Abstract
Mycobacterium bovis bacille Calmette–Guerin (BCG) is one of the great success stories of immunotherapy as a treatment for superficial urothelial carcinoma of the bladder. Despite clinical effectiveness in over 50% of patients, the high incidence of local side effects and presence of nonresponders has led to efforts to improve the therapy. Recent advances have suggested a role for neutrophils and TNF-related apoptosis-inducing ligand (TRAIL) in the antitumor inflammatory response. Cell wall components of mycobacteria alone, lowered doses of BCG, and combination with cytokines have been studied as ways to improve the immune response associated with BCG and/or reduce toxicity. This review will discuss the clinical use of BCG, its proposed mechanism of action, and directions of future research to improve efficacy and decrease side effects.
Keywords: bacillus Calmette, Guerin, BCG, bladder cancer, neutrophil, TRAIL, urothelial carcinoma
Urothelial carcinoma of the bladder is the sixth most common cancer cause of death, and was estimated to be diagnosed in 68,810 people and cause 14,100 deaths in the USA in 2008 [1]. In the USA, bladder cancer is the most expensive malignancy to treat in terms of cost per case from diagnosis to death, and the direct costs of treatment are estimated at US$3.7 billion per year [2]. The cost is disproportionate to its incidence due to the frequent need for follow-up procedures [3]. At initial diagnosis, 20–25% of urothelial carcinoma cases are muscle invasive (stage T2 or higher). The remaining patients have stage Ta (low-grade noninvasive), T1 (invasive into lamina propria), or carcinoma in situ (CIS). These patients are generally treated by resection for diagnosis and local control with subsequent intravesical therapy for elimination of residual tumor and prevention of recurrence.
Bacille Calmette–Guerin (BCG) is the most common intravesical therapy for treating non-muscle-invasive urothelial carcinoma. BCG is a live-attenuated strain of Mycobacterium bovis developed in 1921 as a vaccine for tuberculosis, but the first published reports of its use in the bladder were in 1976 [4]. Advances since that time have lead to improved clinical efficacy and a greater understanding of the immunologic basis for this therapy.
Clinical use of BCG
Intravesical BCG therapy has been demonstrated to reduce the recurrence rate and the risk of progression to muscle-invasive disease in patients with CIS, as well as superficial bladder tumors [5–7]. High-risk patients treated with BCG following transuretheral resection of a bladder tumor (TURBT) have lower cystectomy rates. In addition, some studies have shown a survival advantage for adjuvant BCG when compared with TURBT alone [8]. Comparisons with intravesical chemotherapy used for the same indications have shown BCG to be more effective, but associated with more side effects than mitomycin C, the most commonly used intravesical chemotherapy [9].
Bacille Calmette–Guerin therapy is the gold standard for treatment of urothelial CIS. CIS is more difficult to identify cystoscopically and to resect compared with papillary tumors, and poses a management challenge. Unlike CIS in other organs, urothelial CIS is a high-grade neoplasm that carries a greater than 50% chance of progression to muscle-invasive or metastatic disease at 5 years if untreated [10]. CIS can be present alone or in conjunction with papillary tumors. Current American Urological Association (AUA) guidelines recommend BCG induction as primary therapy for CIS [11]. Response rates vary from 50 to 70% of patients [12], with some late relapses usually within the first 5 years after treatment. Maintenance therapy should also be used to prevent recurrence in patients who respond to induction.
Papillary urothelial tumors are also effectively treated with BCG. TURBT with resection of all visible primary tumor is recommended before beginning BCG therapy. Patients with high-grade T1 papillary tumors, multiple tumors, or with associated CIS are at high risk for recurrence and/or progression to muscle invasion [13]. Despite this risk, BCG is still the preferred initial therapeutic option for high-risk nonmuscle-invasive papillary tumors. Stratification of patients with bladder cancer into low-, intermediate- and high-risk groups for recurrence and progression can be done with tables such as those published by the European Organisation for Research and Treatment of Cancer (EORTC) [14]. Low-risk patients may be successfully managed with intravesical chemotherapy such as mitomycin C only, while patients with high-risk features are best served by a treatment with BCG. Successful initial induction is associated with a recurrence rate of 16–50% [15]. In initial BCG failures, 25–45% can be salvaged with a second induction course [16]. BCG treatment with an induction course only can be used for solitary tumors, but in high-risk patients with multiple papillary tumors or associated CIS, a maintenance course is also recommended.
The most commonly used dosing schedule for BCG consists of six weekly administrations of BCG 2–6weeks following TURBT (induction). Maintenance BCG treatment is superior to an induction course alone for patients with CIS and high-risk superficial tumors [17,18]. Various permutations of maintenance courses have been used, most consisting of three weekly instillations at 3–6 month intervals. Currently at our institution, patients receive three cycles of BCG maintenance (three weekly doses) at 6-month intervals with cystoscopy and cytology at 3-month intervals.
Contraindications to BCG instillation include immunosuppression, history of BCG sepsis, gross hematuria or traumatic catheterization, and active urinary tract infection. Side effects of intravesical BCG are common. Mild (urgency, frequency, low-grade fever and malaise) cystitis occurs in up to 90% of patients [9,19]. More severe reactions are uncommon (<5%), but can include BCG sepsis requiring treatment with long-term antituberculosis medications and even death [20]. Many patients are unable to tolerate BCG; in a large study, 30% of patients discontinued therapy because of side effects [21]. Several reports have suggested that symptoms from BCG are associated with therapeutic effectiveness. Severe symptoms, leukocytosis [22], granulomas [23] and urinary cytokines such as IL-2, -6, -8 and -10, IFN-γ and TNF [24–26], have all shown some correlation with response to BCG in studies – although the cause and effect relationship is disputed [21]. It is clear that induction of an immune response, usually associated with cytokine release and cystitis symptoms, is necessary for the therapeutic effect of BCG.
Mechanism of action of BCG
Bacille Calmette–Guerin induces a massive influx of inflammatory cells and production of cytokines in the bladder mucosa and lumen that leads to an immune response against tumor cells. An intact immune system is a necessary prerequisite to successful BCG therapy. Studies in human patients receiving BCG, as well as in animal models, have provided further elucidation of the mechanism of BCG therapy. When BCG is instilled in the bladder it binds to urothelial cells via fibronectin attachment to fibronectin attachment protein (FAP) on BCG [27,28]. BCG is internalized by both urothelial cells and inflammatory cells, such as neutrophils, and triggers an inflammatory cascade of cytokine release and immune cell recruitment. Early responding cells include primarily neutrophils (75%) as well as macrophages [29]. Later in the immune response, CD4+ T cells are prevalent [30]. Debate exists over the role of various inflammatory cell subsets and most subsets have been implicated in the cascade of events that lead to a response.
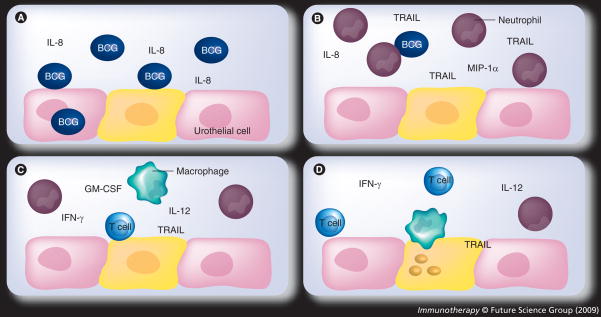
Many studies have examined the cytokines present in the urine of patients treated with BCG and found elevated amounts of IL-1, -2, -6, -8 and -12, TNF, INF-γ and GM-CSF, among others [26,31]. This proinflammatory cytokine profile, especially IL-2, TNF and INF-γ, is a Th1 response. A Th1 cytokine profile, rather than a Th2 profile, is needed for tumor destruction. Studies have been performed looking at specific cytokines found in BCG-induced murine bladder inflammation compared with lipopolysaccharide (LPS)- and TNF-induced inflammation. While levels of many cytokines are altered with BCG, the massive neutrophil infiltration and granuloma formation are two events unique to BCG instillation when compared with the other inflammatory stimuli [32]. Figure 1 is a visual representation of a proposed mechanism for the BCG mechanism of action.
Figure 1. Postulated mechanism of action of bacillus Calmette–Guerin.
(A) BCG infection of urothelial cells leads to the release of cytokines, such as IL-8. (B) Neutrophils compose the early responding cells to BCG; BCG stimulates release of TRAIL in addition to chemotactic factors. (C) Inflammatory effector cells, such as T cells and macrophages, respond to the neutrophil-secreted chemotactic signals. (D) Cytotoxic cells and TRAIL lead to tumor apoptosis.
BCG: Bacille Calmette–Guerin; TRAIL: TNF-related apoptosis-inducing ligand.
Role of TNF-related apoptosis-inducing ligand in the BCG-induced antitumor response
TNF-related apoptosis-inducing ligand (TRAIL) is a member of the TNF family that induces apoptosis in neoplastic cells, but not in normal cells and tissues [33]. TRAIL expression can be induced in many inflammatory cell populations following activation or stimulation with cytokines, especially type I and II interferons [34]. Previous research in our laboratory examined the urinary levels of TRAIL in patients following BCG instillation. BCG responders (>12 months tumor free) had a significantly higher amount of urinary TRAIL than BCG nonresponders [35]. In addition, responders to therapy had increasing amounts of TRAIL in the urine with each subsequent BCG treatment, whereas nonresponders had lower amounts of TRAIL for all treatments. Neutrophils are the primary cells responsible for TRAIL secretion with BCG stimulation. Supernatants of BCG-stimulated neutrophils were able to kill bladder cancer cell lines in vitro in a TRAIL-dependent process [35]. Analysis of urine from patients with urinary tract infections found lower TRAIL levels compared with BCG stimulation, suggesting that the TRAIL response is specific to BCG rather than nonspecific immune activation. Stimulation of peripheral blood neutrophils with various bacterial pathogens showed that BCG was the only bacterial species of those tested able to stimulate TRAIL release [36]. Thus, TRAIL appears to be a contributor to the antineoplastic effect seen with BCG.
Role of neutrophils
Neutrophils are central mediators of innate immunity and recognize bacterial antigens by several mechanisms, including Toll-like receptors (TLRs). Neutrophils are the primary cells present in the urine following BCG instillation and were originally viewed as nonspecific activators of the inflammatory cascade. However, recent demonstration that neutrophils are the primary producers of TRAIL after BCG stimulation suggests a role for neutrophils in the therapeutic effectiveness of BCG in urothelial carcinoma. Indeed, in a murine model of urothelial carcinoma, neutrophils are essential for antitumor responses as their depletion leads to a reduced BCG response [37]. IL-8, an important chemotactic signal for neutrophils, is significantly higher in the urine of patients who respond to BCG compared with nonresponders within 6 h of BCG administration [38,39].
Additional studies from our laboratory revealed that neutrophils possess intracellular stores of TRAIL that are released by stimulation with BCG. The intracellular source of TRAIL appears to be preformed, because neutrophils treated with compounds that inhibit transcription or translation release equal amounts of TRAIL compared with untreated neutrophils when stimulated with BCG [40]. The role of neutrophils in the mechanism of response to BCG therapy appears to be multifaceted. They contribute to the eradication of malignant cells by direct mechanisms, such as secretion of TRAIL. Neutrophils also contribute indirectly to tumor eradication by cytokine production, which amplifies the immune response and leads to the recruitment of effector cells. Further research aimed at identifying the relative contribution of these various mechanisms to the overall success of therapy is warranted.
Improving BCG therapy for urothelial carcinoma
The success of BCG as an immunotherapeutic agent for treating urothelial carcinoma has inspired ways to maintain or improve its therapeutic efficacy, as well as ways to reduce the side-effect profile. Strategies have included administering various inflammatory cytokines in addition to BCG, using lower doses of BCG, and by identifying the mycobacterial components that may be sufficient to induce a therapeutic immune response without leading to severe reactions or infection.
Lower doses of BCG have been studied to reduce side effects while maintaining a therapeutic effect. Initial reports suggested that lower doses may not be as effective, especially in patients with both papillary tumors and CIS [41]. Subsequent studies suggest that low-dose BCG is reasonable in patients with lower-risk tumors [42]. An ongoing clinical trial comparing a third of a dose to a full dose in two different treatment regimens may provide some guidance. A third of the standard dose in patients with intermediate-risk tumors has shown reasonable results that were superior to patients given a sixth of a dose with no difference in toxicity, suggesting a third of a dose is the lowest that should be used [43,44].
Identification of the cytokines that can induce immune responses without the risk of disseminated BCG infection has focused on a number of cytokines – most prominently IFN-α, IL-2, TNF and GM-CSF. Cytokines such as IL-2, IL-12 and IFN-α lead to increased release of IFN-γ from human peripheral blood mononuclear cells alone or in combination with BCG [45]. Experiments showing responses with cytokines provided the rationale for clinical examination of cytokines alone or in combination with BCG for treatment of urothelial carcinoma. However, most clinical trials of single cytokine therapy have not shown improved efficacy [46,47]. The complicated immunologic cascade of BCG may be difficult to replicate even with combination cytokine therapy. Combinations of BCG and cytokines have shown more efficacy; BCG and IFN-α has shown promise for patients who relapse after BCG therapy, as well as BCG-naive patients [48]. Addition of cytokines to BCG may provide benefit for BCG nonresponders or relapsers and studies are ongoing examining combination with other cytokines such as IL-2 and GM-CSF.
The rationale behind cellular component therapy is that live BCG may not be needed to induce the bladder inflammatory cascade and, presumably, the antitumoral effects. Live BCG is responsible for the majority of the serious side effects seen with BCG, such as sepsis. Cellular component therapy could theoretically reduce these complications. Several mycobacterial components have been shown to play specific roles in the immune reaction to BCG. The mycobacterial cell wall has been the best characterized activator of immune response in multiple studies. Fractionation of BCG showed that the cell wall component was responsible for the majority of TRAIL released [40].
Clinical trials of mycobacterial cell wall extract administered intravesically with a similar treatment schedule to BCG showed that the extract had a favorable safety profile with mild side effects comparable with BCG, but no serious side effects such as BCG sepsis. In contrast to whole, viable BCG, the cell wall extract can be given to patients with a disrupted urothelium. Furthermore, administration of the mycobacterial cell wall complex was able to rescue several BCG nonresponders [49]. However, these clinical studies have not been controlled with a BCG arm, preventing a direct comparison of effectiveness. Ongoing clinical trials should provide insight into the value of this therapy. Analysis of the cell wall extract found it contained mycobacterial DNA, and has shown efficacy at inducing apoptosis, as well as leading to clinical responses in in vitro and in vivo models [50]. Further studies (Table 1) are underway to examine this complex in BCG nonresponders, as well as head-to-head comparisons as primary therapy with BCG.
Table 1.
Current US clinical trials involving immunotherapy or BCG.
| Name of trial | Phase | Study compounds | Mechanism of action |
|---|---|---|---|
| Mycobacterial cell wall–DNA Complex in Treatment of BCG-Refractory Patients with Nonmuscle Invasive Bladder Cancer | II, III | Mycobacterial cell wall–DNA complex (Bioniche, Inc.) versus BCG and alone | Mycobacterium phlei cell wall extract with DNA; postulated to work similar to BCG with fewer side effects |
| BCG with or without Gefitinib in Treating Patients with High-risk Bladder Cancer | III | BCG ± gefitinib | EGFR/tyrosine kinase inhibitor |
| Comparative Study of Intravesical BCG in Intermediate and High-Risk Ta, T1 Papillary Carcinoma of the Urinary Bladder | III | Short- versus long-term maintenance; full versus one-third dose | Study aimed at determining ideal dose and maintenance interval for BCG administration |
| Dose-escalation Study of CG0070 for Bladder Cancer after BCG Failure | I | CG0070 (Cell-Genesys) | Conditionally replicating oncolytic adenovirus (in Rb-deficient cells) expressing GM-CSF |
Data obtained from [101].
BCG: Bacillus Calmette–Guerin.
Despite some success with mycobacterial cell wall, other clinical studies have shown decreased efficacy for nonviable BCG [51]. Killed BCG, as well as Toll-like receptor (TLR)2 and TLR4 agonists, stimulate the release of soluble TRAIL from neutrophils at the same level as live BCG in vitro [40]. In addition, murine research has shown that while nonviable BCG does not induce a Th1 response, administration of nonviable BCG following induction with live BCG is sufficient to generate a similar cytokine response to live BCG alone [52]. Further investigation into the clinical effects of various cell components and the use of killed BCG as therapy following initial sensitization with live BCG is needed.
Toll-like receptors are found on innate immune cells and recognize pathogen-associated molecular patterns (PAMPs), such as CpG DNA, dsRNA, and LPS. These PAMPs can be seen in bacteria, viruses and other microbes [53]. TLR ligation induces an inflammatory cascade and influx of effector cells to sites of infection. Mycobacteria activate neutrophils through TLR-dependent and -independent mechanisms [54]. Activation of neutrophils by TLR-dependent mechanisms may be sufficient to generate a robust immune response and antitumor activity. However, various stimuli, such as LPS alone, have failed to induce significant responses. By contrast, CpG DNA, which binds to TLR9, was superior to BCG in an orthotopic murine model of bladder cancer [55]. Thus, TLR may play a role in the antitumor response of BCG, and specific targeting of these receptors has therapeutic potential.
Other components of BCG have been implicated in the elicitation of the antitumor response in the bladder. The FAP on BCG is immunogenic [56] and may serve as a determinant of the immune response in urothelial carcinoma. Immunotherapeutic strategies currently being studied for use in superficial urothelial carcinoma include combination of BCG with gefitinib, an oral EGF receptor (EGFR) kinase blocker with in vitro and in vivo activity in selected cell lines [57]. A selectively replicating adenovirus expressing GM-CSF has also shown in vitro and in vivo activity and a clinical trial is planned [58].
Identification of the patient population most likely to respond to BCG is another frontier for improving BCG therapy. This would allow administration of BCG to those most likely to benefit from the therapy while identifying more appropriate alternatives for those with a high risk of BCG failure. Extensive investigation into immunologic and other parameters has shown several markers with utility in limited studies. A review of the literature in this area from 2003 showed that numerous studies that identified increased urinary cytokine levels, such as IL-2, IL-8 and TNF, as predictors of response to BCG, but none had been translated into routine clinical use [59].
Several recent studies have examined the idea that variations in genes coding for cytokines could be responsible for some portion of the divergent responses seen to BCG administration. One method of variation are single-nucleotide polymorphisms (SNPs), which can lead to variations in cytokine production or function between individuals. Analysis of several cytokines known to be induced by BCG, including IL-2, -6, -10 and IFN-γ, showed that SNPs present in IL-10, TGF-β and IL-4 were associated with progression despite BCG therapy [60]. A specific polymorphism in the gene NRAMP1, which has been implicated in human and murine responses to mycobacteria among other immune stimuli, was found in 2–3% of normal controls and 12% of patients at high risk of recurrence [61]. All of the patients with urothelial carcinoma and this gene polymorphism had rapid (<6 months) recurrence of bladder cancer despite BCG therapy. This association held up in multivariate analysis controlling for other determinants of tumor recurrence. Other polymorphisms have been associated with low risk of recurrence after BCG therapy [62]. Genetic analysis to stratify patients likely to have a response from BCG is far from routine clinical use. However, continued investigation into determinants of host response to BCG could prove fruitful in identifying patients who are likely to benefit from BCG as well as those best served by more aggressive therapy such as early cystectomy.
Conclusion
Bacille Calmette–Guerin for urothelial carcinoma has been the most successful use of immunotherapy to date and represents standard of care for urothelial CIS and superficial bladder tumors. BCG induces a massive influx of cytokines and inflammatory cells into the bladder wall and lumen. Neutrophils are the primary cells involved in the early response and have direct antitumoral activity through secretion of TRAIL. Future research into BCG immunotherapy will focus on improving therapeutic effectiveness while reducing side effects that limit its use in many patients.
Mycobacterial cell wall or other cellular component therapy represents an important avenue for research and clinical trials are underway. In addition, research into optimal cytokine regimens to combine with BCG is ongoing. Patients who do not respond to induction BCG or have contraindications to surgery for recurrent CIS or high-risk superficial disease may benefit the most from addition of cytokines. Cytokines being investigated include GM-CSF and IL-2, in addition to IFN-α. Other research is aimed at determining the most effective dose and maintenance regimen. While in the last 30 years improvements have been made to BCG therapy, the basics of administration remain relatively unchanged – a tribute to the effectiveness of the original therapy. Continuing research is needed to further elaborate the molecular and immunologic basis of BCG therapy and to improve clinical effectiveness while reducing side effects for patients with urothelial carcinoma.
Future perspective
Evolution of the field of BCG immunotherapy will be towards combination therapy and changes in dose and duration based on patient tolerance and tumor characteristics. In the future, patients failing BCG induction will be offered combination therapy with IFN-α as well as other cytokines. Well-defined protocols for patients with different pathology will exist (i.e., a patient with a T1 tumor and CIS will undergo more and longer therapy than a patient with multiple Ta tumors). The effective dose of BCG will be established.
Specific stimulation of an immune response using TLR agonists or mycobacterial cell wall components may be given in patients who have difficulty with serious side effects from BCG in order to replicate the immune response and offer bladder preservation. Compounds administered will be specifically designed to target neutrophils for maximal TRAIL and cytokine release. These agents may be used as maintenance therapies as well as instilled immediately after TURBT where they may add additional benefit to the acute inflammation induced by tumor resection. Tumor and host molecular genetic profiling will identify those patients likely to benefit from BCG treatment and may lead to the development of specific compounds tailored to individual genetic differences. Other unforeseen advances examining the cascade induced by BCG may lead to even more prompt therapy for urothelial carcinoma. The focus will be on replicating and improving the effectiveness of BCG while reducing troubling and harmful side effects.
Executive summary
Clinical use of bacille Calmette–Guerin
Intravesical bacille Calmette–Guerin (BCG) has been shown to reduce recurrence and progression of urothelial carcinoma.
Side effects are common and range from cystitis in approximately 90% of patients to sepsis and death.
Mechanism of action of BCG
BCG elicits a massive influx of inflammatory cells and cytokines that leads to a response against neoplastic cells.
Role of TNF-related apoptosis-inducing ligand
TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in neoplastic cells but not in normal cells; urinary TRAIL levels are increased in BCG responders.
TRAIL is released from neutrophils in a specific manner; TRAIL from patients treated with BCG induces apoptosis in cancer cells.
Role of neutrophils
Neutrophils are the primary cells in the initial response to BCG.
Neutrophil secretion of cytokines and TRAIL is crucial to the antitumor response.
Improving therapy for urothelial carcinoma
Reduced doses of BCG may be as effective as standard dose.
Combination of BCG with cytokines may benefit BCG nonresponders and may be effective as primary therapy.
Neutrophil stimulation by mycobacterial components or Toll-like receptors is a potential therapeutic strategy to reduce systemic effects of BCG.
Molecular approaches have identified specific polymorphisms in cytokine genes, which seem to play a role in the response to BCG.
Acknowledgments
This work was supported by the National Cancer Institute (CA109446).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 3.Konety BR, Joyce GF, Wise M. Bladder and upper tract urothelial cancer. J Urol. 2007;177:1636–1645. doi: 10.1016/j.juro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 4.Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette–Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW, Laudone VP, Badalament RA, et al. Bacillus Calmette–Guerin therapy alters the progression of superficial bladder cancer. J Clin Oncol. 1988;6:1450–1455. doi: 10.1200/JCO.1988.6.9.1450. [DOI] [PubMed] [Google Scholar]
- 6.Pawinski A, Sylvester R, Kurth KH, et al. A combined analysis of European Organization for Research and Treatment of Cancer, and Medical Research Council randomized clinical trials for the prophylactic treatment of stage TaT1 bladder cancer. European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council Working Party on Superficial Bladder Cancer. J Urol. 1996;156:1934–1940. discussion 1940–1941. [PubMed] [Google Scholar]
- 7.Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K. Bacillus Calmette–Guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol. 2005;174:86–91. doi: 10.1097/01.ju.0000162059.64886.1c. discussion 91–92. [DOI] [PubMed] [Google Scholar]
- 8.Herr HW, Schwalb DM, Zhang ZF, et al. Intravesical bacillus Calmette–Guerin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J Clin Oncol. 1995;13:1404–1408. doi: 10.1200/JCO.1995.13.6.1404. [DOI] [PubMed] [Google Scholar]
- 9.Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette–Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 10.Wolf H, Melsen F, Pedersen SE, Nielsen KT. Natural history of carcinoma in situ of the urinary bladder. Scand J Urol Nephrol Suppl. 1994;157:147–151. [PubMed] [Google Scholar]
- 11▪.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. Most recent update of the American Urological Association guidelines for management of superficial bladder cancer. [DOI] [PubMed] [Google Scholar]
- 12.Lamm DL. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992;19:573–580. [PubMed] [Google Scholar]
- 13.Patard J, Moudouni S, Saint F, et al. Tumor progression and survival in patients with T1G3 bladder tumors: multicentric retrospective study comparing 94 patients treated during 17 years. Urology. 2001;58:551–556. doi: 10.1016/s0090-4295(01)01324-3. [DOI] [PubMed] [Google Scholar]
- 14▪.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54:303–314. doi: 10.1016/j.eururo.2008.04.051. European guidelines on superficial bladder cancer including useful in sites for risk stratrification. [DOI] [PubMed] [Google Scholar]
- 15.Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. 1997;158:62–67. doi: 10.1097/00005392-199707000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Haaff EO, Dresner SM, Ratliff TL, Catalona WJ. Two courses of intravesical bacillus Calmette–Guerin for transitional cell carcinoma of the bladder. J Urol. 1986;136:820–824. doi: 10.1016/s0022-5347(17)45091-9. [DOI] [PubMed] [Google Scholar]
- 17.Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette–Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 18.Bohle A, Bock PR. Intravesical bacille Calmette–Guerin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology. 2004;63:682–686. doi: 10.1016/j.urology.2003.11.049. discussion 686–687. [DOI] [PubMed] [Google Scholar]
- 19.Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette–Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992;147:596–600. doi: 10.1016/s0022-5347(17)37316-0. [DOI] [PubMed] [Google Scholar]
- 20.Rawls WH, Lamm DL, Lowe BA, et al. Fatal sepsis following intravesical bacillus Calmette–Guerin administration for bladder cancer. J Urol. 1990;144:1328–1330. doi: 10.1016/s0022-5347(17)39731-8. [DOI] [PubMed] [Google Scholar]
- 21.Sylvester RJ, van der Meijden AP, Oosterlinck W, Hoeltl W, Bono AV. The side effects of bacillus Calmette–Guerin in the treatment of Ta T1 bladder cancer do not predict its efficacy: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. Eur Urol. 2003;44:423–428. doi: 10.1016/s0302-2838(03)00371-3. [DOI] [PubMed] [Google Scholar]
- 22.Saint F, Patard JJ, Irani J, et al. Leukocyturia as a predictor of tolerance and efficacy of intravesical BCG maintenance therapy for superficial bladder cancer. Urology. 2001;57:617–621. doi: 10.1016/s0090-4295(01)00921-9. discussion 621–622. [DOI] [PubMed] [Google Scholar]
- 23.Torrence RJ, Kavoussi LR, Catalona WJ, Ratliff TL. Prognostic factors in patients treated with intravesical bacillus Calmette–Guerin for superficial bladder cancer. J Urol. 1988;139:941–944. doi: 10.1016/s0022-5347(17)42723-6. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe E, Matsuyama H, Matsuda K, et al. Urinary interleukin-2 may predict clinical outcome of intravesical bacillus Calmette–Guerin immunotherapy for carcinoma in situ of the bladder. Cancer Immunol Immunother. 2003;52:481–486. doi: 10.1007/s00262-003-0384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saint F, Patard JJ, Maille P, et al. Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette–Guerin treatment for superficial bladder cancer. J Urol. 2002;167:364–367. [PubMed] [Google Scholar]
- 26.Shintani Y, Sawada Y, Inagaki T, Kohjimoto Y, Uekado Y, Shinka T. Intravesical instillation therapy with bacillus Calmette–Guerin for superficial bladder cancer: study of the mechanism of bacillus Calmette–Guerin immunotherapy. Int J Urol. 2007;14:140–146. doi: 10.1111/j.1442-2042.2007.01696.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhao W, Schorey JS, Groger R, Allen PM, Brown EJ, Ratliff TL. Characterization of the fibronectin binding motif for a unique mycobacterial fibronectin attachment protein, FAP. J Biol Chem. 1999;274:4521–4526. doi: 10.1074/jbc.274.8.4521. [DOI] [PubMed] [Google Scholar]
- 28.Kavoussi LR, Brown EJ, Ritchey JK, Ratliff TL. Fibronectin-mediated Calmette–Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest. 1990;85:62–67. doi: 10.1172/JCI114434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Boer EC, de Jong WH, van der Meijden AP, et al. Leukocytes in the urine after intravesical BCG treatment for superficial bladder cancer. A flow cytofluorometric analysis. Urol Res. 1991;19:45–50. doi: 10.1007/BF00294021. [DOI] [PubMed] [Google Scholar]
- 30.Ponticiello A, Perna F, Maione S, et al. Analysis of local T lymphocyte subsets upon stimulation with intravesical BCG: a model to study tuberculosis immunity. Respir Med. 2004;98:509–514. doi: 10.1016/j.rmed.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Schamhart DH, de Boer EC, de Reijke TM, Kurth K. Urinary cytokines reflecting the immunological response in the urinary bladder to biological response modifiers: their practical use. Eur Urol. 2000;37(Suppl 3):16–23. doi: 10.1159/000052388. [DOI] [PubMed] [Google Scholar]
- 32.Saban MR, Simpson C, Davis C, et al. Discriminators of mouse bladder response to intravesical bacillus Calmette–Guerin (BCG) BMC Immunol. 2007;8:6. doi: 10.1186/1471-2172-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 34.Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: a novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪.Ludwig AT, Moore JM, Luo Y, et al. Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for bacillus Calmette–Guerin-induced antitumor activity. Cancer Res. 2004;64:3386–3390. doi: 10.1158/0008-5472.CAN-04-0374. Urinary TNF-related apoptosis-inducing ligand (TRAIL) levels are increased in bacille Calmette–Guerin (BCG) responders versus nonresponders. The massive influx of neutrophils during BCG therapy is responsible for TRAIL secretion. [DOI] [PubMed] [Google Scholar]
- 36.Simons MP, Nauseef WM, Griffith TS. Neutrophils and TRAIL: insights into BCG immunotherapy for bladder cancer. Immunol Res. 2007;39:79–93. doi: 10.1007/s12026-007-0084-1. [DOI] [PubMed] [Google Scholar]
- 37▪.Suttmann H, Riemensberger J, Bentien G, et al. Neutrophil granulocytes are required for effective bacillus Calmette–Guerin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res. 2006;66:8250–8257. doi: 10.1158/0008-5472.CAN-06-1416. Neutrophils are essential for T-cell migration and BCG therapeutic effect in a mouse model of bladder cancer. [DOI] [PubMed] [Google Scholar]
- 38.Thalmann GN, Sermier A, Rentsch C, Mohrle K, Cecchini MG, Studer UE. Urinary Interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette–Guerin. J Urol. 2000;164:2129–2133. [PubMed] [Google Scholar]
- 39.Kumar A, Dubey D, Bansal P, Mandhani A, Naik S. Urinary interleukin-8 predicts the response of standard and low dose intravesical bacillus Calmette–Guerin (modified Danish 1331 strain) for superficial bladder cancer. J Urol. 2002;168:2232–2235. doi: 10.1016/S0022-5347(05)64361-3. [DOI] [PubMed] [Google Scholar]
- 40.Kemp TJ, Ludwig AT, Earel JK, et al. Neutrophil stimulation with Mycobacterium bovis bacillus Calmette–Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood. 2005;106:3474–3482. doi: 10.1182/blood-2005-03-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morales A, Nickel JC, Wilson JW. Dose–response of bacillus Calmette–Guerin in the treatment of superficial bladder cancer. J Urol. 1992;147:1256–1258. doi: 10.1016/s0022-5347(17)37532-8. [DOI] [PubMed] [Google Scholar]
- 42.Pagano F, Bassi P, Milani C, Meneghini A, Maruzzi D, Garbeglio A. A low dose bacillus Calmette–Guerin regimen in superficial bladder cancer therapy: is it effective? J Urol. 1991;146:32–35. doi: 10.1016/s0022-5347(17)37707-8. [DOI] [PubMed] [Google Scholar]
- 43.Witjes JA. What is the optimal BCG dose in non-muscle-invasive bladder cancer? Eur Urol. 2007;52:1300–1302. doi: 10.1016/j.eururo.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Ojea A, Nogueira JL, Solsona E, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette–Guerin (27 mg) versus very low-dose bacillus Calmette–Guerin (13.5 mg) versus mitomycin C. Eur Urol. 2007;52:1398–1406. doi: 10.1016/j.eururo.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 45.Luo Y, Chen X, O’Donnell MA. Role of Th1 and Th2 cytokines in BCG-induced IFN-γ production: cytokine promotion and simulation of BCG effect. Cytokine. 2003;21:17–26. doi: 10.1016/s1043-4666(02)00490-8. [DOI] [PubMed] [Google Scholar]
- 46.Belldegrun AS, Franklin JR, O’Donnell MA, et al. Superficial bladder cancer: the role of interferon-α. J Urol. 1998;159:1793–1801. doi: 10.1016/S0022-5347(01)63160-4. [DOI] [PubMed] [Google Scholar]
- 47.Weiss GR, O’Donnell MA, Loughlin K, Zonno K, Laliberte RJ, Sherman ML. Phase 1 study of the intravesical administration of recombinant human interleukin-12 in patients with recurrent superficial transitional cell carcinoma of the bladder. J Immunother. 2003;26:343–348. doi: 10.1097/00002371-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 48▪.Joudi FN, Smith BJ, O’Donnell MA. Final results from a national multicenter Phase II trial of combination bacillus Calmette–Guerin plus interferon α-2B for reducing recurrence of superficial bladder cancer. Urol Oncol. 2006;24:344–348. doi: 10.1016/j.urolonc.2005.11.026. BCG plus IFN-α is a reasonably good option for patients who fail previous BCG with 45% of patients recurrence free at a mean follow-up of 24 months. [DOI] [PubMed] [Google Scholar]
- 49.Morales A, Chin JL, Ramsey EW. Mycobacterial cell wall extract for treatment of carcinoma in situ of the bladder. J Urol. 2001;166:1633–1637. discussion 1637–1638. [PubMed] [Google Scholar]
- 50▪.Morales A. Evolution of intravesical immunotherapy for bladder cancer: mycobacterial cell wall preparation as a promising agent. Expert Opin Investig Drugs. 2008;17:1067–1073. doi: 10.1517/13543784.17.7.1067. Highlights preclinical rationale, recent work and future studies for mycobacterial cell wall extract. [DOI] [PubMed] [Google Scholar]
- 51.Kelley DR, Ratliff TL, Catalona WJ, et al. Intravesical bacillus Calmette–Guerin therapy for superficial bladder cancer: effect of bacillus Calmette–Guerin viability on treatment results. J Urol. 1985;134:48–53. doi: 10.1016/s0022-5347(17)46976-x. [DOI] [PubMed] [Google Scholar]
- 52.De Boer EC, Rooijakkers SJ, Schamhart DH, Kurth KH. Cytokine gene expression in a mouse model: the first instillations with viable bacillus Calmette–Guerin determine the succeeding Th1 response. J Urol. 2003;170:2004–2008. doi: 10.1097/01.ju.0000091826.83705.79. [DOI] [PubMed] [Google Scholar]
- 53.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 54.Korf J, Stoltz A, Verschoor J, De Baetselier P, Grooten J. The Mycobacterium tuberculosis cell wall component mycolic acid elicits pathogen-associated host innate immune responses. Eur J Immunol. 2005;35:890–900. doi: 10.1002/eji.200425332. [DOI] [PubMed] [Google Scholar]
- 55.Mangsbo SM, Ninalga C, Essand M, Loskog A, Totterman TH. CpG therapy is superior to BCG in an orthotopic bladder cancer model and generates CD4+ T-cell immunity. J Immunother. 2008;31:34–42. doi: 10.1097/CJI.0b013e3181587d29. [DOI] [PubMed] [Google Scholar]
- 56.Sinn HW, Elzey BD, Jensen RJ, Zhao X, Zhao W, Ratliff TL. The fibronectin attachment protein of bacillus Calmette–Guerin (BCG) mediates antitumor activity. Cancer Immunol Immunother. 2008;57:573–579. doi: 10.1007/s00262-007-0397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dominguez-Escrig JL, Kelly JD, Neal DE, King SM, Davies BR. Evaluation of the therapeutic potential of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib in preclinical models of bladder cancer. Clin Cancer Res. 2004;10:4874–4884. doi: 10.1158/1078-0432.CCR-04-0034. [DOI] [PubMed] [Google Scholar]
- 58.Ramesh N, Ge Y, Ennist DL, et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor – armed oncolytic adenovirus for the treatment of bladder cancer. Clin Cancer Res. 2006;12:305–313. doi: 10.1158/1078-0432.CCR-05-1059. [DOI] [PubMed] [Google Scholar]
- 59.Saint F, Salomon L, Quintela R, et al. Do prognostic parameters of remission versus relapse after bacillus Calmette–Guerin (BCG) immunotherapy exist? Analysis of a quarter century of literature. Eur Urol. 2003;43:351–360. doi: 10.1016/s0302-2838(03)00048-4. discussion 360–361. [DOI] [PubMed] [Google Scholar]
- 60.Basturk B, Yavascaoglu I, Oral B, Goral G, Oktay B. Cytokine gene polymorphisms can alter the effect of bacillus Calmette–Guerin (BCG) immunotherapy. Cytokine. 2006;35:1–5. doi: 10.1016/j.cyto.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Decobert M, Larue H, Bergeron A, et al. Polymorphisms of the human NRAMP1 gene are associated with response to bacillus Calmette–Guerin immunotherapy for superficial bladder cancer. J Urol. 2006;175:1506–1511. doi: 10.1016/S0022-5347(05)00653-1. [DOI] [PubMed] [Google Scholar]
- 62.Ahirwar D, Kesarwani P, Manchanda PK, Mandhani A, Mittal RD. Anti- and proinflammatory cytokine gene polymorphism and genetic predisposition: association with smoking, tumor stage and grade, and bacillus Calmette–Guerin immunotherapy in bladder cancer. Cancer Genet Cytogenet. 2008;184:1–8. doi: 10.1016/j.cancergencyto.2008.02.015. [DOI] [PubMed] [Google Scholar]
Website
- 101.Clinical.Trials.gov . www.clinicaltrials.gov.