Abstract
Context
Physical functioning represents an integrated marker of aging. Whether menopause is associated with an accelerated decline in physical functioning is not known.
Objective
To characterize whether losses in physical functioning are related to the natural or surgical menopause or time, following adjustment for body size and smoking behavior.
Design, Setting and Patients
A longitudinal assessment of physical functioning was conducted from 2000/1 through 2005/06 in a population-based sample of 530 midlife women enrolled in the Michigan Bone Health and Metabolism Study. Longitudinal mixed models were used to relate menopausal status to measures of physical functioning.
Main Outcome Measures
Perception of physical functioning was assessed with the Short-Form 36 10-item physical functioning scale. Performance-based measures included gait, strength (2-lb lift, grip and quadriceps), upper torso flexibility (forward reach distance) and time required for stair climb, 40-foot walk, and sit-to-stand.
Results
Women with surgical menopause (with or without estrogen from ovarian conservation or exogenous replacement) had significantly reduced levels of functioning and greater rates of change in the 2-lb lift (p<0.005), sit-to-stand (p<0.01), timed stair climb (p<0.01), timed walk (p<0.01), velocity (p<0.05) and perception of physical functioning (p<0.01), compared to pre-and perimenopausal women. Data were adjusted for time, body size, and smoking. Diminished functioning in postmenopausal women was observed in hand grip (p<0.005), 2-lb lift (p<0.05), sit-to-stand (p<0.05), velocity (p<0.05) and perceived physical functioning (p<0.05). These differences, based on regression beta coefficients, were not of the magnitude as those in women with surgical menopause.
Conclusions
Surgical menopause, even with availability of an estrogen source, appears to be a “risk” state for diminishing physical function at mid-life and these results suggests that this may be a vulnerable stage for future compromised quality of life.
Keywords: functional limitations, gait analysis, menopause, surgical menopause
INTRODUCTION
Measurement of physical functioning has evolved as a critical element in aging, part of a conceptual framework used to consider the transition of healthy adults from full independence through increasing vulnerability to disability and ultimately death (1,2). Physical functioning represents an integrated marker of aging, influenced by a broad array of physiological and clinical characteristics interacting with behavior and the social environment.
Whether menopause or the menopause transition is associated with an accelerated decline in physical functioning is uncertain. However, it has been projected that by age 45, well within the timeframe of the menopause transition, up to 10–15% of women could be classified as disabled (3). There is a dearth of information about the events of the menopause transition and their relation to physical functioning. One of the few studies, a cross-sectional investigation of 14,427 multi-ethnic women enrolled the Study of Women’s Health Across the Nation (SWAN) found that women reporting substantial functional limitations were significantly more likely to be surgically or naturally postmenopausal compared to those with no limitations, following adjustment for age, race, body size and economic stress (4). SWAN study findings were corroborated by a cross-sectional analyses of 1497 women living on Kinmen Island, just east of mainland China, although this analysis was unadjusted for demographic and health factors (5).
This report describes the evidence for physical functioning losses in a population-based sample of women at the mid-life studied longitudinally over five years. The study, which incorporates both perception of functioning based on an interview, as well as performance measures, characterizes whether losses are related to the natural or surgical menopause or time, following adjustment for body size and smoking behavior.
METHODS
Study population
The Michigan Bone Health and Metabolism Study (MBHMS) is a population-based longitudinal study of musculoskeletal disease and physical functioning in Caucasian women (6). The 664-women sample was identified from two sampling frames, the family records of the now defunct Tecumseh Community Health Study and a 1992 Tecumseh community census. In 1992, 80% of the 24–44 year old female offspring from Tecumseh Community Health Study were recruited. Also recruited in 1992 were 90% of those female residents who were not in the Tecumseh Community Health Study family records but were listed in a community census (Kohl’s Directory). In the 2005/6 annual examination, we collected data from 530 of the still-living 652 enrollees (81%). Over the time period, more than 72% of the cohort members have contributed to at least 8 annual data collection opportunities. Data from the 4 time points included 2017 observations from 544 persons, an average of 3.6 observations per person. Institutional Review Board approval was granted for the study protocol and informed consent was obtained from each participant.
Measurements
Annual measurements from 2000/1 through 2005/6 were included in the analyses for this report. Measurements were selected to characterize perceived and performance-based aspects of physical functioning including muscle strength (upper body and lower body), flexibility and range of motion, balance and coordination. The perception of physical functioning capacity was derived from an interview measure (Medical Outcomes Study SF-36 10-item physical functioning scale), which has been extensively evaluated for construct validity, internal consistency, and test-retest reliability in diverse ethnic groups and age ranges (7–10). The physical functioning scale includes a 3-item response of “limited a lot,” “limited a little,” or “not limited at all” to the following items: vigorous activities; lifting or carrying groceries; climbing several flights of stairs; climbing one flight of stairs; bending, kneeling, or stooping; walking more than one mile; walking several blocks; walking one block; and bathing or dressing. The scale is scored using norm-based methods and transformed to have a mean of 50 (standard deviation =10) in the general US population. Lower scores suggest a perception of greater limitation.
Performance-based measures of strength
A portable isometric chair, a replicate of the chair designed for the Dynamics of Health, Aging and Body Composition Study (11) was used to measure leg strength. Quadriceps strength is measured as torque or the product of force and the torque arm length. Torque (Nm) from three successful trials was averaged. The left leg was tested unless participants reported knee surgery, knee replacement, or pain, conditions under which the alternate leg was tested.
To assess grip strength, a participant was seated in a chair with her forearm at a 90-degree elbow bend and hands placed with fingers and thumb parallel to her body. Three consecutive grip strength (kg) efforts were completed by each participant. The values for each hand were averaged.
Walk and gait analysis
Each participant was timed in two purposeful walks down a 40-foot carpeted (commercial knap) corridor. Measures of gait, including velocity, were obtained from an instrumented mat (Gaitrite, CIR Systems, Clifton, NJ) with an 80 Hz sampling frequency, placed midway in the corridor while women completed the second 40-foot walk. Individual footsteps recorded during the walk were displayed on a computer screen and incomplete footsteps were manually removed.
Timed stair climb and sit-to-stand
Participants were timed during their ascent and descent of three standardized stairs fitted with rubber traction strips. Timing commenced with the toe-off of the leading leg at the start of ascent and ended with the final foot contact of the trailing leg after descent. In addition to time, we identified the participant’s ability to complete the stair climb (i.e., unable because of wheelchair) and the amount and any assistance needed (e.g., none, one or both handrail or personal assistance).
In chair-rise sit-to-stand performance, participants rose from a standard height, armless chair with arms folded over their chest. Movement time was measured from onset of trunk motion on the chair to achievement of an upright standing position. If a participant was unable to rise from the chair or sat back down before achieving full upright stance, it was so noted.
Flexibility and range of motion
In the forward reach measurement, participants were asked to stand, extend their arm at ninety degrees while standing perpendicular to the floor, and then reach the greatest distance (cm) possible forward while maintaining their arm at the same plane established in the perpendicular stance.
Intrarater CV% ranged from <4% to <5% for velocity and leg strength, respectively, and interrater reliability ranged from <4% to <7% for velocity and leg strength, respectively.
Measures of menopausal status
Menopausal status was based on the regularity of menstrual bleeding in the year prior to the study visit. A woman was classified as premenopausal if she had no menstrual irregularity in the previous year, defined as 9 or less cycles in the past 12 months. Perimenopause was defined as having menstrual irregularity. Postmenopause was characterized as having at least twelve consecutive months of amenorrhea associated with no other medical cause. Final menstrual period was defined retrospectively as 12 months of amenorrhea with no alternative explanation.
Hormone therapy (HT) and oral contraceptive use, including duration, was assessed at each visit and hereafter will be referred to as HT because estrogen-based oral contraceptive preparations are used for menopause bleeding or symptoms in addition to contraception. Due to change in formulation use and type of preparation over time, no effort was made to examine type of HT. Surgical menopause including hysterectomy and oophorectomy was verified by medical record abstraction. For purposes of these analyses, separate transition stages based on HT use in surgical or natural menopause were defined. Medical records were requested to corroborate abdominal surgeries, and based on these records, 87 (approximately 70%) hysterectomies with and without unilateral or bilateral oophorectomy were confirmed. In longitudinal mixed models, data were censored at time of surgery if the surgery could not be verified with medical records.
Sex steroid hormones
Specimens from annual visit phlebotomy were held in the MBHMS Repository. Specimens were collected in a fasting state in days 2–7 of the follicular phase of the menstrual cycle. If a woman was postmenopausal, specimens were collected on the anniversary of her study enrollment ± 15 days. Biological samples were aliquoted and stored at −80 degrees Centigrade without thaw until assay. Follicle-stimulating hormone concentrations were measured with a two-site chemiluminometric immunoassay directed to different regions on the beta subunit with CV% of 12.0% and 6.0% and a lower limit of detection of 1.05 mIU/mL. Serum estradiol concentrations were measured with a modified, off-line ACS:180 (E2-6) immunoassay to increase sensitivity, with a lower limit of detection of 1.0 pg/mL and CVs% of 10.6% and 6.4%.
Other measures
Height (cm) and weight (kg) were measured at each annual study visit with a stadiometer and balance-beam scale, respectively. Body mass index (BMI) was calculated by dividing the weight (in kg) by the square of the height (in meters). Annually-assessed BMI was treated as a time-varying covariate in all models.
Smoking history and practice was ascertained annually. Participants who reported current consumption of 7 or more cigarettes per week were classified as current smokers for that examination period; data were then treated as time-varying in analyses.
Statistical analyses
Performance and body size measures were evaluated for presence of outliers and marked deviations from the normal distribution. When necessary, transformations were employed to assure more normal data distributions. Analysis of covariance was used to estimate the least squared means (LS means) of age, physical functioning measures, and hormones, and F-tests were used to evaluate overall statistical significance and pairwise differences. Longitudinal mixed modeling was undertaken using SAS ver. 9. Models were fit using random intercepts and random slopes. We examined plots, residuals, considered potential confounders, and tested models that included time versus those that included age; final models included time since baseline rather than age because it provided a better fit. Table 4 is based on the following longitudinal mixed model (Yij = smoking statusij + logBMIij + time since baselineij + menopausal statusij + errors), with the Yij representing the individual physical function measure.
Table 4.
| Hand grip |
logquad strength |
Forward reach |
log2-lb Lift |
log Sit- stand |
logTimed Stair |
logTimed Walk |
Velocity | SF-36 PF | |
|---|---|---|---|---|---|---|---|---|---|
| β (se) | β (se) | β (se) | β (se) | β (se) | β (se) | β (se) | β (se) | β (se) | |
| intercept | 19.6 | 3.18 | 42.2 | −0.22 | −0.58 | 1.89 | 1.54 | 258.5 | 175 |
| logBMI | 1.91 (0.85) | 0.37 (.05) | −2.05 (.03) | 0.20 (.04) | 0.17 (.03) | 0.25 (.04) | 0.15 (.03) | −25.5 (5.1) | −25.7 (2.9) |
| current smoker | 0.60 (0.56) | 0.04 (.03) | 0.07 (.54) | 0.03 (.03) | 0.02 (.03) | 0.02 (.02) | 0.04 (.01) | −5.20 (2.2) | −0.21 (1.7) |
| time_mo (c) | −0.012 (.003)‡ | −.002(.0004)‡ | −.013(.005)‡ | .002(.0002)‡ | −.0001(.0003) | 0.001(.0002)‡ | 0.001(.0001)‡ | −.021 (.02) | −.012 (.01) |
| Postmenopause | |||||||||
| With HT use | −0.012 (.50) | −0.01 (.04) | −0.65 (.87) | 0.02 (.03) | −.08(.03) | 0.04 (.02) | −0.004 (.02) | 1.14 (3.1) | −1.42 (1.4) |
| No HT | −0.85 (.30)‡ | −0.04 (.02) | 0.13 (.43) | 0.04 (.02)¶ | 0.03 (.03) | 0.02 (.01) | 0.01 (.01) | −3.80(1.7)¶ | −1.87(0.9)¶ |
| Surgical menopause | |||||||||
| Some E2/HT | −0.67 (.98) | −0.05 (.05) | −1.05 ().85 | 0.17 (.06)‡ | 0.13(.04) § | 0.11 (.04)§ | 0.07 (.03)§ | −12.1(4.9)¶ | −6.25(2.2)§ |
| No E2/HT | −0.51 (.72) | −0.08 (.04) | −0.84 (.74) | 0.12 (.04)‡ | 0.18 (.05)§ | 0.09 (.03)§ | 0.06 (.02)‡ | −9.08(3.1)‡ | −7.20(2.6)§ |
| Pre/Peri menopause | |||||||||
| With HT use | 0.30 (.28) | 0.007 (.03) | −0.32 (047) | 0.01 (.02) | −0.01 (.02) | 0.009 (.01) | 0.003 (.01) | −0.60 (1.8) | −0.43 (0.8) |
| No HT | reference | reference | reference | reference | reference | reference | reference | reference | reference |
p<.005
p<0.01
p<0.05
RESULTS
The mean age of the sample was 44.8 (4.8) years in 2000/01 when collection of a more comprehensive battery of physical functioning measures was initiated (Table 1). Seventeen percent of participants reported smoking. Mean (SD) BMI was 28.2 (6.2) kg/m2, a value that had increased to 28.6 (6.3) 5 years later. In 2000/01, 56% of participants considered their health excellent to very good, while 33% and 11% of participants reported good or fair/poor health, respectively; by 2005/06, 51% reported their health excellent to very good (not shown). About 45% (n=226) percent of participants had a final menstrual period by 2005/06.
Table 1.
Mean (SD) values for age, body size, physical functioning, hormone levels, menopausal classification and smoking status
| 2000/01 (n=530) | 2005/06 (n=506) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (y) | 44.8 (4.8) | 49.9 (4.9) |
| Body size measures | ||
| BMI (kg/m2) | 28.2 (6.2) | 28.6 (6.3) |
| Weight (kg) | 74.7 (16.9) | 75.5 (17.3) |
| Perceived physical functioning | ||
| SF-36 physical functioning score | 87.6 (18.1) | 85.3 (19.3) |
| Performance physical function measures | ||
| Grip strength, dominant hand (kg) | 25.7 (5.4) | 24.8 (5.4) |
| Forward reach (cm) | 35.8 (6.0) | 34.7 (5.7) |
| 2-lb lift (sec) | 1.5 (0.5) | 1.8 (0.7) |
| Sit-to-stand (sec) | 1.06 (0.4) | 1.05 (0.3) |
| Leg strength (Nm) | 83.5 (24.6) | 73.7 (26.4) |
| 40-foot timed walk (sec) | 7.6 (1.1) | 8.2 (1.3) |
| Timed stair climb (sec) | 16.2 (3.3) | 17.2 (3.7) |
| Velocity (cm/sec) | 169.9 (25.3) | 166.3 (22.4) |
| Serum hormones | ||
| Follicle-stimulating hormone (mIU/mL) | 13 (13) | 31 (33.8) |
| Estradiol (pg/mL) | 42 (41.6) | 35 (35.6) |
| Menopause status | N (%) | N (%) |
| Pre-perimenopause | 294 (56%) | 144 (28%) |
| Natural postmenopause | 22 (4%) | 164 (32%) |
| Natural menopause with HT | 19 (4%) | 18 (3%) |
| Surgical menopause (no ovarian conservation or HT) | 49 (9%) | 47 (9%) |
| Surgical menopause (ovarian conservation and/or HT) | 34 (6%) | 37 (7%) |
| HT use | 67 (13%) | 29 (6%) |
| Censored due to unconfirmed surgery | 45 (8%) | 67 (13%) |
| Smoking | ||
| Current | 91 (17%) | 79 (16%) |
| Past | 153 (29%) | 158 (31%) |
| Never | 286 (54%) | 269 (53%) |
Table 1 shows values for both interview and performance-based measures at time of their initiation and the more recently ascertained values in 2005/6. The mean (SD) SF-36 physical functioning questionnaire score in 2000/01 was 87.6 (18.1) out of a total possible 100 points while the mean score was 85.3 (19.3) in 2005/06. The mean grip strength values, measured in the dominant hand, was 25.7 (5.4) kg in 2000/01 and 24.8 (5.4) 5 years later.
The annualized rates of change in the physical functioning measures are shown in Table 2. The mean decline in perception of physical functioning was a half point per year. Performance measures with the greater annual changes relative to their baseline values included greater time to lift a 2-pound weight (+2.9%/yr), less leg strength (Nm; −2.6%/yr), and greater time for the stair climb and walk (+1.9%/yr and +1.4%/yr, respectively). Recorded distances were shorter in the forward reach.
Table 2.
Annualized rates of change* in physical functioning measures from 2000/1 to 2005/6
| Functioning measure | Annualized rate of change | |
|---|---|---|
| absolute change | as % of baseline | |
| Grip strength, dominant hand (kg) | −0.20 kg/yr | 0.8 % less/yr |
| Forward reach (cm) | −0.16 cm/yr | 0.4 % less/yr |
| 2-lb lift (sec) | +0.04 sec/yr | 2.9 % longer/yr |
| Sit-to-stand (sec) | 0.003 sec/yr | 0.3 % longer/yr |
| Timed stair climb (sec) | +0.29 sec/yr | 1.9 % longer/yr |
| Leg strength (Nm) | −2.2 Nm/yr | 2.6 % less/yr |
| 40-foot timed walk (sec) | +0.11 sec/yr | 1.4 % longer/yr |
| Velocity (cm/sec) | −0.69 cm/sec/yr | 0.4 % slower/yr |
| Physical functioning perception (SF-36 physical functioning score) | −0.43 points/yr | 0.5% less/yr |
Changes with time are significantly different than zero (p<0.05) except sit-to-stand, velocity, and the SF-36 physical functioning score.
Change with menopause
After adjustment for time, BMI and smoking behavior, two menopause transition groups demonstrated significant 5-year changes in their physical functioning, women with surgical menopause and women with natural menopause. Table 3 shows the performance characteristics in six different menopause groups including two groups of women with surgical menopause, those with and without conserved ovaries/hormone therapy. The LS mean SF-36 physical functioning score was 80 (out of 100) for women with surgical menopause and 77 in women with surgical menopause and an estrogen source, compared to a score of 91 in pre- and perimenopausal women.
Table 3.
LS means (SE) for physical functioning characteristics, age, BMI, and hormones at 2005/06 for 530* MBHMS enrollees
| Menopause classification | |||||||
|---|---|---|---|---|---|---|---|
| Pre or peri-menopause | Post No HT | Post With HT | Surgical No E2/HT† | Surgical With E2/HT† | HT use | Model P | |
| LSM ± SE | LSM ± SE | LSM ± SE | LSM ± SE | LSM ± SE | LSM ± SE | ||
| N | 144 (33%) | 164 (37%) | 18 (4%) | 47 (11%) | 37 (8%) | 29 (7%) | |
| Age (yr) | 46 ± 0.3 | 53 ± 0.3 | 53 ± 0.9 | 50 ± 0.6 | 51 ± 0.6 | 46 ± 0.7 | <.001 |
| BMI (kg/m2) | 29 ± 0.5 | 29 ± 0.5 | 28 ± 1.4 | 30 ± 1.0 | 28 ± 1.1 | 26 ± 1.1 | 0.13 |
| Physical Functioning | |||||||
| Grip strength, dominant hand (kg) | 26.2 ± 0.5 | 23.8 ± 0.4 | 25.4 ± 1.2 | 24.2 ± 0.8 | 23.7 ± 0.9 | 26.9 ± 1.0 | 0.001 |
| Leg strength (Nm) | 80 ± 2.7 | 73 ± 2.2 | 71 ± 6.5 | 69 ± 3.8 | 75 ± 5.0 | 72 ± 5.1 | 0.22 |
| Forward reach (cm) | 35 ± 0.5 | 35 ± 0.5 | 34 ± 1.3 | 34 ± 0.8 | 35 ± 1.0 | 35 ± 1.1 | 0.82 |
| 2-lb lift (sec) | 1.6 ± 0.03 | 1.8 ± 0.3 | 1.7 ± 0.10 | 2.0 ± 0.07 | 2.0 ± 0.09 | 1.7 ± 0.08 | <0.001 |
| Sit-to-stand (sec) | 1.0 ± 0.03 | 1.0 ± 0.03 | 0.9 ± 0.07 | 1.2 ± 0.06 | 1.2 ± 0.07 | 1.0 ± 0.06 | <0.001 |
| 40-foot timed walk (sec) | 8.0 ± 0.1 | 8.3 ± 0.1 | 7.8 ± 0.3 | 8.5 ± 0.2 | 9.1 ± 0.2 | 7.8 ± 0.2 | <0.001 |
| Timed stair climb (sec) | 16.3 ± 0.3 | 17.6 ± 0.3 | 16.0 ± 0.8 | 18.4 ± 0.6 | 19.6 ± 0.7 | 16.1 ± 0.6 | <0.001 |
| Velocity (cm/s) | 170 ± 2.5 | 164 ± 2.2 | 175 ± 6.7 | 162 ± 4.0 | 149 ± 4.9 | 178 ± 5.8 | <0.001 |
| SF-36 physical functioning score | 91 ± 1.5 | 84 ± 1.4 | 92 ± 4.2 | 80 ± 2.6 | 77 ± 3.0 | 91 ± 3.3 | <0.001 |
| Serum hormones | |||||||
| Follicle-stimulating hormone (mIU/mL) | 10.4 ± 0.7 | 66.9 ± 4.0 | 37.8 ± 6.2 | 52.5 ± 5.7 | 37.0 ± 4.8 | 13.9 ± 2.0 | <0.001 |
| Estradiol (pg/mL) | 80.9 ± 6.0 | 19.1 ± 1.3 | 32.4 ± 6.6 | 29.2 ± 3.6 | 41.4 ± 6.1 | 38.8 ± 6.2 | <0.001 |
| N (%) | |||||||
| Health status | |||||||
| Excellent | 25 (17%) | 34 (21%) | 4 (22%) | 1 (2%) | 3 (8%) | 9 (31%) | 0.001‡ |
| Very good | 61 (42%) | 54 (33%) | 6 (33%) | 13 (28%) | 8 (22%) | 11 (38%) | |
| Good | 50 (35%) | 53 (32%) | 7 (39%) | 22 (47%) | 19 (51%) | 5 (17%) | |
| Fair or poor | 8 (5%) | 23 (14%) | 1 (5%) | 11 (23%) | 7 (19%) | 4 (14%) | |
Data censored for N=24 participants in 2005/06, due to unverified surgery.
E2/HT represents ovarian conservation and/or HT use.
Chi-square p-value.
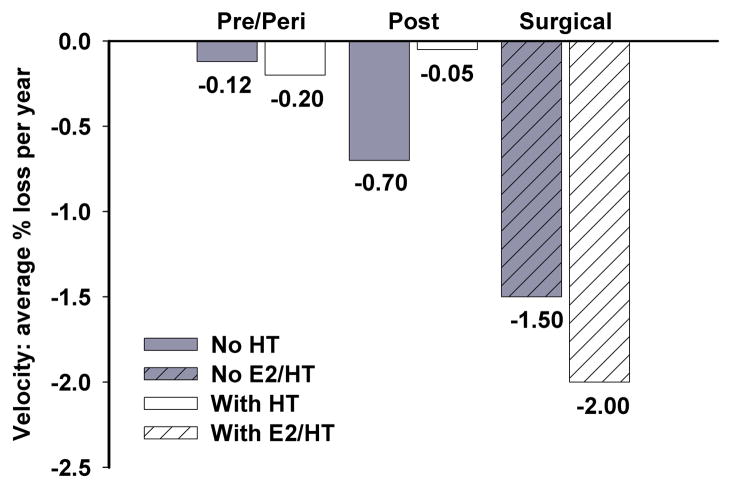
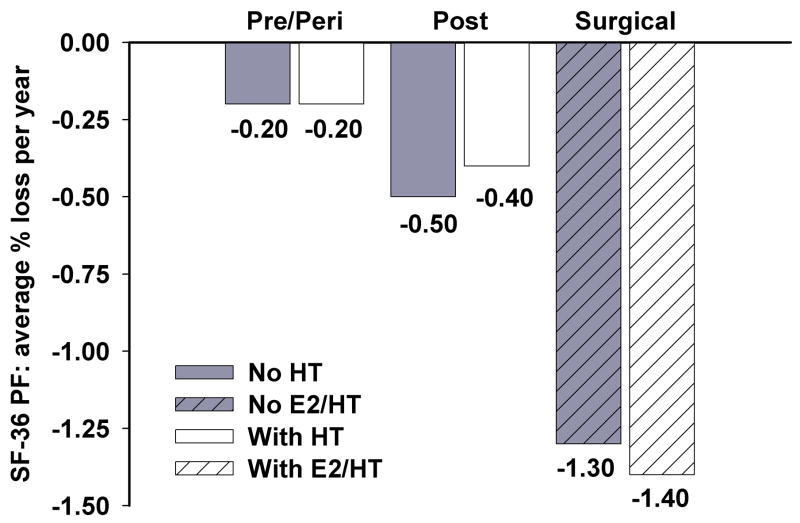
Table 4 shows the changes in physical functioning over time with beta coefficients from longitudinal mixed models. Compared to pre- and perimenopausal women, over the 5-year observation period, women with confirmed surgical menopause and no HT took longer to lift 2 lbs, and required more time for sit-to-stand, walking 40 feet, as well as ascending and descending 3 stairs. They had a greater loss of walking velocity (see Table 4 and Figure 1). Women with surgical menopause perceived themselves as having more functional loss as reported in response to the SF-36 physical functioning questionnaire (Table 4 and Figure 2). Among women in the surgical menopause group without ovarian conservation or HT use, those having higher follicle-stimulating hormone values had diminished functioning in the timed walk, timed stair climb, quadriceps strength and 2-lb lift from the floor (data not shown).
Figure 1.
Comparison of average percent loss of velocity per year from 2000/1 to 2005/6 between women based on menopausal classification. E2/HT represents ovarian conservation and/or HT use.
Figure 2.
Comparison of average percent loss of perceived physical functioning per year from 2000/1 to 2005/6 between women based on menopausal classification. E2/HT represents ovarian conservation and/or HT use.
Women with confirmed surgical menopause with ovarian conservation or using HT had significantly less walking velocity, took longer to lift 2 lbs, and required more time for rising from a chair, walking 40 feet, and ascending and descending 3 stairs, compared to women in the pre-and perimenopausal group (see Table 4). They also perceived themselves as having more functional loss as reported in responses to the SF-36 physical functioning questionnaire.
Women who were naturally postmenopausal and without HT took longer to lift 2-pounds, had less grip strength, walked with less velocity after adjustment for time, BMI, and smoking in comparison to pre- and perimenopausal women (see Tables 3 and 4). They perceived themselves as having more functional loss as reported in responses to the SF-36 physical functioning questionnaire (Table 4).
DISCUSSION
Almost universally, assessment of physical functioning has been associated with describing diminished capacity in elder health. In two studies, female participants have been characterized as having more functional limitation than males (12), experiencing a greater rate of decline in physical functioning and less likelihood of recovery from disability (13). While these studies included women aged 51–61 years (12) and 65 + years (13), they did not consider the contribution of the menopause transition to the decline in physical functioning. In this longitudinal assessment of women at the mid-life, information from both interview and performance based measures of physical functioning showed an increasing vulnerability with time as women age and traverse some aspects of the menopause transition, particularly surgical menopause.
Women at the mid-life were selected for study in anticipation that this time period represented a critical node for change in health status with aging. While there was evidence of increasing compromise as shown in the statistically significant beta coefficients associated with time in the mixed longitudinal models, we specifically evaluated whether elements of the menopause transition contributed to decline in functioning apart from time. We had previously reported that the menopausal transition was associated with lower levels of physical functioning (4); however, those findings were based on a cross-sectional survey, a design that precluded temporal or causal inferences and that did not include performance-based measures.
Surgical menopause was associated with statistically significant loss of physical functioning over a 5-year period in women at mid-life. These losses were observed in both the performance-based measures as well as in the perception of physical functioning. The impact of surgical menopause was still evident even with the evidence of estrogen contribution from ovarian conservation or exogenous hormone use. The observation of more compromise in performance-based physical functioning was corroborated by women’s self-assessment of their overall health status. More than 20% of women with surgical menopause described their health as fair or poor whereas in the group of pre- and perimenopausal women, less than 7% described their health as fair or poor.
The compromise in level of physical functioning occurring in those with surgical menopause may reflect the impact of the events that gave rise to the surgical menopause, the type of surgical procedure, or the impact from an abrupt alteration in hormone levels associated with surgery. Medical records were available for approximately 70% of the procedures reported and we took the very conservative approach of censoring data from those women reporting surgery but for whom we were unsuccessful in securing medical records. While procedures had changed somewhat over time, only a small fraction of procedures were laparoscopic. We considered whether removal of ovaries would result in alterations in estradiol and testosterone concentrations which could affect muscle mass and strength (14) or lead to a shift in the collagen types in cartilage, ligaments and skin (15–17). The similarity in physical functioning loss among women who did or did not retain exposure to an estrogen source through ovarian conservation or exogenous use suggests that having the surgical procedure is the likely explanation for the diminished functioning.
In addition to the loss among women with surgical menopause, there was also loss among the postmenopausal women without hormone use. These losses were substantially less than those observed in women with surgical menopause, both in the number of tasks that reflected compromise and in the magnitude of the beta coefficients from the mixed models. It is noteworthy that among the postmenopausal women, there was substantial loss of hand grip strength, a factor not seen in other groups. Postmenopausal women using hormone therapy had beta coefficients for amount of physical functioning change that were not significantly different from those of the pre- and perimenopausal women.
The greater functional decline observed in women with surgical menopause was found in multiple measures selected to integrate a variety of functioning domains. The time to climb up and down stairs has been used as a performance-based measure of functional status of older adults (18). Stair climbing requires strength; large-joint torque at weight-bearing joints is considerably higher in stair climbing than during walking (19). Stair ascent and descent also requires dynamic balance control, since the body weight is controlled from a single support limb during swing. Grip and pinch strength are used as clinical indicators of strength and dexterity. Sit-to-stand is a task that requires an individual to transfer from a stable position on the chair seat to a much less stable position in upright stance. The task also requires substantial strength, especially of the knee extensor muscles (20–21), to accelerate the body upward. An important, consistently reported difference between the gait patterns of young and old walkers is a decrease in walking velocity and stride length and an increase in double stance time in older walkers (22–23). Not only does the time required to walk increase with age but also the pattern of joint coordination changes; when and how these transitions occur is not known.
This study has notable strengths and limitations. It is a population-based study conducted with very good long-term retention resulting in less likelihood of bias due to loss to follow-up. While the population is younger than is typically reported in this area of research, results are also less likely to reflect morbid and mortal disease events that could contribute to biased findings in the follow-up period. Obviously, the level and rate of change in functioning in this population does not reflect a “frail” subgroup, terminology frequently applied to those who are elderly and functionally limited. Rather, the group with more limitations may reflect an underlying vulnerability that, with time, could predispose them to less successful aging. Given the limited number of studies in this age group, it is important that the study be replicated.
In summary, we identified that in women at mid-life, those with surgical menopause were more vulnerable to having lower levels of physical functioning status as well as a greater decline in functioning. These negative characteristics were retained while women were using hormone therapy. There was some evidence of more physical limitation in the postmenopausal women compared to the pre-menopausal women; but differences were more consistent, more frequent, and of greater magnitude among those with surgical menopause. If these findings are confirmed, it suggests that surgical menopause can lead to characteristics associated with greater “aging” and efforts should be expended to ameliorate those characteristics in addressing the long-term quality of life in these women.
Acknowledgments
Funding sources: R01 AR051384
Footnotes
Dr. MaryFran Sowers had full access to all of the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis
No conflicts of interest exist for any authors for this manuscript.
This manuscript has not been submitted elsewhere for publication in full or in part.
Author contributions: MaryFran R. Sowers is the original architect and principal investigator of the Michigan Bone Health and Metabolism Study, and was involved in every aspect of study design, recruitment, data collection, interpretation, and provided key guidance for manuscript development and statistical interpretation of data; Kristin Tomey analyzed and interpreted data, and participated in drafting the article; Mary Jannausch performed statistical analysis and interpreted data; Aimee Eyvazzadeh analyzed and interpreted data; Mary Crutchfield took the lead on acquiring participant data, and provided administrative and technical support; Bin Nan contributed statistical guidance as well as analyses and interpretation of data; and John Randolph analyzed and interpreted data. All authors contributed to revising the manuscript critically for important intellectual content and gave their final approval for the version of the manuscript being published.
References
- 1.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence R, Jette AM. Disentangling the disablement process. J Gerontol Soc Sci. 1996;51B:S173–S182. doi: 10.1093/geronb/51b.4.s173. [DOI] [PubMed] [Google Scholar]
- 3.Minkler M, Fuller-Thomson E, Guralnik JM. Gradient of disability across the socioeconomic spectrum in the United States. N Engl J Med. 2006;355:695–703. doi: 10.1056/NEJMsa044316. [DOI] [PubMed] [Google Scholar]
- 4.Sowers M, Pope S, Welch G, Sternfeld B, Albrecht G. The association of menopause and physical functioning in women at midlife. J Am Geriatr Soc. 2001;49:1485–92. doi: 10.1046/j.1532-5415.2001.4911241.x. [DOI] [PubMed] [Google Scholar]
- 5.Fuh JL, Wang SJ, Lee SJ, Lu SR, Juang KD. Quality of life and menopausal transition for middle-aged women on Kinmen island. Qual Life Res. 2003;12:53–61. doi: 10.1023/a:1022074602928. [DOI] [PubMed] [Google Scholar]
- 6.Sowers M, Crutchfield M, Bandekar R, et al. Bone mineral density and its change in pre-and perimenopausal white women: the Michigan Bone Health Study. J Bone Miner Res. 1998;13:1134–40. doi: 10.1359/jbmr.1998.13.7.1134. [DOI] [PubMed] [Google Scholar]
- 7.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 8.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–4. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 10.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 12.Clark DO, Stump TE, Wolinsky FD. Predictors of onset of and recovery from mobility difficulty among adults aged 51–61 years. Am J Epidemiol. 1998;148:63–71. doi: 10.1093/oxfordjournals.aje.a009561. [DOI] [PubMed] [Google Scholar]
- 13.Beckett LA, Brock DB, Lemke JH, et al. Analysis of change in self-reported physical function among older persons in four population studies. Am J Epidemiol. 1996;143:766–78. doi: 10.1093/oxfordjournals.aje.a008814. [DOI] [PubMed] [Google Scholar]
- 14.Taaffe DR, Sipila S, Cheng S, Puolakka J, Toivanen J, Suominen H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging. 2005;25:297–304. doi: 10.1111/j.1475-097X.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 15.Brincat MP, Baron YM, Galea R. Estrogens and the skin. Climacteric. 2005;8:110–23. doi: 10.1080/13697130500118100. [DOI] [PubMed] [Google Scholar]
- 16.Richette P, Corvol M, Bardin T. Estrogens, cartilage, and osteoarthritis. Joint Bone Spine. 2003;70:257–62. doi: 10.1016/s1297-319x(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 17.Sowers MR, McConnell D, Jannausch M, Buyuktur AG, Hochberg M, Jamadar DA. Estradiol and its metabolites and their association with knee osteoarthritis. Arthritis Rheum. 2006;54:2481–7. doi: 10.1002/art.22005. [DOI] [PubMed] [Google Scholar]
- 18.West SK, Rubin GS, Munoz B, Abraham D, Fried LP. Assessing functional status: correlation between performance on tasks conducted in a clinic setting and performance on the same task conducted at home. The Salisbury Eye Evaluation Project Team. J Gerontol A Biol Sci Med Sci. 1997;52:M209–17. doi: 10.1093/gerona/52a.4.m209. [DOI] [PubMed] [Google Scholar]
- 19.Andriacchi TP, Andersson GB, Fermier RW, Stern D, Galante JO. A study of lower-limb mechanics during stair-climbing. J Bone Jt Surg Am. 1980;62A:749–757. [PubMed] [Google Scholar]
- 20.Rodosky MW, Andriacchi TP, Andersson GB. The influence of chair height on lower limb mechanics during rising. J Orthop Res. 1989;7:266–71. doi: 10.1002/jor.1100070215. [DOI] [PubMed] [Google Scholar]
- 21.Hughes MA, Myers BS, Schenkman ML. The role of strength in rising from a chair in the functionally impaired elderly. J Biomech. 1996;29:1509–13. [PubMed] [Google Scholar]
- 22.Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther. 1990;70:340–347. doi: 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]
- 23.Murray MP, Kory RC, Clarkson BH. Walking patterns in healthy old men. J Gerontol. 1969;24:169–78. doi: 10.1093/geronj/24.2.169. [DOI] [PubMed] [Google Scholar]