Arterial calcification is one of the potential phenotypes of vascular remodeling and repair in atherosclerosis, diabetes, hyperphosphatemic renal failure, and aging (1-3). Calcification decreases arterial wall compliance (4). Furthermore, deposited crystalline apatite can activate macrophages, resulting in a proinflammatory phenotype. Consequently, arterial calcification localized to the intima is a potential biomarker of atherosclerosis, and is linked to disease progression and cardiovascular mortality, while arterial calcification localized primarily to the tunica media promotes mortality in diabetes and renal failure (4). In addition, calcific stenosis of the aortic valve is a prevalent and highly significant public health problem, and shares such pathophysiological features as ectopic chondro-osseous differentiation in common with arterial calcification (5).
In a study published in the current issue of Arteriosclerosis, Thrombosis, and Vascular Biology, Preusch et al report the effects of lineage-specific deletion of the glucocorticoid receptor (GR) in bone marrow-donor macrophages on chondro-osseous differentiation and calcification in dietary - induced atherosclerotic lesions in lethally irradiated, bone marrow transplant recipient, LDLR knockout mice (6). Arterial calcification appears to be an active and organized multicellular process, which is switched on by chondro-osseous differentiation of a variety of progenitors in the artery wall, and regulated in part by systemic influences. Such influences include the effects of calciotropic hormones and of mineral nucleation promoters and inhibitors (1-4). In the intralesional intercellular dialogue that drives vascular calcification, potential progenitors of calcifying osteoblastic and chondrocytic cells include not only pericytes and resident and recruited vascular stem cells, but also non-terminally differentiated, phenotypically plastic adventitial myofibroblasts and smooth muscle cells (SMCs). Significantly, the latter may undergo chondro-osseous trans-differentiation (1-5,7-12).
Intralesional mechanisms that drive chondro-osseous differentiation in arterial calcification include an excess of inducers of chondro-osseous commitment and maturation, such as BMP2, Pi generation and uptake by SMCs, and signaling stimulated by the wnt beta-catenin axis and by transglutaminase 2 (1-5,7-12). Conversely, intralesional deficiency of physiologic inhibitors of chondro-osseous differentiation also plays a role in arterial calcification, as exemplified by the linkage of spontaneous intra-arterial chondrogenesis and calcification with paucity of the BMP2 inhibitor and matrix calcification inhibitor MGP (13), or of the chondrogenic and matrix calcification inhibitor PPi (9,14).
Paracrine Effects of Macrophage-Driven Inflammation in Arterial Calcification
Certain paracrine effects of inflammation have been observed to promote vascular cell chondro-osseous differentiation and arterial calcification, particularly in atherosclerosis and diabetes (1,2,4,7). For example, certain pro-atherogenic oxidized lipids generated by endothelial cells and macrophages promote calcification by SMCs (15). In a model of diabetic vascular disease, adventitial inflammation mediated by TNFα turns on myofibroblast trans-differentiation via oxidative stress and wnt β-catenin signals (2,7). Inflammation-modulated increases in MMP activity and alterations in extracellular matrix collagen I, elastin (16,17), and osteopontin (3,8) can equally promote calcification of arterial extracellular matrix.
Addressing GR signaling in arterial calcification
Defining how endogenous and synthesized glucocorticoids of therapeuric importance may modulate vascular calcification is a challenging endeavor, since glucocorticoids exert anti-inflammatory effects on endothelial cells and phagocytes, are immunosuppressive (or immunomodulatory), regulate blood pressure, lipoprotein metabolism, and control of blood glucose; equally, they promote osteoporosis. Monocyte/macrophage lineage cells are particularly sensitive to the primary anti-inflammatory effects of GR signaling (18). Moreover, anti-inflammatory synthetic glucocorticoids, despite promoting hyperlipidemia and hypertension, can suppress macrophage accumulation and neointimal proliferation subsequent to certain experimental arterial injuries, (reviewed by Preusch et al), and can suppress experimental atherosclerosis under specific conditions (19). Nevertheless, mice receiving macrophage lineage GR-deficient bone marrow showed no gross change in atherosclerotic lesion size and lesion inflammation (6). In contrast, decrease in calcified areas of atherosclerotic lesions, as assessed by von Kossa staining, was observed; calcium deposition itself was not however specifically quantified.
Importantly, Preusch et al demonstrated that recipients transplanted with macrophage lineage GR-deficient bone marrow showed decreased expression not only of certain chondrocyte lineage-specific genes, but also of BMP2 and Msx2, in atherosclerotic lesions. The homeodomain transcription factor, Msx2, is involved in development and was recently implicated downstream of BMP2 in mediating ectopic osteoblastic differentiation during arterial calcification (2,7). An additional unexpected finding in the study of Preusch et al concerns the observation that GR ligand treatment of a macrophage cell line induced a paracrine stimulatory effect of macrophage-conditioned medium on Pi-stimulated calcification in cultured SMCs (6). GR signaling in pericytes and SMCs is known to promote chondro-osseous differentiation and calcification, an effect which is partly mediated by suppression of both MGP and osteopontin expression in pericytes (20).
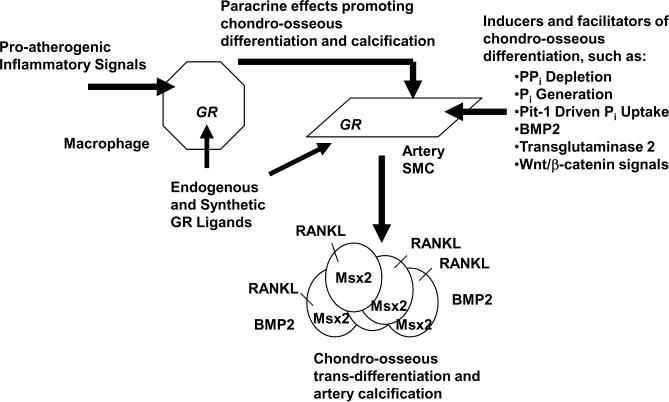
Figure 1 highlights the possible relationship between the above findings on GR signaling and the recognized intercellular dialogue between macrophages and SMCs; this dialogue promotes chondro-ossous trans-differentiation of SMCs and arterial calcification. One may speculate that paracrine effects of macrophage GR signaling that drive calcification may be partly mediated by GR signaling-induced apoptosis in monocyte/macrophages (21), especially as apoptosis can promote calcification by effects that include release of apoptotic bodies with precipitatation of hydroxyapatite (22). In addition, it is noteworthy that GR signaling in osteoblasts induces Receptor Activator for Nuclear Factor-κB Ligand (RANKL).
Figure 1. Where macrophage GR signaling fits in the intercellular dialogue that modulates arterial calcification.
The schematic illustrates how the findings of Preusch et al on macrophage GR effects, discussed in the text, relate with other paracrine effects of macrophages (e.g., pro-atherogenic oxidized lipid and TNFα generation) that modulate the capacity of SMCs (and potentially other phenotypically plastic intra-arterial chondro-osseous progentor cells) to drive arterial calcification. Also depicted here, and implicit to general understanding of how GR signaling can modulate arterial calcification, is the direct promotion of chondro-osseous trans-differentiation by GR signaling in pericytes and SMCs.
Macrophage GR Signaling regulates RANKL in arterial calcification
A striking finding of Preusch et al concerns the reduction in expression of RANKL in lesions, and which occurred in association with macrophage-specific GR knockdown in donor bone marrow (6). Novel attention to RANKL and the bone-vascular axis in arterial calcification has been stimulated by the seminal finding that osteoprotegrin (OPG) knockout mice develop spontaneous arterial calcification (23). In the intercellular dialogue between osteoblasts and osteoclasts in bone metabolism, the osteoblast product OPG inhibits signaling by RANKL that in turn activates osteoclastogenesis by RANK signaling in osteoclast precursors of the macrophage lineage (24). Significantly, SMCs, chondrocytes, and T lymphocytes express RANKL and OPG, and the members of the OPG/RANKL/RANK signaling axis are expressed in atherosclerotic arteries (25). Moreover, OPG treatment of atherogenic diet-fed LDLR knockout mice suppressed atherosclerotic lesion calcification (but not the extent of atherosclerotic lesions) in a recent study (26). One mechanism by which RANKL could promote arterial calcification is by promotion of MMP activity in SMCs (27), and in this regatrd, the data of Preusch et al add to growing evidence that the OPG:RANKL ratio can regulate arterial calcification.
Induction of RANKL by GR signaling, superimposed on GR suppression of osteoblast function, contributes to GR-induced osteoporosis (28). It has not been determined as yet whether macrophage lineage GR expression, (via modulation of osteoclast development and bone turnover), might influence longer-range bone-vascular axis communication loops in arterial calcification (e.g. by alterations in calciotropic hormones, by vascular cell expression of calcium-sensing, PTH and vitamin D receptors and the vitamin D receptor modulator TIF1α, and possibly by release of circulating regulators of mineral nucleation from bone). In addition, Preusch et al focused on ectopic chondrogenic differentiation, which appears central to atherosclerotic calcification in apoE knockout mice (6,25). Though chondrogenesis may be limited in uncalcified and calcified human atherosclerotic lesions (29), chondrocyte-specific gene expression is detected in some forms of calcification of human arterial tunica media (10). Nevertheless, ectopic osteoblastic differentiation is fundamental to other forms of arterial calcification in mice and humans (1-4).
Conclusions
GR signaling inhibits function of bone osteoblasts, promotes osteoclastogenesis and osteoporosis, but paradoxically, GR signaling promotes chondro-osseous differentiation and calcification by pericytes and SMCs. The discovery by Preusch et al of macrophage GR signaling in the dialogue between macrophages and the precursors of calcifying cells in atherosclerotic lesions (6) adds a paracrine twist to the GR story in arterial calcification (Figure 1), a contribution potentially mediated by GR signaling which can promote RANKL expression and apoptosis in cells of the macrophage lineage. How GRs and other nuclear receptors (e.g., vitamin D receptor, estrogen receptors and PPARγ) participate in the short-distance and long-distance communication loops between inflammation, oxidative stress, bone metabolism, and arterial calcification may hold the key to the development of novel therapeutics for inhibition of both osteoporosis and vascular calcification. For the moment, the work of Preusch et al encourages definitive investigation of the net effects of both endogenous and synthetic glucocorticoids on phenotypes of human vascular calcification.
Acknowledgements
None
Supported by the VA Research Service and NIH (HL077360, HL087252).
References
- 1.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 3.El-Abbadi M, Giachelli CM. Mechanisms of vascular calcification. Adv Chronic Kidney Dis. 2007;14:54–66. doi: 10.1053/j.ackd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 5.Moura LM, Maganti K, Puthumana JJ, Rocha-Gonçalves F, Rajamannan NM. New understanding about calcific aortic stenosis and opportunities for pharmacologic intervention. Curr Opin Cardiol. 2007;22:572–577. doi: 10.1097/HCO.0b013e3282f0dae6. [DOI] [PubMed] [Google Scholar]
- 6.Preusch MR, Rattazzi M, Albrecht C, Merle U, Tuckermann J, Schütz G, Blessing G, Zoppelaro G, Pauletto P, Krempien R, Rosenfeld ME, Katus HA, Bea F. Critical role of marophages in glucocorticoid driven vascular calcification in a mouse model of atherosclerosis. Arterisocelr Thromb Vasc Biol. 2008;22:572–577. doi: 10.1161/ATVBAHA.108.174128. [DOI] [PubMed] [Google Scholar]
- 7.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 8.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson K, Polewski M, van Etten D, Terkeltaub R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1-/- mice. Arterioscler Thromb Vasc Biol. 2005;25:686–691. doi: 10.1161/01.ATV.0000154774.71187.f0. [DOI] [PubMed] [Google Scholar]
- 10.Neven E, Dauwe S, De Broe ME, D'Haese PC, Persy V. Endochondral bone formation is involved in media calcification in rats and in men. Kidney Int. 2007;72:574–581. doi: 10.1038/sj.ki.5002353. [DOI] [PubMed] [Google Scholar]
- 11.Fukagawa M, Kazama JJ. The making of a bone in blood vessels: from the soft shell to the hard bone. Kidney Int. 2007;72:533–534. doi: 10.1038/sj.ki.5002440. [DOI] [PubMed] [Google Scholar]
- 12.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 13.El-Maadawy S, Kaartinen MT, Schinke T, Murshed M, Karsenty G, McKee MD. Cartilage formation and calcification in arteries of mice lacking matrix Gla protein. Connect Tissue Res. 2003;44(Suppl 1):272–278. [PubMed] [Google Scholar]
- 14.Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Höhne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nürnberg P. Mutations in ENPP1 are associated with 'idiopathic' infantile arterial calcification. Nat Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 15.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 16.Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation. 2004;110:3480–3487. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:1510–6. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 18.Joyce DA, Gimblett G, Steer JH. Targets of glucocorticoid action on TNF-alpha release by macrophages. Inflamm Res. 2001;50:337–340. doi: 10.1007/PL00012387. [DOI] [PubMed] [Google Scholar]
- 19.Asai K, Funaki C, Hayashi T, Yamada K, Naito M, Kuzuya M, Yoshida F, Yoshimine N, Kuzuya F. Dexamethasone-induced suppression of aortic atherosclerosis in cholesterol-fed rabbits. Possible mechanisms. Arterioscler Thromb. 1993;13:892–899. doi: 10.1161/01.atv.13.6.892. [DOI] [PubMed] [Google Scholar]
- 20.Kirton JP, Wilkinson FL, Canfield AE, Alexander MY. Dexamethasone downregulates calcification-inhibitor molecules and accelerates osteogenic differentiation of vascular pericytes: implications for vascular calcification. Circ Res. 2006;98:1264–1272. doi: 10.1161/01.RES.0000223056.68892.8b. [DOI] [PubMed] [Google Scholar]
- 21.Amsterdam A, Sasson R. The anti-inflammatory action of glucocorticoids is mediated by cell type specific regulation of apoptosis. Mol Cell Endocrinol. 2002;189:1–9. doi: 10.1016/s0303-7207(01)00722-5. [DOI] [PubMed] [Google Scholar]
- 22.Kirsch T, Wang W, Pfander D. Functional differences between growth plate apoptotic bodies and matrix vesicles. J Bone Miner Res. 2003;18:1872–1881. doi: 10.1359/jbmr.2003.18.10.1872. [DOI] [PubMed] [Google Scholar]
- 23.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, Schwartz SM, Giachelli CM, Rosenfeld ME. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE-/- mice. Arterioscler Thromb Vasc Biol. 2006;26:2117–2124. doi: 10.1161/01.ATV.0000236428.91125.e6. [DOI] [PubMed] [Google Scholar]
- 26.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation. 2008;117:411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaden JJ, Dempfle CE, Kiliç R, Sarikoç A, Hagl S, Lang S, Brueckmann M, Borggrefe M. Influence of receptor activator of nuclear factor kappa B on human aortic valve myofibroblasts. Exp Mol Pathol. 2005;78:36–40. doi: 10.1016/j.yexmp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Rehman Q, Lane NE. Effect of glucocorticoids on bone density. Med Pediatr Oncol. 2003;41:212–6. doi: 10.1002/mpo.10339. [DOI] [PubMed] [Google Scholar]
- 29.Aigner T, Neureiter D, Câmpean V, Soder S, Amann K. Expression of cartilage-specific markers in calcified and non-calcified atherosclerotic lesions. Atherosclerosis. 2008;196:37–41. doi: 10.1016/j.atherosclerosis.2007.01.020. [DOI] [PubMed] [Google Scholar]