Abstract
Objectives
Sexual function is an important dimension of adult life and yet very little is known about the relationships between female sexuality and chronic health conditions, including pelvic floor disorders. Our goal was to investigate the hypothesis that pelvic floor disorders are associated with female sexual problems, independent of other related factors.
Methods
The study population included 301 adult women seeking outpatient gynecologic and urogynecologic care. Pelvic floor disorders were assessed with the Pelvic Floor Disorders Inventory-20 (PFDI) and the Pelvic Organ Prolapse Quantification examination. Sexual function was assessed with the Personal Experiences Questionnaire. Using ordinal regression analysis, we identified characteristics and conditions associated with decreased libido, infrequent orgasm, decreased arousal, and dyspareunia.
Results
Sexual function was poorer among 78 women (26%) without a current sexual partner than among 223 with a partner (p<0.01). Among the 223 with a current partner, women with a high PFDI score were significantly more likely to report decreased arousal (p<0.01), infrequent orgasm (p<0.01) and increased dyspareunia (p<0.01). A similar pattern was observed for the urinary, colorectal-anal, and prolapse scales of the PFDI, although some associations were marginally significant. Stage III–IV prolapse was significantly associated with infrequent orgasm (p=0.02), but other sexual complaints were not more common with increasing prolapse stage.
Conclusion
Pelvic floor symptoms are significantly associated with reduced sexual arousal, infrequent orgasm, and dyspareunia. Clinicians who care for women with pelvic floor disorders should be aware of this association and should specifically address sexual concerns with women seeking treatment of incontinence and prolapse.
INTRODUCTION
The American Foundation for Urologic Disease recognizes four types of female sexual dysfunction (1): low libido, problems with sexual arousal, inability to achieve orgasm, and dyspareunia. Studies have found that sexual complaints are common among women with pelvic floor disorders (2–4), but results are not consistent. For example, in an investigation of women planning hysterectomy for benign disease (2), women with urinary symptoms were significantly more likely than those without urinary symptoms to report decreased libido and dyspareunia. However, a recent community-based study (4) found no substantial difference in sexual activity or sexual satisfaction among women with pelvic floor symptoms.
These conflicting results may be a reflection of the populations studied. Clinical populations are likely to have more severe pelvic floor symptoms and more advanced pelvic organ prolapse, with a greater potential for discernable impact on sexual function, while community populations may have mild symptoms and prolapse, with minimal impact. These conflicting results may also be the result of population differences in other related factors, such as age, menopause, or the status of the woman’s intimate relationship. Other challenges in studying the factors associated with sexual function include a limited characterization of female sexual function in some studies and difficulties assessing sexual function among women who do not have intercourse.
The aim of this study to was to improve our understanding of the relationship between pelvic floor disorders and female sexual function through a cross-sectional investigation of women seeking gynecologic care at 5 clinical sites in Metropolitan Baltimore. We included women without any pelvic floor symptoms and with normal pelvic organ support as well as women with severe symptoms and/or severe pelvic organ prolapse. We also sought to characterize other factors that could influence sexual function, such as menopause, relationship factors, depressive symptoms, and generic health status. These data were used to investigate the hypothesis that pelvic floor disorders are associated with female sexual problems, independent of other factors.
MATERIALS AND METHODS
The Johns Hopkins Institutional Review Board approved this study. The target population was adult women with prior sexual experience seeking outpatient gynecologic or urogynecologic care. Our sample came from 5 outpatient sites affiliated with Johns Hopkins Medical Institutions in Metropolitan Baltimore. These sites provide routine gynecologic services for the local community, as well as tertiary care for referred patients. Women over the age of 40 who were scheduled for a gynecologic examination were eligible for this research. Women were asked not to participate if they were intentionally celibate (e.g., if they would not be sexually active in principle). Also excluded were pregnant women, those who could not complete questionnaires in English, and those who had never been sexually active. In order to ensure an adequate number of subjects with and without pelvic floor disorders for this research, subjects were recruited from both gynecologic clinics and urogynecologic clinics. Women were recruited between January 1, 2006 and April 1, 2007. Subjects were recruited from several clinic sessions each week, based on the clinical schedule and the availability of the research coordinator. Potentially eligible research subjects were approached for enrollment prior to the physical examination and the research questionnaire was completed after the clinical visit.
Sample size for this research was based on the observation that 40.4% of women without urinary complaints report sexual complaints and that the odds of sexual complaints are two times higher for women with urinary incontinence (2). Based on this effect size, we estimated that 300 subjects would provide 80% power to detect an effect size of this magnitude with alpha=0.05.
Pelvic floor disorders were assessed using a questionnaire and a standardized gynecologic examination. The Pelvic Floor Distress Inventory-20 (PFDI-20) (5) was used to identify symptoms of pelvic floor disorders, This 20-item validated symptom inventory generates a total score and three scale scores, which include the urinary distress inventory, the colorectal-anal distress inventory, and the pelvic organ prolapse distress inventory. Each scale is scored from 0 to 100, with higher scores indicating greater symptom burden. In addition to the symptom survey and bother score provided by the PFDI, a standardized physical examination was performed to objectively assess pelvic organ support. The examination was performed according to the Pelvic Organ Prolapse Quantification system (6). Twelve physician-investigators demonstrated competency in performing the research examination prior to the study and their competency was reconfirmed throughout the study.
Sexual function was assessed using the short form of the Personal Experiences Questionnaire (SPEQ), (7) a 9-item sexual-function instrument which has been validated among perimenopausal women (7–9). This questionnaire is specifically structured so that the first half is relevant to all women, regardless of partner status (7). We selected this questionnaire in order to include women without a current partner, women with female partners, and women with male partners who could not engage in intercourse. Four domains of the SPEQ were used to describe four aspects of female sexual function. First, sexual desire (libido) was assessed by one item (“…how many times during the last month have you have had sexual thoughts or fantasies”), rated on an ordinal scale, with answers ranging from “never” (0 points) to “several times a day” (5 points). Second, sexual arousal (“responsivity”) was defined by the average of two items: “How enjoyable are sexual activities for you” and “How often during sex do you feel aroused or excited?”, each rated on an ordinal scale, with “1” corresponding to “not at all” and “6” corresponding to “a great deal”. Orgasm was addressed by one item (“Do you currently experience orgasm (climax) during sex activity?”), also measured on a 6-point ordinal scale, with the highest score representing “a great deal”. Finally, dyspareunia was assessed using a single item (“Do you currently experience pain during intercourse?”), rated from 0 (“not at all”) to 5 (”a great deal”), with an option for women who do not have intercourse to mark “not applicable”. Women with partners were also asked “How much passionate love do you feel for your partner?” and “Does your partner(s) experience difficulty in sexual performance?” Each of these items was rated from 1 (“not at all”) to 5 (“a great deal”).
In addition to these assessments for pelvic floor disorders and sexual function, we also obtained demographic information and relevant medical and surgical history. Participants completed the 12-Item Short-Form Health Survey (10) as a measure of overall health-related quality of life. The Center for Epidemiologic Studies--Depression scale (11) was used as a measure of depressive symptoms. Height and weight were measured and body mass index calculated.
To investigate the association between pelvic floor disorders and sexual function, we used proportional odds regression (12), because each sexual outcome was measured on an ordinal scale. Thus, the odds ratios estimated in this study represented the relative odds for a change from one level of sexual function to the next in the direction of worsening function. We anticipated that risk factors would differ between these four sexual complaints and therefore we performed separate analyses for libido, arousal, orgasm and dyspareunia. In each analysis, the primary independent variables were pelvic floor disorders, represented by the PFDI summary score or scale scores. We also considered anatomic prolapse, defined by prolapse stage. Initially, all variables were examined in bivariate analyses. Those found to be significantly associated with sexual function at P< 0.20 were included in the multivariable analysis. For each multivariable comparison, a parsimonious model was obtained by stepwise (backward and forward) variable selection guided by AIC statistics. All analyses were performed using R version 2.5.1 (The R Project for Statistical Computing, 2007). Based on our investigation of factors associated with sexual function in this population, all multivariable models included adjustment for age, menopause, vaginal dryness, education, marital status, depression score, passionate love for partner, and partner difficulty with sexual activities. Dyspareunia models also included adjustment for history of rape or abuse.
We anticipated that some participants would report no sexual partner or no sexual intercourse despite having a partner (3). We used Students t test and chi squared analysis or Fisher’s exact test to compare characteristics of women with and without sexual partners. PFDI scores and scale scores were compared with Mann Whitney U test. Some aspects of sexual function were relevant only to those with a partner (e.g., partner difficulty with sexual function) and were therefore not assessed among those without a current sexual partner. Because of possible difficulty assessing sexual function among women without a partner, our primary analyses were limited to data for women with a current partner (n=223). Once the regression models were developed, we then applied the same regression models to the data for women without a partner (n=78). This allowed us to assess the consistency of the direction and magnitude of associations in these two groups of women.
RESULTS
We approached 420 women for participation in this research and 344 (82%) enrolled. Of the enrolled participants, 305 (89%) completed the questionnaire and 301 answered the question regarding sexual partner status. These 301 women comprised our study population. Of these 301 women, 80 were seeking treatment of pelvic floor conditions (including “problems with bladder function” and “prolapse of uterus or vagina”) and 69 came for treatment of other gynecologic complaints (including menstrual complaints, pelvic pain, menopause, abnormal cytology, and vaginal discharge). The remaining 152 were seeking annual gynecologic care.
Most participants (223 of 301, 74%) reported a current sexual partner (table 1). The 78 women without a sexual partner were older, more likely to be menopausal, and more likely to report prior hysterectomy. Overall, 282 participants (97.6%) were heterosexual, 2 (0.7%) were bisexual and 5 (1.7%) were homosexual.
Table 1.
Characteristics of 301 participants with or without a current sexual partner.
| Variable | With Partner (n=223) | Without Partner (n=78) | P§ |
|---|---|---|---|
| Age (mean, SD) | 53.6 ± 10.1 | 63.2 ± 11.2 | <0.01 |
| Race (n, %) | |||
| White | 179 (81%) | 58 (76%) | 0.70 |
| Black | 32 (14%) | 16 (21%) | |
| Asian | 7 (3%) | 2 (3%) | |
| Other | 2 (1%) | 0 | |
| Nulliparous (n, %) | 35 (16%) | 11 (14%) | 0.91 |
| Currently married (n, %) | 187 (84%) | 15 (19%) | <0.01 |
| Education: High school or less (n, %) | 78 (35%) | 31 (38%) | 0.68 |
| Diabetes (n, %) | 14 (6%) | 10 (13%) | 0.11 |
| Hypertension (n, %) | 55 (25%) | 30 (38%) | 0.03 |
| Current cigarette smoker (n, %) | 16 (7%) | 6 (8%) | 0.90 |
| Menopausal (n, %) | 125 (56%) | 67 (86%) | <0.01 |
| Prior hysterectomy (n, %) | 48 (22%) | 28 (36%) | 0.02 |
| Body mass index (kg/m2) (mean, SD) | 28.5 ± 6.7 | 29.7 ± 7.3 | 0.22 |
| Self-reported health* | |||
| Poor (n, %) | 2 (1%) | 0 | 0.07 |
| Fair (n, %) | 10 (4%) | 11 (14%) | |
| Good (n, %) | 79 (36%) | 28 (36%) | |
| Very good (n, %) | 92 (42%) | 28 (36%) | |
| Excellent (n, %) | 36 (16%) | 11 (14%) | |
| Vaginal dryness score† (mean, SD) | 2.4 ± 1.8 | 1.8 ± 2.0 | 0.05 |
| Depression score‡ (mean, SD) | 11.2 ± 10.5 | 10.9 ± 8.7 | 0.82 |
| Prior rape or sexual abuse (n,%) | 33 (15%) | 9 (12%) | 0.71 |
Self-reported general health: “In general, would you say your health is…”
Higher score indicates a greater “[lack of] …vaginal wetness during sex activities”. Score ranges from 1 (“not at all”) to 6 (“a great deal”)
A higher Center for Epidemiologic Studies--Depression scale score (possible range 0–60) indicates greater depressive symptoms.
P values for statistical comparisons using Students t test for continuous variables and chi squared for categorical variables.
PFDI scores and scale scores are summarized in table 2. Scores for the urinary scale of the PFDI were higher for those without a current partner, indicating a greater burden of urinary symptoms in that group. Otherwise, there were no significant differences in pelvic floor symptoms between women with and without a sexual partner. Anatomic stage of prolapse did not differ significantly between women with and without a partner.
Table 2.
Pelvic floor disorders among women with and without a sexual partner.
| Variable* | With Partner (n=223) | Without Partner (n=78) | P† |
|---|---|---|---|
| Prolapse stage (n, %) | |||
| 0 | 31 (14%) | 8 (11%) | 0.29 |
| I | 103 (47%) | 30 (39%) | |
| II | 63 (29%) | 26 (34%) | |
| III–IV | 21 (10%) | 12 (16%) | |
| PFDI Score (mean, SD) | 51.9 ± 51.0 | 57.5 ± 53.4 | |
| (median) | 33.3 | 39.1 | 0.29 |
| Urinary Score (mean, SD) | 22.2 ± 24.1 | 29.8 ± 26.7 | |
| (median) | 16.7 | 25.0 | 0.02 |
| Colorectal-anal Score (mean, SD) | 15.8 ± 18.8 | 14.3 ± 19.6 | |
| (median) | 9.4 | 8.8 | 0.40 |
| Prolapse Score (mean, SD) | 14.0 ± 18.2 | 13.4 ± 17.6 | |
| (median) | 8.3 | 8.33 | 0.96 |
PFDI = Pelvic floor distress inventory; Urinary score = urinary distress inventory; Colorectal-anal score = colorectal-anal distress inventory; Prolapse Score = pelvic organ prolapse distress inventory. A higher score on the PFDI summary score (range 0 to 300) or any of the scale scores (range 0 to 100) indicates greater symptom burden.
Statistical comparisons with Fisher’s Chi-squared test for POP-Q stage and Mann-Whitney tests otherwise.
Sexual function in this population is summarized in figure 1. Among 223 women with a sexual partner, 28 (13.0%) reported no sexual thoughts or fantasies in the last month. Fifteen women (6.9%) had the lowest arousal score (score=1), indicating no enjoyment from sexual activities and no feeling of arousal during sexual activities. Twenty women (9.3 %) reported no orgasm during sexual activity. Of the 206 who answered the question about dyspareunia, 18 (8.7 %) reported “a great deal” of dyspareunia. Women without a current partner were more likely than those with a partner to report decreased libido (<0.01), arousal (<0.01), and orgasm (<0.01).
Figure 1.
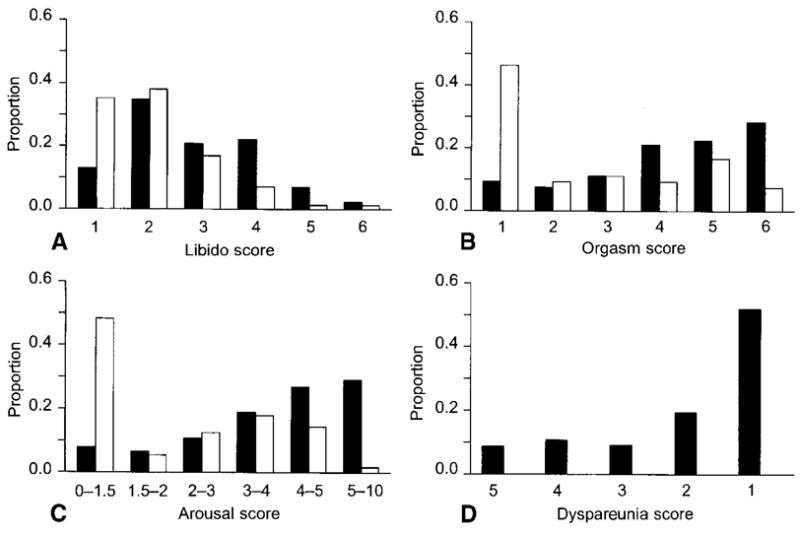
Four domains of sexual function, among 223 women with a current sexual partner (dark grey bars) and 78 women without a partner (light grey bars). The Y axis shows the proportion of women endorsing each response. A. Libido and B. orgasm are rated on a 6-point ordinal scale, with higher scores representing better sexual function. C. The arousal score is an average of two items, with a higher score representing better function. D. Dyspareunia is measured on a 5-point ordinal scale, with the highest score representing the worst function (most dyspareunia).
For the 233 women with a current sexual partner, complete data regarding sexual function was provided by 216 (97%). The characteristics associated with lower scores for libido, arousal and orgasm (table 3) included increasing age, less than college education, marriage, menopause, vaginal dryness and higher depression score. Partner difficulty with sexual function was associated with decreased arousal and orgasm, but not libido. Women who reported a great deal of “passionate love” for partner reported better scores for libido, arousal and orgasm. In contrast, increasing dyspareunia was associated with no college education, vaginal dryness and higher depression score, but not with increasing age, menopause, or marital status. A history of rape or abuse was marginally associated with dyspareunia (p=0.09) but not with other aspects of sexual function.
Table 3.
Relationship between characteristics and self-reported sexual function among 223 women with a current sexual partner. Odds ratios (OR) and 95% confidence intervals from unadjusted proportional odds regression.
| Decreased libido |
Decreased arousal |
Decreased orgasm |
Increased dyspareunia |
|
|---|---|---|---|---|
| Age (per 5-year increase) | 1.30 (1.14, 1.47) | 1.24 (1.09, 1.41) | 1.14 (1.01, 1.29) | 0.97 (0.84, 1.11) |
| No college education (versus college or graduate education) | 1.70 (1.02, 2.81) | 1.92 (1.17, 3.17) | 1.17 (0.71, 1.93) | 1.74 (1.01, 2.99) |
| Nulliparity (versus parity>0) | 0.69 (0.36, 1.32) | 0.43 (0.22, 0.83) | 0.73 (0.40, 1.34) | 1.20 (0.62, 2.33) |
| Currently married (versus unmarried) | 2.14 (1.10, 4.17) | 3.12 (1.55, 6.27) | 2.86 (1.46,5.60) | 1.10 (0.56, 2.17) |
| Post-menopausal (versus pre-menopausal) | 3.14 (1.90, 5.18) | 2.55 (1.56, 4.18) | 2.16 (1.33, 3.51) | 1.62 (0.97, 2.72) |
| Prior hysterectomy (versus no prior hysterectomy) | 1.44 (0.80, 2.61) | 1.72 (0.97, 3.07) | 1.59 (0.87, 2.88) | 1.82 (0.94, 3.52) |
| Body Mass Index (per increase in kg/m2) | 0.98 (0.94, 1.01) | 1.01 (0.97, 1.04) | 1.00 (0.96, 1.04) | 0.99 (0.95, 1.03) |
| General health score* (per unit change) | 1.03 (0.63, 1.68) | 0.67 (0.41, 1.09) | 0.78 (0.47, 1.28) | 1.04 (0.61, 1.77) |
| Depression score† (per unit change) | 1.01 (0.99, 1.04) | 1.04 (1.02, 1.07) | 1.03 (1.00, 1.05) | 1.05 (1.02, 1.08) |
| Love for partner‡ (per unit change) | 0.78 (0.62, 0.98) | 0.51 (0.40, 0.64) | 0.74 (0.60,0.93) | 1.06 (0.83, 1.36) |
| Partner difficulty§ (per unit change) | 1.02 (0.86, 1.20) | 1.26 (1.07, 1.48) | 1.20 (1.02, 1.41) | 0.84 (0.70, 1.00) |
| Vaginal dryness||(per unit change) | 1.18 (1.03, 1.36) | 1.31 (1.14, 1.50) | 1.21 (1.06, 1.39) | 1.57 (1.34, 1.84) |
| History of rape/abuse | 0.75 (0.39, 1.46) | 0.91 (0.47, 1.75) | 1.12 (0.57, 2.20) | 1.84 (0.92, 3.66) |
Self-reported general health: “In general, would you say your health is …” Score ranges from 1 (“poor”) to 5 (“excellent”)
A higher Center for Epidemiologic Studies--Depression scale score (range, 0–60) indicates greater depressive symptoms.
“How much passionate love do you feel for your partner?” Score ranges from 1 (“not at all”) to 5 (“a great deal”)
“Does your partner(s) experience difficulty in sexual performance? (e.g., erectile problems, ejaculation difficulties, low arousal)”; Score ranges from 1 (“not at all”) to 5 (“a great deal”)
Higher score indicates a greater “[lack of] …vaginal wetness during sex activities”. Score ranges from 1 (“not at all”) to 6 (“a great deal”)
Table 4 summarizes the relationship between pelvic floor disorders and sexual function for women with a current partner. A high PFDI summary score, indicating a high burden of pelvic floor symptoms, was significantly associated with poorer sexual function. Specifically, women with a high PFDI score were more likely to report decreased libido, decreased arousal, infrequent orgasm and increased dyspareunia. Adjusting for other characteristics did not substantially affect our estimates of the association between pelvic floor symptoms and sexual function, although adjusting for multiple variables attenuated the statistical significance of these associations. In the final multivariable model, a high PFDI score was significantly associated with decreased arousal (p<0.01), infrequent orgasm (p<0.01), and increased dyspareunia (p<0.01).
Table 4.
Crude and adjusted odds ratios for association between pelvic floor disorders and sexual function for 223 women with a current sexual partner. Odds ratios (OR) and 95% confidence intervals from proportional odds regression.
| Decreased libido | Decreased arousal | Decreased orgasm | Increased dyspareunia | |
|---|---|---|---|---|
| PFDI* (per 10 point increase) | ||||
| Crude | 1.06 (1.01, 1.11) | 1.11 (1.06, 1.16) | 1.10 (1.05, 1.16) | 1.10 (1.05, 1.16) |
| Adjusted† | 1.05 (0.99, 1.11) | 1.09 (1.03, 1.16) | 1.09 (1.03, 1.15) | 1.11 (1.04, 1.18) |
| Urinary score* (per 10 point increase) | ||||
| Crude | 1.09 (0.99, 1.21) | 1.21 (1.09, 1.33) | 1.18 (1.06, 1.30) | 1.16 (1.04, 1.29) |
| Adjusted† | 1.07 (0.95, 1.19) | 1.20 (1.07, 1.33) | 1.16 (1.04, 1.29) | 1.13 (1.00, 1.28) |
| Colorectal-anal score* (per 10 point increase) | ||||
| Crude | 1.20 (1.06, 1.37) | 1.26 (1.10, 1.44) | 1.24 (1.08, 1.42) | 1.23 (1.07, 1.42) |
| Adjusted† | 1.16 (1.00, 1.35) | 1.15 (0.98, 1.34) | 1.16 (1.00, 1.35) | 1.24 (1.05, .147) |
| Prolapse score* (per 10 point increase) | ||||
| Crude | 1.10 (0.96, 1.27) | 1.25 (1.09, 1.42) | 1.26 (1.10, 1.45) | 1.30 (1.12, 1.50) |
| Adjusted† | 1.10 (0.94, 1.28) | 1.24 (1.06, 1.44) | 1.25 (1.08, 1.45) | 1.32 (1.11, 1.57) |
| Prolapse stage | ||||
| Crude | ||||
| 0 | Reference | Reference | Reference | Reference |
| I | 1.42 (0.68, 2.99) | 1.69 (0.82, 3.48) | 1.55 (0.76, 3.18) | 0.89 (0.42, 1.89) |
| II | 1.51 (0.61, 3.35) | 2.04 (0.93, 4.44) | 2.05 (0.94, 4.46) | 0.96 (0.43, 2.13) |
| III–IV | 2.90 (1.04, 8.07) | 2.22 (0.82, 6.03) | 3.57 (1.33, 9.59) | 0.61 (0.21, 1.79) |
| Adjusted† | ||||
| 0 | Reference | Reference | Reference | Reference |
| I | 0.90 (0.40, 2.02) | 1.29 (0.57, 2.89) | 1.49 (0.69, 3.23) | 0.68 (0.29, 1.60) |
| II | 1.41 (0.60, 3.31) | 1.85 (0.78, 4.40) | 2.06 (0.89, 4.77) | 0.98 (0.40, 2.42) |
| III–IV | 1.83 (0.58, 5.77) | 1.36 (0.43, 4.35) | 3.60 (1.20, 10.76) | 0.36 (0.10, 1.33) |
PFDI = Pelvic floor distress inventory; urinary score = urinary distress inventory; colorectal-anal score = colorectal-anal distress inventory; Prolapse score= pelvic organ prolapse distress inventory. A higher score on the PFDI summary score (range 0 to 300) or any of the scale scores (range 0 to 100) indicates greater symptom burden.
Adjusted models include age, menopause, vaginal dryness, education, marital status, depression score, passionate love for partner, and partner difficulty. Dyspareunia models also include additional adjustment for history of rape or abuse.
The same pattern was observed for the urinary, prolapse and colorectal-anal scales of the PFDI. A high score for any of these scale scores was associated with decreased arousal, infrequent orgasm and increased dyspareunia. However, the associations between urinary and prolapse scores with libido were not statistically significant. Adjusting for multiple confounding factors did not have any meaningful effect on the estimated odds ratios but decreased the precision of these measures.
With respect to anatomic prolapse, in the unadjusted analysis, women with Stage III–IV prolapse were more likely to report decreased libido and infrequent orgasm than women with Stage 0 support. Adjustment for other characteristics attenuated the strength of these associations, although the association between prolapse and infrequent orgasm remained statistically significant. In the final adjusted model, the odds of infrequent orgasm was increased >3 times for women with stage III–IV prolapse (p=0.02).
We repeated our analysis in the subgroup of 152 women who were recruited at the time of an annual gynecologic visit. Among the 117 who reported a current partner, a high PFDI summary score was associated with poorer sexual function, including decreased arousal (OR 1.16, 95% CI 1.05, 1.27) and infrequent orgasm (OR 1.14, 95% CI 1.03, 1.25). Associations between PFDI score and measures of libido and dyspareunia were not statistically significant (p=0.24, and p=0.40, respectively). A similar pattern was observed for the urinary, colorectal and prolpase scale scores.
We also conducted a subanalysis among the 78 women who did not report a current sexual partner. The majority (50, 64%) reported no sexual activity in the past month. A large proportion of women without a partner did not complete the sexual function questionnaire: scores for libido were missing in 7 cases (10%), arousal in 22 cases (28%) and orgasm in 24 cases (31%). This limited our power to detect characteristics associated with poor sexual function in this subset of women.
For the 78 women without a current sexual partner, increasing age was associated with decreased libido (OR 1.41 for 5-year increase in age, 95% confidence interval (CI) 1.13, 1.76), as well as decreased arousal (OR 1.3, 95% CI 1.0, 1.67), and infrequent orgasm (OR 1.33, 95% CI 1.03, 1.72). We were not able to identify any other characteristics associated with poor sexual function in this subset of the population. Specifically, among women without a partner, sexual function was not significantly associated with either pelvic floor symptoms or anatomic prolapse.
DISCUSSION
Sexual function is an important dimension of normal adult life and yet very little is known about the relationships between female sexuality and chronic health conditions, including pelvic floor disorders. This study demonstrates that pelvic floor symptoms are associated with low sexual arousal, infrequent orgasm, and dyspareunia in women over 40 years of age. Other studies have reported women with pelvic floor disorders often complain of sexual difficulties (2), but in some cases characterizations of female sexual function in prior studies were limited to a single measure of sexual well-being (4, 13). In this study, we used a validated measure of female sexual function and focused on symptoms related to the four recognized categories of female sexual dysfunction. Thus, this study provides a broad and detailed assessment of female sexual function in this population.
We found that increasing age, low educational attainment, menopause, vaginal dryness, symptoms of depression, and lack of “passionate love” for partner were all associated with infrequent sexual desire, decreased sexual arousal and infrequent orgasm. These are consistent with prior research (14–16). However, in contrast to other studies (16), these characteristics did not confound the association between pelvic floor symptoms and sexual function. In particular, while age and menopause were both associated with poorer sexual function in our study population, these characteristics did not account for the observed relationship between pelvic floor symptom severity and poorer sexual function. This may be because we restricted our research to women over 40 years of age. In this restricted population, age and menopause do not appear to be significant confounders in the relationship between pelvic floor symptoms and sexual complaints.
We intentionally designed this study to include participants with and without clinically significant pelvic floor disorders. This provided an opportunity to assess the impact of pelvic floor disorders across a spectrum of symptoms and severity. Prior research has been limited either to community samples (4) or women seeking gynecologic care (2, 3, 16, 17). In community samples, the women who report pelvic floor disorders are likely to have relatively mild symptoms and therefore the impact on sexual function might therefore be minimal in this setting. Indeed, research based on community samples has concluded that pelvic floor disorders do not have a significant impact on sexual function (4). On the other hand, women seeking treatment of pelvic floor disorders are likely to have severe symptoms, but the absence of a control group without any pelvic floor disorders in such populations limits inferences. Thus, a strength of our study is the inclusion of women without any pelvic floor symptoms and with normal pelvic organ support as well as women with severe symptoms and/or severe pelvic organ prolapse.
This study also included a structured physical examination to classify women with and without prolapse, allowing us to investigate sexual complaints across prolapse stage. We found that women with anatomic prolapse (Stage III–IV) were more likely to report infrequent orgasm but they were not at increased risk of other sexual problems. An important observation is that women with Stage II support were not more likely to report any sexual complaint than women with Stage 0 support. This suggests that the physical presence of stage II prolapse alone is not associated with sexual dysfunction. In contrast, women with prolapse symptoms (as reflected by a high score on the prolapse scale of the PFDI) were much more likely to report sexual complaints. Thus, we conclude that sexual function is worse in women with symptomatic prolapse but not women with asymptomatic prolapse.
Twenty-six percent of participants were not in a sexual relationship. Despite our efforts to evaluate sexual function among such women, we found that women without a current sexual partner were less likely to complete the sexual function questionnaire, leaving a relatively high proportion of missing data for this subgroup. Those women without a partner who did complete the questionnaires reported poorer sexual function than women with a partner. These observations led us to consider sexual function of women without a partner separately in our analysis and prevented us from reaching any strong conclusions about factors that influence sexual function in this subset of women. Although our findings may reflect shortcomings of the SPEQ questionnaire, we are not aware of any other sexual function questionnaires with proven validity among women without a current sexual partner. Thus, the assessment of sexual function in such populations remains a challenge.
A potential limitation of this research is the generalizability of our findings. Johns Hopkins is an academic institution and women seeking care at our clinical sites, even in outpatient suburban clinical offices, may not be representative of all American women. In addition, we recruited women from gynecologic and urogynecologic settings and it is possible that these populations differ by factors not measured in this research. However, our analysis of the subpopulation of women seeking preventative annual gynecologic care is reassuring. These findings mirror the significant association between pelvic floor symptoms and sexual complaints observed in the larger population, albeit with less statistical power. Thus, we conclude that our results were unlikely to have been biased by unmeasured differences between the populations of women with and without gynecologic or urogynecologic complaints. Another possible limitation of this research is that we used the Pelvic Floor Distress Inventory as a measure of pelvic floor symptom burden. This could result in misclassification of some participants. Finally, another potential limitation of this research pertains to the self-administration of questionnaires. This mode of administration may have limited the participation of some women, including those with limited literacy and those who are not fluent in written English. However, self-administered questionnaires are generally preferred for potentially embarrassing or stigmatized conditions and this mode of administration was therefore most appropriate for the content of this investigation.
Our study cannot determine whether the observed associations between pelvic floor disorders and sexual function are causal in nature. However, the disability associated with pelvic floor disorders might cause sexual dysfunction by affecting a woman’s body image or through an impact on her general wellbeing. Alternatively, the association between pelvic floor disorders and certain aspects of female sexual function may suggest a common etiology, such as pelvic muscle weakness or decreased urogenital sensation. Recent studies suggesting that surgical treatment for pelvic floor disorders may improve sexual function (18, 19), but the impact of treatment on sexual function has not been consistently reported (20). Thus, further research is needed to clarify whether certain treatments for pelvic floor disorders are more effective than others in restoring sexual function. Clinicians who care for women with pelvic floor disorders should be aware of this association and should specifically address sexual concerns with women seeking treatment of incontinence and prolapse.
Acknowledgments
Supported by K23HD045806 from the National Institute of Child Health and Human Development.
Footnotes
The authors have no potential conflicts of interest to disclose.
References
- 1.Basson R, Leiblum S, Brotto L, et al. Revised definitions of women’s sexual dysfunction. J Sex Med. 2004;1(1):40–8. doi: 10.1111/j.1743-6109.2004.10107.x. [DOI] [PubMed] [Google Scholar]
- 2.Handa VL, Harvey L, Cundiff GW, Siddique SA, Kjerulff KH. Sexual function among women with urinary incontinence and pelvic organ prolapse. Am J Obstet Gynecol. 2004;191(3):751–6. doi: 10.1016/j.ajog.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99(2):281–9. doi: 10.1016/s0029-7844(01)01727-6. [DOI] [PubMed] [Google Scholar]
- 4.Lukacz ES, Whitcomb EL, Lawrence JM, Nager CW, Contreras R, Luber KM. Are sexual activity and satisfaction affected by pelvic floor disorders? Analysis of a community-based survey. Am J Obstet Gynecol. 2007;197(1):88–6. doi: 10.1016/j.ajog.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 5.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7) Am J Obstet Gynecol. 2005;193(1):103–13. doi: 10.1016/j.ajog.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 7.Dennerstein L, Lehert P, Dudley E. Short scale to measure female sexuality: adapted from McCoy Female Sexuality Questionnaire. J Sex Marital Ther. 2001;27(4):339–51. doi: 10.1080/009262301317081098. [DOI] [PubMed] [Google Scholar]
- 8.Dennerstein L, Anderson-Hunt M, Dudley E. Evaluation of a short scale to assess female sexual functioning. J Sex Marital Ther. 2002;28(5):389–97. doi: 10.1080/00926230290001510. [DOI] [PubMed] [Google Scholar]
- 9.Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77 (Suppl 4):S42–8. doi: 10.1016/s0015-0282(02)03001-7. [DOI] [PubMed] [Google Scholar]
- 10.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Markush R, Favero R. Epidemiologic assessment of stressful life events, depressed mood, and psychological symptoms: a preliminary report. In: Dohrenwend B, Dohrenwend B, editors. Stressful Life Events: Their Nature and Effects. New York: Johns Wiley & Sons; 1973. [Google Scholar]
- 12.Ananth CV, Kleinbaum DG. Regression models for ordinal responses: a review of methods and applications. Int J Epidemiol. 1997;26(6):1323–33. doi: 10.1093/ije/26.6.1323. [DOI] [PubMed] [Google Scholar]
- 13.Rogers RG, Kammerer-Doak D, Darrow A, et al. Does sexual function change after surgery for stress urinary incontinence and/or pelvic organ prolapse? A multicenter prospective study. Am J Obstet Gynecol. 2006;195(5):e1–4. doi: 10.1016/j.ajog.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Gallicchio L, Schilling C, Tomic D, Miller SR, Zacur H, Flaws JA. Correlates of sexual functioning among mid-life women. Climacteric. 2007;10(2):132–42. doi: 10.1080/13697130601167956. [DOI] [PubMed] [Google Scholar]
- 15.Gracia CR, Freeman EW, Sammel MD, Lin H, Mogul M. Hormones and sexuality during transition to menopause. Obstet Gynecol. 2007;109(4):831–40. doi: 10.1097/01.AOG.0000258781.15142.0d. [DOI] [PubMed] [Google Scholar]
- 16.Weber AM, Walters MD, Schover LR, Mitchinson A. Sexual function in women with uterovaginal prolapse and urinary incontinence. Obstet Gynecol. 1995;85(4):483–7. doi: 10.1016/0029-7844(94)00434-F. [DOI] [PubMed] [Google Scholar]
- 17.Ozel B, White T, Urwitz-Lane R, Minaglia S. The impact of pelvic organ prolapse on sexual function in women with urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(1):14–7. doi: 10.1007/s00192-005-1327-0. [DOI] [PubMed] [Google Scholar]
- 18.Handa VL, Zyczynski HM, Brubaker L, et al. Sexual function before and after sacrocolpopexy for pelvic organ prolapse. Am J Obstet Gynecol. 2007;197(6):629–6. doi: 10.1016/j.ajog.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komesu YM, Rogers RG, Kammerer-Doak DN, Barber MD, Olsen AL. Posterior repair and sexual function. Am J Obstet Gynecol. 2007;197(1):101, e1–6. doi: 10.1016/j.ajog.2007.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2007;(3):CD004014. doi: 10.1002/14651858.CD004014.pub3. [DOI] [PubMed] [Google Scholar]


