Abstract
Dendritic cells (DCs) are a heterogeneous group of antigen-presenting leukocytes that play an important role in activation of both the innate and acquired arms of the immune system. While there are several different DC populations in the body, DCs are globally defined by their capacity for potent antigen presentation and naive T cell activation. In non-inflamed human skin during steady-state, there are three main cutaneous DC populations: epidermal Langerhans cells (LCs), dermal myeloid DCs, and dermal plasmacytoid DCs (pDCs). In psoriasis, a model for cutaneous inflammation, there is an additional population of myeloid dermal DCs – “inflammatory DCs” – which appear to be critical for disease pathogenesis.
Introduction
The term “dendritic cell” (DC) was coined in 1973 when DCs in the lymph node were discovered by Ralph Steinman and Zanvil Cohn (Steinman and Cohn, 1973). These DCs were identified as potent antigen presenting cells (APCs) in the mixed leukocyte reaction (MLR). However, it took a number of years for scientists to understand the significance and potential roles of these cells, and we now know that they are central in generating and regulating immune responses. Retrospectively, it was appreciated that Langerhans cells (LCs) described in the epidermis of the skin nearly 140 years ago (Langerhans, 1868), were also DCs.
DCs are a heterogeneous population of cells in the immune system, defined initially by their appearance, but more specifically by their potent ability to present antigen to T cells. Standardized characterization of human cutaneous DC populations is complicated by pleiomorphic phenotype and function during emigration from the skin for ex vivo study, and by the great number of potential surface and intracellular antigens that are present on these leukocytes. In addition, there are differences between human and murine DC networks. DC populations have been historically classified either spatially (circulating blood DCs, draining lymph node DCs, epidermal DCs, and dermal DCs), by their presumed origin (myeloid DCs, plasmacytoid DCs [pDCs]), by physiological or pathophysiological state (steady-state DCs, inflammatory DCs), or by antigen expression (Langerin, DEC-205 etc.). We review our current classification of DC subsets contained within human skin during steady-state and inflammation (Figure 1 and Figure 2). Our main message is that there are three cutaneous DC populations in the steady-state – epidermal LCs, resident dermal myeloid DCs, and pDCs – and during inflammation there appears to be an additional population of myeloid dermal “inflammatory” DCs. Understanding these DCs may lead to new therapeutic targets for augmenting or suppressing inflammation during human disease (Steinman and Banchereau, 2007).
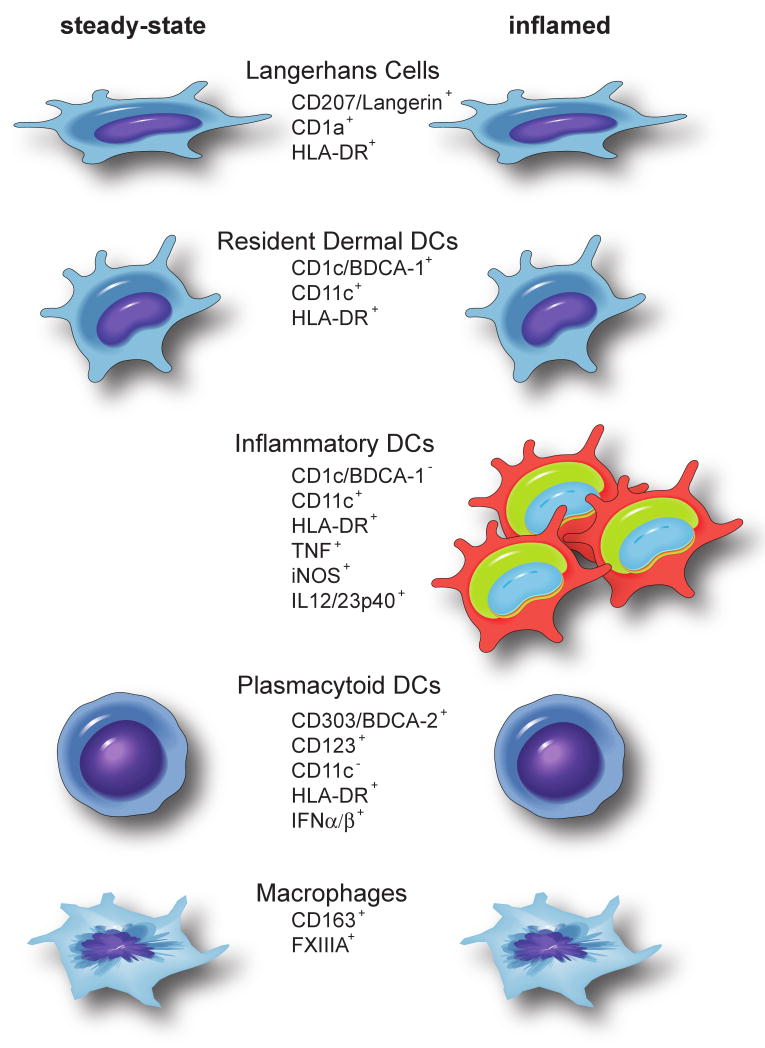
Figure 1. Dendritic cell and macrophage populations in human skin during steady-state and inflammation.
Non-inflamed skin contains epidermal Langerhans cells, CD1c/ BDCA-1+ resident dermal DCs, plasmacytoid DCs, and macrophages. In addition, inflamed skin contains a large population of myeloid “inflammatory” DCs. Common markers used to identify these leukocyte populations are indicated.
Figure 2. Dendritic cell populations in non-lesional and lesional psoriatic skin.

H&E staining demonstrates characteristic features of psoriasis (right), with epidermal acanthosis, elongation of rete ridges, parakeratosis (retention of nuclei in stratum corneum), enlarged blood vessels, and marked leukocytic dermal infiltrate. Immunohistochemistry of non-lesional and lesional psoriatic skin for Langerhans cells (Langerin), resident dermal DCs (CD1c/ BDCA-1+), resident and inflammatory myeloid DCs (CD11c), and plasmacytoid DCs (BDCA-2). Insert is a high power magnification of each cell type and is approximately 60μm length. Size bar = 100μm.
Langerhans cells
LCs reside in the suprabasal layers of the epidermis wedged in between, and in close contact with, keratinocytes. The stellate appearance of these cells led researchers to believe that they were of neural origin until over 100 years later, when their role in antigen presentation was elucidated (Braathen and Thorsby, 1980). LCs were initially identified by the electron-dense organelle, the Birbeck granule, which has a unique tennis-racket appearance. The function of Birbeck granules is still unclear, but likely includes receptor-mediated endocytosis and transport of cellular materials into the extracellular space (Mc Dermott et al., 2002). The first monoclonal antibody that clearly identified LCs bound to CD1a, an MHC-I-like molecule that presents microbial lipids to T cells (Barral and Brenner, 2007; Thomas et al., 1984). More recently, the monoclonal antibody to Langerin/CD207 has been used to specifically recognize LCs (Figure 2). Langerin/CD207 is a membranous C-type lectin discovered by Valladeau et al (Valladeau et al., 2000), that recognizes mannosylated ligands found on the surface of a wide range of pathogens including viruses, bacteria, fungi, and protozoa (Figdor et al., 2002). Following receptor mediated endocytosis, CD1a and Langerin/CD207 traffic to the Birbeck granule where they may participate in antigen processing (Stössel et al., 1990).
Elegant studies by Merad et al. have recently shed light on the origin and trafficking of LCs during steady-state and inflammation (Merad et al., 2004; Merad et al., 2002). It now seems clear that epidermal LCs are continuously replaced from a resident precursor pool (perhaps by self-renewal) throughout life under steady-state conditions (Merad et al., 2002). However following inflammation, LCs are repopulated by blood precursors, most likely monocytes (Ginhoux et al., 2006). In human psoriasis vulgaris, there are variable reports of the number and arrangement of LCs in the epidermis adjacent to non-inflamed epidermis. This may be due to different staining and counting methods used for analysis, or non-equivalent patient populations. In our studies on stable plaque psoriasis, we have found that LCs in lesional and non-lesional epidermis are of similar number per linear surface, with a redistribution to the upper layers of the thickened psoriatic epidermis, and express comparable antigen markers (unpublished data). These observations are supported by studies showing that LC migration in psoriasis is impaired, leading to retention of LCs in psoriatic inflammation (Cumberbatch et al., 2006). Further studies need to be performed to evaluate LCs during human disease states using these new markers.
Interest in LCs has increased with three key observations. Firstly, in mice, there is a newly discovered population of Langerin+ DCs in the dermis and skin draining lymph nodes (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007). These epidermal and dermal LC populations have distinct phenotypic and mitotic capacities, and the dermal Langerin+ LCs do not appear to be simply epidermal LCs en route to the draining lymph node (Douillard et al., 2005; Stoitzner et al., 2003; Stoitzner et al., 2005). Epidermal LCs are Langerin+CCR6+CCR7+/-, radioresistant, slowly self-replicating (Merad et al., 2002), and are highly responsive to CCR6 agonist macrophage inflammatory protein (MIP)-3alpha (CCL20) (Larregina et al., 2001). In contrast, dermal LCs are Langerin+CCR2+CCR7+/-, radiosensitive, rapidly self-replicating, and are responsive to CCR2 agonist monocyte chemoattractant protein (MCP)-1 (CCL2) (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007), although CCR6 expression and responsiveness to CCL20 have not yet been investigated in these dermal Langerin+ CCR2+ cells. Langerin+CD1a+ dermal LCs may also exist in human skin (Angel et al., 2006), although we have not been able to identify many dermal LCs in normal or psoriatic skin in situ (unpublished data and (Lowes et al., 2005b)).
Secondly, there is much debate concerning the pathophysiologial role of LCs. Until recently it was assumed that cutaneous antigens were locally processed by epidermal LCs which then migrate out of the skin into the draining lymph node for efficient antigen presentation to T cells. During this journey to the lymph node, the LCs change their surface phenotype and “mature”, simultaneously down-regulating antigen-processing and acquiring improved ability for T cell co-stimulation (Larregina and Falo, 2005). Recent studies have questioned the biological significance of this pathway as antigen-specific T cell activation remains intact in LC deficient murine models (Bennett et al., 2005; Kaplan et al., 2005; Kissenpfennig et al., 2005a; Mommaas et al., 1994; Ritter et al., 2004; Zhao et al., 2003). However, inconsistent results were obtained in these studies, which may be due to many factors, such as the mode of LC depletion (constitutive or induced), the site of challenge (epidermis versus epidermis and dermis), the dose of antigen, and the timing of contact hypersensitivity schedules. To date, no model claiming to show the redundancy of LCs in the induction of immunity has used a strictly epidermotropic pathogen, e.g papillomavirus. Thirdly, another group recently published a novel role for these cells, describing how LCs expressing ligands for cytotoxic T cells unexpectedly promoted murine carcinogenesis (Strid et al., 2008).
These new observations suggest that there is steady-state migration of LCs to skin-draining lymph nodes, perhaps to induce and maintain tolerance to cutaneous antigens (Steinman and Nussenzweig, 2002). Thus a working hypothesis is emerging whereby DCs that reside in the dermis may be essential for the process of cutaneous immune activation while LCs may play a more important role in sustaining cutanous immunological tolerance.
Dermal DCs
The population of DCs that reside in the dermis of the skin are known as dermal DCs, and these are considered analogous to “interstitial DCs” found in the connective tissue and stroma of other organs (Nestle et al., 1993; Lenz et al., 1993; Shortman and Naik, 2007). These important cells have the capacity to take up cutaneous antigens, mature and migrate to draining local lymph nodes, and present these antigens to T cells and B cells (Dubois et al., 1998; Kissenpfennig et al., 2005b; Nestle et al., 1998). This process may be essential during skin infections, such as herpes simplex, as blockade of dermal DC migration from skin to lymph node may prevent effective cytotoxic T cell activation (Allan et al., 2006).
Because culture methods change the surface cellular phenotype, the purest phenotypic classification of dermal DCs is best performed in situ, followed by ex vivo functional verification of antigen presenting capacity. Unfortunately, there is no single or specific marker for these cells, although the integrin CD11c is probably the best tool we currently have to identify them. For many years, antibodies to Factor XIIIA (FXIIIA) clotting factor were used to identify a dermal population of cells which have multiple dendritic processes protruding from a stellate-shaped cell body, and were thus called “dermal dendrocytes” (Headington, 1986). In 1993 Meunier et al. described a small population of HLA-DR+CD11c+CD1c+FXIIIA+ cells from cultured normal human dermis that had the capacity to stimulate T cells in an MLR (Meunier et al., 1993). In addition, Nestle et al showed that immunostimulatory dermal DC populations were present from psoriatic skin (Nestle et al., 1994). However, it has recently been demonstrated that FXIIIA is induced with culture and is actually a macrophage marker, rather than a specific DC marker (Töröcsik et al., 2005; Zaba et al., 2007b). Thus, more work needs to be performed to re-evaluate the body of literature classifying dermal APCs as FXIIIA+.
Resident dermal DCs
In order to understand dermal DCs and to develop working models to study these cells, we and others have developed the concept that there is a resident population of dermal DCs, as well as an additional group of DCs that appear or develop during inflammation (“inflammatory” dermal DCs) (Shortman and Naik, 2007). A proportion of resident murine steady-state dermal DCs (CD11c+) appear to be able to proliferate in situ to maintain the baseline population (Bogunovic et al., 2006). The major resident population in normal dermis is identified phenotypically with a single monoclonal antibody, CD1c, which is also known as blood dendritic cell antigen (BDCA)-1 (Figure 2). Anti-BDCA antibodies -1 (CD1c), -2 (CD303), -3 (CD141), and -4 (CD304/ neuropilin-1) recognize proteins that were first used to identify circulating DCs (Dzionek et al., 2000; MacDonald et al., 2002). In steady-state, CD1c+ (BDCA-1) DCs are relatively immature with modest T cell stimulatory ability, but their immunostimulatory capacity can be greatly increased with DC maturing stimuli (Zaba et al., 2007b). These CD1c+ (BDCA-1) DCs are also CD11c+HLA-DR+CD45+CD14-, are mostly dendritic cell-specific ICAM-3-grabbing non-integrin (DC-SIGN)/CD209+, and by flow cytometric analysis, are approximately 50% CD1a+ (unpublished data). CD1c+CD14-CD1a+ dermal DCs from normal skin are also CCR7+ and are responsive to the lymph node chemokine CCL19, suggesting that these cells can migrate to draining lymph nodes for antigen presentation (Angel et al., 2006). Prior to these experiments, it had been previously shown that dermal DCs associating with T cells in normal skin display an activated phenotype (McLellan et al., 1999; Pope et al., 1995). It is possible that these immature CD1c+ (BDCA-1) resident DCs are tolerogenic in steady-state, analogous to epidermal Langerhans cells.
CD1a has also been used to specifically identify immunostimulatory dermal DCs in normal skin (Angel et al., 2006; Angel et al., 2007; Lenz et al., 1993; Nestle et al., 1993) and in psoriasis (Nestle et al., 1994). In normal skin, dermal CD1a+ cells appear to be CD1c+ (BDCA-1) in situ and also in single cell suspensions (unpublished data). This suggests that dermal CD1a+ cells may be a subset of the resident myeloid DCs, but CD1c+ (BDCA-1+) may be a more useful marker of these cells as it co-localizes with nearly all the CD11c+ cells. Secondly, there are reports of an additional population of dermal antigen-presenting cells identified by CD14+ (Angel et al., 2007; Morelli et al., 2005). In our studies, there are few CD14+ cells in normal skin in situ (unpublished data) and it is not yet clear if these are indeed dermal residents. When the skin is cultured to produce dermal single cell suspensions, it is possible that there are contaminating monocytes present. Future studies are required to clarify the CD14+ dermal population and the role of the CD1a+CD1c+ dermal DC subset.
While most of these CD1c+ (BDCA-1) cells are relatively immature, there is a small subgroup (∼5%) expressing mature DC markers (Zaba et al., 2007b). This includes dendritic cell-lysosomal-membrane associated protein (DC-LAMP)/CD208, and endocytic receptor DEC-205/CD205, which is present on immature DCs, but markedly upregulated on maturation (Butler et al., 2007; Guo et al., 2000). These rare, phenotypically mature cells often aggregate together in dermal clusters, but their scarcity prevents detailed functional analysis. Perhaps normal skin requires a small population of mature DCs for fast antigen presentation to local T cells, or for ongoing “micro” immune responses.
In normal skin, there is also a population of dermal DCs identified by CD141 (BDCA-3). These CD141+ (BDCA-3) cells constitute approximately 10% of all CD11c+ dermal DCs and do not overlap with CD1c+ (BDCA-1) DCs (Zaba et al., 2007b). Again, functional analysis has not been performed on dermal CD141+ (BDCA-3) cells due to their low frequency. However, it has been established that CD141+ (BDCA-3) DCs in blood are non-overlapping with blood CD1c+ (BDCA-1) DCs and are the least immunostimulatory myeloid blood DC population (MacDonald et al., 2002).
“Inflammatory” dermal DCs
Psoriatic inflammation induces dramatic changes in dermal DC populations, most obviously a 30-fold increase in CD11c+ DCs in the dermis (approximately equal to T cell numbers) (Zaba et al., 2007a) (Figure 2). These DCs return to normal or non-lesional levels with effective treatment (Lowes et al., 2005b; Zaba et al., 2007a). Characterization of these cells during inflammation is not yet complete, but our preliminary studies suggest that they are not CD1c+ (BDCA-1), that is, these “inflammatory” myeloid dermal DCs are CD11c+CD1c-. This indicates that they may be derived from circulating DC- precursors migrating into the skin due to inflammatory and chemotactic signals. Potential precursors include circulating hematopoietic precursor cells (Massberg et al., 2007; Svensson and Kaye, 2006), circulating “pre-DCs” (CD11c+ HLA-DRhi, CD16+) (Piccioli et al., 2007; Randolph et al., 2002; Tacke and Randolph, 2006), monocytes (Serbina et al., 2003), or resident DCs.
In psoriasis, we have been able to determine that dermal DCs produce mediators TNF and intracellular nitric oxide synthase (iNOS), and these have been termed TNF and iNOS-producing DCs (Tip-DCs) (Lowes et al., 2005a). It is likely that these Tip-DCs are contained within the CD11c+CD1c- population of “inflammatory” myeloid DCs in psoriasis lesions. Tip-DCs were first described in a murine model of Listeria monocytogenes infection (Serbina et al., 2003) and have also been found in murine E.coli bladder infection (Engel et al., 2006). In humans, the location and functions of Tip-DCs are emerging: they are present in the lamina propria of the gut where they may be important for IgA production (Tezuka et al., 2007), and they appear to be induced by topical imiquimod treatment of basal cell carcinoma (Stary et al., 2007), so they may participate in tumor rejection. Pathogenicity of these Tip-DCs in psoriasis is suggested by the rapid downmodulation of Tip-DC products TNF, iNOS, IL-20, and IL-23 during treatment with effective therapies (Haider et al., 2008; Zaba et al., 2007a). Tip-DCs can also stimulate the differentiation and activation of Th17 T cells (unpublished data), which are a new set of T cells associated with autoimmune inflammation in many disease models (Zaba et al., 2007a). The potential role of these DCs as sources of inflammatory mediators is in contrast to the classic role of DCs as antigen-presenting cells, and warrants further attention. Because these DCs may actually be the key target of a variety of anti-inflammatory therapies, and may be broadly involved in “autoimmune” inflammation of many different human diseases, we need to better understand the development and activation of these cells.
In another common skin disease, atopic dermatitis, a population of inflammatory DCs have also been described. These cells were initially termed inflammatory dendritic epidermal cells (IDECs) based on flow cytometric analysis of cells from epidermal cell suspensions (Novak and Bieber, 2005; Wollenberg et al., 1996; Wollenberg et al., 2002). IDECs were defined by the following: HLA-DR+Lin-CD11c+CD1a+ and these DCs co-express CD206/macrophage mannose receptor (MMR), CD36, FceRI, IgE, CD1b/c, CD11b, as well as DC-SIGN/CD209 (Guttman-Yassky et al., 2007). There is now appreciation that these DCs are also located in the dermis and appear to produce a different array of cytokines and chemokines (Th2 chemokines CCL17 and CCL18) than do psoriatic inflammatory DCs, and do not have an iNOS signature (Guttman-Yassky et al., 2007). Thus the cutaneous inflammatory milleau may drive the differentiation of different types of inflammatory DCs that contribute specifically to the disease process and even clinical phenotype.
Plasmacytoid DCs
PDCs are an additional unique population of resident cutaneous DCs initially described by their morphology, which is similar to a plasma cell (Corcoran et al., 2003). There are considerable phenotypic and functional differences of PDCs in humans and mice, which may contribute to the confusion in the literature as to their precise role in immune responses (Valladeau and Saeland, 2005). PDCs share many characteristics with B cells, including dependence on a B cell transcription factor (SPI-B), and immunoglobulin gene rearrangements. It now appears that both PDCs and myeloid DCs may actually arise from a bone marrow-derived common DC precursor (Naik et al., 2007; Onai et al., 2007). While both myeloid and plasmacytoid DCs express high levels of HLA-DR and have the capacity to present antigen, pDCs are characterized by their ability to produce large amounts of type 1 interferon (IFN) (IFN-α, β, ω) during viral infection – 10,000 fold more IFN than any other cell type (Ito et al., 2004; Kadowaki et al., 2000). This potent IFN production is induced by viral RNA and DNA containing repeated unmethylated CG nucleotides (CpG) binding to Toll-like receptor (TLR) 7 and TLR9 endosomal receptors, and the CpG class determines the level of IFN produced (Rothenfusser et al., 2002). IFN-α, the most studied of the Type I IFNs, modulates development and maturation of many immune cells, including T cells and myeloid DCs, by binding to abundantly expressed IFN receptor (Theofilopoulos et al., 2005). In addition, different stimulation conditions (eg influenza virus and CpG) may induce distinct activation states in pDC (Iparraguirre et al., 2008). The pleiotropic effects of pDC activation are complicated and not fully understood.
Monoclonal antibodies for pDC identification are well developed and include CD123/IL-3R, BDCA-2, and BDCA-4. BDCA-2 is the only marker that is exclusive to pDCs, as myeloid DCs can express low levels of CD123 and BDCA-4 (Liu, 2005). Thus, a common phenotypic definition of blood and tissue pDCs is HLA-DR+CD11c-CD123hiBDCA-2+. Research on the function of human pDCs during steady-state and disease is limited by low frequency and transitory expression of IFNα (Nestle and Gilliet, 2005).
In studies of normal skin, there are a small number of pDCs (Ebner et al., 2004; Zaba et al., 2007b), and there does not appear to be any marked increase in the frequency of these cells in chronic large plaque psoriasis (Guttman-Yassky et al., 2007), although others have found an increase of these cells in psoriasis (Nestle et al., 2005). However, two recent observations support a role for pDCs during the initiation phase of psoriasis. Firstly, in xenotransplant experiments of non-lesional skin from patients with psoriasis grafted onto immunosuppressed mice, the stress of transplantation is sufficient for psoriasis to develop spontaneously in the graft (Nestle and Nickoloff, 2005). Blockade of IFN-α prevented development of psoriasis, implicating pDCs as they are the primary source of IFN-α in the skin. Secondly, keratinocyte peptide LL37 (also known as cathelicidin or cathelicidin antimicrobial peptide [CAMP]), binds to self-DNA and TLR9 in pDCs, and induces IFN-α (Lande et al., 2007). Expression of this antimicrobial peptide is increased during inflammation such as psoriasis, and downstream effects of this increased IFN-α production may be activation of myeloid DCs and initiation of psoriasis (Nestle and Gilliet, 2005). Patients with psorasis may have pDCs that are particularly sensitive to LL37 and respond in an exaggerated manner with increased IFN-α induction, thus setting in motion psoriatic inflammation.
Summary
DC heterogeneity and complexity has made them difficult to understand, but we are currently developing better tools and models to study these cells. DCs are central orchestrators of innate and cellular immune responses, either by secretion of bioactive cytokines or inflammatory mediators that modulate stromal and local cutaneous immunocytes, or by their ability to directly activate T cells. Currently, we do not know the relative contribution or temporal involvement of DC subsets, T cells, and keratinocytes in initiating and maintaining skin inflammation. The data summarized here indicate that DCs play an important role in cutaneous homeostasis and are likely very central to pathogenic processes in the skin. This information from the skin may also be applicable to other organs, systems, and diseases. Further studies on cutaneous DCs are essential if we are to tap into the enormous therapeutic potential of these fascinating cells.
Acknowledgments
Research was supported by NIH grants R01 AI-49572, AI-49832, and UL1 RR024143. MAL is supported by 1 K23 AR052404-01A1, and LZ is supported by NIH MSTP grant GM07739. The authors would like to thank Dr Ralph Steinman for his continued support and inspiration.
Abbreviations
- DC
dendritic cell
- LC
Langerhans cell
- PDC
plasmacytoid dendritic cell
- APC
antigen presenting cell
- MLR
mixed leukocyte reaction
- MCP-1
monocyte chemotactic protein-1
- FXIIIA
factor XIIIA
- BDCA
blood dendritic cell antigen
- DC-SIGN
dendritic cell-specific ICAM-3-grabbing nonintegrin
- DC-LAMP
DC-lysosomal-associated membrane protein
- INOS
inducible nitric oxide synthase
- Tip-DC
TNF-iNOS producing DC
- IDEC
inflammatory dendritic epidermal cells
- MMR
macrophage mannose receptor
- IFN
interferon
- TLR
toll-like receptor
- CAMP
cyclic adenosine monophosphate
- MIP-3α
macrophage inflammatory protein-3alpha
Footnotes
Conflict Of Interest: The authors state no conflict of interest.
References
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Angel CE, George E, Brooks AE, Ostrovsky LL, Brown TL, Dunbar PR. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J Immunol. 2006;176:5730–5734. doi: 10.4049/jimmunol.176.10.5730. [DOI] [PubMed] [Google Scholar]
- Angel CE, Lala A, Chen CJ, Edgar SG, Ostrovsky LL, Dunbar PR. CD14+ antigen-presenting cells in human dermis are less mature than their CD1a+ counterparts. Int Immunol. 2007;19:1271–1279. doi: 10.1093/intimm/dxm096. [DOI] [PubMed] [Google Scholar]
- Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol. 2007;7:929–941. doi: 10.1038/nri2191. [DOI] [PubMed] [Google Scholar]
- Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Wagers A, Loubeau M, Isola LM, Lubrano L, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med. 2006;203:2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braathen LR, Thorsby E. Studies on human epidermal Langerhans cells. I. Allo-activating and antigen-presenting capacity. Scand J Immunol. 1980;11:401–408. doi: 10.1111/j.1365-3083.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M, Morel AS, Jordan WJ, Eren E, Hue S, Shrimpton RE, et al. Altered expression and endocytic function of CD205 in human dendritic cells, and detection of a CD205-DCL-1 fusion protein upon dendritic cell maturation. Immunology. 2007;120:362–371. doi: 10.1111/j.1365-2567.2006.02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L, Ferrero I, Vremec D, Lucas K, Waithman J, O'Keeffe M, et al. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J Immunol. 2003;170:4926–4932. doi: 10.4049/jimmunol.170.10.4926. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M, Singh M, Dearman RJ, Young HS, Kimber I, Griffiths CE. Impaired Langerhans cell migration in psoriasis. J Exp Med. 2006;203:953–960. doi: 10.1084/jem.20052367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillard P, Stoitzner P, Tripp CH, Clair-Moninot V, Ait-Yahia S, McLellan AD, et al. Mouse lymphoid tissue contains distinct subsets of langerin/CD207 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–994. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- Dubois B, Massacrier C, Vanbervliet B, Fayette J, Briere F, Banchereau J, et al. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol. 1998;161:2223–2231. [PubMed] [Google Scholar]
- Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- Ebner S, Ehammer Z, Holzmann S, Schwingshackl P, Forstner M, Stoitzner P, et al. Expression of C-type lectin receptors by subsets of dendritic cells in human skin. Int Immunol. 2004;16:877–887. doi: 10.1093/intimm/dxh088. [DOI] [PubMed] [Google Scholar]
- Engel D, Dobrindt U, Tittel A, Peters P, Maurer J, Gutgemann I, et al. Tumor necrosis factor alpha- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance. Infect Immun. 2006;74:6100–6107. doi: 10.1128/IAI.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Gong S, Maric S, Misulovin Z, Pack M, Mahnke K, et al. A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Hum Immunol. 2000;61:729–738. doi: 10.1016/s0198-8859(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Whynot J, Novitskaya I, Cardinale I, et al. Major differences in inflammatory dendritic cells and their products distinguish atopic dermatitis from psoriasis. J Allergy Clin Immunol. 2007;119:1210–1217. doi: 10.1016/j.jaci.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Haider AS, Lowes MA, Suarez-Farinas M, Zaba LC, Cardinale I, Khatcherian A, et al. Identification of Cellular Pathways of “Type 1,” Th17 T Cells, and TNF- and Inducible Nitric Oxide Synthase-Producing Dendritic Cells in Autoimmune Inflammation through Pharmacogenomic Study of Cyclosporine A in Psoriasis. J Immunol. 2008;180:1913–1920. doi: 10.4049/jimmunol.180.3.1913. [DOI] [PubMed] [Google Scholar]
- Headington JT. The dermal dendrocyte. Adv Dermatol. 1986;1:159–171. [PubMed] [Google Scholar]
- Iparraguirre A, Tobias JW, Hensley SE, Masek KS, Cavanagh LL, Rendl M, et al. Two distinct activation states of plasmacytoid dendritic cells induced by influenza virus and CpG 1826 oligonucleotide. J Leukoc Biol. 2008;83:610–620. doi: 10.1189/jlb.0807511. [DOI] [PubMed] [Google Scholar]
- Ito T, Amakawa R, Inaba M, Hori T, Ota M, Nakamura K, et al. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J Immunol. 2004;172:4253–4259. doi: 10.4049/jimmunol.172.7.4253. [DOI] [PubMed] [Google Scholar]
- Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Ait-Yahia S, Clair-Moninot V, Stössel H, Badell E, Bordat Y, et al. Disruption of the langerin/CD207 gene abolishes Birbeck granules without a marked loss of Langerhans cell function. Mol Cell Biol. 2005a;25:88–99. doi: 10.1128/MCB.25.1.88-99.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005b;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Langerhans P. Uber die Nerven der menschlichen Haut. Virchows Arch. 1868;A:325–337. [Google Scholar]
- Larregina AT, Falo LD., Jr Changing paradigms in cutaneous immunology: adapting with dendritic cells. J Invest Dermatol. 2005;124:1–12. doi: 10.1111/j.1523-1747.2004.23554.x. [DOI] [PubMed] [Google Scholar]
- Larregina AT, Morelli AE, Spencer LA, Logar AJ, Watkins SC, Thomson AW, et al. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol. 2001;2:1151–1158. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- Lenz A, Heine M, Schuler G, Romani N. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J Clin Invest. 1993;92:2587–2596. doi: 10.1172/JCI116873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) Proc Natl Acad Sci U S A. 2005a;102:19057–19062. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Turton JA, Krueger JG, Barnetson RS. Psoriasis vulgaris flare during efalizumab therapy does not preclude future use: a case series. BMC Dermatol. 2005b;5:9. doi: 10.1186/1471-5945-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, et al. Immunosurveillance by Hematopoietic Progenitor Cells Trafficking through Blood, Lymph, and Peripheral Tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Dermott R, Ziylan U, Spehner D, Bausinger H, Lipsker D, Mommaas M, et al. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where Langerin accumulates. Mol Biol Cell. 2002;13:317–335. doi: 10.1091/mbc.01-06-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AD, Heiser A, Hart DN. Induction of dendritic cell costimulator molecule expression is suppressed by T cells in the absence of antigen-specific signalling: role of cluster formation, CD40 and HLA-class II for dendritic cell activation. Immunology. 1999;98:171–180. doi: 10.1046/j.1365-2567.1999.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier L, Gonzalez-Ramos A, Cooper KD. Heterogeneous populations of class II MHC+ cells in human dermal cell suspensions. Identification of a small subset responsible for potent dermal antigen-presenting cell activity with features analogous to Langerhans cells. J Immunol. 1993;151:4067–4080. [PubMed] [Google Scholar]
- Mommaas M, Mulder A, Vermeer BJ, Koning F. Functional human epidermal Langerhans cells that lack Birbeck granules. J Invest Dermatol. 1994;103:807–810. doi: 10.1111/1523-1747.ep12413456. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Rubin JP, Erdos G, Tkacheva OA, Mathers AR, Zahorchak AF, et al. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells. J Immunol. 2005;175:7905–7915. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Filgueira L, Nickoloff BJ, Burg G. Human dermal dendritic cells process and present soluble protein antigens. J Invest Dermatol. 1998;110:762–766. doi: 10.1046/j.1523-1747.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Gilliet M. Defining upstream elements of psoriasis pathogenesis: an emerging role for interferon alpha. J Invest Dermatol. 2005;125:xiv–xv. doi: 10.1111/j.0022-202X.2005.23923.x. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Nickoloff BJ. From classical mouse models of psoriasis to a spontaneous xenograft model featuring use of AGR mice. Ernst Schering Res Found Workshop. 2005:203–212. doi: 10.1007/3-540-26811-1_11. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines. J Clin Invest. 1994;94:202–209. doi: 10.1172/JCI117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151:6535–6545. [PubMed] [Google Scholar]
- Novak N, Bieber T. The role of dendritic cell subtypes in the pathophysiology of atopic dermatitis. J Am Acad Dermatol. 2005;53:S171–176. doi: 10.1016/j.jaad.2005.04.060. [DOI] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, et al. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood. 2007;109:5371–5379. doi: 10.1182/blood-2006-08-038422. [DOI] [PubMed] [Google Scholar]
- Pope M, Betjes MG, Hirmand H, Hoffman L, Steinman RM. Both dendritic cells and memory T lymphocytes emigrate from organ cultures of human skin and form distinctive dendritic-T-cell conjugates. J Invest Dermatol. 1995;104:11–17. doi: 10.1111/1523-1747.ep12613452. [DOI] [PubMed] [Google Scholar]
- Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med. 2002;196:517–527. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter U, Meissner A, Scheidig C, Körner H. CD8 alpha- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur J Immunol. 2004;34:1542–1550. doi: 10.1002/eji.200324586. [DOI] [PubMed] [Google Scholar]
- Rothenfusser S, Tuma E, Endres S, Hartmann G. Plasmacytoid dendritic cells: the key to CpG. Hum Immunol. 2002;63:1111–1119. doi: 10.1016/s0198-8859(02)00749-8. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoitzner P, Holzmann S, McLellan AD, Ivarsson L, Stössel H, Kapp M, et al. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J Invest Dermatol. 2003;120:266–274. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- Stoitzner P, Tripp CH, Douillard P, Saeland S, Romani N. Migratory Langerhans cells in mouse lymph nodes in steady state and inflammation. J Invest Dermatol. 2005;125:116–125. doi: 10.1111/j.0022-202X.2005.23757.x. [DOI] [PubMed] [Google Scholar]
- Stössel H, Koch F, Kämpgen E, Stöger P, Lenz A, Heufler C, et al. Disappearance of certain acidic organelles (endosomes and Langerhans cell granules) accompanies loss of antigen processing capacity upon culture of epidermal Langerhans cells. J Exp Med. 1990;172:1471–1482. doi: 10.1084/jem.172.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- Svensson M, Kaye PM. Stromal-cell regulation of dendritic-cell differentiation and function. Trends Immunol. 2006;27:580–587. doi: 10.1016/j.it.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Biggerstaff M, Sloane JP, Easton DF. Immunological and histochemical analysis of regional variations of epidermal Langerhans cells in normal human skin. Histochem J. 1984;16:507–519. doi: 10.1007/BF01041351. [DOI] [PubMed] [Google Scholar]
- Töröcsik D, Bardos H, Nagy L, Adany R. Identification of factor XIII-A as a marker of alternative macrophage activation. CMLS. 2005;62:2132–2139. doi: 10.1007/s00018-005-5242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Wollenberg A, Kraft S, Hanau D, Bieber T. Immunomorphological and ultrastructural characterization of Langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J Invest Dermatol. 1996;106:446–453. doi: 10.1111/1523-1747.ep12343596. [DOI] [PubMed] [Google Scholar]
- Wollenberg A, Mommaas M, Oppel T, Schottdorf EM, Gunther S, Moderer M. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J Invest Dermatol. 2002;118:327–334. doi: 10.1046/j.0022-202x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez Farinas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007a;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007b;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]