Abstract
Phosphodiesterases (PDEs) comprise a family of enzymes that regulate the levels of cyclic nucleotides, key second messengers that mediate a diverse array of functions. PDE2A is an evolutionarily conserved cGMP-stimulated cAMP and cGMP PDE. In the present study, the regional and cellular distribution of PDE2A in tissues of rats, mice, cynomolgus monkeys, dogs, and humans was evaluated by immunohistochemistry. A polyclonal antibody directed to the C-terminal portion of PDE2A specifically detected PDE2A by Western blotting and by immunohistochemistry. The pattern of PDE2A immunoreactivity (ir) was consistent across all species. Western blot analysis demonstrated that PDE2A was most abundant in the brain relative to peripheral tissues. PDE2A ir was heterogeneously distributed within brain and was selectively expressed in particular peripheral tissues. In the brain, prominent immunoreactivity was apparent in components of the limbic system, including the isocortex, hippocampus, amygdala, habenula, basal ganglia, and interpeduncular nucleus. Cytoplasmic PDE2A staining was prominent in several peripheral tissues, including the adrenal zona glomerulosa, neurons of enteric ganglia, endothelial cells in all organs, lymphocytes of spleen and lymph nodes, and pituitary. These studies suggest that PDE2A is evolutionarily conserved across mammalian species and support the hypothesis that the enzyme plays a fundamental role in signal transduction. (J Histochem Cytochem 57:933–949, 2009)
Keywords: immunohistochemistry, human, cynomolgus monkey, beagle dog, rat, mouse, cAMP, cGMP, phosphodiesterase
The capacity for multiple signaling cascades to utilize common second-messenger systems is dependent upon a cell's ability to integrate the spatial and temporal specificity of these signals within specific subcellular compartments. Phosphodiesterases (PDEs) comprise a family of enzymes that hydrolyze and terminate the action of cyclic nucleotide signaling, thereby mediating a diverse range of functions, including cytoskeletal rearrangement, gene transcription, and regulation of ion channel function (Manganiello et al. 1990; Soderling and Beavo 2000; Mehats et al. 2002; Conti and Beavo 2007). Eleven major classes of mammalian PDEs encoded by 21 distinct genes subject to significant alternative splicing have been described. PDE gene families differ in substrate affinity, allosteric regulation, tissue expression, and subcellular compartmentalization (Beavo 1995). Each PDE gene possesses a unique peripheral and central expression pattern at the organ, tissue, and cellular level (Bingham et al. 2006).
PDE2A is a dual-substrate enzyme capable of degrading both cGMP and cAMP. A unique property of PDE2A is its activation upon cGMP binding to N-terminal regulatory GAF domains at physiological concentrations, which leads to increased hydrolysis of cAMP. This provides a mechanism by which PDE2A may mediate cross talk between cGMP and cAMP signaling systems (Mehats et al. 2002). Previous studies have demonstrated high levels of PDE2A expression in multiple tissues, including heart, liver, brain, lung, and kidney (Rosman et al. 1997; Sadhu et al. 1999). In situ hybridization of PDE2A mRNA has been described in the developing rat brain (Van Staveren et al. 2003), in the adult rat spinal cord (de Vente et al. 2006), and in human brain (Reyes-Irisarri et al. 2007). Functional PDE2A activity, most often defined by use of rather non-selective inhibitors, has been described in the anterior pituitary (Velardez et al. 2000), parotid gland (Imai et al. 1999), brain (Rosman et al. 1997; van Staveren et al. 2001), adipocytes (Zacher and Carey 1999), retina (Diederen et al. 2007), and heart (Castro et al. 2006). The physiological functions of PDE2A that correspond to tissue-specific expression are emerging. PDE2A has been reported to regulate aldosterone secretion, consistent with the high expression in the zona glomerulosa of the adrenal gland (Gambaryan et al. 2003; Nikolaev et al. 2005). PDE2A has also been reported to selectively regulate microdomains of cAMP in response to β-adrenergic stimulation (Mongillo et al. 2006) and cGMP-produced atrial natriuretic peptide (ANP) or brain natriuretic peptide in rat cardiac myocytes (Castro et al. 2006). PDE2A mRNA and protein are reported to be highest in brain relative to other tissues (Rosman et al. 1997; Sadhu et al. 1999). We have previously reported that PDE2A protein is found in both soluble and membrane-bound fractions of rat brain tissue (Xie et al. 2006), suggesting that PDE2A may regulate multiple subcellular pools of cyclic nucleotides within neurons. The function of PDE2A in the central nervous system (CNS) has been a topic of investigation by several groups. A significant body of evidence suggests that cAMP and cGMP play important roles in memory consolidation processes (Prickaerts 2004; Blokland et al. 2006; Rutten et al. 2007; Reneerkens et al. 2009).
A greater understanding of the anatomical distribution of PDE2A will provide an important context for further investigation of the physiological function of this enzyme, particularly in brain. Multispecies comparison of the distribution of PDE2A expression across central and peripheral tissues has not been described to date. In the present study, we describe the anatomical localization of PDE2A by immunohistochemistry in the central nervous system and in peripheral tissues from rat, mouse, dog, primate, and human.
Materials and Methods
Tissue Specimens
All animals used in this study were housed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International, and used in accordance with an approved animal care and use protocol, the Institute for Laboratory Animal Research Guide for the Care and Use of Laboratory Animals (National Research Council 1996), and all applicable regulations. Human tissues were received with informed consent from the Pfizer Human Tissue Bank. Multiple tissue specimens were also collected from Sprague Dawley rats (Charles River Laboratories; Kingston, NY), cynomolgus macaques (Macaca fascicularis, Charles River Primates; Houston, TX), CD-1 mice (Mus musculis; Charles Rivers Laboratories), and beagle dogs (Canis familiaris; Marshall Farms, North Rose, NY). Rats and mice were euthanized with carbon dioxide gas followed by exsanguination. Primates and dogs were euthanized by intravenous administration of sodium pentobarbital (Nembutal; Abbott Laboratories, North Chicago, IL) and exsanguinated.
PDE2A Knockout Mice
Heterozygous mice containing one PDE2A allele modified by homologous recombination were obtained from Deltagen (San Carlos, CA). Briefly, long-distance polymerase chain reaction (PCR) was used to generate a 2.8-kb 5′ homology arm and a 3.5-kb 3′ homology arm flanking a 28-bp sequence (ACGCCTTCTCTGTCTCTCATTTTTGCTA) in exon 24 of the mouse PDE2A gene (GenBank accession number XM_133606). The 28-bp sequence was replaced with a Lac-Z/Neor selection cassette, which disrupted an early portion of the catalytic domain. 129P2/OlaHsd (E14) mice were transformed by established techniques (Nagy et al. 2003) and G418-resistant colonies were selected. Expanded clones were screened via long-distance PCR and Southern analysis. One clone with the correctly modified PDE2A allele was used to generate the mouse line via standard blastocyst injection (Nagy et al. 2003). The colony was maintained on a mixed 129:C57BL/6J background by intercrossing heterozygous mice. Fetuses from wild-type C57Bl/6 mice and C57BL/6J mice with targeted deletion of the PDE2A gene were evaluated by immunohistochemistry. PDE2A knockout mice die at 17–18 days of gestation; therefore, sections of PDE2A knockout mouse fetuses and wild-type littermates at 15 days of gestation were stained with and without primary antibody and examined using the methods described below.
Tissue Processing
Non-human tissues were fixed immediately after removal by immersion in 10% neutral buffered formalin (NBF) for a period of 24–48 hr. Human tissues were also fixed by immersion in NBF. Formalin-fixed tissues from all species were processed according to established protocols, embedded in paraffin, sectioned at 5 μm, and mounted onto charged glass slides. Frozen specimens were collected from specific target tissues, which included adrenal (rat and cynomolgus monkey) and forebrain (rat). Frozen tissue was prepared by snap freezing excised tissues in dry ice–cooled isopentane. Table 1 lists the tissues of rat, mouse, dog, cynomolgus monkey, and human that were evaluated in the present study.
Table 1.
Tissues evaluated for PDE2A FabGennix expression
| Tissue | Rat | Human | Cynomolgus monkey | Dog | Mouse |
|---|---|---|---|---|---|
| Adrenal | + | + | + | + | + |
| Aorta | + | + | + | + | + |
| Brain | + | + | + | + | + |
| Brown adipose tissue | + | + | NE | NE | + |
| Cervix | + | + | + | + | + |
| Dorsal root ganglion | + | NE | + | + | + |
| Epididymis | + | NE | + | + | + |
| Esophagus | + | + | NE | + | + |
| Eye | + | + | + | + | + |
| Gallbladder | NA | NE | NE | NE | NE |
| Harderian gland | + | NA | NA | NE | NE |
| Heart | + | + | + | + | + |
| Kidney | + | + | + | + | + |
| Large intestine | + | + | + | + | + |
| Liver | + | + | + | + | + |
| Lung | + | + | + | + | + |
| Lymph node | + | + | + | + | + |
| Mammary gland | + | + | + | + | + |
| Ovary and oviduct | + | + | + | + | + |
| Pancreas | + | + | + | + | + |
| Pituitary | + | NE | + | + | + |
| Prostate | + | + | + | + | + |
| Salivary gland | + | NE | + | + | + |
| Sciatic nerve (peripheral nerve) | + | + | + | + | + |
| Seminal vesicle | + | NE | NE | NA | NE |
| Skeletal muscle | + | + | + | + | + |
| Skin | + | + | + | + | + |
| Small intestine | + | + | + | + | + |
| Spinal cord | + | + | + | + | + |
| Spleen | + | + | + | + | + |
| Stomach | + | + | + | + | + |
| Testis | + | + | + | + | + |
| Thymus | + | + | + | + | + |
| Thyroid | + | + | + | + | + |
| Tongue | + | NE | + | + | + |
| Tonsil | NE | + | NE | NE | NE |
| Trachea | + | NE | + | + | + |
| Urinary bladder | + | + | NE | + | + |
| Uterus | + | + | + | + | + |
PDE, phosphodiesterase; +, tissue evaluated; NE, tissue not evaluated; NA, not applicable.
Western Blotting
Western blot analysis was performed on extracts of striatum and peripheral tissues from multiple species as described by Xie et al. (2006). Briefly, striatal tissue was homogenized using a Polytron homogenizer in Roche IP kit lysis buffer containing complete protease inhibitors (Roche Applied Science; Penzberg, Germany) at a concentration of 100 mg tissue per ml. Protein concentration was determined with Pierce BCA protein assay kit 23,225 [Pierce (Thermo Fisher Scientific); Rockford, IL]. Western blots were run using the Invitrogen NuPage system (Invitrogen Life Technologies; Carlsbad, CA) (4–12%–gradient gels) with ∼10 μg total protein per lane. Blots were probed with PDE2A antibody (FabGennix PD2A-101AP, diluted 1:1000) (FabGennix, Inc., International; Frisco, TX) and actin antibody (as loading control) (Chemicon MAP1501, diluted 1:2000) (Chemicon-Millipore; Billerica, MA) and were visualized with anti-rabbit Alexa 680 (Molecular Probes, 1:2000) (Molecular Probes, Invitrogen Life Technologies; Carlsbad, CA) and anti-mouse IR800 (Rockland, 1:5000) (Rockland Immunochemicals, Inc.; Gilbertsville, PA) secondary antibodies, respectively, using the Li-Cor Odyssey system (LI-COR Biosciences; Lincoln, NE). The positive control was a lysate of SF-21 insect cells infected with a baculovirus construct of human full-length PDE2A with N-terminal His tag using the Bac-to-Bac baculovirus expression system [Life Technologies Corporation (formerly Invitrogen); Carlsbad, CA].
Immunoadsorption of the PDE2A antibody was carried out by coincubating the FabGennix PDE2A antibody at a 1:1000 dilution with 300-fold excess immunogenic peptide for 20 min with mixing at room temperature. Parallel immunoblots prepared with 20 μg total protein from human brain samples were probed with PDE2A antibody alone or with the immunadsorbed mixture. Membranes were incubated at 4C overnight, and developed as described above.
Immunohistochemistry
The PDE2A antibody (polyclonal, PD2A-202AP; FabGennix, Inc.) is directed against 18 amino acids from the C-terminal sequence of PDE2A (AA909-926 Ensemble peptide ID ENSP00000334910), which is conserved across species and is not present in other PDE family members.
All immunohistochemical procedures were performed on formalin-fixed paraffin-embedded tissue using an automated immunostainer (Dako; Carpenteria, CA) to ensure consistency across slides. Immunohistochemstry was performed using standard avidin biotin immunoperoxidase methods as previously described (Coskran et al. 2006). To expose antigenic sites, tissue sections were heated in Citra Antigen Retrieval, pH 6 (Biogenex; San Ramon, CA), using the Decloaking Chamber (Biocare Medical; Concord, CA). Immunostaining was performed by incubating tissues with anti-PDE2A antibody at a concentration range of 1.4 to 7.0 μg/ml for 60 min at room temperature. Rabbit IgG (Vector Laboratories; Burlingame, CA) was used as the negative control at the same concentration as anti-PDE2A antibody. The detection method consisted of incubating tissues with a biotinylated secondary goat anti-rabbit antibody (Vector Laboratories) followed by incubation with Elite ABC (Vector Laboratories). Dako Liquid DAB+ (Vector Laboratories) was used to visualize PDE2A staining. Stained slides were counterstained with Mayer's hematoxylin.
Positive and negative controls were run with each immunohistochemical experiment. To determine the optimal PDE2A staining conditions, an initial titration series was performed on rat brain and colon aimed at defining the optimal signal-to-noise ratio. Immunospecificity was determined by preadsorbing the anti-PDE2A antibody with 50 times the concentration of the PDE2A antigenic blocking peptide (P-PD2A100) obtained from FabGennix.
In Situ Hybridization
Chromogenic in situ hybridization was carried out on frozen sections using the Discovery XT automated slide processing system (Ventana Medical Systems, Inc.; Tucson, AZ). Specific target tissues included adrenal and brain. A 310-bp fragment was isolated from a rat brain cDNA library from Clontech. This sequence was BLASTED against the National Center for Biotechnology Information database and yielded 100% homology with the rat sequence (NM_031079) and 97% with the mouse sequence (BC086800.1). This fragment is located between bp 1978–2287 of sequence NM_031079 and is at the N terminus of the catalytic domain. The coding sequence for this gene is at 38-2824. This fragment was isolated with forward and reverse primers containing T7 and T3 polymerases. The sense and anti-sense probes were both labeled with digoxigenin (DIG) nucleotides using the Roche DIG Labeling Kit (Roche; Indianapolis, IN). Additionally, the probes were column purified to remove any unincorporated nucleotides. Cryosections were cut on a cryostat and loaded onto the Discovery XT employing a BlueMAP (Ventana Medical Systems, Inc.) detection kit. Briefly, the slides were pretreated with RiboMAP (Ventana Medical Systems, Inc.) and then permeabilized with protease 3 before the probe was applied. The DIG-labeled PDE2A probe was detected with a biotinylated mouse anti-DIG secondary antibody (Sigma; St. Louis, MO) and visualized by the BlueMAP components (streptavidin-alkaline phosphatase and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium chloride). All slides were counterstained with Nuclear Fast Red.
Evaluation of Immunohistochemistry Slides
PDE2A staining intensity was scored using a semi-quantitative scoring scale as follows: −, no staining; 1+, mild staining clearly differentiated from surrounding tissues; 2+, moderate staining easily visible without obscuring tissue morphology; 3+, marked staining with a dark-brown product. The CNS distribution of PDE2A was evaluated in rat brain in immunostained sections from both coronal and sagittal planes. Neuroanatomical landmarks were defined according to the rat brain atlas of Paxinos and Watson (2007).
Results
Western Blot Analysis
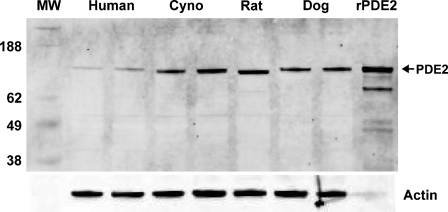
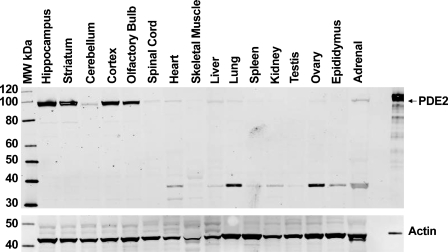
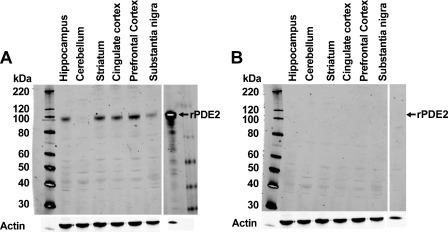
The polyclonal anti-PDE2A antibody directed to the C terminus of PDE2A protein recognized a single ∼100-kDa molecular mass band in Western blots of tissue extracts prepared from the striatum of multiple species (Figure 1). All species tested demonstrated the same band that comigrated at the same molecular mass. The density of the PDE2A 100-kDa band appeared to be less abundant in human striatum as compared with other species. Other tissues in which a similarly sized band was detected included cerebral cortex, hippocampus, adrenal and spinal cord from rat, human, and cynomolgus macaque (not shown). To further confirm antibody specificity, Western blot analysis was carried out on tissue samples following preabsorption of the antibody with the immunogen. Figure 2 demonstrates that all bands recognized by the PDE2A polyclonal antibody in both PDE2A-recombinant cells as well as human tissue extracts were completely abolished following immunoadsorption. Figure 3 illustrates Western blot analysis of PDE2A in tissues derived from rat. At the same tissue concentration and exposure, very little PDE2A was detected in the peripheral tissues, consistent with mRNA distribution. which is most abundantly expressed in brain (Rosman et al. 1997). Prolonged exposure of the blot revealed the presence of an ∼100-kDa band in peripheral tissues that comigrated with the band detected in brain. A second band of ∼40 kDa was observed in the peripheral tissues of rat and was not present following competition with immunogenic peptide. This second band may represent a rat protein that shares some homology with the peptide or a specific proteolytic cleavage product of PDE2A. This band was not observed in multi-tissue blots of dog or human tissues (data not shown) and the molecular mass is much lower than calculated for known splice variants.
Figure 1.
Western blot analysis of human, cynomolgus monkey, rat, and dog brain extracts probed with anti-PDE2A antibody. Left lane is molecular mass standards, whereas the right lane represents the positive control. Each additional lane represents tissue from two individual subjects loaded in adjacent lanes, with the exception of rat, which represented a single animal in one lane. Additional rat samples are illustrated in Figure 2. Recombinant PDE2A with His tag expressed in sf21 cells (rPDE2A) was loaded as a positive control. A primary band of ∼100 kDa is detected in 10 μg of striatal extract from human, with a stronger signal in equally loaded extracts from cynomolgus monkey (Cyno), rat, and dog brain. Equivalent loading was demonstrated by probing the same blot with an anti-actin antibody.
Figure 2.
Western blot analysis of rat central nervous system (CNS) and peripheral tissue extracts probed with anti-PDE2A antibody. Twenty micrograms of total protein per lane was loaded from the various highlighted tissue specimens. Left lane is molecular mass standards, and right lane is PDE2A-positive control. pAb PD2A-101AP recognizes a primary band of ∼100 kDa in CNS tissues that is far less detectable in peripheral tissue extracts. Recombinant PDE2A with His tag expressed in sf21 cells was loaded as a positive control. Equivalent loading was demonstrated by probing the same blot with an anti-actin antibody. Positive control was loaded with less protein to avoid overloading the lane.
Figure 3.
Western blot analysis of human brain extracts probed with anti-PDE2A antibody with (B) and without (A) antigenic peptide competition. Molecular mass standards are shown in left and right lanes and recombinant PDE2A-positive control is shown in the near far-right lane. pAb PD2A-101AP recognizes a primary band of ∼100 kDa in human brain tissues (A, 20 μg sample loaded per lane). The 100-kDa band is prominently observed in human striatum, cingulate cortex, prefrontal cortex, and hippocampus, with less abundance in the substantia nigra and virtually no detectable signal in cerebellum. (B) Identical blot probed with PDE2A-101AP in the presence of the antigenic peptide at a 300× molar excess. Equivalent loading was demonstrated by probing the same blot with an anti-actin antibody, shown on bottom panel.
Immunohistochemistry
Table 1 lists the tissues from rat, mouse, dog, primate, and human that were evaluated by immunohistochemistry. Tables 2 and 3 provide a summary of the positive results for PDE2A immunoreactivity (PDE2A ir) in the tissues examined. PDE2A ir was heterogeneously expressed in multiple regions throughout the CNS (Table 2) and periphery (Table 3). Some areas of the CNS expressed relatively high levels of PDE2A ir whereas a more-restricted subset of peripheral tissues was immunopositive.
Table 2.
Neuroanatomical distribution of PDE2A immunoreactivity in rat brain
| Region | Fibers/Terminals | Cell bodies | Comments |
|---|---|---|---|
| Forebrain | |||
| Olfactory bulb | 2+ | ||
| Isocortex | 2+ | 1+ | Subsets, laminar distribution |
| Caudate | 2+ | MSNs + neuropil | |
| Putamen | 2+ | MSNs + neuropil | |
| Globus pallidus | 3+ | ||
| Olfactory tubercle | 2+ | ||
| Nucleus accumbens | 3+ | ||
| Lateral septum | 2+ | ||
| Bed nucleus of stria terminalis | 3+ | ||
| Hypothalamus | 2+ | Some heterogeneity among subnuclei | |
| Habenula, medial | 3+ | Far less staining in the lateral habenula | |
| Septum, lateral | 1+ | Medial negative | |
| Hippocampus | 3+ | Laminar heterogeneity | |
| Induseum gresium | 3+ | 2+ | |
| Thalamus | 2+ | ||
| AD, VP, Rt, MGB, LGN, CM | 1+ | Not all thalamic nuclei are ir | |
| Midbrain | |||
| Superior colliculus | 2+ | ||
| Mammilary nuclei | 3+ | ||
| Raphe nuclei | 1+ | 2+ | |
| Substantia nigra | 3+ | 1+ | Pars compacta subset |
| Ventral tegmental area | 1+ | 2+ | |
| Periaqueductal gray | 2+ | ||
| Pedunculopontine tegmental nucleus | 1+ | 1+ | |
| Interpeduncular nuclei | 3+ | ||
| Pons | 2+ | ||
| Zona incerta/subincerta | 1+ | ||
| Hindbrain | |||
| Cerebellum | 1+ | Subset of cells in granule cell layer | |
| Cochlear nuclei | 2+ | ||
| Locus coeruleus | Neg | ||
| Vestibular nucleus | 2+ | ||
| Parabrachial nucleus | 2+ | ||
| Lateral dorsal tegmental nucleus | 2+ | 2+ | |
| Nucleus of the solitary tract | 2+ | ||
| Reticular formation | 1+ | Scattered neurons throughout | |
| Cranial motor nuclei | Neg | ||
| White matter | |||
| Fasiculus retroflexus | 2+ | ||
| Fasiculus medialis telencephali | 2+ | ||
| Corpus callosum | 1+ | Subset of fibers | |
| Alveus | 1+ | Subset of fibers | |
| Fornix | Neg | ||
| Inferior cerebellar peduncle | 1+ | ||
1+, Mild staining of tissue components; 2+, moderate staining of tissue components; 3+, marked staining of tissue components; MSN: medium spiny neuron. Key: SON, PVN, VP, AD, Rt, MGB, LGN, CM refer to Paxinos and Watson (2007).
Table 3.
Peripheral tissues and cell types other than endothelium exhibiting positive labeling for PDE2A
| Tissuea | Component | Rat | Mouse | Dog | Human | Cynomolgus monkey | Staining pattern |
|---|---|---|---|---|---|---|---|
| Kidney | Tubular epithelium | 1+ | (−) | 1+ | 1+ | (−) | Rat, dog, human; cytoplasm of tubular epithelia cells |
| Lung | Individual cells | 3+ | 1+ | (−) | (−) | 1+ | Rat; round cells on surface of alveoli; cynomolgus monkey and mouse; bronchial epithelium |
| Ovary | Epithelium | 1+ to 2+ | 2+ | 1+ | (−) | 1+ to 3+ | Rat, mouse, dog, cynomolgus monkey; corpora lutea and follicular epithelium |
| Pituitary | 2+ | 2+ | 2+ | NE | (−) | Rat, mouse, dog; predominantly neuropil staining of pars nervosa with rare cells in pars distalis | |
| Adrenal | 3+ | 3+ | 3+ | 2+ to 3+ | 3+ | Strong cytoplasmic staining in all species | |
| Epididymis | Smooth muscle; interstitium | 3+ | 1+ | (−) | NE | (−) | Rat; interstitial macrophages; mouse; light staining of smooth muscle cells |
| Lymph node | Lymphocytes/macrophages | 2+ | 2+ to 3+ | 1+ | (−) | 1+ to 3+ | Rat, mouse, dog; lymphocyte subsets; cynomolgus monkey; macrophages |
| Dorsal root ganglion | 1+ to 3+ | 1+ to 2+ | 1+ to 2+ | NE | 1+ | Cytoplasmic and nuclear staining of subsets of neurons | |
| Urinary bladder | Urothelium | 1+ | 1+ to 2+ | (−) | (−) | NE | Rat, mouse; cytoplasm of epithelial cells |
| Smooth muscle | 1+ | 1+ to 2+ | (−) | (−) | NE | Rat and mouse; smooth muscle of bladder wall | |
| Tongue | Epithelium | 1+ | (−) | (−) | NE | (−) | Rat; stratum spinosum |
| Uterus | Epithelium | (−) | (−) | (−) | 1+ to 2+ | (−) | Human; cytoplasmic staining of endometrial epithelium |
| Testis | Seminiferous tubules | 1+ | 1+ | (−) | 2+ | 1+ to 2+ | Rat, mouse, human, and cynomolgus monkey; subsets of spermatogenic and Sertoli cells |
| Interstitium | (−) | (−) | (−) | 1+ to 2+ | 1+ | Human, cynomolgus monkey; occasional cytoplasmic staining of interstitial cells | |
| Prostate | Epithelium | 1+ | (−) | (−) | 1+ to 2+ | 1+ | Rat, human; prostatic epithelium; cynomolgus monkey; ganglionic neurons, not epithelium. |
| Stomach | Epithelium | 1+ to 3+ | 1+ to 2+ | 1+ | 2+ to 3+ | 2+ | Cytoplasmic staining of epthelial cells in the gastric pits of all species |
| Ganglia | 2+ to 3+ | 2+ | (−) | NE | 2+ | Rat, mouse, cynomolgus monkey; neurons of myenteric plexi (ganglia) | |
| Small intestine | Epithelium | 1+ to 2+ | 1+ | 1+ to 2+ | 1+ to 2+ | (−) | Cytoplasmic staining of mucosal epithelium in all species except cynomolgus monkey |
| Ganglia | 2+ to 3+ | 2+ to 3+ | 1+ | NE | 1+ to 2+ | Rat, mouse, dog, cynomolgus monkey; neurons of myenteric plexi (ganglia) | |
| Cecum | 2+ to 3+ | 1+ to 2+ | 1+ | NE | 1+ to 2+ | Rat, dog, and cynomolgus monkey; neurons of myenteric plexi (ganglia); mouse; basal epithelium | |
| Colon | Epithelium | 3+ | 1+ to 2+ | 1+ to 2+ | 1+ | (−) | Cytoplasmic staining of mucosal epithelium in all species except cynomolgus monkey |
| Ganglia | 2+ to 3+ | 2+ to 3+ | 1+ | 1+ to 2+ | 1+ to 2+ | All species; neurons of myenteric plexi (ganglia) | |
| Pancreas | Islets | 1+ | (−) | 1+ | 2+ to 3+ | (−) | Rat, dog, human; cytoplasmic staining of islet cells |
| Ganglia | 1+ | 1+ to 2+ | 1+ to 2+ | NE | NE | Rat, mouse, dog; neuronal cytoplasm and nuclei | |
| Salivary gland | Ganglia | (−) | (−) | (−) | NE | 2+ | Cynomolgus monkey; cytoplasm of neurons |
| Trachea | Epithelium | 1+ to 3+ | (−) | 2+ | NE | 2+ | Rat, dog, and cynomolgus monkey; epithelium |
| Spleen | Lymphocytes | 1+ to 2+ | 1+ to 2+ | 1+ | (−) | (−) | Rat, mouse, and dog; lymphocytes of red and white pulp |
| Thymus | Individual cells | (−) | 2+ | (−) | (−) | (−) | Mouse; rare round to polygonal cells in cortex and medulla |
| Thyroid | Epithelium | (−) | (−) | (−) | 1+ to 2+ | (−) | Human; cytoplasmic staining of follicular epithelium |
| Mammary gland | Epithelium | (−) | (−) | (−) | 2+ | (−) | Human; cytoplasmic staining of ductal epithelium |
| Eye | Ciliary epithelium | 1+ | 2+ to 3+ | 1+ | NE | (−) | Rat, mouse, dog; cytoplasm of epithelial cells and some smooth muscle cells |
| Retina | 1+ to 2+ | 2+ to 3+ | 1+ | 1+ to 3+ | Rat, mouse; cytoplasmic staining of rare cells in the inner nuclear cell layer; cynomolgus monkey and dog; nuclei of ganglion cell layer | ||
| Skin | Epithelium | (−) | (−) | 1+ | 2+ | 3+ | Dog, cynomolgus monkey; apocrine gland epithelium; human; basal layer of epidermis |
| GALT or BALT | 1+ | 1+ to 3+ | (−) | (−) | 1+ | Rat GALT; mouse GALT and BALT; cynomolgus monkey GALT |
There is PDE2A staining of endothelium in most tissues from all species, seen consistently in veins and capillaries and in subsets of arteries.
NE, tissue not evaluated; −, negative; 1+, mild staining of tissue components; 2+, moderate staining of tissue components; 3+, marked staining of tissue components; GALT, gut-associated lymphatic tissue; BALT, bronchial-associated lymphatic tissue.
PDE2A Protein Is Heterogeneously Expressed in Multiple Distinct Brain Regions
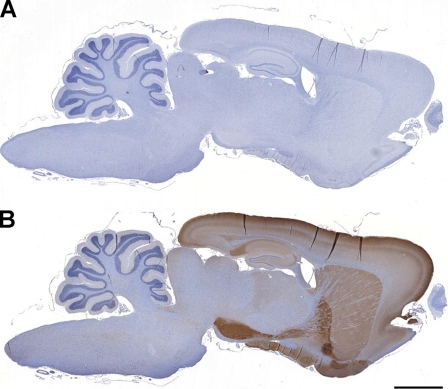
Figure 4B illustrates PDE2A ir in a sagittal section of rat brain. At low magnification, PDE2A ir is observed at high density in forebrain, with very little signal in brainstem and cerebellum. No immunoreactivity was observed with an isotype control antibody in combination with the appropriate secondary antibody (Figure 4A). Highest expression levels were observed in the forebrain, as compared with midbrain and hindbrain, with abundant expression detected in basal ganglia, substantia nigra, isocortex, hippocampus, amygdala, interpeduncular nucleus, and medial habenula. Little to no PDE2A ir was detected in the midbrain, hindbrain, and cerebellum at low magnification. In the isocortex, a laminar distribution of PDE2A ir was observed. Specific subnuclei of the thalamus, such as the lateral dorsal geniculate and medial geniculate nuclei, were immunopositive, whereas the midbrain expressed very little staining. The major brain structures positive for PDE2A ir corresponded to regions comprising the limbic system.
Figure 4.
PDE2A immunoreactivity (ir) is abundantly expressed in mammalian forebrain. (A) Sagittal section of rat brain stained with isotype control showing hematoxylin counterstain and a lack of PDE2A immunoperoxidase staining. (B) Section of rat brain near-adjacent to that shown in A demonstrates strong PDE2A ir prominently in forebrain structures including the cortex, striatum, hippocampus, and substantia nigra. Bar = 2.5 mm.
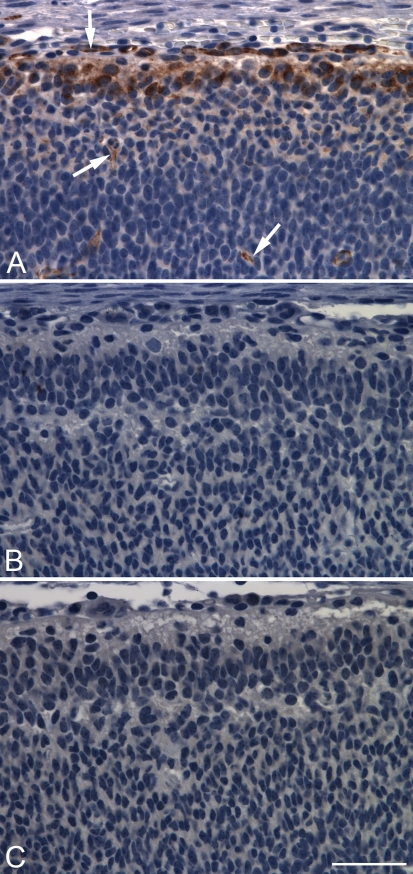
A key experiment to validate antibody specificity was conducted by performing PDE2A immunohistochemistry on sections of fetuses from PDE2A knockout mice and wild-type littermates. PDE2A knockout mouse fetuses were used because PDE2A knockout mice die in utero at 17–18 days of gestation. In embryonic mouse cortex from wild-type fetuses, PDE2A ir consisted of discrete staining of the superficial developing neuroepithelium (Figure 5). In this region, staining was observed both within neurons and in the neuropil; endothelial cells scattered throughout all layers were also stained. Sections from PDE2A knockout mice stained with the antibody and wild-type embryos stained without antibody showed no detectable immunoperoxidase reaction product. The absence of PDE2A ir in PDE2A knockout mice confirms that the polyclonal PDE2A antibody employed in this study is specific for PDE2A protein.
Figure 5.
PDE2A staining is not detected in PDE2A knockout embryos. Developing mouse cortex from wild-type (A,B) and PDE2A knockout (C) embryonic day–15 mouse fetus stained with anti-PDE2A antibody (A,C) or negative control antibody (B). In wild-type embryos, PDE2A staining is present in cerebrovascular endothelium (arrows) and in neurons and neuropil of the superficial layers of the developing neuroepithelium. No detectable immunoperoxidase reaction product is observed in corresponding regions from PDE2A knockout fetuses (C) or in sections incubated with negative-control antibody (B). Bar = 40 μm.
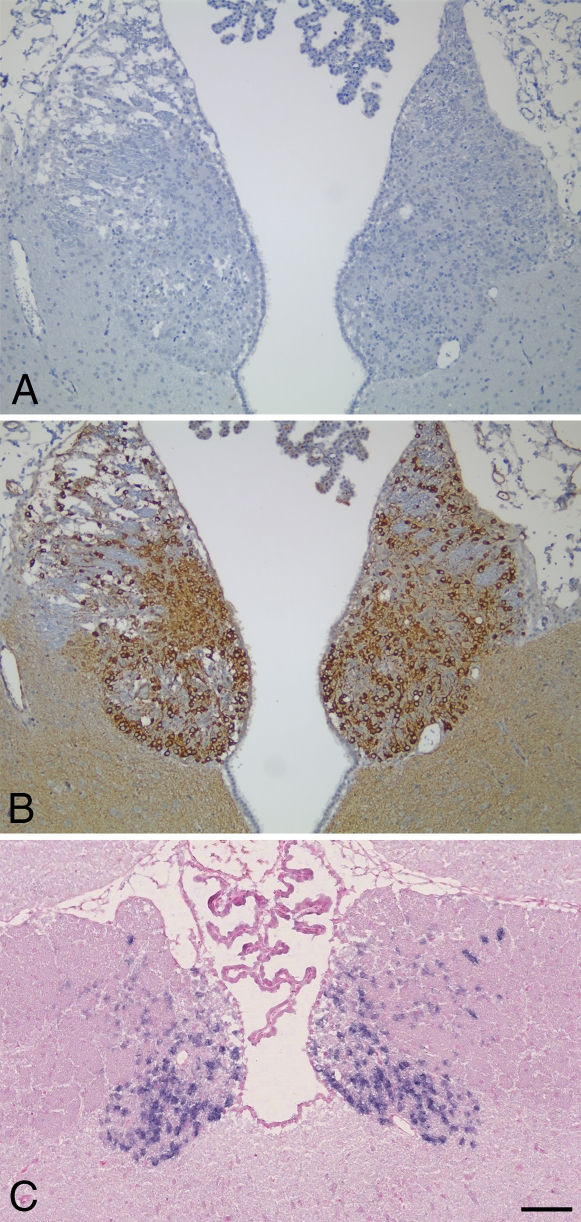
The distribution of PDE2A ir in most immunopositive regions of the CNS consisted of a fine punctate granular pattern that was present throughout the neuropil. Relatively few brain regions exhibited robust staining of neuronal cell bodies. One such region was the medial habenula (Figure 6), where all cell bodies exhibited strong intracellular immunoreactivity that consisted of cytoplasmic intraneuronal staining (Figure 6B). Immunoadsorption with the immunogenic peptide abolished staining observed in the habenula (Figure 6A), as well as in other immunopositive brain regions and peripheral tissues (not shown). In situ hybridization of the forebrain demonstrated that neurons of the medial habenula specifically expressed PDE2A mRNA (Figure 6C), whereas no signal was detected with sense probe (not shown). Other regions that exhibited PDE2A ir within cell bodies included a subset of cortical neurons, a subset of substantia nigra pars compacta (SNPc) neurons, neurons of the raphe nuclei and the lateral dorsal tegmental nucleus, as well as scattered neurons throughout the brainstem reticular formation. In the cerebrovasculature, diffuse PDE2A staining was consistently present in endothelial cells of capillaries and veins. Endothelial staining for PDE2A was inconsistent in arteries.
Figure 6.
PDE2A is highly expressed in rat habenula. (A) Coronal section of rat forebrain incubated with anti-PDE2A antibody immunoadsorbed with PDE2A peptide immunogen. (B) Near-adjacent coronal section demonstrating PDE2A immunoperoxidase staining in the rat habenula. (C) Chromogenic in situ hybridization of PDE2A in rat habenula. Blue reaction product is abundant in neurons of the medial habenula. Sections are counterstained with hemotoxylin (A,B) or Nuclear Fast Red (C). Bar = 100 μm.
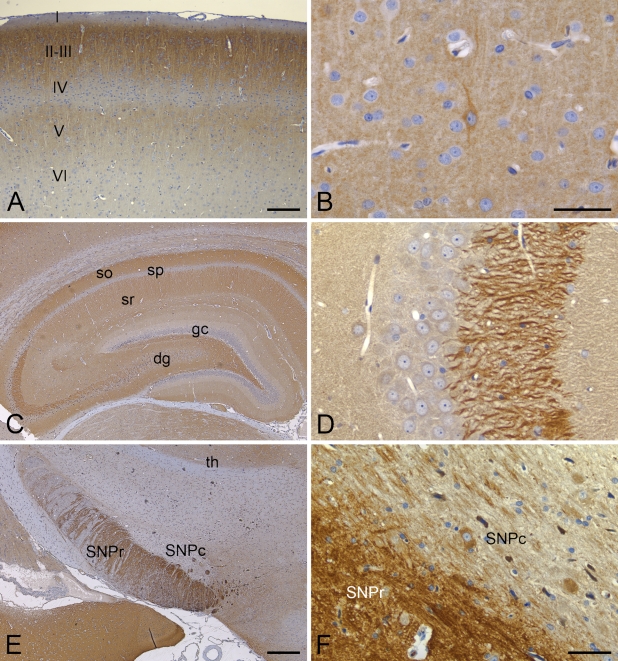
The distribution of PDE2A ir in brain is heterogeneous. Therefore, more-detailed neuroanatomic mapping of PDE2A ir was performed in the rat brain; selected mouse, dog, primate, and human brain regions were examined for interspecies comparisons. PDE2A ir in rat brain was abundant within components of the nigrostriatal pathway, including striatum, globus pallidus, ventral pallidum, medial forebrain bundle, and substantia nigra. In the striatum, PDE2A ir was expressed in the neuropil, whereas strong fiber staining was observed in globus pallidus and substantia nigra pars reticulata (Figures 7E and 7F). Nigrostriatal fiber bundles were intensely immunoreactive. A subset of neurons in the SNPc, particularly those located in the caudal aspects of the SNPc, demonstrated intraneuronal PDE2A ir.
Figure 7.
PDE2A ir is abundantly expressed in rat brain, with discrete staining patterns in many regions. (A) PDE2A ir in the somatosensory cortex shows a distinctive laminar staining distribution (laminae I–VI are indicated). (B) In layer III of the isocortex, fine neuropil staining of terminals and varicosities is present, and isolated immunoreactive non-pyramidal neurons show PDE2A ir in the neuronal cytoplasm. (C) Dorsal hippocampus demonstrates neuropil staining throughout multiple layers, including stratum oriens (so), stratum radiatum (sr), and dentate gyrus (dg). Neuron cell bodies within the CA pyramidal neuron layers (sp) as well as dentate gyrus granule neurons (gc) lack PDE2A ir. (D) High magnification of PDE2A ir in CA3 hippocampal mossy fibers. (E) Low magnification image of midbrain showing intense PDE2A ir fibers with the substantia nigra pars reticulata (SNPr) and neuropil staining in the amygdala and thalamus (th). (F) A subset of substantia nigra pars compacta neurons (SNPc) express intraneuronal PDE2A ir, and strong fiber staining is present in the SNPr. Bars: A,C = 250 μm; B = 40 μm; D,F = 50 μm; E = 500 μm.
In the hippocampus, PDE2A ir was heterogeneously distributed throughout multiple sublayers (Figure 7C). Strong neuropil staining was present in the subiculum, stratum oriens, stratum radiatum, and the dentate gyrus molecular layer and hilus, with little to no signal in the pyramidal cell body layer or in dentate gyrus granule cell bodies. Strong immunoreactivity of PDE2A was also present in the CA3 mossy fibers and in the subiculum (Figure 7D), a major source of input to the hippocampus from the entorhinal cortex. The neuropil of the subiculum exhibited intense PDE2A ir that exceeded the level of staining in other hippocampal regions.
In the isocortex, fine punctate granules of PDE2A ir were observed in the neuropil in a distinctive laminar distribution pattern (Figure 7A). Discrete PDE2A ir sublaminae included the superficial cortical laminae I–II and lamina V, whereas less-abundant staining was present in layer IV and in deeper cortical laminae (layer VI). Such laminar staining pattern was observed throughout the isocortex in both coronal and sagittal orientations. At high magnification, dense cortical PDE2A ir was observed as fine granular staining throughout the synaptic neuropil. Layers with strong neuropil staining did not show detectable PDE2A ir within intraneuronal apical dendrites or cell bodies. Scattered neurons of the isocortex demonstrated intracytoplasmic PDE2A ir. These neurons most often had a bipolar or non-pyramidal morphology and were frequently located in laminae V–VI (Figure 7B).
In mouse, dog, primate, and human cortex, staining was similar to that observed in rat cortex. Human and primate cortical PDE2A ir consisted of distinctive laminar neuropil staining and scattered PDE2A ir neurons, although PDE2A-positive neurons appeared less abundant than observed in rodents. In other brain regions, the pattern of PDE2A ir was similar across species. Such regions included habenula, hippocampus, striatum, amygdala, and substantia nigra.
PDE2A in Spinal Cord and Dorsal Root Ganglia (DRG)
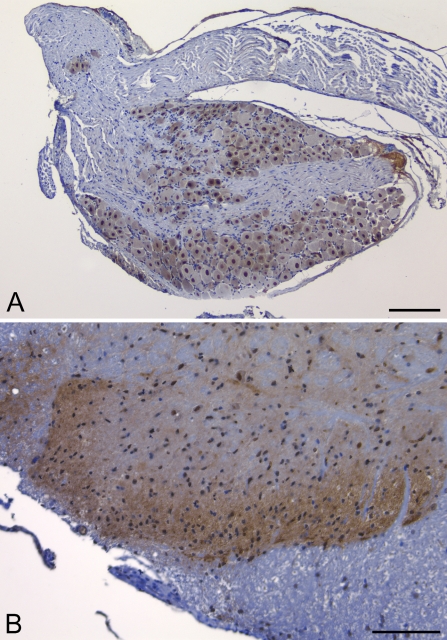
PDE2A ir was observed in the superficial dorsal horn of rat, cynomolgus monkey, dog, and mouse spinal cord. Laminae I–III showed a diffuse punctate pattern of immunoreactivity, whereas no such pattern was observed in the other laminae (Figure 8B). Scattered neurons in the intermediate and ventral horn exhibited intraneuronal PDE2A ir that oftentimes exhibited a nuclear staining pattern. In the rat, mouse, dog, and primate DRG, PDE2A ir was present in nuclei of neurons and absent from dorsal root axons and satellite glia.
Figure 8.
PDE2A ir is observed in rat dorsal root ganglion (A) and dorsal horn of the spinal cord (B). Dorsal root ganglion shows PDE2A ir associated with nearly all ganglion cells in rat. No immunoreactivity is observed in dorsal root and non-ganglionic tissue. (B) Dorsal horn of rat spinal cord exhibiting a diffuse punctate staining pattern throughout the superficial spinal cord laminae. Bars: A = 150 μm; B = 100 μm.
PDE2A Expression in Peripheral Tissues
In the majority of the peripheral tissues, PDE2A ir was detected only in endothelial cells, and not in other cell types. The finding of reduced peripheral PDE2A ir as compared with brain is consistent with the differential expression observed in rats by Western blot analysis.
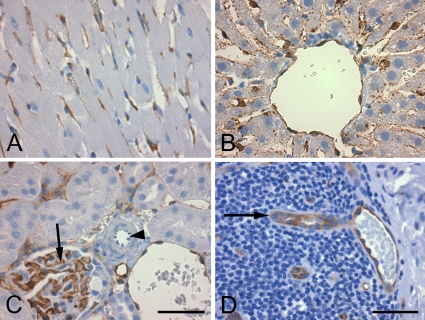
PDE2A ir was present in endothelial cells across multiple tissues from all species (Figure 9). Diffuse cytoplasmic endothelial staining was consistently present in capillaries and veins. As was observed in the brain, cytoplasmic endothelial staining was detected in a subset of muscular arteries and arterioles. For example, in rat kidney, endothelial staining was consistently seen in veins and capillaries, and was present in some but not all arterioles (Figure 9). Figure 9D illustrates staining of high endothelial venules in human gut associated lymphatic tissue. In the heart, PDE2A ir was observed in cardiac endothelium, with no staining present in cardiac myocytes. In some human tissues, weak PDE2A ir was also observed in the tunica media (smooth muscle) of small arteries.
Figure 9.
PDE2A ir is present in endothelium of many tissue types. (A) Rat heart shows lack of cardiac myocyte staining but labeling of endothelial cells. (B) Rat liver shows the presence of PDE2A ir in central vein and sinusoidal endothelium but not in hepatocytes. (C) Rat kidney section shows endothelial staining in glomerulus (arrow) and in neighboring veins. Note the lack of endothelial staining in a small arteriole indicated by the arrowhead. (D) Specialty types of endothelium, such as high endothelial venules, were also shown to express PDE2A ir, as seen in section of human gut-associated lymphoid tissue (arrow). Bars: A–C = 50 μm; D =100 μm.
The strongest immunoreactivity for PDE2A outside the CNS was detected in the adrenal zona glomerulosa and enteric ganglia. In situ hybridization in the rat and cynomolgus monkey adrenal gland showed strong expression of PDE2A mRNA in the zona glomerulosa and not in other cortical areas or in the medulla (not shown). Along the digestive tract from the stomach to the colon, individual neurons within intestinal ganglia exhibited cytoplasmic PDE2A ir. Ganglia observed randomly in other visceral organs also demonstrated neuronal cytoplasmic PDE2A ir. In the gastrointestinal tract, PDE2A ir also was present in mucosal epithelium. In human tissues, moderate PDE2A ir was observed in pancreatic islets. Several types of epithelium from human tissues exhibited mild to moderate PDE2A ir, including uterus, mammary gland, prostate, skin, and thyroid. In the pituitary, PDE2A staining was seen in the pars nervosa and intermediate lobe, whereas subsets of cells were stained in the pars distalis of rat, mouse, and dog. PDE2A ir was observed in lymphocytes and macrophage subsets in lymphoid organs of all species, with the exception of humans.
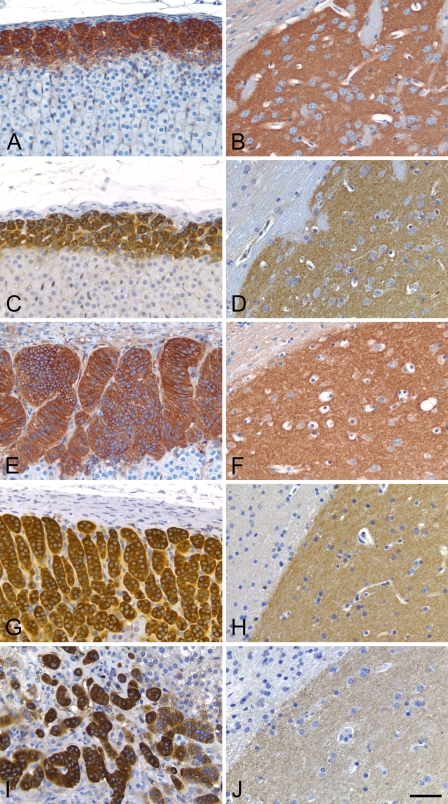
The general patterns of PDE2A immunoreactivity in all tissues were similar among all species. Figure 10 illustrates the consistent distribution of PDE2A ir in the adrenal gland and brain of rat, mouse, dog, cynomolgus monkey, and human. Consistent with what was observed by Western blot (Figure 1), the intensity of PDE2A ir in human striatum was somewhat less than that observed in the striatum of other species. Given that postmortem interval and experimental conditions in humans are different from those in animals, it remains to be determined whether such observed differences reflect true species difference in PDE2A expression levels or whether such observed differences result from some confounding variables inherent in working with human tissue. PDE2A ir was strongly expressed in the adrenal zona glomerulosa and in the striatal neuropil with a cellular distribution pattern that was indistinguishable across the five species, suggesting that the distribution of PDE2A is conserved across mammalian species.
Figure 10.
The distribution of PDE2A ir is conserved across mammalian species. Adrenal cortex from rat (A), mouse (C), dog (E), cynomolgus monkey (G), and human (I) demonstrates strong immunoreaction product within the zona glomerulosa. Brain PDE2A ir in dorsolateral striatum adjacent to the corpus callosum from rat (B), mouse (D), dog (F), cynomolgus monkey (H), and human (J). PDE2A ir is similar in rat, mouse, dog, cynomolgus monkey, and human tissue. Bar = 50 μm.
Discussion
In the present study, we demonstrate that PDE2A is expressed at high levels in brain as compared with peripheral tissues, that PDE2A is heterogeneously distributed in brain by immunohistochemistry, and that PDE2A ir is most consistently observed in endothelial cells across multiple tissues. In the CNS, PDE2A is highly expressed in limbic system structures. Importantly, we report that the distribution of PDE2A ir is similar across mouse, rat, dog, non-human primate, and human species.
The present study utilized a specific polyclonal antibody directed to the C-terminal sequence of PDE2A to evaluate the distribution of the PDE2A protein across multiple tissues from rat, mouse, dog, human, and non-human primates. The 18-aa epitope is expected to be present in all of the three described splice variants of the gene, namely PDE2A1, PDE2A2, and PDE2A3. Evidence of antibody specificity included the recognition of purified PDE2A by Western blot and detection of a band of the appropriate molecular mass in striatal extracts from human, dog, non-human primate, and rat. This cross-species immunoreactivity is consistent with the identical homology within the antigenic peptide used to generate the PDE2A antibody observed across rat, non-human primate, and human sequences. A key validation of the specificity of the PDE2A polyclonal antibody was the absence of immunoreactivity in fetal cerebral cortex from PDE2A knockout mice. Furthermore, immunoadsorption of primary antibody with purified synthetic immunogen eliminated the PDE2A-specific bands in Western blots of rat brain and the histochemical immunoreactivity in tissue sections of rat brain and adrenal gland. A staining pattern identical to that reported in the present study has been described in the adrenal gland by immunohistochemistry using an independent antibody directed to PDE2A1, -A2, and -A3 isoforms (MacFarland et al. 1991).
Previous reports have demonstrated that PDE2A mRNA is expressed in most tissues, with the highest expression present in brain and heart (Rosman et al. 1997; Iffland et al. 2005). Other tissues expressing PDE2A mRNA include lung, kidney, and liver (Rosman et al. 1997), retina (Diederen et al. 2007), adrenal, spleen, thymus, and thyroid (Bingham et al. 2006). The current study describes similar distributions of PDE2A protein expression as assessed by immunohistochemistry profiling with respect to both relative distribution and abundance. By Western blot, the brain appeared to express the highest level of PDE2A protein per wet weight of tissue, similar to what had been previously described in human tissues by Western blot analysis (Sadhu et al. 1999). This study also demonstrated PDE2A protein in endothelial cells in multiple tissues across species, consistent with the finding of PDE2A expression in human venous and capillary endothelial cells in cardiac and renal tissues (Sadhu et al. 1999). PDE2A protein in heart, lung, kidney, liver, and thyroid may be limited to endothelium, because we did not demonstrate PDE2A ir in cardiac myocytes, lung epithelium, renal tubules or glomeruli, hepatocytes, or thyroid follicular epithelium or C-cells.
Given that PDE2A was originally purified from bovine adrenal and heart tissues as a cGMP-stimulated PDE (Martins et al. 1982), it is not surprising that we report very high levels of protein expression in the adrenal zona glomerulosa across species. The precise anatomic localization of PDE2A ir in the adrenal zona glomerulosa is consistent with functional data demonstrating that ANP stimulates cGMP production and that PDE2A activation in the adrenal cortex results in reduced aldosterone secretion (MacFarland et al. 1991; Nikolaev et al. 2005). These data are consistent with the hypothesis that PDE2A regulates the effects of ANP on fluid volume and retention.
Functional PDE2A activity has been reported in cardiomyocytes (Rivet-Bastide et al. 1997; Verde et al. 1999; Yanaka et al. 2003; Castro et al. 2006; Zaccolo 2006). Unexpectedly, we did not detect PDE2A ir in cardiomyocytes from the species we evaluated, yet protein was detectable in extracts of heart by Western blot and in endothelial cells of cardiac tissue by immunohistochemistry. One possible explanation for the discrepancy is the presence of a previously undescribed splice variant that is not recognized by our antibody. Alternatively, cardiac myocytes in vitro may express different properties than in vivo because the majority of published studies describe use of cultured cardiomyocytes in conjunction with rather non-selective PDE2A inhibitors such as erythro-9-(Hydroxy-3-nonyl) adenine HCL (Mery et al. 1995; Rivet-Bastide et al. 1997; Verde et al. 1999). Finally, very low levels of protein expression in cardiac myocytes in vivo may be below the level of detection of the antibody used in our study, but may still be present in sufficient quantities to produce a functional effect mediated by small amounts of active enzyme. The collective data highlight the importance of performing target distribution analyses using a combination of molecular, biochemical, and functional methods.
PDE2A ir was strongly expressed in regions of the spinal cord and particular ganglia of the peripheral nervous system. Immunoreactivity within neuronal cell bodies of colonic ganglia and the dorsal root ganglia was observed across species examined. In the spinal cord, PDE2A immunoreactivity was observed as neuropil labeling in the superficial laminae of the dorsal horn. The pattern of PDE2A ir resembled the distribution of cGMP immunoreactivity in spinal cord dorsal horn that reportedly colocalizes with synapses (de Vente et al. 2006). The spinal cord dorsal horn is known to play a role in pain processing and contains many neuropeptides, such as calcitonin gene-related peptide and Substance P, that regulate nociceptive information processing (Birklein and Schmelz 2008). Other spinal cord neurons also expressed PDE2A immunoreactivity, including scattered neurons within the ventral horn and intermediate laminae, consistent with a previous report describing the distribution of PDE2A, -5, and -9 mRNA in rat spinal cord (de Vente et al. 2006). The distribution of PDE2A ir in neurons of the dorsal root ganglia and not in satellite glia or nerve roots is consistent with the location of known sensory pain fiber pathways and may implicate a role for PDE2A in pain perception and processing.
In the current study, we extended previous studies of PDE2A mRNA distribution in the rodent brain (Juilfs et al. 1997; Van Staveren et al. 2003; Hepp et al. 2007) and human brain (Reyes-Irisarri et al. 2007) by examining the distribution of PDE2A ir in the nervous system across rodent, dog, primate, and human species. At the regional level, there was little detectable PDE2A ir in hindbrain regions such as the brainstem and cerebellum. The forebrain showed high expression in the medial habenula, isocortex, hippocampus, amygdala, interpeduncular nucleus, striatum, and olfactory tubercle, all structures that comprise major components of the limbic system. Limbic system neuroanatomic distribution has been previously reported for PDE2A mRNA (Repaske et al. 1993). We observed a differential expression of PDE2A ir in various cellular compartments, depending on what neuroanatomic region was evaluated. For example, PDE2A ir was abundant in cell bodies of the medial habenula, whereas most other brain regions exhibited staining associated with neuropil, fibers, and terminals. PDE2A ir was also modestly detected in particular thalamic nuclei, consistent with previous data demonstrating PDE2A mRNA in thalamic neurons (Hepp et al. 2007). The heterogeneous localization of PDE2A ir implies that PDE2A protein is differentially compartmentalized within different neuronal populations. Numerous splice isoforms have been described for human PDE2A (Bingham et al. 2006). It is unknown whether PDE2A in different subcellular locations may represent unique splice variants or subserve different functions.
PDE2A has been functionally implicated in regulation of cyclic nucleotide cascades implicated in learning and memory in several systems (Reneerkens et al. 2009). The high expression of PDE2A in the brain relative to the periphery supports the hypothesis that PDE2A functions to integrate cyclic nucleotide signaling in the nervous system. The heterogeneous and diverse expression pattern of PDE2A in the brain has potentially broad implications. The selective distribution of PDE2A ir in forebrain limbic system structures suggests that PDE2A may modulate neuronal signaling involved in complex functions such as emotion, learning, and memory. A role for PDE2A activity in memory consolidation is supported by the finding that the selective PDE2A inhibitor BAY 60-7550 reportedly improves performance of rodents in social and object recognition memory tasks (Boess et al. 2004; Rutten et al. 2007). Further characterization of PDE2A will include investigating the expression and function in conditions of health and disease.
Acknowledgments
The authors thank Alan Opsahl for assistance with photomicrograph preparation, Alison Romegialli and Susan Bove for Western blot analyses, Gary Seitis for immunohistochemical staining support, and Nestor Barrezuetta for in situ hybridization experiments.
References
- Beavo JA (1995) Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev 75:725–748 [DOI] [PubMed] [Google Scholar]
- Bingham J, Sudarsanam S, Srinivasan S (2006) Profiling human phosphodiesterase genes and splice isoforms. Biochem Biophys Res Commun 350:25–32 [DOI] [PubMed] [Google Scholar]
- Birklein F, Schmelz M (2008) Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS). Neurosci Lett 437:199–202 [DOI] [PubMed] [Google Scholar]
- Blokland A, Schreiber R, Prickaerts J (2006) Improving memory: a role for phosphodiesterases. Curr Pharm Des 12:2511–2523 [DOI] [PubMed] [Google Scholar]
- Boess FG, Hendrix M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, de Vente J, et al. (2004) Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology 47:1081–1092 [DOI] [PubMed] [Google Scholar]
- Castro LR, Verde I, Cooper DM, Fischmeister R (2006) Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 113:2221–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Beavo J (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76:481–511 [DOI] [PubMed] [Google Scholar]
- Coskran TM, Morton D, Menniti FS, Adamowicz WO, Kleiman RJ, Ryan AM, Strick CA, et al. (2006) Immunohistochemical localization of phosphodiesterase 10A in multiple mammalian species. J Histochem Cytochem 54:1205–1213 [DOI] [PubMed] [Google Scholar]
- de Vente J, Markerink-van Ittersum M, Vles JS (2006) The role of phosphodiesterase isoforms 2, 5, and 9 in the regulation of NO-dependent and NO-independent cGMP production in the rat cervical spinal cord. J Chem Neuroanat 31:275–303 [DOI] [PubMed] [Google Scholar]
- Diederen RM, La Heij EC, Markerink-van Ittersum M, Kijlstra A, Hendrikse F, de Vente J (2007) Selective blockade of phosphodiesterase types 2, 5 and 9 results in cyclic 3′5′ guanosine monophosphate accumulation in retinal pigment epithelium cells. Br J Ophthalmol 91:379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambaryan S, Butt E, Marcus K, Glazova M, Palmetshofer A, Guillon G, Smolenski A (2003) cGMP-dependent protein kinase type II regulates basal level of aldosterone production by zona glomerulosa cells without increasing expression of the steroidogenic acute regulatory protein gene. J Biol Chem 278:29640–29648 [DOI] [PubMed] [Google Scholar]
- Hepp R, Tricoire L, Hu E, Gervasi N, Paupardin-Tritsch D, Lambolez B, Vincent P (2007) Phosphodiesterase type 2 and the homeostasis of cyclic GMP in living thalamic neurons. J Neurochem 102:1875–1886 [DOI] [PubMed] [Google Scholar]
- Iffland A, Kohls D, Low S, Luan J, Zhang Y, Kothe M, Cao Q, et al. (2005) Structural determinants for inhibitor specificity and selectivity in PDE2A using the wheat germ in vitro translation system. Biochemistry 44:8312–8325 [DOI] [PubMed] [Google Scholar]
- Imai A, Nashida T, Shimomura H (1999) Comparison of phosphodiesterase isozymes in rodent parotid glands. Comp Biochem Physiol B Biochem Mol Biol 124:397–403 [DOI] [PubMed] [Google Scholar]
- Juilfs DM, Fulje H-J, Zhao A, Houslay MD, Garbers DL, Beavo JA (1997) A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2A) and guanylyl cyclase D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci USA 94:3388–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarland RT, Zelus BD, Beavo JA (1991) High concentrations of a cGMP-stimulated phosphodiesterase mediate ANP-induced decreases in cAMP and steroidogenesis in adrenal glomerulosa cells. J Biol Chem 266:136–142 [PubMed] [Google Scholar]
- Manganiello V, Tanaka T, Murashima S (1990) In Beavo JA and Houslay MD, eds. Cyclic Nucleotide Phosphodiesterases: Structure, Regulation and Drug Action. New York, John Wiley & Sons, 61–85
- Martins TJ, Mumy MC, Beavo JA (1982) Purification and characterization of a cyclic GMP-stimulated cyclic nucleotide phosphodiesterase from bovine tissues. J Biol Chem 257:1973–1979 [PubMed] [Google Scholar]
- Mehats C, Andersen CB, Filopanti M, Jin SL, Conti M (2002) Cyclic nucleotide phosphodiesterases and their role in endocrine cell signaling. Trends Endocrinol Metab 13:29–35 [DOI] [PubMed] [Google Scholar]
- Mery P-F, Pavoine C, Pecker F, Rishchmeister R (1995) Erythro-9-(2-hydroxy-3-nonyl)adenine inhibits cyclic GMP-stimulated phosphodiesterase in isolated cardiac myocytes. Mol Pharmacol 48:121–130 [PubMed] [Google Scholar]
- Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, et al. (2006) Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res 98:226–234 [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R (2003) Manipulating the Mouse Embryo, A Laboratory Manual. 3rd ed. Cold Spring Harbor, NY, Cold Spring Harbor Press, 431–453
- National Research Council (1996) Guide for the Care and Use of Laboratory Animals. Washington, D.C., National Academy Press
- Nikolaev VO, Gambaryan S, Engelhardt S, Walter U, Lohse MJ (2005) Real-time monitoring of the PDE2A activity of live cells: hormone-stimulated cAMP hydrolysis is faster than hormone-stimulated cAMP synthesis. J Biol Chem 280:1716–1719 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates. 6th ed. New York, Academic Press
- Prickaerts J (2004) Phosphodiesterase type 5 inhibition improves early memory consolidation of object information. Neurochem Int 45:915–928 [DOI] [PubMed] [Google Scholar]
- Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J (2009) Selective phosophodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl) 202:419–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske DR, Corbin JG, Conti M, Goy MF (1993) A cyclic GMP stimulated cyclic nucleotide phosphodiesterase gene is highly expressed in the limbic system of the rat brain. Neuroscience 56:673–686 [DOI] [PubMed] [Google Scholar]
- Reyes-Irisarri E, Markerink-Van Ittersum M, Mengod G, de Vente J (2007) Expression of the cGMP-specific phosphodiesterases 2 and 9 in normal and Alzheimer's disease human brains. Eur J Neurosci 25:3332–3338 [DOI] [PubMed] [Google Scholar]
- Rivet-Bastide M, Vandecasteele G, Hatem S, Verde I, Benardeau A, Mercadier JJ, Fischmeister R (1997) cGMP-stimulated cyclic nucleotide phosphodiesterase regulates the basal calcium current in human atrial myocytes. J Clin Invest 99:2710–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosman GJ, Martins TJ, Sonnenburg WK, Beavo JA, Ferguson K, Loughney K (1997) Isolation and characterization of human cDNAs encoding a cGMP-stimulated 3′,5′-cyclic nucleotide phosphodiesterase. Gene 191:89–95 [DOI] [PubMed] [Google Scholar]
- Rutten K, Prickaerts J, Hendrix M, van der Staay FJ, Sik A, Blokland A (2007) Time-dependent involvement of cAMP and cGMP in consolidation of object memory: studies using selective phosphodiesterase type 2, 4 and 5 inhibitors. Eur J Pharmacol 558:107–112 [DOI] [PubMed] [Google Scholar]
- Sadhu K, Hensley K, Florio VA, Wolda SL (1999) Differential expression of the cyclic GMP-stimulated phosphodiesterase PDE2A in human venous and capillary endothelial cells. J Histochem Cytochem 47:895–906 [DOI] [PubMed] [Google Scholar]
- Soderling SH, Beavo JA (2000) Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol 12:174–179 [DOI] [PubMed] [Google Scholar]
- van Staveren WC, Markerink-van Ittersum M, Steinbusch HW, de Vente J (2001) The effects of phosphodiesterase inhibition on cyclic GMP and cyclic AMP accumulation in the hippocampus of the rat. Brain Res 888:275–286 [DOI] [PubMed] [Google Scholar]
- Van Staveren WC, Steinbusch HW, Markerink-Van Ittersum M, Repaske DR, Goy MF, Kotera J, Omori K, et al. (2003) mRNA expression patterns of the cGMP-hydrolyzing phosphodiesterases types 2, 5, and 9 during development of the rat brain. J Comp Neurol 467:566–580 [DOI] [PubMed] [Google Scholar]
- Velardez MO, De Laurentiis A, del Carmen Diaz M, Lasaga M, Pisera D, Seilicovich A, Duvilanski BH (2000) Role of phosphodiesterase and protein kinase G on nitric oxide-induced inhibition of prolactin release from the rat anterior pituitary. Eur J Endocrinol 143:279–284 [DOI] [PubMed] [Google Scholar]
- Verde I, Vandecasteele G, Lezoualc'h F, Fischmeister R (1999) Characterization of the cyclic nucleotide phosphodiesterase subtypes involved in the regulation of the L-type Ca2+ current in rat ventricular myocytes. Br J Pharmacol 127:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Adamowicz W, Eldred WD, Jakowski AB, Kleiman RJ, Morton DG, Stephenson DT, et al. (2006) Cellular and subcellular localization of PDE10A, a striatum-enriched phosphodiesterase. Neuroscience 139:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaka N, Kurosawa Y, Minami K, Kawai E, Omori K (2003) cGMP-phosphodiesterase activity is up-regulated in response to pressure overload of rat ventricles. Biosci Biotechnol Biochem 67:973–979 [DOI] [PubMed] [Google Scholar]
- Zaccolo M (2006) Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur J Cell Biol 85:693–697 [DOI] [PubMed] [Google Scholar]
- Zacher LA, Carey GB (1999) Cyclic AMP metabolism by swine adipocyte microsomal and plasma membranes. Comp Biochem Physiol B Biochem Mol Biol 124:61–71 [DOI] [PubMed] [Google Scholar]