Abstract
While immunotherapy for cancer has become increasingly popular, clinical benefits for such approaches remain limited. This is likely due to tumor-associated immune suppression, particularly in the advanced-disease setting. Thus, a major goal of novel immunotherapeutic design has become the coordinate reversal of existing immune dysfunction and promotion of specific tumoricidal T-cell function. Costimulatory members of the TNF-receptor family are important regulators of T-cell-mediated immunity. Notably, agonist ligation of these receptors restores potent antitumor immunity in the tumor-bearing host. Current Phase I/II evaluation of TNF-receptor agonists as single-modality therapies will illuminate their safety, mechanism(s) of action, and best use in prospective combinational immunotherapy approaches capable of yielding superior benefits to cancer patients.
Keywords: combinational therapeutics, costimulation, cytotoxic T cell, immunotherapy, regulatory T cell, T cell, TNF-receptor
Despite overwhelming evidence that tumor-bearing hosts can mount antitumor immune responses either spontaneously or following vaccination or other immunotherapeutic interventions [1,2], numerous suppressive factors in the tumor microenvironment appear to contribute to tumor immune evasion and loss of therapy-associated benefits [3,4]. Peripheral self-tolerance mechanisms adopted by tumors to prevent their immune-mediated destruction include tumor cell downregulation of MHC or costimulatory molecules, thereby limiting tumor cell recognition by T cells, and the induction or elaboration of dominant suppressive elements (i.e., regulatory T cells [Tregs], indoleamine 2,3-dioxygenase [IDO] or TGF-β). Accumulating preclinical data suggests that engagement of costimulatory members of the TNF receptor (TNFR) family may counteract tumor-mediated immunosuppression by directly reactivating tumor-specific T cells or inhibiting dominant suppressive mechanisms that prevent T-cell effector function(s) in vivo. These data support the use of TNFR-based agonistic modalities alone or in combination with other immunotherapies for the treatment of cancer. This review will focus on the molecular, cellular and treatment-associated consequences of engagement of three costimulatory TNFRs, 4-1BB (CD137), OX40 (CD134) and the glucocorticoid-induced TNFR (GITR), using agonist agents in the cancer setting.
Costimulatory members of the TNFR family: immunobiology
Costimulation is classically defined as the engagement of receptors secondary to T-cell receptor (TCR) signaling that functions to lower the activation threshold of naive T cells [5,6]. The primary costimulatory receptor on T cells is CD28, which upon binding of its ligands B7.1 (CD80) or B7.2 (CD86) initiates high-level IL-2 production and clonal T-cell expansion. Additional costimulatory molecules, including members of the TNFR family, function subsequent to CD28 engagement to enhance the activation, survival and differentiation of T-effector and memory cells [7]. Unlike CD28, which is constitutively expressed by Tcells, TNFR co stimulatory molecules are upregulated shortly after activation and downregulated soon thereafter. TNFR ligands are biologically active as trimers, which induce corresponding trimerization of their receptors upon binding. This has been observed for 4-1BB, OX40 and GITR ligation in both murine and human cells [7]. Recently, it was reported that murine GITR ligand (GITRL) may exert stronger activity when in a dimer conformation [8], while human GITRL can exist and signal effectively in multiple states of oligomerization [9], suggesting that structural diversity can exist among TNFR ligands. Upon ligand engagement, multiple TNFR-associated factor (TRAF) molecules are recruited to the intracellular domain of costimulatory TNFRs and initiate JNK, p38 and NF-κB signaling pathways [7]. Alterations in signaling cascades elicited by various TNFR family members may be attributed to divergent affinities for TRAF proteins and the existence of at least six unique TRAF isoforms [7].
4-1BB
4-1BB, or CD137, has been well characterized over the past two decades and is expressed on activated T, natural killer (NK) and NKT cells [10], dendritic cells (DCs) [11] and natural Tregs [7,12]. During T-cell activation, 4-1BB expression peaks less than 48 h after antigen stimulation and appears to be upregulated faster on CD8+ versus CD4+ T cells [13,14]. Despite such differences in the kinetics of 4-1BB expression, similar proliferative responses between CD4+ and CD8+ T cells have been observed in both mice and humans [15,16], indicating that 4-1BB signaling enhances the primary expansion of both T-cell subsets to a similar extent. In a model of ovalbumin (OVA) peptide-induced tolerance, Wilcox and colleagues reported that CD8+ T-cell anergy can be reversed in vivo by systemic administration of an agonistic monoclonal antibody (mAb) to 4-1BB [17]. Furthermore, the production of both type 1 and type 2 effector cytokines (IFN-γ and IL-4, respectively) is also augmented by engagement of 4-1BB on CD4+ and CD8+ T cells [15], while the cytotoxic activity of mouse and human CD8+ T cells can be enhanced in response to 4-1BB ligation [15,16], suggesting that 4-1BB signaling plays a significant role in activation and optimal effector function of CD4+ and CD8+ T cells.
Costimulatory TNFR signaling is also thought to play a dominant role in T-cell survival and the generation of memory following initial clonal expansion; this topic has been comprehensively discussed in recent reviews [7,18]. Sustained survival of activated T cells upon 4-1BB engagement appears to involve the increased expression of antiapoptotic Bcl family members [15,19], while 4-1BB ligation can rescue CD8+ T cells from activation-induced cell death (AICD) following superantigen exposure [20]. Regarding the role of 4-1BB in generating T-cell memory, Zhu et al. recently reported that triggering 4-1BB through an agonistic mAb induces the expansion of memory CD4+ and CD8+ T cells in the absence of cognate antigen [21]. IL-15 appears to be required for 4-1BB-dependent maintenance of the CD8 memory pool in particular, as this cytokine induces 4-1BB upregulation and may contribute to a unique ‘CD8 memory niche’, which may be further potentiated by GITR-mediated signals [18]. 4-1BB–4-1BBL interactions may also affect cells of the innate immune system. Triggering of 4-1BB on murine splenic and bone marrow-derived DC enhances the secretion of proinflammatory cytokines [11], while engagement of 4-1BB on activated NKT cells induces IL-4, IL-13 and IFN-γ production and secretion [10].
Despite its observed immunostimulatory effects in vitro and in vivo, 4-1BB signaling has somewhat counterintuitively been noted to abrogate autoimmunity, a property that is unique among costimulatory TNFR members [22]. For example, 4-1BB ligation is therapeutic in animal models of rheumatoid arthritis, Type 1 diabetes, multiple sclerosis and systemic lupus erythmatosus (SLE) [23]. In these systems, it is believed that 4-1BB-mediated signaling leads to the induction of effector CD4+ T-cell AICD [24] and/or the expansion of regulatory CD4+ [25] or CD8+ T cells [26]. As for the effect of 4-1BB ligation on Tregs, 4-1BB−/− mice exhibit similar numbers of naturally occurring Foxp3+ Treg cells when compared with wild-type mice [27]. This suggests that 4-1BB signaling may exert, at best, a minor impact on the maintenance of these cells in the periphery. However, it has been consistently shown in both mouse and human models that ligation of 4-1BB induces Treg proliferation, with expanded Treg cells retaining suppressor function [28,29]. The dichotomous nature of 4-1BB signaling in T cells remains an area of research controversy that must be resolved prior to 4-1BB agonistic reagents entering the clinic.
OX40
Unlike 4-1BB, which is widely expressed by diverse immune cell types, OX40 is restricted to naturally occurring Tregs and activated effector T cells (preferentially CD4+), with expression typically peaking 48–72 h after TCR engagement [7,12,30]. Interestingly, activated human T cells have been found to express OX40–4-1BB heterodimers [31]. Although the biologic relevance of these structures requires further characterization, signaling through these heterodimers appears to suppress T-cell activation and induce apoptosis, defining a unique mechanism of costimulatory regulation. Administration of agonist OX40 mAb enhances the primary clonal expansion of both CD4+ and CD8+ TCR transgenic T cells in vivo [32–34], perhaps owing to the OX40-dependent downregulation of the inhibitory costimulatory receptor CTLA-4 [35]. While early studies suggested that OX40 ligation preferentially yielded type 2 effector cytokine production by CD4+ T cells, accumulating evidence now indicates that OX40–OX40L interactions enhance ongoing type 1 or type 2 responses and do not bias the functional polarity of CD4+ effector T cells [7]. Experimentally induced CD4+ T-cell anergy can also be abrogated by OX40 engagement [36], and it has recently been reported that genetic mutations leading to OX40 overexpression predispose individuals to SLE [37], suggesting that OX40 ligation can potently reverse established T-cell tolerance.
Similar to other costimulatory members of the TNFR family, the primary role of OX40 signaling appears to involve the extension of T-cell survival following initial clonal expansion and differentiation into memory T cells. OX40-deficient CD4+ and CD8+ T cells undergo premature apoptosis in vivo [34,38], while agonist mAb-mediated OX40 costimulation prolongs CD8+ T-cell survival that can be further augmented by cotreatments incorporating 4-1BB agonist mAb [39,40]. Furthermore, the lack of OX40 signaling impairs the maintenance of CD8+ memory and CD4+ effector but not central memory T cells [41,42]. In contrast to the 4-1BB- and IL-15-dependent maintenance of CD8+ memory Tcells, OX40–OX40L interactions may help to establish a ‘CD4 memory niche’ involving the IL-7-dependent upregulation of OX40 expression on CD4+ T cells [18]. Finally, the role of OX40 in both natural and inducible Tregs (nTreg and iTreg, respectively) has recently received significant attention [43]. OX40 signaling does not appear to play a significant role in the peripheral maintenance of nTreg, as similar numbers of these cells are observed in the peripheral tissues of OX40−/− and wild-type mice [44]. Similar to 4-1BB, OX40 ligation on nTreg can induce their proliferation, but it has not been conclusively demonstrated that these expanded nTregs retain their suppressor function. It does appear, however, that OX40 engagement may antagonize nTreg function in the absence of cell proliferation [44,45]. Naive CD4+CD25− T cells can also acquire regulatory activity upon culture with, for example, low-dose antigen and TGF-β [46]. Recent reports suggest, however, that the induction of Foxp3 gene expression (a transactivator protein crucial to Treg function [47]) can be inhibited through OX40–OX40L interactions that may involve the manipulation of TGF-β signaling pathways [44,46,48]. Alternatively, OX40 signaling may indirectly subvert Treg function by rendering effector T cells refractory to the action of Tregs [45]. Hence, OX40 appears to interface a number of divergent mechanisms that limit Treg-mediated immune suppression, including the prevention of iTreg generation, direct inhibition of nTreg function, and by conferring resistance of effector cells to Treg-dependent inhibition.
GITR
GITR and its human ortholog AITR are expressed on nTregs and activated B cells, macrophages, DCs, NK cells and effector T cells [7,12,49]. On effector T cells, GITR is upregulated earlier than 4-1BB and OX40, peaking approximately 24 h after T-cell activation. Interestingly, it was recently shown that GITR expression is induced in Tregs by a complex between Foxp3 and the transcription factor NFAT [50], whereas in activated effector T cells, NFAT inhibits while NF-κB induces GITR upregulation [51], suggesting that distinct regulatory mechanisms control GITR expression in different cell subsets. Similar to 4-1BB and OX40, GITR ligation enhances primary T-cell expansion and effector cytokine secretion in the presence of low-dose antigen [52,53]. Likewise, GITR–GITRL interactions can reverse T-cell tolerance via a Treg-independent mechanism, as depletion of CD25hi Treg does not yield a similar phenotype [54]. GITR engagement has also been reported to sustain the survival of activated T cells and to prevent AICD in wild-type versus GITR−/− T cells [55,56]. As noted previously, GITR appears to play a role in the ‘CD8 memory niche’, as GITR is upregulated in CD8+ memory T cells upon provision of IL-15 [18].
Despite accumulating evidence that GITR signaling directly augments the immune function of effector T cells, considerably more attention has been paid to investigating the impact of GITR on regulatory T-cell activity. In this regard, only a limited role for GITR in the development, maintenance and function of nTregs has been demonstrated in GITR−/− mice [57]. As with OX40, GITR ligation can induce the proliferation of both murine and human nTregs, but conflicting reports exist regarding the function of these expanded Tregs [58–60]. A recent study by Wang and colleagues suggests that GITR engagement can prevent the conversion of naive CD4+ T cells into Foxp3+ iTregs in the presence of TGF-β [61]. Although GITR–GITRL interactions may contribute directly to the regulation of Treg subsets, elegant experiments from the Shevach group using combinations of wild-type or GITR−/− effector cell/Treg cocultures indicate that effector T cells are the primary target of GITR costimulation [57,62]. While GITR−/− CD4+CD25+ Tregs exhibit similar suppressive capacity as wild-type Tregs, the proliferation of GITR−/− but not wild-type CD4+CD25− effector T cells is inhibited upon stimulation with GITR agonist mAbs, suggesting that GITR ligation renders effector T cells resistant to Treg-mediated suppression. Finally, although GITR is expressed by activated human NK cells, it is unclear whether GITR signaling mediates activating or inhibitory signals in these cells [63,64], despite clear evidence that GITR stimulation enhances the proliferation and effector cytokine production of murine NKT cells in vitro and in vivo [65].
TNFR-based cancer immunotherapies: preclinical efficacy & mechanisms of action
The National Cancer Institute recently published a priority list of immunotherapy agents with anticipated antitumor potential, placing an emphasis on agents that are not commercially available or fully developed for testing in humans at this time [201]. Agonist antibodies targeting 4-1BB, OX40 and GITR were among the 20 agents emphasized from a list of over 100 novel modalities. Based on the aggregate conclusions from a large volume of murine tumor studies, it is clear that TNFR-targeting therapeutics have tremendous potential to augment antitumor immunity and, ultimately, the survival of cancer patients. The results of such preclinical investigations will now be summarized, along with the proposed biologic mechanisms supporting the efficacy of these reagents applied to tumor-bearing hosts.
4-1BB
As discussed previously, TNFR ligation results in a broad range of downstream molecular effects that have been deduced from in vitro studies and observations made in mouse models of autoimmunity and transplantation. In contrast to such models, however, progressively growing tumors display a unique microenvironment characterized by enrichment in immunosuppressive cells and cytokines, downregulation of tumor-expressed MHCs, and dysfunctional antigen-presenting cells (APCs) [3]. Therefore, the cellular and molecular consequences of TNFR engagement in tumor models may differ significantly from those reported in other disease models. Most mechanistic studies have utilized depleting antibodies specific for various immune cell subsets and immunodeficient mice to define requisite cell types linked to drug impact. For example, a number of groups have reported that the therapeutic efficacy of 4-1BB agonists in tumor-bearing mice is abrogated by CD8+ T-cell depletion prior to 4-1BB-targeted treatment [66–71]. Furthermore, IFN-γ appears to play an essential role upon 4-1BB engagement, as tumors in 4-1BB-treated IFN-γ-deficient mice progress with similar kinetics to those established in untreated animals [68,71,72]; however, it is unlikely that IFN-γ is acting directly on the tumor because expression of a nonsignaling IFN-γ decoy receptor by tumor cells did not alter the therapeutic effect of treatment [72]. Fewer studies have indicated that CD4+ T cells [66,67,73] or NK cells [71,73,74] are required for 4-1BB-mediated therapy, although NK-cell depletion appears to abrogate cytotoxic activity mediated by CD8+ T cells isolated from treated mice [75]. In adoptive T-cell transfer models, tumor-reactive TCR-transgenic CD8+ T cells stimulated ex vivo or in vivo with 4-1BB agonists exhibited enhanced survival in peripheral lymphoid organs and within the tumor microenvironment of tumor-bearing mice versus control untreated CD8+ Tcells [76,77]. The function of such tumor-specific CD8+ T cells (transgenic and endogenous) also appears to be enhanced by 4-1BB treatment, with a type 1 polarized cytokine profile observed in the lymph nodes and spleens of treated animals [69]. In such animals, tumor-infiltrating lymphocytes (TILs) were enriched in CD8+ T cells exhibiting a CD107a+FasL+ phenotype [78]. Although costimulatory engagement certainly appears to have a direct effect on tumor-reactive CD8+ T cells, 4-1BB ligation on CD4+ helper T cells and NK cells may also indirectly enhance the activity of cytotoxic Tcells through redundant mechanisms. First, CD4+ T cells and NK cells both serve to ‘license’ DC-mediated priming of CD8+ effector T cells in the lymph nodes via CD40–CD40L interactions or the secretion of IFN-γ, respectively [79,80]. Indeed, 4-1BB therapy was recently reported to increase NK cell production of IFN-γ in tumor-draining lymph nodes [71]. NK cell and CD4+ T-cell ‘help’ may also potentiate the effector function of CD8+ cytotoxic T cells through their direct production of IFN-γ [81]. Wilcox and colleagues observed that significantly less CD8+ T cells infiltrate the tumors of IFN-γ−/− mice compared with wild-type mice after 4-1BB treatment [72], consistent with an earlier report indicating that IFN-γ regulates T-cell migration to the tumor site [82].
The therapeutic efficacy of numerous 4-1BB-stimulating modalities, including agonist antibodies, 4-1BBL–Fc fusion proteins, 4-1BB aptamers [83], adoptive transfer of costimulated T cells, ligand- or agonist mAb-transduced tumor cell vaccines, and ligand-expressing viral vectors, have now been studied in a range of murine tumor models (Table 1). Melero et al. first demonstrated that poorly immunogenic, subcutaneous tumors can be rejected when agonist 4-1BB mAbs were administered up to 12 days after tumor inoculation, whereas disseminated metastases could be cleared as long as the antibody was applied within 3 days after intravenous tumor injection [66]. 4-1BB-targeting therapies have also been successfully combined as an ‘adjuvant’ with therapeutic tumor vaccines to elicit enhanced antitumor immunity. Two independent groups effectively treated tumor-bearing mice with vaccines consisting of tumor cells transduced to express the Fv fragment of agonistic 4-1BB mAbs, leading to enhanced tumor-specific immunity as a result of the coordinate provision of cognate antigen and costimulation by these engineered APCs [73,84]. Several reports suggest that 4-1BB-mediated therapy is synergistically enhanced when combined with IL-12 gene therapy [85–90]. It has been proposed that IL-12 initially activates NK cells to secrete IFN-γ, inducing DCs to upregulate 4-1BB and mature into efficient APCs upon administration of 4-1BB agonist cotherapy [87]. More recently, the promotion of tumor cell death by chemotherapy, radiotherapy or engagement of the apoptotic TNFR family member DR5 on tumor cells has been shown to enhance the benefits of 4-1BB-based therapy [91–96]. Finally, the efficacy of 4-1BB agonists in the treatment of progressing tumors can be enhanced in combination with other costimulatory agonists [97] or agents that block the T-cell inhibitory receptors CTLA-4 and PD-1 [98,99].
Table 1.
Superior efficacy of combinational therapies versus TNF receptor-based agonistic monotherapies (i.e., monoclonal antibodies, ligand-transduced vaccines and recombinant viruses) in the treatment of progressing murine tumors.
| Treatment | Tumor model | Costimulator modality type | Refs |
|---|---|---|---|
| 4-1BB agonist | |||
| + Vaccination | Sarcoma, melanoma | mAb | [69] |
| Melanoma | mAb | [73,78,84] | |
| Breast carcinoma | mAb | [116] | |
| Lymphoma | Ligand-transduced vaccine | [148] | |
| Lung carcinoma, melanoma | mAb | [149] | |
| + Adoptive cell transfer | Lung carcinoma | Recombinant virus | [76] |
| Lymphoma | mAb | [77] | |
| Melanoma | mAb | [150] | |
| Sarcoma | mAb | [151] | |
| + IL-12 | Colorectal carcinoma | Recombinant virus, mAb, fusion protein | [85,86,89,90,107] |
| Melanoma, lymphoma | mAb | [88] | |
| Breast carcinoma | mAb | [152] | |
| + Chemo/radiotherapy | Sarcoma | mAb | [91] |
| Lung and breast carcinomas | mAb | [92] | |
| Renal cell carcinoma | mAb | [93] | |
| Colorectal carcinoma | mAb | [94] | |
| + Coinhibitory blockade | Colorectal carcinoma | mAb | [98] |
| Lymphoma, breast carcinoma | mAb | [99] | |
| Costimulatory combinations | Breast and renal carcinomas, sarcoma | mAb | [95] |
| Colorectal carcinoma | Recombinant virus, mAb | [97,107] | |
| Sarcoma | mAb | [115] | |
| Breast carcinoma | mAb | [116,152] | |
| OX40 agonist | |||
| + Vaccination | Lymphoma, melanoma | Ligand-transduced vaccine | [104] |
| Breast carcinoma | mAb | [109,116] | |
| Sarcoma, prostate carcinoma | mAb | [113] | |
| Melanoma | Ligand-transduced vaccine | [153] | |
| Colorectal carcinoma | Ligand-transduced vaccine | [154] | |
| + Adoptive cell transfer | Sarcoma, melanoma, glioma | mAb | [101] |
| Lymphoma | mAb | [108] | |
| Prostate carcinoma | mAb | [155] | |
| + IL-12 | Colorectal carcinoma | mAb | [107] |
| Sarcoma, prostate carcinoma | mAb | [113] | |
| + Chemo/radiotherapy | Lung carcinoma | mAb | [114] |
| + Coinhibitory blockade | Lymphoma | mAb | [156] |
| Costimulatory combinations | Colorectal carcinoma | mAb | [107] |
| Sarcoma | mAb | [115] | |
| Breast carcinoma | mAb | [116] | |
| GITR agonist | |||
| + Vaccination | Colorectal carcinoma | mAb | [122] |
| + IL-12 | Breast carcinoma | Fusion protein | [152] |
| + Chemo/radiotherapy | Colorectal carcinoma | mAb | [122] |
| + Coinhibitory blockade | Sarcoma | mAb | [118] |
| Costimulatory combinations | Breast carcinoma | Fusion protein | [152] |
GITR: Glucocorticoid-induced TNF receptor; mAb: Monoclonal antibody.
OX40
Similar to preclinical studies employing 4-1BB agonists, OX40-mediated immunotherapy appears to rely heavily on CD8+ T cells, as tumors rapidly progress upon depletion of this immune cell subset [100–106]. In contrast to 4-1BB-based models, CD4+ T cells appear to play an even greater role in treatment outcomes associated with OX40-based therapy [100–104]. Pan and colleagues reported that coadministration of agonist OX40 significantly enhances the tumor infiltration and ex vivo tumoricidal activity of CD8+ T cells isolated from mice treated with anti-4-1BB and IL-12 gene therapy, while CD4+ T-cell depletion abrogates this effect [107]. Moreover, a recent study using OVA-transduced tumors and adoptive transfer of OX40-stimulated OT1 (anti-OVA CD8+) T cells suggests that the accumulation of type 1, tumor-specific CD8+ T cells in the tumor microenvironment is dependent on endogenous CD4+ T cells, as CD8+ TILs are absent in homologous models established in MHC class II-deficient (CD4+ T-cell-deficient) mice [108]. OX40-mediated signals thus appear to augment the helper function of CD4+ T cells, thereby indirectly promoting the optimal effector function of antitumor CD8+ T cells. However, this notion has not been universally observed in experimental models, since OX40-dependent, CD8+ T-cell-mediated therapeutic efficacy is preserved in CD4+ T-cell-deficient mice [34,109]. This suggests that OX40-associated signals may directly result in the stimulation of CD8+ T cells under such conditions, or that an alternative cell type may provide OX40-dependent ‘help’ to developing CD8+ T cells in the CD4-deficient host. Support for the latter was recently provided by Zaini and colleagues, who found that OX40-mediated therapy of B16 melanoma is abrogated in NKT cell-deficient mice [104]. In this regard, it is important to note that although OX40 expression is thought to be restricted to T cells, 20% of OX40+ TILs in this model exhibit a NKT cell phenotype. The authors propose that OX40L-transduced DCs activate tumor-localized NKT cells to secrete IFN-γ, which acts to facilitate the priming of type 1 T cells via the maturation and functional ‘licensing’ of DCs [80] and/or via direct induction of CD8+ T effector cell differentiation [81,82]. Two additional recent studies suggest that OX40 agonists may abrogate tumor-induced immunosuppression by either preventing the conversion of naive CD4+ Tcells into Foxp3 + Treg [106], or by limiting the frequency and function of myeloid-derived suppressor cells (MDSCs) [110].
A variety of OX40-targeted modalities exist as potential therapeutic agents, including agonist antibodies, ligand–Fc fusion proteins [111], ligand-expressing viral vectors, and ligand-transduced DC or tumor cell vaccines (Table 1). Weinberg and colleagues initially assessed the therapeutic efficacy of agonist OX40 mAbs and recombinant OX40L–Fc fusion protein, concluding that when administered early after tumor inoculation (i.e., by day 3), overall tumor-free survival was significantly enhanced in model systems employing a range of tumor cell lines exhibiting variable inherent immunogenicities [112]. We have similarly observed that well-established day-17 tumors are completely rejected by BALB/c mice upon treatment with OX40L–Fc as well as GITRL–Fc reagents [Pardee AD et al., Unpublished Data]. Using a clinically relevant model of tumor antigen tolerance, transgenic neu-N mice bearing established neu-expressing tumors were immunized with GM-CSF-transduced versions of the same tumor as a vaccine [109]. Notably, enhanced survival of these mice could only be achieved when OX40 mAb was coadministered along with the vaccine. As in the case of 4-1BB-based therapies, combinational treatments with either IL-12 or radiotherapy have recently been shown to enhance the efficacy of OX40-based regimens [113,114]. Of particular significance to this discussion, combinations of 4-1BB and OX40 agonists have been investigated in preclinical tumor models by numerous groups [115,116]. In such cases, the survival rate of mice bearing late-stage hepatic metastases was doubled in the two agent-based design when compared with each monotherapy [107], indicating that multi-costimulatory approaches may hold significant potential in the treatment of malignant disease.
GITR
As GITR was characterized more recently than 4-1BB and OX40, it is not surprising that substantially less data exist regarding the functional mechanisms and therapeutic efficacy of GITR agonists in preclinical tumor models [62]. Ramirez-Montagut et al. showed that GITR-mediated tumor rejection is abrogated upon depletion of CD4+, CD8+ and NK1.1+ cell subsets and in mice deficient in expression of IFN-γ and FasL [117], consistent with results reported by other groups using different tumor models [118–120]. Treatment of progressing CT26 colon carcinomas with agonist GITR mAbs led to increased numbers of activated CD4+ Tcells, CD8 + Tcells and NK cells in the tumor-draining lymph nodes [119]. However, this effect was abrogated when CD4+ T cells were depleted prior to treatment, indicating that CD4+ T cells play an indispensable role in the GITR-dependent priming of therapeutic antitumor CD8+ Tcells and NK cells. Following expansion in the draining lymph node, it appears that GITR- and OX40-stimulated effector T cells exhibiting a type 1-polarized gene expression profile accumulate in established tumors as a prelude to disease resolution [Pardee AD et al., Unpublished Data]. Not unexpectedly, increased numbers of Foxp3+ Tregs are observed in the spleen and tumor-draining lymph nodes of GITR-treated mice [117,119], yet despite the ability of these Treg to secrete IL-10, responder antitumor CD4+CD25− T cells in these animals appear refractory to Treg-mediated suppression [119]. This observation is likely due to the direct costimulatory effect of GITR ligation on effector CD4+ Tcells, rather than via the abrogation of Treg function [57]. Further support for this conclusion is provided by studies performed by Ramirez-Montagut and colleagues, who reported that GITR-mediated tumor rejection is enhanced by CD25 depletion, indicating that the abrogation of Treg function is not the primary mechanism of GITR-based therapy [117]. Thus, despite the availability of only a limited data set, GITR agonists appear to foster antitumor immunity in vivo by primarily targeting fully differentiated CD8+ T cells and by augmenting the ability of CD4+ T-helper cells to activate secondary waves of tumoricidal T cells.
The agonist GITR mAb, clone DTA-1, has been the most common GITR-stimulating reagent utilized in murine tumor models to date (Table 1). BALB/c mice treated with DTA-1 within 8days of syngeneic MethA fibrosarcoma inoculation rapidly reject their tumors and exhibit specific resistance to tumor rechallenge [118]. GITRL–Fc fusion proteins have also demonstrated potent agonist activity in Colon 26 (colorectal carcinoma) and RENCA (renal carcinoma) models [120]. Similarly, intratumoral injection of recombinant adenovirus encoding GITRL leads to significant inhibition of poorly immunogenic B16 melanoma growth in C57BL/6 mice [121]. As in the cases for 4-1BB- and OX40-targeted modalities, GITR agonists have also been recently shown to enhance the efficacy of vaccination and chemotherapy regimens when administered in combination therapy schemes [122].
Clinical application of TNFR-based immunotherapy: status & potential
4-1BB
Evaluation of clinical specimens support the likely utility of 4-1BB-based agonist therapies in humans. For example, tumor-reactive CD8+ T cells in the PBMCs of patients with Ewing’s sarcoma have been shown to express 4-1BB, with these T cells mediating tumoricidal activity upon in vitro stimulation with anti-CD3 and anti-4-1BB [123]. In addition, immunofluorescence imaging of tumor biopsies from patients with hepatocellular carcinoma indicate that CD4+ and CD8+ TILs express 4-1BB, while 4-1BB expression could not be detected in normal liver tissues [124]. Together, these studies suggest that tumor-reactive T cells in cancer patients are likely competent to respond to 4-1BB stimulation. In a translational model, Stephan and colleagues used a Hu-SCID xenograft model to investigate the therapeutic antitumor potential of transgenic T cells engineered to express CD80 and 4-1BBL [125]. Such T cells would be susceptible to ‘autocostimulation’, thereby preventing the predicted induction of anergy upon antigen encounter within the tumor microenvironment in vivo. Primary human T cells specific for the prostate-specific membrane antigen (PSMA) tumor antigen were generated in vitro and transduced to express CD80 and 4-1BBL using recombinant retroviruses. Adoptive transfer of these gene-modified T cells into immunodeficient mice with metastatic PSMA+ prostate tumor lesions resulted in the complete eradication of disseminated disease. Impressively, therapeutic regression of tumors occurred even if treatment was withheld until 1 month after tumor inoculation. Humanized agonist anti-4-1BB antibodies have recently been developed in both academic and industrial laboratories for use in clinical trials [126]. BMS-663513 is a fully human agonist 4-1BB antibody developed by Medarex Inc. (Princeton, NJ, USA) and currently being tested by Bristol-Myers Squibb in Phase I/II studies in patients with various types of advanced cancer. Preliminary results indicate that BMS-663513 is well tolerated with 17% of melanoma patients remaining progression free for more than 6 months [127]. Patients are currently being recruited for BMS-663513 treatment in combination with chemotherapy, radiotherapy or chemoradiation (NIH clinical trials database NCT00351325 and NCT00461110).
OX40
Substantial literature exists regarding the expression of OX40 in cancer patients [112,128,129]. For example, OX40 was found to be expressed by approximately 30% of TILs and draining lymph node cells in patients with head and neck squamous cell carcinoma (HNSCC) or melanoma [130]. Furthermore, high levels of OX40 expression in the TILs of colorectal cancer or melanoma patients have been positively correlated with prolonged patient survival [131,132]. Initial screening of an agonist OX40 mAb of murine origin in nonhuman primates revealed no overt toxicities despite its potent immunostimulatory properties [133]. In addition, a recombinant human OX40L–Fc fusion protein has recently been developed that appears capable of stimulating peripheral blood T cells in a manner comparable with that noted for agonist mAbs [134]. While Phase I studies are currently being conducted with the murine antibody, repeated dosing may be restricted due to the generation of immunity (i.e., human antimouse antibody [HAMA] responses) against the murine sequences of the antibody [135].
GITR
Recent reports suggest that human tumors are enriched in CD4+ TILs expressing a CD25hiFoxp3+ phenotype, consistent with Treg cells [136]. Such Tregs also express GITR and exhibit strong suppressor function ex vivo, mediated primarily through IL-10 and TGF-β, while Tregs in the peripheral blood of patients do not express GITR and exhibit minimal suppressor activity [136]. Tumor-localized GITR+ Tregs may, thus, be more sensitive to GITR ligation than circulating Tregs. One mechanism that tumor cells employ to evade immune-mediated destruction is the downregulation or ‘shedding’ of costimulatory ligands and the subsequent induction of tumor-specific T-cell anergy [4,137]. In fact, soluble tumor-shed GITRL has been reported in the sera of patients with various malignancies but not healthy controls [138,139], where it has been shown to blunt the function of NK cells [139,140]. However, GITR engagement activates human T lymphocytes in vitro [9], suggesting that GITRL signals may only be inhibitory to NK cell-mediated, rather than T-cell-mediated anti-tumor immunity. While clinical-grade GITR agonists are in their early stages of development, a fully humanized agonist GITR mAb, TRX518, has recently been developed by Tolerx Inc. (Cambridge, MA, USA). Initial characterization studies indicate that TRX518 is agonistic for human PBMC in vitro and that it fails to bind Fc receptors, thus limiting the theoretical likelihood of antibody- or complement-mediated deletion of GITR+ cells in vivo [141].
Conclusion
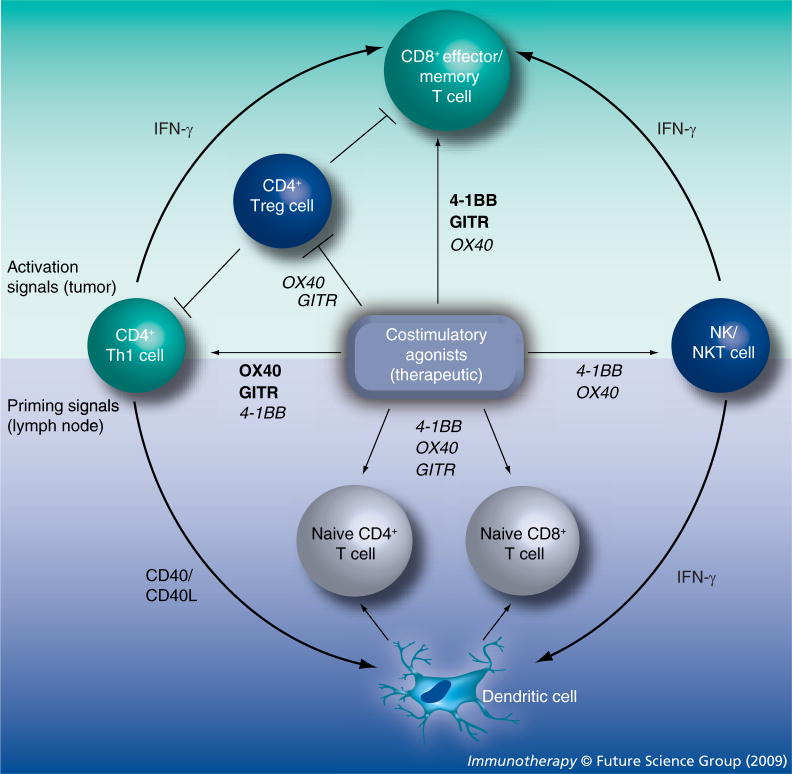
Costimulatory members of the TNFR family are essential components of functional immunity, regulating T-cell survival and activation at naive, effector, and memory stages of the adaptive immune response [7]. Cumulative data from in vitro studies and in nontumor disease models indicate that 4-1BB, OX40 and GITR signaling can enhance T-cell activation (proliferation and effector function), reverse anergy, promote survival and augment the generation of long-lasting T-cell-mediated immune memory. Furthermore, stimulation of all three of these receptors alters the operational activity of natural and inducible Tregs, directly or indirectly [142]. Unique to 4-1BB is the ability of agonist therapy to reverse autoimmunity in murine models, while a role for 4-1BB and GITR, but not OX40, in innate immunity appears to exist, probably as a result of the broader expression of these two receptors [7]. It can also be argued that 4-1BB and OX40 agonists preferentially target CD8+ or CD4+ T-cell subsets, respectively [13,18,30], although results have differed between experimental models. In tumor-bearing mice, engagement of all three of these receptors provides activation signals to fully differentiated, potentially nonresponsive or anergic CD8+ effector/memory T cells (Figure 1). This appears to represent a dominant mechanism through which 4-1BB mediates antitumor activity. Secondary effects of 4-1BB ligation include the enhancement of IFN-γ-dependent NK cell and CD4+ T-cell help. Antitumor immunity elicited by OX40 stimulation, on the other hand, is achieved primarily through the optimization of CD4+ T-cell help manifest in both the priming and effector phases of the adaptive immune response. Enhanced NKT cell function and the inhibition of iTreg generation appear to play secondary roles in OX40-based therapy. Data from in vitro studies and murine tumor models collectively indicate that GITR agonists promote tumor-specific immunity mainly by rendering effector T cells resistant to Treg suppressor function. However, this conclusion is based on a rather limited number of reports, supporting the clear need for further investigation into the mechanisms of GITR-based therapy.
Figure 1. TNF-receptor-mediated immunomodulation contributing to the antitumor effects of agonist therapies.
The antitumor activity of TNF receptor (TNFR)-based therapy is executed through activation signals (upper half) to effector and memory T cells, which create an initial wave of tumor killing mediated by CD8+ cytolytic T cells and the indirect tumoricidal activity of IFN-γ [147]. Antitumor immunity can also be enhanced at this stage by the abrogation of regulatory T cell (Treg) function. Priming signals (lower half) to naive T cells in the tumor-draining lymph node also contribute to the TNFR-mediated response by generating secondary, delayed waves of tumor-specific effector cells. CD4+ Th1 cells and NK and NKT cells play essential roles in both stages, via IFN-γ-dependent CD8+ T cell ‘help’ in the effector phase (upper half) or by ‘licensing’ dendritic cell-mediated priming of naive T cells (lower half). The central box represents a source of exogenously administered agonist reagents, as would be applied therapeutically. The impact of costimulatory molecules expressed naturally by host DC is purposely discounted in the current figure. Dominant routes of TNFR-dependent immunomodulation are indicated in bold; secondary actions are italicized. GITR: Glucocorticoid-induced TNF receptor; NK: Natural killer.
The efficacy of TNFR-based therapy has been consistently demonstrated in preclinical tumor models in the absence of pathologic inflammatory responses, probably attributable to the action of inducible rather than constitutively expressed TNFRs on T cells [135]. Although efficacious as single treatment modalities, TNFR agonist reagents appear to mediate enhanced therapeutic benefit when used in combination with each other or with alternate forms of cancer therapy (Table 1). In particular, synergistic effects have been observed for the application of TNFR agonists in the setting of tumor-specific vaccination. Since TNFR engagement occurs in an antigen-independent manner, provision of signal 1 (tumor antigen) by vaccination allows therapeutic costimulation to preferentially enhance the development of specific antitumor T cells. Cancer treatments that induce ‘immunogenic’ tumor cell death, such as radiotherapy, DR5 antibody, and some types of chemotherapy are conceptually similar to active vaccination and, not surprisingly, appear to yield synergistic benefits when combined with costimulatory therapies [93,95,122]. Addition of IL-12 gene therapy to TNFR regimens has also proven to be an effective combination, probably via its coordinate promotion of both innate and adaptive immune responses. Clinical trials designed to assess the safety and efficacy of antihuman 4-1BB and OX40 agonist agents are ongoing at the current time with promising preliminary results thus far reported. By contrast, clinical application of GITR agonist therapies has been hindered owing to the lack of suitable reagents. Significant progress has recently been made however, and it is expected that patient recruitment for the first in-human studies of GITR agonists will be initiated within the coming year. Furthermore, the combination of a 4-1BB agonist and chemotherapy or radiotherapy is currently being studied in cancer patients with advanced-stage disease (NCT00351325 and NCT00461110), an encouraging sign for future combinational therapy designs that are forecast to yield improved clinical benefits.
Future perspective
The majority of preclinical studies referenced in this review have been executed in poorly immunogenic, transplantable tumor models, while others have used models of carcinogen-dependent primary tumorigenesis, which arguably more closely resemble human disease [95]. In coming years, it is expected that additional insights for the translational efficacy of TNFR agonist-based curative approaches may be garnered from the study of transgenic mice predisposed to develop spontaneous tumors. Furthermore, it is expected that recent insights into the mechanism(s) of action associated with effective TNFR-based immune modulation will yield novel combinational therapies to be evaluated in such models, as a prelude to their transition into clinical trials. As an example of possible novel combinational therapies, IL-15 has recently been shown to upregulate 4-1BB expression on CD8+ T cells, while IL-7-mediated upregulation of OX40 was observed in CD4+ T cells, leading in both cases to an expanded T-cell memory pool [18]. However, to our knowledge, the combination of 4-1BB agonists and IL-15 or OX40 agonists and IL-7 has not been studied as a therapy regimen in preclinical tumor models. Since the abrogation of Treg activity does not appear to represent the dominant mechanism underlying the benefits associated with TNFR-based therapies (Figure 1), coadministration of Treg-depleting reagents, such as denileukin diftitox (Ontak) [143], may yield a more effective combination therapy for cancer patients.
There will be great interest to discern whether disease relapse will be prevented in patients effectively treated in Phase I trials of 4-1BB and OX40 agonists, due to the well-established role of TNFR costimulation in the generation of T-cell memory [78,107]. In this regard, the optimal benefits associated with administration of agonist anti-OX40 mAb will unlikely be determined until a fully humanized reagent has been developed, thereby allowing for repeated patient dosing. Indeed, as preclinical studies suggest that multiple waves of antitumor immunity can be induced by TNFR ligation, multidose regimens will invariably represent a clinically preferred strategy (Figure 1). Recombinant human TNFR ligand–Fc fusion proteins should also be assessed in the clinic, given the comparable, or in some cases enhanced, degree of efficacy observed for ligand–Fc reagents versus agonist antibodies in mouse models (i.e., Epstein and colleagues have recently reported that a novel OX40L fusion protein but not the OX40 agonist antibody can elicit long-term survival of tumor-bearing mice using molar equivalent doses of each reagent) [105]. Due to their lower avidity and bioavailability, ligand–Fc fusion proteins may also represent a safer alternative to costimulatory agonist antibodies, which can elicit significant collateral toxicity [144].
Furthermore, as numerous studies suggest that tumor-specific T cells successfully traffic to the tumor microenvironment where they are subsequently rendered nonresponsive [3], a reasonable approach to focus intervention on such T cells would be to conjugate costimulatory agonists to tumor-targeting antibodies. For example, Zhang et al. demonstrated that 4-1BBL fused to the variable region of monoclonal antibody TNT-3 (directed against a nuclear antigen present in necrotic tumor cell debris) prolongs survival of tumor-bearing mice versus animals treated with an untargeted 4-1BBL construct [70]. Such ‘localized’ approaches would also have the expectation of limiting systemic side effects in treated patients.
Although clinical trials assessing the combination of 4-1BB agonist antibody administration along with chemotherapy or radiotherapy are already in development, additional therapeutic combination modalities can be readily envisioned for clinical translation based on existing animal models (Table 1). In addition, with the recent reported success of adoptive cell therapies and anti-CTLA-4 blocking regimens in the clinic [145,146], and given the anticipated nonoverlapping nature of how these and TNFR-based therapies work, it is highly anticipated that these modalities define additional ‘building blocks’ with which to construct novel and effective combinational regimens for the treatment of malignant disease.
Executive summary
Failure of current immunotherapies & rationale for TNF-receptor-based therapies
The therapeutic benefits of clinically approved immune-based regimens have been limited by tumor-induced immunosuppression.
TNF-receptor (TNFR)-targeting agonists break immune tolerance and induce the rejection of established tumors in mice.
Immunobiology of costimulatory TNFR family members
TNF–TNFR interactions play a role in all phases of an immune response through both redundant and distinct mechanisms.
Amplification of antitumor immunity by TNFR-based therapies
An initial wave of tumoricidal immunity is elicited through the (re)activation of CD8+ cytolytic T cells, CD4+ helper T cells, and natural killer (NK) or NKT cells, and the simultaneous abrogation of Treg function.
Naive antitumor effector T cells are subsequently primed to generate corollary waves of TNFR-dependent antitumor immunity.
Additional mechanistic insight into TNFR-based activity in humans should come from the immunomonitoring of cancer patients accrued to ongoing Phase I clinical trials.
Efficacy in preclinical tumor models
TNFR agonist agents have proven to be effective alone or in combination with tumor-specific vaccination, adoptive T-cell therapy, IL-12 gene therapy, chemo- or radio-therapy, coinhibitory blockade, and other costimulatory agonists in the treatment of established murine tumors.
The translational potential of TNFR-based therapies may be further illuminated in animal models of spontaneous tumor development.
Novel combinational approaches, such as the coadministration of IL-15 or IL-7 with TNFR agonists to optimize antitumor T-cell memory, will probably be investigated in the near future.
Transition into humans
Antihuman agonist 4-1BB and OX40 monoclonal antibodies have already entered Phase I clinical trials; however, fully humanized reagents will be required to allow repeated dosing of such therapeutics, where optimal clinical benefit may be anticipated.
Ligand fusion proteins may represent a preferred modality as they represent ‘natural’ agonists with potentially reduced concerns for collateral toxicity.
Given expectations of greatest immune dysfunction in the tumor microenvironment and its nodal basin, approaches focusing treatment to these sites may exhibit the greatest treatment benefit-to-collateral side-effect index.
It is expected that combinational approaches incorporating TNFR-based therapy will soon be ready for testing in humans.
Conclusion
The potential of TNFR-targeting therapeutics as a monotherapy or in combinational regimens for the treatment of cancer has recently become well recognized and it is anticipated that these modalities will shape the design of cancer immunotherapies in the coming years.
Acknowledgments
The authors wish to thank Louis D Falo for careful review and helpful comments provided during the preparation of this review.
Financial & competing interests disclosure
This work was supported by NIH P01 grant CA109688 (to Walter J Storkus) and T32 predoctoral training grant CA082084 (to Angela D Pardee). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Boon T, Coulie PG, Van den Eynde BJ, et al. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 2.Dunn GP, Old LJ, Schreiber RD, Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 3.Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 4.Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher P, Cohn M. A theory of self–nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7 ▪▪.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. Comprehensive review of costimulatory TNF-receptor signaling in normal, pathologic and therapeutic immune responses. [DOI] [PubMed] [Google Scholar]
- 8.Chattopadhyay K, Ramagopal UA, Brenowitz M, Nathenson SG, Almo SC. Evolution of GITRL immune function: murine GITRL exhibits unique structural and biochemical properties within the TNF superfamily. Proc Natl Acad Sci USA. 2008;105:635–640. doi: 10.1073/pnas.0710529105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Song X, Berezov A, et al. Human glucocorticoid-induced TNF receptor ligand regulates its signaling activity through multiple oligomerization states. Proc Natl Acad Sci USA. 2008;105:5465–5470. doi: 10.1073/pnas.0711350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DH, Chang WS, Lee YS, et al. 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J Immunol. 2008;180:2062–2068. doi: 10.4049/jimmunol.180.4.2062. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox RA, Chapoval AI, Gorski KS, et al. Cutting edge: Expression of functional CD137 receptor by dendritic cells. J Immunol. 2002;168:4262–4267. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 12.McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 13.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 14.Lee SW, Park Y, Song A, Cheroutre H, Kwon BS, Croft M. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol. 2006;177:4464–4472. doi: 10.4049/jimmunol.177.7.4464. [DOI] [PubMed] [Google Scholar]
- 15.Cannons JL, Lau P, Ghumman B, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 16.Wen T, Bukczynski J, Watts TH. 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J Immunol. 2002;168:4897–4906. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox RA, Tamada K, Flies DB, et al. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood. 2004;103:177–184. doi: 10.1182/blood-2003-06-2184. [DOI] [PubMed] [Google Scholar]
- 18.Sabbagh L, Snell LM, Watts TH. TNF family ligands define niches for T cell memory. Trends Immunol. 2007;28:333–339. doi: 10.1016/j.it.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169:4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 21.Zhu Y, Zhu G, Luo L, et al. CD137 stimulation delivers an antigen-independent growth signal for T lymphocytes with memory phenotype. Blood. 2007;109:4882–4889. doi: 10.1182/blood-2006-10-043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melero I, Murillo O, Dubrot J, Hervas-Stubbs S, Perez-Gracia JL. Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol Sci. 2008;29:383–390. doi: 10.1016/j.tips.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Myers LM, Vella AT. Interfacing T-cell effector and regulatory function through CD137 (4-1BB) co-stimulation. Trends Immunol. 2005;26:440–446. doi: 10.1016/j.it.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Choi WS, La S, et al. Stimulation with 4-1BB (CD137) inhibits chronic graft-versus-host disease by inducing activation-induced cell death of donor CD4+ T cells. Blood. 2005;105:2206–2213. doi: 10.1182/blood-2004-06-2080. [DOI] [PubMed] [Google Scholar]
- 25.Irie J, Wu Y, Kachapati K, et al. Modulating protective and pathogenic CD4+ subsets via CD137 in Type 1 diabetes. Diabetes. 2007;56:186–196. doi: 10.2337/db06-0793. [DOI] [PubMed] [Google Scholar]
- 26.Myers L, Takahashi C, Mittler RS, et al. Effector CD8 T cells possess suppressor function after 4-1BB and Toll-like receptor triggering. Proc Natl Acad Sci USA. 2003;100:5348–5353. doi: 10.1073/pnas.0837611100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi BK, Bae JS, Choi EM, et al. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol. 2004;75:785–791. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
- 28.Elpek KG, Yolcu ES, Franke DD, et al. Ex vivo expansion of CD4 +CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol. 2007;179:7295–7304. doi: 10.4049/jimmunol.179.11.7295. [DOI] [PubMed] [Google Scholar]
- 29.Hippen KL, Harker-Murray P, Porter SB, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847–2857. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taraban VY, Rowley TF, O’Brien L, et al. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur J Immunol. 2002;32:3617–3627. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 31.Ma BY, Mikolajczak SA, Danesh A, et al. The expression and the regulatory role of OX40 and 4-1BB heterodimer in activated human T cells. Blood. 2005;106:2002–2010. doi: 10.1182/blood-2004-04-1622. [DOI] [PubMed] [Google Scholar]
- 32.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 33.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4+ T cell mobilization/memory development and humoral immunity: comparison of αOX-40 with alphaCTLA-4. J Immunol. 2001;167:6804–6811. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 34.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172:4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 35.Prell RA, Evans DE, Thalhofer C, Shi T, Funatake C, Weinberg AD. OX40-mediated memory T cell generation is TNF receptor-associated factor 2 dependent. J Immunol. 2003;171:5997–6005. doi: 10.4049/jimmunol.171.11.5997. [DOI] [PubMed] [Google Scholar]
- 36.Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7:907–912. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 37.Graham DS, Graham RR, Manku H, et al. Polymorphism at the TNF superfamily gene TNFSF4 confers susceptibility to systemic lupus erythematosus. Nat Genet. 2008;40:83–89. doi: 10.1038/ng.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song J, Salek-Ardakani S, Rogers PR, Cheng M, Van Parijs L, Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat Immunol. 2004;5:150–158. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 39.Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8+ memory T cell survival and significantly increases recall responses. Eur J Immunol. 2007;37:157–166. doi: 10.1002/eji.200636428. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Rossi RJ, Lee SK, et al. CD134 costimulation couples the CD137 pathway to induce production of supereffector CD8 T cells that become IL-7 dependent. J Immunol. 2007;179:2203–2214. doi: 10.4049/jimmunol.179.4.2203. [DOI] [PubMed] [Google Scholar]
- 41.Mousavi SF, Soroosh P, Takahashi T, et al. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soroosh P, Ine S, Sugamura K, Ishii N. Differential requirements for OX40 signals on generation of effector and central memory CD4+ T cells. J Immunol. 2007;179:5014–5023. doi: 10.4049/jimmunol.179.8.5014. [DOI] [PubMed] [Google Scholar]
- 43.So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev. 2008;19:253–262. doi: 10.1016/j.cytogfr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda I, Ine S, Killeen N, et al. Distinct roles for the OX40–OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 46.So T, Croft M. Cutting edge: OX40 inhibits TGF-β- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 47.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 48.Ito T, Wang YH, Duramad O, et al. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci USA. 2006;103:13138–13143. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 51.Zhan Y, Gerondakis S, Coghill E, et al. Glucocorticoid-induced TNF receptor expression by T cells is reciprocally regulated by NF-κB and NFAT. J Immunol. 2008;181:5405–5413. doi: 10.4049/jimmunol.181.8.5405. [DOI] [PubMed] [Google Scholar]
- 52.Kanamaru F, Youngnak P, Hashiguchi M, et al. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–7314. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 53.Ronchetti S, Zollo O, Bruscoli S, et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 54.Kohm AP, Williams JS, Miller SD. Cutting edge: ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004;172:4686–4690. doi: 10.4049/jimmunol.172.8.4686. [DOI] [PubMed] [Google Scholar]
- 55.Esparza EM, Arch RH. Glucocorticoid-induced TNF receptor functions as a costimulatory receptor that promotes survival in early phases of T cell activation. J Immunol. 2005;174:7869–7874. doi: 10.4049/jimmunol.174.12.7869. [DOI] [PubMed] [Google Scholar]
- 56.Ronchetti S, Nocentini G, Riccardi C, Pandolfi PP. Role of GITR in activation response of T lymphocytes. Blood. 2002;100:350–352. doi: 10.1182/blood-2001-12-0276. [DOI] [PubMed] [Google Scholar]
- 57.Stephens GL, McHugh RS, Whitters MJ, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 58.Ji HB, Liao G, Faubion WA, et al. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol. 2004;172:5823–5827. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- 59.Igarashi H, Cao Y, Iwai H, et al. GITR ligand-costimulation activates effector and regulatory functions of CD4+ T cells. Biochem Biophys Res Commun. 2008;369:1134–1138. doi: 10.1016/j.bbrc.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 60.Tuyaerts S, Van Meirvenne S, Bonehill A, et al. Expression of human GITRL on myeloid dendritic cells enhances their immunostimulatory function but does not abrogate the suppressive effect of CD4+CD25+ regulatory T cells. J Leukoc Biol. 2007;82:93–105. doi: 10.1189/jlb.0906568. [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shevach EM, Stephens GL. The GITR–GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 63.Liu B, Li Z, Mahesh SP, et al. Glucocorticoid-induced tumor necrosis factor receptor negatively regulates activation of human primary natural killer (NK) cells by blocking proliferative signals and increasing NK cell apoptosis. J Biol Chem. 2008;283:8202–8210. doi: 10.1074/jbc.M708944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanabuchi S, Watanabe N, Wang YH, et al. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL) Blood. 2006;107:3617–3623. doi: 10.1182/blood-2005-08-3419. [DOI] [PubMed] [Google Scholar]
- 65.Kim HJ, Kim HY, Kim BK, Kim S, Chung DH. Engagement of glucocorticoid-induced TNF receptor costimulates NKT cell activation in vitro and in vivo. J Immunol. 2006;176:3507–3515. doi: 10.4049/jimmunol.176.6.3507. [DOI] [PubMed] [Google Scholar]
- 66 ▪.Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. First use of agonist modalities targeting 4-1BB, OX40 or glucocorticoid-induced TNF receptor in murine tumor models. [DOI] [PubMed] [Google Scholar]
- 67.Kim JA, Averbook BJ, Chambers K, et al. Divergent effects of 4-1BB antibodies on antitumor immunity and on tumor-reactive T-cell generation. Cancer Res. 2001;61:2031–2037. [PubMed] [Google Scholar]
- 68.Miller RE, Jones J, Le T, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–1800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 69.Ito F, Li Q, Shreiner AB, et al. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64:8411–8419. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
- 70.Zhang N, Sadun RE, Arias RS, et al. Targeted and untargeted CD137L fusion proteins for the immunotherapy of experimental solid tumors. Clin Cancer Res. 2007;13:2758–2767. doi: 10.1158/1078-0432.CCR-06-2343. [DOI] [PubMed] [Google Scholar]
- 71.Murillo O, Arina A, Hervas-Stubbs S, et al. Therapeutic antitumor efficacy of anti-CD137 agonistic monoclonal antibody in mouse models of myeloma. Clin Cancer Res. 2008;14:6895–6906. doi: 10.1158/1078-0432.CCR-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilcox RA, Flies DB, Wang H, et al. Impaired infiltration of tumor-specific cytolytic T cells in the absence of interferon-γ despite their normal maturation in lymphoid organs during CD137 monoclonal antibody therapy. Cancer Res. 2002;62:4413–4418. [PubMed] [Google Scholar]
- 73.Ye Z, Hellstrom I, Hayden-Ledbetter M, et al. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med. 2002;8:343–348. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- 74.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 75.Wilcox RA, Tamada K, Strome SE, et al. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol. 2002;169:4230–4236. doi: 10.4049/jimmunol.169.8.4230. [DOI] [PubMed] [Google Scholar]
- 76.Yi KH, Nechushtan H, Bowers WJ, et al. Adoptively transferred tumor-specific T cells stimulated ex vivo using herpes simplex virus amplicons encoding 4-1BBL persist in the host and show antitumor activity in vivo. Cancer Res. 2007;67:10027–10037. doi: 10.1158/0008-5472.CAN-06-2391. [DOI] [PubMed] [Google Scholar]
- 77.May KF, Jr, Chen L, Zheng P, Liu Y. Anti-4-1BB monoclonal antibody enhances rejection of large tumor burden by promoting survival but not clonal expansion of tumor-specific CD8+ T cells. Cancer Res. 2002;62:3459–3465. [PubMed] [Google Scholar]
- 78.Li B, Lin J, Vanroey M, et al. Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb. Clin Immunol. 2007;125:76–87. doi: 10.1016/j.clim.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 80.Kalinski P, Giermasz A, Nakamura Y, et al. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol Immunol. 2005;42:535–539. doi: 10.1016/j.molimm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 81.Fallarino F, Gajewski TF. Cutting edge: differentiation of antitumor CTL in vivo requires host expression of Stat1. J Immunol. 1999;163:4109–4113. [PubMed] [Google Scholar]
- 82.Nakajima C, Uekusa Y, Iwasaki M, et al. A role of interferon-γ (IFN-γ) in tumor immunity: T cells with the capacity to reject tumor cells are generated but fail to migrate to tumor sites in IFN-γ-deficient mice. Cancer Res. 2001;61:3399–3405. [PubMed] [Google Scholar]
- 83 ▪.McNamara JO, Kolonias D, Pastor F, et al. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J Clin Invest. 2008;118:376–386. doi: 10.1172/JCI33365. Oligonucleotide-based 4-1BB agonist therapy as a potentially safer and more cost-effective therapy option versus conventional antibody-based therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang S, Yang Y, Raycraft J, et al. Melanoma cells transfected to express CD83 induce antitumor immunity that can be increased by also engaging CD137. Proc Natl Acad Sci USA. 2004;101:4990–4995. doi: 10.1073/pnas.0400880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinet O, Ermekova V, Qiao JQ, et al. Immunomodulatory gene therapy with interleukin 12 and 4-1BB ligand: long- term remission of liver metastases in a mouse model. J Natl Cancer Inst. 2000;92:931–936. doi: 10.1093/jnci/92.11.931. [DOI] [PubMed] [Google Scholar]
- 86.Chen SH, Pham-Nguyen KB, Martinet O, et al. Rejection of disseminated metastases of colon carcinoma by synergism of IL-12 gene therapy and 4-1BB costimulation. Mol Ther. 2000;2:39–46. doi: 10.1006/mthe.2000.0086. [DOI] [PubMed] [Google Scholar]
- 87.Pan PY, Gu P, Li Q, et al. Regulation of dendritic cell function by NK cells: mechanisms underlying the synergism in the combination therapy of IL-12 and 4-1BB activation. J Immunol. 2004;172:4779–4789. doi: 10.4049/jimmunol.172.8.4779. [DOI] [PubMed] [Google Scholar]
- 88.Xu D, Gu P, Pan PY, et al. NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation. Int J Cancer. 2004;109:499–506. doi: 10.1002/ijc.11696. [DOI] [PubMed] [Google Scholar]
- 89.Tirapu I, Arina A, Mazzolini G, et al. Improving efficacy of interleukin-12-transfected dendritic cells injected into murine colon cancer with anti-CD137 monoclonal antibodies and alloantigens. Int J Cancer. 2004;110:51–60. doi: 10.1002/ijc.20093. [DOI] [PubMed] [Google Scholar]
- 90.Xu DP, Sauter BV, Huang TG, Meseck M, Woo SL, Chen SH. The systemic administration of Ig-4-1BB ligand in combination with IL-12 gene transfer eradicates hepatic colon carcinoma. Gene Ther. 2005;12:1526–1533. doi: 10.1038/sj.gt.3302556. [DOI] [PubMed] [Google Scholar]
- 91.McMillin DW, Hewes B, Gangadharan B, et al. Complete regression of large solid tumors using engineered drug-resistant hematopoietic cells and anti-CD137 immunotherapy. Hum Gene Ther. 2006;17:798–806. doi: 10.1089/hum.2006.17.798. [DOI] [PubMed] [Google Scholar]
- 92.Shi W, Siemann DW. Augmented antitumor effects of radiation therapy by 4-1BB antibody (BMS-469492) treatment. Anticancer Res. 2006;26:3445–3453. [PubMed] [Google Scholar]
- 93.Ju SA, Cheon SH, Park SM, et al. Eradication of established renal cell carcinoma by a combination of 5-fluorouracil and anti-4-1BB monoclonal antibody in mice. Int J Cancer. 2008;122:2784–2790. doi: 10.1002/ijc.23457. [DOI] [PubMed] [Google Scholar]
- 94.Kim YH, Choi BK, Kim KH, Kang SW, Kwon BS. Combination therapy with cisplatin and anti-4-1BB: synergistic anticancer effects and amelioration of cisplatin-induced nephrotoxicity. Cancer Res. 2008;68:7264–7269. doi: 10.1158/0008-5472.CAN-08-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95 ▪.Uno T, Takeda K, Kojima Y, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. Combinational regimen incorporating three monoclonal antibodies to simultaneously induce tumor cell death and to stimulate the resultant antitumor adaptive immune response. [DOI] [PubMed] [Google Scholar]
- 96.Teng MW, Westwood JA, Darcy PK, et al. Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res. 2007;67:7495–7504. doi: 10.1158/0008-5472.CAN-07-0941. [DOI] [PubMed] [Google Scholar]
- 97.Kudo-Saito C, Hodge JW, Kwak H, et al. 4-1BB ligand enhances tumor-specific immunity of poxvirus vaccines. Vaccine. 2006;24:4975–4986. doi: 10.1016/j.vaccine.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kocak E, Lute K, Chang X, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66:7276–7284. doi: 10.1158/0008-5472.CAN-05-2128. [DOI] [PubMed] [Google Scholar]
- 99.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 100.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 101.Kjaergaard J, Peng L, Cohen PA, Drazba JA, Weinberg AD, Shu S. Augmentation versus inhibition: effects of conjunctional OX-40 receptor monoclonal antibody and IL-2 treatment on adoptive immunotherapy of advanced tumor. J Immunol. 2001;167:6669–6677. doi: 10.4049/jimmunol.167.11.6669. [DOI] [PubMed] [Google Scholar]
- 102.Andarini S, Kikuchi T, Nukiwa M, et al. Adenovirus vector-mediated in vivo gene transfer of OX40 ligand to tumor cells enhances antitumor immunity of tumor-bearing hosts. Cancer Res. 2004;64:3281–3287. doi: 10.1158/0008-5472.can-03-3911. [DOI] [PubMed] [Google Scholar]
- 103.Ali SA, Ahmad M, Lynam J, et al. Anti-tumour therapeutic efficacy of OX40L in murine tumour model. Vaccine. 2004;22:3585–3594. doi: 10.1016/j.vaccine.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 104.Zaini J, Andarini S, Tahara M, et al. OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J Clin Invest. 2007;117:3330–3338. doi: 10.1172/JCI32693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sadun RE, Hsu WE, Zhang N, et al. Fc-mOX40L fusion protein produces complete remission and enhanced survival in 2 murine tumor models. J Immunother. 2008;31:235–245. doi: 10.1097/CJI.0b013e31816a88e0. [DOI] [PubMed] [Google Scholar]
- 106.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pan PY, Zang Y, Weber K, Meseck ML, Chen SH. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther. 2002;6:528–536. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- 108.Song A, Song J, Tang X, Croft M. Cooperation between CD4 and CD8 T cells for anti-tumor activity is enhanced by OX40 signals. Eur J Immunol. 2007;37:1224–1232. doi: 10.1002/eji.200636957. [DOI] [PubMed] [Google Scholar]
- 109 ▪▪.Murata S, Ladle BH, Kim PS, et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176:974–983. doi: 10.4049/jimmunol.176.2.974. Utilizes a translational tumor model to demonstrate the synergistic enhancement of tumor-specific vaccination by OX40 agonist treatment. [DOI] [PubMed] [Google Scholar]
- 110.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 111.Morris A, Vetto JT, Ramstad T, et al. Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo. Breast Cancer Res Treat. 2001;67:71–80. doi: 10.1023/a:1010649303056. [DOI] [PubMed] [Google Scholar]
- 112.Weinberg AD, Rivera MM, Prell R, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 113.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J Immunol. 2008;180:2140–2148. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 114.Yokouchi H, Yamazaki K, Chamoto K, et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci. 2008;99:361–367. doi: 10.1111/j.1349-7006.2007.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee SJ, Myers L, Muralimohan G, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–3012. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 116.Cuadros C, Dominguez AL, Lollini PL, et al. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116:934–943. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 117.Ramirez-Montagut T, Chow A, Hirschhorn-Cymerman D, et al. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J Immunol. 2006;176:6434–6442. doi: 10.4049/jimmunol.176.11.6434. [DOI] [PubMed] [Google Scholar]
- 118.Ko K, Yamazaki S, Nakamura K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119 ▪.Zhou P, L’Italien L, Hodges D, Schebye XM. Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced-immune activation and tumor immunity in CT26 tumors. J Immunol. 2007;179:7365–7375. doi: 10.4049/jimmunol.179.11.7365. One of few studies to investigate the role of effector T cells in the GITR-dependent augmentation of antitumor immunity. [DOI] [PubMed] [Google Scholar]
- 120.Hu P, Arias RS, Sadun RE, et al. Construction and preclinical characterization of Fc-mGITRL for the immunotherapy of cancer. Clin Cancer Res. 2008;14:579–588. doi: 10.1158/1078-0432.CCR-07-0940. [DOI] [PubMed] [Google Scholar]
- 121.Calmels B, Paul S, Futin N, Ledoux C, Stoeckel F, Acres B. Bypassing tumor-associated immune suppression with recombinant adenovirus constructs expressing membrane bound or secreted GITR-L. Cancer Gene Ther. 2005;12:198–205. doi: 10.1038/sj.cgt.7700781. [DOI] [PubMed] [Google Scholar]
- 122.Ko HJ, Kim YJ, Kim YS, et al. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 123.Zhang H, Merchant MS, Chua KS, et al. Tumor expression of 4-1BB ligand sustains tumor lytic T cells. Cancer Biol Ther. 2003;2:579–586. doi: 10.4161/cbt.2.5.545. [DOI] [PubMed] [Google Scholar]
- 124.Wan YL, Zheng SS, Zhao ZC, et al. Expression of co-stimulator 4-1BB molecule in hepatocellular carcinoma and adjacent non-tumor liver tissue, and its possible role in tumor immunity. World J Gastroenterol. 2004;10:195–199. doi: 10.3748/wjg.v10.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125 ▪▪.Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. Novel combinational approach integrating adoptive T-cell transfer and TNF-receptor-based therapies as a prelude to clinical translation. [DOI] [PubMed] [Google Scholar]
- 126.Son JH, Lee UH, Lee JJ, et al. Humanization of agonistic anti-human 4-1BB monoclonal antibody using a phage-displayed combinatorial library. J Immunol Methods. 2004;286:187–201. doi: 10.1016/j.jim.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 127.Logan T, Hodi F, Margolin K, et al. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients with advanced cancer; Presented at: International Society for Biological Therapy of Cancer 23rd Annual Meeting; San Diego, CA, USA. 31 October–2 November; 2008. [Google Scholar]
- 128.Ramstad T, Lawnicki L, Vetto J, Weinberg A. Immunohistochemical analysis of primary breast tumors and tumor-draining lymph nodes by means of the T-cell costimulatory molecule OX-40. Am J Surg. 2000;179:400–406. doi: 10.1016/s0002-9610(00)00361-5. [DOI] [PubMed] [Google Scholar]
- 129.Imura A, Hori T, Imada K, et al. OX40 expressed on fresh leukemic cells from adult T-cell leukemia patients mediates cell adhesion to vascular endothelial cells: implication for the possible involvement of OX40 in leukemic cell infiltration. Blood. 1997;89:2951–2958. [PubMed] [Google Scholar]
- 130.Vetto JT, Lum S, Morris A, et al. Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am J Surg. 1997;174:258–265. doi: 10.1016/s0002-9610(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 131 ▪▪.Petty JK, He K, Corless CL, Vetto JT, Weinberg AD. Survival in human colorectal cancer correlates with expression of the T-cell costimulatory molecule OX-40 (CD134) Am J Surg. 2002;183:512–518. doi: 10.1016/s0002-9610(02)00831-0. Clinical correlation of OX40 expression in the tumor microenvironment with prolonged survival. [DOI] [PubMed] [Google Scholar]
- 132.Ladanyi A, Somlai B, Gilde K, Fejos Z, Gaudi I, Timar J. T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res. 2004;10:521–530. doi: 10.1158/1078-0432.ccr-1161-03. [DOI] [PubMed] [Google Scholar]
- 133.Weinberg AD, Thalhofer C, Morris N, et al. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J Immunother. 2006;29:575–585. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]
- 134.Morris NP, Peters C, Montler R, et al. Development and characterization of recombinant human Fc:OX40L fusion protein linked via a coiled-coil trimerization domain. Mol Immunol. 2007;44:3112–3121. doi: 10.1016/j.molimm.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Melero I, Hervas-Stubbs S, Glennie M, et al. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 136.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-β1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 137.Salih HR, Schmetzer HM, Burke C, et al. Soluble CD137 (4-1BB) ligand is released following leukocyte activation and is found in sera of patients with hematological malignancies. J Immunol. 2001;167:4059–4066. doi: 10.4049/jimmunol.167.7.4059. [DOI] [PubMed] [Google Scholar]
- 138.Kim YS, Jung HW, Choi J, et al. Expression of AITR and AITR ligand in breast cancer patients. Oncol Rep. 2007;18:1189–1194. [PubMed] [Google Scholar]
- 139.Baltz KM, Krusch M, Baessler T, et al. Neutralization of tumor-derived soluble glucocorticoid-induced TNFR-related protein ligand increases NK cell anti-tumor reactivity. Blood. 2008;112:3735–3743. doi: 10.1182/blood-2008-03-143016. [DOI] [PubMed] [Google Scholar]
- 140.Baltz KM, Krusch M, Bringmann A, et al. Cancer immunoediting by GITR (glucocorticoid-induced TNF-related protein) ligand in humans: NK cell/tumor cell interactions. FASEB J. 2007;21:2442–2454. doi: 10.1096/fj.06-7724com. [DOI] [PubMed] [Google Scholar]
- 141.Ponte J, Apostolou I, Doty D, et al. Characterization of a humanized anti-hGITR monoclonal antibody, TRX518. Presented at: International Society for Biological Therapy of Cancer 23rd Annual Meeting; San Diego, CA, USA. 31 October–2 November 2008. [Google Scholar]
- 142.Ndhlovu LC, Takeda I, Sugamura K, et al. Expanding role of T-cell costimulators in regulatory T-cell function: recent advances in accessory molecules expressed on both regulatory and nonregulatory T cells. Crit Rev Immunol. 2004;24:251–266. doi: 10.1615/critrevimmunol.v24.i4.30. [DOI] [PubMed] [Google Scholar]
- 143.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 144.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a Phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 145.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weber JS, O’Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 147.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 148.Guinn BA, DeBenedette MA, Watts TH, Berinstein NL. 4-1BBL cooperates with B7–1 and B7–2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J Immunol. 1999;162:5003–5010. [PubMed] [Google Scholar]
- 149.Wilcox RA, Flies DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Strome SE, Martin B, Flies D, et al. Enhanced therapeutic potential of adoptive immunotherapy by in vitro CD28/4-1BB costimulation of tumor-reactive T cells against a poorly immunogenic, major histocompatibility complex class I-negative A9P melanoma. J Immunother. 2000;23:430–437. doi: 10.1097/00002371-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 151.Li Q, Iuchi T, Jure-Kunkel MN, Chang AE. Adjuvant effect of anti-4-1BB mAb administration in adoptive T cell therapy of cancer. Int J Biol Sci. 2007;3:455–462. doi: 10.7150/ijbs.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen L, Huang TG, Meseck M, et al. Rejection of metastatic 4T1 breast cancer by attenuation of Treg cells in combination with immune stimulation. Mol Ther. 2007;15:2194–2202. doi: 10.1038/sj.mt.6300310. [DOI] [PubMed] [Google Scholar]
- 153.Dannull J, Nair S, Su Z, et al. Enhancing the immunostimulatory function of dendritic cells by transfection with mRNA encoding OX40 ligand. Blood. 2005;105:3206–3213. doi: 10.1182/blood-2004-10-3944. [DOI] [PubMed] [Google Scholar]
- 154.Gri G, Gallo E, Di Carlo E, Musiani P, Colombo MP. OX40 ligand-transduced tumor cell vaccine synergizes with GM-CSF and requires CD40–APC signaling to boost the host T cell antitumor response. J Immunol. 2003;170:99–106. doi: 10.4049/jimmunol.170.1.99. [DOI] [PubMed] [Google Scholar]
- 155.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the acquisition of CD8 T cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. J Immunol. 2007;179:7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 156.Houot R, Levy R. T cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2008 doi: 10.1182/blood-2008-07-170274. DOI: 0: blood-2008-07-170274v1 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 201.Final rankings of agents with high potential for use in treating cancer. National Cancer Institute Immunotherapy Agent Workshop. 2007 July 12; http://dcb.nci.nih.gov/immunagentwork/final_rankings_of_agents_with_high_potential_for_use_in_treating_cancer.cfm.