The role of positron emission tomography (PET) with [F-18]-fluorodeoxyglucose (FDG) for the imaging evaluation of patients with cancer has expanded rapidly around the world. The development of hybrid PET-CT imaging systems that can precisely localize metabolic abnormalities and characterize the metabolic activity of normal and abnormal structures, commercial regional distribution of FDG, growing clinical experience, recently spearheaded by National Oncologic PET Registry (NOPR; http://www.cancerPETregistry.org/), and improved understanding of clinical indications and reimbursement have all facilitated the increasing use of FDG PET and PET-CT in cancer imaging. FDG PET has been employed for diagnosis, initial staging, restaging, prediction and monitoring of treatment response, surveillance and prognostication in a variety of cancers and leads to improved clinical decision-making and cost-effective management changes in considerable number of patients (1–3).
The exact clinical use of PET and PET-CT in prostate cancer is currently being explored. The recent publications summarizing the findings of NOPR demonstrated that prostate cancer was one of the most common cancers with patient enrollment (4, 5). Here we first review the available relatively limited data on the molecular biology basis of FDG accumulation in prostate cancer followed by a brief review of the current clinical experience with the use of PET with FDG in the imaging evaluation of prostate cancer.
Glucose Metabolism in Prostate Cancer
Several hallmarks of cancer have been described that include self-sufficiency in growth signals, insensitivity to anti-growth signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, tissue invasion and launch of metastasis envoys, evasion of tumors from the immune system and increased glucose metabolism (6, 7). The ability of FDG PET to detect cancer is based on the latter hallmark (Warburg effect). The relationship between tumor growth and the inefficient energy production by glucose metabolism is not well understood but may be explained in terms of adaptation to hypoxia through upregulation of glucose transporters and increased enzymatic activity of hexokinase (8, 9). Glucose transporter - the currently approved gene symbol is “SLC2Ax” but here we will use the familiar symbol “GLUTx” - is the first rate-limiting step for glucose metabolism that allows energy-independent glucose transport across the cell membrane down the concentration gradient while hexokinase-II phosphorylates glucose to glucose-6-phosphate. Similarly, FDG is phosphorylated to FDG-6-phosphate but contrary to glucose-6-phosphate, it cannot be metabolized further in the glycolytic pathway and becomes trapped in the cell due to its negative charge and the very low activity of the reverse enzyme, glucose-6-phophatase, in most cancers (10–15).
The GLUT1 mRNA expression has been assessed in the androgen-independent cell lines, DU145 and PC-3, and the androgen-sensitive cell line LNCaP (16). The poorly differentiated androgen-independent cell lines showed higher mRNA expression than the well-differentiated androgen-sensitive cell line suggesting that GLUT1 expression directly related to the malignancy grade. One study evaluated the expression of a number of hypoxia-associated genes within benign prostatic hyperplasia (BPH) and prostate cancer (Gleason score 5 to 10) human tissue specimen (17). GLUT1 gene expression was not only significantly higher in the tumor than in BPH but also was correlated directly with Gleason score (R=0.274, p=0.026), corroborating the direct relationship between GLUT1 expression and tumor grade. Other in-vitro studies have also shown that FDG uptake increases with hypoxic condition in both androgen-sensitive and androgen-independent prostate cancer cell lines (18). Moreover, hypoxia resulted in upregulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha that could be inhibited with the nonsteroidal anti-inflammatory drug Ibuprofen leading to cyclooxygenase-2 independent down-regulation of HIF-regulated proteins vascular endothelial growth factor (VEGF) and GLUT1 (19). The glucose metabolism of prostate cancer may not be limited to GLUT1 as another investigation showed that GLUT12 may also be involved (20). The modulatory effect of androgen on tumor glucose metabolism has also been investigated. Higher FDG accumulation has been observed in androgen-independent tumors than in androgen-sensitive tumors (21). Accordingly, it has been observed that castration reduces glucose metabolism in the prostate tumor (21–23). In summary, despite very limited data, it appears that glucose metabolism in prostate cancer is GLUT-mediated but the relationship is complex and may be affected by many factors such as hypoxia and androgen level.
Clinical Utility of FDG PET in Prostate Cancer
There is considerable heterogeneity in the current clinical experience with FDG PET inprostate cancer. This is due to variability in disease states, validation criteria, and end points among studies (24–26). Additional complicating factors include the inherent biological and clinical heterogeneity of the disease as well as technical and image processing factors (e.g. filtered-back projection in comparison to iterative reconstruction with segmented attenuation) irrespective of the underlying biological heterogeneity (18, 27–29).
Normal Prostate
We recently reported on the FDG accumulation in the normal prostate gland in relation to age and prostate size on PET-CT studies that were performed on 145 men (age range 22-97 years) with no clinical and laboratory evidence of prostate pathology (30). The population average and range of the normal prostate size were 4.3 +/− 0.5 cm (mean +/− SD) and 2.9–5.5 cm, respectively. The population average of mean and maximum SUV (standardized uptake value as semi-quantitative measure of the amount of tracer accumulation in a region of interest normalized to body weight and injected tracer dose) was 1.3 +/− 0.4 (range 0.1–2.7) and 1.6 +/− 0.4 (range 1.1–3.7), respectively. Mean SUV tended to decrease as the prostate size increased (r = −0.16, P = 0.058) while the prostate tended to get larger as age increased (r = 0.32, P < 0.001). Despite the generally low FDG uptake by the normal prostate gland, however, other studies have shown that there can be significant overlap among FDG accumulation in normal prostate, BPH and prostate cancer (25, 26, 31–34).
Diagnosis and Initial Staging
FDG PET may not be useful in diagnosis and staging of clinically organ-confined disease and in detection of locally recurrent disease due to overlap of tumor uptake with scar tissue and also because of the obscuring that can occur due to intense urine activity in the adjacent urinary bladder (32, 35). FDG is also not specific for cancer and false positives may occur with inflammatory condition such as prostatitis (36). However, despite these less enthusiastic observations, several animal-based translational and human-based clinical studies have shown that FDG PET can be useful in specific clinical circumstances in prostate cancer. FDG uptake in prostate cancer increases directly with increasing Gleason grade, clinical stage and serum PSA level (37, 38). In an early experience with 34 men with prostate cancer and known or suspected metastatic disease, FDG PET demonstrated a SUV range of 2.1 to 5.7 for the metastatic lesions with the notation that PET was less sensitive than bone scintigraphy and that detection of pelvic lymph node metastases was limited due to intense bladder urine activity (39). However in patients with known osseous metastatic disease, FDG PET may distinguish the metabolically active lesions from the metabolically quiescent lesions (40–42). Also, recent data from our laboratory suggest that FDG PET concordance rate with other imaging studies appears to be higher in the castrate-resistant disease in comparison to castrate-sensitive disease and also higher for lymph nodes and visceral lesions than for osseous lesions (43).
PSA Relapse Only
A large group of men present with “PSA relapse only” (biochemical failure) at some point after definitive therapy in whom by definition there is no standard imaging evidence of disease. In this group of men, “non-standard” imaging detection of disease can direct appropriate treatment such as salvage radiation therapy for local recurrence in the prostate bed or systemic therapy for metastatic disease (Fig. 1, Fig. 2). In one study of 24 patients with a rising serum PSA level after treatment for localized prostate cancer, FDG PET was performed before pelvic lymph node dissection (44). In accordance with “PSA relapse only” disease, all men had negative findings on whole body bone scan and equivocal pelvic CT results. Validation was by histology of the pelvic lymph nodes harvested at surgery in 67% of patients. The authors reported a sensitivity of 75%, specificity of 100%, accuracy of 83.3%, positive predictive value of 100%, and negative predictive value of 67.7% in detecting metastatic pelvic lymph nodes in this relatively homogeneous state of disease. In a similar investigation of 91 patients with PSA relapse following prostatectomy and validation by biopsy or clinical and imaging follow-up, the researchers reported that mean PSA level was higher in PET-positive than in PET-negative patients (9.5 +/− 2.2 vs. 2.1 +/− 3.3 ng/mL) (45). Using a receiver operating characteristic (ROC) curve analysis, the best tradeoff between sensitivity and specificity was found at a PSA level of 2.4 ng/mL (80%, 73%, respectively) and PSA velocity of 1.3 ng/mL/y (71%, 77%, respectively). The authors reported that FDG PET detected local or systemic disease in 31% of patients with PSA relapse. However, confidence in the accuracy and relevance of this figure is guarded in view of the definition of PSA relapse and the heterogeneity of the validation criteria, frequent with other similar studies (46). Other studies have shown that FDG PET may be particularly useful in men with rising PSA level despite treatment and that in the clinical setting of PSA relapse, it is more sensitive than In-111 capromab pendetide scintigraphy (47, 48).
Fig. 1.
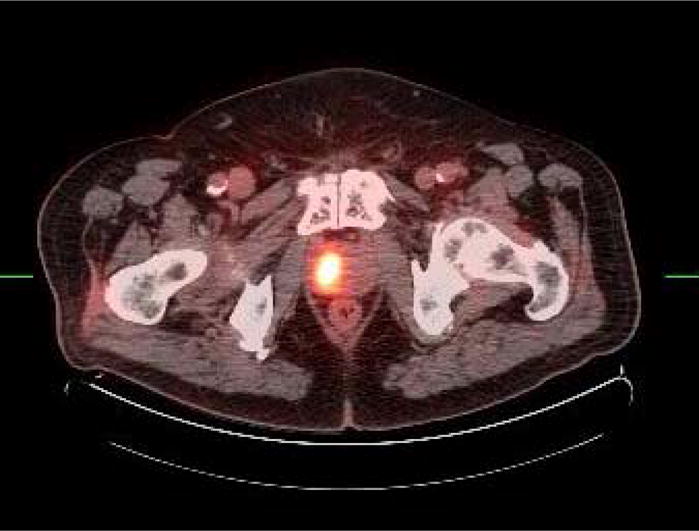
Axial fused FDG PET-CT of locally recurrent disease in right prostate lobe 11 years after radiation therapy for primary moderately differentiated prostate cancer with Gleason sum score of 4.
Fig. 2.
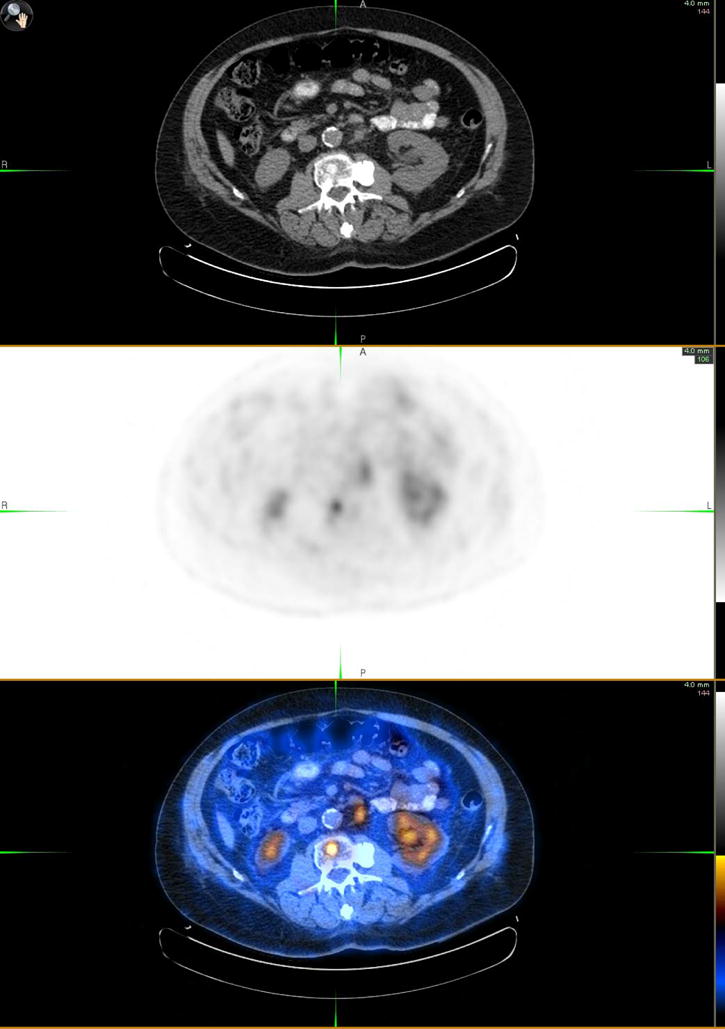
Axial CT (top panel), FDG PET (middle panel) and fused PET-CT (bottom panel) of metastatic prostate cancer involving L2 vertebral body (SUVmax = 4.8) and a conglomerate of subcentimeter left paraaortic nodes (SUVmax = 3.3, largest single node 9 mm).
Evaluation of Treatment Response
FDG PET has also been investigated for its utility in the assessment of response to treatment. An earlier rat prostate cancer study was disappointing in showing that the global FDG uptake was unchanged after treatment with gemcitabine (49). However a later investigation demonstrated that FDG accumulation in the primary prostate cancer and metastatic sites decreased 1–5 months after initiation of androgen deprivation therapy similar in principle to the results of the animal xenograft studies (21, 50, 51). Our preliminary results with both androgen deprivation and various chemotherapy regimens have shown that the tumor accumulation of FDG uptake decreases with successful treatment in concordance with other measures of response such as decline in serum PSA level (Fig. 3).
Fig. 3.
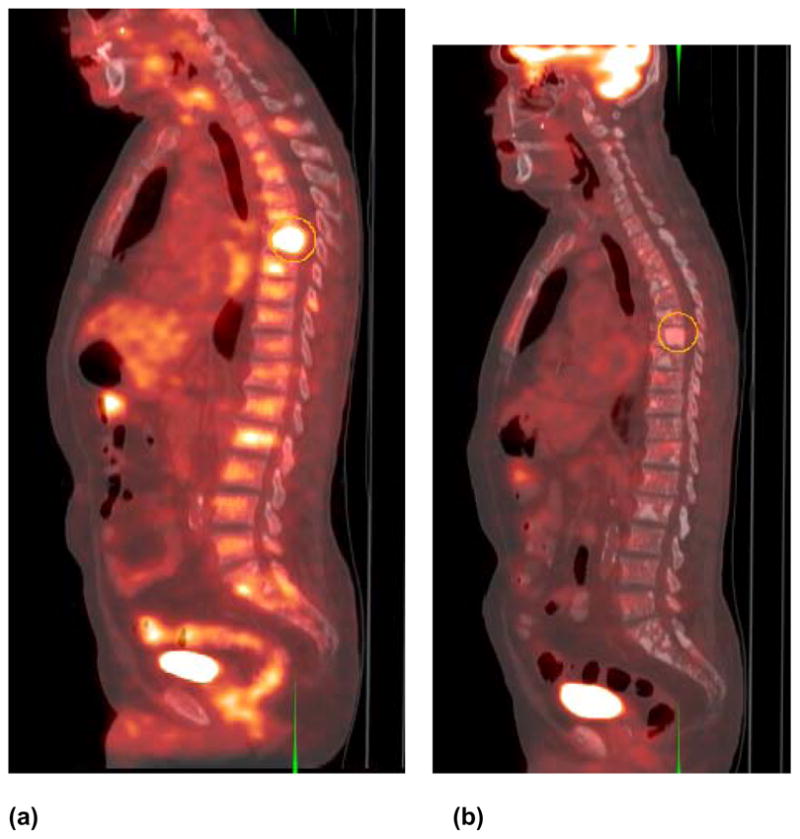
Sagittal fused FDG PET-CT in a man with castrate-sensitive metastatic prostate cancer (a) before and (b) five months after androgen deprivation therapy. Note the decline in the hypermetabolic activity of the sclerotic mid thoracic spine vertebral body in response to therapy. Lesion maximum SUV declined from 5.0 before therapy to 2.7 after therapy corresponding to serum PSA decline from 98 to 21 ng/mL, respectively.
Prognostication
It has also been shown that the level and extent of FDG accumulation in metastatic lesions may also provide information on prognosis. One investigation reported that >33% increase in average SUVmax of up to 5 lesions or appearance of new lesions could dichotomize castrate metastatic prostate cancer patients treated with antimicrotubule chemotherapy as progressors or non-progressors (52). Similar prognostic utility was demonstrated in that men with highly FDG-avid primary prostate tumors had a poorer prognosis in comparison to those with low SUV (53). Since FDG uptake in the prostate tumor appears to be initially dependent on androgen, FDG PET may also be useful in the prediction of the time to androgen refractory state (e.g. early increase in tumor FDG uptake during castrate state) that may allow an early intervention in order to avert or delay this clinical state and improve overall patient outcome (21).
NOPR and Prostate Cancer
The NOPR was established in May 2006 to collect and analyze data on the clinical utility of FDG PET to provide evidence for reimbursement coverage by the Centers for Medicare & Medicaid Services (CMS)(4). The results for the first 2 years of data from 40,863 PET scans for staging, restaging, or detection of suspected recurrence in patients with pathologically-proven cancers were recently reported (5). The most number of FDG PET scans (5,309 scans equivalent to 13% of all scans) was performed in patients with prostate cancer. PET findings changed clinical management in 35.1% of prostate cancer cases (95% CI: 33.8%−36.4%) although the odds ratio for change in management compared with that for other cancers in the NOPR trial was less than 1 at 0.86 (95% CI: 0.81–0.92) implying that chance of changing management was lower for prostate cancer than that for all the other cancer types. The change in management was from non-treatment to treatment in 25.3% and from treatment to non-treatment in 9.7% of cases with a major change in type of treatment relative to the plan before PET in 8.5% of cases. The PET-directed change in management was nearly equal in relation to the testing indications with 32.0% (30.0–34.1%) for initial staging (n=2042 cases), 34.0% (95% CI: 31.6–36.4%) for restaging (n=1477 cases), and 39.4% (95% CI: 37.2–41.7%) for detection of suspected recurrence (n=1790 cases).
Conclusion
The current clinical experience with FDG PET in prostate cancer is afflicted by considerable variability in disease states, validation criteria, and end points. Despite this significant limitation, however, it appears that FDG PET may be useful for diagnosis and staging of known or suspected high Gleason score primary tumors, in detection of locally recurrent and/or metastatic disease in a portion of patients with “PSA relapse only” with scan sensitivity that increases with increasing PSA level, in assessment of the extent of metabolically active castrate-resistant disease, in monitoring response to androgen deprivation and other therapies, and in prognostication As suggested by NOPR trial, FDG PET can impact the clinical management of men with prostate cancer although this impact may be lower than that for other cancers. FDG PET may be limited in diagnosis and staging of clinically organ-confined disease and can be falsely negative due to overlap of tumor uptake with normal, BPH, and scar tissue as well as be falsely positive with inflammatory conditions. More extensive experience obtained through well-designed clinical trials based on well-defined clinical states of disease and hard end-points can help to determine the exact role of FDG PET in prostate cancer. A prospective clinical trial is currently underway to help define the role of FDG PET-CT in therapy assessment and in outcome prediction (time to hormone refractory state and survival) in men with androgen-naïve and androgen refractory metastatic prostate cancer (54).
Acknowledgments
National Institutes of Health – National Cancer Institute Grant No. R01-CA111613.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Phelps ME. PET: the merging of biology and imaging into molecular imaging. J Nucl Med. 2000;41:661–681. [PubMed] [Google Scholar]
- 2.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 3.Basu S, Alavi A. Unparalleled contribution of 18F-FDG PET to medicine over 3 decades. J Nucl Med. 2008;49:17N–21N. 37N. [PubMed] [Google Scholar]
- 4.Hillner BE, Siegel BA, Liu D, et al. Impact of positron emission tomography/computed tomography and positron mission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry. J Clin Oncol. 2008;26:4229. doi: 10.1200/JCO.2007.14.5631. [DOI] [PubMed] [Google Scholar]
- 5.Hillner BE, Siegel BA, Shields AF, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the National Oncologic PET Registry. J Nucl Med. 2008;49:1928–1935. doi: 10.2967/jnumed.108.056713. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Gambhir SS. Molecular imaging of cancer: from molecules to humans. Introduction. J Nucl Med. 2008;49(Suppl 2):1S–4S. doi: 10.2967/jnumed.108.053751. [DOI] [PubMed] [Google Scholar]
- 8.Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49(6 Suppl):24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 9.Plathow C, Weber WA. Tumor cell metabolism imaging. J Nucl Med. 2008;49(6 Suppl):43S–63S. doi: 10.2967/jnumed.107.045930. [DOI] [PubMed] [Google Scholar]
- 10.Medina RA, Owen GI. Glucosetransporters: expression, regulation and cancer. Biol Res. 2002;35:9–26. doi: 10.4067/s0716-97602002000100004. [DOI] [PubMed] [Google Scholar]
- 11.Smith TA. Facilitative glucose transporter expression in human cancer tissue. Br J Biomed Sci. 1999;56:285–92. [PubMed] [Google Scholar]
- 12.Macheda ML, Rogers S, Bets JD. Molecular and cellular regulation of glucose transport (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 13.Wilson JE. Isoenzymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206(Pt 12):2049–57. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 14.Smith TA. Mammalian hexokinases and their abnormal expression in cancer. Br J Biomed Sci. 2000;57:170–8. [PubMed] [Google Scholar]
- 15.Pastorino JG, Hoek JB. Hexokinase II: the integration of energy metabolism and control of apoptosis. Curr Med Chem. 2003;10:1535–51. doi: 10.2174/0929867033457269. [DOI] [PubMed] [Google Scholar]
- 16.Effert P, Beniers AJ, Tamimi Y, et al. Expression of glucose transporter 1 (GLUT-1) in cell lines and clinical specimen from human prostate adenocarcinoma. Anticancer Res. 2004;24(5A):3057–3063. [PubMed] [Google Scholar]
- 17.Stewardt GD, Gray K, Pennington CJ, et al. Analysis of hypoxia-associated gene expression in prostate cancer: lysyl oxidase and glucose transporter 1 expression correlate with Gleason score. Oncol Rep. 2008;20:1561–1567. [PubMed] [Google Scholar]
- 18.Hara T, Bansal A, DeGrado TR. Effect of hypoxia on the uptake of [methyl-3H]choline, [1–14C]acetate and [18F]FDG in cultured prostate cancer cells. Nucl Med Biol. 2006;33:977–984. doi: 10.1016/j.nucmedbio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Palayoor ST, Tofilon PJ, Coleman CN. Ibuprofen-mediated reduction of hypoxia-inducible factors HIF-1alpha and HIF-2alpha in prostate cancer cells. Clin Can Res. 2003;9:3150–3157. [PubMed] [Google Scholar]
- 20.Chandler JD, Williams ED, Slavin JL, et al. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer. 2003;97:2035–2042. doi: 10.1002/cncr.11293. [DOI] [PubMed] [Google Scholar]
- 21.Jadvar H, Li X, Shahinian A, et al. Glucose metabolism of human prostate cancer mouse xenografts. Mol Imaging. 2005;4:91–97. doi: 10.1162/15353500200505118. [DOI] [PubMed] [Google Scholar]
- 22.Oyama N, Kim J, Jones LA, et al. MicroPET assessment of androgenic control of glucose and acetate uptake in the rat prostate and a prostate cancer tumor model. Nuc Med Biol. 2002;29:783–90. doi: 10.1016/s0969-8051(02)00346-3. [DOI] [PubMed] [Google Scholar]
- 23.Agus DB, Golde DW, Squouros G, et al. Positron emission tomography of a human prostate cancer xenograft: association of changes in deoxyglucose accumulation with other measures of outcome following androgen withdrawal. Cancer Res. 1998;58:3009–3014. [PubMed] [Google Scholar]
- 24.Apolo AB, Pandit-Taskar N, Morris MJ. Novel tracers and their development for the imaging of metastatic prostate cancer. J Nucl Med. 2008;49:2031–2041. doi: 10.2967/jnumed.108.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi N, Inoue T, Lee J, et al. The roles of PET and PET/CT in the diagnosis and management of prostate cancer. Oncology. 2007;72:226–233. doi: 10.1159/000112946. [DOI] [PubMed] [Google Scholar]
- 26.Salminen E, Hogg A, Binns D, et al. Investigations with FDG-PET scanning in prostate cancer show limited value for clinical practice. Acta Oncol. 2002;41(5):425–429. doi: 10.1080/028418602320405005. [DOI] [PubMed] [Google Scholar]
- 27.Pugachev A, Ruan S, Carlin S, et al. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys. 2005;62:545–553. doi: 10.1016/j.ijrobp.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Etchebehere EC, Macapinlac HA, Gonen M, et al. Qualitative and quantitative comparison between images obtained with filtered back projection and iterative reconstruction in prostate cancer lesions of 18F-FDG PET. Q J Nucl Med. 2002;46:122–130. [PubMed] [Google Scholar]
- 29.Turlakow A, Larson SM, Coakley F, et al. local detection of prostate cancer by positron emission tomography with 2-fluorodeoxyglucose: comparison of filtered back projection and iterative reconstruction with segmented attenuation correction. Q J Nucl Med. 2001;45:235–244. [PubMed] [Google Scholar]
- 30.Jadvar H, Ye W, Groshen S, et al. [F-8]-fluorodeoxyglucose PET-CT of the normal prostate gland. Ann Nucl Med. 2008;22:787–793. doi: 10.1007/s12149-008-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Effert PJ, Bares R, Handt S, et al. Metabolic imaging of untreated prostate cancer by positron emission tomography with 18fluorine-labeled deoxyglucose. J Urol. 1996;155:994–998. [PubMed] [Google Scholar]
- 32.Hofer C, Laubenbacher C, Block T, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur Urol. 1999;36:31–35. doi: 10.1159/000019923. [DOI] [PubMed] [Google Scholar]
- 33.Patel P, Cohade C, DeWeese T, et al. Evaluation of metabolic activity of prostate gland with PET-CT. J Nucl Med. 2002;43(5 Suppl):119P. [Google Scholar]
- 34.von Mallek D, Backhaus B, Muller SC, et al. Technical limits of PET/CT with 18FDG in prostate cancer. Aktuelle Urol. 2006;37:218–221. doi: 10.1055/s-2006-932129. [DOI] [PubMed] [Google Scholar]
- 35.Liu IJ, Zafar MB, Lai YH, et al. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology. 2001;57:108–111. doi: 10.1016/s0090-4295(00)00896-7. [DOI] [PubMed] [Google Scholar]
- 36.Kao PF, Chou YH, lai CW. Diffuse FDG uptake in acute prostatitis. Clin Nucl Med. 2008;33:308–310. doi: 10.1097/RLU.0b013e3181662f8b. [DOI] [PubMed] [Google Scholar]
- 37.Oyama N, Akino H, Suzuki Y, et al. The increased accumulation of [18F]fluorodeoxyglucose in untreated prostate cancer. Jpn J Clin Oncol. 1999;29:623–9. doi: 10.1093/jjco/29.12.623. [DOI] [PubMed] [Google Scholar]
- 38.Kanamaru H, Oyama N, Akino H, et al. Evaluation of prostate cancer using FDG-PET. Hinyokika Kiyo. 2000;46:851–853. [PubMed] [Google Scholar]
- 39.Shreve PD, Grossman HB, Gross MD, et al. Metastatic prostate cancer: initial findings of PET with FDG. Radiology. 1996;199:751–756. doi: 10.1148/radiology.199.3.8638000. [DOI] [PubMed] [Google Scholar]
- 40.Morris NJ, Akhurst T, Osman I, et al. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. 2002;59:913–918. doi: 10.1016/s0090-4295(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 41.Yeh SD, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23:693–697. doi: 10.1016/0969-8051(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 42.Jadvar H, Pinski J, Conti P. FDG PET in suspected recurrent and metastatic prostate cancer. Oncol Rep. 2003;10(5):1485–1488. [PubMed] [Google Scholar]
- 43.Jadvar H, Pinski J, Quinn D, Ye W, et al. Concordance among FDG PET, CT and bone scan in men with metastatic prostate cancer. Proc SNM 55th Ann Meeting; New Orleans, LA. 2008. p. 373P. [Google Scholar]
- 44.Chang CH, Wu HC, Tsai JJ, et al. Detecting metastatic pelvic lymph nodes by (18)f-2-deoxyglucose positron emission tomography in patients with prostate- specific antigen relapse after treatment for localized prostate cancer. Urol Int. 2003;70(4):311–315. doi: 10.1159/000070141. [DOI] [PubMed] [Google Scholar]
- 45.Schoder H, Herrmann K, Gonen M, et al. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res. 2005;11:4761–4769. doi: 10.1158/1078-0432.CCR-05-0249. [DOI] [PubMed] [Google Scholar]
- 46.Sanz G, Robles JE, Gimenez M, et al. Positron emission tomography with 18fluorine-labelled deoxyglucose: utility in localized and advanced prostate cancer. BJU Int. 1999;84:1028–1031. doi: 10.1046/j.1464-410x.1999.00349.x. [DOI] [PubMed] [Google Scholar]
- 47.Sung J, Espiritu JI, Segall GM, et al. Fluorodeoxyglucose positron emission tomography studies in the diagnosis and staging of clinically advanced prostate cancer. BJU Int. 2003;92:24–27. doi: 10.1046/j.1464-410x.2003.04297.x. [DOI] [PubMed] [Google Scholar]
- 48.Seltzer MA, Barbaric Z, Belldegrun A, et al. Comparison of helical computerized tomography, positron emission tomography and monoclonal antibody scans for evaluation of lymph node metastases in patients with prostate specific antigen relapse after treatment for localized prostate cancer. J Urol. 1999;162:1322–1328. [PubMed] [Google Scholar]
- 49.Haberkorn U, Bellemann ME, Altmann A, et al. PET 2-fluoro-2-deoxyglucoseuptake in rat prostate adenocarcinoma during chemotherapy with gemcitabine. J Nucl Med. 1997;38:1215–1221. [PubMed] [Google Scholar]
- 50.Oyama N, Akino H, Suzuki Y, et al. FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun. 2001;22:963–969. doi: 10.1097/00006231-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Saylor M, Wen S, et al. Longitudinally quantitative 2-deoxy-2-[18F]fluoro-D-glucose micro positron emission tomography imaging for efficacy of new anticancer drugs: a case study with bortezomib in prostate cancer murine model. Mol Imaging Biol. 2006;8:300–308. doi: 10.1007/s11307-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 52.Morris MJ, Akhurst T, Larson SM, et al. Fluorodeoxyglucose positron emission tomography as an outcome measure for castrate metastatic prostate cancer treated with antimicrotubule chemotherapy. Clin Cancer Res. 2005;11:3210–6. doi: 10.1158/1078-0432.CCR-04-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oyama N, Akino H, Suzuki Y, et al. Prognostic value of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography imaging for patients with prostate cancer. Mol Imaging Biol. 2002;4:99–104. doi: 10.1016/s1095-0397(01)00065-6. [DOI] [PubMed] [Google Scholar]
- 54.Jadvar H. [F-18]-Fluorodeoxyglucose (FDG) Positron Emission Tomography and Computed Tomography (PET-CT) in Metastatic Prostate Cancer. USC Norris Comprehensive Cancer Center. 2008 ClinicalTrials.gov Identifier: NCT00282906. [Google Scholar]


