Abstract
Bovine lactadherin holds a stereo-specific affinity for phosphatidylserine (PS) membrane domains and binds at PS concentrations lower than the benchmark PS probe, annexin V. Accordingly, lactadherin has recognized PS exposure on preapoptotic immortalized leukemia cells at an earlier time point than has annexin V. In the present study, the cervical cancer cell line HeLa has been employed as a model system to compare the topographic distribution of PS with the two PS binding proteins as adherent cells enter the apoptotic program. HeLa cells were cultured on glass-bottom Petri dishes, and apoptosis was induced by staurosporine. Fluorescence-labeled lactadherin and/or annexin V were used to detect PS exposure by confocal microscopy. Both lactadherin and annexin V staining revealed PS localized to plasma membrane rim and blebs. In addition, lactadherin identified PS exposure on long filopodia-like extensions, whereas annexin V internalized in granule-like structures. All in all, the data further delineate the differences in PS binding patterns of lactadherin and annexin V. (J Histochem Cytochem 57:907–914, 2009)
Keywords: lactadherin, annexin V, HeLa, phosphatidyserine, confocal microscopy, MFG-E8
Phosphatidylserine (PS) is one of the four major phospholipids that predominate in the plasma membranes of mammalian cells, typically comprising 8–15 mol% of the total phospholipid content (Devaux and Zachowski 1994). Under normal conditions, PS is exclusively located in the cytosolic inner membrane leaflet. This non-random distribution is maintained by an energy-dependent enzymatic process that translocates PS against its concentration gradient in the membrane (Zwaal et al. 2005). In most cases, the non-random orientation of PS is preserved during the life span of the cell. However, loss of cell membrane asymmetry resulting in PS exposure on the outer membrane leaflet has been reported in platelet and erythrocyte activation (Shi et al. 2008), as well as apoptotic cells (Fadok et al. 1992; Wolfs et al. 2005; Lang et al. 2006). In the blood, the encounter with PS surfaces promotes assembly and catalytic activity of factor Xa and thrombin, thereby shifting the hemostatic balance toward coagulation (Zwaal et al. 1998). Apoptosis is a controlled shutdown of cellular processes characterized by cell shrinkage, nuclear condensation, and membrane vesiculation/blebbing (Kerr et al. 1972; Fadok et al. 1992; Wolfs et al. 2005). The typical fate of apoptotic cells is rapid engulfment and degradation by phagocytes, thereby avoiding the risk of necrosis and inflammation (Fadok et al. 2001). The surface-exposed PS is directly involved in this process by participation in the coordinating of phagocyte recognition (Wu et al. 2006).
It has previously been reported that the C-terminal C1C2 domains of the protein lactadherin (also known as PAS-6/7 or MFG-E8) bind PS in a stereospecific, calcium-independent manner (Shi and Gilbert 2003). In addition, the N-terminal epidermal growth factor–like domains of the protein contain one Arg-Gly-Asp (RGD) amino acid sequence that mediates cell adhesion through the recognition of αVβ5 and αVβ3 integrins. The dual binding affinities led to the notion that lactadherin acts as an opsonin that facilitates PS-dependent phagocytosis of apoptotic cells (Hvarregaard et al. 1996; Andersen et al. 1997,2000), as later confirmed in vivo (Hanayama et al. 2002).
Annexin V, another PS binding protein of yet-unclear specific physiological function, is currently used as the benchmark probe for assessment of PS exposure in imaging techniques (Koopman et al. 1994; Vermes et al. 1995). However, recent flow cytometric and confocal microscopic studies suggest that lactadherin enables detection of surface exposure of PS in apoptotic suspension cells at an earlier time point than does annexin V (Dasgupta et al. 2006; Shi et al. 2007). In addition, lactadherin and annexin V are known to diverge in their specificity toward PS. Annexin V requires Ca2+ and has enhanced binding to membranes also containing phosphatidylethanolamine (Swairjo et al. 1995), whereas lactadherin is affected by neither Ca2+ nor phosphatidylethanolamine (Shi and Gilbert 2003). Furthermore, lactadherin–PS binding is enhanced by increased membrane curvature, whereas annexin V preferentially binds to flat membrane patches (Andree et al. 1992; Shi et al. 2004). Lactadherin binding is evident in a steep-positive manner in spherical membranes with PS content at 0–2%. In contrast, annexin V binds the same membranes in a sigmoidal manner with a threshold above 4% PS (Andree et al. 1990; Tait and Gibson 1992). Using confocal microscopy, it has been shown that annexin V can be pinocytosed by viable and apoptotic cells (Kenis et al. 2004). In the latter example, annexin V can bind to membrane blebs, and reverses them to form invaginations. The research presented in this study compares staining patterns of lactadherin and annexin V on apoptotic HeLa cells. The results reveal topographical differences in PS recognition between lactadherin and annexin V, respectively.
Materials and Methods
Chemicals
All buffer salts (analytical grade) were supplied by Sigma-Aldrich Corp. (St. Louis, MO) or Merck and Co., Inc. (Whitehouse Station, NJ), and DMSO extra pure was purchased from Ferak (Berlin, Germany). Propidium iodide (PI) was from Sigma-Aldrich Corp. Fatty acid–free bovine serum albumine (BSA), fraction V, was from Calbiochem (San Diego, CA). Alexa Fluor 488, Alexa Fluor 633, cell culture media, FBS, and Pen/Strep were from Invitrogen (Paisley, UK). Annexin was from BD Pharmingen (San Jose, CA). Antibody against the bovine C2 domain of lactadherin was produced in-house (Shi et al. 2008). Ser-His-Arg-Gly-Asp-Val-Phe (SHRGDVF) peptide was produced by Schafer-N (Copenhagen, Denmark).
Cell Culture
HeLa cells were maintained in DMEM media with GlutaMax (Invitrogen) with 10% FBS and 1% Pen/Strep at 37C in 5% CO2. For microscopy, cells were grown on 35-mm Petri dishes, 14-mm Microwell, No. 1.5 coverglass, (0.16–0.19 mm) from the MatTek Corp. (Ashland, MA).
Protein Purification and Labeling
Bovine lactadherin was purified as previously described (Hvarregaard et al. 1996) and stored in 75 mM sodium phosphate, pH 7.0 (−80C). Purity was verified to be >95%, in two glycosylation forms, by SDS-PAGE and N-terminal amino acid sequencing. Protein concentrations were determined by amino acid analysis using post column o-phthaldialdehyde derivatization.
Labeling of either annexin V or lactadherin with either Alexa Fluor 488 or Alexa Fluor 633 followed the same protocol. Immediately before labeling, the dye was dissolved in DMSO. Ten times molar excess of dye and protein was mixed (75 mM NaHPO4, pH 7.0) and allowed to react under constant mild agitation for 1 hr in the dark at room temperature. Unreacted dye was removed by dialysis twice in a SpinDialyser (Sialomed, Inc.; Columbia, MD) against 1 liter of 60 mM NaHPO4, pH 7.0, 140 mM NaCl. Following dialysis, 0.02% NaN3 was added, and the labeled protein was kept in the dark at 4C. Protein concentration was validated by amino acid analysis. Labeling in this manner has no or minimal effect of membrane binding (Shi et al. 2004).
Confocal Microscopy
HeLa cells were grown to 50–75% confluence on glass-bottom Petri dishes. To induce apoptosis, 10 μM staurosporine in DMSO was added and cells were incubated at 37C in 5% CO2 for 3 hr. After 3 hr, the medium was aspirated and cells were gently washed twice in sterile filtered (0.2 μm) Tyrode's buffer (15 mM HEPES, 3.3 mM NaH2PO4, pH 7.4, 138 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 5.5 mM dextrose, and 0.2% fatty acid–free BSA). During experiments involving annexin V staining, 1.5 mM CaCl2 was added to the buffer. For experiments at low magnification, cells were incubated with 2-ml solutions of Tyrode's buffer containing 5 nM Alexa Fluor 488–labeled lactadherin and 0.5 μM PI. In experiments performed at high magnification, cells were incubated with 2-ml solutions of Tyrode's buffer containing 10 nM Alexa Fluor 488– or Alexa Fluor 633–labeled lactadherin. Furthermore, 10 nM of Alexa Fluor 488–labeled annexin V was used for costaining experiments. In addition, for inhibition experiments, 250 μM of the SHRGDVF peptide or 200 nM goat antibody against the C2 domain of lactadherin was added to the staining solution prior to addition to cell culture.
For all annexin V or lactadherin staining experiments, the staining solution was allowed to incubate with the cells for 15 min at 37C in 5% CO2 in the dark. After incubation, cells were gently washed twice with Tyrode's buffer, and 2 ml Tyrode's buffer was added to avoid cells drying during imaging. Cells were immediately taken to the laser scanning confocal microscope, and z-stacks were taken. Microscopy was done at room temperature in the dark using an LSM 510 META equipped with an LSM 510 laser module and controlled by the LSM 510 3.2 SP2 software from Zeiss (Thornwood, NY). A 40× NA 1.2 W water objective and a 100× NA 1.3 W oil objective were used.
To image membranes in general, phospholipid bilayer staining was performed on staurosporine-induced cells using the CellBrite green cytoplasmic membrane staining kit (Biotium, Inc.; Hayward, CA) following the description given by the manufacturers. This kit utilizes the NeuroDiO stain, which is characterized as a lipid-soluble, long-carbon-chain carbocyanine dye capable of staining bilayer membranes in a non-uniform manner without an apparent effect on adhesion (Honig and Hume 1986; Kreft et al. 1997). The incubation time for staining was selected to be 20 min.
Results
Staining Patterns of Lactadherin Upon Staurosporine-induced Apoptosis
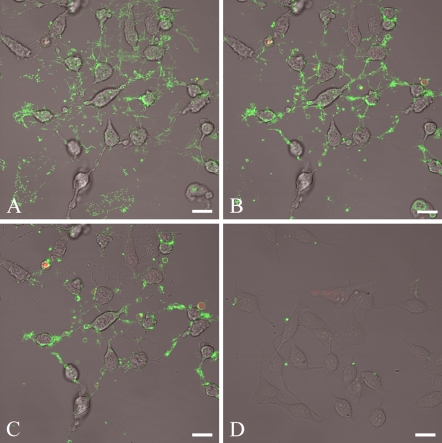
HeLa cells treated with staurosporine were used to illustrate the binding pattern of lactadherin to exposed PS. Representative images of confocal slices taken throughout the z axis at low magnification are given in Figure 1. The staurosporine-induced apoptosis changed the morphology of the HeLa cells by reduction of cell volume, ruffling and vesiculation/blebbing of the plasma membrane, and concomitant appearance of large amounts of thin, elongated filopodia-like areas. PI staining revealed that only a minor fraction (<10%) of the HeLa cells became necrotic during the experimental period. The z-stack closest to the plane of cell attachment showed lactadherin staining of elongated thin filopodia-like structures (Figure 1A). Areas with remnants of detached cells also stained brightly. Moving upward along the basal–apical axis, the dominant lactadherin staining features are small blebs and the narrow rim of the plasma membrane (Figures 1B and 1C). As a control, viable non-apoptotic HeLa cells were stained with labeled lactadherin and PI (Figure 1D). Approximately one third of control cells exhibited occasional rounded protuberances and rare patches that stained with lactadherin. However, most cells had no lactadherin staining. Similar staining of occasional scattered protuberances has been observed on suspended leukemia cells (Shi J, et al., unpublished data).
Figure 1.
Lactadherin staining of apoptotic cells at low magnification. Cells are stained with 5 nM Alexa Fluor 488–labeled bovine lactadherin and propidium iodide (PI). (A–C) HeLa cells induced for 3 hr to apoptosis by 10 μM staurosporine. The images represent selected slides of a confocal z-stack of the same area, with A located nearest to the glass surface. (D) Non-apoptotic HeLa cells stained with 5 nM of Alexa Fluor 488–labeled bovine lactadherin. The observed morphology of staurosporine-induced Hela cells is characterized by reduction of cell volume, ruffling, and vesiculation. In addition, a relatively large amount of thin, elongated filopodia-like areas appears. Little PI staining is evident, suggesting that few cells have entered secondary necrosis. Lactadherin stains avidly in the thin, elongated filopodia-like areas, as well as cell rims and vesicles. Bar = 20 μm.
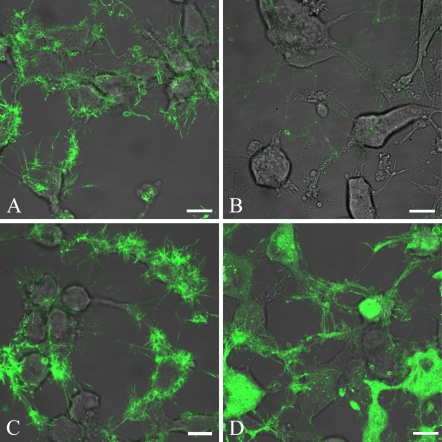
The patterns described at low magnification become clearer at high magnification. Expressive lactadherin binding on the thin, elongated filopodia-like areas in apoptotic cells is easily seen (Figure 2A). Staining seems to be focused on these and on protruding vesicles and membrane ruffles attached to the cells. Labeled lactadherin was coincubated with 20-fold molar excess of a polyclonal goat antibody directed against the C2 domain of bovine lactadherin, to confirm that lactadherin binds to the surface of the cells by C2 domain–mediated PS recognition. The antibody inhibited lactadherin binding extensively, but not completely (Figure 2B). This is in accordance with prior studies utilizing this antibody, where 80% inhibition of binding to stimulated platelets could be achieved (Shi et al. 2008). To exclude that part of the lactadherin binding relates to RGD-dependent integrin interaction, experiments were performed with 250× molar excess of a heptapeptide (SHRGDVF). Besides the RGD sequence, this peptide also holds flanking regions that correspond to the equivalent region in bovine lactadherin, and it is therefore a highly specific inhibitor of lactadherin–integrin binding. It turned out that inclusion of the heptapeptide did not have any recognizable effect upon the pattern or intensity of the lactadherin staining (Figure 2C), indicating that lactadherin interaction with integrins is negligible under the experimental conditions chosen.
Figure 2.
Validation of lactadherin-specific binding at high magnification. HeLa cells are induced for 3 hr to apoptosis by 10 μM staurosporine and stained with fluorescently labeled lactadherin. Lactadherin staining is normalized to appear green. (A) Cells are stained with 10 nM Alexa Fluor 633–labeled lactadherin. (B) Cells are stained with 10 nM Alexa Fluor 488–labeled lactadherin and 200 nM goat antibody against the C2 domain of lactadherin. (C) Cells are stained with 10 nM Alexa Fluor 633 coincubated with 250× molar surplus of Ser-His-Arg-Gly-Asp-Val-Phe peptide. (D) Cells stained with CellBrite Green cytoplasmic membrane staining kit. The observed lactadherin staining is independent of Arg-Gly-Asp–mediated adhesion, whereas antibodies against the PS binding C2 domain result in clear staining inhibition. Furthermore, staining with the NeuroDIO membrane stain available in the Cellbrite Green cytoplasmic membrane staining kit confirms that the observed affinities of lactadherin staining can indeed be assigned to membrane-covered areas. Bar = 10 μm.
Finally, experiments were performed with a fluorescent lipophilic membrane dye (NeuroDiO) to determine whether the lactadherin staining found on the thin, elongated filopodia-like structures arose from membrane structures (Figure 2D). It is evident that the lactadherin and the membrane dye locate to equivalent areas on the elongated filopodia-like areas. However, as expected, the membrane dye stains the elongated cell extensions less selectively. Costaining of apoptotic HeLa cells with labeled lactadherin and NeuroDiO failed presumably because the NeuroDiO staining abrogated lactadherin binding (data not shown).
Costaining Patterns of Lactadherin and Annexin V
The preceding data document that lactadherin locates to the plasma membrane on attached apoptotic cells. The next move was to compare the topological distribution of annexin V and lactadherin on adherent cells. Accordingly, staurosporine-induced apoptotic cells were coincubated with fluorescence-labeled lactadherin (green) and annexin V (red). Figure 3 shows representative images of a confocal z-stack of apoptotic HeLa cells at high magnification.
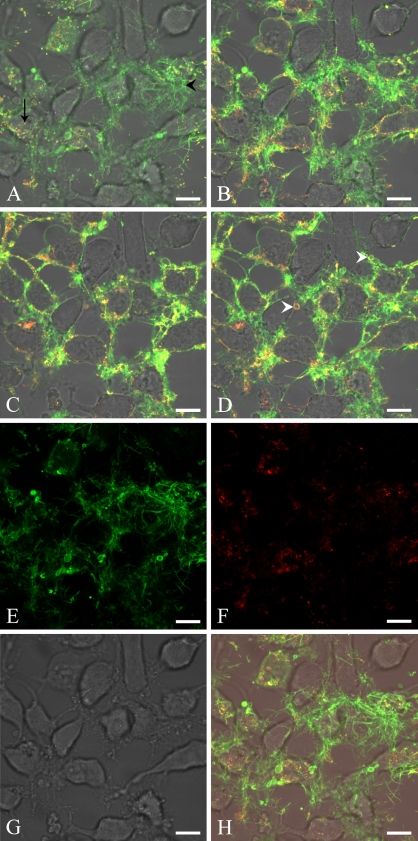
Figure 3.
Lactadherin and annexin V costaining of apoptotic cells at high magnification. Cells are stained with 10 nM Alexa Fluor 633–labeled bovine lactadherin and 10 nM Alexa Fluor 488–labeled annexin V. Lactadherin is normalized to appear green, and annexin V is normalized to appear red. Areas with costaining appear yellow. (A–D) HeLa cells induced for 3 hr to apoptosis by 10 μM staurosporine. The images represent selected slides of a confocal z-stack of the same area, with A located nearest to the glass surface. There is a markedly different binding pattern of lactadherin and annexin V. Lactadherin mainly performs staining of the cellular rim and of blebbing vesicles (white arrowhead), as well as staining of thin filopodia-like cell appendages (black arrowhead). Annexin V stains mainly as bright spots (black arrow), consistent with internalized annexin V, or on blebbing membranes and the rim of cells. (E–G) Images represent one unmerged z-stack near the plane of cell attachment, with E being lactadherin staining, F being annexin V staining, and G showing the phase contrast image. (H) The merged image of E–G. Bar = 10 μm.
A comparison of cellular layers by z-stacks revealed different binding patterns of the two ligand proteins. In close proximity to the cell attachment sites, lactadherin interacts mainly in the elongated filopodia-like structures, whereas annexin V binding mainly occurs in rounded invaginations in the plasma membrane. Presumably, these are on the basal-lateral surface of the cells. Some overlapping of the two PS probes is seen in the plasma membrane region (Figure 3A). Moving upward in the z-stack, lactadherin becomes increasingly widespread in the thin filopodia-like areas and on curved vesicles (Figure 3B). In addition, some lactadherin staining is also apparent on the cellular rim. In the same z-stack interval, annexin V stains mainly at the cellular rim, on curved vesicles or as intracellular bright spots. Moving upward along the basal–apical axis, staining of both proteins results in more-pronounced staining of the cellular rim areas (Figure 3C). Both annexin V and lactadherin recognize curved vesicles. In the apical z-stack, the staining pattern by both proteins is mainly characterized by overlapping rim staining of the cells. However, lactadherin staining is more widespread than annexin V staining (Figure 3D). An unmerged z-stack of an image taken near the bottom plane (closest to the glass) clearly shows a markedly differing staining pattern of lactadherin and annexin V. Lactadherin intensely stains the filopodia-like structures, whereas annexin V shows no discernable attachment to these (Figures 3E–3H). In summary, marked differences were observed in the way the two proteins stain apoptotic HeLa cells. Lactadherin staining was seen to be brightest on the elongated filopodia-like areas, curved vesicles, and general cell rim staining. Conversely, annexin V interacts weakly with the elongated thin filopodia-like areas, whereas the main staining by annexin V can be seen as bleb staining or rim staining or as bright intracellular spots on cells.
Discussion
The present study confirms prior observations that staining cells with lactadherin reveals PS-exposed features not previously detectable. This demonstrates that an early step in the HeLa apoptosis program is the extension of a network of filopod-like extensions with exposed PS. The function of PS exposure on these extensions is unknown.
Lactadherin is an effective PS-staining reagent for microscopy-based study designs describing phenotypes of staurosporine-induced apoptotic HeLa cells when compared with annexin V. Lactadherin particularly locates to the thin, elongated filopodia-like appendages presented by many of the cells. It seems likely that the thin filopodia-like structures appear as a consequence of either cell shrinkage or the apoptosis mechanism induced by staurosporine. It is also likely that the filopodia-like appendages are present in normal living cells, but owing to their size, they are very hard to discern at the magnifications technically viable in our laboratory. Staurosporine is a microbial alkaloid originally isolated from Streptomyces staurospores that provokes several cellular effects including inhibition of protein kinases, e.g., protein kinase C and the cyclin-dependent kinases (Tamaoki et al. 1986; Ward and O'Brian 1992; Nishi et al. 2002). The resulting morphological consequences of staurosporine treatment have been described on numerous occasions, and among the best described effects is the thinning and dissolution of the actin microfilament bundles in the cytoskeleton (Hedberg et al. 1990; Yang et al. 1997). Presently, there are clear and marked differences between the binding patterns of lactadherin and annexin V toward the elongated filopodia-like appendices derived from apoptotic HeLa cells. Lactadherin shows an intense staining capacity toward thin, elongated filopodia-like membranes, whereas annexin V binding is negligible. This is consistent with findings showing that lactadherin binds preferentially toward highly curved membranes, whereas annexin V favors relatively flat membrane areas (Andree et al. 1992; Shi et al. 2004).
The specificity of lactadherin toward apoptotic membranes via its C2 domain has been validated in three ways. First, it could be seen that lactadherin-stained membrane areas correspond to the regions marked by the lipophilic membrane dye NeuroDiO. Incubation of NeuroDiO-stained apoptotic HeLa cells with lactadherin unambiguously reduced lactadherin staining, suggesting that the NeuroDiO blocks the recognition sites for lactadherin within the membrane. Second, competitive inhibition experiments showed that the lactadherin attachment to the membrane could not be inhibited by a soluble RGD peptide, indicating that the observed lactadherin binding is unrelated to the integrin receptors. Finally, the involvement of PS binding was confirmed, inasmuch as admittance of a polyclonal antibody directed against the C2 PS binding domain of lactadherin quenched the staining capability of lactadherin. Collectively these experiments provide direct evidence that lactadherin cell attachment is mediated by PS binding alone and is not the result of specific or nonspecific interactions with other plasma membrane components, thus confirming earlier studies characterizing these binding properties (Andersen et al. 2000; Shi et al. 2004,2007).
Staining cells with annexin V is not always the optimal method for detecting apoptosis. For example, whereas annexin V requires the presence of near physiologic levels of calcium for binding, lactadherin binds to PS in a calcium-independent manner. This makes lactadherin an attractive probe for the detection of PS-positive cells in assays in which cells are sensitive to the high calcium concentrations required for annexin V binding, e.g., in blood platelet analysis. (Tracy et al. 1988; Dasgupta et al. 2006; Shi et al. 2008; Albanyan et al. 2009).
In the present experiments, annexin V staining of apopotic HeLa cells frequently gave rise to punctate staining in close proximity to the edge of the plasma membrane. This staining pattern has previously been attributed to pinocytic annexin V–positive compartments, which can also be found in viable annexin V–stained cells (Kenis et al. 2004). Eventually, this could imply potential situational weaknesses in the reliability of using annexin V as a selective probe for cells progressing through the cell death program. Another issue relates to the observation that annexin V tends to reverse the blebbing of PS-expressing cells, thus inducing invagination and thereby interfering with the creation of apoptotic bodies (Kenis et al. 2004). Lactadherin, on the other hand, provides two important advantages over annexin V for the assessment of PS exposure. First, it does not interfere with the morphological hallmarks of apoptosis. Second, lactadherin is more sensitive than annexin V when PS is minimally exposed or at an early stage of apoptosis. As seen in this study, the higher sensitivity did influence the ability of fluorescent lactadherin conjugates to detect new PS-exposing cellular features.
Acknowledgments
Funding was received from The Milk Protein Consortium and the PhD School for Industrial-related Biotechnology, University of Aarhus, Denmark.
We thank Dr. Anna M. Jurkiewicz for technical assistance with confocal microscopy and Margit S. Rasmussen for purification assistance.
References
- Albanyan A, Murphy MF, Rasmussen JT, Heegaard CW, Harrison P (2009) Measurement of phosphatidylserine exposure during storage of platelet concentrates using the novel probe lactadherin: a comparison study with annexin V. Transfusion 39:99–107 [DOI] [PubMed] [Google Scholar]
- Andersen MH, Berglund L, Rasmussen JT, Petersen TE (1997) Bovine PAS-6/7 binds αVβ5 integrin and anionic phospholipids through two domains. Biochemistry 36:5441–5446 [DOI] [PubMed] [Google Scholar]
- Andersen MH, Graversen H, Fedosov SN, Petersen TE, Rasmussen JT (2000) Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry 39:6200–6206 [DOI] [PubMed] [Google Scholar]
- Andree H, Reutelingsperger C, Hauptmann R, Hemker H, Hermens W, Willems G (1990) Binding of vascular anticoagulant α (VACa) to planar phospholipid bilayers. J Biol Chem 265:4923–4928 [PubMed] [Google Scholar]
- Andree HA, Stuart MC, Hermens WT, Reutelingsperger CP, Hemker HC, Frederik PM, Willems GM (1992) Clustering of lipid-bound annexin V may explain its anticoagulant effect. J Biol Chem 267:17907–17912 [PubMed] [Google Scholar]
- Dasgupta SK, Guchhait P, Thiagarajan P (2006) Lactadherin binding and PS expression on cell surface-comparison with annexin A5. Transl Res 148:19–25 [DOI] [PubMed] [Google Scholar]
- Devaux PF, Zachowski A (1994) Maintenance and consequences of membrane phospholipid asymmetry. Chem Phys Lipids 73:107–120 [Google Scholar]
- Fadok VA, Bratton DL, Guthrie L, Henson PM (2001) Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol 166:6847–6854 [DOI] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 48:2207–2216 [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417:182–187 [DOI] [PubMed] [Google Scholar]
- Hedberg KK, Birrell GB, Habliston DL, Griffith OH (1990) Staurosporine induces dissolution of microfilament bundles by a protein kinase C-independent pathway. Exp Cell Res 188:199–208 [DOI] [PubMed] [Google Scholar]
- Honig MG, Hume RI (1986) Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol 103:171–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvarregaard J, Andersen MH, Berglund L, Rasmussen JT, Petersen TE (1996) Characterization of glycoprotein PAS-6/7 from membranes of bovine milk fat globules. Eur J Biochem 240:628–636 [DOI] [PubMed] [Google Scholar]
- Kenis H, van Genderen H, Bennaghmouch A, Rinia HA, Frederik P, Narula J, Hofstra L, et al. (2004) Cell surface-expressed phosphatidylserine and annexin A5 open a novel portal of cell entry. J Biol Chem 50:52623–52629 [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis. A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman G, Reutelingsperger CP, Kujiten GA, Keehnen RM, Pals ST, van Oers MH (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415–1420 [PubMed] [Google Scholar]
- Kreft B, Berndorff D, Böttinger A, Finnemann S, Wedlich D, Hortsch M, Tauber R, et al. (1997) LI-cadherin-mediated cell-cell adhesion does not require cytoplasmic interactions. J Cell Biol 136:1109–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F, Lang KS, Lang PA, Huber SM, Wieder T (2006) Mechanisms and significance of eryptosis. Antioxid Redox Signal 8:1183–1192 [DOI] [PubMed] [Google Scholar]
- Nishi K, Schnier JB, Bradbury EM (2002) Cell shape change precedes staurosporine-induced stabilization and accumulation of p27kip1. Exp Cell Res 280:233–243 [DOI] [PubMed] [Google Scholar]
- Shi J, Gilbert GE (2003) Lactadherin inhibits enzyme complexes of blood coagulation by competing for phospholipid binding sites. Blood 101:2628–2636 [DOI] [PubMed] [Google Scholar]
- Shi J, Heegaard CW, Rasmussen JT, Gilbert GE (2004) Lactadherin binds selectively to membranes containing phosphatidyl-L-serine and increased curvature. Biochim Biophys Acta 1667:82–90 [DOI] [PubMed] [Google Scholar]
- Shi J, Pipe SW, Rasmussen JT, Heegaard CW, Gilbert GE (2008) Lactadherin blocks thrombosis and hemostasis in vivo: correlation with platelet phosphatidylserine exposure. J Thromb Haemost 6:1167–1174 [DOI] [PubMed] [Google Scholar]
- Shi J, Shi Y, Waehrens LN, Rasmussen JT, Heegaard CW, Gilbert GE (2007) Lactadherin detects early phosphatidylserine exposure on immortalized leukemia cells undergoing programmed cell death. Cytometry A 69:1193–1201 [DOI] [PubMed] [Google Scholar]
- Swairjo MA, Concha NO, Kaetzel MA, Dedman JR, Seaton BA (1995) Ca2+-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat Struct Biol 2:968–974 [DOI] [PubMed] [Google Scholar]
- Tait JF, Gibson T (1992) Phospholipid binding of annexin V: effects of calcium and membrane phosphatidylserine content. Arch Biochem Biophys 298:187–191 [DOI] [PubMed] [Google Scholar]
- Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F (1986) Staurosporine, a potent inhibitior of phospholipid/Ca++ dependent protein kinase. Biochem Biophys Res Commun 135:397–402 [DOI] [PubMed] [Google Scholar]
- Tracy RBE, Stump D, Lin T, Gomol T, Colleen D, Mann K (1988) Reduction of in vitro artifact during blood collection in TIMI II. Blood 72:376A [Google Scholar]
- Vermes I, Hannen C, Steffens-Nakken H, Reutelingsperger CP (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods 184:39–51 [DOI] [PubMed] [Google Scholar]
- Ward NE, O'Brian CA (1992) Kinetic analysis of protein kinase C inhibition by staurosporine: evidence that inhibition entails inhibitor binding at a conserved region of the catalytic domain but not competition with substrates. Mol Pharmacol 41:387–392 [PubMed] [Google Scholar]
- Wolfs JLN, Comfurius P, Rasmussen JT, Keuren JFW, Lindhout T, Zwaal RFA, Bevers EM (2005) Activated scramblase and inhibited aminophospholipid translocase cause phosphatidylserine exposure in a distinct platelet fraction. Cell Mol Life Sci 62:1514–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Tibrewal N, Birge RB (2006) Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol 16:189–197 [DOI] [PubMed] [Google Scholar]
- Yang R, Fu W, Wang S, Liu T, Lin-Shiau S (1997) Mechanism of the morphological changes induced by staurosporine in rat osteoblasts. Calcif Tissue Int 61:68–73 [DOI] [PubMed] [Google Scholar]
- Zwaal RFA, Comfurius P, Bevers EM (1998) Lipid-protein interactions in blood coagulation. Biochim Biophys Acta 1376:433–453 [DOI] [PubMed] [Google Scholar]
- Zwaal RFA, Comfurius P, Bevers EM (2005) Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci 62:971–988 [DOI] [PubMed] [Google Scholar]