Abstract
The ability to simultaneously visualize expression of multiple antigens in cells and tissues can provide powerful insights into cellular and organismal biology. However, standard methods are limited to the use of just two or three simultaneous probes and have not been widely adopted for routine use in paraffin-embedded tissue. We have developed a novel approach called sequential immunoperoxidase labeling and erasing (SIMPLE) that enables the simultaneous visualization of at least five markers within a single tissue section. Utilizing the alcohol-soluble peroxidase substrate 3-amino-9-ethylcarbazole, combined with a rapid non-destructive method for antibody–antigen dissociation, we demonstrate the ability to erase the results of a single immunohistochemical stain while preserving tissue antigenicity for repeated rounds of labeling. SIMPLE is greatly facilitated by the use of a whole-slide scanner, which can capture the results of each sequential stain without any information loss. (J Histochem Cytochem 57:899–905, 2009)
Keywords: immunohistochemistry, colocalization, multiple, antigens
Visual colocalization of molecular species within sectioned tissue can provide insights into cellular biochemistry and can serve as the basis for further study of protein–organelle and protein–protein interactions. Visualization of abnormal protein coexpression in neoplastic tissue may elucidate components of oncogenic signaling pathways. Colocalization of COX2 and laminin-5, for example, has been observed at the cancer–stromal interface of lung adenocarcinoma and may be associated with abnormalities in p53 expression (Niki et al. 2002). Additionally, colocalization of β-catenin with compartment-specific markers revealed prognostic significance that was not found using traditional single-stain methods (Camp et al. 2002).
Current colocalization methods, however, have several limitations. The most commonly used method, multicolor immunofluorescence, is limited by the number of viable combinations of available fluorescent tags and can be negatively affected by spectral bleed-through, antibody cross-reactivity, photo-bleaching, and autofluorescence of paraffin-embedded tissue (Robertson et al. 2008). Because of these limitations, this method is usually restricted to the simultaneous visualization of just two antigens plus a nuclear counterstain. Peroxidase or alkaline phosphatase–linked multicolor immunostaining is possible, but the maximum number of chromogenic substrates that can be differentiated within a single section is typically two or three (Dandrea et al. 2001). Moreover, multiple chromogenic substrates do not allow colocalization in the same cellular compartment, owing to the obscuring of lighter colors by darker chromogens. Additionally, the use of more than one primary antibody from the same species (i.e., mouse or rabbit) is usually impossible, owing to secondary antibody cross-reactivity. A recent report of a multiplex immunostaining chip demonstrated simultaneous staining of a large section with a grid of many different antibodies, but because different portions of the tissue section are stained with each probe, no colocalization information is obtained (Furuya et al. 2004).
To overcome these limitations, we have developed a novel approach called sequential immunoperoxidase labeling and erasing (SIMPLE) that enables the simultaneous visualization of multiple markers within a single tissue section. By combining the use of an alcohol-soluble immunoperoxidase substrate, 3-amino-9-ethylcarbazole (AEC), with a previously described antibody–antigen dissociation method (Tramu et al. 1978), we have performed up to five serial rounds of staining on a single tissue section without loss of tissue antigenicity. This method is accessible to any histology lab. The use of a whole-slide digital scanning microscope greatly increases the power of SIMPLE, because it facilitates permanent archiving of each round of labeling. In addition, from a practical standpoint, the method enables multiple immunohistochemical analyses to be performed on otherwise-limited tissue samples, such as very small biopsies and tissue microarrays (TMAs).
Materials and Methods
Tissue Samples
All experiments using mice were approved by the Animal Care and Use Committee at the University of Virginia. Mice were perfused intracardially with 4% paraformaldehyde after deep anesthetization with xylazine/ketamine. After overnight fixation in 4% paraformaldehyde, brains were then embedded in paraffin and sectioned at 4 μm. Human pituitary tissue was obtained from autopsy-procured archival specimens under an approved University of Virginia protocol.
Antibodies
The following primary antibodies were used: GFAP (DAKO rabbit polyclonal Z0334, 1:5000; DakoCytomation, Carpinteria, CA); S100-β (DAKO rabbit polyclonal Z0311, 1:500); calbindin (Sigma mouse monoclonal C8666, clone CL300, 1:1000; Sigma, St. Louis, MO); neurofilament-M (monoclonal antibody, clone 5B8, Developmental Studies Hybridoma Bank, University of Iowa, supernatant used undiluted); MAP2 (NeoMarkers monoclonal antibody AP18, 1:500; NeoMarkers, Fremont, CA); adrenocorticotropic hormone (ACTH; DAKO mouse monoclonal antibody clone 02A3, 1:4000); human chorionic gonadotropin alpha subunit (hCG; mouse monoclonal antibody, Biogenix clone F23, 1:25, Biogenix, San Ramon, CA); luteinizing hormone (LH; DAKO mouse monoclonal antibody clone C93, 1:400); thyroid-stimulating hormone (TSH; Biogenix mouse monoclonal antibody clone 5404, 1:400).
Immunohistochemistry
Paraffin sections mounted on slides were placed in a 60C oven for 1 hr, then dewaxed in xylenes (5 min ×2) and rehydrated in a series of graded alcohols to distilled water. Prior to immunostaining, the tissue was stained with hematoxylin followed by bluing in 0.5% ammonium hydroxide. Slides were then coverslipped in aqueous mountant (70% glycerol in PBS or GelMount; Genetex, San Antonio, TX). After slide imaging (see below), slides were decoverslipped by immersion in distilled water and rinsed in TBST. One round of antigen retrieval was then performed, consisting of three cycles of 5 min (high power) in a commercial microwave in 10 mM sodium citrate buffer (pH 6.0), followed by cooling to room temperature. Endogenous peroxidase was quenched in 3% hydrogen peroxide for 10 min, followed by rinsing in TBST. Tissue was then blocked in 2.5% normal horse blocking serum (Vector Laboratories; Burlingame, CA) for 20 min, followed by a 1-hr incubation of primary antibody at room temperature. Detection of primary antibody was performed with the ImmPress reagents for either mouse or rabbit primaries (DakoCytomation) and AEC (Vector Laboratories), and slides were again coverslipped in aqueous mounting medium.
AEC Destaining and Antibody Stripping
After high-resolution scanning, slides were decoverslipped in distilled water, and stained slides were dehydrated in an alcohol gradient to 95% ethanol. Slides were incubated until no visible AEC reaction product remained. Following rehydration, antibodies were eluted by incubating sections in 0.15 M KMnO4/0.01 M H2SO4 solution for 2 min, followed immediately by a distilled water wash (Tramu et al. 1978). Tissue was then restained, beginning with the blocking step, as described above.
Microscopy and Image Analysis
Full-slide scans of stained tissue were obtained after each round of staining on an Aperio ScanScope at 40× magnification (Aperio Technologies; Vista, CA). To make multicolor composite images, digital snapshots of tissue section subregions were selected and overlaid as separate layers in Adobe Photoshop CS2 (Adobe Systems, Inc.; San Jose, CA). The hematoxylin-stained image was used as the background layer. Working with a single layer at a time, subsequent layers were aligned to the background image by selecting the “difference” blending mode and using the “move tool” to nudge the layer into proper alignment. Aligned layers were then linked to prevent further movement. Alignment of all layers was confirmed by visual inspection using the “normal” blending mode and toggling between layers. The “select color” tool was then used to copy the stained regions of each image into a new layer. The “replace color” tool was then used to selectively apply pseudocolors to replace the AEC precipitate color. These psuedocolored layers were overlaid using the “normal” blending mode to produce the final composite images.
Results
SIMPLE Strategy
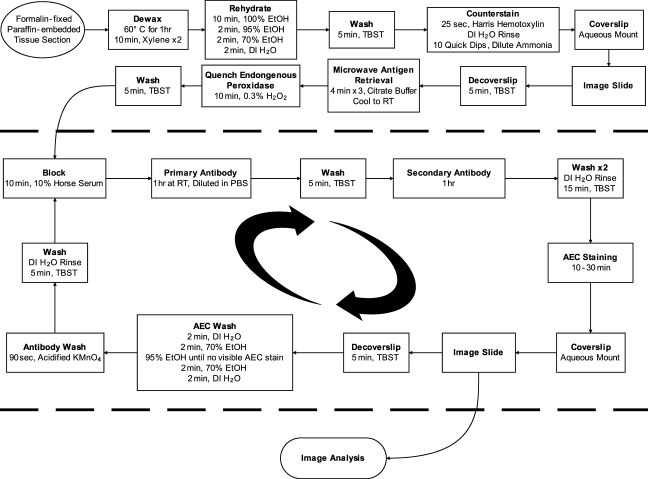
The general protocol of the SIMPLE method is illustrated in Figure 1. Formalin-fixed, paraffin-embedded tissue is dewaxed and rehydrated using standard procedures. The tissue is subsequently counterstained with hematoxylin, and a counterstain-only reference image is obtained. Following imaging, antigen retrieval is performed, which also removes the counterstain. This retrieval is followed by immunohistochemical staining with the red peroxidase substrate AEC. After imaging, the AEC precipitate is washed away in 95% ethanol, and bound antibody is removed in an elution solution of acidified KMnO4. The staining process is then repeated as desired, with subsequent images analyzed separately or overlaid digitally using pseudocoloring to form a single composite image. The method is greatly facilitated by use of a whole-slide scanning microscope, which provides permanent archiving of all labeling and allows any region of each stain to be viewed at a variety of magnifications.
Figure 1.
SIMPLE strategy. Formalin-fixed, paraffin-embedded sections are dewaxed, rehydrated, and counterstained before initial probing. Tissue is imaged and then subjected to antigen retrieval, removing the counterstain. Each staining round is conducted using standard immunohistochemical protocols with the alcohol-soluble red peroxidase substrate 3-amino-9-ethylcarbazole (AEC). After each round of staining, the tissue is imaged and then stripped of AEC precipitate in ethanol. Antibody is then eluted in acidified permanganate, and the tissue is subjected to the next round of staining.
Validation of Antibody Removal and Antigen Preservation
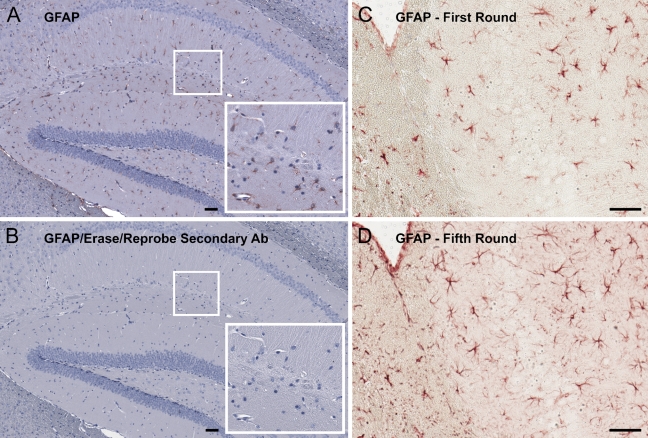
To validate the efficacy of acidified permanganate as an antibody-stripping agent, an adult mouse brain section was immunostained with a rabbit anti-GFAP polyclonal antibody, imaged, and then treated with acidified permanganate. The section was then blocked and incubated in peroxidase-conjugated anti-rabbit secondary antibody for 1 hr, followed by 30 min of incubation in AEC substrate. After re-counterstaining, the tissue was imaged again and analyzed for the presence of any residual anti-GFAP antibody. A complete lack of staining was observed, indicating a complete removal of bound primary and secondary antibody (Figures 2A and 2B). A test of antigen stability was performed by subjecting a single mouse brain section to five stain/strip cycles, reprobing for GFAP each time. GFAP immunoreactivity actually became more intense following several rounds of staining and elution, possibly due to an antigen retrieval effect of the acid treatment (Figures 2C and 2D).
Figure 2.
Demonstration of antibody elution efficacy and preservation of tissue antigenicity after five rounds of SIMPLE. Adult mouse brain was probed for GFAP and imaged (A). After stripping and antibody elution, the tissue was incubated in secondary antibody and AEC according to standard immunohistochemical protocol. Corner inset shows enlargement of small boxed region. Counterstaining in hemotoxylin was performed, and the tissue was then imaged again (B). A complete lack of staining was observed, indicating that the primary anti-GFAP antibody had been completely eluted. Corner inset shows enlargement of small boxed region. A section of adult mouse brain was probed for GFAP, both initially (C) and again after four previous rounds of SIMPLE (D). GFAP immunostaining was completely retained and in fact appeared more intense. Bar = 50 μm.
Multiple Antibody Visualization
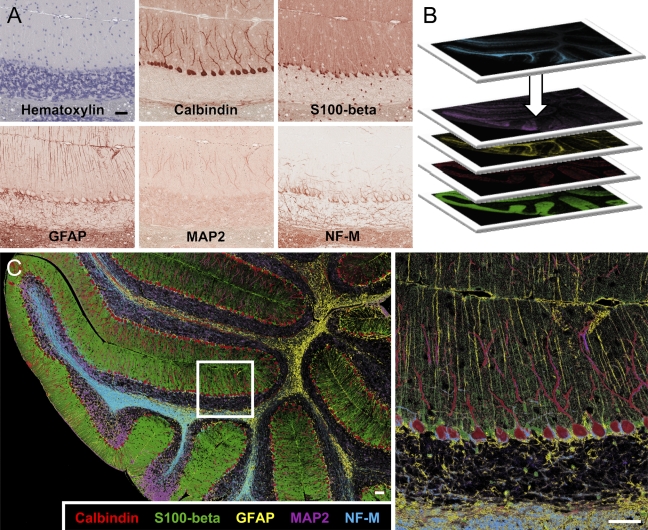
To demonstrate the ability to probe for multiple antigens on a single tissue section, a sagittal mouse brain section was stained with antibodies recognizing calbindin, S100-β (rabbit polyclonal), GFAP (rabbit polyclonal), MAP2 (mouse mAb AP18), and neurofilament (mouse mAb 2H3) (Figure 3A). Digital snapshots of the same brain regions were taken from each slide scan. Snapshots were imported into Adobe Photoshop (Adobe Systems, Inc.) and overlaid, and the “replace color” tool was used to create a false-color composite allowing for simultaneous visualization of all five probes (Figures 3B and 3C). In the cerebellum, Purkinje cells stained strongly for calbindin, and they also contained dendritic MAP2. S100-β and GFAP were seen to colocalize in astrocytes and Bergmann glia, with robust expression in the nucleus and processes, respectively. Neurofilament staining revealed axons, especially those of basket cells terminating on Purkinje cell bodies.
Figure 3.
Simultaneous visualization of five antigens in mouse cerebellum. (A) Adult mouse brain was counterstained with hemotoxylin, then sequentially probed with polyclonal antibodies to calbindin, S100-β, and GFAP, and monoclonal antibodies to MAP2 (AP18) and neurofilament (NF-M) 2H3. (B) The images were individually pseudocolored and overlaid. (C) The small boxed area in the left panel is shown magnified at right. The resultant image reveals the morphology of different cell types and fine details of interactions of Purkinje cells, Bergmann glia, astrocytes, and basket cell terminals that would not be obvious with single or dual labeling. Bar = 50 μm.
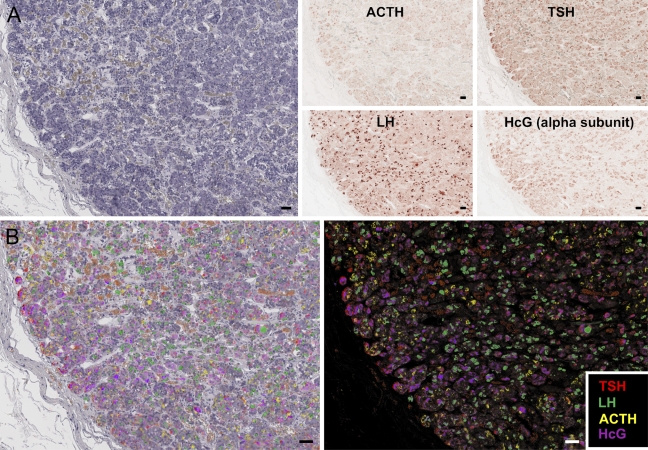
To ascertain the utility of the SIMPLE method for use on human archival tissue, autopsy pituitary tissue was obtained. Autopsy tissue, especially from aged individuals, frequently has high autofluorescence, making two- or three-color immunofluorescence difficult. SIMPLE imaging for the hormones ACTH, hCG, LH, and TSH was performed on a single paraffin section (Figure 4A). The pseudocolored image allows clear visualization of all probes, revealing patterns of unique and overlapping hormone expression (Figures 4B and 4C).
Figure 4.
SIMPLE labeling of hormone expression in human anterior pituitary. Paraffin-embedded autopsy-acquired human anterior pituitary gland was probed for adrenocorticotropic hormone (ACTH), luteinizing hormone (LH), human chorionic gonadotropin alpha subunit (hCG), and thyroid-stimulating hormone (TSH), respectively. Hemotoxylin counterstain (A, left) is used to align subsequent images of hormone labeling (A, right panels) for multicolor display (B, left), or images can be displayed on a black background to create pseudoimmunofluorescence (B, right panel). Bar = 50 μm.
Discussion
The technique of stripping bound antibody from antigen is widely used to reprobe Western blots and has been used previously to improve multistain immunoenzyme methods (Tramu et al. 1978). However, SIMPLE is a novel approach to eliminate the problems associated with multiprobe color compatibility and antigens located in the same cellular compartment. The advent of new, whole-slide scanning technologies, combined with the use of alcohol-soluble peroxidase substrates such as AEC, creates a powerful tool for multiple antigen colocalization in paraffin-embedded tissue. Here we have validated antibody removal and antigen preservation, and demonstrated the ability to apply SIMPLE on single paraffin sections from mouse or human. A comparison of SIMPLE with some existing methods of multiple antigen colocalization is shown in Table 1.
Table 1.
Comparison of SIMPLE with existing multiprobe immunolabeling methods
| SIMPLE | Traditional multi-chromagen IHC | Multiplex-immunostain chip | Multicolor IF | |
|---|---|---|---|---|
| Maximum labels per section | 5+ | 2–3 | 50 | 3 |
| Use on paraffin-embedded archival tissue | + | + | + | −/+ |
| Ability to overcome autofluorescence/photobleaching | + | + | + | − |
| Colocalization within a single cellular compartment | + | − | − | + |
| Compatible with primary antibodies from same species | + | − | + | − |
SIMPLE is compared with traditional two- or three-color multichromagen immunohistochemistry (IHC), the multiplex immunostain chip method (Furuya et al. 2004), and multicolor immunofluorescence (IF) methods.
Traditional multiple immunofluorescent staining is generally limited by the number of different primary antibody species (i.e., mouse, rabbit, goat) that would allow specific labeling of each primary. As a consequence, typically only two or three distinct colors may be stained. Recent approaches have allowed multiple labeling with antibodies from the same species, although they have not found widespread utility (Wang and Larsson 1985). For multiple immunoperoxidase labeling, there is the additional limitation that opaque substrates do not allow simultaneous visualization within the same subcellular compartment, such as the nucleus.
A related method, termed MELC (multi-epitope-ligand cartography) offers the ability to image the localization of dozens of antigens in the same cell or tissue preparation, using a sequential immunofluorescence method with a photobleaching erasing step (Schubert et al. 2006). MELC is a powerful technique that will likely provide novel insights into multiple protein colocalization patterns. However, an important disadvantage of MELC as compared with SIMPLE is that the photobleaching step in MELC can only be applied to the microscope's field of view, meaning that the multiprobe image is limited to a single microscopic medium-to-high power field. In contrast, SIMPLE, when imaged using a whole-slide scanner, provides a multiprobe image for the entire tissue section, which encompasses thousands of high-power fields. Another disadvantage of MELC is cost: the method requires a robotic staining apparatus integrated with an inverted fluorescence microscope. Finally, the reliance of MELC on immunofluorescence can be a problem in some archival fixed human tissues that contain high levels of tissue autofluorescence.
Situations in which tissue specimens are limiting in quantity could benefit from SIMPLE. In clinical consultations, pathologists are frequently provided inadequate numbers of unstained slides to perform a full array of immunohistochemical stains. Likewise, TMAs are often limited in availability, and when they are obtained commercially, are often very costly. In both of these instances, the tissue samples could be probed with multiple antibodies using SIMPLE. An important advantage of a serial immunohistochemical approach is that the same cells or tissue feature can be analyzed for expression of multiple antigens, which is impossible when staining near-adjacent sections. Our experience has shown SIMPLE to be a useful tool for up to six repeated reprobes. Beyond this, some physical tissue degradation becomes apparent and image quality is reduced. We believe that this degradation may be associated with either the physical handling of slides or the repeated dehydration of tissue in ethanol, inasmuch as extended exposure to the acidic permanganate solution itself (up to 30 min) caused no obvious deleterious effects on tissue quality (data not shown). Thus, development of gentler slide-handling methods should allow increased numbers of probes to be applied.
SIMPLE will have multiple applications. For example, simultaneous visualization of different phosphorylation sites, as well as total protein expression within specific cell types, could reveal important information about signaling pathway activation status in normal and neoplastic tissues. SIMPLE can be easily performed by any lab already conducting traditional immunohistochemistry methods with paraffin sections, and it should find a number of practical uses in both research and diagnostic laboratories.
This work was supported by a Coulter Foundation Translational Research award and a University of Virgina Cancer Center Craig Foundation Grant (to JP and JWM)..
References
- Camp RL, Chung GG, Rimm DL (2002) Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med 8:1323–1327 [DOI] [PubMed] [Google Scholar]
- Dandrea MR, Reiser PA, Gumula NA, Hertzog BM, Andrade-Gordon P (2001) Application of triple immunohistochemistry to characterize amyloid plaque-associated inflammation in brains with Alzheimer's disease. Biotech Histochem 76:97–106 [PubMed] [Google Scholar]
- Furuya T, Ikemoto K, Kawauchi S, Oga A, Tsunoda S, Hirano T, Sasaki K (2004) A novel technology allowing immunohistochemical staining of a tissue section with 50 different antibodies in a single experiment. J Histochem Cytochem 52:205–210 [DOI] [PubMed] [Google Scholar]
- Niki T, Kohno T, Iba S, Moriya Y, Takahashi Y, Saito M, Maeshima A, et al. (2002) Frequent co-localization of Cox-2 and laminin-5 gamma2 chain at the invasive front of early-stage lung adenocarcinomas. Am J Pathol 160:1129–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Savage K, Reis-Filho JS, Isacke CM (2008) Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol 9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert W, Bonnekoh B, Pommer AJ, Philipsen L, Bockelmann R, Malykh Y, Gollnick H, et al. (2006) Analyzing proteome topology and function by automated multidimensional fluorescence microscopy. Nat Biotechnol 24:1270–1278 [DOI] [PubMed] [Google Scholar]
- Tramu G, Pillez A, Leonardelli J (1978) An efficient method of antibody elution for the successive or simultaneous localization of two antigens by immunocytochemistry. J Histochem Cytochem 26:322–324 [DOI] [PubMed] [Google Scholar]
- Wang BL, Larsson LI (1985) Simultaneous demonstration of multiple antigens by indirect immunofluorescence or immunogold staining. Novel light and electron microscopical double and triple staining method employing primary antibodies from the same species. Histochemistry 83:47–56 [DOI] [PubMed] [Google Scholar]