Abstract
In the pallid bat auditory cortex and inferior colliculus (IC), the majority of neurons tuned in the echolocation range is selective for the direction and rate of frequency-modulated (FM) sweeps used in echolocation. Such selectivity is shaped mainly by spectrotemporal asymmetries in sideband inhibition. An early-arriving, low-frequency inhibition (LFI) shapes direction selectivity. A delayed, high-frequency inhibition (HFI) shapes rate selectivity for downward sweeps. Using iontophoretic blockade of GABAa receptors, we show that cortical FM sweep selectivity is at least partially shaped locally. GABAa receptor antagonists, bicuculline or gabazine, reduced or eliminated direction and rate selectivity in ∼50% of neurons. Intracortical GABA shapes FM sweep selectivity by either creating the underlying sideband inhibition or by advancing the arrival time of inhibition relative to excitation. Given that FM sweep selectivity and asymmetries in sideband inhibition are already present in the IC, these data suggest a refinement or recreation of similar response properties at the cortical level.
INTRODUCTION
Although there is considerable subcortical processing of receptive fields in the auditory system, recent studies indicate that intracortical inhibition nonetheless plays an important role in refining the receptive fields of neurons in the auditory cortex. Miller et al. (2001), in a study of thalamocortical transference of receptive fields, found that while thalamic excitatory fields are largely unchanged at the cortical level, inhibitory components can be added at the cortical level. Whole cell recording has also revealed that intracortical inhibition shapes the spectrotemporal properties of cortical neurons (Kaur et al. 2004; Wehr and Zador 2003). Zhang et al. (2003) reported that inhibitory currents recorded in cortical neurons enhance their selectivity for frequency-modulated (FM) sweep direction by differentially suppressing responses to the nonpreferred direction. The blockade of GABAergic receptors has revealed that the response patterns and frequency tuning of cortical neurons are also shaped by intracortical inhibition (Chen and Jen 2000; Kurt et al. 2006; Schulze and Langner 1999; Wang et al. 2000, 2002).
The role of auditory cortex in creating or refining response selectivity is of particular interest because most response properties of cortical neurons have also been found at lower levels of the auditory system. An excellent example is the pulse-echo delay tuning of cortical neurons in the mustached bat (Suga and O'Neill 1979). This response selectivity is already present at the level of the inferior colliculus (IC) (Portfors and Wenstrup 1999), leading to one possible conclusion that the auditory cortex is simply inheriting selectivity from lower levels (Nelken et al. 2003).
To address this issue, we have examined response selectivity for FM sweeps in both the IC (Fuzessery 1994; Fuzessery et al. 2006) and auditory cortex (Razak and Fuzessery 2006) in the pallid bat, a species that possesses an unusually high percentage of neurons with a marked selectivity for FM sweeps used in echolocation. Selectivity for the rate and direction of the FM sweeps are very similar at both levels of the system. To determine if the cortex inherited FM sweep selectivity from subcortical nuclei or if intracortical inhibition was involved in refinement of selectivity, the present study used the iontophoresis of GABAa receptor antagonists. Given that FM sweep selectivity has been found at both the collicular (Andoni et al. 2007; Casseday et al. 1997; Felsheim and Ostwald 1996; Ferragamo et al. 1998) and cortical (Heil et al. 1992; Mendelson et al. 1993; Nelken and Versnel 2000; Suga 1965; Tian and Rauschecker 2004) levels in other species, these results may have broad relevance. More fundamentally, these results are relevant to the debate on the relative contributions of thalamocortical feed-forward convergence and intracortical inhibition in shaping cortical response properties (Crook and Eysel 1992; Ferster and Miller 2000).
An important advantage offered by the pallid bat auditory system in this endeavor is that the inhibitory mechanisms that shape selectivity for FM sweeps are known (Fuzessery et al. 2006; Razak and Fuzessery 2006). In the cortex, the arrival times and strength of low- and high-frequency sideband inhibition play a major role. We therefore offer the working hypothesis that, if intracortical GABAergic circuits play a role, the blockade of receptors will alter the timing and strength of inhibitory inputs and thereby change or eliminate FM sweep rate and/or direction selectivity.
METHODS
Recordings were obtained from the auditory cortex of 14 adult pallid bats. These bats were captured in New Mexico and housed within a 16 × 21 ft2 room, where they were free to fly and obtain mealworms raised on vitamin-enriched ground Purina rat chow. The room was maintained on a reversed 12:12 h light:dark cycle. All procedures followed the animal welfare guidelines required by the Society for Neuroscience, National Institutes of Health and the Institutional Animal Care and Use Committee.
Surgical procedures
Recordings were obtained from bats that were anesthetized with Metofane (methoxyflurane) inhalation, followed by an intraperitoneal injection of pentobarbital sodium (30 μg/g body wt) or urethan (0.7 g/ml, 0.03 ml/kg body wt) and acepromazine (2 μg/g body wt). The surgery to expose the auditory cortex was identical to that reported previously (Razak and Fuzessery 2002, 2006, 2007). The location of the auditory cortex was determined relative to the rostrocaudal extent of the midsagittal sinus, the distance laterally from the midsagittal sinus, and the location of a prominent lateral blood vessel that travels parallel to the midsagittal sinus (Razak and Fuzessery 2002). The size of the exposure was usually ∼2 mm2. Exposed muscle was covered with petroleum jelly (Vaseline) to prevent desiccation.
Recording and iontophoresis procedures
Experiments were conducted in a heated (85–90°F) soundproofed chamber lined with anechoic foam. Bats were kept anesthetized throughout the course of the experiments, with sedation maintained by additional pentobarbital sodium (1/3 of presurgical dose) injections. Stimuli were generated using Modular Instruments and Tucker Davis Technologies digital hardware, and custom-written software (Fuzessery et al. 1991). The waveforms were amplified with a stereo amplifier and presented as closed-field stimuli through Infinity emit-K ribbon tweeters fitted with funnels that were inserted into the bat's pinnae and sealed there with petroleum jelly. The speaker-funnel frequency response curve showed a gradual increase of 20 dB from 6 to 70 kHz, as measured with a Bruel and Kjaer 1/8 in microphone placed at the tip of the funnel.
Single-unit recording and iontophoresis of GABAa receptor antagonists were accomplished using a three-barrel glass pipette (WPI). The tip of the electrode was broken to an outer diameter of 5–10 μm. One barrel was used for recording action potentials (1 M NaCl, 2–5 MΩ impedance). The second barrel was used for injection of BIC (bicuculline methiodide, Sigma) or GZ (Gabazine/SR95531, Sigma). BIC (10 mM concentration, pH 3.0 with 0.1 N HCl) and GZ (3 mM, pH 3.0 with 0.1 N HCl) were prepared fresh in isotonic saline. Iontophoretic currents were presented and monitored with a BH-2 Neurophore System (Medical Systems). Once a stable change in response magnitude to the preferred stimulus [usually a downward sweep centered at the neuron's best frequency (BF), with durations between 3 and 10 ms] was recorded, response selectivity data were obtained. This typically occurred 10–15 min after the ejection current was turned on. The third barrel was used as the balance electrode (1 M NaCl). A retaining current between −15 and −20 nA was used during the search phase and the predrug (control) recording phase. Currents between +5 and +40 nA were used to eject BIC and GZ.
Iontophoresis controls
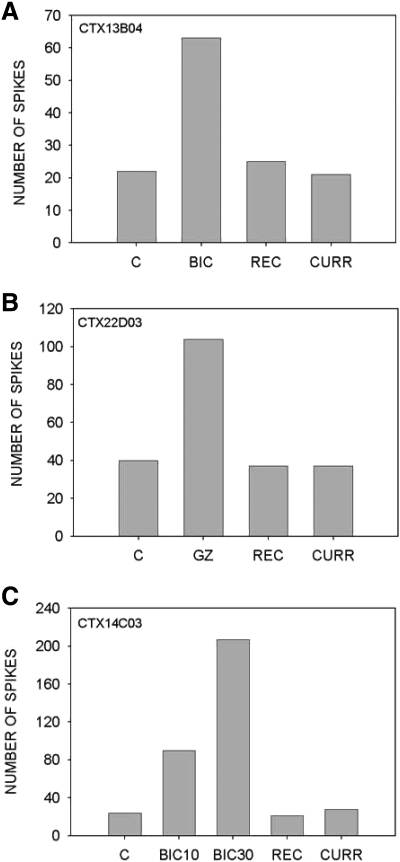
Three types of tests were performed to ensure that the effects observed were due to the antagonists. Recovery data were obtained from 51% (34/67) of neurons tested at 5 min intervals after the ejection current was turned off to determine if neurons regained their control response magnitudes. In the examples shown in Fig. 1, BIC (A and C) or GZ (B) enhanced responses to downward FM sweeps. As was seen in every neuron tested, the response magnitudes of these three neurons recovered to predrug (control) levels between 10 and 30 min after the injection current was turned off.
FIG. 1.
Tests used to validate iontophoresis data. A: the response of this neuron to a downward FM sweep (60–30 kHz) was enhanced approximately 3-fold by bicuculline (BIC) iontophoresis (20 nA) ejection current. Fifteen minutes after the current was turned off, the response recovered (REC) to control level. Injection of 20 nA current (CURR) through the balance barrel did not significantly alter the response magnitude. C, control (predrug). B: A ∼2.5-fold increase in response to a 80–40 kHz downward sweep was observed in this neuron (BF = 51 kHz) in the presence of gabazine (GZ, 15 nA ejection current). Twenty minutes after the current was turned off, the response recovered to control levels. A 25 nA current through the balance barrel did not have an effect on response to the stimulus. C: in the control condition, the neuron (BF = 46 kHz) responded with 20 spikes (stimulus-downward FM sweep 60–30 kHz, 10-ms duration). BIC ejected with a 10 nA current increased the response to 90 spikes. BIC ejected with a 30 nA current increased the response to 200 spikes. Fifteen minutes after the current was turned off, the response recovered to control value. The response was not altered by a 50 nA current through the balance barrel.
Current was passed through the balance barrel to observe if the current by itself had an effect on responses. This procedure did not impact response magnitude in any of the 31 neurons tested (e.g., Fig. 1, A–C, CURR), indicating that the effects were caused by the antagonists. In addition, the onset and offset of iontophoresis effects on responses did not occur immediately after the ejection current was turned on or off, respectively. In every neuron, there was a time delay (typically >30 s) before responses increased or decreased also indicating no effect of the current by itself.
A potential concern with iontophoresis studies is response saturation. Selectivity may appear to be reduced if disinhibition increases responses equally for all stimuli, but firing rate limitations prevent the response to the preferred stimulus from increasing more than the responses to nonpreferred stimuli. Response saturation is an unlikely explanation for decreased selectivity if the antagonist-induced increase in responses can be further enhanced by a larger injection current. To test for response saturation, the ejection current was increased (to between 20 and 50 nA) following a smaller injection current (5–20 nA) in 8 neurons (e.g., Fig. 1C). In the example shown, BIC caused a 4-fold increase in response magnitude for a +10 nA ejection current and a 10-fold increase for a +30 nA ejection current. In 7/8 neurons tested, the response magnitude was higher for the larger current showing that ejection currents <30 nA do not cause saturation of responses in most neurons. In one neuron, the response magnitude was the same for both 20 and 40 nA currents indicating saturation may occur for currents ≤20 nA in a small number of neurons.
Ejection currents between +5 to +20 nA were used in 55/67 (82%) neurons, making it likely that response magnitudes in the presence of the antagonists were below saturation levels in most neurons. This is also well below the current range that may cause BIC to have effects through non-GABAergic mechanisms (Kurt et al. 2006). This range of ejection currents caused a 2- to 5-fold increase in response magnitude to the preferred stimulus (downward FM sweep) in 64/67 (96%) of neurons.
Another potential concern is that pentobarbital anesthesia potentiates GABAergic inhibition. However, if BIC/GZ is capable of reducing selectivity even under potentiated GABAergic inhibition, then the effects can actually be considered an underestimation of the role of GABA in shaping cortical response selectivity. We have shown previously that the majority of cortical neurons with BF >25 kHz are selective for the downward direction and a narrow range of rates present in the echolocation calls (Razak and Fuzessery 2006). Such strong selectivity is unlikely to be an artifact of pentobarbital anesthesia because similar selectivity is observed in pallid bats injected only with butorphenol, an analgesic (unpublished observations). In the present study, we also present data obtained from urethan-anesthetized pallid bat cortex. Urethan potentiates GABAa receptors to a much lower extent than barbiturates (Hara and Harris 2002), and a comparison of data from urethan- versus barbiturate-anesthetized bats can be used, albeit indirectly, to address if the selectivity observed in the pallid bat cortex is an artifact of barbiturate-induced enhancement of GABA function.
Data acquisition
Electrode penetrations were made orthogonal to the surface of the cortex. All results are based on single-unit recordings, identified by the constancy of amplitude and waveform displayed on an oscilloscope. Poststimulus time histograms were constructed from the timing of action potentials relative to stimulus onset. Responses were quantified as the total number of spikes elicited over 20 stimulus presentations.
In this study, we focused exclusively on the high-frequency FM sweep-selective region (Razak and Fuzessery 2002). Once a neuron with BF >25 kHz, and with strong response to FM sweeps, but not to noise, was isolated, we attempted to determine as many of the following response properties possible before, during, and after drug iontophoresis.
Excitatory frequency tuning curve
Pure tones (5–75 kHz, 3–5 ms duration, 1 ms rise/fall times, 1 Hz repetition rate) were used to determine BF and frequency tuning. BF was defined as the frequency that elicited action potentials to at least five successive stimulus repetitions at the lowest intensity. The intensity was then increased in 10 dB steps to record the frequency-intensity combinations that produce excitatory responses.
FM duration, rate, and direction selectivity
The first step in determining FM rate selectivity was recording responses to FM sweeps of various durations (0.5–70 ms) and bandwidths centered near the BF of the neuron. The second step was plotting the selectivity of a neuron as a function of FM rate (kHz/ms) by dividing the spectral bandwidth by the duration of the sweep. This procedure allowed us to discriminate between tuning for sweep duration and sweep rate. A neuron was defined as rate-selective if the response declined to 50% of maximum as the sweep rate was decreased. The 50% cutoff rate (50% CO) was defined as the rate at which response declined to 50% of maximum for slower rates. Some neurons had fast-pass rate functions (e.g., Fig. 7C) and therefore had no 50% CO for fast rates, but all rate-selective neurons had a 50% CO rate for slow rates. The role of GABAergic inhibition in shaping cortical FM rate selectivity was quantified by comparing the 50% CO before and during iontophoresis.
To test for direction selectivity, responses to upward FM sweeps with the same range of duration and bandwidth as the downward sweeps were recorded. A direction selectivity index (DSI) was calculated to quantify direction selectivity (O'Neill and Brimijoin 2002). The formula used was: DSI = (D − U)/(D + U), where D and U are the maximum response magnitudes for downward and upward sweeps, respectively. The DSI was not necessarily calculated at the same sweep rate for the two directions as the maximum responses could occur at different durations for the two sweep directions. Values of DSI can range between −1 and +1 with more positive values indicating higher selectivity for the downward direction. DSI > 0.6 indicates that the maximum response to the upward sweep was ≥75% lower than the maximum response to the downward sweep. The effect of GABAa receptor antagonists on direction selectivity was determined by comparing the DSI before (DSI-C) and during (DSI-A) iontophoresis of the antagonist.
Arrival time of sideband inhibition
To determine the properties of sideband inhibition such as relative arrival time and bandwidth, a “two-tone inhibition over time” (TTI over time) paradigm was used (Brosch and Schreiner 1997; Calford and Semple 1995; Fuzessery et al. 2006; Gordon and O'Neill 1998; Razak and Fuzessery 2006, 2008). In this method, the delay between an excitatory and inhibitory frequency tone was varied, while the intensity of the two tones was kept constant. The frequency of the excitatory tone was set at the BF of the neuron, and was presented at an intensity of 10–20 dB above threshold and with a duration of either 3 or 5 ms. A second tone (10 ms duration) with frequencies ranging from 5 to 70 kHz was presented before, beginning with, or after the onset of the excitatory tone. The delay between the onset of the two tones was varied to determine the delay-frequency combinations that resulted in inhibition of response to the excitatory tone for at least four of five (80% inhibition) consecutive presentations. A delay-inhibitory frequency tuning curve was constructed based on the delay-frequency combinations that caused 80% inhibition. The two-tone procedure was repeated with the best inhibitory frequency (center frequency of the inhibitory tuning curve) and the BF tone to determine the delay at which response magnitude decreased to 50% of excitatory tone-alone response. In all TTI graphs, negative delays denote that the onset of the excitatory tone was before the onset of the inhibitory tone. Positive delays denote a temporal advantage to the inhibitory tone.
RESULTS
This study explored the effects of GABAa receptors antagonists on FM rate and direction selectivity and on the underlying inhibitory sideband properties. The data presented were obtained from 58 neurons exposed to bicuculline (BIC) and 9 neurons exposed to gabazine (GZ).
GABA shapes FM direction selectivity in the majority of cortical neurons
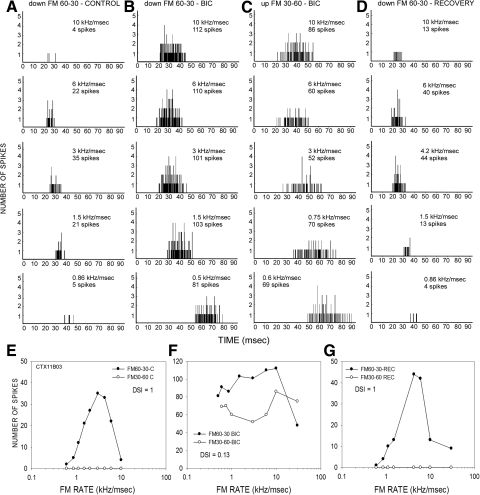
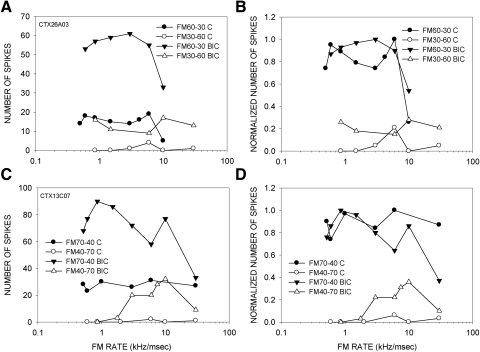
The contribution of GABAergic inhibition to direction selectivity was tested in 42 neurons. Figure 2 shows data from a neuron that was completely direction selective (DSI = 1) in the control (predrug) condition. The neuron responded in a rate selective manner to a 60 to 30 kHz downward sweep (Fig. 2, A and E). There was no response to a 30 to 60 kHz upward sweep at any sweep rate (Fig. 2E). In the presence of BIC, the neuron responded strongly to both downward (Fig. 2, B and F) and upward (Fig. 2, C and F) FM sweeps. The DSI decreased to 0.13 in the presence of BIC. Figure 2, D and G, shows that the neuron's response magnitudes and direction selectivity (DSI = 1) recovered to control level 20 min after the BIC injection current was turned off. These data indicate that intracortical GABA made a significant contribution to direction selectivity in this neuron.
FIG. 2.
A neuron in which BIC eliminated direction selectivity in the auditory cortex. A: poststimulus time histographs (PSTHs) showing the responses to downward FM sweep (60–30 kHz) at different sweep rates. B and C: PSTHs of response to downward (B) and upward (C) sweeps in the presence of BIC (20 nA ejection current). D: PSTHs of responses to downward FM sweeps in the recovery condition. E: in the control condition, this neuron responded to a 60 to 30 kHz downward sweep, but not to a 30 to 60 kHz upward sweep (DSI = 1). F: injection of BIC caused an increase in response magnitude for both up- and downward sweeps (note different scales on y axis compared with E). The responses to upward sweeps were increased at all sweep rates. The DSI in the presence of the antagonist was 0.13, indicating a considerable decrease in direction selectivity. G: control DSI was recovered ∼15 min after the ejection current was turned off.
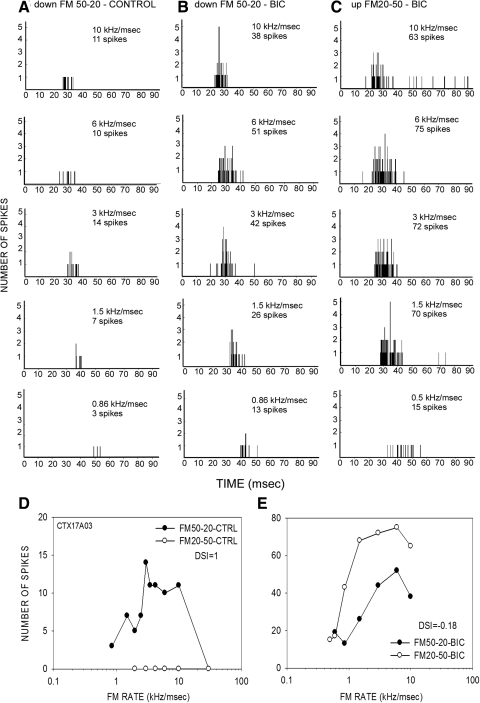
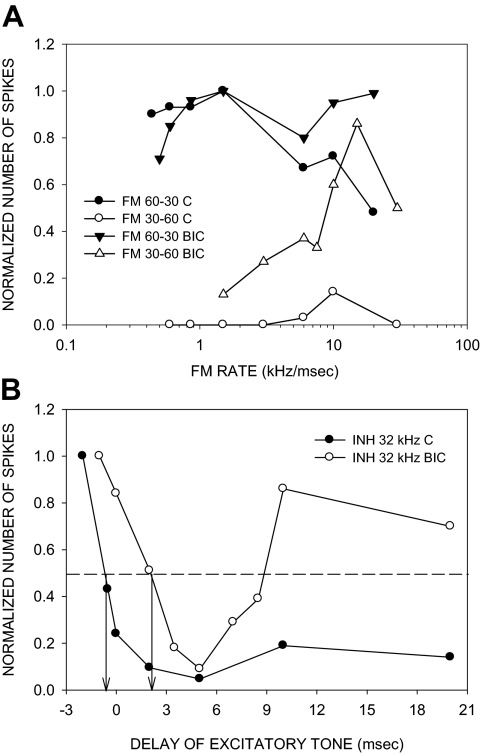
In the previous example, the antagonist-induced increase in response to upward FM sweeps was seen at all sweep rates tested (Fig. 2F). Such rate-independent increase in response to upward FM sweeps was seen in 13/42 (31%) of neurons. Another example of this type of an effect is shown in Fig. 10. A second type of effect was more common (29/42, 69%), and entailed a selective increase in response to fast upward sweeps compared with slow upward sweeps (e.g., Figs. 3 and 4). The neuron shown in Fig. 3 was completely direction selective (DSI = 1) for the downward sweep in the control condition (Fig. 3A). In the presence of BIC, the neuron responded to both sweep directions (Fig. 3, B and C). The DSI decreased to −0.18, indicating a slightly stronger response to upward sweeps. The increase in response to upward FM sweeps was seen only for sweep rates faster than 1 kHz/ms (Fig. 3E). Figure 4 shows another example in which response to upward sweeps was only seen for fast sweeps. In this neuron, GZ caused a rate-dependent loss of direction selectivity. An additional example of antagonist-induced rate-dependent increase in response to upward sweeps can be seen in Fig. 8, A and B.
FIG. 3.
A neuron in which BIC reduced direction selectivity in a rate-selective manner. PSTHs of response to downward FM sweep (50–20 kHz) in the control (A) and BIC (B) condition. C: PSTHs of response to upward FM (20–50 kHz) in the BIC condition. D: responses to up- and downward sweeps in the control condition (DSI = 1). E: responses during BIC application; note increases in response only at rates faster than 1 kHz/ms.
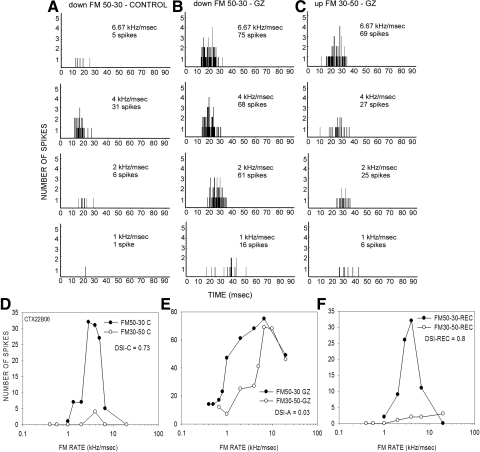
FIG. 4.
A neuron in which GZ reduced direction selectivity in a rate-dependent manner. PSTH of response to downward FM sweep (50–30 kHz) in the control (A) and GZ (B) condition. C: response to upward FM sweep (30–50 kHz) in the GZ condition. D: the DSI in the control condition was 0.73. E: GZ injection reduced DSI to 0.03. F: the neuron recovered DSI values near control levels after the ejection current was turned off.
The effects of antagonists on direction selectivity across the population ranged from loss of direction selectivity (e.g., Figs. 2–4) to no effect. Figure 5 A shows the only neuron in this study in which direction selectivity was not affected by the antagonists. The DSI-C and DSI-A were identical although the response magnitude for the sweeps increased almost 4-fold. Figure 5B shows a second neuron in which an intermediate level of reduction in DSI (0.88-0.5) was observed.
FIG. 5.
A: in this neuron there was no change in DSI during BIC application (15 nA) despite a 4-fold increase in response magnitude. B: normalized plot of the data shown in A shows relative changes in response during BIC application. C: a 2nd neuron in which BIC (25 nA ejection current) produced a decrease in DSI (from 0.88 to 0.5) but did not eliminate direction selectivity. D: normalized plot of data in C.
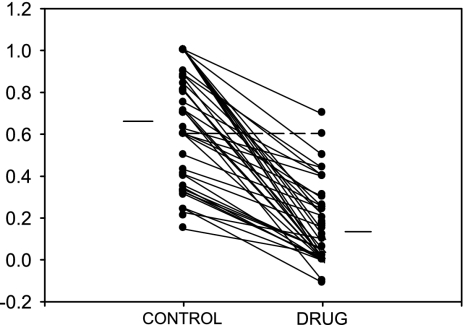
The range of effects is summarized in Fig. 6, which shows a comparison of DSI values in the control and iontophoresis conditions for the entire population. It can be seen that DSI values decreased in the antagonist condition compared with the control condition in all except 1 neuron (- - -, the neuron shown in Fig. 5A). DSI decreased by up to 4-fold in the majority of neurons (23/42, 55%). DSI decreased to ≤0 in 17/42 (40%) neurons, indicating a loss of selectivity for the downward sweep direction due to disinhibition. The mean DSI decreased significantly from 0.65 ± 0.04 in the control condition to 0.17 ± 0.03 in the drug condition (paired t-test, P < 0.001). Taken together, these data show that direction selectivity is at least partially shaped by intracortical GABA in the majority of cortical neurons. In the majority of neurons, intracortical inhibition appears to play a greater role in suppressing responses to upward sweeps at faster sweep rates. Over half of the neurons remained direction-selective at slower sweep rates, suggesting that this directionality is shaped at subcortical levels and is therefore not affected by intracortical receptor blockade.
FIG. 6.
Summary of BIC/GZ effects on DSI in all direction-selective neurons. The mean DSI values before and during antagonist iontophoresis are shown as horizontal bars off to the sides. - - -, the sole neuron in which there was no change in DSI.
GABA shapes FM rate selectivity
The effect of GABAa receptor antagonists on rate selectivity for downward FM sweeps was tested in 49 neurons. As with direction selectivity, a wide range of effects was observed. Rate selectivity was eliminated in 14/49 (28.6%) neurons. In the examples shown in Fig. 7A, rate selectivity of the neurons was eliminated in the presence of BIC. The neuron recovered rate selectivity at the predrug values 15–20 min after the ejection current was turned off.
FIG. 7.
Effects of GABAa receptor antagonists on FM rate selectivity. A and B: a neuron in which rate selectivity was eliminated by BIC (20 nA ejection current). The normalized plots show that the neuron was rate tuned at the 2 FM bandwidths tested with a best rate of 4 kHz/ms and a 50% CO rate of 1.5 kHz/ms (arrow). When BIC was applied, the neuron's rate selectivity was eliminated. (- - -, in this and all other normalized plots, 50% response.) Maximum response magnitudes for 20 stimulus repetitions were as follows: control condition, FM 70–40: 20 spikes; FM 70–50: 19 spikes, BIC condition, FM 70–40: 100 spikes; FM 70–50: 96 spikes, REC condition, FM 70–50: 20 spikes. B: a neuron in which rate selectivity was reduced by GZ (10 nA ejection current). The normalized plots show that the 50% CO rate was reduced from 2.5 kHz/ms to 0.35 kHz/ms by GZ. Maximum response magnitudes for 20 stimulus repetitions were as follows: control condition, FM 50–30: 19 spikes, GZ condition, FM 50–30: 67 spikes. C: a neuron in which BIC (20 nA ejection current) did not affect rate selectivity. The 50% CO rate was around 0.8 kHz/ms in both the control and BIC conditions. Maximum response magnitudes for 20 stimulus repetitions were as follows: control condition, FM 50–30: 18 spikes; BIC condition, FM 50-30: 109 spikes.
The remaining neurons were rate-selective in the presence of the antagonists, with varying degrees of effects on the 50% CO rate. In 10/49 (20%) of neurons, there was at least a 2-fold decrease in the 50% CO rate indicating that the neuron responded >50% of maximum to a broader range of rates during iontophoresis than in the control condition. In the example in Fig. 7B, GZ reduced the 50% CO from 3 to 0.35 kHz/ms, suggesting that GABA was involved in making this neuron respond selectively to higher sweep rates. In 25/49 (51%) neurons, iontophoresis of antagonists had no effect on rate selectivity. That is, in these neurons the 50% CO before and during iontophoresis was similar (the percent change was <25%). In the example neuron shown in Fig. 7C, the 50% CO in the control condition was ∼0.8 kHz/ms. The neuron was rate-selective in the presence of BIC. The 50% CO was relatively unchanged even though the response magnitude increased nearly 4-fold.
The effect of antagonists on both direction and rate selectivity was tested in 19 neurons. In 47% (9/19) of these neurons, direction selectivity was reduced by the drug without an effect on rate selectivity. In 10/19 neurons (53%), both direction and rate selectivity were reduced. These data suggest that cortical GABAergic interactions can shape response selectivity for one property of the FM sweep, while selectivity for another property of the sweep is unaffected, suggesting inheritance from subcortical nuclei. In most cortical neurons, it appears that direction selectivity is more dependent on local GABAergic circuits than rate selectivity.
GABA shapes the LFI underlying direction selectivity
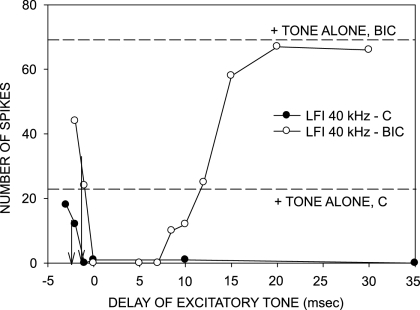
We have previously shown that the arrival time of inhibition relative to excitation explains rate and direction selectivity in the vast majority (∼90%) of cortical neurons (Razak and Fuzessery 2006, 2008). Low-frequency inhibition (LFI) arrives early relative to excitation and shapes direction selectivity for downward sweeps. The majority of neurons with early LFI are selective for downward sweeps. For all upward sweeps, regardless of rate, LFI is triggered before excitation and its early arrival results in reduced responses to upward FM sweeps. Almost all neurons with delayed LFI respond to sweeps in either direction. The delayed arrival of LFI allows excitatory input to be triggered earlier than inhibitory input by fast upward sweeps. As sweep rate slows, the delayed inhibition arrives before or together with the excitation, suppressing the response. This suggests that blocking inhibition may have the effect of delaying the arrival and/or reducing the strength of LFI. In addition, a rate-independent increase in response to upward sweeps may occur due to a complete loss of LFI.
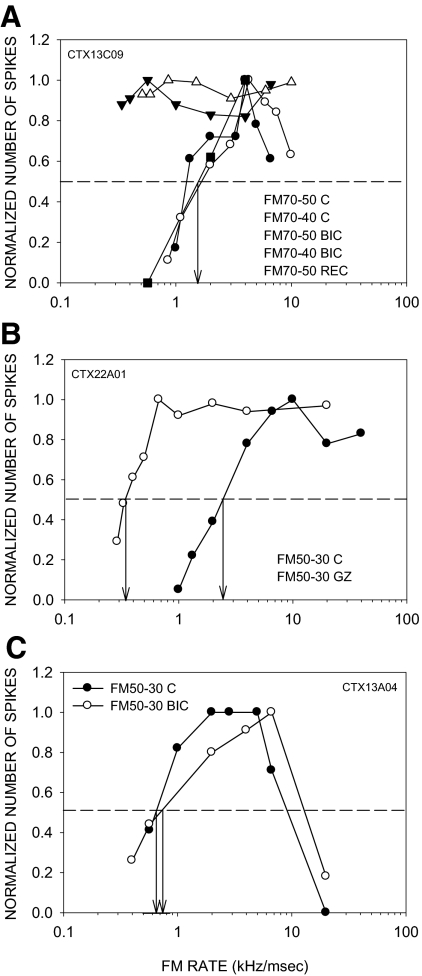
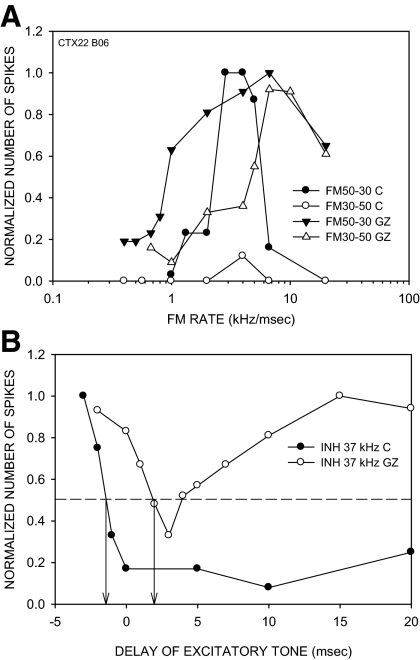
The effects of BIC/GZ on LFI were tested in 16 neurons. In 9/16 (56%) neurons, LFI was delayed but not eliminated by the antagonist. In all (9/9) of these neurons, direction selectivity was reduced due to an increase in response to fast upward sweeps. The neuron shown in Fig. 8 had a DSI of 0.71 (Fig. 8A) and an LFI centered at 32 kHz in the control condition. The LFI arrived early, with a 50% arrival time of −1 ms (Fig. 8B). Receptor blockade reduced direction selectivity, mainly due to an increase in response to upward sweeps at 10 kHz/ms (Fig. 8A). It also retarded the 50% arrival time of LFI to +2 ms (Fig. 8B). The delay in LFI allowed fast upward sweeps to excite the neuron before inhibition arrived. As the upward FM sweep rate decreased, LFI and excitation arrived closer together in time until LFI arrived together with or before excitation and reduced responses to upward FM sweeps.
FIG. 8.
A neuron in which BIC reduced direction selectivity in a rate-dependent manner and delayed the arrival time of low-frequency inhibition (LFI). A: in the control condition, the neuron was direction-selective with a DSI of 0.71. In the presence of BIC (20 nA ejection current), the DSI was reduced to 0.09 due to increased responses to upward sweeps primarily at rates faster than 10 kHz/ms. Maximum response magnitudes for 20 stimulus repetitions were as follows: control condition, FM 60–30: 28 spikes, FM 30–60: 4 spikes; BIC condition, FM 60–30: 118 spikes, FM 30–60: 100 spikes. B: in the control condition, the neuron exhibited LFI centered at 32 kHz. BIC did not eliminate LFI but delayed the arrival time. ↓, the delay of the excitatory tone at which the two-tone response declined to 50% of the BF response (defined as 50% arrival time). BIC caused a delay in 50% arrival time of LFI from −0.5 to +2.5 ms. The maximum responses to 20 repetitions of the excitatory tone alone were as follows: control condition, 21 spikes; BIC condition, 100 spikes.
Figure 9 shows an example of GZ-induced delay in LFI and a rate-dependent decrease in direction selectivity (same neuron as Fig. 4). The DSI decreased due to a rate-selective increase in response to upward sweeps (Fig. 9A). In the control condition, LFI was centered at 37 kHz and arrived early (50% arrival time of −2 ms, Fig. 9B). GZ did not eliminate LFI but delayed it (50% arrival time of +2 ms, Fig. 9B). Across the population of neurons showing a selective increase in response to fast upward sweeps, the 50% arrival time of LFI showed a significant change (P < 0.05, paired t-test) from −0.5 ± 0.33 to +1.2 ± 0.67 ms due to BIC/GZ.
FIG. 9.
A neuron in which GZ caused a rate-dependent change in direction selectivity and a delay in LFI arrival time. The raw data for direction selectivity of this neuron are shown in Fig. 4. A: normalized direction selectivity plot shows that direction selectivity is reduced by GZ (20 nA ejection current) primarily due to an increase in response to upward FM sweeps of rates faster than 5 kHz/ms. B: the neuron exhibited LFI centered at 37 kHz. GZ did not eliminate LFI. The normalized plot shows that GZ delayed the LFI from −2 ms in the control condition to +2 ms. Conventions for dashed lines and arrows are the same as in Fig. 7. The maximum responses to 20 repetitions of the excitatory tone alone were as follows: control condition, 12 spikes: BIC condition, 50 spikes.
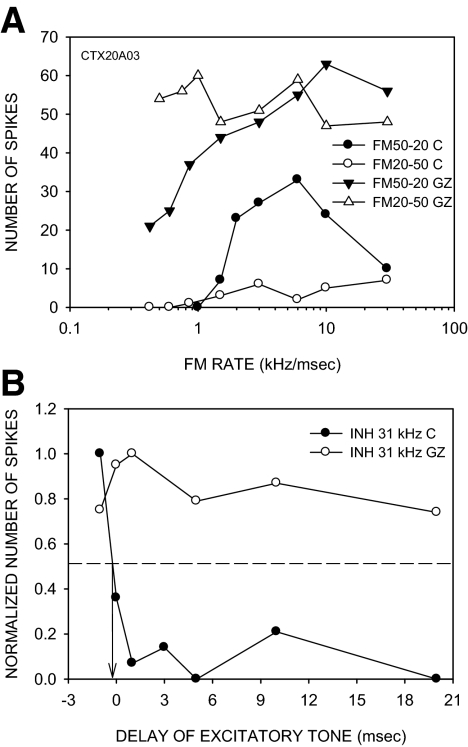
In 5/16 (31%) neurons, LFI was eliminated by the antagonists. In four of these neurons, direction selectivity was reduced due to an increase in response to upward sweeps at all rates. In the example shown in Fig. 10, the neuron lost direction selectivity and responded to upward sweeps at all rates tested (Fig. 10A). In the control condition, the neuron exhibited an early LFI with a 50% arrival time of −0.5 ms (Fig. 10B). GZ eliminated LFI (Fig. 10B). LFI arrival time recovered to control values (Fig. 10B). In the remaining neuron in this category, the response to upward FM was rate-selective even though the LFI was eliminated, suggesting that mechanisms other than delayed LFI may shape rate-selective responses to upward sweeps (Razak and Fuzessery 2008).
FIG. 10.
A neuron in which GZ caused a rate-independent loss of direction selectivity and a loss of LFI. A: the neuron was direction-selective in the control condition (DSI = 0.7). GZ (20 nA ejection current) eliminated direction selectivity with the neuron showing enhanced responses to upward sweeps at all rates tested. B: in the control condition, LFI centered at 31 kHz arrived early (50% arrival time = −0.5 ms). In the presence of GZ, LFI was abolished. The maximum responses to 20 repetitions of the excitatory tone alone were as follows: control condition, 17 spikes; BIC condition, 62 spikes.
In 2/16 (13%) neurons, LFI arrival times were only slightly altered by the antagonists, and arrival times of LFI remained negative or zero. In both neurons, there was little change in DSI. The example TTI plots shown in Fig. 11 are from the same neuron shown in Fig. 5, A and B. LFI arrived early in both control and antagonist conditions. The early arrival of LFI even during receptor blockade likely underlies the low response to upward sweeps and the preservation of direction selectivity.
FIG. 11.
An example in which the LFI arrival time was minimally affected by BIC. In the control and BIC (15 nA ejection current) conditions, LFI (centered at 40 kHz) arrival time was −2.5 and −1.5 ms, respectively. In both cases, the arrival time was negative, indicating earlier arrival than excitation. This is the same neuron as shown in Fig. 5, C and D, where it can be seen that direction selectivity was also unaffected by BIC.
These data show a strong correlation between the type of effect on LFI and direction selectivity. Neurons in which LFI was delayed, but not eliminated, by disinhibition showed a relatively larger increase in response to fast upward sweeps compared with slow upward sweeps. Most neurons in which LFI was eliminated by the drug showed a rate-independent increase in responses to upward sweeps. These data suggest that in the majority of auditory cortex neurons, direction selectivity is increased by the hastening the arrival time of LFI relative to excitation by intracortical inhibitory circuits.
GABA shapes the HFI underlying rate selectivity in the majority of cortical neurons
Delayed HFI shapes rate selectivity for downward FM sweeps (Razak and Fuzessery 2006, 2007). Neurons without HFI are not rate-selective. The arrival time and bandwidth of HFI accurately predict the 50% CO rate. The loss or reduction of rate selectivity because of the antagonists may occur through the loss or a delay in the arrival time of HFI, respectively. This prediction was tested in nine neurons. HFI was eliminated in seven/nine neurons by the antagonists. In the remaining two neurons, there was no effect on HFI timing.
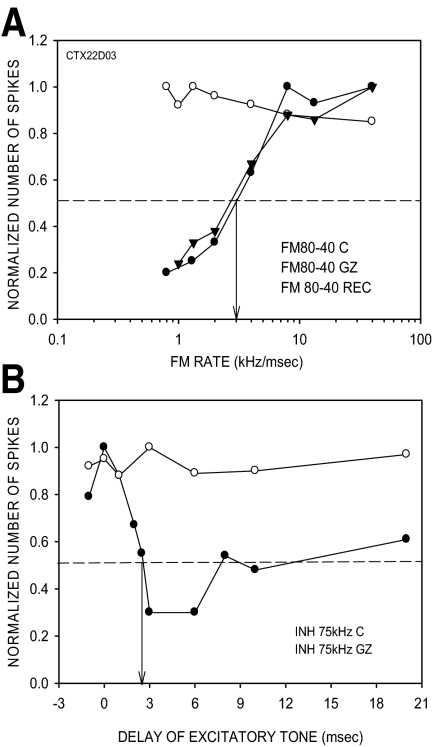
Rate selectivity was eliminated in five/seven neurons in which HFI was also eliminated. The example neuron shown was rate-selective with a 50% CO rate of 3.5 kHz/ms in the control condition (Fig. 12A). The neuron exhibited delayed HFI centered at 75 kHz. The 50% arrival time of HFI was +2.5 ms (Fig. 12B). GZ eliminated both rate selectivity (Fig. 12A) and HFI (Fig. 12B). In the other two neurons in which HFI was eliminated, rate selectivity was broadened but still present, suggesting that mechanisms other than HFI may contribute to rate selectivity for downward sweeps (Razak and Fuzessery 2008).
FIG. 12.
A neuron in which GZ eliminated both HFI and FM rate selectivity. A: in the control condition, the neuron was rate-selective for downward FM sweeps with a 50% CO rate of 3 kHz/ms. This panel also shows the recovery data for rate selectivity. The rate selectivity was eliminated by GZ (20 nA ejection current). The maximum responses to 20 stimulus repetitions were as follows: control condition, 42 spikes; GZ condition, 120 spikes; REC condition, 42 spikes. B: high-frequency inhibition (HFI) centered at 75 kHz was delayed with the 50% arrival time of +2.5 ms. The HFI was eliminated by GZ. The responses to 20 repetitions of the excitatory tone alone were as follows: control condition, 32 spikes; GZ condition, 102 spikes.
In the two neurons in which the antagonists did not affect HFI timing, the 50% CO rate was also not affected. In the example shown in Fig. 13, the 50% CO in the control condition was 2 kHz/ms (A). The arrival time of HFI was +1.2 ms (Fig. 13B). During receptor blockade, there was little effect on rate selectivity; the 50% CO was 1.5 kHz/ms (Fig. 13A). HFI was still present, although inhibition was not as strong as in the control condition. The 50% arrival time was +2 ms (Fig. 13B). Taken together, these data show that the effects of BIC/GZ on downward FM rate selectivity occur through changes in the properties of HFI, suggesting that, in some neurons, the HFI sideband is at least partially created in the cortex.
FIG. 13.
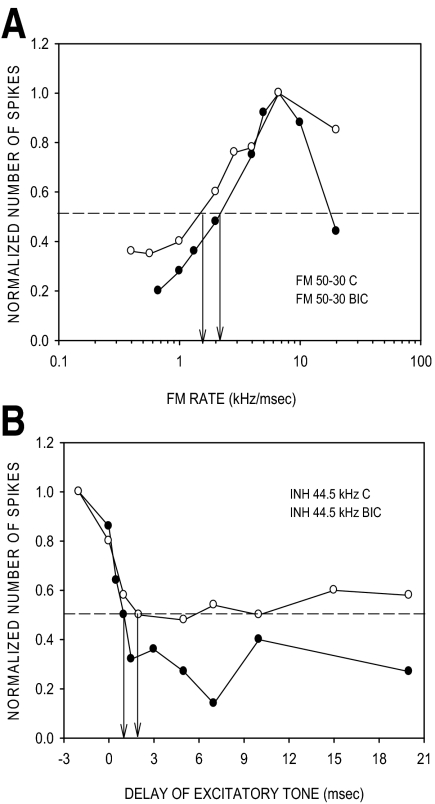
A neuron in which BIC had minimal effect on HFI arrival time and 50% CO rate. A: in the control condition, the neuron was rate-selective with a 50% CO rate of 2 kHz/ms. In the presence of BIC, the neuron was still rate-selective with a 50% CO rate of 1.5 kHz/ms. The maximum responses to 20 stimulus repetitions were as follows: control condition, 24 spikes; BIC condition, 90 spikes. B: in the control condition, HFI centered at 44 kHz had a 50% arrival time of 1 ms. Although the percent magnitude of inhibition was reduced in the presence of BIC (20 nA ejection current), the 50% arrival time of HFI was similar to the control value (1.8 vs. 2.2 ms). The responses to 20 repetitions of the excitatory tone alone were as follows: control condition, 22 spikes; GZ condition, 88 spikes.
Comparison of BIC and GZ
Because the objective of this study did not involve directly comparing GZ and BIC effects, we did not test the same neurons with both antagonists. We used both BIC and GZ due to recent studies that suggest BIC injected at high currents may affect responses through non-GABAa receptor-mediated mechanisms (Johansson et al. 2001; Kurt et al. 2006). However, perhaps not surprisingly due to the low currents (<40 nA) used in this study, a comparison of the effects in different neurons shows no major difference between BIC and GZ. The reduction in DSI was similar between the two antagonists (t-test, P > 0.05). BIC reduced DSI from 0.65 ± 0.04 to 0.17 ± 0.03. GZ reduced DSI from 0.66 ± 0.09 to 0.17 ± 0.1. Increase in response to fast upward sweeps compared with slow sweeps was observed with both antagonists (e.g., Figs. 8 and 9). The effects on FM rate selectivity were also similar. Two of eight (25%) neurons lost rate selectivity due to GZ. Two (25%) neurons showed a broadening of selectivity, while the remaining four (50%) showed no effects. With BIC, 12/41 (29%) neurons lost rate selectivity, 8/41 (20%) showed a broadening of selectivity and 21 (51%) showed no effects. The similarity in the effects of BIC and GZ, a more specific GABAa receptor antagonist, provides further support for the role of GABAa receptors in shaping cortical FM sweep selectivity.
Comparison of data obtained from urethan- and barbiturate-anesthetized bats
FM sweep selectivity and two-tone inhibition data were also obtained from pallid bats anesthetized with urethan. Urethan affects GABA transmission to a much lower extent than barbiturates (Hara and Harris 2002). Although iontophoresis data were not obtained in urethan-anesthetized (UA) bats, comparison of control data between barbiturate-anesthetized (BA) (from Razak and Fuzessery 2006) and UA bats can be used to address if the response properties of FM sweep-selective neurons are artifacts of barbiturate-induced enhancement of GABA.
Table 1 summarizes the results of this comparison. Bandwidth (BW) of excitatory tuning curves at 10 and 20 dB above threshold, arrival time of LFI and HFI, bandwidth of LFI and HFI and measures of FM sweep selectivity such as DSI, best and 50% FM rate were not significantly different between neurons recorded in UA and BA bats. However, the durations of both LFI and HFI were significantly longer in BA compared with UA neurons. The duration of inhibition in BA neurons was ∼128 ms for LFI and ∼149 ms for HFI indicating that inhibition lasted much longer than the 10 ms inhibitory tone durations used. This is similar to duration of inhibition in BA cat cortex (∼143 ms determined with 30 ms duration inhibitory tones) (Brosch and Schreiner 1997). In UA pallid bat cortex, inhibition was significantly shorter (∼21 and 14 ms for LFI and HFI, respectively), indicting that the duration of inhibition is similar to the duration of the inhibitory tone. This observation is similar to ketamine-anesthetized cat cortex in which inhibition duration was similar to the inhibitory tone duration (Volkov and Galazyuk 1992). Thus it can be concluded that the barbiturates enhance the duration of inhibition in auditory cortex compared with ketamine or urethane anesthesia. This is consistent with previous reports that barbiturates enhance inhibitory postsynaptic potential (IPSP) duration in the brain (Scholfied 1978; Serkov 1984). FM sweep selectivity and arrival time and bandwidth of inhibition are unlikely to be artifacts of barbiturate-induced enhancement of GABA transmission.
TABLE 1.
Comparison of excitatory and inhibitory tuning and FM sweep selectivity between urethan- and barbiturate-anesthetized bats
| Urethane | Barbiturate | P-value | |
|---|---|---|---|
| BW 10 dB, kHz | 5.2 ± 0.5 (39) | 5 ± 0.4 (66) | 0.74 |
| BW 20 dB | 7.2 ± 0.9 (39) | 6.7 ± 0.6 (66) | 0.66 |
| 50% arrival time | |||
| LFI | −0.45 ± 0.04 (21) | −0.34 ± 0.19 (40) | 0.76 |
| HFI | 2.5 ± 0.34 (12) | 3.2 ± 0.5 (26) | 0.29 |
| Bandwidth | |||
| LFI | 9.1 ± 1.4 kHz (14) | 12.4 ± 1.7 kHz (36) | 0.23 |
| HFI | 2.4 ± 0.2 kHz (8) | 2.8 ± 0.4 kHz (21) | 0.57 |
| Duration | |||
| LFI | 20.75 ± 4.8 msec (20) | 128.1 ± 16 msec (25) | <0.0001* |
| HFI | 14.9 ± 1.2 msec (12) | 149.7 ± 17 msec (21) | <0.0001* |
| FM 50% cut-off rate | 1.5 ± 0.1 kHz/msec (32) | 1.51 ± 0.2 kHz/msec (41) | 0.90 |
| Best FM rate | 4.1 ± 0.3 kHz/msec (32) | 3.6 ± 0.3 kHz/msec (29) | 0.23 |
| DSI | 0.56 ± 0.06 (38) | 0.62 ± 0.06 (51) | 0.44 |
Numbers indicate means ± SE. n values in parentheses. LFI and HFI, low- and high-frequency inhibition; DSI, direction selectivity index.
DISCUSSION
The main finding of this study is that intracortical GABAa receptor activity sharpens FM rate and direction selectivity of cortical neurons. One of the mechanisms through which intracortical inhibition shapes FM selectivity is modification of timing of inhibition. The main implication of these findings is that intracortical inhibition may be required to recover response selectivity even when this selectivity is already present at lower levels of the system.
The percentage of FM rate and direction-selective neurons is similar between the IC (Fuzessery et al. 2006) and cortex (Razak and Fuzessery 2006) of the pallid bat. Various measures of FM selectivity show no change from the IC to the cortex (Razak and Fuzessery 2006). FM direction selectivity is, at least in part, created by GABAergic inhibition in the IC (Fuzessery and Hall 1996). Taken together with the present results, it appears that FM sweep selectivity is shaped by GABAergic inhibition at multiple levels of the auditory system without an increase in selectivity from one level to the next. This is similar to the presence of neurons tuned to pulse-echo time delays in the mustached bat cortex, thalamus, and IC without any apparent differences in selectivity of neurons across different levels of the system (Olsen and Suga 1991; Portfors and Wenstrup 1999, 2001; Suga and O'Neill 1979). While it is known that inhibitory mechanisms play a crucial role in the IC (Nataraj and Wenstrup 2005), it remains untested if delay selectivity is further refined or reshaped in the cortex of the mustached bat.
Another example of shaping of response properties at multiple levels in the auditory system is selectivity for interaural intensity difference (IID) in the lateral superior olive (LSO) and the IC. IID sensitivity is created de novo in many IC neurons without a significant difference in sensitivity from the LSO and the IC (Park and Pollak 1993). In the visual system, direction selectivity is synthesized in both primary visual cortex (V1) and middle temporal (MT) area, although MT depends on V1 for most of its inputs (Thiele et al. 2004). Iontophoretic blockade of GABAergic receptors causes a deterioration of direction selectivity in MT during the early response period, without affecting selectivity later in the response period. This suggests that MT refines direction selectivity locally although its V1 input is already direction-selective. Thus the reshaping of response selectivity without an increase in selectivity across multiple levels may be common in sensory systems.
Studies in the visual cortex have shown the importance of intracortical inhibition in shaping movement velocity (analogous to FM rate) and direction (analogous to FM direction) selectivity (Murthy and Humphrey 1999; Patel and Sillito 1978; Sato et al. 1996). Similar to the results presented here, intracortical inhibition shapes direction selectivity in the visual cortex by refining the spatiotemporal structure of the receptive field (RF) (Murthy and Humphrey 1999). Thus a common mechanism across sensory cortices may be the influence of intracortical circuits in establishing the appropriate timing of inhibitory input.
Also similar to the visual cortex studies, we observed a wide range of effects of the antagonists on FM sweep selectivity, from no change to complete loss. Part of the reason may be methodological in that sufficient antagonists may not have been injected to completely block GABAa receptors. However, the observation that response magnitudes were increased to similar extents in neurons that lost selectivity and those that showed no change for similar ejection currents suggests that genuine differences exist in the extent to which intracortical GABA shapes sweep selectivity. Given that iontophoresis studies typically have to find a compromise between insufficient antagonist and saturation effects, the results presented here suggest that intracortical GABA at least partially shapes cortical FM sweep selectivity.
One reason for the need to refine FM sweep selectivity at multiple levels may be the convergence of selective and nonselective inputs. Anatomical data show considerable convergence and divergence of thalamocortical connections (McMullen and de Vencia 1993; Middlebrooks and Zook 1983). By recording from connected thalamic and cortical neurons, Miller et al. (2001) showed that convergence underlies thalamocortical transformation of spectral and temporal properties. Particularly pertinent to present results, they showed that inhibitory RF properties are transmitted with less fidelity compared with excitatory properties. Because the interactions between inhibitory and excitatory receptive field properties shape FM sweep selectivity (O'Neill and Brimijoin 2002; Razak and Fuzessery 2006; Suga 1965), the cortex may have to refine these properties using local inhibition. The main conclusion of this study, that cortical inhibition is important for FM sweep selectivity, is similar to conclusions of Zhang et al. (2003). The traditional view of sensory systems has been dominated by the idea that if two levels show similar response selectivity, then the higher level must inherit it from the lower level. However, given the accumulating evidence for corticofugal pathways, it may be more reasonable to consider different levels reciprocally influencing each other with properties shaped locally.
A striking result of this study is the delay in arrival time of LFI induced by disinhibition that allows a preferential increase in response to fast upward sweeps, thereby reducing direction selectivity for downward sweeps. The fast LFI is, however, already present in the IC and shapes FM direction selectivity there (Fuzessery et al. 2006). Therefore the addition of early LFI in the cortex may reflect the need to recover a loss in the temporal fidelity of inhibitory inputs relative to excitation in the IC-thalamus-cortex pathway (Miller et al. 2001). One caveat to this interpretation is the fact that the TTI paradigm measures arrival time of inhibition relative to excitation. It is unclear if GABAa antagonists affect the rise time of excitatory postsynaptic potentials and therefore the arrival time of excitatory input. A simple measure of excitatory spiking latency is not sufficient to address this question as GABAergic inhibition may play a role in shaping excitatory latencies. Thus a possible alternate explanation to the delayed arrival of inhibition is the advance of excitation.
A second issue to consider is the prominence of sideband inhibition in shaping FM sweep selectivity in the cortex. At least three mechanisms have been proposed to shape FM direction and rate tuning in the auditory system namely, duration tuning (Fuzessery et al. 2006; Gordon and O'Neill 1998), sideband inhibition (Fuzessery et al. 2006; O'Neill and Brimijoin 2002; Razak and Fuzessery 2006, 2007; Suga 1965) and facilitation (Razak and Fuzessery 2008). Duration tuning is not involved in shaping FM sweep selectivity in the auditory cortex of the pallid bat (see following text). Facilitation accounts for FM sweep selectivity in ∼15% of cortical neurons. The remaining 85% of neurons depend primarily on arrival time and bandwidth of sideband inhibition for FM selectivity. Thus the proposed role of intracortical GABA in shaping arrival time and strength of LFI and HFI applies to the vast majority of cortical neurons in the pallid bat.
An interesting example of a loss of response selectivity between the IC and cortex in the pallid bat is that of duration tuning (Fuzessery et al. 2006; Razak and Fuzessery 2006). Nearly 50% of IC neurons tuned to the echolocation pulse are duration tuned (Fuzessery 1994). In addition to delayed HFI, duration tuning creates FM rate selectivity in the IC (Fuzessery et al. 2006). In the cortex, however, duration tuning is rare (10% of neurons) and is not involved in FM rate selectivity. Despite the loss of a mechanism, the percentage of FM rate-selective neurons is similar between IC and cortex. Local GABA may be required to increase the percentage of FM rate-selective neurons in the cortex. This idea is supported by the finding that a large percentage of cortical neurons lost rate selectivity when disinhibited by GABA antagonists.
Possible mechanisms of GABA effect on timing of inhibition
In vivo patch-clamp recordings from the rat auditory cortex showed that local inhibition shapes cortical direction selectivity by enhancing the strength of inhibition to the nonpreferred direction compared with the preferred direction (Zhang et al. 2003). The strength and timing of inhibition may be related. Strong inhibitory synapses have a steeper rising slope of the IPSP, reaching a given level earlier than in weaker synapses. Support for this relationship between strength and timing of inhibition has been observed in the auditory system. In rat auditory cortical neurons with monotonic intensity/rate functions, the timing of inhibitory conductance advances with sound intensity, maintaining a balance with excitatory conductance (Wu et al. 2006). A similar conclusion was reached in a study showing a consistency in duration tuning in the inferior colliculus with changes in sound intensity; a balance of excitatory and inhibitory conductance is required to maintain best duration at different intensities (Fremouw et al. 2005). Thus neurons in which inhibitory sidebands are eliminated and those in which timing of inhibition is delayed by the antagonists may represent a single mechanism. In the former case, the entire IPSP arises cortically. In the latter case, local GABA adds to the strength of the IPSP and thereby advances timing.
Implications for development of the auditory cortex
During development, direction selectivity is present in only 25% of the neurons at postnatal day (p) 14 compared with ∼70% in the adults (Razak and Fuzessery 2007). Between p14 and p60, most pup neurons exhibit delayed LFI. As expected, most pup neurons exhibit poor direction selectivity due to responses to fast upward sweeps. This is similar to responses in adult neurons following iontophoresis of GABAa receptor antagonists. Thus iontophoresis in adult cortex appears to unmask the delayed LFI observed in early development. The increase in the incidence of direction selectivity occurs due to progressive decrease in the arrival times of LFI during development.
The development of LFI arrival time and direction selectivity is experience-dependent (Razak et al. 2008). In bats without normal experience with echolocation calls, direction selectivity is dramatically reduced at p90. In most of these neurons, LFI is delayed. Taken together with the iontophoresis data, it appears that the experience-dependent decrease in LFI arrival times, and development of direction selectivity occurs through mechanisms intrinsic to the cortex. Studies in the rat auditory system also suggest that the development of inhibitory sidebands in cortical neurons was due to a maturation of cortical GABAergic mechanism (Chang et al. 2005). Thus experience-dependent plasticity of cortical inhibitory circuits may include local changes and changes inherited from lower levels.
That a trace of the properties that develop in an experience-independent manner may be present even after experience-dependent modifications was first suggested by Zheng and Knudsen (1999) based on work in prism-reared barn owls. The interaural intensity difference (ITD) sensitivity of neurons in the optic tectum of owls shifts in an adaptive direction dictated by prism-induced changes in visual locations. Application of BIC unmasks the previous ITD sensitivity, suggesting that the original ITD sensitivity was masked by new adaptive inhibitory input. The continued “covert” persistence of early developmental response properties after experience-dependent plasticity may therefore be a general principle of neural development.
GRANTS
This research was supported by National Institute of Deafness and Othe Communicaiton Disorders Grant DC-05202 Z. M. Fuzessery and INBRE P20 RR0 16474-04.
Acknowledgments
We thank Dr. Jeff Wenstrup and T. Zumsteg for valuable comments on the manuscript, D. Richardson for participating in various stages of this study, and G. McLellan for programming the software required for this study.
Present address of K. A. Razak: Department of Psychology, University of California, Riverside, CA 92521.
REFERENCES
- Andoni et al. 2007.Andoni S, Li N, Pollak GD. Spectrotemporal receptive fields in the inferior colliculus revealing selectivity for spectral motion in conspecific vocalizations. J Neurosci 27: 4882–4893, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch and Schreiner 1997.Brosch M, Schreiner CE. Time course of forward masking tuning curves in cat primary auditory cortex. J Neurophysiol 77: 923–943, 1997. [DOI] [PubMed] [Google Scholar]
- Calford and Semple 1995.Calford MB, Semple MN. Monaural inhibition in cat auditory cortex. J Neurophysiol 73: 1876–1891, 1995. [DOI] [PubMed] [Google Scholar]
- Casseday et al. 1997.Casseday JH, Covey E, Grothe B. Neural selectivity and tuning for sinusoidal frequency modulations in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Neurophysiol 77: 1595–1605, 1997. [DOI] [PubMed] [Google Scholar]
- Chang et al. 2005.Chang EF, Bao S, Imaizumi K, Schreiner C, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci USA 102: 16460–16465, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen and Jen 2000.Chen QC, Jen PH. Bicuculline application affects discharge patterns, rate-intensity functions, and frequency tuning characteristics of bat auditory cortical neurons. Hear Res 150: 161–174, 2000. [DOI] [PubMed] [Google Scholar]
- Crook and Eysel 1992.Crook JM, Eysel UT. GABA-induced inactivation of functionally characterized sites in cat visual cortex (area 18): effects on orientation tuning. J Neurosci 12: 1816–1825, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans and Smith 1982.Evans RH, Smith DA. Effect of urethane on synaptic and amino acid-induced excitation in isolated spinal cord preparations. Neuropharmacology 21: 857–860, 1982. [DOI] [PubMed] [Google Scholar]
- Felsheim and Ostwald 1996.Felsheim C, Ostwald J. Responses to exponential frequency modulations in the rat inferior colliculus. Hear Res 98: 137–151, 1996. [DOI] [PubMed] [Google Scholar]
- Ferster and Miller 2000.Ferster D, Miller KD. Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci 23: 441–471, 2000. [DOI] [PubMed] [Google Scholar]
- Ferragamo et al. 1998.Ferragamo MJ, Haresign T, Simmons JA. Frequency tuning, latencies, and responses to frequency-modulated sweeps in the inferior colliculus of the echolocating bat, Eptesicus fuscus. J Comp Physiol [A] 182: 65–79, 1998. [DOI] [PubMed] [Google Scholar]
- Fremouw et al. 2005.Fremouw T, Faure PA, Casseday JH, Covey E. Duration selectivity of neurons in the inferior colliculus of the Big Brown Bat: tolerance to changes in sound level. J Neurophysiol 94: 1869–1878, 2005. [DOI] [PubMed] [Google Scholar]
- Fuzessery 1994.Fuzessery ZM. Response selectivity for multiple dimensions of frequency sweeps in the pallid bat inferior colliculus. J Neurophysiol 72: 1061–1079, 1994. [DOI] [PubMed] [Google Scholar]
- Fuzessery et al. 1991.Fuzessery ZM, Gumtow RG, Lane R. A microcomputer-controlled system for use in auditory physiology. J Neurosci Methods 36: 45–52, 1991. [DOI] [PubMed] [Google Scholar]
- Fuzessery and Hall 1996.Fuzessery ZM, Hall JC. The role of GABA in shaping frequency tuning and selectivity for FM sweep direction in the inferior colliculus. J Neurophysiol 76: 1059–1073, 1996. [DOI] [PubMed] [Google Scholar]
- Fuzessery et al. 2006.Fuzessery ZM, Richardson MD, Coburn MS. Neural mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the inferior colliculus of the pallid bat. J Neurophysiol 96: 1320–1336, 2006. [DOI] [PubMed] [Google Scholar]
- Gordon and O'Neill 1998.Gordon M, O'Neill WE. Temporal processing across frequency channels by FM selective auditory neurons can account for direction and rate selectivity. Hear Res 122: 97–108, 1998. [DOI] [PubMed] [Google Scholar]
- Hara and Harris 2002.Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 94: 313–318, 2002. [DOI] [PubMed] [Google Scholar]
- Heil et al. 1992.Heil P, Rajan R, Irvine DRF. Sensitivity of neurons in cat primary auditory cortex to tones and frequency modulated stimuli. II. Organization of response properties along the “isofrequency” dimension. Hear Res 63: 135–156, 1992. [DOI] [PubMed] [Google Scholar]
- Johansson et al. 2001.Johansson S, Druzin M, Haage D, Wang M-D. The functional role of a bicuculline-sensitive Ca2+-activated K+ current in rat medial preoptic neurons. J Physiol 532: 625–635, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur et al. 2004.Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol 91: 2551–2567, 2004. [DOI] [PubMed] [Google Scholar]
- Kurt et al. 2006.Kurt S, Crook JM, Ohl FW, Scheich H, Schulze H. Differential effects of iontophoretic in vivo application of the GABA(A)-antagonists bicuculline and gabazine in sensory cortex. Hear Res 212: 224–235, 2006. [DOI] [PubMed] [Google Scholar]
- McMullen and de Venecia 1993.McMullen NT, de Venecia RK. Thalamocortical patches in auditory neocortex. Brain Res 620: 317–322, 1993. [DOI] [PubMed] [Google Scholar]
- Mendelson et al. 1993.Mendelson JR, Schreiner CE, Sutter ML, Grasse KL. Functional topography of cat primary auditory cortex: responses to frequency-modulated sweeps. Exp Brain Res 94: 65–87, 1993. [DOI] [PubMed] [Google Scholar]
- Middlebrooks and Zook 1983.Middlebrooks JC, Zook JM. Intrinsic organization of the cat's medial geniculate body identified by projections to binaural response-specific bands in the primary auditory cortex. J Neurosci 3: 203–224, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller et al. 2001.Miller LM, Escabi MA, Read HL, Schreiner CE. Functional convergence of response properties in the auditory thalamocortical system. Neuron 32: 151–160, 2001. [DOI] [PubMed] [Google Scholar]
- Murthy and Humphry 1999.Murthy A, Humphry AL. Inhibitory contributions to spatiotemporal receptive-field structure and direction selectivity in simple cells of cat area 17. J Neurophysiol 81: 1212–1244, 1999. [DOI] [PubMed] [Google Scholar]
- Nataraj and Wenstrup 2005.Nataraj K, Wenstrup JJ. Roles of inhibition in creating complex auditory responses in the inferior colliculus: facilitated combination-sensitive neurons. J Neurophysiol 93: 3294–3312, 2005. [DOI] [PubMed] [Google Scholar]
- Nelken et al. 2003.Nelken I, Fishbach A, Las L, Ulanovsky N, Farkas D. Primary auditory cortex of cats: feature detection or something else? Biol Cybern 89: 397–406, 2003. [DOI] [PubMed] [Google Scholar]
- Nelken and Versnel 2000.Nelken I, Versnel H. Responses to linear and logarithmic frequency-modulated sweeps in ferret primary auditory cortex. Eur J Neurosci 12: 549–562, 2000. [DOI] [PubMed] [Google Scholar]
- Olsen and Suga 1991.Olsen JF, Suga N. Combination-sensitive neurons in the medial geniculate body of the mustached bat: encoding of target range information. J Neurophysiol 65: 1275–1296, 1991. [DOI] [PubMed] [Google Scholar]
- O'Neill and Brimijoin 2002.O'Neill WE, Brimijoin WO. Directional selectivity for FM sweeps in the suprageniculate nucleus of the mustached bat medial geniculate body. J Neurophysiol 88: 172–187, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park and Pollak 1993.Park TJ, Pollak GD. GABA shapes sensitivity to interaural intensity disparities in the mustache bat's inferior colliculus: implications for encoding sound location. J Neurosci 13: 2050–2067, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel and Sillito 1978.Patel HH, Sillito AM. Inhibition and velocity tuning in the cat visual cortex. J Physiol 284: 113P–114P, 1978. [PubMed] [Google Scholar]
- Portfors and Wenstrup 1999.Portfors CV, Wenstrup JJ. Delay-tuned neurons in the inferior colliculus of the mustached bat: implications for analyses of target distance. J Neurophysiol 82: 1326–1338, 1999. [DOI] [PubMed] [Google Scholar]
- Portfors and Wenstrup 2001.Portfors CV, Wenstrup JJ. Topographical distribution of delay-tuned responses in the mustached bat inferior colliculus. Hear Res 151: 95–105, 2001. [DOI] [PubMed] [Google Scholar]
- Razak and Fuzessery 2002.Razak KA, Fuzessery ZM. Functional organization of the pallid bat auditory cortex: emphasis on binural organization. J Neurophysiol 87: 72–86, 2002. [DOI] [PubMed] [Google Scholar]
- Razak and Fuzessery 2006.Razak KA, Fuzessery ZM. Neural mechanisms underlying selectivity for the rate and direction of frequency modulated sweeps in the auditory cortex of the pallid bat. J Neurophysiol 96: 1303–1319, 2006. [DOI] [PubMed] [Google Scholar]
- Razak and Fuzessery 2007.Razak KA, Fuzessery ZM. Development of inhibitory mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex. J Neurosci 27: 1769–1781, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak and Fuzessery 2008.Razak KA, Fuzessery ZM. Facilitatory mechanisms underlying selectivity for the direction and rate of frequency modulated sweeps in the auditory cortex. J Neurosci 28: 9806–9816, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak et al. 2008.Razak KA, Richardson MD, Fuzessery ZM. Experience is required for the maintenance and refinement of FM sweep selectivity in the developing auditory cortex. Proc Natl Acad Sci USA 105: 4465–4470, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato et al. 1996.Sato H, Katsuyama N, Tamura H, Hata Y, Tsumoto T. Mechanisms underlying orientation selectivity of neurons in the primary visual cortex of the macaque. J Physiol 494: 757–771, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield 1978.Scholfield CN. A barbiturate induced intensification of the inhibitory potential in slices of guinea-pig olfactory cortex. J Physiol 275: 559–566, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze and Langner 1999.Schulze H, Langner G. Auditory cortical responses to amplitude modulations with spectra above frequency receptive fields: evidence for wide spectral integration. J Comp Physiol [A] 185: 493–508, 1999. [DOI] [PubMed] [Google Scholar]
- Serkov 1984.Serkov FN. Neuronal and synaptic mechanisms of cortical inhibition. Neirofiziologiia 16: 394–403, 1984. [PubMed] [Google Scholar]
- Suga 1965.Suga N. Responses of cortical auditory neurons to frequency modulated sounds in echo-locating bats. Nature 206: 890–891, 1965. [DOI] [PubMed] [Google Scholar]
- Suga and O'Neill 1979.Suga N, O'Neill WE. Neural axis representing target range in the auditory cortex of the mustache bat. Science 206: 351–353, 1979. [DOI] [PubMed] [Google Scholar]
- Tian and Rauschecker 2004.Tian B, Rauschecker JP. Processing of frequency-modulated sounds in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol 92: 2993–3013, 2004. [DOI] [PubMed] [Google Scholar]
- Thiele et al. 2004.Thiele A, Distler C, Korbmacher H, Hoffmann K-P. Contribution of inhibitory mechanisms to direction selectivity and response normalization in macaque middle temporal area. Proc Natl Acad Sci USA 101: 9810–9815, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov and Galazyuk 1992.Volkov IO, Galazyuk AV. Peculiarities of inhibition in cat auditory neurons evoked by tonal stimuli of various durations. Exp Brain Res 91: 115–120, 1992. [DOI] [PubMed] [Google Scholar]
- Wang et al. 2000.Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport 11: 1137–1140, 2000. [DOI] [PubMed] [Google Scholar]
- Wang et al. 2002.Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res 944: 219–231, 2002. [DOI] [PubMed] [Google Scholar]
- Wehr and Zador 2003.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426: 442–446, 2003. [DOI] [PubMed] [Google Scholar]
- Wehr and Zador 2005.Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron 47: 437–445, 2005. [DOI] [PubMed] [Google Scholar]
- Wu et al. 2006.Wu GK, Li P, Tao HW, Zhang LI. Nonmonotonic synaptic excitation and imbalanced inhibition underlying cortical intensity tuning. Neuron 52: 705–715, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. 2003.Zhang LI, Tan AYY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature 424: 201–205, 2003. [DOI] [PubMed] [Google Scholar]
- Zheng and Knudsen 1999.Zheng W, Knudsen EI. Functional selection of adaptive auditory space map by GABAA-mediated inhibition. Science 284: 962–965, 1999. [DOI] [PubMed] [Google Scholar]