Abstract
During embryonic development, axons are guided to their target by patterning proteins encountered along their trajectory. These cues can be linked to the cells that produce them or secreted into the extracellular matrix. Whether secreted cues, like netrin-1, provide traction for the growth cone when they become attached to the extracellular matrix is unclear. Here, advancing spinal commissural neuron growth cones were shown to generate local forces of 4−15pN but, when confronted with immobilized netrin-1, generated traction forces in excess of 63pN on netrin-1 that can redirect the trajectory of the axon.
During embryonic development, axons are guided to their target by patterning proteins encountered along their trajectory (1). These cues can be linked to the cells that produce them or secreted into the extracellular matrix. Whether secreted cues provide traction for the growth cone when attached to the extracellular matrix is unclear. For instance, the secreted cue netrin can diffuse through collagen to induce axon outgrowth at a distance and its release from a micropipette can influence the extension of axons in culture (2, 3). However, netrins are also known to bind to extracellular matrix components and can function when attached directly to or in close proximity to the cells that produce them (4, 5). The substrate associations of netrin may simply buffer their concentration distribution by slowing their dispersion. Here we tested whether growth cones exert a traction force directly on netrin-1 that is sufficient to reorient their axons.
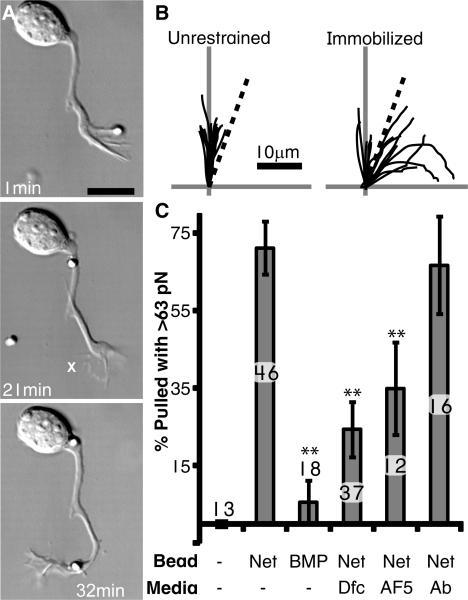
As they extend ventrally in the spinal cord, spinal commissural neuron (SCN) axons are attracted to netrin-1 released by the floor plate. When dissociated SCNs were presented with an unrestrained silica bead with covalently attached netrin-1 (6), their growth cones captured and transported it down the axon (Fig. 1A; Movie S1). However, when presented with a netrin-1 coated bead immobilized to the substrate, a distinct reorientation of the axon was observed (Fig. 1A,B; Movie S2, S3). Thus immobilized netrin-1 was sufficient to reorient the axon. Moreover, the retrograde transport of an unrestrained bead, as well as the reorientation towards immobilized beads indicated that the growth cone generated force on netrin-1. To test this possibility, we measured the pulling forces on netrin-1 by presenting SCN growth cones with optically trapped beads. Growth cones rapidly (∼4.5min) generated pulling forces in excess of 63 pN (Fig. 1C; Movie S4). Such pulling forces were not observed on uncoated beads (Fig. 1C). Similarly, when growth cones encountered beads coated with the repellent cue Bone Morphogenetic Protein 7 (BMP) (7), they rapidly collapsed and rarely exerted forces above 25 pN (Fig. 1C; Movie S5). Chemoattraction to netrin-1 required the transmembrane receptor Deleted in Colorectal Cancer (DCC) (8). When the interaction between netrin-1 and DCC was decreased by competition with a recombinant soluble form of the extracellular domain of DCC (Dfc) or when DCC function was disrupted with an antibody (AF5), pulling of netrin-1 coated beads was disrupted (Fig. 1C). Thus SCN growth cones exerted a specific pulling force that appeared to be directly on netrin-1 and to require the receptor DCC.
Fig. 1.
(A) A netrin-1 bead is presented and released upon contact on one side of the growth cone. After 22 min., another bead is presented, but this time it is attached to the substrate (marked ‘X’). Scale bar = 10μm. (B) Traces of axons confronted with unrestrained or immobilized netrin-1 beads. Vertical axis is the initial trajectory of the axon, while the dotted line marks a 20° angle. None of the 13 axons assayed turned at an angle >20° when unrestrained, while 10 of the 15 axons reoriented to immobilized beads. (C) Percent of beads pulled with >63 pN within 8 min (Ab = unspecific antibodies). The number of growth cones assayed is indicated above or within each bar. Error bars are S.E.M and ** = p<0.01 relative to netrin-1 (Tukey).
To understand what the magnitude of these pulling forces represented, it was necessary to compare them to the normal pulling events of an advancing growth cone. Growth cones generated a forward locomotive force (9), however, the distribution and magnitude of these forces within a growth cone was unclear. Using the calibrated deflections of micro-fabricated polyacrylamide pillars, we measured the simultaneous, local pulling events of extending SCN axons (Fig. S1; Movie S6). Pulling forces ranged from 2 to 37 pN, although the majority (>90%) occurred between 4 and 15 pN and the average was 9 pN (n=2789, Fig. S1E). Thus, the pulling forces observed on netrin-1 beads were more than 7-fold higher than the average local pulling forces of SCN growth cones.
The mechanical interaction between the growth cone and netrin-1 may play a role in other aspects of the response to netrin-1. For instance, mechanical extension of adhesion site proteins can expose buried sequences that transduce mechanical force into intracellular signaling cascades (10, 11). Future studies are needed to define how netrin-1 triggers the intracellular signaling required to coordinate the reorientation of the axon.
Supplementary Material
Footnotes
References and Notes
- 1.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Annu. Rev. Neurosci. 2003;26:509. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy TE, Serafini T, de l. T., Jr., Tessier-Lavigne M. Cell. 1994;78:425. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 3.de la Torre, et al. Neuron. 1997;19:1211. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 4.Deiner MS, et al. Neuron. 1997;19:575. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 5.Brankatschk M, Dickson BJ. Nat. Neurosci. 2006;9:188. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- 6.See Material and Methods on Science online
- 7.Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S. Neuron. 1999;24:127. doi: 10.1016/s0896-6273(00)80827-2. [DOI] [PubMed] [Google Scholar]
- 8.Keino-Masu K, et al. Cell. 1996;87:175. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 9.Lamoureux P, Buxbaum RE, Heidemann SR. Nature. 1989;340:159. doi: 10.1038/340159a0. [DOI] [PubMed] [Google Scholar]
- 10.Sawada Y, et al. Cell. 2006;127:1015. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del RA, et al. Science. 2009;323:638. [Google Scholar]
- 12.We thank T.E. Kennedy for critical reading, N. Gauthier for discussion and support from NIH grants PN2 EY016586-02 (S.W.M. & M.P.S.) and AI079030 (N.B.).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.