Abstract
The occurrence of cardiovascular dysfunction in sepsis is associated with a significantly increased mortality rate of 70% to 90% compared with 20% in septic patients without cardiovascular impairment. Thus, rectification or blockade of myocardial depressant factors should partly ameliorate sepsis progression. Heat shock protein 20 (Hsp20) has been shown to enhance myocardial contractile function and protect against doxorubicin-induced cardiotoxicity. To investigate the possible role of Hsp20 in sepsis-mediated cardiac injury, we first examined the expression profiles of five major Hsps in response to lipopolysaccharide (LPS) challenge, and observed that only the expression of Hsp20 was downregulated in LPS-treated myocardium, suggesting that this decrease might be one of mechanisms contributing to LPS-induced cardiovascular defects. Further studies using loss-of-function and gain-of function approaches in adult rat cardiomyocytes verified that reduced Hsp20 levels were indeed correlated with the impaired contractile function. In fact, overexpression of Hsp20 significantly enhanced cardiomyocyte contractility upon LPS treatment. Moreover, after administration of LPS (25μg/g) in vivo, Hsp20 transgenic mice (10-fold overexpression) displayed: 1) an improvement in myocardial function; 2) reduced the degree of cardiac apoptosis; and 3) decreased NF-κB activity, accompanied with reduced myocardial cytokines IL-1β and TNF-α production, compared to the LPS-treated non-transgenic littermate controls. Thus, the increases in Hsp20 levels can protect against LPS-induced cardiac apoptosis and dysfunction, associated with inhibition of NF-κB activity, suggesting that Hsp20 may be a new therapeutic agent for the treatment of sepsis.
Keywords: small heat shock protein, sepsis, myocardial function, NF-κB
Introduction
Sepsis is one of the main consequences of injury and disease and is responsible for ~250, 000 deaths annually in the United States. Furthermore, the incidence of sepsis and sepsis-related deaths is increasing by 1.5% per year [1–3]. One of the causes of death among patients afflicted with sepsis is severe hypotension associated with a decrease in cardiac output [3, 4]. Indeed, accumulating evidence has indicated that myocardial depression is a common feature of sepsis in both patients and experimental models of lipopolysacharide(LPS)-induced endotoxemia [3–8]. Although the precise mechanisms that cause myocardial dysfunction during sepsis remain elusive, a multitude of studies demonstrate that the overproduction of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β), as well as their secretion by cardiomyocytes act as early signaling events which lead to sepsis-related cardiac dysfunction [3, 4]. Furthermore, it has been well documented that nuclear factor-κB (NF-κB) modulates these proinflammatory cytokine synthesis during sepsis [7–9]. Accordingly, inhibition of the NF-κB signaling pathway is believed to be beneficial in the setting of septic shock [10, 11].
The expression of heat-shock proteins (Hsps) has recently become a major interest of clinicians because Hsps have been suggested to be cytoprotective in a variety of clinical settings such as ischemia/reperfusion and shock [12]. Stimulation with LPS was found to increase the expression of Hsp70 and Hsp60, but not Hsp27 and αB-crystallin in animal’s spleen, lung, and heart [13–15]. A prior hyperthermic treatment, which induces Hsp synthesis, leads to a significant improvement in the survival rate after experimentally induced endotoxic or septic shock [16, 17]. These beneficial effects appear to be mediated through inhibition of cytokine pathways. Indeed, overexpression of Hsp70 in the heart confers resistance to endotoxemic myocardial depression via inhibition of NF-κB activation [18]. Furthermore, Hsp expression attenuates plasma concentrations of the proinflammatory cytokines, such as TNF-α and IL-1βin vitro and in vivo, which appears to correlate with improved survival from septic insult [19]. We have recently found that a new member of the Hsp family, Hsp20, protects against ischemia/reperfusion-induced cardiac injury [20], and elevated Hsp20 in cardiomyocytes renders protection against chronic β–agonist- and doxorubicin-triggered apoptosis in vitro and in vivo, leading to improved myocardial function [21–23]. However, the potential benefits of Hsp20 action on endotoxin-induced cardiac injury and its underlying mechanism(s) are largely unknown.
To address the role of Hsp20 in the myocardial depression occurred in endotoxin-induced septic shock, we employed in vitro adenoviral-mediated gene delivery and in vivo cardiac-specific overexpression approaches. We found that Hsp20 overexpression significantly attenuated LPS-induced cardiac dysfunction and reduced the degree of apoptosis, relative to controls. In addition, Hsp20-TG hearts displayed an inhibition of LPS-triggered NF-κB activation, leading to reduced levels of the proinflammatory cytokines IL-1β and TNF-α, compared with non-transgenic controls. Thus, increases in Hsp20 levels protect the heart against LPS challenge, through inhibition of the NF-κB signaling pathway.
Methods and Materials
Adult Rat Ventricular Myocyte Isolation and Adenovirus-Mediated Gene Transfer
Rat ventricular myocytes were isolated from adult male Sprague-Dawley rats (6–8-wk old) and plated on laminin-coated glass coverslips or dishes, as described previously [24]. Recombinant adenoviruses Ad.GFP encoding GFP, Ad.Hsp20 containing both Hsp20 and GFP, and Ad.Hsp20-AS containing both antisense Hsp20 and GFP were generated by using the AdEasy-1 expression system, as described previously [21, 22]. Cardiomyocytes seeded on coverslips or dishes were infected with adenovirus in diluted media, at a multiplicity of infection of 500, for 2 h before the addition of a suitable volume of culture media containing 1 μg/ml of lipopolysacharide (LPS, E.coli 055:B5, Sigma-Aldrich).
Contractile Parameter Measurements
LPS-treated myocytes that adhered to the coverslips were bathed in temperature (37°C) -equilibrated KHB containing 1 mM Ca for 20 min. The myocyte suspension was then placed in a Plexiglas chamber, which was positioned on the stage of an inverted epifluorescence microscope (Diaphot 200; Nikon). Myocyte contraction was field-stimulated by a Grass S5 stimulator (0.5 Hz, square waves; Grass Technologies, An Astro-Med, Inc.), and contractions were videotaped and digitized on a computer. A video edge motion detector (Crescent Electronics, Windsor, ON, Canada) was used to measure myocyte length and cell shortening, from which the percent fractional shortening (% FS) and maximal rates of contraction and relaxation (±dL/dt) were calculated. All data were analyzed using Felix 1.1 (Photon Technology International, Birmingham, NJ, USA) and IonWizard software (IonOptix Corp., Milton, MA, USA).
Animal Preparation and LPS treatment
All protocols conformed to the Guidelines for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health, and were approved by the University of Cincinnati Animal Care and Use Committee. Generation of cardiac-specific overexpressed Hsp20 mice has been previously described [20]. Male non-transgenic (NTG) and transgenic (TG) mice, inbred on a FVB/N background, were studied at 8 to 10 weeks. Both NTG and TG mice were randomly assigned to either the vehicle group or the LPS-treated group. LPS was administered by intraperitoneal (ip) injection at one dose of 25μg/g (100μl). NTG mice received injections of saline to a comparable volume (100μl).
Determination of cardiac function after LPS challenge
Left ventricular (LV) function variables were assessed by transthoracic echocardiography, which was performed at 6 hours after LPS administration using the iE33 Ultrasound System (Phillips) with a 15-MHz probe [25]. After the induction of light general anesthesia, hearts were imaged two-dimensionally in long-axis view at the level of the greatest LV diameter. The LV end-diastolic diameters (LVEDd) and LV end-systolic diameters (LVESd) were measured from M-mode recordings according to the leading-edge method. LV ejection fraction (LVEF) was calculated as: LVEF (%) = [left ventricular end-diastolic dimension (LVEDd) 3 − left ventricular end-systolic dimension (LVESd) 3]/(LVEDd) 3 × 100. Data from three to five consecutive selected cardiac cycles were analyzed and averaged.
Electrophoretic Mobility Shift Assay
Electrophoretic mobility shift assays (EMSAs) were performed as previously described [26, 27]. An oligonucleotide probe corresponding to the NF-κB consensus sequence (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) was labeled with γ-[32P]ATP using T4 polynucleotide kinase and purified in Bio-Spin chromatography columns (Bio-Rad). Densitometric analysis was performed using ImageQuant (Molecular Dynamics).
Measurement of Myocardial Cytokines and Serum Lactate Dehydrogenase (LDH) levels
Myocardial levels of TNF-α and IL-1β were evaluated by commercially available solid-phase sandwich ELISA kits (R&D Systems), using the protocol recommended by the manufacturer. Serum LDH levels were determined using an in vitro Toxicology Assay Kit (Sigma) and expressed as units per ml (U/ml).
Measurement of Cell Viability and Apoptosis
The cardiomyocyte viability was determined by the CellTiter 96 AQueous One Solution Cell Proliferation Assay Kit (Promega), as the manufacturer’s instructions. Cardiomyocyte nuclear fragmentation was determined by Hoechst staining and visualized under a fluorescent microscope, as previous described [21]. In situ DNA fragmentation was assessed using the DeadEnd Fluorometric TUNEL system (Promega), followed by staining with an anti–α-sarcomeric actin antibody (Sigma-Aldrich) and 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) [22]. TUNEL-positive (green) nuclei were counted from 10 randomly-chosen microscopic fields of the midventricular section and were expressed as a percentage of total nuclei (both blue and green staining nuclei) in the same fields. For more accurate quantification of apoptosis in the LPS-treated hearts, DNA fragmentation was determined by a cell-death-detection ELISA kit (Roche Applied Science), which measures the content of cytosolic mono- and oligo-nucleosomes (180 base pair nucleotides or multiples) by employing a sandwich enzyme immunoassay technique. Results were normalized to the standard provided in the kit and expressed as a fold increase over control.
Determination of Caspase-3 Activation
Heart samples were homogenized with ice-cold lysis buffer as previously described [22]. After centrifuged at 14,000 × g for 10 min, the supernatants were used for caspase-3 activity assay. Briefly, 100 μg of proteins were diluted with assay buffer and incubated at 25° C with the colorimetric substrates (Biomol, Plymouth, PA): Ac-DEVD-pNA, 200 μM final concentration, in 96-well microtiter plates. Cleavage of the p-nitroaniline (pNA) dye from the peptide substrate was determined by the measure of absorbance of pNA at 405 nm in a microplate reader.
Immunoblotting
Protein samples from heart homogenates or cell lysis were fractionated by 10% or 12% SDS-PAGE, as described in detail elsewhere [22, 23]. Binding of the primary antibody was detected by peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Amersham), and bands were quantified with densitometry. The source of the antibodies and dilutions used were as follows: Rabbit anti-Hsp70 (1:1000 dilution), mouse anti-Hsp60 (1:4000 dilution), mouse anti-Hsp27 (1:1000 dilution) and rabbit anti-αB-crystallin (1:1000 dilution) (Affinity BioReagents), mouse Hsp20 antibodies (1:5000 dilution, Research Diagnostics Inc.), rabbit anti-IκB-α (1:200 dilution), and mouse anti-p- IκB-α (1:200 dilution) (Santa Cruz Bitotech. Inc). α-actin (1:1000 dilution, Sigma) was probed in each membrane as a loading control.
Statistical Analysis
All values are expressed as mean ± SD. Statistical analysis was performed using a 2-tailed Student t-test for unpaired observations and ANOVA followed by the Bonferroni post hoc test for multiple comparisons (Systat 11). P<0.05 was considered statistically significant.
Results
Expression Patterns of Hsps in Murine Hearts upon LPS Treatment
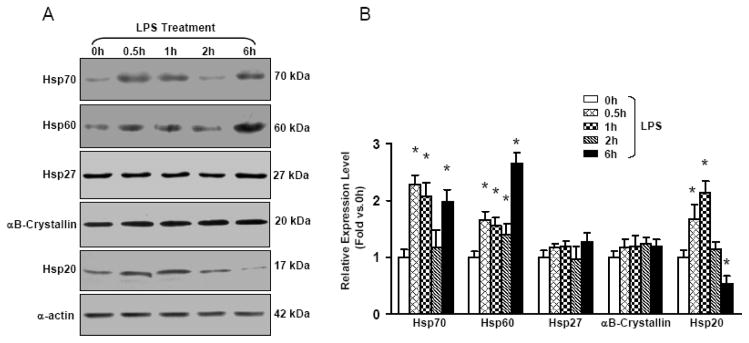
To elucidate the functional significance of Hsp20 in the heart upon LPS challenge, we first determined the expression profiles of the five major Hsps (Hsp70, Hsp60, Hsp27, αB-crystallin, and Hsp20) at different time points following LPS insults (25 μg/g, i.p. injection). Expression levels of both Hsp70 and Hsp60 were strongly upregulated in the heart at 30 min, 1 h and 6 h after LPS administration, as demonstrated by Western blot analysis (Figure 1). Hsp27 and αB-crystallin levels were not significantly induced in the heart upon LPS stimulation. Interestingly, Hsp20 expression was transiently increased at 30 min and 1 h, returned to basal levels at 2 h, and significantly decreased by ~50% at 6 h after LPS injection. These results indicate that the expression patterns of various Hsps are differentially regulated in the heart upon LPS challenge, suggesting that these Hsps may be instrumental in providing cardioprotection under these stress conditions.
Figure 1.
Time course of major Hsps’ expression in the mouse heart after i.p. administration of LPS(25 μg/g). (A) At the indicated intervals, mouse hearts were excised and homogenized to assess major Hsps’ expression by Western blot analysis. (B) The quantitative results from the Western-blots. α-actin was used as an internal control (n = 4 hearts for each time point, *P < 0.05 versus control 0 h ).
Knock-Down of Endogenous Hsp20 Decreases Contractility in Adult Rat Cardiomyocytes
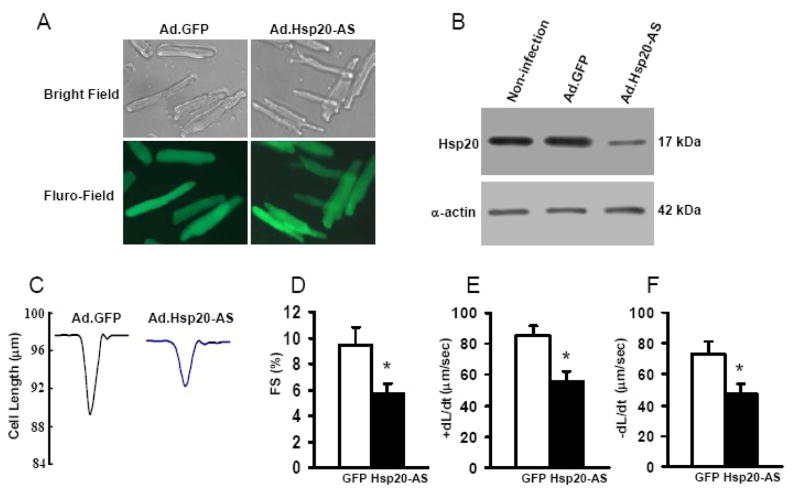
Since cardiac Hsp20 protein levels were specifically down-regulated at 6 h after LPS treatment, we next examined whether a drop of Hsp20 protein expression was associated with LPS-induced myocardial dysfunction (SIMD). Recombinant adenoviral vector Ad.Hsp20-AS, which contains antisense cDNA of Hsp20, was generated to knock-down the endogenous Hsp20. Ventricular myocytes were isolated from adult rat hearts and infected with Ad.GFP or Ad.Hsp20-AS for 48 h. The infection efficiency reached more than 95%, and there were no apparent morphological alterations or differences between the Ad.Hsp20-AS- and Ad.GFP-infected myocytes (Figure 2A). Endogenous Hsp20 protein levels were decreased by ~40% using this approach (Figure 2B). As shown in Figure 2C–F, down-regulation of Hsp20 significantly decreased the fractional shortening (FS%: by 40%), as well as the maximal rates of contraction (+dL/dt: by 34%) and relaxation (−dL/dt: by 36%). These results indicate that downregulation of Hsp20 is correlated with depressed cardiac contractility.
Figure 2.
Effect of decreased Hsp20 expression on cardiaomyocyte contractility.(A) Photomicrographs of Ad.Hsp20-AS-infected or Ad.GFP-infected adult rat cardiomyocytes (500 MOI)) were taken after infection for 48 h. (B) Expression of Hsp20 in infected adult rat cardiomyocytes. The endogenous Hsp20 protein was down-regulated in Ad.Hsp20-AS-infected myocytes by 40% compared with Ad.GFP-infected myocytes. α-Actin was used as a loading control. (C) Representative traces of cardiomyocyte mechanics in Ad.GFP-infected and Ad.Hsp20-AS-infected adult rat cardiomyocytes. (D–F) Decreased myocyte percent fractional shortening (FS%; B) and decreased maximal rates of contraction and relaxation (±dL/dt; E and F) in Ad.Hsp20-AS-infected myocytes compared with Ad.GFP-infected cells. n = 5 hearts for each group and 15–25 myocytes/heart. Values are means ± SD. *P < 0.05.
Hsp20 Overexpression Improves Contractility and Cell Viability in LPS-Treated Rat Cardiomyocytes
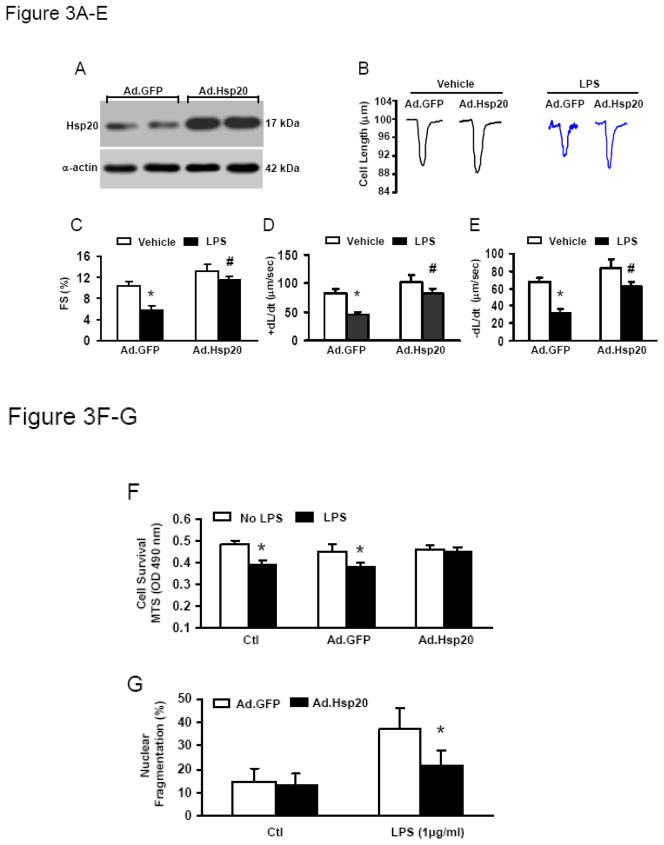
To further investigate whether increased Hsp20 levels could prevent LPS-induced detrimental effects on cardiomyocyte contractility, we infected adult rat cardiomyocytes with Ad.Hsp20 for 24 h, and then treated with LPS (1μg/ml) overnight. Infection with Ad.Hsp20 resulted in 2.5-fold increases in total Hsp20 protein levels (Figure 3A). LPS exposure significantly decreased contractility in the Ad.GFP group (FS%: by 47%, +dL/dt: by 45%, and −dL/dt: by 53%) (Figure 3B–E). However, Hsp20 overexpression greatly attenuated the reduction of these contractile parameters (Figure 3B–E), indicating that increased Hsp20 protein levels could improve LPS-induced contractile defects.
Figure 3.
Effect of increased Hsp20 expression on cardiaomyocyte contractility and viability after LPS treatment. (A) Expression of Hsp20 in infected adult rat cardiomyocytes. The total Hsp20 protein was up-regulated in Ad.Hsp20- infected myocytes by 2.5 fold compared with Ad.GFP-infected myocytes. α-Actin was used as a loading control. (B) Representative traces of cardiomyocyte mechanics in Ad.GFP-infected and Ad.Hsp20-infected adult rat cardiomyocytes with or without LPS treatment (1 μg/ml). (C–E) LPS treatment resulted in a marked decrease in FS% (C) and maximal rates of contraction and relaxation (±dL/dt; D and E) in Ad.GFP-infected myocytes, whereas these decreases were greatly attenuated in Ad.Hsp20-infected myocytes (C–E). LPS-induced cell death (F) and apoptosis (G) were significantly reduced in Ad.Hsp20-infected cardiomyocytes. n = 6 hearts for each group and 15–25 myocytes/heart for contractility measurement. For assessment of cell viability and apoptosis, similar results were observed in three additional, independent experiments. Values are means ± SD. *P < 0.001, # P < 0.05 vs. vehicle.
In addition, we observed that LPS significantly induced cell death in both control and GFP-groups, as measured by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS)-based cell incorporation (Figure 3F). On the contrary, Ad.Hsp20-cardiomyocytes were resistant to LPS-induced cell death (Figure 3F). Accordingly, the degree of LPS-induced apoptosis was greatly reduced in Ad.Hsp20-cardiomyocytes, compared with the Ad.GFP-group (Figure 3G).
Cardiac-Specific Overexpression of Hsp20 Attenuates LPS-Induced Myocardial Dysfunction
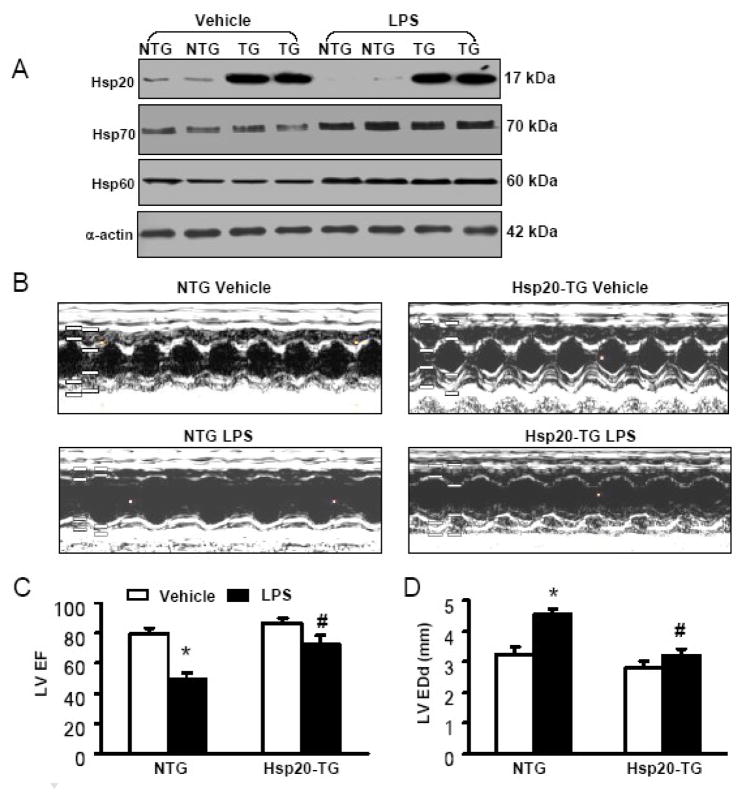
To determine whether Hsp20 overexpression prevents endotoxin-triggered cardiac dysfunction in vivo, TG mice overexpressing Hsp20, under the control of the cardiac-specific α-myosin heavy chain promoter, were analyzed. TG hearts showed a 10-fold increase in total (transgenic and endogenous) Hsp20 protein compared with non-transgenic (NTG) mouse hearts (Figure 4A). Eight-week-old NTG and TG mice were injected i.p. with LPS at one dose of 25μg/g or a comparable volume of saline (100 μl) as a control. Six hours after LPS injection, cardiac function was assessed by echocardiography in mice. Compared with vehicle-treated animals, the administration of LPS caused a 39% increase in left ventricle end-diastolic diameters (LVEDD) and a 43% decrease in LV ejection fraction (LVEF) (Figure 4B–D), indicating depressed myocardial contractility in NTG mice after LPS challenge. However, Hsp20 mice showed only a slight increase (14%) in LVEDD and much less decrease (16%) in LVEF after LPS injection (Figure 4B–D), demonstrating that cardiac-specific overexpression of Hsp20 attenuated LPS-induced myocardial dysfunction. Of note, while LPS treatment significantly upregulated the expression of Hsp70 and Hsp60 in the murine heart, there were no differences in the levels of Hsp70 and Hsp60 between NTG and Hsp20-TG hearts (Figure 4A). Therefore, it is not likely that Hsp70 and Hsp60 play some compensatory roles in improvement of cardiac function exerted by Hsp20 overexpression upon LPS challenge.
Figure 4.
Overexpression of Hsp20 in vivo enhances cardiac function (A and B) and improves LPS-induced myocardial dysfunction. (A) 10-fold overexpression of Hsp20 protein was observed in Hsp20-hearts. LPS treatment (25μg/g) for 6 h resulted in reduced levels of Hsp20 in Non-transgenic (NTG) hearts, whereas no significant alteration was observed in Hsp20-hearts. In addition, Hsp20 overexpression did not alter the levels of Hsp70 and Hsp60 in the heart under either control conditions or LPS treatment for 6 h._ (B) Representative M-mode echocardiograms are shown in NTG mice and Hsp20 mice with vehicle or LPS treatment for 6 h. (C) Left ventricular ejection fraction (LVEF) and (D) left ventricular end diastolic diameters (LVEDD) were dramatically depressed in NTG-hearts treated with LPS. By contrast, LPS-induced cardiac depression was significantly attenuated in Hsp20-hearts (C and D). *P < 0.001, # P < 0.05 verse vehicle. n=6 for each group.
Overexpression of Hsp20 Suppresses LPS-Induced Inflammatory Cytokines Production
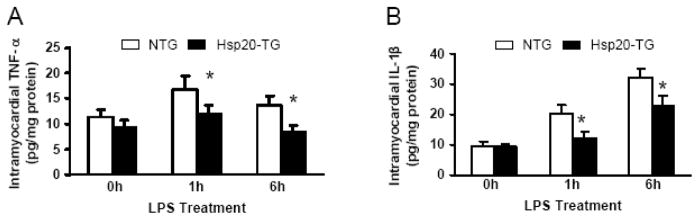
Since enhanced formation of proinflammatory cytokines has been implicated as the major mediator of myocardial depression in septic shock [2, 3], we evaluated myocardial TNF-α and IL-1β production in LPS-treated NTG and Hsp20 mice using an ELISA method. The TNF-α and IL-1β levels were significantly increased in the myocardium from the LPS-treated NTG mice at either 1 h or 6 h after LPS administration, compared with pre-treated groups (0 h) (Figure 5A and B). However, the induction of TNF-α and IL-1β in the LPS-treated Hsp20 mice was prevented in comparison with those of the LPS-treated NTG mice (Figure 5A and B). These results indicate that the benefits of increased Hsp20 levels could be at least partly attributed to reduction of LPS-stimulated myocardial proinflammatory cytokine production.
Figure 5.
Myocardial TNF-α (A) and IL-1β (B) protein production after LPS challenge. NTG and Hsp20-TG mice were administered an injection of LPS i.p. (25μg/g). Values represent mean ± SD from 3 different animals at each time point (*P<0.05 vs NTG).
Hsp20 Protects Against LPS-Induced NF-κB Activation
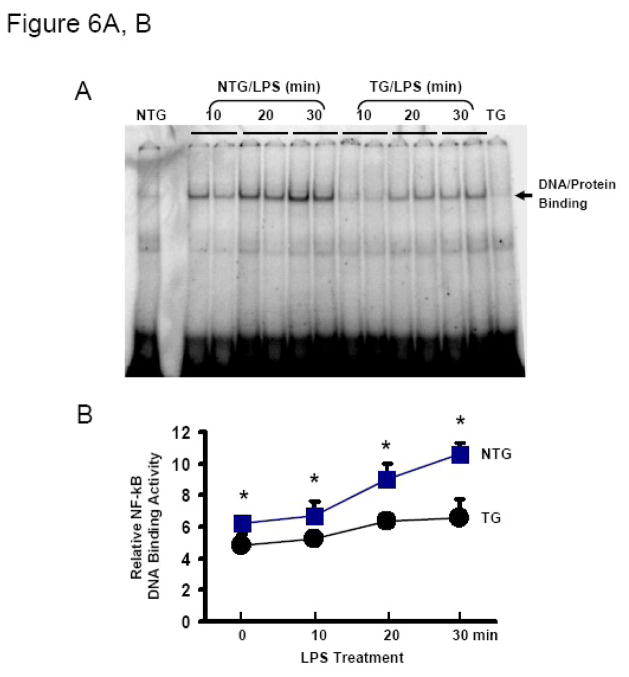
Since LPS is known to activate NF-κB leading to stimulation of proinflammatory cytokine expression in a variety of cells [28, 29], we sought to determine whether Hsp20 inhibits LPS-induced NF-κB activation in the myocardium. As such, we examined NF-κB DNA binding activity in myocardial nuclear lysates from LPS-treated mice at different time points. As shown in Figure 6A, NF-κB activity was obviously increased in NTG hearts following administration of LPS, whereas only slightly increased in Hsp20-hearts upon LPS challenge. Quantification of DNA/protein binding density demonstrated that NF-κB activity was significantly reduced in the myocardium of LPS-treated Hsp20 mice as compared to NTG animals treated with LPS (Figure 6B).
Figure 6.
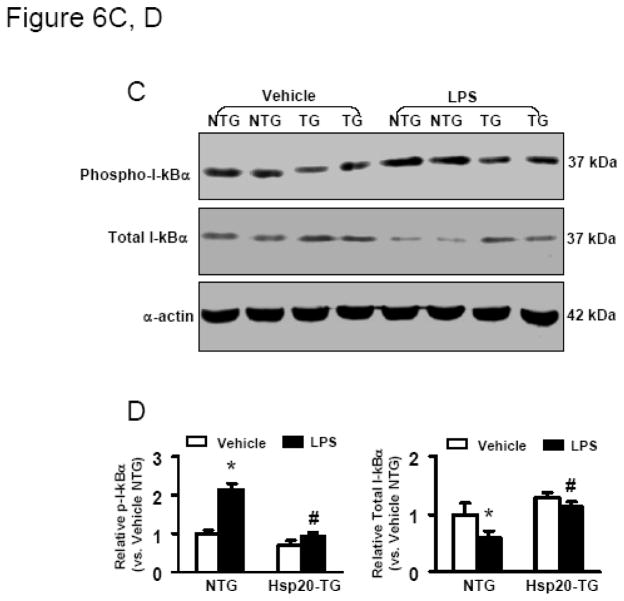
Cardiac-specific expression of Hsp20 inhibits NF-κB activation. (A) Representative autoradiographs of an EMSA for NF-κB, and (B) Image analysis of activity of NF-κB. Results are representative of 3 separate time course experiments. *P < 0.05 vs. NTG mice. C) Representative Western blot analysis of phosphorylated and total IκBα in NTG and Hsp20-TG mice with or without LPS treatment (25μg/g) for 30 min. α-Actin was used as a loading control. D) Image analysis of phosphorylated and total IκBα determined by densitometry. Fold increase was calculated vs. respective NTG value (vehicle) set to 1.0. *P < 0.001, # P < 0.05 vs. vehicle mice, n=4 per group.
Because degradation of IκBα by a phosphorylation- and ubiquitination-dependent pathways represent an important event for NF-κB nuclear translocation and the initiation of transcription [29], we further determined the LPS-induced phosphorylation and degradation of IκBα in NTG and Hsp20 mice. LPS treatment for 30 min led to a marked increase in phosphorylation of IκBα in the myocardium of NTG mice, consequently, the content of IκBα was dramatically decreased (Figure 6C, D), consistent with previous reports [7, 10]. However, the levels of IκBα phosphorylation were slightly increased in the myocardium of Hsp20 mice following LPS treatment (Figure 6C, D), indicating that cardiac-specific expression of Hsp20 inhibited LPS-triggered NF-κB complex dissociation/activation.
Overexpression of Hsp20 Reduces LPS-Induced Myocardial Apoptosis and Caspase-3 Activity
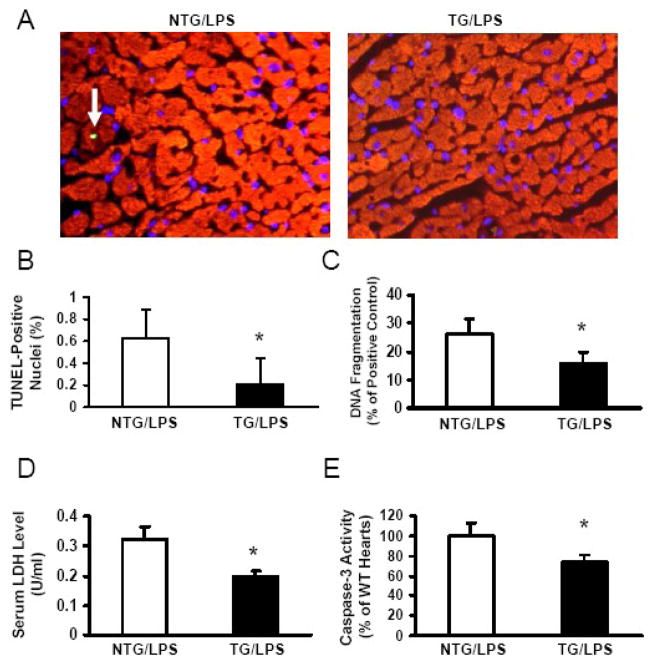
Recent studies have shown that endotoxin-induced activation of apoptosis pathways may directly induce myocardial dysfunction, which can be improved by administration of apoptosis inhibitors [30, 31]. To investigate whether cardioprotective effects of Hsp20 against LPS are associated with inhibition of myocardial apoptosis, we analyzed myocardial sections for apoptosis by TUNEL staining. Few apoptotic cells were observed in the myocardium of vehicle-treated NTG mice and Hsp20 mice (data not shown). At 6 h after LPS administration, extensive apoptotic nuclei (~6/per 1000 nuclei) were observed in the myocardium of LPS-treated NTG mice. In contrast, much less TUNEL-positive nuclei (~2/per 1000 nuclei) were present in the hearts of LPS-treated Hsp20 mice (Figure 7A and B). These results were further confirmed by myocardial DNA fragmentation, measured by an ELISA method, which was reduced by 40% in LPS-treated Hsp20 hearts, compared with NTG controls (Figure 7C). LPS-induced cardiac injury was also assessed by measuring lactate dehydrogenase (LDH) release, and we observed that serum LDH levels were markedly reduced by 40% in Hsp20 hearts relative to NTG controls (Figure 7D). In addition, Hsp20-hearts displayed a significant reduction of caspase-3 activity upon LPS stimulation, compared with that of LPS-treated NTG hearts (Figure 7E), suggesting that improvement of myocardial function in LPS-challenged Hsp20 mice is associated with the inhibition of caspase-3 activity.
Figure 7.
Cardiac-specific expression of Hsp20 suppresses LPS-induced myocardial apoptosis and caspase-3 activity. (A) Triple-staining with anti–α-sarcomeric actin antibody (red), DAPI (blue), and TUNEL (green, see arrow) to determine apoptosis in the myocardium of LPS-treated Hsp20 mice compared with LPS-treated NTG mice. (B) LPS-treated Hsp20 TG hearts exhibited significantly reduced number of TUNEL-positive (green fluorescence) nuclei (n=5, with 3 sections per heart). *P<0.05, vs LPS-treated NTG hearts. (C) The extent of apoptosis was further assessed using an ELISA kit, which measures DNA fragmentation. *P<0.05 vs LPS-treated NTG hearts (n=6). (D) Serum LDH levels were significantly reduced in Hsp20-TG mice after LPS treatment for 6h, compared with those of NTG mice (n=5, *P<0.05). (E) Caspase-3 activity measurement in the myocardium of LPS-treated NTG and Hsp20-TG mice. Fold change was calculated vs. NTG value set to 100%. * P < 0.05 vs. LPS-treated NTG mice, n=6 per group.
Discussion
Myocardial depression is a major clinical abnormality in patients with sepsis [2, 3]. Importantly, a 50–70% increase in sepsis-associated mortality has been observed in septic patients with myocardial defects, as compared to those patients without cardiovascular impairment [2]. Currently, β-agonists are commonly prescribed to augment cardiac output and thereby maintain tissue perfusion pressure and oxygen delivery. However, β-adrenergic signal transduction has been shown to be impaired during severe sepsis in humans [32–34]. Thus, it is necessary to identify potential clinical alternatives for management of myocardial dysfunction associated with sepsis. Interestingly, exposure of cardiomyocytes with β-agonist significantly upregulated Hsp20 expression, accompanied with enhanced cardiac contractility [21, 35]. These observations suggest that Hsp20 may prevent sepsis-mediated cardiac dysfunction. Indeed, the present study further demonstrates that Hsp20 was down-regulated in the heart following LPS treatment (Figure 1), leading to contractile dysfunction (Figure 2). On the contrary, overexpression of Hsp20 attenuated LPS-induced cardiac dysfunction (Figure 3 and 4). Supportively, several studies have shown that the abundance of Hsp20 was mostly increased in the rat heart proteome after exercise training [36, 37], a well-known model of dampening the detrimental effects of sepsis [38, 39]. Taken together, these results indicate that upregulation of Hsp20 may be beneficial for sepsis patients. Therefore, it would be very important to identify any pharmacological agent that selectively upregulates Hsp20 expression. Recently, we reported that injection of cholesterol-modified antagomiR-320 increased Hsp20 expression in the murine heart, which resulted in protection against ischemia-reperfusion-induced cardiac injury [40]. Future studies will be helpful to investigate the therapeutic role for antagomiR-320 in sepsis.
There are two main mechanisms underlying the cardioprotection of Hsp20 against LPS- induced myocardial dysfunction. The first mechanism is through the inhibition of NF-κB activation, which controls production of proinflammatory cytokines. NF-κB is usually sequestered as an inactive state in the cytoplasm through its interaction with the inhibitory κB (IκB) [29]. Under stress conditions, IκB is phosphorylated by IκB kinase (IKK), which leads to degradation of IκB and disruption of the NF-κB/IκB complex [29]. The dissociated NF-κB subsequently translocates from the cytoplasm to the nucleus, where this transcription factor binds to the κB promoter region of target genes, including proinflammatory cytokines [2, 3, 28]. Accordingly, prevention of NF-κB activation inhibits proinflammatory cytokine production, which consequently improves myocardial function [10, 11]. Both CD14 and TLR-4 deficient mice showed that the signaling of NF-κB/proinflammatory cytokine was blunted in the myocardium upon LPS stimulation, and further protected from LPS-induced myocardial depression, suggesting that TLR4/CD14-NF-κB signaling is necessary for the development of LV dysfunction during endotoxic shock [41–43]. In the present study, we demonstrate that, after LPS administration, the activation of NF-κB and a substantial increase in the levels of myocardial TNF-α and IL-1β were impaired in Hsp20-TG animals. These results may provide one explanation for the reduction of LV dysfunction in the LPS-treated Hsp20 mice (Figure 8). At present, it is not entirely clear how Hsp20 inhibits NF-κB activation. Several studies have considered LPS-induced oxidative stress as a major source of NF-κB activation [44], and Hsp20 has been shown to reduce doxorubicin-triggered oxidative stress in cardiomyocytes [23]. Therefore, suppression of NF-κB activation by Hsp20 may be through attenuation of oxidative stress. In addition, binding of Hsp20 with 14-3-3γ could have wide-ranging cellular effects including regulation of NF-κB activity [45]. While there are several lines of evidence showing that activation of NF-κB requires 14-3-3 proteins [46, 47], more experiments need to be done for further elucidation of the molecular mechanisms involved in Hsp20-mediated inhibition of NF-κB activity.
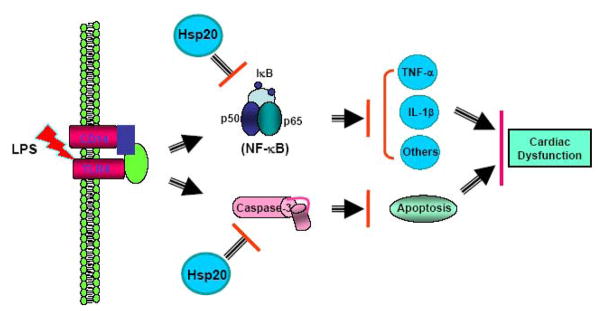
Figure 8.
Proposed scheme for protection of Hsp20 against LPS-triggered myocardial dysfunction and apoptosis. Overexpression of Hsp20 suppresses NF-κB activation and reduces caspase-3 activity triggered by LPS stimulation, leading to decreased production of myocardial proinflammatory cytokines including TNF-α and IL-1β, and inhibition of cardiac apoptosis. Consequently, LPS-induced myocardial dysfunction is attenuated in Hsp20-mice.
Secondly, recent studies indicate that endotoxin-induced activation of apoptotic pathways may directly lead to myocardial dysfunction [31, 48], and inhibition of cardiac apoptosis using modulators of caspases or other components of the cell-death pathway has shown striking beneficial efficacy in clinically relevant animal models of sepsis [28, 30, 31]. Therefore, identifying and inhibiting the initiating apoptotic stimuli or blocking a common cell death pathway will provide a new therapeutic approach for treating sepsis. The data presented here indicate that the TUNEL-positive nuclei were reduced by 3 fold in the myocardium of LPS-challenged Hsp20-mice, compared to that of LPS-treated NTG mice (Figure 7). Moreover, the activity of caspase-3, one of the key executioners of apoptosis, was significantly decreased in the myocardial tissues from LPS-challenged Hsp20 mice, relative to that of the LPS-challenged NTG mice. Our findings demonstrate that the inhibition of caspase-3 activity by Hsp20 may be associated with protection against LPS-induced myocardial dysfunction (Figure 8). These data further support the importance of apoptotic signaling in LPS-induced myocardial damage, and therefore strategies to block apoptosis might be an effective means to cope with this highly lethal disorder.
In summary, our study demonstrates that increased levels of cardiac Hsp20 lead to attenuation of LPS-induced myocardial dysfunction. The underlying mechanisms are associated with: 1) reduced caspase-3 activity and myocardial apoptosis; and 2) inhibition of NF-κB activation, resulting in reduction of proinflammatory cytokine levels and improvement of cardiac function in response to LPS challenge. These findings suggest that Hsp20 may be a potential therapeutic agent for preventing sepsis-associated cardiovascular dysfunction.
Acknowledgments
This study was supported by NIH grant HL-087861 (Dr. G. C. Fan).
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 4.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 5.Chagnon F, Metz CN, Bucala R, Lesur O. Endotoxin-induced myocardial dysfunction: effects of macrophage migration inhibitory factor neutralization. Circ Res. 2005;96:1095–1102. doi: 10.1161/01.RES.0000168327.22888.4d. [DOI] [PubMed] [Google Scholar]
- 6.Layland J, Cave AC, Warren C, Grieve DJ, Sparks E, Kentish JC, et al. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J. 2005;19:1137–1139. doi: 10.1096/fj.04-2519fje. [DOI] [PubMed] [Google Scholar]
- 7.Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, et al. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;114:1838–1846. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- 8.Niu J, Azfer A, Kolattukudy PE. Protection against lipopolysaccharide-induced myocardial dysfunction in mice by cardiac-specific expression of soluble Fas. J Mol Cell Cardiol. 2008;44:160–169. doi: 10.1016/j.yjmcc.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Wright G, Singh IS, Hasday JD, Farrance IK, Hall G, Cross AS, et al. Endotoxin stress-response in cardiomyocytes: NF-kappaB activation and tumor necrosis factor-alpha expression. Am J Physiol Heart Circ Physiol. 2002;282:H872–879. doi: 10.1152/ajpheart.00256.2001. [DOI] [PubMed] [Google Scholar]
- 10.Hall G, Singh IS, Hester L, Hasday JD, Rogers TB. Inhibitor-kappaB kinase-beta regulates LPS-induced TNF-alpha production in cardiac myocytes through modulation of NF-kappaB p65 subunit phosphorylation. Am J Physiol Heart Circ Physiol. 2005;289:H2103–2111. doi: 10.1152/ajpheart.00393.2005. [DOI] [PubMed] [Google Scholar]
- 11.Haudek SB, Spencer E, Bryant DD, White DJ, Maass D, Horton JW, et al. Overexpression of cardiac I-kappaBalpha prevents endotoxin-induced myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2001;280:H962–968. doi: 10.1152/ajpheart.2001.280.3.H962. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- 13.Meng X, Brown JM, Ao L, Nordeen SK, Franklin W, Harken AH, et al. Endotoxin induces cardiac HSP70 and resistance to endotoxemic myocardial depression in rats. Am J Physiol. 1996;271:C1316–1324. doi: 10.1152/ajpcell.1996.271.4.C1316. [DOI] [PubMed] [Google Scholar]
- 14.McComb MA, Spurlock ME. Expression of stress proteins in porcine tissues: developmental changes and effect of immunological challenge. J Anim Sci. 1997;75:195–201. doi: 10.2527/1997.751195x. [DOI] [PubMed] [Google Scholar]
- 15.Huey KA, Meador BM. Contribution of IL-6 to the Hsp72, Hsp25, and aB-crystallin responses to inflammation and exercise training in mouse skeletal and cardiac muscle. J Appl Physiol. 2008;105:1830–1836. doi: 10.1152/japplphysiol.90955.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan AJ, Flanagan SW, Moseley PL, Gisolfi CV. Acute heat stress protects rats against endotoxin shock. J Appl Physiol. 1992;73:1517–1522. doi: 10.1152/jappl.1992.73.4.1517. [DOI] [PubMed] [Google Scholar]
- 17.Chu EK, Ribeiro SP, Slutsky AS. Heat stress increases survival rates in lipopolysaccharide-stimulated rats. Crit Care Med. 1997;25:1727–1732. doi: 10.1097/00003246-199710000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Chan JY, Ou CC, Wang LL, Chan SH. Heat shock protein 70 confers cardiovascular protection during endotoxemia via inhibition of nuclear factor-kappaB activation and inducible nitric oxide synthase expression in the rostral ventrolateral medulla. Circulation. 2004;110:3560–3566. doi: 10.1161/01.CIR.0000143082.63063.33. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro SP, Villar J, Downey GP, Edelson JD, Slutsky AS. Effects of the stress response in septic rats and LPS-stimulated alveolar macrophages: evidence for TNF-alpha posttranslational regulation. Am J Respir Crit Care Med. 1996;154:1843–1850. doi: 10.1164/ajrccm.154.6.8970379. [DOI] [PubMed] [Google Scholar]
- 20.Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, et al. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111:1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- 21.Fan GC, Chu G, Mitton B, Song Q, Yuan Q, Kranias EG. Small heat-shock protein Hsp20 phosphorylation inhibits beta-agonist-induced cardiac apoptosis. Circ Res. 2004;94:1474–1482. doi: 10.1161/01.RES.0000129179.66631.00. [DOI] [PubMed] [Google Scholar]
- 22.Fan GC, Yuan Q, Song G, Wang Y, Chen G, Qian J, et al. Small heat-shock protein Hsp20 attenuates beta-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ Res. 2006;99:1233–1242. doi: 10.1161/01.RES.0000251074.19348.af. [DOI] [PubMed] [Google Scholar]
- 23.Fan GC, Zhou X, Wang X, Song G, Qian J, Nicolaou P, et al. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–1279. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan GC, Yuan Q, Zhao W, Chu G, Kranias EG. Junctin is a prominent regulator of contractility in cardiomyocytes. Biochem Biophys Res Commun. 2007;352(3):617–22. doi: 10.1016/j.bbrc.2006.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, et al. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zingarelli B, Hake PW, Yang Z, O’Connor M, Denenberg A, Wong HR. Absence of inducible nitric oxide synthase modulates early reperfusion-induced NF-kappaB and AP-1 activation and enhances myocardial damage. FASEB J. 2002;16:327–342. doi: 10.1096/fj.01-0533com. [DOI] [PubMed] [Google Scholar]
- 27.Chima RS, Hake PW, Piraino G, Mangeshkar P, Denenberg A, Zingarelli B. Ciglitazone ameliorates lung inflammation by modulating the inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. Crit Care Med. 2008;36:2849–2857. doi: 10.1097/ccm.0b013e318187810e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 29.Hall G, Hasday JD, Rogers TB. Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell Cardiol. 2006;41:580–591. doi: 10.1016/j.yjmcc.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Fauvel H, Marchetti P, Chopin C, Formstecher P, Nevière R. Differential effects of caspase inhibitors on endotoxin-induced myocardial dysfunction and heart apoptosis. Am J Physiol Heart Circ Physiol. 2001;280:H1608–1614. doi: 10.1152/ajpheart.2001.280.4.H1608. [DOI] [PubMed] [Google Scholar]
- 31.Lancel S, Joulin O, Favory R, Goossens JF, Kluza J, Chopin C, et al. Ventricular myocyte caspases are directly responsible for endotoxin-induced cardiac dysfunction. Circulation. 2005;111:2596–2604. doi: 10.1161/CIRCULATIONAHA.104.490979. [DOI] [PubMed] [Google Scholar]
- 32.Silverman HJ, Penaranda R, Orens JB, Lee NH. Impaired beta-adrenergic receptor stimulation of cyclic adenosine monophosphate in human septic shock: association with myocardial hyporesponsiveness to catecholamines. Crit Care Med. 1993;21:31–39. doi: 10.1097/00003246-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Bernardin G, Strosberg AD, Bernard A, Mattei M, Marullo S. Beta-adrenergic receptor-dependent and -independent stimulation of adenylate cyclase is impaired during severe sepsis in humans. Intensive Care Med. 1998;24:1315–1322. doi: 10.1007/s001340050768. [DOI] [PubMed] [Google Scholar]
- 34.Eisenhut M. Impairment of beta-adrenergic receptor signaling as a cause of both pulmonary oedema and cardiovascular failure in patients with septicaemia. Pathology. 2007;39:611–612. doi: 10.1080/00313020701684433. [DOI] [PubMed] [Google Scholar]
- 35.Chu G, Egnaczyk GF, Zhao W, Jo SH, Fan GC, Maggio JE, et al. Phosphoproteome analysis of cardiomyocytes subjected to beta-adrenergic stimulation: identification and characterization of a cardiac heat shock protein p20. Circ Res. 2004;94:184–193. doi: 10.1161/01.RES.0000107198.90218.21. [DOI] [PubMed] [Google Scholar]
- 36.Burniston JG. Adaptation of the rat cardiac proteome in response to intensity-controlled endurance exercise. Proteomics. 2009;9:106–115. doi: 10.1002/pmic.200800268. [DOI] [PubMed] [Google Scholar]
- 37.Boluyt MO, Brevick JL, Rogers DS, Randall MJ, Scalia AF, Li ZB. Changes in the rat heart proteome induced by exercise training: Increased abundance of heat shock protein hsp20. Proteomics. 2006;6:3154–3169. doi: 10.1002/pmic.200401356. [DOI] [PubMed] [Google Scholar]
- 38.DeBlieux PM, McDonough KH, Barbee RW, Shepherd RE. Exercise training attenuates the myocardial dysfunction induced by endotoxin. J Appl Physiol. 1989;66:2805–2810. doi: 10.1152/jappl.1989.66.6.2805. [DOI] [PubMed] [Google Scholar]
- 39.Shek PN, Shephard RJ. Physical exercise as a human model of limited inflammatory response. Can J Physiol Pharmacol. 1998;76:589–597. doi: 10.1139/cjpp-76-5-589. [DOI] [PubMed] [Google Scholar]
- 40.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knuefermann P, Nemoto S, Misra A, Nozaki N, Defreitas G, Goyert SM, et al. CD14-deficient mice are protected against lipopolysaccharide-induced cardiac inflammation and left ventricular dysfunction. Circulation. 2002;106:2608–2615. doi: 10.1161/01.cir.0000038110.69369.4c. [DOI] [PubMed] [Google Scholar]
- 42.Nemoto S, Vallejo JG, Knuefermann P, Misra A, Defreitas G, Carabello BA, et al. Escherichia coli LPS-induced LV dysfunction: role of toll-like receptor-4 in the adult heart. Am J Physiol Heart Circ Physiol. 2002;282:H2316–2323. doi: 10.1152/ajpheart.00763.2001. [DOI] [PubMed] [Google Scholar]
- 43.Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res. 2004;95:700–707. doi: 10.1161/01.RES.0000144175.70140.8c. [DOI] [PubMed] [Google Scholar]
- 44.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Chernik IS, Seit-Nebi AS, Marston SB, Gusev NB. Small heat shock protein Hsp20 (HspB6) as a partner of 14-3-3gamma. Mol Cell Biochem. 2007;295:9–17. doi: 10.1007/s11010-006-9266-8. [DOI] [PubMed] [Google Scholar]
- 46.Aguilera C, Fernández-Majada V, Inglés-Esteve J, Rodilla V, Bigas A, Espinosa L. Efficient nuclear export of p65-IkappaBalpha complexes requires 14-3-3 proteins. J Cell Sci. 2006;119:3695–3704. doi: 10.1242/jcs.03086. [DOI] [PubMed] [Google Scholar]
- 47.Matitau AE, Scheid MP. Phosphorylation of MEKK3 at threonine 294 promotes 14-3-3 association to inhibit nuclear factor kappaB activation. J Biol Chem. 2008;283:13261–13268. doi: 10.1074/jbc.M801474200. [DOI] [PubMed] [Google Scholar]
- 48.Li HL, Suzuki J, Bayna E, Zhang FM, Dalle Molle E, Clark A, et al. Lipopolysaccharide induces apoptosis in adult rat ventricular myocytes via cardiac AT(1) receptors. Am J Physiol Heart Circ Physiol. 2002;283:H461–467. doi: 10.1152/ajpheart.00701.2001. [DOI] [PubMed] [Google Scholar]