Abstract
Emerging reports on human islets emphasize distinct differences from the widely accepted prototype of rodent islets, raising questions over their suitability for human studies. Here we aim at elucidating architectural differences and similarities of human versus rodent islets. The cellular composition and architecture of human and rodent islets were compared through three-dimensional (3D) reconstructions. Physiological and pathological changes were examined using islets from various mouse models such as non-obese diabetic (NOD), ob/ob, db/db mice and during pregnancy. A subpopulation of human islets is composed of clusters of alpha-cells within the central beta-cell cores, while the overall proportion of alpha-cells varies among islets. In mouse islets under normal conditions, alpha-cells are localized in the islet periphery, but they do not envelop the entire beta-cell core, so that beta-cells are exposed on the outer layer of the islet, as in most human islets. Also, an increased proportion of alpha-cells within the central core is observed in the pancreas of mouse models exhibiting increased demand for insulin. In summary, human and mouse islets share common architectural features as endocrine micro-organs. Since these may hold a key to better understanding islet plasticity, our concept of the prototypic islet should be revised.
Keywords: pancreatic beta-cells, alpha-cells, islets, pregnancy, insulin resistance, diabetes
INTRODUCTION
Insulin-secreting beta-cells play a key role in glucose homeostasis and pathogenesis of diabetes mellitus. However, individual beta-cells are not an isolated functional unit, but rather part of a micro-organ, the islet of Langerhans. The pancreatic islet is comprised of multiple cell types, including beta-cells and glucagon-secreting alpha-cells that work together to maintain normoglycemia.
Recently, Brissova et al. (1) and Cabrera et al. (2) have reported intriguing observations based on comparative studies of islet composition, architecture and function among mouse, monkey and human islets. Their results (based on 2D analysis) suggested that human and monkey islets have an increased proportion of alpha-cells and that alpha-cells appear to be dispersed throughout the islet, whereas in mouse islets, there are fewer alpha-cells relative to beta-cells and they are found in the periphery of the islet forming a mantle-like structure. The “disorganized islet composition” of human and monkey islets compared to rodent islets may alter the functional properties of primate and rodent islets. For example, primate islets are more sensitive to stimulation by low concentrations of glucose (1 mM) to which mouse islets are blind (2). The implication of these studies is that results obtained from studies carried out using rodent islets need to be confirmed using human islets.
The increase in body size and resulting increased demand for insulin in man compared to mice is accompanied by a proportionate expansion of pancreas size and beta-cell mass. However, there is no significant change in the size distribution of islets, which average 100–150 μm in diameter in both mice and man, suggesting that this micro-organ has a certain limit in size to be functional. Then, a possible compensation could have been occurred within an islet, i.e. in islet composition and architecture, to be functionally more efficient.
In the present study, we aim to explore how human islets are different from those of rodents, if they are truly different as an endocrine organ. This information has a potentially significant impact on the interpretation of a wealth of knowledge accumulated in the field based on rodent studies, and further on the direction of pancreatic islet studies per se in the immediate future. Here we report that human and mouse islets are more similar than previously implied, and the change of islet composition and architecture may reflect the islet adaptation to the increased body demand rather than species differences.
MATERIALS AND METHODS
Human islets
Human islets were obtained from Islet Cell Resource Centers. Use of human islets has been approved in advance by the University of Chicago Institutional Review Board.
Mice
CD-1 (20-wk for islet studies and pregnancy, 6-wk for wild-type control in morphometric analysis of the ratio of alpha-cells to beta-cells), non-obese diabetic (NOD; prediabetic 6-wk, diabetic 40-wk), ob/ob (15-wk), and db/db (20-wk) mice were used. All the strains of mice were obtained from Jackson Laboratory (Bar Harbor, ME) except CD-1 mice. All the procedures involving animals were approved by the University of Chicago Institutional Animal Care and Use Committee.
Immunohistochemistry
Human islets were fixed in 4% paraformardehyde (PFA), permeabilized with 1% Triton X in phosphate buffer solution (PBS) and whole-mount stained with antibodies against insulin (1:100, DAKO, Carpinteria, CA) and glucagon (1:100, Sigma-Aldrich, St. Louis, MO). Mouse pancreata were partially digested with collagenase type X (Sigma-Aldrich) and small pieces of pancreatic tissues containing islets were wholemount-stained as described above. Paraffin-embedded pancreata were cut 6 μm in thickness and stained using insulin (1:500), glucagon (1:500) and somatostatin (1:500, Santa Cruz Biotechnology, Santa Cruz, CA). Images were taken using an Olympus IX80 DSU confocal microscope (New York, NY) with SlideBook software (Minneapolis, MN).
Three-dimensional (3D) image analysis
A stack of images (3 μm interval) was reconstructed in 3D and surface plot using Slidebook software. Volume of insulin-positive beta-cells and glucagon-positive alpha-cells was measured using the voxel counting plug-in developed for ImageJ (http://rsbweb.nih.gov/iij/). Data are expressed as mean ± SEM.
Statistical analysis
Statistical analyses were performed using Student’s t test. Differences were considered to be significant at P < 0.05.
RESULTS
Human and mouse islets are more similar than previously suggested
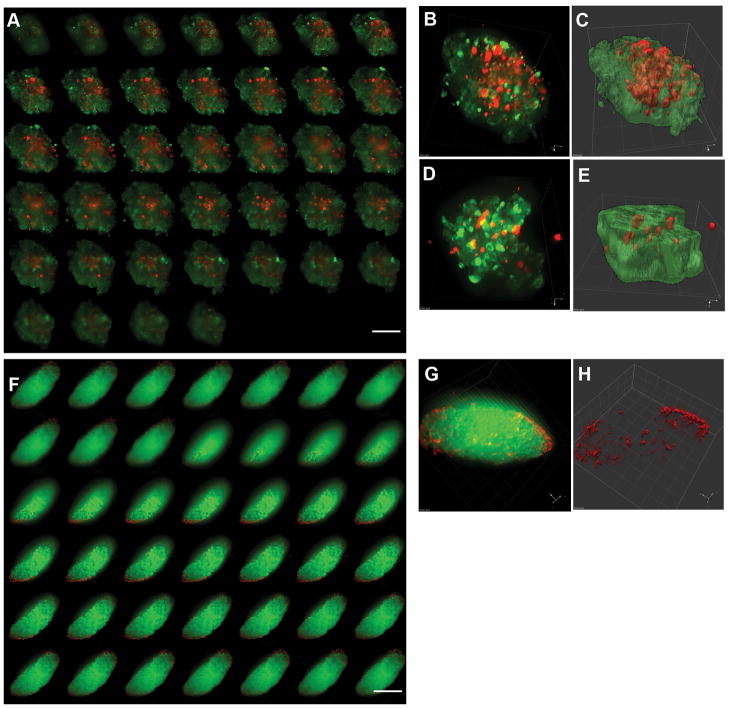
A subpopulation of human islets is composed of alpha-cells located within the central core. The alpha-cells appear to be “intermingled” in a top-down view of a stack of confocal optical images of an isolated human islet (Fig. 1A). This organization is similar to that reported previously for “representative human islets” (1, 2). Three-dimensional reconstruction reveals that alpha-cells are not scattered singly throughout the islet (Fig. 1B), but the surface plot (in which the luminance is interpreted as height for the plot) shows that the alpha-cells are clustered (Fig. 1C). The percent volume of alpha-cells to beta-cells in the particular islet shown in Fig. 1 is 45.5% (by voxel measurement). However, other islets contained fewer alpha-cells (8.5%; Fig. 1D and 1E). The average volume of alpha-cells in human islets studied was 23.6% (Table 1; n=69 from 3 donors: age range 25–45 years).
Fig. 1. Human versus mouse islets.
A: A stack of optical images of an isolated human islet. Scale bar = 100 μm. B: 3D reconstruction of A. Note that the intensity in insulin staining is reduced (by increasing transparency) to show an internal view of an islet in 3D. C: Surface plot of B demonstrating cell clustering. D–E: Three-dimensional reconstruction of another human islet. Note that overall the proportion of alpha-cells varies among islets. F: A stack of optical images of a representative mouse islet in situ. Scale bar = 100 μm. G: 3D reconstruction of F. H: Surface plot of G. Note that alpha-cells segregate in the periphery, however, the islet surface is primarily composed of beta-cells as seen in human islets.
Table I.
Comparison of alpha-cell/beta-cell volume ratio between human and rodent islets
| Alpha-cell/Beta-cell volume (%) | Number of islets | |
|---|---|---|
| human | 23.6 ± 1.8 % | 69 (3 humans) |
| mouse | 13.4 ± 1.5 %* | 33 (5 mice) |
| rat | 15.7 ± 2.5 %* | 25 (5 rats) |
P < 0.05 compared with human
Isolated islets from normal adult mice showed the distinct segregation of alpha-cells in the periphery of the islet, however, no “mantle” formation was observed (n=33 from 5 mouse pancreata). Figure 1F shows a representative mouse islet in situ. The percent volume of alpha-cells to beta-cells in this islet is 8.7%. Three-dimensional reconstruction shows that the islet surface is primarily composed of beta-cells with a few local clusters of alpha-cells (Fig. 1G and 1H). The average volume of alpha-cells in mouse islets studied was 13.4% (Table 1), which is consistent with reported data (3–5). Rat islets showed similar alpha-cell volume (15.7%; Table 1).
Physiological and pathological changes of islet composition and architecture in mice
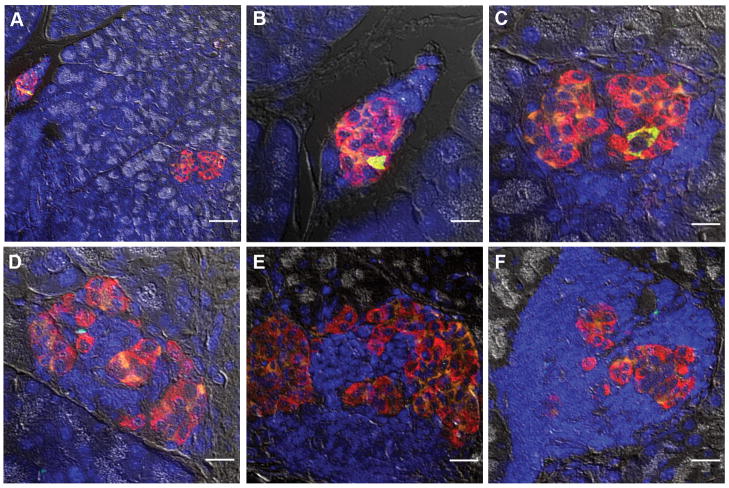
An increased ratio of alpha-cells to beta-cells in the core of the islet as observed in isolated normal human islets (Fig. 1) is also observed in mouse islets in the state of increased demand for insulin such as inflammation (prediabetic NOD), pregnancy and insulin resistance (db/db mice) (Fig. 2 and Table II). Note that in ob/ob mice, although some islets exhibit a mixed composition of alpha- and beta-cells, the average ratio is similar to that of wild-type.
Fig. 2. Alpha-cell hyperplasia in inflammation, pregnancy and insulin resistance.
A: Prediabetic NOD mouse islet. Scale bar = 50 μm. B: Pregnant mouse islet at day 14. Scale bar = 50 μm. C: ob/ob mouse islet (15-wk). Scale bar = 20 μm. D: db/db mouse islet (20-wk). Scale bar = 20 μm. Left: Insulin (green), glucagon (red), and somatostatin (orange). Right: Merged with nuclei staining (DAPI, blue) and bright-field. Note morphological changes with alpha- and delta-cells present in the central core of the islet in all four mouse models.
Table II.
Comparison of alpha-cell area between wild-type and mice with pathophysiological conditions
| Alpha-cell area (%) | Number of islets | |
|---|---|---|
| wild-type mice | 7.5 ± 0.8 % | 104 (3 mice) |
| prediabetic NOD mice | 21.1 ± 0.8 %* | 120 (3 mice) |
| pregnant mice | 13.2 ± 2.3 %* | 105 (3 mice) |
| db/db mice | 30.9 ± 2.1 %* | 104 (3 mice) |
| ob/ob mice | 6.2 ± 0.7 % | 158 (3 mice) |
P < 0.05 compared with wild-type mouse islets
Collapse of central beta-cell core in mice with overt diabetes
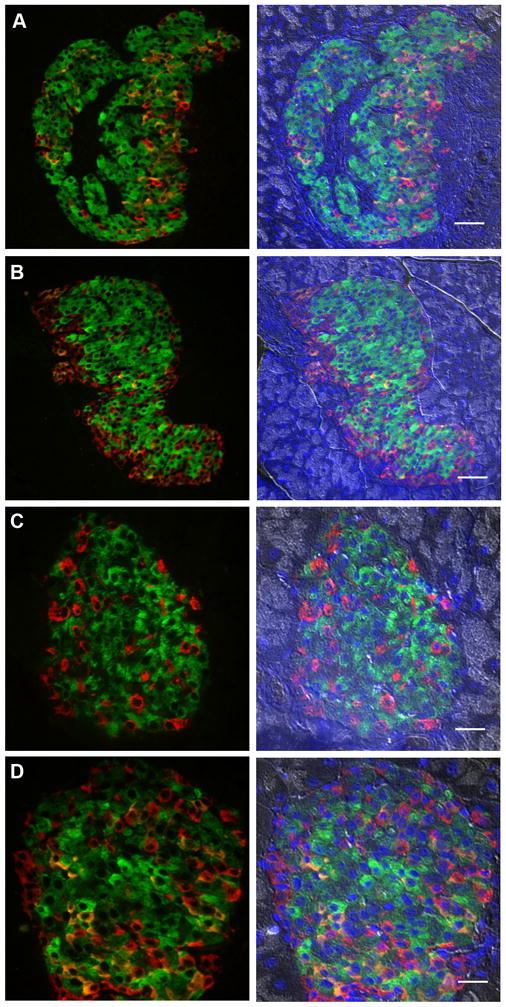
A relative increase of alpha-cells within the central core of an islet could be due to “the collapse of beta-cell core”. However, severe beta-cell loss in the state of longstanding hyperglycemia results in the overall reduced islet volume with a small cluster of alpha- and delta-cells with a trace of beta-cells (Fig. 3A–3C; a representative image of pancreas out of eight NOD mice whose blood glucose levels at the time of harvesting tissues: > 350 mg/dL). The remaining islet structure is often filled with infiltrated lymphocytes (Fig. 3D–3F).
Fig. 3. Collapse of the central beta-cell core.
A: A representative image of reduced size of islets scattered in the pancreas of NOD mice with long-standing hyperglycemia. Stained for insulin (green), glucagon (red), somatostatin (orange) and neuclei (DAPI, blue) and combined with a bright field image. Scale bar = 50 μm. B–C: Enlarged images of islets in A. Note that only a trace of beta-cells is remained. Scale bar = 20 μm. D–E: Infiltrated lymphocytes often replaced beta-cells. Scale bar = 20 μm.
DISCUSSION
Rodent islets do not have a mantle
Three-dimensional reconstruction of a stack of optical images of whole-mount stained islets showed that a subpopulation of human islets was composed of a cluster of alpha-cells within the central beta-cell core of an islet, while overall the proportion of alpha-cells varies among islets and endocrine-cells are not intermingled at a single cell level as often demonstrated using 2-D analysis. In mouse islets under normal conditions, alpha-cells are localized in the periphery of the islet. However, they do not envelop the entire beta-cell core, therefore beta-cells are exposed to the outer layer of the islet similar to human islets. This explains a long-standing question in the field of islet physiology: that is, why intracellular calcium measurements using acetomethyl-esterified (AM) calcium indicators such as Fura2-AM work. It is widely recognized that Fura2-AM does not penetrate into the core of an islet, but rather is only taken up by the outer layer of cells. If a rodent islet has a mantle of alpha-cells (including other non-beta-cells), the recorded responses should have been from these cells, not from beta-cells. However, beta-cell physiology reveals otherwise. Investigators have speculated that somehow “the mantle cells” fall off in the process of islet isolation so that beta-cells are exposed to the surface. For this reason, we carried out the whole-mount staining of mouse islets in situ.
Alpha-cell hyperplasia and beta-cell proliferation
A relative increase in alpha-cells present in the central core of the islet has been reported in many animal models of beta-cell deficiency (6–11) as well as in patients with type 2 diabetes (12, 13), and is often described as “disorganized islet architecture” based on “the prototype” structure. The disorganized architecture is thought to result from the collapse of the central beta-cell core due to beta-cell loss rather than alpha-cell hyperplasia. While this is obviously not the case during pregnancy and in obesity, in which the increased alpha-cell ratio is accompanied with the increased beta-cell mass, the progressive autoimmune beta-cell destruction which occurs in NOD mice could theoretically obscure observations in changes of such islet architecture. However, the loss of beta-cells leads to the overall shrinkage of islet size distribution, and the remaining islets consisting of a small cluster of alpha- and delta-cells are often accompanied with massive lymphocyte infiltration. In fact, whether hyperglycemia per se is a major factor that triggers beta-cell compensation (or so-called regeneration) is controversial (14, 15). Development of overt diabetes, i.e. chronic hyperglycemia, is a long process. It occurs when the overall functional beta-cell mass drops below a threshold necessary to maintain euglycemia (10–30% of total beta-cell mass in various species; 16–22). Studies on NOD mice, for example, show that compensatory beta-cell proliferation precedes the onset of hyperglycemia (23, 24). In this regard, we have observed an increase in the ratio of alpha-cells to beta-cells preceding autoimmune destruction of beta-cells (from neonates up to 8-wk of age; Hara et al., unpublished data). This prolonged alpha-cell hyperplasia is not observed in other strains of mice (i.e. CD-1, C57BL/6 and 129). It may reflect a previously unappreciated feature of the pathophysiology of type 1 diabetes in the NOD model. During pregnancy (CD-1 mice), there is also an increase in the ratio of alpha-cells to beta-cells that peaks around day 15 and then declines to normal levels just prior to birth. The pattern of change in the ratio of alpha-cells to beta-cells appears to mirror the time-course of the increase in beta-cell replication that occurs during pregnancy (25).
The present study demonstrates that the difference between human and mouse islets is whether these “specialized islets” consisting of increased proportions of alpha-and delta-cells in the central core are observed in the normal condition in humans or in the states of increased demand of insulin in mice such as pregnancy, insulin resistance, and inflammation. The composition and architecture of these islets accompanied with increased intra-islet vascularization may lead to more efficient cell-cell communication resulting in enhanced endocrine function such as increased sensitivity to changes of external glucose concentrations (2).
In summary, we have shown that rodent islets do not have “the mantle” to envelop the beta-cell core and the prototype of the islet should be revisited. Islet composition and architecture vary among species. They also vary within the same species depending on physiological and pathological states. Emerging observations from studies on human islets, in which any differences from rodent islets tend to be more emphasized than the similarities, should be extrapolated carefully for the following considerations: [1] the paucity of human islets/pancreatic tissues for research; [2] variability of the quality of islets including purity and functionality; and [3] the difficulty of normalizing data generated from specimens from various donors of different ages and health states, and in most cases, from an undetermined region of the pancreas. We propose that the overall differences and similarities of islet architecture and functions among species should be interpreted in a more global fashion by taking into account of possible evolutionary changes in terms of islet adaptation to compensate the increased body demand.
Acknowledgments
This research was supported in part by US Public Health Service Grant DK-081527, DK-20595 to the University of Chicago Diabetes Research and Training Center (Animals Models Core), and a gift from the Kovler Family Foundation.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 2.Cabrera O, Berman DM, Kenyon NS, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonner-Weir S. Anatomy of islet of Langerhans. In: Samols E, editor. The endocrine pancreas. Raven; New York: 1991. pp. 15–27. [Google Scholar]
- 4.Bauer GE. Islets of Langrhans. In: Weiss L, editor. Cell and tissue biology, a textbook of histology. Urban & Schwarzenberg; Baltimore: 1988. pp. 738–749. [Google Scholar]
- 5.Masharani U, Karam JH, German MS. Pancreatic hormones and diabetes mellitus. In: Greenspan FS, Gardner DG, editors. Basic and clinical endocrinology. Lange medical Books/McGraw-Hill; New York: 2001. pp. 658–746. [Google Scholar]
- 6.Epstein PN, Overbeek PA, Means AR. Calmodulin-induced early-onset diabetes in transgenic mice. Cell. 1989;58:1067–1073. doi: 10.1016/0092-8674(89)90505-9. [DOI] [PubMed] [Google Scholar]
- 7.Dahl U, Sjødin A, Semb H. Cadherins regulate aggregation of pancreatic beta-cells in vivo. Development. 1996;122:2895–2902. doi: 10.1242/dev.122.9.2895. [DOI] [PubMed] [Google Scholar]
- 8.Krakowski ML, Kritzik MR, Jones EM, et al. Transgenic expression of epidermal growth factor and keratinocyte growth factor in beta-cells results in substantial morphological changes. J Endocrinol. 1999;162:167–75. doi: 10.1677/joe.0.1620167. [DOI] [PubMed] [Google Scholar]
- 9.Inada A, Hamamoto Y, Tsuura Y, et al. Overexpression of inducible cyclic AMP early repressor inhibits transactivation of genes and cell proliferation in pancreatic beta cells. Mol Cell Biol. 2004;24:2831–2841. doi: 10.1128/MCB.24.7.2831-2841.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fazio EN, Everest M, Colman R, et al. Altered Glut-2 accumulation and beta-cell function in mice lacking the exocrine-specific transcription factor, Mist1. J Endocrinol. 2005;187:407–418. doi: 10.1677/joe.1.06376. [DOI] [PubMed] [Google Scholar]
- 11.Bates HE, Sirek A, Kiraly MA, et al. Adaptation to intermittent stress promotes maintenance of beta-cell compensation: comparison with food restriction. Am J Physiol Endocrinol Metab. 2008;295:E947–E958. doi: 10.1152/ajpendo.90378.2008. [DOI] [PubMed] [Google Scholar]
- 12.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 13.Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 14.Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab. 2008;10(Suppl 4):23–31. doi: 10.1111/j.1463-1326.2008.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchetti P, Dotta F, Lauro D, et al. An overview of pancreatic beta-cell defects in human type 2 diabetes: implications for treatment. Regul Pept. 2008;146:4–11. doi: 10.1016/j.regpep.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- 17.Kjems LL, Kirby BM, Welsh E, et al. Decrease in beta-cell mass leads to impaired pulsatile insulin secretion, reduced postprandial hepatic insulin clearance, and relative hyperglucagonemia in the minipig. Diabetes. 2001;50:2001–2012. doi: 10.2337/diabetes.50.9.2001. [DOI] [PubMed] [Google Scholar]
- 18.Larsen MO, Rolin B, Wilken M, et al. Measurements of insulin secretory capacity and glucose tolerance to predict pancreatic beta-cell mass in vivo in the nicotinamide/streptozotocin gottingen minipig, a model of moderate insulin deficiency and diabetes. Diabetes. 2003;52:118–123. doi: 10.2337/diabetes.52.1.118. [DOI] [PubMed] [Google Scholar]
- 19.Sreenan S, Pick AJ, Levisetti M, et al. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes. 1999;48:989–996. doi: 10.2337/diabetes.48.5.989. [DOI] [PubMed] [Google Scholar]
- 20.Saito K, Yaginuma N, Takahashi T. Differential volumetry of a, b and d cells in the pancreatic islets of diabetic and nondiabetic subjects. Tohoku J Exp Med. 1979;129:273–283. doi: 10.1620/tjem.129.273. [DOI] [PubMed] [Google Scholar]
- 21.Bonner-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983;71:1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tominaga M, Maruyama H, Bolli G, et al. Simulation of the normal glucopenia-induced decline in insulin partially restores the glucagon response to glucopenia in isolated perfused pancreata of streptozotocin-diabetic rats. Endocrinology. 1986;118:886–887. doi: 10.1210/endo-118-2-886. [DOI] [PubMed] [Google Scholar]
- 23.Sreenan S, Pick AJ, Levisetti M, et al. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes. 1999;48:989–996. doi: 10.2337/diabetes.48.5.989. [DOI] [PubMed] [Google Scholar]
- 24.Sherry NA, Kushner JA, Glandt M, et al. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes. 2006;55:3238–3245. doi: 10.2337/db05-1034. [DOI] [PubMed] [Google Scholar]
- 25.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]