Abstract
A monumental task of the mammalian retina is to encode an enormous range (>109-fold) of light intensities experienced by the animal in natural environments. Retinal neurons carry out this task by dividing labor into many parallel rod and cone synaptic pathways. Here we study the operational plan of various rod- and cone-mediated pathways by analyzing electroretinograms (ERGs), primarily b-wave responses, in dark-adapted wildtype, connexin36 knockout, depolarizing rod–bipolar cell (DBCR) knockout, and rod transducin alpha-subunit knockout mice [WT, Cx36(−/−), Bhlhb4(−/−), and Trα(−/−)]. To provide additional insight into the cellular origins of various components of the ERG, we compared dark-adapted ERG responses with response dynamic ranges of individual retinal cells recorded with patch electrodes from dark-adapted mouse retinas published from other studies. Our results suggest that the connexin36-mediated rod–cone coupling is weak when light stimulation is weak and becomes stronger as light stimulation increases in strength and that rod signals may be transmitted to some DBCCs via direct chemical synapses. Moreover, our analysis indicates that DBCR responses contribute about 80% of the overall DBC response to scotopic light and that rod and cone signals contribute almost equally to the overall DBC responses when stimuli are strong enough to saturate the rod bipolar cell response. Furthermore, our study demonstrates that analysis of ERG b-wave of dark-adapted, pathway-specific mutants can be used as an in vivo tool for dissecting rod and cone synaptic pathways and for studying the functions of pathway-specific gene products in the retina.
INTRODUCTION
The mammalian retina must encode an enormous range (>109-fold) of light intensity as encountered in the natural environment, from few photons in the starlit sky to >108 photons reflected from sunlit snow (Rodieck 1998). This is a monumental task because there are only limited numbers of photoreceptor subtypes and synaptic pathways in the retina and each retinal neuron has a limited dynamic range for registering visual signals of various strengths (e.g., for a study in mouse see Pang et al. 2004). The first-order visual neurons are rod and cone photoreceptors. Rods, which are more sensitive than cones, encode dim light but saturate at lower light levels, whereas cones have low light sensitivity and increase in amplitude as stimulus strength is increased to much higher levels. Rod and cone signals, which under certain conditions can spread to each other through gap junctions, are transmitted to second-order retinal cells, the bipolar cells, via glutamatergic chemical synapses, as reviewed in Dowling (1987). In mammalian retinas, rod signals are conveyed to the inner retina through at least three bipolar cell pathways. The first (primary) rod pathway transmits hyperpolarizing rod response through a sign-inversing synapse to the on or depolarizing rod bipolar cells (DBCRs) (Kolb and Famiglietti 1974) and DBCRs send the signal through an excitatory glutamate synapse to AII amacrine cells (AIIACs). AIIACs signal to cone depolarizing bipolar cells (DBCCs) through a heterodimeric electrical synapse, consisting of connexin36 (Cx36) on the AIIAC side and Cx45 on the DBCC side (Dedek et al. 2006; Maxeiner et al. 2005). AIIACs also signal to cone off, or hyperpolarizing bipolar cells (HBCCs), through a sign-inverting glycinergic synapse (Crooks and Kolb 1992). DBCCs and HBCCs send signals to on and off ganglion cells (ONGCs and OFFGCs), respectively (Kolb and Nelson 1983).
In the second (secondary) rod pathway, rod signals spread to cones, through an electrical synapse, at least partially mediated by Cx36 (Deans et al. 2002; Zhang and Wu 2005), after which cones convey these rod signals to DBCCs and HBCCs via glutamatergic synapses. The third (tertiary) rod pathway, initially described for contacts between rods and HBCCs (Soucy et al. 1998; Tsukamoto et al. 2001), has more recently been shown to also include synaptic contacts between rod photoreceptors and a certain subset of DBCCs (Tsukamoto et al. 2007).
In addition to the three rod pathways, a fourth pathway for on and off responses, perhaps overlapping with the secondary rod pathway, is the cone bipolar cell pathway, which conveys hyperpolarizing cone responses to DBCCs and HBCCs and, subsequently, to on, on–off, and off GCs (Kolb and Famiglietti 1974).
Although the basic rod and cone bipolar cell synaptic pathways in mammalian retina (described earlier) have been identified, many pieces of the puzzle are still missing. For example, it is not clear how much each of the bipolar cell pathways contribute to the overall job of the bipolar cells, to relay signals to retinal ganglion cells (RGCs). Moreover, because the pathways consist of many key elements, such as gap junction channels, specific cells (e.g., DBCRs and DBCCs), and specific inputs (e.g., rod or cone inputs), it is important to determine how each of these elements affects individual pathway functions and the overall operation of the retina.
In this study, we examine full-field electroretinograms (ERGs), mainly the b-wave responses, of dark-adapted mice to brief 500-nm flashes that ranged from flashes weak enough to just elicit an ERG response to stimuli that were 9 log units stronger. Since the ERG b-wave represents the overall bipolar cell light responses of the retina (Robson et al. 2004; Stockton and Slaughter 1989; Tian and Slaughter 1995), these responses provided an in vivo tool for analyzing bipolar cell functional pathways over the entire operating range of the visual system for flashes presented from darkness. The more common situation in nature, when there is the transition from rod-dominated to cone-dominated vision, is for the mean level of illumination to change as well—i.e., from starlight to daylight. Using light flashes from darkness, rather than imposing them on backgrounds of increasing mean illumination, isolates the responses of the pathways from the effects of adaptational mechanisms that would otherwise be activated (Dunn and Rieke 2008; Dunn et al. 2006). By comparing the dark-adapted b-wave responses with the response dynamic ranges of individual retinal neurons recorded with patch electrodes in the dark-adapted mouse retina published from our laboratory and others, we identified plausible cellular origins of b-wave responses for different light zones. Moreover, we took advantage of three pathway-specific knockout mice to study how the different bipolar pathways affect the b-wave response to various ranges of light stimulation and the corresponding bipolar cell pathways involved. To eliminate the primary rod pathway, we used the basic helix–loop–helix transcription factor Bhlhb4 knockout mice, Bhlhb4(−/−), which lose their DBCRs at postnatal day 8 (P8) to P12 (Bramblett et al. 2004). To eliminate the secondary rod pathway (via rod–cone coupling), we used the connnexin36 knockout mouse, Cx36(−/−). These mice lack a gap junction that is expressed in the outer and inner plexiform layers and is thought to mediate rod–cone coupling (Deans et al. 2002; Volgyi et al. 2004). To isolate the cone pathway, we used the rod transducin alpha-subunit knockout mouse, Trα(−/−), in which rod phototransduction is effectively eliminated, without appreciable retinal degeneration at 13 wk of age (Calvert et al. 2000). To find evidence of the tertiary rod pathway (rod → DBCCs), we compared responses of the Bhlhb4(−/−) mouse, in which the tertiary pathway should be intact [because DBCC populations have been shown to be intact (Kim et al. 2008)] and the Trα(−/−) mouse, which should not have any functional rod pathways.
In this way, we were able to provide new insights into the functional organization of rod and cone bipolar cell pathways in the dark-adapted mouse retina and show how these signaling pathways operate together in forming parallel information channels to encode the wide range light that is encountered in nature.
METHODS
Ethical approval
Mice were cared for and handled following approved protocols from the Animal Care and Use Committees of Baylor College of Medicine and in compliance with National Institutes of Health guidelines for the care and use of experimental animals.
Animals
Wildtype (WT) mice (C57BL/6) from Jackson Laboratories (Bar Harbor, ME), between 12 and 18 wk old, were used for experiments. Bhlhb4(−/−) mice (99% C57BL/6 genetic background) (Bramblett et al. 2004), Cx36(−/−) (C57BL6/12SvEV hybrids) (Deans et al. 2002), and Trα(−/−) (C57BL6/12SvEV hybrids) (Calvert et al. 2000), between 12 and 18 wk old, were used for experiments. Preliminary evidence suggested that these strains were adequately backcrossed to ensure that strain differences did not affect these recordings (Supplemental Fig. S1).1
Electroretinograms
Before testing, mice were allowed to adapt to the dark overnight. Under dim red light, mice were anesthetized with a solution of ketamine (95 mg/ml) and xylazine (5 mg/ml). The pupils were dilated with a single drop of 1% tropicamide and 2.5% phenylephrine. A drop of 0.5% proparacaine hydrochloride was applied for corneal anesthesia. Mice were placed on a heating pad maintained at 39°C, inside a Ganzfeld dome coated with highly reflective white paint (Munsell Paint, New Windsor, NY). A small amount of 2.5% methylcellulose gel was applied to the eye and a platinum needle electrode was placed in contact with the center of the cornea. Similar platinum reference and ground electrodes were placed in the forehead and tail, respectively. After placement in the dome, mice were allowed to remain in complete darkness for 5 min. Signals were amplified with a Grass P122 amplifier (band-pass 0.1 to 1,000 Hz; Grass Instruments, West Warwick, RI). Data were acquired with a National Instruments Lab personal computer data acquisition board (sampling rate: 10,000 Hz; National Instruments, Austin, TX). Traces were averaged and analyzed with custom software written in Matlab (The MathWorks, Natick, MA).
Flashes were calibrated using a photometer (Model IL1700; International Light, Newburyport, MA) fitted with a scotopic filter in integrating mode that gave results as scotopic (sc) candela second (cd·s) m−2. To convert these units to photoisomerizations/rod, we used the same conversion factors as reported previously (Saszik et al. 2002): 1 sc td s = 121.6Rh* per rod. This calibration was also appropriate for the mouse M-cone, with a peak near that of rods (Lyubarsky et al. 1999). For the area of the dilated pupil, we use 3.14 mm2, as reported in Pennesi et al. (1998). Flashes for scotopic measurements were generated by a Grass PS-33 photostimulator (Grass Instruments). Light was spectrally filtered with a 500-nm interference filter (Edmund Industrial Optics, Barrington, NJ). A series of metal plates with holes of varying diameters and glass neutral density filters were used to attenuate the flash. As the strength of the flash increased, the number of trials was decreased and the time between each flash was increased. To remove oscillatory potentials before fitting, the scotopic b-wave was digitally filtered using the filtfilt function in Matlab (low-pass filter; FC = 60 Hz). The relationship between b-wave amplitude and flash intensity can be described by a saturating hyperbolic function (Naka–Rushton) with the form (Naka and Rushton 1966)
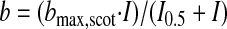 |
(1) |
where bmax,scot is the saturated scotopic b-wave amplitude and I0.5 is the intensity that provides half saturation. The baseline (from the trough of the unfiltered a-wave) and peak of each filtered trace were measured and the data for multiple intensities were fitted to Eq. 1. For purposes of finding the local maximum, a time window of 10 to 200 ms after the flash was used. To find the negative scotopic threshold response (nSTR), a local minimum was found in a time window 150 to 250 ms after the flash was used. For purposes of illustrating the positive scotopic threshold response (pSTR) and the nSTR, both a digital 60-Hz notch filter and a Gaussian smoothing filter (IGOR Pro, WaveMetrics, Lake Oswego, OR) were used.
For stronger stimuli, 1500-W Novatron (Dallas, TX) xenon flash lamps provided intense illumination. For these light levels, time between flashes was increased to ≤2 min to allow the retina to dark adapt.
Immunohistochemistry
Immunohistochemistry was performed using the indirect antibody method. For vibratome immunolabeling experiments, free-floating 40-μm vibratome sections were blocked with 10% normal donkey serum in phosphate-buffered saline (PBS) with 0.5% Triton X-100 and 0.1% sodium azide for 2 h to overnight to reduce nonspecific labeling. The tissues were incubated in primary antibodies in the presence of 3% donkey serum and PBS with 0.5% Triton X-100 and 0.1% sodium azide for 1–5 days at 4°C. Tissues were then washed with PBS containing 0.5% Triton X-100 and 0.1% sodium azide; tissue immunoreactivity was visualized by incubating with fluorescent secondary antibodies overnight. After extensive rinsing, the tissue was mounted with Vectashield (Vector Laboratories, Burlingame, CA). Mounted slides were observed with a confocal laser scanning microscope (LSM) with a krypton–argon laser (LSM 510; Zeiss, Thornwood, NY). Images were acquired using ×40 and ×63 oil-immersion objectives and processed with Zeiss LSM personal computer software and Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA). The mouse monoclonal antibody used was Connexin36 (1:500; Chemicon, Temecula, CA). The rabbit polyclonal antibody used was the alpha isoform of protein kinase C (PKCα, 1:500; Sigma–Aldrich, St. Louis, MO). Secondary antibodies used were Cy3 donkey anti-mouse IgG (1:200; Jackson ImmunoResearch, West Grove, PA) and Alexa Fluor 488 donkey anti-rabbit IgG (Molecular Probes, Eugene, OR).
RESULTS
Anatomic pathways as probed by qualitative immunofluorescence imaging
In the wildtype mouse, the rod bipolar cell (DBCR) pathway can be probed using immunocytochemistry. The DBCRs, the primary postsynaptic cells through which rod photoreceptors signal, can be visualized by using PKCα (Fig. 1 A). Connexin36 staining is seen in the outer plexiform layer (OPL), consistent with a possible role in photoreceptor coupling, and diffusely in the inner plexiform layer (IPL) (Fig. 1A), consistent with AIIAC–AIIAC and AIIAC–DBCC coupling (Veruki and Hartveit 2002). We previously published our immunohistochemical results in the retinas of the Cx36(−/−) mice, where we show loss of connexin36 staining in both the OPL and IPL, but normal staining of PKCα. We also showed that the Bhlhb4(−/−) mice exhibited normal staining for connexin36, but loss of staining for PKCα (Bramblett et al. 2004; Pang et al. 2007). Here, we show that in the rod transducin α-subunit knockout [Trα(−/−)] mouse, staining for connexin36 in both the OPL and IPL and PKCα in DBCRs (Fig. 1B). This suggests that loss of effective rod signaling does not eliminate formation of electrical synapses by connexin36 in either the outer or inner retina and that the downstream rod pathways such as the DBCRs are similarly not eliminated.
FIG. 1.
Left: connexin36 (Cx36) staining in the outer plexiform layer (OPL) and the inner plexiform layer (IPL) in the wildtype (WT, top) and rod transducin alpha-subunit knockout [Trα(−/−), bottom] mouse retinal sections. Left: Cx35/36 immunostaining is seen in both the OPL and IPL of WT and Trα(−/−) mouse retinal sections. Right: the alpha isoform of protein kinase C (PKCα) stains depolarizing rod–bipolar cells (DBCRs) in the WT (top) and Trα(−/−) (bottom) mouse retinal sections. Scale bar: 10 μm.
In vivo analysis of bipolar cell signaling pathways with dark-adapted ERG
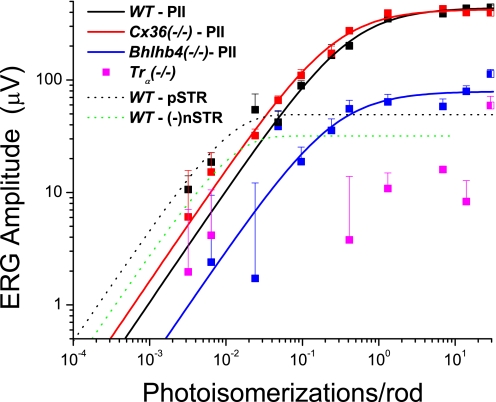
It is well established that the b-wave of the electroretinogram (ERG) represents the overall extracellular current responses of retinal bipolar cells to light flashes (Robson et al. 2004; Stockton and Slaughter 1989; Tian and Slaughter 1995) and responses to brief flashes in mice are dominated by responses of on, or depolarizing bipolar cells (DBCs) (Robson et al. 2004), although there is some evidence that photoreceptor activity may have minimal effects on these amplitudes (Hood and Birch 1996). To analyze how bipolar cell pathways function in vivo, we examined dark-adapted ERG b-waves of the WT, Cx36(−/−), Bhlhb4(−/−), and Trα(−/−) mice over the entire operation range of the dark-adapted mouse retina (>9 log units; 500-nm monochromatic flashes) (Fig. 2). (See Supplemental Fig. S1 to compare C57/Bl6 responses to a small number of wildtype littermates, showing that these strains have been sufficiently backcrossed that strain differences in these recordings are negligible.) The response–intensity relations (Fig. 3 A) of the ERG b-waves reveal four distinct light zones of increasing stimulus intensity, in which notable differences between b-wave amplitudes of the four mouse stains could be identified. Since each of the three mutant mouse strains has a known pathway-specific deletion, we were able not only to determine relative contributions of these deleted pathways to ERG responses for each of the four light zones, but also to compare ERG results to previously published suction and whole cell voltage-clamp experiments. These four zones were defined as follows: zone I, <0.1 photoisomerizations/rod; zone II, between 0.1 and 30 photoisomerizations/rod; zone III, between 30 and 103 photoisomerizations/rod; and zone IV, >103 photoisomerizations/rod.
FIG. 2.
Raw electroretinogram (ERG) waveforms of WT, connexin36 knockout [Cx36(−/−)], depolarizing rod–bipolar cell (DBCR) knockout [Bhlhb4(−/−)], and Trα(−/−) mice using a wide light stimulus range (1.25 × 10−3 to 1.9 × 106 photoisomerizations/rod). All stimuli were 500 nm, except the 2 strongest stimuli, which were white light.
FIG. 3.
A: plots of the filtered maximum b-wave amplitudes for a range of increasing stimulus strengths for WT (black), Cx36(−/−) (red), Bhlhb4(−/−) (blue), and Trα(−/−) (pink) mice reveal 4 distinct zones of differing b-waves for the different mice. Black, red, and blue lines are Naka–Rushton fits for the b-wave (see Table 1). Numbers in parentheses indicate the number of light flashes at that intensity, as well as the time, in seconds, between flashes (#flashes, seconds between flashes). B: dynamic range (defined as 0.05–0.95 of maximum response) of different retinal neurons from single-cell recordings. Rod data are from suction-electrode recordings from outer segments (Field and Rieke 2002). M- and S-cone data are suction electrode data from Nikonov et al. (2006) who recorded from cone outer segments of wildtype (WT) mice. M/S-cone dynamic range is extrapolated from the fact that many cones coexpress both pigments (Applebury et al. 2000). Dynamic range for rod depolarizing bipolar cells (DBCRs), cone depolarizing bipolar cells (DBCCs), and AII amacrine cells (AIIACs) from light-evoked voltage-clamp experiments (Pang et al. 2004). These are excitatory currents (ΔIc) elicited at ECl (−60 mV) (Pang et al. 2004).
In zone II (between 0.1 and 30 photoisomerizations/rod), the WT mice showed a characteristic b-wave that was well fit with a saturating hyperbolic function (Naka–Rushton; see Eq. 1) with a bmax,scot of 451 μV and an I0.5 of 0.31 photoisomerization/rod (solid black line in Fig. 3A and Table 1). Cx36(−/−) mice had very similar b-waves with similar saturating values and sensitivities (solid red line in Fig. 3A and Table 1), suggesting that deletion of connexin36 results in only negligible changes in bipolar cell responses in this light zone. The Bhlhb4(−/−) mice have a diminished b-wave in this zone, consistent with the b-wave in this zone observed in WT and Cx36(−/−) being largely mediated by rod bipolar cells; however, the Bhlhb4(−/−) mice did exhibit a saturating, though much smaller positive-going response (blue squares and blue solid line in Fig. 3A), which is likely to represent the responses of the high-sensitivity cone DBCs (DBCC1s) (Pang et al. 2004). The Trα(−/−) mice had a response that could not be differentiated from background noise, consistent with the b-wave in this zone being derived from rod-mediated responses (including the rod bipolar cell responses; Robson et al. 2004). This response has commonly been given the name “PII” after Granit (1933).
TABLE 1.
Naka–Rushton fits of b-wave (PII) in zone II
| Genotype | bmax, μV | I0.5, Rh*/rod | R2 |
|---|---|---|---|
| WT (n = 12) | 439 ± 11 | 0.40 ± 0.05 | 0.99 |
| Cx36(−/−) (n = 10) | 426 ± 17 | 0.26 ± 0.05 | 0.96 |
| Bhlhb4(−/−) (n = 10) | 79 ± 10 | 0.26 ± 0.09 | 0.95 |
Values are means ± SD.
In the first light zone (zone I, <0.1 photoisomerization/rod), the WT ERG exhibited positive and negative scotopic threshold responses (pSTRs and nSTRs; Fig. 4 A), which have been described previously in mice (Saszik et al. 2002). pSTRs and nSTRs are distinct from the stereotypic b-wave in that they are much more sensitive, they saturate at lower light levels, and are thought to arise from third-order retinal cells. We found that the different mouse strains exhibited different responses in this zone (Fig. 4, A and B).
FIG. 4.
A: in zone I (<0.1 photoisomerizations/rod), very dim stimuli were used to elicit positive and negative scotopic threshold responses (pSTRs and nSTRs). B: all mice had a positive-going response in zone 1; however, only WT mice had nSTR, which can also be fit with an exponential equation.
nSTRs were observed in WT mice, becoming maximal about 150 ms after the pSTR peak, but was not found in Cx36(−/−) and Bhlhb4(−/−) mice (Fig. 4, A and B). WT nSTR data points can be fit by an exponential function of the following form (solid black line, Fig. 4B and Table 2)
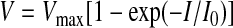 |
(2) |
TABLE 2.
Exponential fits of pSTR and nSTR in zone I
| Genotype | Vmax, μV | I0 | R2 |
|---|---|---|---|
| WT pSTR (n = 10) | 49 ± 9 | 1 ± 1 × 10−2 | 0.87 |
| WT nSTR (n = 10) | −50 ± 8 | 4 ± 2 × 10−2 | 0.86 |
Values are means ± SD.
The WT mouse had a robust pSTR, whereas the Cx36(−/−) and Bhlhb4(−/−) mice had positive-going responses with lower amplitudes and different sensitivity. Although the Naka–Rushton equation fit the data for the WT and Cx36(−/−) mice in zone II (PII) (Fig. 3A), the positive-going responses of WT mice in zone I were clearly positively deviated from this hyberbolic function, as previously shown in WT mice (Saszik et al. 2002). For the responses in zone I, Eq. 2 was found to be an appropriate fit (the pSTR, black dotted line in Fig. 5 and Table 2).
FIG. 5.
Analysis of the b-wave reveals 2 components: a sensitive pSTR shown as a dashed line and a less sensitive PII component, shown as solid lines. WT mice had a robust pSTR, which could be fit by an exponential function (see Table 2), whereas responses of Cx36(−/−) and Bhlhb4(−/−) mice in the range that produced the pSTR were not fit to the exponential function. For PII, WT and Cx36(−/−) had very similar maximum amplitudes and sensitivity (see Table 1).
Data points from the Cx36(−/−) and Bhlhb4(−/−) mice in this zone, on the other hand, were not consistently larger than the extension of the fit to the b-wave in zone II, although the Cx36(−/−) did have some relatively large responses (e.g., Fig. 4A) that appeared to deviate from the PII fit (Fig. 5). Due to the fact that the Cx36(−/−) lacked an nSTR, it was felt that the positive-going responses in this zone could be due to underlying PII being revealed when nSTR was lost and any residual pSTR that was present. Because of the limitations of our stimulus, we were unable to differentiate between a very sensitive pSTR and a slightly less sensitive positive scotopic response (pSR) described by Saszik et al. (2002); the sensitivity and maximum amplitude of our pSTR is similar to the pSR of the previous study.
In zone III (between 30 and 1 × 103 photoisomerizations/rod), the WT ERG b-wave amplitude had a slight plateau, but increased in amplitude as stimulus strength was further increased (Fig. 3A). However, the response of the Cx36(−/−) mice had a distinct dip in amplitude in this zone, recovering to their zone II saturated amplitudes only at the end of zone III. This reduction in response in Cx36(−/−) mice suggests that the plateau and rise in WT b-wave in this zone required that connexin36 must be functional. In contrast, for Bhlhb4(−/−) mice the b-wave amplitude in zone III grew at a similar rate and to an amplitude similar to that of the WT mice (but with lower starting point). In the Trα(−/−) mice, an increase in the b-wave amplitudes with similar rate (with even lower starting point) are seen starting at zone III, although their b-wave amplitudes do not reach the level of the Bhlhb4(−/−) mice until zone IV.
In zone IV (>1 × 103 photoisomerizations/rod), WT ERG b-waves continued to grow and eventually saturated at about 1 × 106 photoisomerizations/rod. ERG b-waves of Cx36(−/−) mice in the final zone began to increase in amplitude at very similar rates, but did not reach WT amplitudes until the two strongest stimuli were used (Fig. 3A). ERG b-wave amplitudes of Bhlhb4(−/−) mice continued the increase started in zone III, but did not reach the maximum amplitudes of WT or Cx36(−/−) mice. Responses of Trα(−/−) mice also continued to increase in amplitude in zone IV, reaching the level of the Bhlhb4(−/−) mice for the strongest light stimulus.
Comparison of single-cell response dynamic ranges of WT mice with ERG responses of different mouse strains reveals functional organization of bipolar cell pathways
Single-cell light responses of various types of mouse retinal neurons in dark-adapted WT mice, published previously from our laboratory and others, can be useful in determining cellular origins of ERG responses in different light zones, as well as in understanding the functional organization of various bipolar cell pathways. In Fig. 3B, we replotted the response dynamic ranges [light range needed to produce 5% (threshold) to 95% of the cells' maximum responses] of rod photocurrents (Field and Rieke 2002), M-cones, S-cones photocurrents (Nikonov et al. 2006), and the results of current recordings under voltage clamp from rod and cone DBCs and AIIACs (Pang et al. 2004). The light range in zone I is clearly below the average sensitivity of individual rods. However, due to signal convergence from rods to rod bipolar cells (DBCRs) (Smith et al. 1986), DBCRs in the mouse retina start to show light responses at about 0.1 photoisomerization/rod (Pang et al. 2004) and the ERG b-wave, which sums over bipolar cells, will be present at even lower light levels, although small in amplitude, as predicted by the Naka–Rushton fit to the data in zone II. A cone DBC (DBCC1), with the cone DBC morphology, a DBCR-like response threshold, and a higher saturation point, has been found in dark-adapted WT mouse retina (Pang et al. 2004) and its dynamic range is also given in Fig. 3B. Moreover, a second level of convergence and high synaptic gains from DBCs to AII amacrine cells (AIIACs) led to a response threshold of about 1 × 10−3 photoisomerizations/rod in AIIACs (Pang et al. 2004).
The single-cell response range plots in Fig. 3B indicate that the highly sensitive pSTR and nSTR in zone I fall into the same dynamic ranges of rod-driven third-order cells in the proximal retina. It is important to note that nSTR is a negative voltage response, suggesting that it is a radial current flow from the proximal retina to the distal retina, in the opposite direction as the radial current underlying the stereotypic b-wave. Because the stereotypic b-wave represents the radial current flow in DBCs injected into the dendrites from the photoreceptor output synapses, the nSTR would represent a radial current injected into the axonal end of the bipolar cells and/or contributions of Mueller cells (see following text). An excellent neuronal candidate for the nSTR source is the gap junction between AIIACs and DBCCs, since light responses of AIIACs lie in the same dynamic range of the nSTR (Figs. 3 and 4), and AIIACs are connected to DBCCs through gap junctions in the proximal retina (Veruki and Hartveit 2002), so that light-evoked depolarizing AIIAC current may enter the DBCC axon terminals and form a proximal-to-distal flow. The absence of the nSTR in the Cx36(−/−) and Bhlhb4(−/−) mice supports this idea, since the gap junctions between AIIAC and DBCCs are mediated by connexin36 (Deans et al. 2002) and AIIAC response sensitivity is greatly reduced in the absence of DBCRs [in the Bhlhb4(−/−) mice] (Pang et al. 2007).
The precise origin of the pSTR is not clear. It may be present with reduced amplitude in Cx36(−/−) mice and is not clearly adding to PII in Bhlhb4(−/−) mice (Fig. 4), consistent with the third-order retinal neuron generating the pSTR receiving substantial inputs from Cx36-dependent pathways and DBCRs. It has also been shown that RGC survival is essential for the presence of the pSTR in rat (Bui and Fortune 2004) and mouse ERGs (Moshiri et al. 2008); thus high-sensitivity (rod-dominated) ganglion cells are the top candidates for the pSTR source.
In zone II (between 0.1 and 30 photoisomerizations/rod), dark-adapted WT mice show b-wave responses that follow a hyperbolic saturating response–intensity curve (Fig. 3A, black line, Naka–Rushton equation) of shape and dynamic range similar to those of the DBCRs and DBCC1s (Fig. 3B) (Pang et al. 2004). The b-wave of the Cx36(−/−) exhibits a nearly identical response–intensity curve (red line in Fig. 3A), although the Bhlhb4(−/−) mice have much smaller (though saturating) b-waves (blue line in Fig. 3A). These results suggest that the dark-adapted b-wave responses in this zone represent predominantly DBCR responses (Robson et al. 2004), which are unchanged in the Cx36(−/−) mice and absent in the Trα(−/−) mice. The residual small (and saturating) b-waves in the Bhlhb4(−/−) mice are likely to reflect DBCC1 responses (see following text).
In zone III (between 30 and 1 × 103 photoisomerizations/rod), rod light responses still have not saturated, although DBCR responses have saturated (Nikonov et al. 2006; Pang et al. 2004), and cones have not started to respond to light (Nikonov et al. 2006) (see dynamic range plots in Fig. 3B). Therefore the most likely source for the increasing b-wave responses in this zone is the signal from rods to DBCCs because saturated DBCRs cannot be responsible for the growing b-wave. Moreover, in Bhlhb4(−/−) mice, where rod–cone coupling and the rod–DBCC chemical synapses are presumably intact, the DBCC1-mediated b-wave (although much smaller due to lack of DBCRs) continues to grow in zone III. In Cx36(−/−) mice, b-wave amplitudes do not grow, but instead dip in this zone, indicating that the b-wave increase in the WT mice is subserved by the connexin36-mediated rod–cone coupling, which becomes crucial for continued growth of the b-wave in this light zone (thus rod signal → cone → DBCC) (Yang and Wu 1989). The b-wave of Cx36(−/−) mice does not plateau, as expected, due to the saturation of the DBCRs, but rather dips at the top of this zone, indicating that some adaptation of the DBCR response may be occurring here and is uncovered in these mutants.
In zone IV (stimuli stronger than 1 × 103 photoisomerizations/rod), cones (most cones have mixed M and S pigments; Applebury et al. 2000) begin to be sensitive to 500-nm light flashes (Nikonov et al. 2006) (see dynamic range plots in Fig. 3B) and start to send signals to various types of DBCCs, resulting in increases in the b-wave amplitude. Moreover, as light becomes brighter, more and more DBCCs (such as the S-cone–driven DBCCs, which are very insensitive to 500-nm light and respond only to the strongest stimuli) are recruited to contribute to the b-wave, although the contribution of the photoreceptors cannot be totally ignored and have been shown to contribute minimally in other models (Hood and Birch 1996). In Fig. 3A, b-waves of Cx36(−/−) mice in zone IV are smaller than the WT b-waves for all but the strongest stimuli, where they show the same saturation amplitude. This suggests that connexin36 is involved in mediating the b-wave increase in this zone. It is possible, for example, that part of the b-wave increment is mediated by an M-cone → S-cone → S-cone DBC pathway; if the M-cone–S-cone coupling is mediated by connexin36, then the b-wave increase should be reduced in Cx36(−/−) mice. Our results also show that the Bhlhb4(−/−) and Trα(−/−) response–intensity plots have slopes similar to those (although with different starting points) of the WT mice in this zone, suggesting that the cone → DBCC pathways in these mutant mice are scarcely altered.
DISCUSSION
Rod and cone synaptic pathways in dark-adapted mouse retina
By analyzing scotopic ERG b-waves of WT, Cx36(−/−), Bhlhb4(−/−), and Trα(−/−) mice over a wide light range (>9 log units in four zones) and comparing with previously published single-cell responses in dark-adapted WT mice, we propose that various scotopic b-wave components in the four intensity zones are mediated by six rod and cone synaptic pathways (Fig. 6). Pathways 1 and 4 are two well-known rod and cone bipolar cell information channels established by previous anatomical and physiological studies of the mammalian retina (Feigl et al. 2005; Field and Rieke 2002; Kolb and Nelson 1983). Pathways 2 and 3 have been proposed since a DBC subtype with cone DBC morphology and DBCR-like sensitivity (DBCC1) was found in the dark-adapted mouse retina (Pang et al. 2004). The rod-mediated high sensitivity in DBCC1s is thought to result from rod–cone coupling (Pang et al. 2004) and/or direct rod → DBCC1 chemical synapses (Tsukamoto et al. 2007). Our observation that the Bhlhb4(−/−) mice have a robust (though smaller) b-wave in zone II supports the presence of these DBCC1 pathways. Another possible origin of this small b-wave may be residual DBCRs that have not been totally knocked out. However, a previous publication (Bramblett et al. 2004) shows that staining for PKCα, a known marker for DBCR cells, was reduced by ≥92% and that the remaining PKCα-staining cells costained with Pax6, a pan-amacrine marker, making them most likely amacrine cells (see also Kim et al. 2008). Moreover, in an analysis of amacrine II cell (AIIAC) light responses, we have shown that AIIACs have identical responses in wildtype mice treated with the ionotropic glutamate receptor antagonist DNQX (which effectively blocks the DBCR to AIIAC synapse) and Bhlhb4(−/−) AIIACs (Pang et al. 2007).
FIG. 6.
Schematic diagram of the 6 rod and cone synaptic pathways mediating various ERG b-wave components in dark-adapted mouse retina. R, rod; C, cone; DBCR, rod depolarizing bipolar cell; DBCC1, high-sensitivity cone depolarizing bipolar cell; DBCC2, low-sensitivity cone depolarizing bipolar cell; AII, AII amacrine cell; ONGCs: on ganglion cells; thick red arrows (±: sign-preserving/sign-inverting), glutamatergic synapses; red zigzags, electrical synapses; PRL, photoreceptor layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Additionally, Fig. 3A shows that the scotopic b-wave in Cx36(−/−) mice is essentially indistinguishable from the WT b-wave in zone II (between 0.1 and 30 photoisomerizations/rod), suggesting that pathway 3 is more prominent than pathway 2 in mediating rod signals in the DBCC1s, and thus rod–cone coupling (presumably mediated by connexin36) is weak at these light levels. At higher light levels (zone III), however, the b-wave increase disappeared in Cx36(−/−) mice, indicating that pathway 2 (rod–cone coupling) is operational in WT mice in this range. Pathways 5 and 6, as previously suggested, are proposed for the pSTR and/or nSTR in zone I, because only third-order retinal cells, such as the high-sensitivity GCs and AIIACs, have such high sensitivity (Pang et al. 2002), consistent with evidence from early studies, initially for the nSTR in the cat (Naarendorp and Sieving 1991; Robson and Frishman 1995; Sieving et al. 1986) and later for both nSTR and pSTR in rat and mouse (Naarendorp et al. 2001; Saszik et al. 2002). The amplitudes of pSTR and nSTR are relatively small, partially because they both saturate at such low light levels and because GCs and AIIACs do not have the long processes extending radially from outer to inner retina that the bipolar cells do (Pang et al. 2002, 2004).
The time course of the nSTR is rather slow compared with that of the b-wave and previous studies in the cat have proposed a Mueller cell involvement in generating the nSTR (Frishman and Steinberg 1989). Another piece of evidence that the origin of the nSTR is not the RGCs is a recent study where RGC loss in the mouse did not cause loss of the nSTR (Moshiri et al. 2008).
The analysis in this study provides an in vivo tool for dissecting various rod and cone signaling pathways of the mammalian retina. Since each of the three mutant mice has pathway-specific deletions [connexin36, DBCR, and rod photocurrent for the Cx36(−/−), Bhlhb4(−/−), and Trα(−/−) mice, respectively], we were able to estimate the relative contributions of each of these deletions to the overall responses. For example, by deleting DBCRs in the Bhlhb4(−/−) mice, the overall responses (b-wave) in zone II were reduced to about one fifth of the control value, which represents largely the DBCC1 contribution. By taking out rod photocurrent in Trα(−/−) mice, the overall bipolar cell responses are absent in zones I and II, consistent with the notion that zones I and II are mediated by rod signals. Trα(−/−) b-wave starts to appear in zone III, where cones begin to function (Fig. 3B), and reach a maximum in zone IV (near 103 photoisomerizations/rod) of about half the size as the maximum b-wave in the WT mice, suggesting that rod and cone inputs to DBCs contribute near equally to the overall DBC response to very bright flashes. The absence of connexin36 in AIIACs and photoreceptors [in the Cx36(−/−) mice], on the other hand, caused little change in DBCR and DBCC1 responses (zone II), but it reduced pSTR and abolished nSTR, in agreement with the idea that these high-sensitivity response components are mediated by Cx36 gap junctions in AIIACs (Deans et al. 2002).
Deletion of Cx36 also reduced the overall DBC response to bright flashes by about 10% in all but the largest stimuli of zone IV, suggesting that Cx36 is involved in the cone → DBC signaling pathways (or, perhaps, cone–cone coupling).
Operational plan of the dark-adapted mouse retina for encoding a large range of light intensities
In this study, we have gained insight into the operational plan that the mouse retina uses to encode a large range of stimulus strengths in the dark-adapted state. At the lowest light levels (<0.1 photoisomerization/rod, zone I), large amplification of rod signals is needed in third-order retinal neurons to provide reliable responses to dim light information. Based on modeling of the passive electrotonic properties of rods and cones, it was proposed that in the “low-scotopic” range, sharing of current between the rods and cones would hinder efficient transfer of signal between the rods and DBCRs (Smith et al. 1986), which would not allow the retina to maintain its exquisite sensitivity (Hecht et al. 1941). It has also been demonstrated in amphibian retina that rod–cone coupling is very weak at low light conditions (Yang and Wu 1989). Voltage recordings in macaque monkey cones have shown that only about 15% of the rod signal is present in cone pedicles, making the influence of weak rod signals small (Schneeweis and Schnapf 1995). Therefore in zone I, rod signals are carried by the rod–DBCR pathway (pathway 1) and/or rod–DBCC1 pathway (pathway 3) only.
At photon-counting levels (0.1 to 30 photoisomerizations/rod, zone II), rod–cone coupling is also weak, with rods conveying their responses to the DBCRs in a faithful manner, not allowing for any loss of signal to the cone photoreceptors. Our results show that scotopic b-waves in WT and Cx36(−/−) mice are indistinguishable, which indicates that rod responses in this zone are transmitted either directly to DBCRs or directly to DBCC1s, without significant passive spread into cones (through Cx36-mediated gap junctions) (Deans et al. 2002; Pang et al. 2004). Had passive spread to other rods or cones been a major pathway under this low-light conditions, we would have expected the DBCR response in the Cx36(−/−) mouse to be more sensitive and the DBCC1 response in the Cx36(−/−) mouse to be less sensitive than the corresponding responses in the WT. Preliminary single-cell studies show that dark-adapted DBCRs and DBCC1s in Cx36(−/−) mice have the same sensitivity as their counterparts in WT (Pang and Wu, unpublished results), supporting the idea that rod–cone coupling is weak in zone II.
The deviation in the b-wave between Cx36(−/−) mice and the WT mice in zone III suggests that the Cx36-dependent rod–cone coupling (rod → cone → DBCC pathway) begins to function in the WT in this light range. An explanation as to why positive-going responses were recorded in Trα(−/−) mice in zone III, where we predict cones are not sensitive, comes from the observation that cone responses in this mouse model appear to be slightly more sensitive than WT cone responses in retinal preparations, mostly due to the lack of an interfering rod dark current and not due to intrinsic differences in the cone responses (Nikonov et al. 2006). At levels where rods and cones are active (30–103 photoisomerizations/rod), zone III DBCR responses saturate, whereas rod photoreceptor responses have not (Fig. 3B). Instead, electrical synapses between rods and cones become important in allowing rod signals access to the parallel cone bipolar pathways. Although cones represent only 3% of the mouse photoreceptors (Carter-Dawson and LaVail 1979), cone bipolar cells constitute roughly 50% of all the bipolar cells in the mouse retina (Ghosh et al. 2004)—making them robust channels for signal transmission to the inner retina. Our results showing that the Cx36-dependent rod–cone coupling is active when stimuli are strong provides in vivo support for previous reports that gap junctions are not simply passive-resistive components, but rather have significant active properties (Zhang and Wu 2005) and can be modulated by light (Yang and Wu 1989) or activity-dependent processes (Kothmann et al. 2007; Sitaramayya et al. 2003). This is also reminiscent of observations in cat cone horizontal cell bodies, in which the threshold of rod responses was found to be approximately −0.5 log sc td, corresponding to 38Rh*/rod (presumably mediated by the rod → cone → HC pathway; Steinberg 1969), similar to the stimulus strength at which the b-wave indicates that Cx36-dependent rod–cone coupling was necessary for the b-wave plateau (zone III). Further evidence of rod–cone coupling modulation was seen in recording from B-type horizontal cell bodies in WT and Cx36(−/−) mice (Trumpler et al. 2008).
Finally, for zone IV (>1 × 103 photoisomerizations/rod), M-cone and S-cone signals are transmitted to DBCCs (pathway 4) and our data showing that b-waves of WT mice are larger with a steeper slope to saturation than those in Cx36(−/−) mice (Fig. 3A) suggest that a Cx36-dependent cone–cone coupling may be active in this zone in WT mice. Although other studies have shown that M-cone and S-cone pathways may be independent at the level of the photoreceptors (Li and DeVries 2004), the coexpression of M- and S-opsin in mouse retinas (Applebury et al. 2000; Nikonov et al. 2006) suggests that this may not be the case; thus further work may elucidate the existence and characteristics of such coupling.
Use of knockout mouse models to isolate retinal pathways
We have shown the utility of using specific knockout mouse models in trying to understand the overall signaling paradigms of the mammalian retina in this study. This is a powerful technique because it is not always possible to isolate certain responses using pharmacology or different stimuli. In a previous study, we show the close correspondence between using two of these knockouts [Cx36(−/−) and Bhlhb4(−/−)] and pharmacologic isolation of the different rod and cone pathways on the responses of the AIIAC (Pang et al. 2007). It is important to note that neuronal reorganization and rewiring have been shown to occur in some mouse models of retinal degeneration (Marc et al. 2007). However, immunohistochemical (see, e.g., Fig. 1 and Pang et al. 2007) and electrophysiological (Pang et al. 2007) evidence suggests that remodeling is not a major confounding factor in the mouse models we have used. Specifically, in the Bhlhb4(−/−) mouse, although there is a complete loss of DBCRs, subsequent studies have shown the maintenance of DBCC, amacrine cell, and ganglion cell populations (Kim et al. 2008).
For the Trα(−/−) mouse, morphological studies showed maintenance of ≥90% of the outer nuclear layer (ONL) at 13 wk of age and only scant changes in the secondary and tertiary neurons of the retina (Calvert et al. 2000). For the Cx36(−/−) mouse, marker distribution in Cx36(−/−) and KO knockout animals showed no loss of cells that express Cx36 in WT mice and there were no changes in the number of ganglion cells (Deans et al. 2002), although it is entirely possible that any effects of knocking out specific gene products are subtle and not discovered by these techniques.
Comparison with other studies
Two studies of fast-responding RGCs (Deans et al. 2002; Volgyi et al. 2004) showed, using extracellular recordings, the existence of at least three on pathways by recording from WT and Cx36(−/−) mice. In Cx36(−/−) mice, the primary and secondary pathways were lost. Our results are consistent with this finding. The loss of the primary pathway response is most likely due to the dependence of the AIIAC–DBCC electrical synapse on Cx36 (Deans et al. 2002; Maxeiner et al. 2005). The loss of the secondary pathway predicts the loss of growing b-wave in zone III observed in our study. These studies did not find evidence of the tertiary rod pathway (rod → DBCC1), but characteristics of ganglion cells potentially receiving input from this novel pathway are presently unknown.
Human studies have shown the presence of two distinct rod pathways, reviewed in Sharpe and Stockman (1999). Much of the evidence for the existence of at least two different rod pathways in humans comes from flicker ERGs, which show a loss of ERG amplitudes at about 15 Hz. It is hypothesized that this loss is due to destructive interference of the two canonical rod pathways. Interestingly, in the human it is hypothesized that the secondary rod pathway may by extremely robust, exceeding the primary pathway response amplitudes by a factor of two. Mouse flicker ERGs show a very similar decline in amplitudes at frequencies of about 15 Hz, although the hypothesized signal coming from rod–cone coupling was approximately half of the signal from the primary pathway (Nusinowitz et al. 2007).
Instead of observing the effects of eliminating rod signals, other studies have examined the effect of eliminating cone signals on the ERG. Ideally, this would allow a look at the extent of the rod signaling in all of its pathways. Two recent studies of the GNAT2cpfl3 mutant mouse (which is a mutation in the gene that encodes the cone α-transducin) have taken this approach. The first study (Chang et al. 2006) showed that using standard ERG protocols, the GNAT2cpfl3 mutant did not exhibit a significant change in b-wave amplitudes compared with that of wildtype. The second study (Nusinowitz et al. 2007) used flicker ERGs and did show a selective loss of the second amplitude maximum, which they attributed to loss of the secondary pathway. They hypothesized that rod–cone coupling was disrupted, although this was not directly tested in immunohistochemical, biochemical, or anatomical studies. However, other mutations in mice that eliminate cone function, such as elimination of the cyclic nucleotide-gated channel CHGA3, have been shown to lead to cone degeneration (Michalakis et al. 2005).
As stated by Nusinowitz et al. (2007) the apparent discrepancies between these two ERG studies of the GNAT2cpfl3 mouse can be attributed to the fact that with standard ERG recordings (stated to be 1 Hz; Nusinowitz et al. 2007) the primary rod pathway is the major throughput of visual information to secondary neurons. As the frequency is increased, the secondary pathway becomes functional. This is a plausible hypothesis, in that we have shown that rod–rod coupling in the tiger salamander is frequency dependent (Zhang and Wu 2005) as is rod–cone coupling (Wu, unpublished data) and rod–cone coupling is light dependent (Yang and Wu 1989).
However, similar to our results, Nusinowitz et al. (2007) showed that light level also played an important role in separating these pathways, with the local minimum in response amplitudes occurring at about 30 photoisomerizations/rod which is in our zone III, or where we predict that rod–cone coupling through Cx36 in the mouse is of functional consequence.
The frequency dependence of rod–cone coupling can partially explain the discrepancies of our findings of normal scotopic b-waves in both Cx36 KOs (zone II) and the dramatic loss of b-wave described by others (Güldenagel et al, 2001). In that study, ERGs were recorded using a longer-duration light stimulus (10 ms) and shorter intervals between flashes (20 s) compared with the standard microsecond flash stimulus and intervals sometimes exceeding 2 min in the present study. The shorter intervals between flashes will not allow the retina to remain dark-adapted for strong flashes, although it could be argued that the same desensitization would carry over to the wildtype. However, the recent molecular and biochemical evidence that electrical synapses are capable of being phosphorylated (Kothmann et al. 2007; Urschel et al. 2006) could raise the point that this rapid activity may be stimulating such pathways for modulation of the rod–cone coupling.
A recent study by Ribelayga et al. (2008) suggested that rod–cone coupling (as assessed by neurobiotin tracer diffusion) in the CBA/CaJ mouse is controlled by an intrinsic circadian clock in the retina and is much stronger at night. We have not made systematic studies to look for evidence of this phenomenon, under tightly controlled conditions; however, we have found evidence during daytime hours of rod–cone coupling in ERGs, as well as in single-cell recordings in strains of mice other than CBA/CaJ, characterized by low b-wave amplitude and sensitivity (Pinto et al. 2007).
In an interesting study to assess contrast sensitivities of mice using an optomotor response, Umino et al. (2008) showed that GNAT2cpfl3 mice have contrast sensitivities that are very similar to those of WT (C57BL/6) mice at low light levels, consistent with our results that the rod visual pathways are the major determinants of visual function at the lowest light levels. Similarly, using the rod–transducin knockout Trα(−/−), they showed that these mice do not start to display contrast sensitivity until a mean luminance equivalent to 10−3 photoisomerizations/rod. Comparing the contrast sensitivities across a 109 unit mean luminance range, they also show that using only the rod-vision mice and cone-vision mice, there is a dip in contrast sensitivities compared with WT for about 2 log units of mean luminance. From the results of our study, this dip might be explained by loss of the additive value of rod vision entering cone pathways through Cx36, although it is difficult to compare studies directly because adaptational effects will play a major role in affecting neuronal function in such constant luminance experiments (Dunn and Rieke 2008; Dunn et al. 2006).
In summary, the dark-adapted mouse retina encodes visual images over a very wide light range (>9 log units) by several parallel DBC synaptic pathways, as illustrated in Fig. 6. The rod → DBCR/DBCC1 → AIIAC/high sensitivity GC pathway (weak rod–cone coupling) provides sensitive responses, whereas the additional rod → cone → DBCC1 → AIIAC/GC pathway provides intermediate signals and the additional cone(→ cone) → DBCC1/DBCC → AIIAC/GC signals very strong stimuli. The light-dependent additional pathways are controlled by switching on and off of the Cx36-mediated gap junctions between rods and cones, by the relative sensitivity of rod, M-cone and S-cone pigments, and perhaps by other light-dependent processes in the retina. In the presence of steady mean illumination, as occurs in nature, additional mechanisms of adaptation also would be present. The interplay between “hard-wire” synaptic pathways and synaptic modulation therefore dictate the overall operation of the mammalian retina.
GRANTS
This work was supported by National Eye Institute (NEI) Grant EY-04446 to S. M. Wu, National Institute of General Medical Services Grant GM-37751 and NEI Grant EY-014127 to D. Paul, EY-012008 to J. Lem and grant EY-06671 to L. Frishman, NEI Vision Core Grant EY-02520, National Institute of Child Health and Human Development Grant HD-04260, Retina Research Foundation (Houston), International Retinal Research Foundation, and Foundation Fighting Blindness and Research to Prevent Blindness, Inc.
Supplementary Material
Acknowledgments
We thank C. Cowan for carefully reading manuscript and helpful comments.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Applebury et al. 2000.Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27: 513–523, 2000. [DOI] [PubMed] [Google Scholar]
- Bramblett et al. 2004.Bramblett DE, Pennesi ME, Wu SM, Tsai MJ. The transcription factor Bhlhb4 is required for rod bipolar cell maturation. Neuron 43: 779–793, 2004. [DOI] [PubMed] [Google Scholar]
- Bui and Fortune 2004.Bui BV, Fortune B. Ganglion cell contributions to the rat full-field electroretinogram. J Physiol 555: 153–173, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert et al. 2000.Calvert PD, Krasnoperova NV, Lyubarsky AL, Isayama T, Nicolo M, Kosaras B, Wong G, Gannon KS, Margolskee RF, Sidman RL, Pugh EN Jr, Makino CL, Lem J. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha-subunit. Proc Natl Acad Sci USA 97: 13913–13918, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson and LaVail 1979.Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol 188: 245–262, 1979. [DOI] [PubMed] [Google Scholar]
- Chang et al. 2006.Chang B, Dacey MS, Hawes NL, Hitchcock PF, Milam AH, Atmaca-Sonmez P, Nusinowitz S, Heckenlively JR. Cone photoreceptor function loss-3, a novel mouse model of achromatopsia due to a mutation in Gnat2. Invest Ophthalmol Vis Sci 47: 5017–5021, 2006. [DOI] [PubMed] [Google Scholar]
- Crooks and Kolb 1992.Crooks J, Kolb H. Localization of GABA, glycine, glutamate and tyrosine hydroxylase in the human retina. J Comp Neurol 315: 287–302, 1992. [DOI] [PubMed] [Google Scholar]
- Deans et al. 2002.Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron 36: 703–712, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek et al. 2006.Dedek K, Schultz K, Pieper M, Dirks P, Maxeiner S, Willecke K, Weiler R, Janssen-Bienhold U. Localization of heterotypic gap junctions composed of connexin45 and connexin36 in the rod pathway of the mouse retina. Eur J Neurosci 24: 1675–1686, 2006. [DOI] [PubMed] [Google Scholar]
- Dowling 1987.Dowling JE. The Retina, an Approachable Part of the Brain. Cambridge, MA: Harvard Univ. Press, 1987.
- Dunn et al. 2006.Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the mammalian retina. J Neurosci 26: 3959–3970, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn and Rieke 2008.Dunn FA, Rieke F. Single-photon absorptions evoke synaptic depression in the retina to extend the operational range of rod vision. Neuron 57: 894–904, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigl et al. 2005.Feigl B, Brown B, Lovie-Kitchin J, Swann P. Monitoring retinal function in early age-related maculopathy: visual performance after 1 year. Eye 19: 1169–1177, 2005. [DOI] [PubMed] [Google Scholar]
- Field and Rieke 2002.Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron 34: 773–785, 2002. [DOI] [PubMed] [Google Scholar]
- Frishman and Steinberg 1989.Frishman LJ, Steinberg RH. Intraretinal analysis of the threshold dark-adapted ERG of cat retina. J Neurophysiol 61: 1221–1232, 1989. [DOI] [PubMed] [Google Scholar]
- Ghosh et al. 2004.Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol 469: 70–82, 2004. [DOI] [PubMed] [Google Scholar]
- Granit 1933.Granit R. The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol 77: 207–239, 1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güledenagel et al. 2001.Güldenagel M, Ammermüller J, Feigenspan A, Teubner B, Degen J, Söhl G, Willecke K, Weiler R. Visual transmission deficits in mice with targeted disruption of the gap junction gene connexin36. J Neurosci 21: 6036–6044, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht et al. 1941.Hecht S, Shlaer S, Pirenne MH. Energy at the threshold of vision. Science 93: 585–587, 1941. [DOI] [PubMed] [Google Scholar]
- Hood and Birch 1996.Hood DC, Birch DG. Beta wave of the scotopic (rod) electroretinogram as a measure of the activity of human on-bipolar cells. J Opt Soc Am A Opt Image Sci Vis 13: 623–633, 1996. [DOI] [PubMed] [Google Scholar]
- Kim et al. 2008.Kim DS, Ross SE, Trimarchi JM, Aach J, Greenberg ME, Cepko CL. Identification of molecular markers of bipolar cells in the murine retina. J Comp Neurol 507: 1795–1810, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb and Famiglietti 1974.Kolb H, Famiglietti EV. Rod and cone pathways in the inner plexiform layer of cat retina. Science 186: 47–49, 1974. [DOI] [PubMed] [Google Scholar]
- Kolb and Nelson 1983.Kolb H, Nelson R. Rod pathways in the retina of the cat. Vision Res 23: 301–312, 1983. [DOI] [PubMed] [Google Scholar]
- Kothmann et al. 2007.Kothmann WW, Li X, Burr GS, O'Brien J. Connexin 35/36 is phosphorylated at regulatory sites in the retina. Vis Neurosci 24: 363–375, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li and DeVries 2004.Li W, DeVries SH. Separate blue and green cone networks in the mammalian retina. Nat Neurosci 7: 751–756, 2004. [DOI] [PubMed] [Google Scholar]
- Lyubarsky et al. 1999.Lyubarsky AL, Falsini B, Pennesi ME, Valentini P, Pugh EN Jr. UV- and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. J Neurosci 19: 442–455, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc et al. 2007.Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, Wensel T, Lucas RJ. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci 48: 3364–3371, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxeiner et al. 2005.Maxeiner S, Dedek K, Janssen-Bienhold U, Ammermuller J, Brune H, Kirsch T, Pieper M, Degen J, Kruger O, Willecke K, Weiler R. Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission 5. J Neurosci 25: 566–576, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis et al. 2005.Michalakis S, Geiger H, Haverkamp S, Hofmann F, Gerstner A, Biel M. Impaired opsin targeting and cone photoreceptor migration in the retina of mice lacking the cyclic nucleotide-gated channel CNGA3. Invest Ophthalmol Vis Sci 46: 1516–1524, 2005. [DOI] [PubMed] [Google Scholar]
- Moshiri et al. 2008.Moshiri A, Gonzalez E, Tagawa K, Maeda H, Wang M, Frishman LJ, Wang SW. Near complete loss of retinal ganglion cells in the math5/brn3b double knockout elicits severe reductions of other cell types during retinal development. Dev Biol 316: 214–227, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naarendorp et al. 2001.Naarendorp F, Sato Y, Cajdric A, Hubbard NP. Absolute and relative sensitivity of the scotopic system of rat: electroretinography and behavior. Vis Neurosci 18: 641–656, 2001. [DOI] [PubMed] [Google Scholar]
- Naarendorp and Sieving 1991.Naarendorp F, Sieving PA. The scotopic threshold response of the cat ERG is suppressed selectively by GABA and glycine. Vision Res 31: 1–15, 1991. [DOI] [PubMed] [Google Scholar]
- Naka and Rushton 1966.Naka KI, Rushton WA. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol 185: 536–555, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov et al. 2006.Nikonov SS, Kholodenko R, Lem J, Pugh EN Jr. Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol 127: 359–374, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusinowitz et al. 2007.Nusinowitz S, Ridder WH 3rd, Ramirez J. Temporal response properties of the primary and secondary rod-signaling pathways in normal and Gnat2 mutant mice. Exp Eye Res 84: 1104–1114, 2007. [DOI] [PubMed] [Google Scholar]
- Pang et al. 2007.Pang J-J, Abd-El-Barr MM, Gao F, Bramblett DE, Paul DL, Wu SM. Relative contributions of rod and cone bipolar cell inputs to AII amacrine cell light responses in the mouse retina. J Physiol 580: 397–410, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang et al. 2002.Pang J-J, Gao F, Wu SM. Relative contributions of bipolar cell and amacrine cell inputs to light responses of ON, OFF and ON–OFF retinal ganglion cells. Vision Res 42: 19–27, 2002. [DOI] [PubMed] [Google Scholar]
- Pang et al. 2004.Pang J-J, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol 558: 897–912, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennesi et al. 1998.Pennesi ME, Lyubarsky AL, Pugh EN Jr. Extreme responsiveness of the pupil of the dark-adapted mouse to steady retinal illumination. Invest Ophthalmol Vis Sci 39: 2148–2156, 1998. [PubMed] [Google Scholar]
- Pinto et al. 2007.Pinto LH, Invergo B, Shimomura K, Takahashi JS, Troy JB. Interpretation of the mouse electroretinogram. Doc Ophthalmol 115: 127–136, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga et al. 2008.Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod–cone coupling. Neuron 59: 790–801, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson and Frishman 1995.Robson JG, Frishman LJ. Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Vis Neurosci 12: 837–850, 1995. [DOI] [PubMed] [Google Scholar]
- Robson et al. 2004.Robson JG, Maeda H, Saszik SM, Frishman LJ. In vivo studies of signaling in rod pathways of the mouse using the electroretinogram. Vision Res 44: 3253–3268, 2004. [DOI] [PubMed] [Google Scholar]
- Rodieck 1998.Rodieck R. The First Steps in Seeing. Sunderland, MA: Sinauer, 1998.
- Saszik et al. 2002.Saszik SM, Robson JG, Frishman LJ. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J Physiol 543: 899–916, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeweis and Schnapf 1995.Schneeweis DM, Schnapf JL. Photovoltage of rods and cones in the macaque retina. Science 268: 1053–1056, 1995. [DOI] [PubMed] [Google Scholar]
- Sharpe and Stockman 1999.Sharpe LT, Stockman A. Rod pathways: the importance of seeing nothing. Trends Neurosci 22: 497–504, 1999. [DOI] [PubMed] [Google Scholar]
- Sieving et al. 1986.Sieving PA, Frishman LJ, Steinberg RH. Scotopic threshold response of proximal retina in cat. J Neurophysiol 56: 1049–1061, 1986. [DOI] [PubMed] [Google Scholar]
- Sitaramayya et al. 2003.Sitaramayya A, Crabb JW, Matesic DF, Margulis A, Singh V, Pulukuri S, Dang L. Connexin 36 in bovine retina: lack of phosphorylation but evidence for association with phosphorylated proteins. Vis Neurosci 20: 385–395, 2003. [DOI] [PubMed] [Google Scholar]
- Smith et al. 1986.Smith RG, Freed MA, Sterling P. Microcircuitry of the dark-adapted cat retina: functional architecture of the rod–cone network. J Neurosci 6: 3505–3517, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy et al. 1998.Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron 21: 481–493, 1998. [DOI] [PubMed] [Google Scholar]
- Steinberg 1969.Steinberg RH. Rod–cone interaction in S-potentials from the cat retina. Vision Res 9: 1331–1344, 1969. [DOI] [PubMed] [Google Scholar]
- Stockton and Slaughter 1989.Stockton RA, Slaughter MM. B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J Gen Physiol 93: 101–122, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian and Slaughter 1995.Tian N, Slaughter MM. Correlation of dynamic responses in the ON bipolar neuron and the b-wave of the electroretinogram. Vision Res 35: 1359–1364, 1995. [DOI] [PubMed] [Google Scholar]
- Trumpler et al. 2008.Trumpler J, Dedek K, Schubert T, De Sevilla Muller LP, Seeliger M, Humphries P, Biel M, Weiler R. Rod and cone contributions to horizontal cell light responses in the mouse retina. J Neurosci 28: 6818–6825, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto et al. 2007.Tsukamoto Y, Morigiwa K, Ishii M, Takao M, Iwatsuki K, Nakanishi S, Fukuda Y. A novel connection between rods and ON cone bipolar cells revealed by ectopic metabotropic glutamate receptor 7 (mGluR7) in mGluR6-deficient mouse retinas. J Neurosci 27: 6261–6267, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto et al. 2001.Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci 21: 8616–8623, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umino et al. 2008.Umino Y, Solessio E, Barlow RB. Speed, spatial, and temporal tuning of rod and cone vision in mouse. J Neurosci 28: 189–198, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urschel et al. 2006.Urschel S, Hoher T, Schubert T, Alev C, Sohl G, Worsdorfer P, Asahara T, Dermietzel R, Weiler R, Willecke K. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem 281: 33163–33171, 2006. [DOI] [PubMed] [Google Scholar]
- Veruki and Hartveit 2002.Veruki ML, Hartveit E. AII (rod) amacrine cells form a network of electrically coupled interneurons in the mammalian retina. Neuron 33: 935–946, 2002. [DOI] [PubMed] [Google Scholar]
- Volgyi et al. 2004.Volgyi B, Deans MR, Paul DL, Bloomfield SA. Convergence and segregation of the multiple rod pathways in mammalian retina. J Neurosci 24: 11182–11192, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang and Wu 1989.Yang XL, Wu SM. Modulation of rod–cone coupling by light. Science 244: 352–354, 1989. [DOI] [PubMed] [Google Scholar]
- Zhang and Wu 2005.Zhang J, Wu SM. Physiological properties of rod photoreceptor electrical coupling in the tiger salamander retina. J Physiol 564: 849–862, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.