Abstract
Activity is thought to guide the patterning of synaptic connections in the developing nervous system. Specifically, differences in the activity of converging inputs are thought to cause the elimination of synapses from less active inputs and increase connectivity with more active inputs1,2. Here we present findings that challenge the generality of this notion and offer a novel view of the role of activity in synapse development. To imbalance neurotransmission from different sets of inputs in vivo, we generated transgenic mice in which ON but not OFF types of bipolar cells (BCs) in the retina express tetanus toxin (TeNT). During development, retinal ganglion cells (RGCs) select between ON and OFF BC inputs (ON or OFF RGCs) or establish a similar number of synapses with both on separate dendritic arbors (ON-OFF RGCs). In TeNT retinas, ON RGCs correctly selected the silenced ON BC inputs over the transmitting OFF BCs, but were connected with them through fewer synapses at maturity. Time-lapse imaging revealed that this was caused by a reduced rate of synapse formation rather than an increase in synapse elimination. Similarly, TeNT-expressing ON BC axons generated fewer presynaptic active zones. The remaining active zones often recruited multiple, instead of single, synaptic ribbons. ON-OFF RGCs in TeNT mice maintained convergence of ON and OFF BCs inputs and had fewer synapses on their ON arbor without changes to OFF arbor synapses. Our results reveal an unexpected and remarkably selective role for activity in circuit development in vivo, regulating synapse formation but not elimination, affecting synapse number but not dendritic or axonal patterning, and mediating independently the refinement of connections from parallel (ON and OFF) processing streams even where they converge onto the same postsynaptic cell.
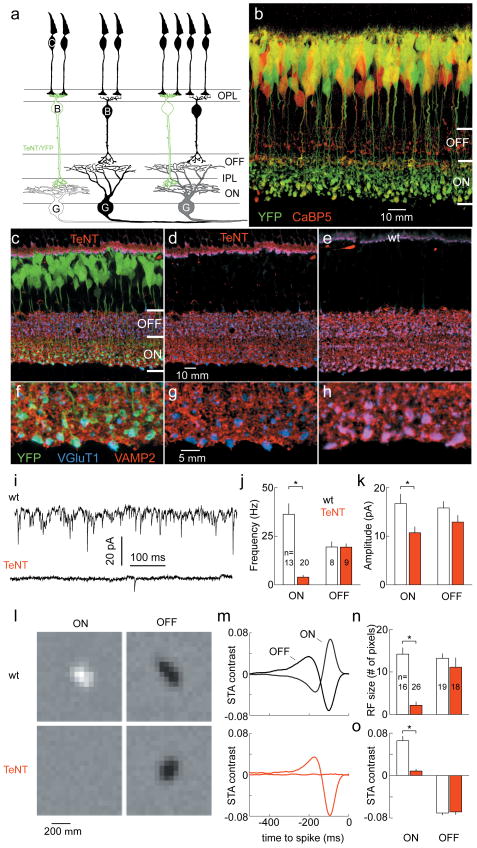
ON BCs, which depolarize in response to light, receive and invert photoreceptor signals through metabotropic glutamate receptors (mGluR6) on their dendrites. OFF BCs, instead, express ionotropic glutamate receptors and hyperpolarize upon illumination3,4. Thus, the parallel processing of light increment (ON) and decrement (OFF) signals that is central to visual system function is initiated at the first synapse in the retina. To create an imbalance of glutamate release from ON and OFF BCs in the inner plexiform layer (IPL) where their axons relay signals to RGCs, we generated transgenic mice in which a promoter fragment of mGluR65 drove expression of the light chain of TeNT and YFP (mGluR6-YFP/TeNT, Fig. 1a). TeNT, a bacterial protease, cleaves vesicle associated membrane protein 2 (VAMP2) and inhibits vesicle fusion 6. In mGluR6-YFP/TeNT mice, transgene expression was limited to ON BCs which ramify their axons in the inner half of the IPL (Fig. 1b), and was present throughout the period of BC - RGC synaptogenesis (Supplementary Fig. 1). We tested the proteolytic activity of TeNT using an antibody against VAMP27. Double-labeling for the vesicular glutamate transporter 1 (VGluT1), which is present in all BC axon terminals, verified that VAMP2 was selectively depleted from ON BCs (Fig. 1c - h). To examine glutamate release, we recorded spontaneous excitatory postsynaptic currents (sEPSCs) from large monostratified RGCs in retinal flat mount preparations (Fig. 1i). ON RGCs from mGluR6-YFP/TeNT mice showed very few and lower amplitude sEPSCs compared to wildtype, whereas OFF RGC sEPSCs were normal in frequency and amplitude (Fig. 1i - k). Focal application of the glutamate receptor agonist kainate (100 μM) near the soma of ON RGCs elicited excitatory currents of similar amplitude and without failure in wildtype and mGluR6-YFP/TeNT mice (Supplementary Fig. 2), arguing that the reduction in spontaneous events likely reflects a blockade of presynaptic transmitter release.
Figure 1. Selective blockade of glutamate release from retinal ON BCs.
a, ON and OFF pathways. C, cone; B, bipolar cell; G, ganglion cell; O / IPL, outer / inner plexiform layer. ON (white), OFF (black) and ON-OFF responsiveness (gray) indicated by fill color. b, YFP expression in mGluR6-YFP/TeNT mouse at postnatal day 21 (P21) colocalizes with anti-CaBP5 labeling in ON but not OFF BCs 15. c - h, Overview (c - e) and enlargements of ON sublamina (f - h) from P21 mGluR6-YFP/TeNT (c, d, f, g) and wildtype retinas (e, h). YFP not shown in d and g. i - k, Representative traces (i, ON RGCs) and population data (mean ± s.e.m., j, k) for sEPSC recorded from RGCs at P21. l, m, Spatial profile (l, 100 ms before spike) and time course (m, average of ten pixels with highest SD) of STAs from representative RGCs. n, o, Summary data (mean ± s.e.m.) of receptive field extent (n, # pixels whose SD exceeded that of background pixels more than fourfold), and sensitivity (o, peak of average STA time course exemplified in m). * indicates p < 0.01.
To examine ON and OFF responses, which are driven by glutamate release from BCs, we used multielectrode arrays to record from ensembles of RGCs in wildtype and mGluR6-YFP/TeNT mice during visual stimulation. Receptive fields were characterized by the spike-triggered average (STA) stimulus during white noise presentation8. In wildtype retinas, all RGCs were light responsive, and ON and OFF RGCs displayed similar receptive field size and sensitivity (Fig. 1l - o). By contrast, in mGluR6-YFP/TeNT mice OFF RGC showed normal receptive field properties, but ON responses were greatly reduced or absent (Fig. 1l - o). Taken together, these results suggest that in mGluR6-YFP/TeNT mice both spontaneous and evoked glutamate release were diminished from ON but not OFF BCs.
Early in development, monostratified RGCs elaborate dendrites throughout the depth of the IPL9,10 and appear to be contacted by ON and OFF BCs11. With maturation, dendritic branches are pruned and a single narrowly stratified arbor connected to either ON or OFF BCs is maintained12. To visualize dendrites and excitatory synapses, we biolistically labeled single RGCs with tdTomato and PSD95-CFP13. Figure 2 shows that despite the selective reduction of input activity from ON BCs in mGluR6-YFP/TeNT mice, ON RGC dendrites stratified normally and their lateral branching patterns were indistinguishable from wildtype littermates (Fig. 2a, b, e, f, and Supplementary Fig. 3). Excitatory postsynaptic sites marked by PSD95-CFP, on ON RGC dendrites were localized precisely to appositions with TeNT-expressing ON BCs (Fig. 2c, Supplementary Fig. 4, Supplementary Movie 1) but were reduced in density by ∼50% compared to wildtype at P21 (Fig. 2d, g and h). The remaining synapses showed similar centroperipheral distributions across dendritic fields as in wildtype (Fig. 2d, i and j). These results indicate that input selection and dendritic stratification of postsynaptic RGCs in mice occur independent of BC activity (see Supplementary Information). Neurotransmission, however, is crucial for normal synaptic connectivity with the selected inputs.
Figure 2. Silencing ON BCs reduces synapse number on RGC dendrites in an input specific manner without changes to laminar targeting or branching.
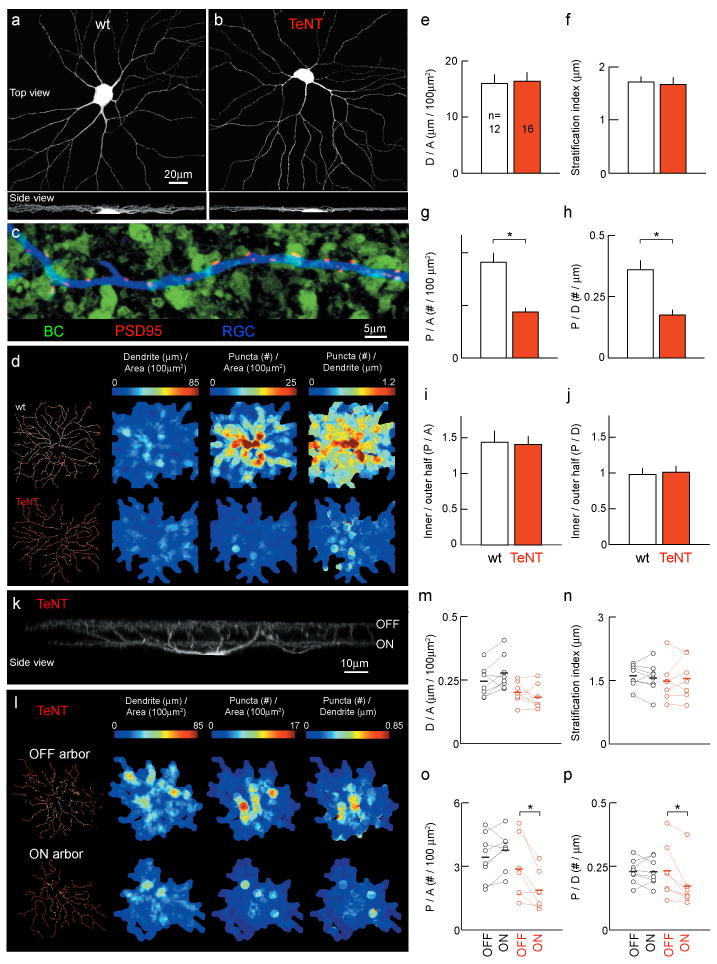
a, b, Large ON RGCs (tdTomato, P21). c, RGC dendrite (tdTomato) in a TeNT retina (P21) illustrating the localization of PSD95-CFP puncta to BC (YFP) contacts (see also Supplementary Movie 1 and Supplementary Fig. 4). d, Maps of dendritic and glutamatergic postsynaptic densities for representative P21 ON RGCs. e - j, Mean (± s.e.m.) RGC dendritic density (e, D/A) and stratification (f, see also Supplementary Fig. 3), areal (g, P/A) and linear (h, P/D) density of PSD95-CFP puncta, and centroperipheral gradients (i and j) of PSD95-CFP puncta across dendritic fields for large ON RGCs13. k, Bistratified ON-OFF RGC (tdTomato, P21) in TeNT retina. l, Maps of dendritic and glutamatergic postsynaptic densities for OFF and ON arbor for a representative bistratified RGC in an mGluR6-YFP/TeNT mouse. m - p, Comparison of dendritic density (m, D/A) and stratification (n), and areal (o, P/A) and linear (p, P/D) density of PSD95-CFP puncta between the OFF and ON arbor of bistratified RGCs from wildtype (black) and mGluR6-YFP/TeNT (red) mice . Mean indicated by bold horizontal lines. * indicates p < 0.01.
In contrast to monostratified RGCs, bistratified RGCs maintain two narrowly stratified arbors at distinct depths of the IPL (Fig. 2k). The inner arbor is contacted by ON BCs, the outer by OFF BCs3,4. In wildtype mice, synaptic densities across both arbors are correlated (R = 0.86; p < 0.01, n = 8 cells), presumably to balance ON and OFF light responses for each cell. If this balance were achieved by matching ON and OFF input activity, one would predict that a lower density of synapses on the ON arbor of bistratified RGCs in mGluR6-YFP/TeNT mice would be accompanied by a decrease in synapses on its OFF arbor. Alternatively, if ON and OFF BCs engaged in synaptic competition, one might expect the density of OFF arbor synapses to increase1,2. Instead, we found that synaptic densities on the ON dendrites of bistratified RGCs were reduced (p < 0.04) without changes to OFF dendrites (p > 0.4, Fig. 2k - p). These results argue that synaptic connections on the two arbors are established independent of each other, and are sensitive to transmitter release locally but not to differences between inputs onto separate arbors of the same cell.
Several presynaptic changes could accompany the reduced density of synapses on RGC dendrites in mGluR6-YFP/TeNT mice: i) There could be fewer ON BCs, ii) their axonal arbors could be smaller and less complex, and iii) the density of synapses along axonal arbors could be reduced. Comparison with mGluR6-GFP mice14 showed that the density of ON BCs in mGluR6-YFP/TeNT mice was unchanged (mGluR6-YFP/TeNT: 32045 ± 1173 cells/mm2; mGluR6-GFP: 30820 ± 610 cells/mm2; p > 0.3, Supplementary Fig. 5). To assess the structure of single BC axons, we generated mGluR6-tdTomato mice, selected founders in which isolated ON BCs were labeled and crossed them to mGluR6-YFP/TeNT mice. Most of the tdTomato expressing BCs were type 6 and type 7 ON cone BCs or rod BCs (Supplementary Fig. 6)15. Axonal arbors of these cell types retained their characteristic stratification and branching patterns in mGluR6-YFP/TeNT compared to wildtype background (Fig. 3a - f). However, when we labeled presynaptic sites in BC axons with an antibody against C-terminal binding protein 2 (CtBP2), we found that their density in mGluR6-YFP/TeNT mice was reduced (Fig. 3g and h).
Figure 3. Axonal morphology is normal, but multiple ribbons accumulate at fewer synapses in TeNT-expressing ON BCs.
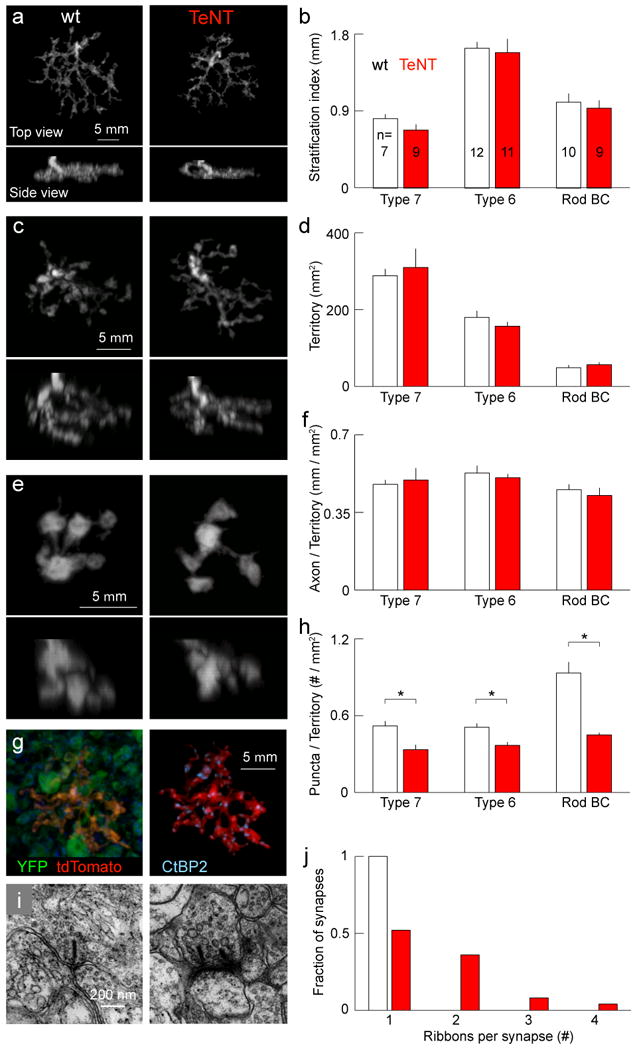
a-f, Axon arbors of representative type 7 (a) and type 6 (c) ON cone BCs, and rod BCs (e) labeled in an mGluR6-tdTomato mouse in wildtype (left) or mGluR6-YFP/TeNT (right) background at P21. TdTomato signal was used to mask arbors of single BCs. Summary data (mean ± s.e.m.) comparing stratification (b), lateral territories (d), and axonal density (f) between wildtype and mGluR6-YFP/TeNT background. g, Synapses on BC terminals labeled with anti-CtBP2. TdTomato signal shown in mGluR6-YFP/TeNT background (left) was used as a mask to isolate ribbon synapses of an individual axonal arbor (right). h, Mean (± s.e.m.) density of synapses for the different BC types. * indicates p < 0.01. i, Electron micrographs of ON BC ribbon synapses of in wildtype (right) and mGluR6-YFP/TeNT (left) littermates at P21. j, Histogram of the number of ribbons per synapse for wildtype (white, n = 25) and mGluR6-YFP/TeNT (red, n = 25) mice.
To determine whether the remaining synapses were structurally normal, we obtained electron micrographs from retinas of mGluR6-YFP/TeNT mice and wildtype littermates. Vesicles at BC active zones are tethered to synaptic ribbons, a common feature of neurons using graded voltage signals rather than action potentials16. Each BC synapse normally contains a single ribbon, but the signals that regulate ribbon localization and number remain unknown16. As expected, all BC active zones (25 of 25) in wildtype mice contained single ribbons (Fig. 3i and j, Supplementary Fig.7 and 8). By contrast, at about half the ON BC active zones (12 of 25) in mGluR6-YFP/TeNT mice, we found more than one and up to four ribbons (Fig. 3i and j). This phenotype was restricted to ON BCs; only single ribbons (11 of 11) were found in the OFF sublamina of mGluR6-YFP/TeNT mice (Supplementary Fig. 7). Since active zones and ribbons assemble by insertion of Piccolo-Bassoon transport vesicles and/or the arrival of precursor spheres, both of which do not contain VAMP217,18, it seems unlikely that TeNT interferes directly with this process. Instead, we propose that transmitter release normally elicits a signal which prohibits the placement of further ribbons at this active zone (see Supplementary Information).
Because the number of BCs, their axons and the RGC dendrites they contact were normal in TeNT retinas, it remained unclear which developmental mechanisms account for the lower density of BC - RGC synapses observed at maturity (Fig. 2). To begin to address this question, we measured the number and density of excitatory synapses on ON RGC dendrites at different times during development (Fig. 4a and b). At P7, when synaptic transmission between BCs and RGCs is first observed19, synapse numbers and densities were indistinguishable between wildtype and mGluR6-YFP/TeNT mice (p > 0.4) and gradually diverged over the following days toward the differences observed at maturity (Fig. 4a and b). To assess the dynamics which underlie this divergent development, we carried out live imaging experiments of RGCs biolistically labeled with tdTomato and PSD95-CFP in mGluR6-YFP/TeNT mice and wildtype littermates at P920,21. Time-lapse imaging revealed frequent synapse formation (Fig. 4c - e and Supplementary Fig. 9) and elimination (Fig. 4f - h) events. The synaptic turnover rate was 27 ± 5.8% per day with a net increase in synapses of 11 ± 6.5% per day in wildtype mice. Surprisingly, in mGluR6-YFP/TeNT mice the rate of synapse formation was reduced several fold (Fig. 4i), whereas synapse elimination was indistinguishable from wildtype littermates (Fig. 4j). We verified that > 90% of newly formed synapses were present in more than one time-lapse image to make sure that the reduced rate of synapse formation we report was not caused by a shortening of synapse lifetimes below our sampling interval. Moreover, the difference in the rate of synapse formation between TeNT and wildtype retinas (36 ± 13%; TeNT / wt in %, Fig. 4i), was similar to the difference in synapse density increase estimated by linear regression from P7 to P21 (23% ± 13%; TeNT / wt in %, Fig. 4a). Together these results suggest that inhibition of glutamate release from TeNT-expressing ON BCs selectively lowers the rate at which new synapses are established during development, thus accounting for the reduced density of BC - RGC synapses observed in mature mGluR6-YFP/TeNT mice.
Figure 4. Transmitter release regulates synapse formation but not elimination, causing a gradual divergence of synaptic development between wildtype and mGluR6-YFP/TeNT mice.
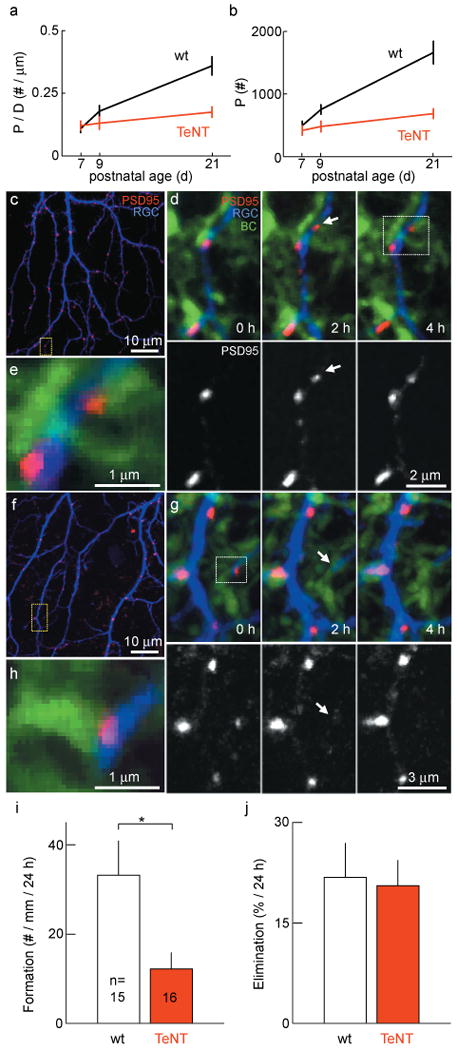
a, b, Developmental increase in density (a) and number (b) of PSD95-CFP puncta on ON RGCs (5 -16 cells per time point and genotype). c, First image of a time-lapse series of a P9 RGC labeled with td-Tomato (blue) and glutamatergic postsynaptic densities labeled with PSD95-CFP (red). d, Time series of the region indicated by a yellow box in c in which a PSD95 cluster forms (arrow). YFP expression in BCs shown in green. PSD95-CFP channel isolated in the lower panels. e, Higher magnification of a single plane of the region indicated by the white box in d showing that the new punctum formed at an apposition of BC axon and RGC dendrite. f - h, analogous to c - e for loss of a PSD95 cluster. i, j, Summary data (mean ± s.e.m) for the rate of PSD95-FP cluster formation (i) and elimination (j). * indicates p < 0.05.
The self-organization of neurons into complex circuits during development is thought to depend in part on neurotransmission. The most prominent model of activity-dependent circuit development proposes that converging inputs engage in activity-mediated competitions, the outcome of which is determined by differences in synapse elimination1,2. This is supported by studies of the neuromuscular junction (NMJ). During development, each NMJ receives convergent innervation from multiple axons of which all but one are eventually retracted. When transmitter release from a subset of these axons is inhibited the outcome of competition is biased towards removal of silenced axons22,23. By contrast, studies of RGC axons in the zebrafish tectum showed that activity could regulate branch addition rather than retraction24, suggesting that different rules might apply in different circuits. In this study, we modified synaptic activity directly while imaging synaptic dynamics in the developing retina. In doing so we find that during the assembly of retinal circuits, glutamate release regulates the formation but not elimination of synapses between BCs and RGCs. In addition, we find that rather than engaging in competition, the axons of ON and OFF BCs refine their connectivity with RGCs independent of one another even when they converge stably onto a single postsynaptic cell. Finally, neurotransmission seems to selectively affect synaptic but not axonal or dendritic development in this system. Which factors determine the different rules that appear to guide activity-dependent development of different neural circuits? Part of the answer might be found in the distinct architectures of early neural circuits which are set up prior to neurotransmission. Accordingly, the early spatial separation of ON and OFF BC axons in the IPL might serve to constrain the role of activity in synaptic remodeling. It is tempting to speculate that laminar circuit architecture throughout the nervous system25 might similarly serve to limit activity-dependent competition between parallel circuits that process distinct information, while allowing activity to fine-tune wiring within each information channel.
Methods Summary
Transgenic mice
We generated two transgenic mouse lines. In one (mGluR6-YFP/TeNT) a ∼9kb fragment of the mGluR6 promoter was used to express yellow fluorescent protein (YFP) and the light chain of tetanus toxin (TeNT) selectively in retinal ON BCs. In the other (mGluR6-tdTomato) the ∼9kb mGluR6 promoter fragment drove expression of the red fluorescent protein tandem dimer Tomato (tdTomato).
Immunohistochemistry
Paraformaldehyde (4%) fixed retinal flat mount preparations or vibratome slices were labeled by standard immunohistochemistry procedures.
Electrophysiology
We performed whole-cell patch clamp recordings from RGCs in retinal flat mount preparations to analyze spontaneous excitatory synaptic transmission and used multielectrode array recordings to characterize light responses during white noise presentation from an organic light emitting display (OLED).
Imaging and analysis
Images of fixed tissue were acquired on an Olympus FV1000 confocal microscope using a 1.35 NA 60× oil objective. Time lapse imaging experiments on retinas of mGluR6-YFP/TeNT mice and wildtype littermates were performed in parallel on an Olympus FV1000 confocal microscope (mGluR6-YFP/TeNT) and either an Olympus FV300 confocal or a custom built two photon microscope (wildtype) using 1.1 NA 60× water objectives. Image stacks were analyzed using Amira (Mercury Computer System Inc.), Imaris (Bitplane) and software custom written in Matlab (The Mathworks).
Electron microscopy
Retinas were fixed using standard EM fixation techniques, cut into pieces and en bloc stained in uranyl acetate. Thin sections were cut and stained with Reynolds lead citrate.
Supplementary Material
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Acknowledgments
We are grateful to J. Sanes and R.W. Burgess for TeNT-CFP, S. Naganishi for the mGluR6 promoter fragment, A.M. Craig for PSD95-CFP and R.Y. Tsien for tdTomato. We thank F. Soto, L. Godinho, A. Lewis, F. Dunn and T. Misgeld for comments on the manuscript. This work was supported by the National Institutes of Health (R.O.W, EY10699, J.L.M T32 EY07031), the McDonnell Foundation at Washington University (R.O.W), National Eye Institute core grant (E.D.P, EY01730) and the Deutsche Forschungsgemeinschaft (D.K, KE 1466/1-1).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions D.K. and R.O.W. conceived the experiments. D.K and R.M.L generated transgenic constructs. D.K. performed and analyzed patch-clamp and multielectrode array recordings, and imaging experiments on fixed tissue. D.K and J.L.M. performed and analyzed live imaging experiments. D.K., E.D.P. and R.O.W. carried out the ultrastructural analysis. D.K and R.O.W. wrote the paper.
Author Information Reprints and permissions information is available at www.nature.com/reprints
The authors declare no competing financial interest.
References
- 1.Wong RO, Lichtman JW. In: Fundamental Neuroscience. Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Academic Press; San Diego: 2002. pp. 533–554. [Google Scholar]
- 2.Miller KD. Synaptic economics: competition and cooperation in synaptic plasticity. Neuron. 1996;17:371–374. doi: 10.1016/s0896-6273(00)80169-5. [DOI] [PubMed] [Google Scholar]
- 3.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 4.Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 5.Ueda Y, Iwakabe H, Masu M, Suzuki M, Nakanishi S. The mGluR6 5′ upstream transgene sequence directs a cell-specific and developmentally regulated expression in retinal rod and ON-type cone bipolar cells. J Neurosci. 1997;17:3014–3023. doi: 10.1523/JNEUROSCI.17-09-03014.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiavo G, et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 7.Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J Comp Neurol. 2005;490:72–84. doi: 10.1002/cne.20635. [DOI] [PubMed] [Google Scholar]
- 8.Chichilnisky EJ. A simple white noise analysis of neuronal light responses. Network. 2001;12:199–213. [PubMed] [Google Scholar]
- 9.Maslim J, Webster M, Stone J. Stages in the structural differentiation of retinal ganglion cells. J Comp Neurol. 1986;254:382–402. doi: 10.1002/cne.902540310. [DOI] [PubMed] [Google Scholar]
- 10.Coombs JL, Van Der List D, Chalupa LM. Morphological properties of mouse retinal ganglion cells during postnatal development. J Comp Neurol. 2007;503:803–814. doi: 10.1002/cne.21429. [DOI] [PubMed] [Google Scholar]
- 11.Wang GY, Liets LC, Chalupa LM. Unique functional properties of on and off pathways in the developing mammalian retina. J Neurosci. 2001;21:4310–4317. doi: 10.1523/JNEUROSCI.21-12-04310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson R, Famiglietti EV, Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- 13.Morgan JL, Schubert T, Wong RO. Developmental patterning of glutamatergic synapses onto retinal ganglion cells. Neural Develop. 2008;3:8. doi: 10.1186/1749-8104-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci. 2006;9:85–92. doi: 10.1038/nn1615. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- 16.Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Regus-Leidig H, Tom Dieck S, Specht D, Meyer L, Brandstatter JH. Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. J Comp Neurol. 2009;512:814–824. doi: 10.1002/cne.21915. [DOI] [PubMed] [Google Scholar]
- 18.Zhai RG, et al. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 19.Johnson J, et al. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J Neurosci. 2003;23:518–529. doi: 10.1523/JNEUROSCI.23-02-00518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okabe S, Miwa A, Okado H. Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J Neurosci. 2001;21:6105–6114. doi: 10.1523/JNEUROSCI.21-16-06105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- 22.Buffelli M, et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 23.Kasthuri N, Lichtman JW. The role of neuronal identity in synaptic competition. Nature. 2003;424:426–430. doi: 10.1038/nature01836. [DOI] [PubMed] [Google Scholar]
- 24.Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- 25.Sanes JR, Yamagata M. Formation of lamina-specific synaptic connections. Curr Opin Neurobiol. 1999;9:79–87. doi: 10.1016/s0959-4388(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 26.Shaner NC, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 27.Marquardt T, et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 28.Kerschensteiner D, et al. Genetic control of circuit function: Vsx1 and Irx5 transcription factors regulate contrast adaptation in the mouse retina. J Neurosci. 2008;28:2342–2352. doi: 10.1523/JNEUROSCI.4784-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.