Abstract
Stimulation of the trigeminal nerve evokes a dramatic decrease in heart rate and blood pressure, and this reflex has generally been termed the trigeminocardiac reflex. A subset of the trigeminocardiac reflex is the diving reflex in which the nasal mucosa is stimulated with water or air-borne chemical irritants. Activation of the diving reflex evokes a pronounced bradycardia, mediated by increased parasympathetic cardiac activity, and is the most powerful autonomic reflex. However, exaggeration of this protective response could be detrimental and has been implicated in Sudden Infant Death Syndrome (SIDS). Despite the importance and strength of the trigeminocardiac reflex, there is little information about the cellular mechanisms and brain stem pathways that constitute this reflex. To address these issues, stimulation of trigeminal afferent fibers and the evoked excitatory postsynaptic currents were recorded in cardiac vagal neurons (CVNs) in an in vitro brain stem slice preparation. This synaptic pathway is robust and activation of the trigeminal pathway often evoked action potentials in CVNs. Application of the serotonin (5-HT) reuptake inhibitor citalopram significantly enhanced these responses. Consistent with the hypothesis this pathway is endogenously modulated by 5-HT receptors the 5-HT1A receptor antagonist, WAY 100635 inhibited, whereas the 5-HT2A/C receptor antagonist, ketanserin facilitated the excitatory neurotransmission to CVNs. The 5-HT1A receptor agonist 8-hydroxy-2-(dipropylamino)tetralin hydrobromide increased, whereas the 5-HT2 receptor agonist, α-methylserotonin maleate salt inhibited this reflex pathway. These results indicate stimulation of trigeminal fibers evokes a powerful excitatory and polysynaptic pathway to CVNs, and this pathway is endogenously modulated and differentially enhanced and depressed, by 5-HT1A and 5-HT2 receptors, respectively.
INTRODUCTION
Electrical or mechanical stimulation of the trigeminal nerve evokes a dramatic decrease in heart rate, marked fall in blood pressure, and apnea in both experimental animals and man (Kumada et al. 1977, 1978; Schaller 2005; Schaller et al. 1999). The reflex responses evoked on stimulation of the trigeminal nerve have often been termed the “trigeminocardiac reflex” in the clinical literature and “trigeminal depressor responses” in the earliest animal studies of this reflex using the rabbit model (Kumada et al. 1977, 1978; Schaller 2004, 2005, 2007; Schaller and Buchfelder 2006; Schaller et al. 1999). Trigeminal depressor responses can be evoked by electrical stimulation of the root entry of the trigeminal nerve as well as from many branches of the peripheral trigeminal nerve, including the supra- and infraorbital (Kumada et al. 1977) as well as the ethmoidal nerve (McCulloch et al. 1999).
Stimulation of the trigeminal nerve likely excites sensory afferent fibers involved in a variety of functions and reflexes (Schaller 2004). Trigeminal afferent neurons can be activated by fluid in the nasal cavity, craniofacial pain, and mechanical stimulation of the ocular and peri-ocular structures (oculocardiac reflex) as well as during oro-facial and maxillofacial surgery (Barnard and Bainton 1990; Schaller 2004; Schaller and Buchfelder 2006; Schaller et al. 1999). Interestingly, however, stimulation of any of these trigeminal afferent sensory fibers elicits the same pattern of cardiovascular responses consisting of a pronounced bradycardia and hypotension accompanied by apnea (Barnard and Bainton 1990; Schaller 2004; Schaller and Buchfelder 2006; Schaller et al. 1999).
A subset of the trigeminocardiac reflex is the diving reflex, which is the most powerful autonomic reflex (Schaller 2004). Stimulation of the diving reflex by activation of nasotrigeminal sensory nerve fibers evokes a pronounced bradycardia, mediated by increased parasympathetic cardiac activity, in both humans and all mammals examined including rats, rabbits, cats, muskrats, and dogs (Elsner et al. 1971; Gandevia et al. 1978; Gooden 1994; Gooden et al. 1974; Martner et al. 1977; McCulloch and Panneton 1997; Nalivaiko et al. 2003; Panneton et al. 2000; White et al. 1974). The diving reflex is highly pronounced in man, particularly in infants, with heart rate decreasing in the range from 5 to 51% on a single facial submersion (Goksor et al. 2002). Similarly, in muskrats, heart rate decreases by an average of 54% following the onset of the diving reflex (McCulloch et al. 1999). The diving reflex is normally elicited when the nasal mucosa is stimulated with water or air-borne chemical irritants and can be prevented by anesthetizing the tip of the nose or nasal mucosa with local anesthetics (Dykes 1974; Yavari et al. 1996).
Exaggeration of this protective response, however, could be detrimental. Sudden Infant Death Syndrome (SIDS) is the leading cause of infant death in the post neonatal period and occurs in 0.3 cases per 1,000 live births in the United States (Hunt and Hauck 2006). Infants that succumb to SIDS typically experience a severe bradycardia that precedes or is accompanied by centrally mediated apnea (Fewell et al. 2001; Meny et al. 1994; Nachmanoff et al. 1998; Poets et al. 1999). Bradycardia is also the most prevalent and predictive event in infants monitored for apparent life-threatening events (Cote et al. 1998). Although the cause(s) for SIDS remains unknown, it has been speculated that an exaggeration of cardiorespiratory control, and in particular the parasympathetic control of cardiac function, may be involved (Divon et al. 1986; Harper and Bandler 1998; Meny et al. 1994; Schechtman et al. 1992; Spyer and Gilbey 1988). An abnormal or exaggerated response to trigeminal sensory nerve stimulation has been implicated in SIDS (Lobban 1991, 1995; Morpurgo et al. 2004). For example, a recent report describes a case of SIDS that was delayed, but triggered by, temporarily plunging an infant's face under water during bathing (Matturri et al. 2005). SIDS is also strongly associated with abnormalities in brain stem serotonergic (5-HT) function (Hunt 2005; Hunt and Hauck 2006; Paterson et al. 2006).
The clinical correlations of 5-HT dysfunction with SIDS are handicapped by a lack of information on the role of 5-HT, and different 5-HT receptors, in brain stem cardiovascular network function. There is also little information about the cellular mechanisms and properties of the trigeminocardiac reflex and in particular the synaptic pathways to parasympathetic cardioinhibitory neurons in the brain stem that dominate the neural control of heart rate. The goal of this study is to characterize the fundamental cellular electrophysiological properties of the trigeminocardiac reflex evoked inputs to brain stem parasympathetic cardiac vagal neurons and the modulation of this reflex by 5-HT1 and 5-HT2 receptors.
METHODS
Neonatal Sprague-Dawley rats (P3–P7; Hilltop, Scottdale, PA) were anesthetized and cooled to 4°C to slow the heart rate. A right thoracotomy was performed, and the retrograde fluorescent tracer X-rhodamine-5-(and-6)-isothiocyanate (Molecular Probes, Eugene, OR) was injected into the fat pads at the base of the heart. After 24–48 h of recovery, animals were anesthetized with isoflurane and killed by cervical dislocation, and the brain tissue was placed in a 4°C physiologic saline solution [containing (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 5 glucose, and 10 HEPES] bubbled with 100% O2 (pH 7.4). All animal procedures were performed with the approval of the Animal Care and Use Committee of The George Washington University in accordance with the recommendations of the panel on euthanasia of the American Veterinary Medical Association and the National Institutes of Health publication, “Guide for the Care and Use of Laboratory Animals.”
The medulla was removed with care to preserve the trigeminal cranial nerve rootlet and was mounted on a cutting block and placed into a vibrating blade microtome (Leica, Nussloch, Germany). A modified horizontal slice (800–900 μm) was obtained to stimulate the trigeminal nerve rootlet and record synaptic responses in cardiac vagal neurons, as shown in Fig. 1. The tissue was submerged in a recording chamber that allowed perfusion (4 ml/min) above and below the slice with artificial cerebrospinal fluid [ACSF; containing (in mM) 125 NaCl, 3 KCl, 2 CaCl2, 26 NaHCO3, 5 glucose, and 5 HEPES] equilibrated with 95% O2-5% CO2 (pH 7.4).
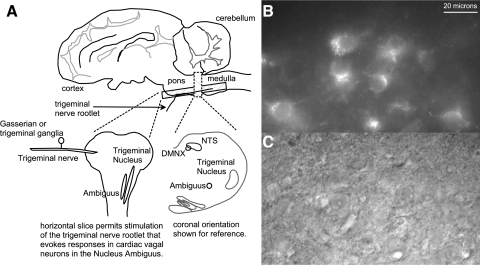
FIG. 1.
A horizontal slice was obtained that permits the electrical stimulation of the trigeminal nerve rootlet while keeping the pathway to cardiac vagal neurons in the nucleus ambiguus intact as illustrated in A. Cardiac vagal neurons in the nucleus ambiguus were identified by the presence of the fluorescent tracer, XRITC (B) previously injected into the pericardial sac. Patch electrodes were positioned onto the surface of the cardiac vagal neurons for whole cell recording using IR light and an IR sensitive camera as illustrated in C.
CVNs in the nucleus ambiguus (NA) were identified by the presence of the fluorescent tracer as described previously (Mendelowitz and Kunze 1991) and as shown in Fig. 1. Briefly, slices were viewed with infrared illumination and differential interference optics (Zeiss, Oberkochen, Germany) and under fluorescent illumination with an infrared-sensitive cooled charged-coupled device camera (Photometrics, Tucson, AZ). Neurons that contained the fluorescent tracer were identified by superimposing the fluorescent and infrared images on a video monitor (Sony, Tokyo, Japan). Patch pipettes (2.5–3.5 MΩ) were visually guided to the surface of individual CVNs using differential interference optics and infrared illumination (Zeiss). CVNs were voltage-clamped at a holding potential of –80 mV. The patch pipettes were filled with a solution that consisted of (in mM) 135 K-gluconic acid, 10 HEPES, 10 EGTA, 1 CaCl2, 1 MgCl2, and 0.005 QX314 at a pH of 7.3, except in current-clamp experiments QX314 was omitted.
Afferent fibers in the trigeminal nerve were stimulated, 1- to 2-ms duration, 1-Hz frequency, with a bipolar concentric stimulating electrode (W.P.I., Sarasota, FL) using a flexible stimulus isolator (A.M.P.I., Jerusalem, Israel). Stimulus intensity was increased in each experiment until the synaptic pathway was evoked consistently during control conditions and then maintained at that intensity and duration of stimulation throughout the experiment. A series of 10 consecutive stimulations in each neuron was averaged in all series of experiments with the exception that in studies using citalopram, the 5-HT reuptake inhibitor, only the last 3 stimulations within each condition were used. The mean value from each neuron in the population was then averaged to create a summary of results for each condition. Excitatory events were measured using pClamp 8 software (Molecular Devices, Sunnyvale, CA), which analyzes EPSC amplitudes as peak currents subtracted from baseline currents. Results are presented as means ± SE and statistically compared with a paired Student's t-test (for significance of difference, P < 0.05). Only one experiment was performed per preparation.
The following drugs were applied by inclusion in the perfusate: the 5-HT reuptake inhibitor citalopram hydrobromide (citalopram, 1 μM), the 5-HT1A receptor agonist 8-hydroxy-2-(dipropylamino) tetralin hydrobromide (DPAT, 1 μM), the 5-HT1A receptor antagonist WAY-100635 maleate salt (WAY, 100 μM), the 5-HT2 receptor agonist α-methylserotonin maleate salt (α-methyl-5-HT, 10 μM), the 5-HT2a/c receptor antagonist ketanserin tartrate salt (ketanserin, 10 μM). N-methyl-d-aspartate (NMDA) and non-NMDA receptor antagonists d(−)-2-amino-5-phosphonopentanoic acid (AP5, 50 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 50 μM), respectively, were focally applied using a WPI Pneumatic Picopump pressure delivery system from a patch pipette positioned within 30 μm from the patched cardiac vagal neuron. All drugs were purchased from Sigma Aldrich (St. Louis, MO).
RESULTS
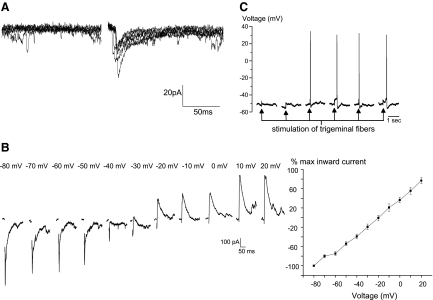
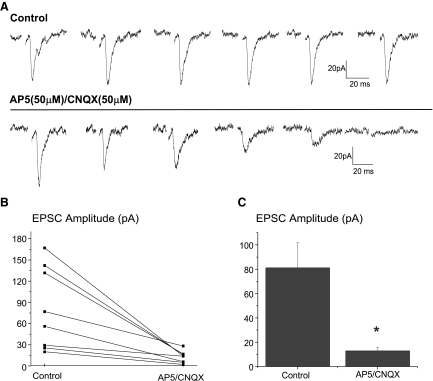
Stimulation of trigeminal afferent fibers evoked a single excitatory postsynaptic current (EPSC, average peak current of −30.0 ± 4.1 pA) in cardiac vagal neurons that was occasionally followed by multiple EPSCs with a longer latency. The latency of the initial evoked EPSC ranged from 3.3 to 30.75 ms with an average of 16.6 ± 1.7 ms (n = 54), suggesting this reflex pathway consists of a di- or polysynaptic pathway, see Fig. 2A. The EPSCs reversed at a holding voltage (−20.4 ± 2.8 mV, n = 8), consistent with an excitatory glutamatergic synapse, see Fig. 2B. In current-clamp mode, stimulation of trigeminal fibers depolarized cardiac vagal neurons, often sufficient to reach the threshold for an action potential as shown in Fig. 2C. Repeated stimulations of the trigeminal fibers evoked consistent synaptic responses in cardiac vagal neurons as shown in Fig. 3A. Focal application of the NMDA and non-NMDA receptor blockers AP5 (50 μM) and CNQX (50 μM), respectively, significantly decreased the excitatory events by 87.4 ± 1.1%, almost completely abolishing these responses (n = 7, P < .05), as shown for each individual experiment in Fig. 3B and in the summary of these experiments, Fig. 3C.
FIG. 2.
Stimulation of trigeminal afferent fibers evoked a single excitatory postsynaptic current (EPSC) in cardiac vagal neurons as shown in a typical example of 6 consecutive stimulations in A. Evoked EPSCs had an average amplitude of −30.0 ± 4.1 pA with latencies ranging from 3.3 to 30.75 ms with an average of 16.6 ± 1.7 ms (n = 54). A series of evoked EPSCs, recorded at voltage between −80 and 20 mV (B) indicates the EPSCs reversed at a holding voltage (−20.4 ± 2.8 mV, n = 8), consistent with an excitatory glutamatergic synapse. In current-clamp mode (C) 6 consecutive stimulations of trigeminal fibers depolarized cardiac vagal neurons, often sufficient to reach the threshold for an action potential, as shown in the last 4 stimulations.
FIG. 3.
Repeated stimulations of the trigeminal fibers evoked consistent synaptic responses in cardiac vagal neurons as shown in (A). Focal application of the N-methyl-d-aspartate (NMDA) and non-NMDA receptor blockers d(−)-2-amino-5-phosphonopentanoic acid (AP5, 50 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 50 μM), respectively, nearly abolished the activation of this synaptic pathway, decreasing the excitatory events by 87.4 ± 1.1%. The results from each experiment (n = 7), is shown in B with the summary of these results in C.
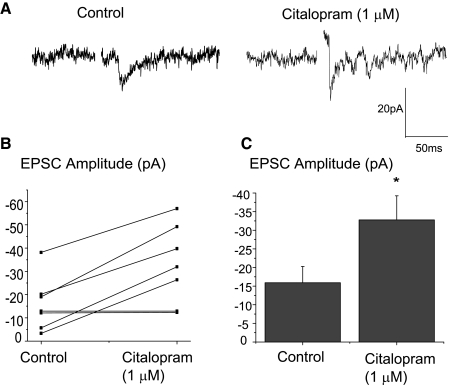
Application of the 5-HT reuptake inhibitor citalopram (1 μM) significantly increased the peak amplitude of the excitatory synaptic response in cardiac vagal neurons evoked by the stimulation of trigeminal afferents by 106.2 ± 6.9% from −15.9 ± 4.4 to −32.8 ± 6.5 pA (n = 7, P < 0.05), as shown in a typical experiment, Fig. 4A, as well as in the results from seven experiments, B and C, indicating this reflex pathway is endogenously enhanced by 5-HT.
FIG. 4.
Application of the serotonin (5-HT) reuptake inhibitor citalopram (1 μM) significantly increased the peak amplitude of the excitatory synaptic response in cardiac vagal neurons. A typical experiment is illustrated in the top traces, A, and the summary results from 7 experiments are shown in B and C. Citalopram (1 μM) increased the responses evoked by the stimulation of trigeminal afferents by an average of 106.2 ± 6.9% from −15.9 ± 4.4 to −32.8 ± 6.5 pA (n = 7, P < 0.05).
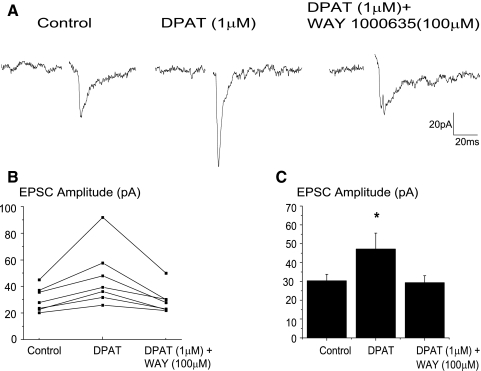
To identify the specific 5-HT receptors involved and test whether 5-HT1A and 5-HT2 receptors modulate this reflex pathway, the effects of different 5-HT1A and 5-HT2 receptor agonists and antagonists were tested. Application of the 5-HT1A receptor agonist, DPAT (1 μM), significantly increased the peak amplitude of the synaptic response (from −30.3 ± 3.5 to −47.2.6 ± 8.4 pA, n = 7) as shown in a typical experiment in Fig. 5A with the results from all seven experiments shown in B and C. Addition of the 5-HT1A receptor antagonist, WAY 100635 (100 μM) restored the responses to their previous magnitude (Fig. 5, A–C).
FIG. 5.
To identify the specific 5-HT receptors involved the effects of different 5-HT1A and 5-HT2 receptor agonists and antagonists were tested. Application of the 5-HT1A receptor agonist, 8-hydroxy-2-(dipropylamino)tetralin hydrobromide (DPAT, 1 μM), significantly increased (from −30.3 ± 3.5 to −47.2.6 ± 8.4 pA, n = 7) the peak amplitude of the synaptic response as shown in a typical experiment (A) and in the summary from 7 experiments (B and C). Addition of the 5-HT1A receptor antagonist, WAY 100635 (100 μM) blocked the facilitation of the synaptic pathway that occurred with DPAT, A–C.
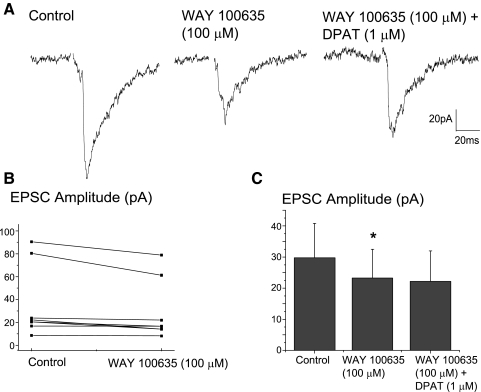
To examine if this pathway is endogenously modulated by 5-HT1A receptors, the 5-HT1A receptor antagonist, WAY 100635 (100 μM) was applied in additional experiments prior to application of DPAT (1 μM). The 5-HT1A receptor antagonist, WAY 100635 (100 μM) significantly inhibited the excitatory neurotransmission to CVNs by 21.6 ± 9.2% (n = 7, P < 0.05), see Fig. 6, as well as blocked the facilitation previously shown to be evoked by DPAT (1 μM). A typical experiment is shown in Fig. 6A with the summary data from seven experiments illustrated in B and C.
FIG. 6.
Consistent with the hypothesis that 5-HT1A receptors are endogenously active and enhance this synaptic pathway application of the 5-HT1A receptor antagonist, WAY 100635 (100 μM) significantly inhibited the excitatory neurotransmission to cardiac vagal neurons (CVNs) by 21.6 ± 9.2% (n = 7, P < 0.05). A typical experiment is illustrated in A, and the summary results from 7 experiments are shown in B and C. WAY 100635 also blocked the facilitation evoked by DPAT (1 μM; A and C).
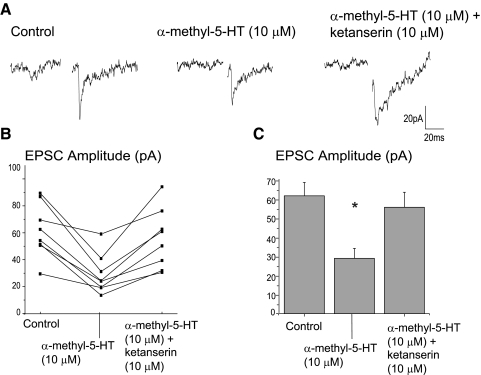
Application of the 5-HT2 receptor agonist, α-methyl-5-HT (10 μM) induced a significant (P < .05) inhibition of 53.0 ± 5.2% of the excitatory neurotransmission, Fig. 7, and this inhibition was reversed by application of the 5-HT2A/C receptor antagonist, ketanserin (10 μM). A typical experiment is shown in Fig. 7A with the summary data from eight experiments illustrated in B and C.
FIG. 7.
Application of the 5-HT2 receptor agonist, α-methyl-5-HT (10 μM) induced a significant inhibition (n = 8, P < 0.05) of 53.0 ± 5.2% of the excitatory neurotransmission. This inhibition was reversed by application of the 5-HT2A/C receptor antagonist, ketanserin (10 μM). A typical experiment is illustrated in A with the summary results from 8 experiments shown in B and C.
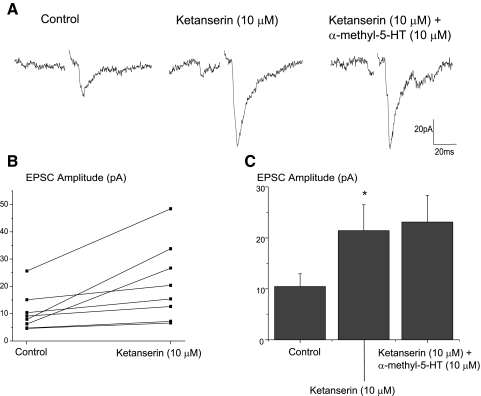
To examine if this modulation is endogenously active, another series of experiments were conducted in which ketanserin (10 μM) was applied before any receptor agonists. Application of the 5-HT2A/C receptor antagonist significantly facilitated (105.4 ± 5.1%, P < 0.05) the synaptic excitatory response in CVNs elicited by trigeminal afferent stimulation. A typical experiment is shown in Fig. 8A with the summary data from eight experiments illustrated in B and C. Ketanserin also prevented the inhibitory actions of α-methyl-5-HT on the trigeminal evoked EPSCs in cardiac vagal neurons, see Fig. 8, A and C.
FIG. 8.
The 5-HT2A/C receptor antagonist, ketanserin (10 μM), significantly facilitated (105.4 ± 5.1%, P < 0.05) the synaptic excitatory response in CVNs elicited by trigeminal afferent stimulation (n = 8, P < .05). A typical experiment is illustrated in A with the summary results shown in B and C. Ketanserin prevented the inhibitory actions of α-methyl-5-HT on the trigeminal evoked EPSCs in cardiac vagal neurons (A and C).
DISCUSSION
This study provides three major conclusions: 1) stimulation of trigeminal nerve rootlets evokes a polysynaptic excitatory glutamatergic pathway to cardiac vagal neurons in the NA, 2) the 5-HT reuptake inhibitor citalopram significantly enhanced these responses, and 3) this facilitation is likely mediated by 5-HT1A receptors as the 5-HT1A receptor antagonist, WAY 100635 inhibited and the 5-HT1A receptor agonist DPAT increased the excitatory neurotransmission to CVNs. However, 5-HT modulation of this reflex pathway is also complex as the 5-HT2A/C receptor antagonist, ketanserin facilitated and the 5-HT2 receptor agonist, α-methyl-5-HT inhibited this synaptic transmission. Taken together these results indicate stimulation of trigeminal fibers evokes a powerful excitatory and polysynaptic pathway to CVNs, and this pathway is endogenously modulated and differentially enhanced, and depressed, by 5-HT1A and 5-HT2 receptors, respectively.
The in vitro electrophysiological results obtained in this study are generally consistent with in vivo and anatomical studies of the trigeminocardiac and diving reflexes. The anatomical substrate for the trigeminocardiac and diving reflexes arises in the sensory afferent fibers in the trigeminal nerve, including those in the ethmoidal nerve, which project via the Gasserian, also known as the trigeminal, ganglia to the sensory nucleus of the trigeminal nerve (McCulloch et al. 1999; Schaller 2004). Second-order neurons in the ventral trigeminal nucleus that receive this sensory information have been identified using Fos immunohistochemistry either after nasal stimulation or during voluntary diving (McCulloch 2005; McCulloch and Panneton 1997). These second-order neurons are mostly lateral and slightly dorsal to the nucleus ambiguus in the ventral trigeminal nucleus (McCulloch 2005; McCulloch and Panneton 1997). In close agreement with this anatomical framework, bilateral inhibition of glutamatergic neurotransmission in similar locations in the ventral trigeminal complex blocks the cardiorespiratory responses normally evoked on stimulation of the nasal sensory neurons (McCulloch et al. 1995; Panneton and Yavari 1995).
The pathway continues from the ventral trigeminal nucleus through the short internuncial nerve fibers in the reticular formation in the brain stem to finally synapse on efferent premotor parasympathetic cardioinhibitory neurons in the nucleus ambiguus (Schaller 2004). Bilateral electrolytic lesions of the nucleus tractus solitarius (NTS) that abolish the baroreceptor reflex fail to alter the responses to stimulation of the trigeminal nerve, indicating these reflex pathways do not synapse or transverse through the NTS but instead utilize afferent nerve rootlets, nuclei, and fibers outside of the NT/DMNX region and likely contained within the ventral brain stem (Kumada et al. 1977). Because the circuitry for the trigeminocardiac and diving reflexes are intrinsic to the brain stem, it can be readily studied in vitro without the confounding effects and depression of the reflex that has been observed with anesthetics (Dutschmann and Paton 2002; Schaller 2004). Our results also suggest that some cardiac vagal neurons may be more responsive than other cardiac vagal neurons to stimulation of trigeminal sensory fibers. Future experiments would be helpful in determining if these differences are due to differences in cardiac ganglia innervations and/or preferential control of chronotropic, dromotropic, or ionotropic functions of the heart.
The results in this study, that 5-HT1A receptors are endogenously active and facilitate this reflex pathway in the brain stem, may have significant clinical implications particularly in relation to SIDS, which is strongly associated with abnormalities in brain stem 5-HT function (Hunt 2005; Hunt and Hauck 2006; Paterson et al. 2006). SIDS victims have a higher number of 5-HT neurons in the medulla compared with a control population, and the increased number of 5-HT neurons have been localized to the midline raphe, lateral extraraphe, and at the ventral surface of the medulla (Paterson et al. 2006). The cerebrospinal fluids of infant victims of sudden death show a very significant increase in the metabolites of 5-HT (Caroff et al. 1992). If there is an augmented 5-HT function in SIDS victims, this could lead to an exaggerated endogenous facilitation of the trigeminocardiac or diving reflex in these individuals.
However, while the 5-HT neuron density is increased, the 5-HT1A ligand binding density is significantly reduced in SIDS cases compared with controls (Paterson et al. 2006). In an attempt to reconcile these different studies, it has been proposed that there may be an increased number of 5-HT neurons and an exaggerated level of 5-HT activity, which results in a compensatory downregulation of 5-HT receptors in SIDS victims(Paterson et al. 2006). Alternatively, the autoradiographic evidence that 5-HT1A ligand binding density is significantly reduced in SIDS victims could be caused by decreased affinity of the receptor for the ligand and/or increased turnover of receptor protein rather than a decreased number of 5-HT1A receptors.
In conclusion, the results in this study demonstrate the 5-HT reuptake inhibitor citalopram (1 μM) exaggerates the trigeminocardiac reflex and further shows that 5-HT pathways are endogenously active and facilitate this reflex pathway. The facilitation by selective activation of 5-HT1A receptors therefore likely exerts a greater effect that the 5-HT2 receptor-mediated inhibition of the trigeminocardiac reflex. However, it seems likely the response to exaggerated 5-HT activity, such that might occur in SIDS victims, would have complex and often competing influences on different 5-HT receptors and the trigeminocardiac and diving reflexes.
GRANTS
Supported by grants National Heart, Lung, and Blood Institute Grants HL-59895 and HL-72006 to D. Mendelowitz.
REFERENCES
- Barnard 1990.Barnard NA, Bainton R. Bradycardia and the trigeminal nerve. J Craniomaxillofac Surg 18: 359–360, 1990. [DOI] [PubMed] [Google Scholar]
- Caroff 1992.Caroff J, Girin E, Alix D, Cann-Moisan C, Sizun J, Barthelemy L. Neurotransmission and sudden infant death. Study of cerebrospinal fluid. C R Acad Sci III 314: 451–454, 1992. [PubMed] [Google Scholar]
- Cote 1998.Cote A, Hum C, Brouillette RT, Themens M. Frequency and timing of recurrent events in infants using home cardiorespiratory monitors. J Pediatr 132: 783–789, 1998. [DOI] [PubMed] [Google Scholar]
- Divon 1986.Divon MY, Winkler H, Yeh SY, Platt LD, Langer O, Merkatz IR. Diminished respiratory sinus arrhythmia in asphyxiated term infants. Am J Obstet Gynecol 155: 1263–1266, 1986. [DOI] [PubMed] [Google Scholar]
- Dutschmann 2002.Dutschmann M, Paton JF. Influence of nasotrigeminal afferents on medullary respiratory neurones and upper airway patency in the rat. Pfluegers 444: 227–235, 2002. [DOI] [PubMed] [Google Scholar]
- Dykes 1974.Dykes RW. Factors related to the dive reflex in harbor seals: sensory contributions from the trigeminal region. Can J Physiol Pharmacol 52: 259–265, 1974. [DOI] [PubMed] [Google Scholar]
- Elsner 1971.Elsner R, Gooden BA, Robinson SM. Arterial blood gas changes and the diving response in man. Aust J Exp Biol Med Sci 49: 435–444, 1971. [DOI] [PubMed] [Google Scholar]
- Fewell 2001.Fewell JE, Smith FG, Vienna KYN. Prenatal exposure to nicotine impairs protective responses of rat pups to hypoxia in an age dependent manner. Respir Physiol 127: 61–73, 2001. [DOI] [PubMed] [Google Scholar]
- Gandevia 1978.Gandevia SC, McCloskey DI, Potter EK. Reflex bradycardia occurring in response to diving, nasopharyngeal stimulation and ocular pressure, and its modification by respiration and swallowing. J Physiol 276: 383–394, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goksor 2002.Goksor E, Rosengren L, Wennergren G. Bradycardic response during submersion in infant swimming. Acta Paediatr 91: 307–312, 2002. [DOI] [PubMed] [Google Scholar]
- Gooden 1994.Gooden BA. Mechanism of the human diving response. Integr Physiol Behav Sci 29: 6–16, 1994. [DOI] [PubMed] [Google Scholar]
- Gooden 1974.Gooden BA, Jones CL, Stone HL, Young S. Proceedings: cardiac responses to diving in trained dogs. J Physiol 239: 17P–19P, 1974. [PubMed] [Google Scholar]
- Harper 1998.Harper RM, Bandler R. Finding the failure mechanism in Sudden Infant Death Syndrome. Nat Med 4: 157–158, 1998. [DOI] [PubMed] [Google Scholar]
- Hunt 2005.Hunt CE. Gene-environment interactions: implications for sudden unexpected deaths in infancy. Arch Dis Child 90: 48–53, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt 2006.Hunt CE, Hauck FR. Sudden infant death syndrome. Can Med Assoc J 174: 1861–1869, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada 1977.Kumada M, Dampney RA, Reis DJ. The trigeminal depressor response: a novel vasodepressor response originating from the trigeminal system. Brain Res 119: 305–326, 1977. [DOI] [PubMed] [Google Scholar]
- Kumada 1978.Kumada M, Dampney RA, Whitnall MH, Reis DJ. Hemodynamic similarities between the trigeminal and aortic vasodepressor responses. Am J Physiol Heart Circ Physiol 234: H67–73, 1978. [DOI] [PubMed] [Google Scholar]
- Lobban 1991.Lobban CD. The human dive reflex as a primary cause of SIDS. A review of the literature. Med J Aust 155: 561–563, 1991. [PubMed] [Google Scholar]
- Lobban 1995.Lobban CD. The oxygen-conserving dive reflex re-examined as the principal contributory factor in sudden infant death. Med Hypotheses 44: 273–277, 1995. [DOI] [PubMed] [Google Scholar]
- Martner 1977.Martner J, Wadenvik H, Lisander B. Apnea and bradycardia from submersion in “chronically” decerebrated cats. Acta Physiol Scand 101: 476–480, 1977. [DOI] [PubMed] [Google Scholar]
- Matturri 2005.Matturri L, Ottaviani G, Lavezzi AM. Sudden infant death triggered by dive reflex. J Clin Pathol 58: 77–80, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch 2005.McCulloch PF. Activation of the trigeminal medullary dorsal horn during voluntary diving in rats. Brain Res 1051: 194–198, 2005. [DOI] [PubMed] [Google Scholar]
- McCulloch 1999.McCulloch PF, Faber KM, Panneton WM. Electrical stimulation of the anterior ethmoidal nerve produces the diving response. Brain Res 830: 24–31, 1999. [DOI] [PubMed] [Google Scholar]
- McCulloch 1997.McCulloch PF, Panneton WM. Fos immunohistochemical determination of brain stem neuronal activation in the muskrat after nasal stimulation. Neuroscience 78: 913–925, 1997. [DOI] [PubMed] [Google Scholar]
- McCulloch 1995.McCulloch PF, Paterson IA, West NH. An intact glutamatergic trigeminal pathway is essential for the cardiac response to simulated diving. Am J Physiol Regulatory Integrative Comp Physiol 269: R669–677, 1995. [DOI] [PubMed] [Google Scholar]
- Meny 1994.Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics 93: 44–49, 1994. [PubMed] [Google Scholar]
- Morpurgo 2004.Morpurgo CV, Lavezzi AM, Ottaviani G, Rossi L. Bulbo-spinal pathology and sudden respiratory infant death syndrome. Eur J Anaesthesiol 21: 589–593, 2004. [DOI] [PubMed] [Google Scholar]
- Nachmanoff 1998.Nachmanoff DB, Panigrahy A, Filiano JJ, Mandell F, Sleeper LA, Valdes-Dapena M, Krous HF, White WF, Kinney HC. Brain stem 3H-nicotine receptor binding in the sudden infant death syndrome. J Neuropathol Exp Neurol 57: 1018–1025, 1998. [DOI] [PubMed] [Google Scholar]
- Nalivaiko 2003.Nalivaiko E, De Pasquale CG, Blessing WW. Electrocardiographic changes associated with the nasopharyngeal reflex in conscious rabbits: vago-sympathetic co-activation. Auton Neurosci 105: 101–104, 2003. [DOI] [PubMed] [Google Scholar]
- Panneton 2000.Panneton WM, McCulloch PF, Sun W. Trigemino-autonomic connections in the muskrat: the neural substrate for the diving response. Brain Res 874: 48–65, 2000. [DOI] [PubMed] [Google Scholar]
- Panneton 1995.Panneton WM, Yavari P. A medullary dorsal horn relay for the cardiorespiratory responses evoked by stimulation of the nasal mucosa in the muskrat Ondatra zibethicus: evidence for excitatory amino acid transmission. Brain Res 691: 37–45, 1995. [DOI] [PubMed] [Google Scholar]
- Paterson 2006.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brain stem abnormalities in sudden infant death syndrome. J Am Med Assoc 296: 2124–2132, 2006. [DOI] [PubMed] [Google Scholar]
- Poets 1999.Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res 45: 350–354, 1999. [DOI] [PubMed] [Google Scholar]
- Schaller 2004.Schaller B. Trigeminocardiac reflex. A clinical phenomenon or a new physiological entity? J Neurol 251: 658–665, 2004. [DOI] [PubMed] [Google Scholar]
- Schaller 2005.Schaller B. Trigemino-cardiac reflex during microvascular trigeminal decompression in cases of trigeminal neuralgia. J Neurosurg Anesthesiol 17: 45–48, 2005. [PubMed] [Google Scholar]
- Schaller 2007.Schaller BJ. Trigeminocardiac reflex. J Neurosurg 107: 243; author reply 243–244, 2007. [DOI] [PubMed] [Google Scholar]
- Schaller 2006.Schaller BJ, Buchfelder M. Delayed trigeminocardiac reflex induced by an intraorbital foreign body. Ophthalmologica 220: 348, 2006. [DOI] [PubMed] [Google Scholar]
- Schaller 1999.Schaller B, Probst R, Strebel S, Gratzl O. Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg 90: 215–220, 1999. [DOI] [PubMed] [Google Scholar]
- Schechtman 1992.Schechtman VL, Raetz SL, Harper RK, Garfinkel A, Wilson AJ, Southall DP, Harper RM. Dynamic analysis of cardiac R-R intervals in normal infants and in infants who subsequently succumbed to the sudden infant death syndrome. Pediatr Res 31: 606–612, 1992. [DOI] [PubMed] [Google Scholar]
- Spyer 1988.Spyer KM, Gilbey MP. Cardiorespiratory interactions in heart-rate control. Ann NY Acad Sci 533: 350–357, 1988. [DOI] [PubMed] [Google Scholar]
- White 1974.White SW, McRitchie RJ, Franklin DL. Autonomic cardiovascular effects of nasal inhalation of cigarette smoke in the rabbit. Aust J Exp Biol Med Sci 52: 111–126, 1974. [DOI] [PubMed] [Google Scholar]
- Yavari 1996.Yavari P, McCulloch PF, Panneton WM. Trigeminally-mediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J Auton Nerv Syst 61: 195–200, 1996. [DOI] [PubMed] [Google Scholar]