation and precipitation by transglutaminase-catalyzed intermolecular cross-linking. J. Biol. Chem. 2005;280:17520–17525. doi: 10.1074/jbc.M413988200. [DOI] [PubMed] [Google Scholar]
- 16.Fink ML, Folk JE. γ-Glutamylamine cyclotransferase. An enzyme involved in the catabolism of ε-(γ-glutamyl)lysine and other γ-glutamylamines. Mol. Cell. Biochem. 1981;38:59–67. doi: 10.1007/BF00235688. [DOI] [PubMed] [Google Scholar]
- 17.Cordella-Miele E, Miele L, Beninati S, et al. Transglutaminase-catalyzed incorporation of polyamines into phospholipase A2. J. Biochem. 1993;113:164–173. doi: 10.1093/oxfordjournals.jbchem.a124021. [DOI] [PubMed] [Google Scholar]
- 18.Masuda M, Betancourt L, Matsuzawa T, et al. Activation of rho through a cross-link with polyamines catalyzed by Bordetella dermonecrotizing toxin. EMBO J. 2000;19:521–530. doi: 10.1093/emboj/19.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuda M, Minami M, Shime H, et al. In vivo modifications of small GTPase Rac and Cdc42 by Bordetella dermonecrotic toxin. Infect. Immun. 2002;70:998–1001. doi: 10.1128/iai.70.2.998-1001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20■.Folk JE, Mullooly JP, Cole PW. Mechanism of action of guinea pig liver transglutaminase. II. The role of metal in enzyme activation. J. Biol. Chem. 1967;242:1838–1844. [■ of interestPaper of historical interest.] [PubMed] [Google Scholar]
- 21.Martinet N, Beninati S, Nigra TP, et al. N1N8-Bis(γ-glutamyl)spermidine cross-linking in epidermal-cell envelopes. Comparison of cross-link levels in normal and psoriatic cell envelopes. Biochem. J. 1990;271:305–308. doi: 10.1042/bj2710305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murthy SN, Wilson J, Guy SL, et al. Intramolecular crosslinking of monomeric fibrinogen by tissue transglutaminase. Proc. Natl Acad. Sci. USA. 1991;88:10601–10604. doi: 10.1073/pnas.88.23.10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson J, Rickles FR, Armstrong PB, et al. Nε(γ-glutamyl)lysine crosslinks in the blood clot of the horseshoe crab, Limulus polyphemus. Biochem. Biophys. Res. Commun. 1992;188:655–661. doi: 10.1016/0006-291x(92)91106-z. [DOI] [PubMed] [Google Scholar]
- 24.Kuchibhotla KV, Goldman ST, Lattarulo CR, et al. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Lesort M, Guttmann RP, et al. Modulation of the in situ activity of tissue transglutaminase by calcium and GTP. J. Biol. Chem. 1998;273:2288–2295. doi: 10.1074/jbc.273.4.2288. [DOI] [PubMed] [Google Scholar]
- 26.Tucholski J, Kuret J, Johnson GVW. Tau is modified by tissue transglutaminase in situ: possible functional and metabolic effects of polyamination. J. Neurochem. 1999;73:1871–1880. [PubMed] [Google Scholar]
- 27.Casadio R, Polverini E, Mariani P, et al. The structural basis for the regulation of tissue transglutaminase by calcium ions. Eur. J. Biochem. 1999;262:672–679. doi: 10.1046/j.1432-1327.1999.00437.x. [DOI] [PubMed] [Google Scholar]
- 28■.Ahvazi B, Kim HC, Kee SH, et al. Three-dimensional structure of the human transglutaminase 3 enzyme: binding of calcium ions changes structure for activation. EMBO J. 2002;21:2055–2067. doi: 10.1093/emboj/21.9.2055. [■ of interestImportant x-ray crystallographic study of a TG.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Cerione RA, Clardy J. Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc. Natl Acad. Sci. USA. 2002;99:2743–2747. doi: 10.1073/pnas.042454899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahvazi B, Boeshans KM, Idler W, et al. Roles of calcium ions in the activation and activity of the transglutaminase 3 enzyme. J. Biol. Chem. 2003;278:23834–23841. doi: 10.1074/jbc.M301162200. [DOI] [PubMed] [Google Scholar]
- 31.Boeshans KM, Mueser TC, Ahvazi B. A three-dimensional model of the human transglutaminase 1: insights into the understanding of lamellar ichthyosis. J. Mol. Model. 2007;13:233–246. doi: 10.1007/s00894-006-0144-9. [DOI] [PubMed] [Google Scholar]
- 32■.Pinkas DM, Strop P, Brunger AT, et al. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:E327. doi: 10.1371/journal.pbio.0050327. [■ of interestDemonstrates the major changes in TG 2 topology during the catalytic cycle.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreotti AH. Native state proline isomerization: an intrinsic molecular switch. Biochemistry. 2003;42:9515–9524. doi: 10.1021/bi0350710. [DOI] [PubMed] [Google Scholar]
- 34.Andreotti AH. Opening the pore hinges on proline. Nat. Chem. Biol. 2006;2:13–14. doi: 10.1038/nchembio0106-13. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar P, Reichman C, Saleh T, et al. Proline cis–trans isomerization controls autoinhibition of a signaling protein. Mol. Cell. 2007;25:413–426. doi: 10.1016/j.molcel.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chazin WJ, Kördel J, Drakenberg T, et al. Proline isomerism leads to multiple folded conformations of calbindin D9k: direct evidence from two-dimensional 1H NMR spectroscopy. Proc. Natl Acad. Sci. USA. 1989;86:2195–2198. doi: 10.1073/pnas.86.7.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datta S, Antonyak MA, Cerione RA. Importance of Ca2+-dependent transamidation activity in the protection afforded by tissue transglutaminase against doxorubicin-induced apoptosis. Biochemistry. 2006;45:13163–13174. doi: 10.1021/bi0606795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugimura Y, Hosono M, Wada F, et al. Screening for the preferred substrate sequence of transglutaminase using a phage-displayed peptide library: identification of peptide substrates for TGASE 2 and factor XIIIA. J. Biol. Chem. 2006;281:17699–17706. doi: 10.1074/jbc.M513538200. [DOI] [PubMed] [Google Scholar]
- 39■.Iismaa SE, Holman S, Wouters MA, et al. Evolutionary specialization of a tryptophan indole group for transition-state stabilization by eukaryotic transglutaminases. Proc. Natl Acad. Sci. USA. 2003;100:12636–12641. doi: 10.1073/pnas.1635052100. [■ of interestImportant study of TG mechanism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eigel WN, Randolph HE. Preparation of whole γ-casein by treatment with calcium phosphate gel. J. Dairy. Sci. 1974;57:1444–1447. doi: 10.3168/jds.S0022-0302(74)85087-3. [DOI] [PubMed] [Google Scholar]
- 41.Bungay PJ, Owen RA, Coutts IC, et al. A role for transglutaminase in glucose-stimulated insulin release from the pancreatic β-cell. Biochem. J. 1986;235:269–278. doi: 10.1042/bj2350269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiraboschi P, Hansen LA, Thal LJ, et al. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62:1984–1989. doi: 10.1212/01.wnl.0000129697.01779.0a. [DOI] [PubMed] [Google Scholar]
- 43.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 45.Nistor M, Don M, Parekh M, et al. α- and β-secretase activity as a function of age and β-amyloid in Down syndrome and normal brain. Neurobiol. Aging. 2007;28:1493–1506. doi: 10.1016/j.neurobiolaging.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Aβ42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled Phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz C, Rutten BP, Pielen A, et al. Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer's disease. Am. J. Pathol. 2004;164:1495–1502. doi: 10.1016/S0002-9440(10)63235-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mudher A, Lovestone S. Alzheimer's disease – do tauists and baptists finally shake hands? Trends Neurosci. 2002;25:22–26. doi: 10.1016/s0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- 49.Johnson GVW, Cox TM, Lockhart JP, et al. Transglutaminase activity is increased in Alzheimer's disease brain. Brain Res. 1997;751:323–329. doi: 10.1016/s0006-8993(96)01431-x. [DOI] [PubMed] [Google Scholar]
- 50.Singer SM, Norlund MA, Lee JM, et al. Transglutaminase cross-links in neurofibrillary tangles and paired helical filament tau early in Alzheimer's disease. Neurochem. Int. 2002;40:17–30. doi: 10.1016/s0197-0186(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 51.Phillips MA, Jessen BA, Lu Y, et al. A distal region of the human TGM1 promoter is required for expression in transgenic mice and cultured keratinocytes. BMC Dermatol. 2004;4:2. doi: 10.1186/1471-5945-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ritter SJ, Davies PJ. Identification of a transforming growth factor-β1/bone morphogenetic protein 4 (TGF-β1/BMP4) response element within the mouse tissue transglutaminase gene promoter. J. Biol. Chem. 1998;273:12798–12806. doi: 10.1074/jbc.273.21.12798. [DOI] [PubMed] [Google Scholar]
- 53.Kuncio GS, Tsyganskaya M, Zhu J, et al. TNF-α modulates expression of the tissue transglutaminase gene in liver cells. Am. J. Physiol. 1998;274:G240–G245. doi: 10.1152/ajpgi.1998.274.2.G240. [DOI] [PubMed] [Google Scholar]
- 54.Park KC, Chung KC, Kim YS, et al. Transglutaminase 2 induces nitric oxide synthesis in BV-2 microglia. Biochem. Biophys. Res. Commun. 2004;323:1055–1062. doi: 10.1016/j.bbrc.2004.08.204. [DOI] [PubMed] [Google Scholar]
- 55.Campisi A, Caccamo D, Li Volti G, et al. Glutamate-evoked redox state alterations are involved in tissue transglutaminase upregulation in primary astrocyte cultures. FEBS. Lett. 2004;578:80–84. doi: 10.1016/j.febslet.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 56.Kim Y, Park YW, Lee YS, et al. Hyaluronic acid induces transglutaminase II to enhance cell motility; role of Rac1 and FAK in the induction of transglutaminase II. Biotechnol. Lett. 2008;30:31–39. doi: 10.1007/s10529-007-9496-1. [DOI] [PubMed] [Google Scholar]
- 57■.Festoff BW, SantaCruz K, Arnold PM, et al. Injury-induced ‘switch’ from GTP-regulated to novel GTP-independent isoform of tissue transglutaminase in the rat spinal cord. J. Neurochem. 2002;81:708–718. doi: 10.1046/j.1471-4159.2002.00850.x. [■ of interestA novel truncated form of TG 2.] [DOI] [PubMed] [Google Scholar]
- 58.Antonyak MA, Jansen JM, Miller AM, et al. Two isoforms of tissue transglutaminase mediate opposing cellular fates. Proc. Natl Acad. Sci. USA. 2006;103:18609–18614. doi: 10.1073/pnas.0604844103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonelli RM, Aschoff A, Niederwieser G, et al. Cerebrospinal fluid tissue transglutaminase as a biochemical marker for Alzheimer's disease. Neurobiol. Dis. 2002;11:106–110. doi: 10.1006/nbdi.2002.0535. [DOI] [PubMed] [Google Scholar]
- 60.Vermes I, Steur EN, Jirikowski GF, et al. Elevated concentration of cerebrospinal fluid tissue transglutaminase in Parkinson's disease indicating apoptosis. Mov. Disord. 2004;19:1252–1254. doi: 10.1002/mds.20197. [DOI] [PubMed] [Google Scholar]
- 61■.Aeschlimann D, Mosher D, Paulsson M. Tissue transglutaminase and factor XIII in cartilage and bone remodeling. Semin. Thromb. Hemost. 1996;22:437–443. doi: 10.1055/s-2007-999043. [■ of interestGood review of some of the physiological roles of TG2 and factor XIII.] [DOI] [PubMed] [Google Scholar]
- 62.Zemskov EA, Janiak A, Hang J, et al. The role of tissue transglutaminase in cell–matrix interactions. Front. Biosci. 2006;11:1057–1176. doi: 10.2741/1863. [DOI] [PubMed] [Google Scholar]
- 63.El Nahas AM, Abo-Zenah H, Skill NJ, et al. Elevated ε-(γ-glutamyl)lysine in human diabetic nephropathy results from increased expression and cellular release of tissue transglutaminase. Nephron Clin. Pract. 2004;97:C108–C117. doi: 10.1159/000078639. [DOI] [PubMed] [Google Scholar]
- 64.Siegel M, Strnad P, Watts RE, et al. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS ONE. 2008;3:E1861. doi: 10.1371/journal.pone.0001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jensen PH, Sørensen ES, Petersen TE, et al. Residues in the synuclein consensus motif of the α-synuclein fragment, NAC, participate in transglutaminase-catalysed cross-linking to Alzheimer-disease amyloid βA4 peptide. Biochem. J. 1995;310:91–94. doi: 10.1042/bj3100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dudek SM, Johnson GVW. Transglutaminase facilitates the formation of polymers of the β-amyloid peptide. Brain Res. 1994;651:129–133. doi: 10.1016/0006-8993(94)90688-2. [DOI] [PubMed] [Google Scholar]
- 67.Hartley DM, Zhao C, Speier AC, et al. Transglutaminase induces protofibril-like β-protein assemblies that are protease-resistant and inhibit long-term potentiation. J. Biol. Chem. 2008;283:16790–16800. doi: 10.1074/jbc.M802215200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdalla S, Lother H, El Missiry A, et al. Angiotensin II AT2 receptor oligomers mediate G-protein dysfunction in an animal model of Alzheimer disease. J. Biol. Chem. 2009;284:6554–6565. doi: 10.1074/jbc.M807746200. [DOI] [PubMed] [Google Scholar]
- 69.Abdalla S, Lother H, El Missiry A, et al. Dominant negative AT2 receptor oligomers induce G-protein arrest and symptoms of neurodegeneration. J. Biol. Chem. 2009;284:6566–6574. doi: 10.1074/jbc.M808277200. [DOI] [PubMed] [Google Scholar]
- 70.Cooper AJL, Sheu KR, Burke JR, et al. Transglutaminase-catalyzed inactivation of glyceraldehyde 3-phosphate dehydrogenase and α-ketoglutarate dehydrogenase complex by polyglutamine domains of pathological length. Proc. Natl Acad. Sci. USA. 1997;94:12604–12609. doi: 10.1073/pnas.94.23.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gentile V, Sepe C, Calvani M, et al. Tissue transglutaminase-catalyzed formation of high-molecular-weight aggregates in vitro is favored with long polyglutamine domains: a possible mechanism contributing to CAG-triplet diseases. Arch. Biochem. Biophys. 1998;352:314–321. doi: 10.1006/abbi.1998.0592. [DOI] [PubMed] [Google Scholar]
- 72.Cooper AJL, Wang J, Pasternack R, et al. Lysine-rich histone (H1) is a lysyl substrate of tissue transglutaminase: possible involvement of transglutaminase in the formation of nuclear aggregates in (CAG)n/Qn expansion diseases. Dev. Neurosci. 2000;22:404–417. doi: 10.1159/000017470. [DOI] [PubMed] [Google Scholar]
- 73■.Nemes Z, Devreese B, Steinert PM, et al. Cross-linking of ubiquitin, HSP27, parkin, and α-synuclein by γ-glutamyl-ε-lysine bonds in Alzheimer's neurofibrillary tangles. FASEB J. 2004;18:1135–1137. doi: 10.1096/fj.04-1493fje. [■ of interestIdentification of major crosslinked proteins in AD brain.] [DOI] [PubMed] [Google Scholar]
- 74.Norlund MA, Zainelli G, Lee J, et al. Elevated transglutaminase-induced bonds in Alzheimers PHF tau. Brain Res. 1999;851:154–163. doi: 10.1016/s0006-8993(99)02179-4. [DOI] [PubMed] [Google Scholar]
- 75.Appelt DM, Balin BJ. The association of tissue transglutaminase with human recombinant tau results in the formation of insoluble filamentous structures. Brain Res. 1997;745:21–31. doi: 10.1016/s0006-8993(96)01121-3. [DOI] [PubMed] [Google Scholar]
- 76.Dudek SM, Johnson GVW. Transglutaminase catalyzes the formation of sodium dodecyl sulfate-insoluble, Alz-50-reactive polymers of tau. J. Neurochem. 1993;61:1159–1162. doi: 10.1111/j.1471-4159.1993.tb03636.x. [DOI] [PubMed] [Google Scholar]
- 77.Miller ML, Johnson GVW. Transglutaminase cross-linking of the tau protein. J. Neurochem. 1995;65:1760–1770. doi: 10.1046/j.1471-4159.1995.65041760.x. [DOI] [PubMed] [Google Scholar]
- 78.Murthy SN, Wilson JH, Lukas TJ, et al. Cross-linking sites of the human tau protein, probed by reactions with human transglutaminase. J. Neurochem. 1998;71:2607–2614. doi: 10.1046/j.1471-4159.1998.71062607.x. [DOI] [PubMed] [Google Scholar]
- 79.Wang DS, Dickson DW, Malter JS. Tissue Transglutaminase, protein cross-linking and Alzheimer's disease: review and views. Int. J. Clin. Exp. Pathol. 2008;1:5–18. [PMC free article] [PubMed] [Google Scholar]
- 80.Junn E, Ronchetti RD, Quezado MM, et al. Tissue transglutaminase-induced aggregation of α-synuclein: implications for Lewy body formation in Parkinson's disease and dementia with Lewy bodies. Proc. Natl Acad. Sci. USA. 2003;100:2047–2052. doi: 10.1073/pnas.0438021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmid AW, Chiappe D, Pignat V, et al. Dissecting the mechanisms of tissue transglutaminase-induced cross-linking of α-synuclein: implications for the pathogenesis of Parkinson's disease. J. Biol. Chem. 2009 doi: 10.1074/jbc.M809067200. DOI: 10.1074/jbc.M809067200. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Segers-Nolten IM, Wilhelmus MM, Veldhuis G, et al. Tissue transglutaminase modulates α-synuclein oligomerization. Protein Sci. 2008;17:1395–1402. doi: 10.1110/ps.036103.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steinert PM, Kim SY, Chung SI, et al. The transglutaminase 1 enzyme is variably acylated by myristate and palmitate during differentiation in epidermal keratinocytes. J. Biol. Chem. 1996;271:26242–26250. doi: 10.1074/jbc.271.42.26242. [DOI] [PubMed] [Google Scholar]
- 84.Kim SY, Chung SI, Steinert PM. Highly active soluble processed forms of the transglutaminase 1 enzyme in epidermal keratinocytes. J. Biol. Chem. 1995;270:18026–18035. doi: 10.1074/jbc.270.30.18026. [DOI] [PubMed] [Google Scholar]
- 85.Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp. Mol. Med. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- 86.Busche MA, Eichhoff G, Adelsberger H, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 87.Tandon A, Fraser P. The presenilins. Genome Biol. 2002;3:3014. doi: 10.1186/gb-2002-3-11-reviews3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tu H, Nelson O, Bezprozvanny A, et al. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nelson O, Tu H, Lei T, et al. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J. Clin. Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stutzmann GE, Caccamo A, LaFerla FM, et al. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer's-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J. Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stutzmann GE, Smith I, Caccamo A, et al. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J. Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang SQ, Song LS, Lakatta EG, et al. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- 94.Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 95.Gibson GE, Zhang H, Toral-Barza L, et al. Calcium stores in cultured fibroblasts and their changes with Alzheimer's disease. Biochim. Biophys. Acta. 1996;1316:71–77. doi: 10.1016/0925-4439(96)00002-6. [DOI] [PubMed] [Google Scholar]
- 96.Gusella JF, MacDonald ME. Hunting for Huntington's disease. Mol. Genet. Med. 1993;3:139–158. doi: 10.1016/b978-0-12-462003-2.50009-2. [DOI] [PubMed] [Google Scholar]
- 97.Stine OC, Pleasant N, Franz ML, et al. Correlation between the onset age of Huntington's disease and length of the trinucleotide repeat in IT-15. Hum. Mol. Genet. 1993;2:1547–1549. doi: 10.1093/hmg/2.10.1547. [DOI] [PubMed] [Google Scholar]
- 98.Brandt J, Bylsma FW, Gross R, et al. Trinucleotide repeat length and clinical progression in Huntington's disease. Neurology. 1996;46:527–531. doi: 10.1212/wnl.46.2.527. [DOI] [PubMed] [Google Scholar]
- 99.DiFiglia M, Sapp E, Chase K, et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995;14:1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 100.Chen S, Berthelier V, Yang W, et al. Polyglutamine aggregation behavior in vitro supports a recruitment mechanism of cytotoxicity. J. Mol. Biol. 2001;311:173–182. doi: 10.1006/jmbi.2001.4850. [DOI] [PubMed] [Google Scholar]
- 101■.Green H. Human genetic diseases due to codon reiteration: relationship to an evolutionary mechanism. Cell. 1993;74:955–956. doi: 10.1016/0092-8674(93)90718-6. [■ of interestFirst suggestion that expanded Qn domains may be good TG substrates and thereby contribute to HD pathology.] [DOI] [PubMed] [Google Scholar]
- 102.Kahlem P, Terré C, Green H, et al. Peptides containing glutamine repeats as substrates for transglutaminase-catalyzed cross-linking: relevance to diseases of the nervous system. Proc. Natl Acad. Sci. USA. 1996;93:14580–14585. doi: 10.1073/pnas.93.25.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kahlem P, Green H, Djian P. Transglutaminase action imitates Huntington's disease: selective polymerization of Huntingtin containing expanded polyglutamine. Mol. Cell. 1998;1:595–601. doi: 10.1016/s1097-2765(00)80059-3. [DOI] [PubMed] [Google Scholar]
- 104.Karpuj MV, Garren H, Slunt H, et al. Transglutaminase aggregates huntingtin into nonamyloidogenic polymers, and its enzymatic activity increases in Huntington's disease brain nuclei. Proc. Natl Acad. Sci. USA. 1999;96:7388–7393. doi: 10.1073/pnas.96.13.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cooper AJL, Jeitner TM, Gentile V, et al. Cross linking of polyglutamine domains catalyzed by tissue transglutaminase is greatly favored with pathological-length repeats: does transglutaminase activity play a role in (CAG) n/Qn-expansion diseases? Neurochem. Int. 2002;40:53–67. doi: 10.1016/s0197-0186(01)00058-4. [DOI] [PubMed] [Google Scholar]
- 106■.Zainelli GM, Dudek NL, Ross CA, et al. Mutant huntingtin protein: a substrate for transglutaminase 1, 2, and 3. J. Neuropathol. Exp. Neurol. 2005;64:58–65. doi: 10.1093/jnen/64.1.58. [■ of interestDemonstration that mutant htt is substrate of three TGs in human brain.] [DOI] [PubMed] [Google Scholar]
- 107.Zainelli GM, Ross CA, Troncoso JC, et al. Transglutaminase cross-links in intranuclear inclusions in Huntington disease. J. Neuropathol. Exp. Neurol. 2003;62:14–24. doi: 10.1093/jnen/62.1.14. [DOI] [PubMed] [Google Scholar]
- 108.Lesort M, Chun W, Johnson GVW, et al. Tissue transglutaminase is increased in Huntington's disease brain. J. Neurochem. 1999;73:2018–2027. [PubMed] [Google Scholar]
- 109.Lee J, Kim YS, Choi DH. Transglutaminase 2 induces nuclear factor-κB activation via a novel pathway in BV-2 microglia. J. Biol. Chem. 2004;279:53725–53735. doi: 10.1074/jbc.M407627200. [DOI] [PubMed] [Google Scholar]
- 110.Zhang H, Das S, Li QZ, et al. Elucidating a normal function of huntingtin by functional and microarray analysis of huntingtin-null mouse embryonic fibroblasts. BMC Neurosci. 2008;9:38. doi: 10.1186/1471-2202-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tang TS, Tu H, Chan EY, et al. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cepeda C, Ariano MA, Calvert CR, et al. NMDA receptor function in mouse models of Huntington disease. J. Neurosci Res. 2001;66:525–539. doi: 10.1002/jnr.1244. [DOI] [PubMed] [Google Scholar]
- 113.Zeron MM, Hansson O, Chen N, et al. Increased sensitivity to N-methyl-d-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron. 2002;33:849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 114.Tang TS, Chen X, Liu J, et al. Dopaminergic signaling and striatal neurodegeneration in Huntington's disease. J. Neurosci. 2007;27:7899–7910. doi: 10.1523/JNEUROSCI.1396-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hodgson JG, Agopyan N, Gutekunst CA, et al. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron. 1999;23:181–192. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- 116.Romero E, Cha GH, Verstreken P, et al. Suppression of neurodegeneration and increased neurotransmission caused by expanded full-length huntingtin accumulating in the cytoplasm. Neuron. 2008;57:27–40. doi: 10.1016/j.neuron.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Panov AV, Lund S, Greenamyre JT. Ca2+-induced permeability transition in human lymphoblastoid cell mitochondria from normal and Huntington's disease individuals. Mol. Cell Biochem. 2005;269:143–152. doi: 10.1007/s11010-005-3454-9. [DOI] [PubMed] [Google Scholar]
- 118.Panov AV, Gutekunst CA, Leavitt BR, et al. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat. Neurosci. 2005;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 119.Milakovic T, Quintanilla RA, Johnson GVW. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. J. Biol. Chem. 2006;281:34785–34795. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- 120.Lim D, Fedrizzi L, Tartari M, et al. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J. Biol. Chem. 2008;283:5780–5789. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- 121.Puszkin EG, Raghuraman V. Catalytic properties of a calmodulin-regulated transglutaminase from human platelet and chicken gizzard. J. Biol. Chem. 1985;260:16012–16020. [PubMed] [Google Scholar]
- 122.Billett HH, Puszkin EG. The red cell membrane contains calmodulin-regulated crosslinking and proteolytic activity. Hematol. Pathol. 1991;5:185–193. [PubMed] [Google Scholar]
- 123.Zainelli GM, Ross CA, Troncoso JC, et al. Calmodulin regulates transglutaminase 2 cross-linking of Huntingtin. J. Neurosci. 2004;24:1954–1961. doi: 10.1523/JNEUROSCI.4424-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dudek NL, Dai Y, Muma NA. Protective effects of interrupting the binding of calmodulin to mutant huntingtin. J. Neuropathol. Exp. Neurol. 2008;67:355–365. doi: 10.1097/NEN.0b013e31816a9e60. [DOI] [PubMed] [Google Scholar]
- 125.Dudek NL, Dai Y, Muma NA. Neuroprotective effects of calmodulin peptide 76−121aa: disruption of calmodulin binding to mutant huntingtin. Brain Pathol. 2008 doi: 10.1111/j.1750-3639.2008.00258.x. DOI: 10.1111/j.1750−3639.2008.00258.x. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126■■.Mastroberardino PG, Iannicola C, Nardacci R, et al. ‘Tissue’ transglutaminase ablation reduces neuronal death and prolongs survival in a mouse model of Huntington's disease. Cell Death Differ. 2002;9:873–880. doi: 10.1038/sj.cdd.4401093. [■■ of considerable interestCompelling evidence that TG 2 contributes to HD pathology.] [DOI] [PubMed] [Google Scholar]
- 127.Liu P, Gupta N, Jing Y, et al. Age-related changes in polyamines in memory-associated brain structures in rats. Neuroscience. 2008;155:789–796. doi: 10.1016/j.neuroscience.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 128.Kim SY, Jeitner TM, Steinert PM. Transglutaminases in disease. Neurochem. Int. 2002;40:85–103. doi: 10.1016/s0197-0186(01)00064-x. [DOI] [PubMed] [Google Scholar]
- 129■.Muma NA. Transglutaminase is linked to neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2007;66:258–263. doi: 10.1097/nen.0b013e31803d3b02. [■ of interestRecent review of TGs in neurodegeneration.] [DOI] [PubMed] [Google Scholar]
- 130.Lees AJ. The Parkinson chimera. Neurology. 2009;72(Suppl 7):S2–S11. doi: 10.1212/WNL.0b013e318198daec. [DOI] [PubMed] [Google Scholar]
- 131.Mezey E, Dehejia A, Harta G, et al. α-synuclein in neurodegenerative disorders: murderer or accomplice? Nat. Med. 1998;4:755–757. doi: 10.1038/nm0798-755. [DOI] [PubMed] [Google Scholar]
- 132.Spillantini MG, Schmidt ML, Lee VM, et al. α-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 133.Arima K, Uéda K, Sunohara N, et al. Immunoelectron-microscopic demonstration of NACP/α-synuclein-epitopes on the filamentous component of Lewy bodies in Parkinson's disease and in dementia with Lewy bodies. Brain Res. 1998;808:93–100. doi: 10.1016/s0006-8993(98)00734-3. [DOI] [PubMed] [Google Scholar]
- 134.Wirths O, Weickert S, Majtenyi K, et al. Lewy body variant of Alzheimer's disease: α-synuclein in dystrophic neurites of Aβ plaques. Neuroreport. 2000;11:3737–3741. doi: 10.1097/00001756-200011270-00029. [DOI] [PubMed] [Google Scholar]
- 135.Emadi S, Kasturirangan S, Wang MS, et al. Detecting morphologically distinct oligomeric forms of α-synuclein. J. Biol. Chem. 2009 doi: 10.1074/jbc.M806559200. DOI: 10.1074/jbc.M806559200. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kragh CL, Lund LB, Febbraro F, et al. α-Synuclein aggregation and Ser129 phosphorylation dependent cell death in oligodendroglial cells. J. Biol. Chem. 2009 doi: 10.1074/jbc.M809671200. DOI: 10.1074/jbc.M809671200. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Masliah E, Rockenstein E, Veinbergs I, et al. Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 138.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 139.Krüger R, Kuhn W, Müller T, et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 140.Uversky VN. α-synuclein misfolding and neurodegenerative diseases. Curr. Protein Pept. Sci. 2008;9:507–540. doi: 10.2174/138920308785915218. [DOI] [PubMed] [Google Scholar]
- 141.Andringa G, Lam KY, Chegary M, Wang X, et al. Tissue transglutaminase catalyzes the formation of α-synuclein crosslinks in Parkinson's disease. FASEB J. 2004;18:932–934. doi: 10.1096/fj.03-0829fje. [DOI] [PubMed] [Google Scholar]
- 142.Bennett EJ, Shaler TA, Woodman B, et al. Global changes to the ubiquitin system in Huntington's disease. Nature. 2007;448:704–708. doi: 10.1038/nature06022. [DOI] [PubMed] [Google Scholar]
- 143.Wang J, Wang CE, Orr A, et al. Impaired ubiquitin-proteasome system activity in the synapses of Huntington's disease mice. J. Cell Biol. 2008;180:1177–1189. doi: 10.1083/jcb.200709080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mandrusiak LM, Beitel LK, Wang X, et al. Transglutaminase potentiates ligand-dependent proteasome dysfunction induced by polyglutamine-expanded androgen receptor. Hum. Mol. Genet. 2003;12:1497–1506. doi: 10.1093/hmg/ddg161. [DOI] [PubMed] [Google Scholar]
- 145.Surmeier DJ. Calcium, ageing, and neuronal vulnerability in Parkinson's disease. Lancet Neurol. 2007;6:933–938. doi: 10.1016/S1474-4422(07)70246-6. [DOI] [PubMed] [Google Scholar]
- 146.Ibáñez-Sandoval O, Carrillo-Reid L, Galarraga E, et al. Bursting in substantia nigra pars reticulata neurons in vitro: possible relevance for Parkinson disease. J. Neurophysiol. 2007;98:2311–2323. doi: 10.1152/jn.00620.2007. [DOI] [PubMed] [Google Scholar]
- 147.Choi YM, Kim SH, Uhm DY, et al. Glutamate-mediated [Ca2+]c dynamics in spontaneously firing dopamine neurons of the rat substantia nigra pars compacta. J. Cell Sci. 2003;116:2665–2675. doi: 10.1242/jcs.00481. [DOI] [PubMed] [Google Scholar]
- 148.McMahon A, Wong BS, Iacopino AM, et al. Calbindin-D28k buffers intracellular calcium and promotes resistance to degeneration in PC12 cells. Brain Res. Mol. Brain Res. 1998;54:56–63. doi: 10.1016/s0169-328x(97)00305-7. [DOI] [PubMed] [Google Scholar]
- 149.Houghton DJ, Litvan I. Unraveling progressive supranuclear palsy: from the bedside back to the bench. Parkinsonism Relat. Disord. 2007;13(Suppl 3):S336–S340. doi: 10.1016/S1353-8020(08)70028-2. [DOI] [PubMed] [Google Scholar]
- 150.Zemaitaitis MO, Lee JM, Troncoso JC, et al. Transglutaminase-induced cross-linking of tau proteins in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 2000;59:983–989. doi: 10.1093/jnen/59.11.983. [DOI] [PubMed] [Google Scholar]
- 151.Zemaitaitis MO, Kim SY, Halverson RA, et al. Transglutaminase activity, protein, and mRNA expression are increased in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 2003;62:173–184. doi: 10.1093/jnen/62.2.173. [DOI] [PubMed] [Google Scholar]
- 152.Albers DS, Augood SJ. New insights into progressive supranuclear palsy. Trends Neurosci. 2001;24:347–353. doi: 10.1016/s0166-2236(00)01794-x. [DOI] [PubMed] [Google Scholar]
- 153.Walther DJ, Peter JU, Winter S, et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet α-granule release. Cell. 2003;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- 154.Dai Y, Dudek NL, Patel TB, et al. Transglutaminase-catalyzed transamidation: a novel mechanism for Rac1 activation by 5-hydroxytryptamine2A receptor stimulation. J. Pharmacol. Exp. Ther. 2008;326:153–162. doi: 10.1124/jpet.107.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lorand L, Conrad SM. Transglutaminases. Mol. Cell Biochem. 1984;58:9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- 156.Jeitner TM, Delikatny EJ, Ahlqvist J, et al. Mechanism for the inhibition of transglutaminase 2 by cystamine. Biochem. Pharmacol. 2005;69:961–970. doi: 10.1016/j.bcp.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 157.Karpuj MV, Becher MW, Springer JE, et al. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat. Med. 2002;8:143–149. doi: 10.1038/nm0202-143. [DOI] [PubMed] [Google Scholar]
- 158.Van Raamsdonk JM, Pearson J, Bailey CDC, et al. Cystamine treatment is neuroprotective in the YAC128 mouse model of Huntington disease. J. Neurochem. 2005;95:210–220. doi: 10.1111/j.1471-4159.2005.03357.x. [DOI] [PubMed] [Google Scholar]
- 159.Stack EC, Ferro JL, Kim J, et al. Therapeutic attenuation of mitochondrial dysfunction and oxidative stress in neurotoxin models of Parkinson's disease. Biochim. Biophys. Acta. 2008;1782:151–162. doi: 10.1016/j.bbadis.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 160.Fox JH, Barber DS, Singh B, et al. Cystamine increases l-cysteine levels in Huntington's disease transgenic mouse brain and in a PC12 model of polyglutamine aggregation. J. Neurochem. 2004;91:413–422. doi: 10.1111/j.1471-4159.2004.02726.x. [DOI] [PubMed] [Google Scholar]
- 161.Pinto JT, Van Raamsdonk JM, Leavitt BR, et al. Treatment of YAC128 mice and their wild-type littermates with cystamine does not lead to its accumulation in plasma or brain: implications for the treatment of Huntington disease. J. Neurochem. 2005;94:1087–1101. doi: 10.1111/j.1471-4159.2005.03255.x. [DOI] [PubMed] [Google Scholar]
- 162.Borrell-Pagès M, Canals JM, Cordelières FP, et al. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J. Clin. Invest. 2006;116:1410–1424. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jeitner TM, Pinto JT, Kranikov BF, et al. Transglutaminases and neurodegeneration. J. Neurochem. 2009;109(Suppl 1):160–166. doi: 10.1111/j.1471-4159.2009.05843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lesort M, Lee M, Tucholski J, et al. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J. Biol. Chem. 2003;278:3825–3830. doi: 10.1074/jbc.M205812200. [DOI] [PubMed] [Google Scholar]
- 165.Bailey CDC, Johnson GVW. The protective effects of cystamine in the R6/2 Huntington's disease mouse involves mechanisms other than the inhibition of tissue transglutaminase. Neurobiol. Aging. 2006;27:871–879. doi: 10.1016/j.neurobiolaging.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 166.Goldstone SD, Fragonas JC, Jeitner TM, et al. Transcription factors as targets for oxidative signalling during lymphocyte activation. Biochim. Biophys. Acta. 1995;1263:114–122. doi: 10.1016/0167-4781(95)00088-x. [DOI] [PubMed] [Google Scholar]
- 167.Medvedev A, Saunders NA, Matsuura H, et al. Regulation of the transglutaminase I gene. Identification of DNA elements involved in its transcriptional control in tracheobronchial epithelial cells. J. Biol. Chem. 1999;274:3887–3896. doi: 10.1074/jbc.274.6.3887. [DOI] [PubMed] [Google Scholar]
- 168.Lorand L, Rule NG, Ong HH, et al. Amine specificity in transpeptidation. Inhibition of fibrin cross-linking. Biochemistry. 1968;7:1214–1223. doi: 10.1021/bi00843a043. [DOI] [PubMed] [Google Scholar]
- 169.Curtis CG, Brown KL, Credo RB, et al. Calcium-dependent unmasking of active center cysteine during activation of fibrin stabilizing factor. Biochemistry. 1974;13:3774–3780. doi: 10.1021/bi00715a024. [DOI] [PubMed] [Google Scholar]
- 170.Gross M, Whetzel NK, Folk JE. Alkyl isocyanates as active site-directed inactivators of guinea pig liver transglutaminase. J. Biol. Chem. 1975;250:7693–7699. [PubMed] [Google Scholar]
- 171.Lee KN, Fésüs L, Yancey ST, et al. Development of selective inhibitors of transglutaminase. Phenylthiourea derivatives. J. Biol. Chem. 1985;260:14689–14694. [PubMed] [Google Scholar]
- 172.Lee KN, Chung SI, Girard JE, et al. Evaluation of phenylthiourea derivatives as inhibitors of transglutaminase-catalyzed reaction in Chinese hamster ovary cells. Biochim. Biophys. Acta. 1988;972:120–130. doi: 10.1016/0167-4889(88)90110-3. [DOI] [PubMed] [Google Scholar]
- 173.Auger M, McDermott AE, Robinson V, et al. Solid-state carbon-13 NMR study of a transglutaminase-inhibitor adduct. Biochemistry. 1993;32:3930–3934. doi: 10.1021/bi00066a012. [DOI] [PubMed] [Google Scholar]
- 174.Choi K, Siegel M, Piper JL, et al. Chemistry and biology of dihydroisoxazole derivatives: selective inhibitors of human transglutaminase 2. Chem. Biol. 2005;12:469–475. doi: 10.1016/j.chembiol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 175.Watts RE, Siegel M, Khosla C. Structure-activity relationship analysis of the selective inhibition of transglutaminase 2 by dihydroisoxazoles. J. Med. Chem. 2009;49:7493–7501. doi: 10.1021/jm060839a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Killackey JJ, Bonaventura BJ, Castelhano AL, et al. A new class of mechanism-based inhibitors of transglutaminase enzymes inhibits the formation of cross-linked envelopes by human malignant keratinocytes. Mol. Pharmacol. 1989;35:701–706. [PubMed] [Google Scholar]
- 177.Pliura DH, Bonaventura BJ, Pauls HW, et al. Irreversible inhibition of transglutaminases by sulfonium methylketones: optimization of specificity and potency with Ω-aminoacyl spacers. Enzyme Inhib. 1992;6:181–194. doi: 10.3109/14756369209020168. [DOI] [PubMed] [Google Scholar]
- 178.Freund KF, Doshi KP, Gaul SL, et al. Transglutaminase inhibition by 2-[(2-oxopropyl)thio]imidazolium derivatives: mechanism of factor XIIIa inactivation. Biochemistry. 1994;33:10109–10119. doi: 10.1021/bi00199a039. [DOI] [PubMed] [Google Scholar]
- 179.Marrano C, de Macédo P, Keillor JW. Evaluation of novel dipeptide-bound α,β-unsaturated amides and epoxides as irreversible inhibitors of guinea pig liver transglutaminase. Bioorgan. Med. Chem. 2001;9:1923–1928. doi: 10.1016/s0968-0896(01)00101-8. [DOI] [PubMed] [Google Scholar]
- 180.Marrano C, de Macédo P, Gagnon P, et al. Synthesis and evaluation of novel dipeptide-bound 1,2,4-thiadiazoles as irreversible inhibitors of guinea pig liver transglutaminase. Bioorgan. Med. Chem. 2001;9:3231–3241. doi: 10.1016/s0968-0896(01)00228-0. [DOI] [PubMed] [Google Scholar]
- 181.de Macédo P, Marrano C, Keillor JW. Synthesis of dipeptide-bound epoxides and α,β-unsaturated amides as potential irreversible transglutaminase inhibitors. Bioorgan. Med. Chem. 2002;10:355–360. doi: 10.1016/s0968-0896(01)00292-9. [DOI] [PubMed] [Google Scholar]
- 182.Case A, Ni J, Yeh LA, et al. Development of a mechanism-based assay for tissue transglutaminase – results of a high-throughput screen and discovery of inhibitors. Anal. Biochem. 2005;338:237–244. doi: 10.1016/j.ab.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 183.Case A, Stein RL. Kinetic analysis of the interaction of tissue transglutaminase with a nonpeptide slow-binding inhibitor. Biochemistry. 2007;46:1106–1115. doi: 10.1021/bi061787u. [DOI] [PubMed] [Google Scholar]
- 184.Duval E, Case A, Stein RL, et al. Structure–activity relationship study of novel tissue transglutaminase inhibitors. Bioorg. Med. Lett. 2005;15:185–189. doi: 10.1016/j.bmcl.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 185.Lai T-S, Liu Y, Tucker T, et al. Identification of chemical inhibitors to human tissue transglutaminase by screening existing drug libraries. Chem. Biol. 2008;15:969–978. doi: 10.1016/j.chembiol.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sohn J, Kim TI, Yoon YH, et al. Novel transglutaminase inhibitors reverse the inflammation of allergic conjunctivitis. J. Clin. Invest. 2003;111:121–128. doi: 10.1172/JCI15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.McConoughey SJ, Chavez JC, Cooper AJL, et al. Release of transcriptional repression created by transglutaminase is protective in mutant huntingtin expressing neurons.. Presented at: Annual Meeting of the Society for Neuroscience.; San Diego, CA, USA. 3−7 November 2007. [Google Scholar]
- 188.McConoughey SJ, Nietsetskaya Z, Karpisheva K, et al. Transglutaminase 2 inhibition ameliorates transcriptional dysregulation in Huntington disease.. Presented at: Annual Meeting of the Society for Neuroscience.; Washington, DC, USA. 15−19 November 2008. [Google Scholar]
- 189.Gines S, Seong IS, Fossale E, et al. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington's disease knock-in mice. Hum. Mol. Gentet. 2003;12:497–508. doi: 10.1093/hmg/ddg046. [DOI] [PubMed] [Google Scholar]
- 190■.Yuan L, Choi K, Khosla C. Tissue transglutaminase 2 inhibition promotes cell death and chemosensitivity in glioblastomas. Mol. Cancer Ther. 2005;4:1293–1302. doi: 10.1158/1535-7163.MCT-04-0328. [■ of interestImportant demonstration of the possibility of using TG inhibitors as therapeutics.] [DOI] [PubMed] [Google Scholar]
- 191.Yuan L, Siegel M, Choi K, et al. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthoptic glioblastomas to chemotherapy. Oncogene. 2007;26:2563–2573. doi: 10.1038/sj.onc.1210048. [DOI] [PubMed] [Google Scholar]