Abstract
Endothelial cell (EC) loss and subsequent angiogenesis occurs over the first week after spinal cord injury (SCI). To identify molecular mechanisms that could be targeted with intravenous (i.v.) treatments we determined whether transmembrane A Disintegrin And Metalloprotease (ADAM) proteins are expressed in ECs of the injured spinal cord. ADAMs bind to integrins which are important for EC survival and angiogenesis. Female adult C57Bl/6 mice with a spinal cord contusion had progressively more ADAM8 (CD156) immunostaining in blood vessels and individual ECs between 1 and 28 days following injury. Uninjured spinal cords had little ADAM8 staining. The increase in ADAM8 mRNA and protein was confirmed in spinal cord lysates, and ADAM8 mRNA was present in FACS-enriched ECs. ADAM8 co-localized extensively and exclusively with the EC marker PECAM and also with i.v. injected lectins. I.v. injected isolectin B4 (IB4) labels a subpopulation of blood vessels at and within the injury epicenter 3-7 days after injury, coincident with angiogenesis. Both ADAM8 and the proliferation marker Ki-67 were present in IB4-positive microvessels. ADAM8-positive proliferating cells were seen at the leading end of IB4-positive blood vessels. Angiogenesis was confirmed by BrdU incorporation, binding of i.v. injected nucleolin antibodies, and MT1-MMP immunostaining in a subset of blood vessels. These data suggest that ADAM8 is vascular-selective and plays a role in proliferation and/or migration of ECs during angiogenesis following SCI.
Keywords: angiogenesis, blood vessel, contusion, disintegrin, metalloprotease, neovascularization, nucleolin, proliferation
Introduction
SCI causes immediate tissue loss as well as rapidly progressing secondary degeneration over the first two weeks (Hall and Springer, 2004; Hagg and Oudega, 2006; Bramlett and Dietrich, 2007; Donnelly and Popovich, 2008). In rodents, EC dysfunction and cell death occurs over the first post-injury day (Loy et al., 2002; Casella et al., 2002, 2006; Benton et al., 2008a) most likely leading to ischemia, edema and initiation of detrimental infiltration of leukocytes. It is, therefore, conceivable that i.v. treatments that target the ECs to reduce their dysfunction and death would be neuroprotective. I.v. infusion is readily performed in most medical facilities and is therefore a good route to deliver treatments soon after the injury. The interaction between extracellular matrix molecules and integrin receptors is important for attachment and survival of cells, including ECs (Hynes, 1992, 2002; Ruoslahti and Engvall, 1997; Giancotti and Ruoslathi, 1999). EC attachment is disrupted early after SCI in mice, rats and primates (Goodman et al 1979; Koyanagi et al.,. 1993; Benton et al., 2008a). We are interested to find integrin-interacting proteins that would be present in the EC surface facing the blood vessel lumen after injury as they would be accessible to manipulation by i.v. agents (e.g., Murphy et al., 2008). After the acute loss of ECs, some blood vessels at the injury epicenter apparently undergo angiogenesis between 3 and 7 days following injury (Loy et al., 2002; Casella et al., 2002; Whetstone et al., 2003; Benton et al., 2008a). However, EC proliferation and migration after SCI remains to be shown, and it is still unclear whether angiogenesis is beneficial or detrimental. We are interested to identify molecules that would specifically be expressed in angiogenic ECs as this would facilitate investigation of mechanism(s) that could be targeted with i.v. agents. Integrins, notably αvβ3 integrin, are involved in EC survival and angiogenesis during development and in tumor growth (Brooks et al, 1994a,b). We have reported the vascular-specific increase in expression of α1β1 integrin in the spinal cord after a contusion in adult rats (Baker and Hagg, 2007).
ADAMs are transmembrane proteins whose disintegrin domain binds integrins (Primakoff and Myles, 2000; Evans, 2001; White, 2003; Blobel, 2005; Huovila et al.2005; Yang et al., 2006). Most ADAMs also have an active metalloprotease domain and they are mainly responsible for activating cleavage-dependent transmembrane proteins. Among such proteins are Notch, Tie1, and TNFα, which are involved in angiogenesis (van Hinsbergh and Koolwijk, 2008; Sainson et al., 2008a,b) and ICAM and TNFα, involved in leukocyte migration into tissues (Worthylake and Burridge, 2001; Tsakadze et al., 2006; Bell et al., 2007; Chandrasekharan et al., 2007). The dual integrin-binding and metalloprotease activities of ADAMs put them in a unique position to orchestrate biological processes involved in secondary degeneration or repair after SCI. ADAM17 has been found in ECs of the normal CNS (Goddard et al., 2001). ADAM15 is present in developing vasculature and plays a role in pathological angiogenesis in the eye (Horiuchi et al., 2003) and ADAM8 and 15 have been detected in angiogenic tumor blood vessels (Wildeboer et al., 2006). ADAM10, 17 and 19 null mice have defects in cardiovascular development, suggesting that these ADAMs might also affect angiogenesis (Blobel, 2005). ADAM11, 19 and 22 have been found in the uninjured spinal cord, but not in blood vessels (Kurisaki et al., 1998; Sagane et al. 1999; Rybnikova et al., 2002; Sagane et al., 2005). Nothing is known about ADAM expression after SCI. A preliminary screening of commercially available antibodies identified ADAM8 in blood vessels.
Here, we determined whether ADAM8 was present in blood vessels and/or showed selective changes in blood vessels after SCI in adult mice. We also determined whether such changes were related to the angiogenesis detected at the epicenter after SCI.
Materials & Methods
Experimental overview and animals
All animal experiments were conducted in accordance with the National Institutes of Health's guidelines on the care and use of laboratory animals and were approved by the Animal Care Committee of the University of Louisville. A total of 78 young adult female C57Bl/6 mice (Jackson Laboratory, Bar Harbor, ME; 18-20 g) were used. To assess ADAM8 immunostaining in the spinal cord, uninjured mice and mice 1, 3, 7, 14, or 28 days following a contusion at thoracic level 9 were used (n = 4 each). To determine changes in ADAM8 protein levels, spinal cords from 3 uninjured mice and 3 mice with a 7 day injury were processed for Western blotting. In addition, uninjured, and mice 1 and 3 days post-injury (n = 2 each) were used to extract RNA for analysis of ADAM8 mRNA in whole spinal cord homogenate. Thirty-six additional mice were contused and 3 days later injected i.v. with FITC-conjugated Lycospersicon esculentum agglutinin (LEA) lectin to FACS-purify their microvessels, containing predominantly ECs, to detect ADAM8 mRNA. To assess angiogenic markers in the spinal cord, mice were contused and 2 days later were injected with 5-bromo2′-deoxy-uridine (BrdU; n = 3) and processed for histology the following day. Other mice with a contusion received an i.v. injection of antibodies against nucleolin (n = 3) 1 hour prior to processing 3 days post-injury.
Spinal cord contusion and intravenous injections
Mice were anesthetized with an intraperitoneal injection of Avertin (0.4 mg/g body weight 2,2,2-tribromoethanol in 0.2 ml of 1.25% (v/v) 2-methyl-2-butanol in saline; Sigma-Aldrich, St. Louis, MO; Papaioannou and Fox, 1993). Their backs were shaved and cleansed with betadine, and Lacriblube opthalamic ointment (Allergen, Irvine, CA) was placed on their eyes to prevent drying. In addition, the mice received an intramuscular injection of 50 mg/kg gentamicin sulfate antibiotic (Boehringer Ingelheim, St. Joseph, MO) to prevent infection and a 2 cc bolus of saline, administered subcutaneously. After a laminectomy at spinal cord level T9, a moderate spinal cord contusion was delivered through the intact dura with the IH impactor (Infinite Horizons Inc., Lexington, KY) set at a force of 50 kdyn (400-700 μm displacement). After contusion, the muscle and skin were sutured in layers and Bacitracin zinc antibiotic ointment (Altana Inc., Melville, NY) was applied to the incision area. Mice were housed 4 per cage and recovered on a 37°C heating pad overnight. Post-operative analgesia was achieved by subcutaneous injections of buprenorphine (Bupronex; 0.075 mg/kg) every 12 hours up to 72 hours. Their bladders were expressed manually twice daily until onset of reflexive voiding (7-10 days post-injury).
To label perfused blood vessels containing ECs, anesthetized mice received an injection into the jugular vein of 100 μg in 100 μl of FITC-conjugated Griffonia simplicifolia isolectin B4 (IB4, Sigma) or FITC-LEA (Sigma), 15-20 minutes before euthanasia. IB4 was only injected in mice that were processed at 3, 7 and 14 days post-injury. IB4 binds a subset of ECs within the lesion epicenter at these times when angiogenesis occurs and is absent at 1 and 28 days (Benton et al., 2008a). FITC-LEA labels most perfused blood vessels in the spinal cord (Yamauchi et al., 2004) and was used for FACS purification of ECs 3 days after injury. Nucleolin antibodies (rabbit polyclonal, 150 μg in 100 μl; a gift from Dr. Ruoslahti) were injected into one jugular vein and allowed to circulate for 1 hour prior to euthanasia. They bind to angiogenic blood vessels in tumors implanted in mice (Christian et al., 2003). To label proliferating cells, BrdU (B5002; Sigma; dissolved in Tris-buffered saline or TBS, pH 7.4) was injected intraperitoneally (60 mg/kg) at 48 and 60 h after SCI and the mice were euthanized at 72 h.
Histological procedures
For histological analyses, all mice were deeply anesthetized and transcardially perfused with ice-cold 0.1M phosphate buffer and fresh, non-fixed tissue was collected. Their spinal cords were dissected and a 10 mm long segment spanning the injury epicenter was cyroprotected in 30% sucrose for 48h at 4°C. Multiple spinal cords were cast flat on the bottom of a single cutting block with their epicenters aligned. For the time-course experiments, at least one mouse at each time point was represented in a block. The spinal cord blocks were then sectioned longitudinally at 20 μm on a cryostat. Sections were thaw-mounted onto charged microscope slides (#12-550-15, Fisher, Pittsburgh, PA) and stored at -20°C. For immunohistochemical processing, slides were warmed at 37°C for 20 min, the mounting matrix removed, and the tissue post-fixed in ice-cold methanol for 10 min. Non-specific binding in the sections was blocked with 0.5% BSA and 10% normal donkey serum in 0.1M TBS, containing 0.3% TritonX-100 (Sigma), for 1 h at room temperature. Sections were immunostained using primary antibodies (see below) diluted in 0.1M TBS containing 0.3% TritonX-100, 0.5% BSA and 5% normal donkey serum overnight at 4°C. For double immunofluorescence staining, sections were incubated with two primary antibodies made in different species. Next, sections were incubated for 1 h in secondary antibodies conjugated with Alexa Fluor 594 (Red, 1:300; Molecular Probes, Eugene, OR) or AMCA (Blue; 1:100; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted in TBS. In between steps, sections were rinsed 3 × 5 min with TBS. Finally, sections were dried and cover-slipped with antifade Gel/Mount aqueous mounting media (SouthernBiotech, Birmingham, AL).
Primary antibodies and dilutions used are shown in Table 1. ADAM8 was identified with a polyclonal rabbit antibody made against a synthetic peptide representing amino acids 795-824 of the end of the cytoplasmic C-terminus of human ADAM8 (PGPAEGAVGPKVALKPPIQRKQGAGAPTAP; Chemicon/Millipore, Temecula, CA) and was characterized in this study. ECs were identified with a monoclonal rat anti-PECAM-1 antibody (clone MEC13.3; BD Pharmingen, Franklin Lakes, NJ) made against cell membrane fractions from 129/Sv mouse-derived polyoma middle T transformed endothelial cell tEnd.1. The specificity was shown by immunodepletion analysis with PECAM and exclusive labeling of vascular endothelium in vitro and in vivo (Vecchi et al., 1994), also in the normal and injured adult mouse spinal cord (Hsu et al., 2006; Benton et al., 2008). Proliferating cells were identified by using a polyclonal rabbit Ki-67 antibody (Novocastra Laboratories, Newcastle upon Tyne, United Kingdom). The antibody is against a recombinant protein containing the so-called Ki-67 motif (TPKEKAQALEDLAGFKELFQTP; Gerdes et al., 1991; Schluter et al., 1993) and reacts only with the expected 345 and 395 kDa bands in Western blots. It gives the same pattern of staining as other Ki-67 antibodies in tumors (Lindboe and Torp, 2002). This antibody recognizes only proliferating cells, including endothelial cells, in the adult mouse brain and spinal cord (Baker et al., 2006; Quinones-Hinojosa et al., 2006; Seki et al., 2007; Benton et al., 2008). Injected BrdU incorporated in nuclei of proliferating cells was identified by using a monoclonal mouse antibody (clone BU-1; Chemicon/Millipore, Temecula, CA). This antibody specifically binds to BrdU, as defined by competition ELISA experiments, and does not cross-react with any endogenous cellular components such as thymidine or uridine (Gonchoroff et al., 1985). Tips of migrating cells were detected by an MT1-MMP polyclonal rabbit antibody (Chemicon/Millipore) made against a peptide overlapping with the hemopexin-like domain of human MT1-MMP (CDGNFDTVAMLRGEM; Rathke-Hartlieb S. et al., 2000). It shows the expected 50-60 kDa molecular weight bands in Western blots of CNS plasma membrane preparations from mice compared to MT1-MMP transfected cells (Rathke-Hartlieb S. et al., 2000) and recognizes MT1-MMP in Western blots of mouse retina/choroid tissues (Yu et al., 2008).
Table 1. Primary Antibodies used.
| Antigen | Immunogen | Manufacturer, species, type, catalog number | Dilution used |
|---|---|---|---|
| ADAM8 | last 30 amino acids of human C-terminus | Chemicon/Millipore, affinity-purified polyclonal rabbit IgG, AB19017 | 1:300 |
| PECAM or CD31 | mouse endothelial cell line tEnd.1 | BD Pharmingen, affinity-purified monoclonal rat IgG, 550274 | 1:1,000 |
| Ki-67 | recombinant protein containing the so-called Ki-67 motif | Novocastra Laboratories, polyclonal rabbit IgG, NCL-Ki67p | 1:500 |
| BrdU | BrdU | Chemicon/Millipore, culture supernatant monoclonal mouse IgG, MAB3510 | 1:30,000 |
| MT1-MMP or MMP14 | human hemopexin-like domain peptide | Chemicon/Millipore, affinity-purified polyclonal rabbit IgG, AB8104 | 1:200 |
The immunization antigens and specificity of the antibodies are described in Materials and Methods.
As controls, the primary antibodies were replaced by a non-immune IgG of the same species, which gave no staining. To determine the working dilutions, the ADAM8 antibody was tested in serial dilutions between 1:100 and 1:1000 and all other ADAM8 staining was done at 1:300. For double BrdU-platelet-derived EC adhesion molecule (PECAM) immunostaining, spinal cord sections were first incubated with antibodies against PECAM overnight at 4°C followed by 1 h in Alexa 594-conjugated secondary antibodies (1:300; Molecular Probes). After 3 × 10 min washing with TBS, PECAM-immunostained sections were fixed in 4% paraformaldehyde for 15 minutes and then processed for BrdU staining according to a modified protocol (Kuhn et al., 1996). Sections were incubated in 50% formamide in 2× standard saline citrate (SSC) at 65° C for 45 min. After rinsing in 2× SSC for 5 min, sections were incubated at 2N HCl at 37° C for 30 min, neutralized in 0.1M boric acid (pH = 8.5) for 10 min and washed in 0.1M TBS (3 × 5 min). Sections were quenched in 1% hydrogen peroxide in TBS for 30 min and washed in TBS with 0.3% TritonX-100 (2 × 10 min). Afterwards, sections were immunostained as described above using primary antibodies against BrdU and secondary antibodies conjugated with Alexa 488 (Green; 1:300; Molecular Probes). We were unable to perform BrdU/ADAM8 double-labeling due to technical difficulties including the degradation of ADAM8 epitopes during the denaturing step necessary for the BrdU staining.
Western blotting
To confirm that the ADAM8 antibodies were specific and that increased ADAM8 immunostaining reflected protein increases, mice were transcardially perfused with cold PBS and spinal cord tissue (4 mm rostral and caudal to the injury epicenter) was quickly dissected and homogenized in PBS on ice. After centrifugation, pellets were lysed in the buffer containing 50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 nM phenylmethylsulfonyl fluoride (PMSF), 1 μg/ml aprotinin, leupeptin, and pepstatin, 1 mM Na3VO4, and 1 mM NaF (Sigma) on ice for 30 min. After centrifugation (12,000 × g, 10 min), supernatants were collected and protein concentrations measured with a Lowry protein assay kit according to the manufacturer's instructions (P5656, Sigma). Individual lysates were aliquoted and stored at -80°C until further use. Proteins were separated with 7% SDS-PAGE gels (150V, 50 min, room temperature) and transferred to 0.2-μm PVDF membranes (Bio-Rad, Hercules, CA; 95V, 28 min, 4°C). The membranes were blocked with 5% reconstituted dry milk and incubated with primary (overnight, 4°C) and secondary antibodies (60-90 min, room temperature), and then visualized with an ECL Plus chemiluminescence detection kit (Amersham Biosciences, Piscataway, NJ). The density of ADAM8 bands from uninjured and 7 day contused mice (n=3 each from 3 different experiments) were measured using a Kodak Image Station 4000R with Molecular Imaging Software (Eastman Kodak Company, Rochester, NY).
ADAM8 mRNA detection in whole spinal cord and FACS-purified ECs
Total RNA was isolated from whole spinal cords by sonicating the tissue in TRIZOL® Reagent (Invitrogen, Inc., Carlsbad, CA) and extraction following the manufacturer's protocol. Total RNA (1 μg) was reverse transcribed into cDNA in reactions containing M-MLV RT (100 U, Promega), DTT (5 mM), dNTPs (1 mM each), random hexamers (4 μg), and RNAse inhibitor (20 u, Boehringer Mannheim). PCR was performed with primers for ADAM8 (forward: 5′-CTT ATG GAC ACC AGC AGT AG-3′; reverse: 5′-ATG CTT TGC CTG ATA CAT CGC-3′), or cyclophilin A (forward: 5′-CGG AGA GAA ATT TGA GGA TGA GA-3′; reverse: 5′-AGT CTT GGC AGT GCA GAT AAA A-3′) (Invitrogen). Optimum annealing temperatures, cycle numbers, and RT input were determined by finding a single amplicon at the appropriate molecular weight. All RT and PCR reactions were performed on a PTC-200 gradient thermocycler (MJ Research, Reno, NV). End-point PCR products were separated by native polyacrylamide gel electrophoresis followed by detection using SYBR gold staining (Molecular Probes, Eugene OR).
Spinal cord microvascular ECs (scmECs) were enriched using a previously described method which provides >90% purity as confirmed by FACS and real-time RT-PCR of EC-specific and non-EC mRNAs (Benton et al., 2008b). In short, a 3 mm length of the spinal cords of LEA-injected mice, including the injury epicenter, was quickly dissected, and homogenized in HBSS on ice for 3 minutes. After sequential trituration through 26 and 30 gauge needles, the samples were sieved using a 70 μm mesh tube insert (BD Falcon, Bedford, MA) and purified by FACS (MoFlo system; DAKO, Ft. Collins, CO). Events were triggered by FITC-LEA bound fragments (488 nm excitation, 530/40 nm detection). The brightest events were selected, as only this population contains the microvessels. The purified vessels were pelleted by centrifugation (16,000 × g, 4°C, 15 min) and RNA isolated using the PicoPure™ RNA Isolation Kit (Arcturus Bioscience, Inc., Mountain View, CA) according to the manufacturer's protocol. Total RNA (1 μg) was reverse transcribed with the ReactionReady™ First Strand cDNA Synthesis Kit (SuperArray Bioscience Corp., Frederick, MD) according to the manufacturer's protocol. PCR and analysis were performed as described above.
Image Processing and quantification
All fluorescent photomicrographs were taken using either a Leica DMIRE2 inverted microscope with an attached Spot RTKE digital camera and associated Spot software for Windows (Version 4.0.8; Diagnostic Instruments Inc., Sterling Heights, MI) or a Nikon D-Eclipse C1 laser scanning confocal microscope (Nikon Instruments, Dallas, TX) with EZC1 software. The final figures were produced with Adobe Photoshop® and, when necessary, brightness adjusted to reflect the natural view through the microscope. The area of ADAM8-positive blood vessels between 1 mm rostral and caudal of the middle of the injury epicenter in longitudinal sections was measured using digitized images and Image-Pro Plus software (version 6.2, Media Cybernetics Inc., Bethesda, MD). The threshold method was used to select only positively stained blood vessels. The total area per mouse was measured in every 5th section for approximately 12 sections through the dorso-ventral axis of the spinal cord. The ADAM8-positive area was expressed as a percentage of the total area analyzed. A one-way ANOVA was performed to distinguish potential differences in percentage of ADAM8 immunopositive area in injured mice vs. uninjured, with significance being defined at p < 0.05.
Results
ADAM8 is increased after SCI in adult mice
In the spinal cords of uninjured mice, ADAM8 immunostaining was only seen in a few structures, which appeared to be blood vessels (Fig. 1A). In mice, tissue that is lost after SCI is replaced by a fibroblast-rich cellular mass (Zhang et al., 1996, Kuhn and Wrathall, 1998; Ma et al., 2001; Whetstone et al., 2003). At 1 day following SCI, the injury epicenter appeared essentially devoid of ADAM8 immunostaining (Fig. 1B). ADAM8 staining increased around the injured epicenter as early as 3 days following SCI (Fig. 1C) with progressively more immunoreactive structures appearing within the epicenter at later times (Fig. 1D-F). Much of the ADAM8 immunostaining in the penumbra around the injury center appeared to be associated with blood vessels (see insets in Fig. 1). Quantification of the area of ADAM8 staining showed a significant increase at 7, 14 and 28 days post-injury compared to the uninjured controls (Fig. 1G).
Figure 1. ADAM8 immunostaining increases following SCI in adult mice.
A-F) Shown are horizontal (longitudinal) sections through the center of the spinal cords of uninjured mice (0) and at progressively increasing times after injury. Left is rostral. In uninjured mice (A) very little ADAM8 immunostaining is seen. Positive structures appeared to be blood vessels as shown in the inset. At 1 and 3 days post-injury (B,C), some ADAM8-positive structures can be seen around the epicenter (*), which itself appears devoid of staining. From 7 days onward, progressively more staining appears, particularly in the epicenter (D-F), the site where lost tissue is replaced by a fibroblast- and laminin-rich cell matrix. At all post-injury times ADAM8-positive blood vessels could be seen often times in the penumbra (insets). G) The area occupied by ADAM8-positive structures in horizontal sections throughout the dorso-ventral extent of the spinal cord shows that the increase becomes significantly different from the uninjured controls by 7 days post-injury. Data are means plus standard deviation; n = 4 each.
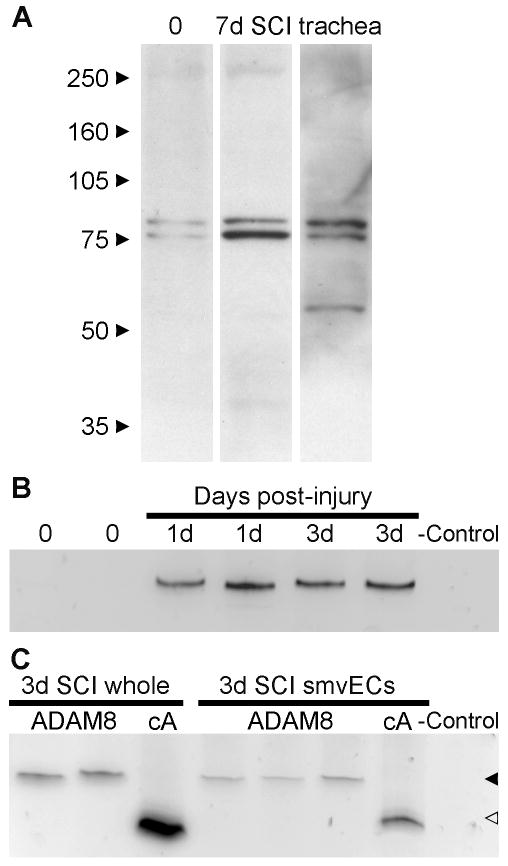
The specificity of the ADAM8 antibody was shown in whole spinal cord extracts obtained from uninjured mice and mice 7 days after SCI (Fig. 2A). Only two bands were seen at around 80 kDa, an Mr that is in agreement with the findings of others (Schlomann et al., 2000, 2002; Kelly et al., 2005) and with the predicted Mr of mature ADAM8 (UniProt KnowledgeBase; www.expasy.org/sprot). The two bands most likely represent different states of post-translational modification. The human ADAM8 C-terminus peptide used to produce the antibody (position 795-824: PGPAEGAVGPKVALKPPIQRKQGAGAPTAP) has homology over 6 consecutive amino acids required for antigenicity (underlined) with the corresponding region in the mouse ADAM8 C-terminus (position 806-826: PGATQGAGEPKVALKVPIQKR) but not in other regions of mouse ADAM8. The predicted rat ADAM8 (position 805-825: PGVTQGAGGSKVALKVPIQKR) has homology for only 5 consecutive amino acids, suggesting that this antibody might not react with rat ADAM8. The immunogen also has such 6 amino acid homology with 18 mouse proteins other than ADAM8, as determined by BLAST search. Only one protein, nuclear factor erythroid derived 2-like 1 (gene Nfe2l1) has a predicted size in the range of 80 kDa (UniProt KnowledgeBase), but it has a nuclear localization. This suggests that the ADAM8 antibody is specific. Trachea, a source of ADAM8 (NCBI Unigene expression profile), also showed blood vessel immunostaining in sections (not shown) and was used as a positive control in the Western blots (Fig. 2A). Tracheal extracts had an additional band at around 60 kDa, which represents an ADAM8 “remnant” seen in many other tissues (Schlomann et al., 2000, 2002; Kelly et al., 2005). The ADAM8-positive bands showed a marked increase 7 days following SCI compared to uninjured (Fig. 2A) in each of the 3 experiments, as measured by densitometry. This suggests that the increased immunostaining in tissue sections represents an increase in protein levels.
Figure 2. ADAM8 mRNA and protein is increased following SCI.
A) Western blots show that of whole spinal cord extract from uninjured (0) and 7 day injured (7d SCI) mice probed with ADAM8 antibody shows two bands around the expected 80 kDa size of mature ADAM8. The doublet probably represents different post-translationally modified forms. The lack of other bands shows the specificity of the antibody. A blot of trachea extract is shown as a positive control. Note the increase of ADAM8 7 days after injury compared to uninjured control, which was seen in 3 different experiments. B) End-point RT-PCR shows the absence of ADAM8 mRNA in 2 uninjured spinal cord extracts (0) and the increased expression in individual mice at both 1 and 3 days post-injury. The bands are at 459 bp, the expected amplicon size for the primer set used. For the negative control, reverse transcriptase was replaced by water. C) RT-PCR of whole spinal cord at 3 days post-injury (3d SCI whole; 2 lanes = 2 mice) was compared to that of FACS-purified spinal microvascular ECs (smvECs) pooled from 12 mice per lane 3 days following SCI. The household gene cyclophilin A (cA) is shown for comparison. Despite the much reduced mRNA levels in the isolated preparation, judged by the reduced cA mRNA, the ADAM8 mRNA remains detectable, suggesting an enrichment of ADAM8 in blood vessels. Solid arrowhead indicates the expected amplicon size of ADAM8 and the open arrowhead the expected amplicon size of cA.
RT-PCR analysis of whole spinal cord tissue demonstrated that levels of ADAM8 mRNA were increased at 1 and 3 days post-injury compared to that of uninjured spinal cord tissue (Fig 2B; 31 PCR cycles). When uninjured spinal cord tissue was analyzed with 40 PCR cycles, only a light band appeared, despite a robust signal of the GFAP mRNA control. This suggests that the increase in immunostaining in the sections and Western blots represents that of ADAM8. It also suggests that expression of ADAM8 mRNA starts during the first day after SCI. To definitively identify ADAM8 expression in blood vessels, FACS-purified scmECs from contused spinal cord were assessed (Benton et al., 2008b). Three days after SCI, scmECs contained ADAM8 mRNA as shown by RT-PCR (Fig. 2C; 30 PCR cycles). Using cyclophilin A as an internal normalizer, we extrapolate that the ADAM8 mRNA levels in the isolated vessels were comparable, if not higher, on a per cell basis than those seen in RNA extracts of whole spinal cord, both 3 days following the contusion.
ADAM8 immunostaining is exclusively present in ECs after SCI
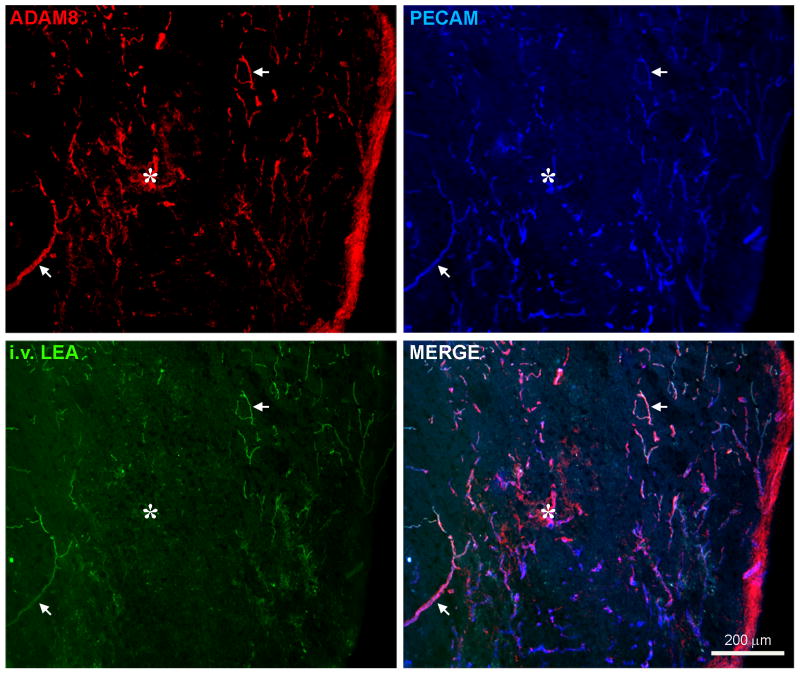
In sections through the injury epicenter, ADAM8 immunostaining was seen in microvascular structures where it co-localized with PECAM, a well-known marker of the luminal side of blood vessels (Fig. 3). ADAM8 also co-localized with i.v. injected FITC-conjugated LEA which binds to essentially all perfused blood vessels (Yamauchi et al., 2004). In the center of the injury epicenter, which is generally devoid of perfused blood vessels, ADAM8 was present in LEA-negative, PECAM-positive cells and structures (asterisk in Fig. 3). These could represent obstructed, degenerating and/or angiogenic blood vessels. We had previously shown that a subset of blood vessels in and immediately around these aberrant tissue areas bound i.v. injected FITC-IB4 (Benton et al., 2008a). Such vessels had dysfunctional tight junctions and had detached from the basement membrane. Here, ADAM8 co-localized with PECAM in IB4-labeled blood vessels within the injury epicenter at 7 days after SCI (Fig. 4A-D). The ADAM8 staining was present in the luminal membrane as well as in a rim around the PECAM staining and luminal bound IB4, most likely representing the EC cytoplasm and/or abluminal membrane facing the basement membrane.
Figure 3. ADAM8 is present in perfused blood vessels and within ECs of the injury epicenter following SCI.
Shown is a horizontal section through the center of the spinal cord at 7 days post-injury. The lateral side is on the right, the rostral side at the top and the central canal would be ∼1/3 from the left. ADAM8 shows extensive co-localization with PECAM, a luminal marker for ECs. ADAM8 is seen not only in penumbral blood vessels (arrows) around the injury epicenter (*), many of which are labeled by i.v. injection of LEA, but also within EC structures within the epicenter, which typically is devoid of perfused blood vessels. Arrows indicate clear examples of perfused blood vessels that also have ADAM8 and PECAM staining.
Figure 4. ADAM8 is present in angiogenic blood vessels following SCI.
A-D) Seven days post-injury, ADAM8 (A) is seen within several blood vessels overlapping with PECAM (B) and i.v. injected IB4 lectin (C) both of which are known to be present on the luminal side of ECs. The arrows in the merged confocal image (D) indicate the abluminal rim of ADAM8 staining, suggesting the interaction with the region between the ECs and the basement membrane. The circular structures on the bottom left are probably cross sections through three blood vessel branches. Note also that more structures are positive for ADAM8 and PECAM than for IB4 suggesting that these are non-perfused blood vessels. E-H) Seven days following injury, ADAM8 immunostaining (E) can be seen associated with a blood vessel that was labeled by i.v. injection of IB4 (F). Ki-67-positive (G), proliferating, ECs (arrows) are seen in the thinner IB4-positive branch as well as in an ADAM8-positive cell emanating from the leading non-perfused end (H).
ADAM8 is present in blood vessels undergoing angiogenesis after SCI
We had previous shown that a sub-set of blood vessels in and around the injury epicenter can be labeled by i.v. IB4 and show signs of activation consistent with angiogenesis (Benton et al., 2008a). ADAM8 is also present in a subset of IB4-positive microvessels that express Ki-67 (Fig. 4E-H), a nuclear protein marker for proliferating cells which is highly upregulated from late G1 phase until and including mitosis (Scholzen and Gerdes, 2000; Kee et al., 2002). Moreover, Ki-67 can also be seen in ADAM8-positive cells at the leading ends of capillaries and ADAM8-positive cells with leading processes can be seen extending beyond the perfused part of the blood vessels. This suggests that ADAM8 is present in proliferating and migrating ECs and might play a role in angiogenesis. Previously, the existence of angiogenesis has only been inferred from data showing an increase in the number of blood vessels. Here, we added several pieces of evidence for the existence of angiogenesis after SCI. First, we injected BrdU over the third day following SCI. BrdU is incorporated into the nucleus during the S-phase of proliferation and is widely used as a mitotic marker. Within and immediately around the injury epicenter, many PECAM-positive ECs had BrdU-positive nuclei (Fig. 5A-C). We could not confirm that ADAM8 was co-localized with BrdU, as the BrdU staining procedure was not compatible with ADAM8 staining. Blood vessels at the injury epicenter labeled by i.v. injection of LEA also bound i.v. injected antibodies to nucleolin (Fig. 5D-F), which is newly expressed by blood vessels undergoing angiogenesis (Christian et al., 2003). In addition, PECAM-positive structures often had patches of MT1-MMP staining (Fig. 5G-I), a marker for the leading end of migrating ECs during angiogenesis in other systems (van Hinsbergh and Koolwijk, 2008). In fact, many of these patches were located at the tips of the blood vessels. These results suggest that angiogenesis occurs after SCI in a similar manner as in other systems, with ECs expressing the same angiogenic markers.
Figure 5. Angiogenic markers are present following SCI.
A-C) To label proliferating cells, BrdU was injected systemically over the last 24 hours up to 3 days post-injury. BrdU-positive nuclei can be seen throughout a small region of the spinal cord. Some of the BrdU-positive nuclei co-localize with PECAM-positive ECs (B) as is evident in the merged image (C). D-F) Some perfused blood vessels, identified by i.v. injected LEA (D), can also be labeled by i.v. injection of antibodies against nucleolin (E), a marker for angiogenic blood vessels in tumors, as is evident from the merged confocal image (F). G-I) Some tips of PECAM-positive blood vessels (G) contain MT1-MMP (arrows in H), a marker for migrating ECs, as shown in the merged confocal image (I).
Discussion
The current data suggest that ADAM8 is increased only in ECs and plays a role in angiogenesis following SCI. We also provide additional evidence for the existence of angiogenesis following SCI, using proliferation markers such as BrdU, Ki-67 and i.v. nucleolin antibodies, as well as the EC migration marker MT1-MMP. This study also reinforces the utility of using i.v. injection of lectins (Benton et al., 2008a) and antibodies, and their combination with FACS (Benton et al., 2008b), in the investigation of vascular responses following SCI.
ADAM8 expression in the uninjured spinal cord is very low. Immunostaining was observed in only a few blood vessels, perhaps explaining why ADAM8 was not detected by RT-PCR in extracts of whole spinal cord from uninjured mice. This finding is in general agreement with a previous report where Western blots and RT-PCR of spinal cord tissue showed very little ADAM8 in wild-type mice (Schlomann et al., 2000). However, increases in ADAM8 mRNA and immunostaining occur in the wobbler mice CNS in association with ongoing neurodegeneration (Schlomann et al. 2000). Those increases were also seen in the spinal cord, particularly in reactive astrocytes and microglia, but no mention was made of blood vessels. The presence of ADAM8 in angiogenic blood vessels is consistent with its presence in the vasculature of some human primary brain tumors (Wildeboer et al., 2006). Our finding that ADAM8 is exclusively present on ECs following SCI suggests that the pathophysiological processes are different between acute SCI and chronic wobbler degeneration, where a vascular response might not occur. However, some of the underlying mechanisms might also be similar. For example, in the wobbler mouse, TNFα seems to be related to the increases in ADAM8 and TNFα can induce ADAM8 expression. TNFα also plays an important role following SCI to regulate the inflammatory response and cellular damage (Sharma et al., 2003). ADAM8 could perform the cleavage-dependent activation TNFα (Naus et al., 2006) raising the possibility of a TNFα-ADAM8 feed-forward mechanism. In summary, it is clear that ADAM8 plays a role in the nervous system during pathological events. This is also suggested by the findings that ADAM8 null mice do not have an overt phenotype (Kelly et al., 2005).
The role of ADAM8 in the vascular responses following SCI remains to be investigated. Most blood vessels within the epicenter die over the first post-injury day in both rats and mice (Loy et al., 2002, Casella et al., 2002, 2006; Benton et al., 2008a). Here, ADAM8 mRNA was increased after 1 day but ADAM8 immunostaining was not seen at the core of the injury. This suggests that ADAM8 does not play a role during the acute phase of the injury, i.e., not in the death or survival responses of the ECs. Such a role was conceivable given the fact that the cytoplasmic tail has a consensus Src homology 3 (SH3)-binding domain which could interact with intracellular signaling molecules affecting cell survival (Thomas and Brugge, 1997; Pawson, 2004). The temporal and spatial expression pattern of ADAM8 and its presence of in leading tips of proliferating and growing vascular ECs suggest that it plays a role in angiogenesis. The increases in ADAM8 mRNA and protein overlapped in time with the reported angiogenic response from day 3 to 14 in mice (Whetstone et al., 2003; Benton et al., 2008a). The finding that ADAM8 was present in ECs within the area of tissue damage in the absence of perfused capillaries also suggests that it is associated with angiogenesis. Our and other's data in support of the existence of angiogenesis following SCI have included increases in measurements of the area of EC markers and blood vessels i.v. labeled with lectins (Loy et al., 2002; Casella et al., 2002; Whetstone et al., 2003; Benton et al., 2008a). Intravascular labeling with IB4 was associated with blood vessels undergoing remodeling, including loss of tight junctions and detachment from the basement membrane (Benton et al., 2008a). Here, more direct evidence for the existence of angiogenesis in and around the injury epicenter was the incorporation of BrdU in PECAM-positive ECs in the injury epicenter. Cell surface expression of nucleolin is known to be associated with proliferating, angiogenic, blood vessels in tumors and has become a target for cancer drugs (Christian et al., 2003). In the current study, blood vessels that were labeled with i.v. injected antibodies against nucleolin co-localized with intravascular injected LEA around the injury epicenter. Another marker for proliferation, Ki-67, was present in IB4-positive blood vessels. The finding that ADAM8 was also present in IB4-positive blood vessels that contained Ki-67-positive cells, suggests that it is also present in proliferating ECs. Lastly, leading ends of some blood vessels contained MT1-MMP which is important for digestion of extracellular matrix molecules and penetration of leading EC membranes into tissue in other systems (van Hinsberg and Koolwijk, 2008). This supports the idea that ECs migrate into the injured spinal cord tissue from the blood vessels. ADAM8 also seems to play a role in this process as it was present in single ECs at the non-perfused ends of IB4-perfused capillaries.
The role of ADAM8 in the molecular mechanism(s) regulating vascular responses after SCI remain to be determined. It is unclear which integrins and to which cells the disintegrin domain of ADAM8 might bind. One interesting possibility would be the α1β1 integrin, which is also selectively increased in the vasculature following SCI (Baker and Hagg, 2007) and co-localizes with ADAM8 (unpublished data). The finding that a disintegrin peptide has anti-inflammatory properties (Schlüsener, 1998) suggests that this domain has biological activity. The role of the metalloprotease domain of ADAM8 is similarly unclear. However, it might well be involved in the cleavage-dependent activation of TNFα, which has been shown to induce tip cell phenotype in ECs, priming them for angiogenesis (Sainson et al., 2008a).
ADAM8 immunostaining was still greatly increased in vascular structures 28 days following SCI in the epicenter, penumbra and over several mm from the injury site. Overt angiogenesis has ceased by then (Whetstone et al., 2003; Benton et al., 2008a), suggesting that ADAM8 expression does not only occur in angiogenic ECs. Administration of a peptide representing the disintegrin domain of ADAM8 is protective in experimental autoimmune encephalitis, neuritis, and uveitis (Schlüsener, 1998). This raises the possibility that ADAM8 is involved in regulating inflammatory responses after SCI. In fact, leukocyte infiltration occurs over several millimeters beyond the immediate injury epicenter similar to the widespread upregulation of ADAM8. Also, chronic inflammation is seen until at least 6 weeks following SCI in mice (Kigerl et al., 2006). Thus the persistent ADAM8 expression could contribute to the maintenance or resolution of chronic inflammation. Our confocal microscopy data suggest that ADAM8 is present in the luminal EC membrane where it could directly affect leukocyte transmigration. ADAM8 can cleave and activate a number of proteins involved in inflammatory responses, including TNFα, VCAM-1 and fractalkine (Amour et al., 2002; Fourie et al., 2003; Naus et al., 2006; Matsuno et al., 2006). However, it most likely is but one of the molecules regulating inflammation after SCI (Donnelly and Popovich, 2008; Popovich and Longbrake, 2008). ADAM8 appeared to be present in the abluminal membrane where it could also interact with the basement membrane surrounding the blood vessels or with astrocyte endfeet, pericytes and smooth muscle cells in the neurovascular unit. Thus, it is possible that ADAM8 plays a role in the ongoing remodeling of the neurovascular unit and the glial scar seen several weeks following SCI, perhaps similar to MMP2 (Goussev et al., 2003).
While it remains to be determined what specific mechanism(s) ADAM8 affects and whether it is unique amongst other ADAMs, the present data indicate that it most likely plays an important role in vascular responses, including angiogenesis, after SCI.
Acknowledgments
We thank Sheher Sun for technical assistance, Aaron Puckett for animal care, Dr. Erkki Ruoslahti (UC Santa Barbara) for the nucleolin antibody, Dr. Peng Yang for advice on the Western blotting and Dr. Stanley D'Souza for advice on the use of the MT1-MMP marker. We are grateful for the gift of antibodies from Chemicon International. Supported by the Kentucky Spinal Cord and Head Injury Research Trust, NS045734, RR015576, Norton Healthcare, and the Commonwealth of Kentucky Challenge for Excellence (SRW, TH).
References
- Amour A, Knight CG, English WR, Webster A, Slocombe PM, Knauper V, Docherty AJ, Becherer JD, Blobel CP, Murphy G. The enzymatic activity of ADAM8 and ADAM9 is not regulated by TIMPs. FEBS Lett. 2002;524:154–158. doi: 10.1016/s0014-5793(02)03047-8. [DOI] [PubMed] [Google Scholar]
- Baker KA, Hagg T. Developmental and injury-induced expression of alpha1beta1 and alpha6beta1 integrins in the rat spinal cord. Brain Res. 2007;1130:54–66. doi: 10.1016/j.brainres.2006.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KL, Daniels SB, Lennington JB, Lardaro T, Czap A, Notti RQ, Cooper O, Isacson O, Frasca S, Jr, Conover JC. Neuroblast protuberances in the subventricular zone of the regenerative MRL/MpJ mouse. J Comp Neurol. 2006;498:747–761. doi: 10.1002/cne.21090. [DOI] [PubMed] [Google Scholar]
- Bell JH, Herrera AH, Li Y, Walcheck B. Role of ADAM17 in the ectodomain shedding of TNF-alpha and its receptors by neutrophils and macrophages. J Leukoc Biol. 2007;82:173–176. doi: 10.1189/jlb.0307193. [DOI] [PubMed] [Google Scholar]
- Benton RL, Maddie MA, Minnillo DR, Hagg T, Whittemore SR. Griffonia simplicifolia isolectin B4 identifies a specific subpopulation of angiogenic blood vessels following contusive spinal cord injury in the adult mouse. J Comp Neurol. 2008a;507:1031–1052. doi: 10.1002/cne.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton RL, Maddie MA, Worth CA, Mahoney ET, Hagg T, Whittemore SR. Transcriptomic screening of microvascular endothelial cells implicates novel molecular regulators of vascular dysfunction after spinal cord injury. J Cereb Blood Flow Metab. 2008b doi: 10.1038/jcbfm.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994b;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994a;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Casella GT, Bunge MB, Wood PM. Endothelial cell loss is not a major cause of neuronal and glial cell death following contusion injury of the spinal cord. Exp Neurol. 2006;202:8–20. doi: 10.1016/j.expneurol.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Casella GT, Marcillo A, Bunge MB, Wood PM. New vascular tissue rapidly replaces neural parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Exp Neurol. 2002;173:63–76. doi: 10.1006/exnr.2001.7827. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan UM, Siemionow M, Unsal M, Yang L, Poptic E, Bohn J, Ozer K, Zhou Z, Howe PH, Penn M, DiCorleto PE. Tumor necrosis factor alpha (TNF-alpha) receptor-II is required for TNF-alpha-induced leukocyte-endothelial interaction in vivo. Blood. 2007;109:1938–1944. doi: 10.1182/blood-2006-05-020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie AM, Coles F, Moreno V, Karlsson L. Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J Biol Chem. 2003;278:30469–30477. doi: 10.1074/jbc.M213157200. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E, Flad HD. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;138:867–873. [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Goddard DR, Bunning RA, Woodroofe MN. Astrocyte and endothelial cell expression of ADAM 17 (TACE) in adult human CNS. Glia. 2001;34:267–271. doi: 10.1002/glia.1060. [DOI] [PubMed] [Google Scholar]
- Gonchoroff NJ, Greipp PR, Kyle RA, Katzmann JA. A monoclonal antibody reactive with 5-bromo-2-deoxyuridine that does not require DNA denaturation. Cytometry. 1985;6:506–512. doi: 10.1002/cyto.990060604. [DOI] [PubMed] [Google Scholar]
- Goodman JH, Bingham WG, Jr, Hunt WE. Platelet aggregation in experimental spinal cord injury. Ultrastructural observations. Arch Neurol. 1979;36:197–201. doi: 10.1001/archneur.1979.00500400051006. [DOI] [PubMed] [Google Scholar]
- Goussev S, Hsu JY, Lin Y, Tjoa T, Maida N, Werb Z, Noble-Haeusslein LJ. Differential temporal expression of matrix metalloproteinases after spinal cord injury: relationship to revascularization and wound healing. J Neurosurg. 2003;99:188–197. doi: 10.3171/spi.2003.99.2.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006;23:264–280. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K, Weskamp G, Lum L, Hammes HP, Cai H, Brodie TA, Ludwig T, Chiusaroli R, Baron R, Preissner KT, Manova K, Blobel CP. Potential role for ADAM15 in pathological neovascularization in mice. Mol Cell Biol. 2003;23:5614–5624. doi: 10.1128/MCB.23.16.5614-5624.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, Noble-Haeusslein LJ. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 2006;26:9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends Biochem Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. A reevaluation of integrins as regulators of angiogenesis. Nat Med. 2002;8:918–921. doi: 10.1038/nm0902-918. [DOI] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kelly K, Hutchinson G, Nebenius-Oosthuizen D, Smith AJ, Bartsch JW, Horiuchi K, Rittger A, Manova K, Docherty AJ, Blobel CP. Metalloprotease-disintegrin ADAM8: expression analysis and targeted deletion in mice. Dev Dyn. 2005;232:221–231. doi: 10.1002/dvdy.20221. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, McGaughy VM, Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol. 2006;494:578–594. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi I, Tator CH, Lea PJ. Three-dimensional analysis of the vascular system in the rat spinal cord with scanning electron microscopy of vascular corrosion casts. Part 2: Acute spinal cord injury. Neurosurgery. 1993;33:285–291. [PubMed] [Google Scholar]
- Kuhn PL, Wrathall JR. A mouse model of graded contusive spinal cord injury. J Neurotrauma. 1998;15:125–140. doi: 10.1089/neu.1998.15.125. [DOI] [PubMed] [Google Scholar]
- Kurisaki T, Masuda A, Osumi N, Nabeshima Y, Fujisawa-Sehara A. Spatially- and temporally-restricted expression of meltrin alpha (ADAM12) and beta (ADAM19) in mouse embryo. Mech Dev. 1998;73:211–215. doi: 10.1016/s0925-4773(98)00043-4. [DOI] [PubMed] [Google Scholar]
- Lindboe CF, Torp SH. Comparison of Ki-67 equivalent antibodies. J Clin Pathol. 2002;55:467–471. doi: 10.1136/jcp.55.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Crawford CH, Darnall JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002;445:308–324. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- Ma M, Basso DM, Walters P, Stokes BT, Jakeman LB. Behavioral and histological outcomes following graded spinal cord contusion injury in the C57Bl/6 mouse. Exp Neurol. 2001;169:239–254. doi: 10.1006/exnr.2001.7679. [DOI] [PubMed] [Google Scholar]
- Matsuno O, Miyazaki E, Nureki S, Ueno T, Kumamoto T, Higuchi Y. Role of ADAM8 in experimental asthma. Immunol Lett. 2006;102:67–73. doi: 10.1016/j.imlet.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci U S A. 2008;105:9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naus S, Reipschlager S, Wildeboer D, Lichtenthaler SF, Mitterreiter S, Guan Z, Moss ML, Bartsch JW. Identification of candidate substrates for ectodomain shedding by the metalloprotease-disintegrin ADAM8. Biol Chem. 2006;387:337–346. doi: 10.1515/BC.2006.045. [DOI] [PubMed] [Google Scholar]
- Papaioannou VE, Fox JG. Efficacy of tribromoethanol anesthesia in mice. Lab Anim Sci. 1993;43:189–192. [PubMed] [Google Scholar]
- Pawson T. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell. 2004;116:191–203. doi: 10.1016/s0092-8674(03)01077-8. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- Primakoff P, Myles DG. The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–7. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, varez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Rathke-Hartlieb S, Budde P, Ewert S, Schlomann U, Staege MS, Jockusch H, Bartsch JW, Frey J. Elevated expression of membrane type 1 metalloproteinase (MT1-MMP) in reactive astrocytes following neurodegeneration in mouse central nervous system. FEBS Lett. 2000;481:227–234. doi: 10.1016/s0014-5793(00)02011-1. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Engvall E. Integrins and vascular extracellular matrix assembly. J Clin Invest. 1997;99:1149–1152. doi: 10.1172/JCI119269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybnikova E, Karkkainen I, Pelto-Huikko M, Huovila AP. Developmental regulation and neuronal expression of the cellular disintegrin ADAM11 gene in mouse nervous system. Neuroscience. 2002;112:921–934. doi: 10.1016/s0306-4522(02)00124-0. [DOI] [PubMed] [Google Scholar]
- Sagane K, Hayakawa K, Kai J, Hirohashi T, Takahashi E, Miyamoto N, Ino M, Oki T, Yamazaki K, Nagasu T. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 2005;6:33. doi: 10.1186/1471-2202-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagane K, Yamazaki K, Mizui Y, Tanaka I. Cloning and chromosomal mapping of mouse ADAM11, ADAM22 and ADAM23. Gene. 1999;236:79–86. doi: 10.1016/s0378-1119(99)00253-x. [DOI] [PubMed] [Google Scholar]
- Sainson RC, Harris AL. Regulation of angiogenesis by homotypic and heterotypic notch signalling in endothelial cells and pericytes: from basic research to potential therapies. Angiogenesis. 2008b;11:41–51. doi: 10.1007/s10456-008-9098-0. [DOI] [PubMed] [Google Scholar]
- Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, Hughes CC. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008a;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlomann U, Rathke-Hartlieb S, Yamamoto S, Jockusch H, Bartsch JW. Tumor necrosis factor alpha induces a metalloprotease-disintegrin, ADAM8 (CD 156): implications for neuron-glia interactions during neurodegeneration. J Neurosci. 2000;20:7964–7971. doi: 10.1523/JNEUROSCI.20-21-07964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlomann U, Wildeboer D, Webster A, Antropova O, Zeuschner D, Knight CG, Docherty AJ, Lambert M, Skelton L, Jockusch H, Bartsch JW. The metalloprotease disintegrin ADAM8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. J Biol Chem. 2002;277:48210–48219. doi: 10.1074/jbc.M203355200. [DOI] [PubMed] [Google Scholar]
- Schluesener HJ. The disintegrin domain of ADAM 8 enhances protection against rat experimental autoimmune encephalomyelitis, neuritis and uveitis by a polyvalent autoantigen vaccine. J Neuroimmunol. 1998;87:197–202. doi: 10.1016/s0165-5728(98)00080-0. [DOI] [PubMed] [Google Scholar]
- Schluter C, Duchrow M, Wohlenberg C, Becker MH, Key G, Flad HD, Gerdes J. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993;123:513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Seki T, Namba T, Mochizuki H, Onodera M. Clustering, migration, and neurite formation of neural precursor cells in the adult rat hippocampus. J Comp Neurol. 2007;502:275–290. doi: 10.1002/cne.21301. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Winkler T, Stalberg E, Gordh T, Alm P, Westman J. Topical application of TNF-alpha antiserum attenuates spinal cord trauma induced edema formation, microvascular permeability disturbances and cell injury in the rat. Acta Neurochir Suppl. 2003;86:407–413. doi: 10.1007/978-3-7091-0651-8_85. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Tsakadze NL, Sithu SD, Sen U, English WR, Murphy G, D'Souza SE. Tumor necrosis factor-alpha-converting enzyme (TACE/ADAM-17) mediates the ectodomain cleavage of intercellular adhesion molecule-1 (ICAM-1) J Biol Chem. 2006;281:3157–3164. doi: 10.1074/jbc.M510797200. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res. 2008;78:203–212. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- Vecchi A, Garlanda C, Lampugnani MG, Resnati M, Matteucci C, Stoppacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A. Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol. 1994;63:247–254. [PubMed] [Google Scholar]
- Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM. ADAMs: modulators of cell-cell and cell-matrix interactions. Curr Opin Cell Biol. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Wildeboer D, Naus S, my Sang QX, Bartsch JW, Pagenstecher A. Metalloproteinase disintegrins ADAM8 and ADAM19 are highly regulated in human primary brain tumors and their expression levels and activities are associated with invasiveness. J Neuropathol Exp Neurol. 2006;65:516–527. doi: 10.1097/01.jnen.0000229240.51490.d3. [DOI] [PubMed] [Google Scholar]
- Worthylake RA, Burridge K. Leukocyte transendothelial migration: orchestrating the underlying molecular machinery. Curr Opin Cell Biol. 2001;13:569–577. doi: 10.1016/s0955-0674(00)00253-2. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Lin Y, Sharp FR, Noble-Haeusslein LJ. Hemin induces heme oxygenase-1 in spinal cord vasculature and attenuates barrier disruption and neutrophil infiltration in the injured murine spinal cord. J Neurotrauma. 2004;21:1017–1030. doi: 10.1089/0897715041651042. [DOI] [PubMed] [Google Scholar]
- Yang P, Baker KA, Hagg T. A disintegrin and metalloprotease 21 (ADAM21) is associated with neurogenesis and axonal growth in developing and adult CNS. Journal of Comparative Neurology. 2005;490:163–179. doi: 10.1002/cne.20659. [DOI] [PubMed] [Google Scholar]
- Yang P, Baker KA, Hagg T. The ADAMs family: coordinators of nervous system development, plasticity and repair. Prog Neurobiol. 2006;79:73–94. doi: 10.1016/j.pneurobio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Yu HG, Liu X, Kiss S, Connolly E, Gragoudas ES, Michaud NA, Bulgakov OV, Adamian M, DeAngelis MM, Miller JW, Li T, Kim IK. Increased choroidal neovascularization following laser induction in mice lacking lysyl oxidase-like 1. Invest Ophthalmol Vis Sci. 2008;49:2599–2605. doi: 10.1167/iovs.07-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Fujiki M, Guth L, Steward O. Genetic influences on cellular reactions to spinal cord injury: a wound-healing response present in normal mice is impaired in mice carrying a mutation (WldS) that causes delayed Wallerian degeneration. J Comp Neurol. 1996;371:485–495. doi: 10.1002/(SICI)1096-9861(19960729)371:3<485::AID-CNE10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]