Abstract
We used epifluorescence microscopy and a voltage-sensitive dye, di-8-ANEPPS, to study changes in membrane potential during hypercapnia with or without synaptic blockade in chemosensory brain stem nuclei: the locus coeruleus (LC), the nucleus of the solitary tract, lateral paragigantocellularis nucleus, raphé pallidus, and raphé obscurus and, in putative nonchemosensitive nuclei, the gigantocellularis reticular nucleus and the spinotrigeminal nucleus. We studied the response to hypercapnia in LC cells to evaluate the performance characteristics of the voltage-sensitive dye. Hypercapnia depolarized many LC cells and the voltage responses to hypercapnia were diminished, but not eradicated, by synaptic blockade (there were intrinsically CO2-sensitive cells in the LC). The voltage response to hypercapnia was substantially diminished after inhibiting fast Na+ channels with tetrodotoxin. Thus action potential–related activity was responsible for most of the optical signal that we detected. We systematically examined CO2 sensitivity among cells in brain stem nuclei to test the hypothesis that CO2 sensitivity is a ubiquitous phenomenon, not restricted to nominally CO2 chemosensory nuclei. We found intrinsically CO2 sensitive neurons in all the nuclei that we examined; even the nonchemosensory nuclei had small numbers of intrinsically CO2 sensitive neurons. However, synaptic blockade significantly altered the distribution of CO2-sensitive cells in all of the nuclei so that the cellular response to CO2 in more intact preparations may be difficult to predict based on studies of intrinsic neuronal activity. Thus CO2-sensitive neurons are widely distributed in chemosensory and nonchemosensory nuclei and CO2 sensitivity is dependent on inhibitory and excitatory synaptic activity even within brain slices. Neuronal CO2 sensitivity important for the behavioral response to CO2 in intact animals will thus be determined as much by synaptic mechanisms and patterns of connectivity throughout the brain as by intrinsic CO2 sensitivity.
INTRODUCTION
Multiple nuclei within the medulla contain CO2-sensitive neurons and contribute to the ventilatory response to CO2 (Coates et al. 1993; Putnam et al. 2004). Putative central chemosensory nuclei include the locus coeruleus (LC), the nucleus of the solitary tract (NTS), the retrotrapezoid nucleus (RTN), the lateral paragigantocellularis nucleus (PGL), the caudal medullary raphé nuclei, the fastigial cerebellar nucleus, and possibly the rostral elements of the nucleus ambiguus (Putnam et al. 2004; Solomon 2003). Different investigators have championed the primacy of particular nuclei among these multiple putative chemosensory nuclei based on the relative CO2 sensitivity of the constituent neurons and/or the unique connectivity of specific nuclei to the neural respiratory control system (Feldman et al. 2003; Guyenet et al. 2008; Loeschcke 1973; Parisian et al. 2004; Putnam et al. 2004; Severson et al. 2003; Solomon 2003; Xu et al. 2001). To address the question of the relative importance and the distribution of CO2-sensitive chemosensory cells within these nuclei, three approaches have been used: anatomical, electrophysiological, and immunohistochemical.
The anatomical studies have depended on lesions or injections of acidic stimuli (Putnam et al. 2004). The lesions have ranged from physical destruction of a site to physical destruction of a subset of specific neurons within a site. Electrophysiological studies of individual neurons in brain slices have been conducted using either sharp electrodes or patch-clamp methods (Putnam et al. 2004). These studies allow investigators to analyze the ionic basis of CO2 chemosensitivity and explore the stimulus–response characteristics of individual neurons (Dean et al. 1989; Erlichman and Leiter 1997; Filosa et al. 2002; Mulkey et al. 2004; Ritucci et al. 2005; Wang and Richerson 1999). Furthermore, interneurons, which may be activated by synaptic mechanisms, can be differentiated from intrinsically CO2 sensitive neurons, which retain CO2/pH sensitivity in the absence of synaptic activity, by using solutions that block synaptic transmission. Last, investigators have used hypercapnia-induced expression of c-fos, an early-response gene activated by increased neuronal activity, to map the number and location of CO2-activated neurons within the brain stem (Belegu et al. 1999; Douglas et al. 2001; Okada et al. 2002; Sica et al. 1999; Teppema et al. 1997; Wickström et al. 2002). The results of anatomical, electrophysiological, and c-fos studies have generally been concordant; nuclei that contain fos positive neurons after exposure to hypercapnia also contain neurons with electrophysiological responses to hypercapnic acidosis and destruction of these nuclei often reduces the respiratory response to hypercapnia in the whole animal. The methods diverge most in the prediction of the number, distribution and relative sensitivity of CO2-sensitive cells within nuclei (Belegu et al. 1999; Mulkey et al. 2004; Ritucci et al. 2005; Wang and Richerson 1999; Wickström et al. 2002).
Optical techniques using potentiometric probes provide a powerful method to simultaneously study the spatial and temporal distribution of cellular activity at multiple sites (Albowitz and Kuhnt 1993; Ito et al. 2004; Nakagami et al. 1997; Prechtl et al. 1997; Salzberg et al. 1977; Tominaga et al. 2000; Zochowski et al. 2000). The potentiometric dye di-8-ANEPPS intercalates into the outer leaflet of the plasma membrane, where octyl hydrocarbon chains in the dye anchor it to the membrane (Loew 1996). Changes in transmembrane voltage affect the molecular orbitals of this electrochromic probe (Loew 1996) and the change in molecular orbitals leads to an emission shift when the dye is excited. We developed optical methods using di-8-ANEPPS to study the voltage responses of neurons within multiple chemosensory regions of the brain stem. Our study consists of two parts. In the first section, we validated the use of di-8ANEPPS in brain slices and developed a calibration routine. In the second part of the study, we systematically analyzed CO2 chemosensitivity before and after synaptic blockade in multiple neurons in individual brain slices taken from putative CO2 chemosensory sites in the brain stem and in two nonchemosensory sites. The use of potentiometric dyes permitted us to analyze the heterogeneity of cellular voltage responses with far greater resolution than extracellular recordings and greater efficiency and yield than those of intracellular recordings. Furthermore, the optical method retains the benefits of electrophysiological studies in that one may identify intrinsically CO2 sensitive neurons that are either excited or inhibited by CO2 by studying cells both with and without synaptic blockade, but one may also identify the entire population of CO2-sensitive neurons within a slice rather than just one or two cells. Finally, previous electrophysiological studies of CO2 chemosensitivity used a variety of different methods among the multiple CO2 sensitive nuclei, whereas we applied a consistent protocol to all nuclei to facilitate meaningful comparisons of CO2 sensitivity among nuclei. The overarching hypotheses being tested in this work were that chemosensory responses to CO2 are widespread within the brain stem, heterogeneous within nuclei, and dynamically determined by both inhibitory and excitatory synaptic inputs.
METHODS
Slice preparation
We studied brain slices obtained from 50 Sprague–Dawley rats of either sex ranging in age from postnatal day 4 (P4) to P23. Multiple medullary brain slices were prepared from each animal, but data were obtained from no more than two tissue slices from any single animal. Procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals from the Public Health Service, National Institutes of Health, and approved by the institutional animal care committees at St. Lawrence University, Wright State University, and Dartmouth College. Each animal was killed by rapid decapitation and the brain was removed from the skull and submerged in chilled (4°C) choline-substituted slicing solutions (described in the following text) for 2–3 min. The caudal cerebellum was removed and a tissue block extending from the obex caudally to the rostral portion of the pons was prepared. Transverse medullary slices (400 μm thick) were sectioned in chilled, oxygenated choline-substituted slicing solution using a vibratome (Series 1000; Pelco, Redding, CA). Following sectioning, slices were transferred to a holding chamber containing oxygenated normal artificial cerebrospinal fluid (described in the following text) at room temperature.
Solutions
Artificial cerebrospinal fluid (aCSF) contained (in mM): 124 NaCl, 3 KCl, 2.4 CaCl2, 1.3 MgSO4, 1.24 KH2PO4, 26 NaHCO3, and 10 glucose. Control aCSF was equilibrated with 95% O2-5% CO2 to maintain a pH of 7.48 at 37°C. Hypercapnic solutions were equilibrated with 90% O2-10% CO2. To reduce cell death, a choline-modified solution was used while cutting each brain slice. In this solution choline was substituted for sodium; calcium was reduced and magnesium was elevated; and kynurenic acid, a glutamate antagonist, and ascorbic acid, an antioxidant, were added. The solution contained (in mM): 135 choline chloride, 1 KCl, 0.5 CaCl2, 20 MgCl2, 1.4 NaH2PO4, 24 choline bicarbonate, 20 kynurenic acid, 5 ascorbic acid, and 10 glucose. The solution was chilled to 4°C and equilibrated with 95% O2-5% CO2, causing the pH of the solution to fall to about 6.9 (Erlichman et al. 2004). Several additional modified solutions were used in the experiments. For the elevated K+/valinomycin calibration experiments, solutions were made with increased K+ (20 mM) and an equimolar reduction in Na+ to keep the solution isosmotic. Valinomycin (10 μM), a potassium ionophore, was added to facilitate equilibration of potassium inside and outside the cell. A reduced calcium/elevated magnesium solution was used to reduce Ca2+-dependent synaptic transmission. In high Mg2+/low Ca2+ solutions, Mg2+ was increased to 10.4 mM and Ca2+ reduced to 0.6 mM. Tetrodotoxin (TTX, 500 nM) was used to block the generation of action potentials (APs). Both APs and calcium oscillations were blocked using aCSF that included 500 nM TTX and 2 mM cobalt. The various saline solutions were kept at 37°C using a servo-controlled syringe heater block (Model SW-707; Warner Instruments, New Haven, CT) and in-line heaters (Model SH-27B; Warner Instruments, New Haven, CT).
Optical membrane potential measurements
The stock voltage-sensitive dye, 1-(3-sulfonatopropyl)-8-vinylpyridium betaine (di-8-ANEPPS; Molecular Probes, Eugene, OR) was dissolved in 20% pluronic F-127 in DMSO (Invitrogen), and individual slices were incubated in an aCSF solution containing 7.5 μM di-8-ANEPPS for 20 min at room temperature. After incubation, the slice was placed in a closed chamber and perfused at a constant flow with aCSF at 37°C. The perfusion chamber was placed on the light microscope (Eclipse TE2000-S; Nikon, Melville, NY) on an air table. Membrane potential (Vm) measurements were collected sequentially during the experiment every 20–30 s. The Vm of individual cells was measured by alternately exciting the tissue with light from a 150-W xenon lamp (OPTI Quip, Highland Mills, NY) at 440 ± 30- and 535 ± 10-nm wavelengths for 0.5–1.2 s. The excitation filters were switched using a computer-controlled filter wheel (Lambda 10-B; Sutter Instruments, Novato, CA). The emitted fluorescence from the di-8-ANEPPS was focused with a ×20 objective lens (PlanFluor; Nikon, Melville, NY), filtered by a 565-nm dichroic mirror (Chroma Technology, Bennington, VT), directed through an emission filter (605 nm) and measured with a cooled charge-coupled device (CCD) camera (Hamamatsu ORCA, Hamamatsu City, Japan). During depolarization, the emitted fluorescence after 440-nm excitation decreased, whereas the emitted fluorescence after 530-nm excitation increased. The digital images at each excitation wavelength were acquired and processed with Compix software (C Imaging Systems, Cranberry Township, PA). A fluorescence ratio (RFL) was obtained by dividing sequential fluorescence values obtained from 440- and 530-nm excitation (RFL = FL440/FL530). The change in membrane potential was calculated from the measured RFL using a calibration equation described in the following text.
Di-8-ANEPPS loading and photobleaching properties
The di-8-ANEPPS loaded medullary brain slices well and provided consistent and reliable staining. We observed two patterns of staining in neurons from brain stem slices. A halo-like loading pattern that clearly outlines the cell membrane could be identified in some neurons, as shown in Fig. 1, A and C. These cells were large and it may be that they permitted better optical sectioning through the cell. In the other pattern of staining, the neurons appeared as solid orbs or discs (Fig. 1B). These cells were smaller than those that stained with a halo pattern and it seemed that the entire cell was contained within the focal plane of the image. The fluorescence responses to changes in membrane potential were independent of the different di-8-ANEPPS dye loading patterns.
FIG. 1.
Typical di-8-ANEPPS [1-(3-sulfonatopropyl)-8-vinylpyridium betaine] staining patterns from the locus coeruleus (LC) in the juvenile rat medulla are shown above. In A, note the halo-like pattern of the cell membrane staining (×100 magnification; scale bar = 200 μm). The disc-like pattern of di-8-ANEPPS staining is shown in B (white diagonal arrow; ×200 magnification; upright arrow scale bar = 50 μm). A single digitally enlarged and enhanced di-8-ANEPPS stained halo-like neuron in the LC is shown in C (diagonal arrow; scale bar = 15 μm). Di-8-ANEPPS acts like a vital dye and fluoresces only in membranes with a potential difference between the extracellular and intracellular spaces.
The ratiometric fluorescence values (RFL) of di-8-ANEPPS increased during membrane depolarization and decreased in response to hyperpolarization. The RFL values were stable over time and resistant to internalization. Photobleaching did occur at each excitation wavelength, FL440 and FL530, but the rate of photobleaching was similar at both wavelengths, as previously noted (Bullen and Saggau 1999; Kao et al. 2001). Thus the RFL values were stable (mean = 1.454 ± 0.001) for >90 min after almost 1,000 exposures (Fig. 2).
FIG. 2.
The change in fluorescence at each excitation wavelength (bottom) and in the ratio of fluorescence at the 2 excitation wavelengths (top) are shown as a function of time in cells from the LC (n = 47). Fluorescence at both FL440 and FL530 was measured under constant perfusion conditions for 958 exposures over the course of 1.5 h. The emitted fluorescence of both wavelengths bleached at nearly the same rate and thus photobleaching had no significant effect on the fluorescence ratio (RFL). The mean value of RFL was 1.454 ± 0.001 and it was stable over the course of this study.
Whole cell recordings
Whole cell patch-clamp recordings were made in cells in the locus coeruleus (LC) in brain slices prepared as described earlier. Whole cell pipettes (5 MΩ) were fabricated from borosilicate glass (TW150-3, World Precision Instruments) using a Narishige PP-830 dual-stage pipette puller and were filled with a solution containing (in mM): 130 K-gluconate, 1 MgCl2, 10 HEPES, 0.4 EGTA [ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid], 2 Na2ATP, 0.3 Na2GTP, and 0.2 pyranine (pH ∼7.35 at 37°C). The filling solution had no added Ca2+ and low levels of EGTA. The pipette holder contained Ag-AgCl wire and the circuit was completed with Ag-AgCl wire placed (downstream to the brain slice) in the perfusion bath. A gigaohm seal was created by applying negative pressure to the patch pipette once the pipette touched the cell surface. The tight seal was ruptured to achieve the whole cell configuration. Electrophysiological recordings were conducted in current-clamp mode and Vm was measured with a Dagan BVC-700 amplifier. Vm was saved to both a digital VCR (model 400; Vetter) and to Axoscope software (version 8.0) for later analysis. Vm represents a time-averaged value of all potentials over ≥1 min. Only neurons with a resting Vm between −30 and −60 mV were analyzed.
Di-8-ANEPPS calibration
We constructed a calibration curve that allowed us to calculate the change in membrane potential (ΔVm) given any particular change in RFL (ΔRFL) of the di-8-ANEPPS dye in LC neurons. The following K+ solutions were used to calibrate the change in RFL from the baseline value measured at an extracellular K+ of 3 mM (the number of cells studied at each K+ concentration is given in parentheses): 5 mM (n = 88), 10 mM (n = 76), 15 mM (n = 127), and 20 mM (n = 77). The change in RFL was expressed as a function of the change in extracellular K+ concentration using linear regression as shown in Fig. 3 (top left). Similar techniques were used in separate experiments to correlate the change in extracellular K+ concentrations with changes in Vm measured using patch-clamp techniques. The K+ solutions used and numbers of cells studied in these experiments were as follows: 5 mM (n = 4), 10 mM (n = 5), 15 mM (n = 5), and 20 mM (n = 5). These data were also fitted by linear regression (Fig. 3, top right). The results from these two studies were combined to derive the relationship between Vm and di-8-ANEPPS RFL. The final calibration relationship was as follows
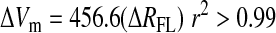 |
The linear relationship between RFL and Vm that we observed is consistent with previous studies using di-8-ANEPPS in cell culture (Zhang et al. 1998).
FIG. 3.
In the top left panel, a calibration curve was generated for di-8-ANEPPS by changing the extracellular K+ concentration from 3 mM (control) to 5–20 mM while measuring the RFL. These results were fit by linear regression (n = 157 cells). In the top right panel, membrane potential was measured using conventional patch-clamp techniques while the extracellular K+ concentration was changed from 3 to 20 mM (n = 16). The results were fit with linear regression. The regression equations from these 2 studies were combined and the following calibration equation in terms of membrane potential (Vm) and RFL was derived: ΔVm = 456.6(ΔRFL); r2 > 0.99. Voltage changes in LC neurons during repeated exposure to elevated K+ (15 mM; blue bars) are shown in the bottom panel. Note the depolarization on introduction of elevated K+ solution followed by the return to baseline levels when these solutions were replaced with normal artificial cerebrospinal fluid (aCSF).
Depolarization can also occur as an artifact of neuronal cell death. To ensure that elevated K+ solutions caused a repeatable depolarization without lethal effects, we exposed a subset of cells (n = 16) to 15 mM K+ pulses interspersed with normal aCSF (K+ = 3 mM). These studies were done without added valinomycin. Switching between solutions of different extracellular K+ concentration resulted in consistent depolarization and repolarization (Fig. 3, bottom). The fluorescence of di-8-ANEPPS changed reversibly and tracked Vm during prolonged periods of study.
Data analysis and statistics
Each study of CO2 sensitivity consisted of a control, normocapnic exposure period and a hypercapnic test period with or without synaptic blockade. Each condition lasted about 15 min and data were obtained in the last 2–3 min of each exposure period. No more than one set of measurements was made in each brain slice. To measure the fluorescence of individual cells, regions of interest restricted to the cell membrane in the focal plane of the image were created. These constrained regions of the image were selected to limit the effect of fluorescence from adjacent cells. Di-8-ANEPPS is nonfluorescent in aqueous solution, but fluoresces when intercalated in the cell membrane (Obaid et al. 2004; Rohr and Salzberg 1994); thus there is limited stray light except from adjacent membranes. Data were reported as means ± SD. Comparisons between treatments were made using unpaired t-test or a one-way ANOVA followed by preplanned comparisons using the Bonferroni correction when the ANOVA indicated that significant differences existed among treatment groups.
RESULTS
Origin of optical signals at slow temporal resolution
We used the RFL measurements with low temporal resolution; we could not track the morphology of individual APs given the speed and sensitivity of our CCD camera and our need to use a ratiometric dye. At a low acquisition rate and long exposure times, it was our hypothesis that the sequential images captured the average change in Vm over time and provided an index of aggregate activity over minutes rather than seconds. To confirm this hypothesis, we studied the hypercapnic response of neurons within the LC, which contains a homogeneous population of CO2-sensitive neurons with well-defined electrophysiological responses to specific treatment conditions (Filosa and Putnam 2003; Oyamada et al. 1998; Pineda and Aghajanian 1997). In Fig. 4, examples of fluorescence ratios from single cells exposed to normocapnic and hypercapnic aCSF (Fig. 4A) and exposed to hypercapnia alone, hypercapnia plus TTX (500 nM) treatment, or hypercapnia plus TTX plus cobalt (2 mM, to block calcium channels) are shown. The average responses to these treatments are shown in the bottom panels of Fig. 4. Hypercapnia alone significantly depolarized LC neurons (Fig. 4C, P < 0.01) and synaptic blockade (low Ca2+/high Mg2+ = 0.6 and 10.4 mM, respectively) reduced, but did not eliminate, the depolarizing effect of hypercapnia (Fig. 4C: the degree of depolarization was less than that of hypercapnia alone, P < 0.05, but greater than zero, P < 0.05). Thus many LC neurons were intrinsically CO2 sensitive, as shown previously (Filosa et al. 2002). Tetrodotoxin, which is known to inhibit hypercapnia-induced APs in LC neurons (Filosa et al. 2002), also reduced the magnitude of the hypercapnic depolarization (P < 0.01), but a slight depolarization remained on average that was still different from zero (P < 0.01). Thus APs made a significant contribution to the signal that we measured in the absence of TTX, although this signal was not entirely dependent on the generation of APs since a small residual depolarization persisted during hypercapnic perfusion after TTX treatment. Hypercapnia also activates calcium channels in the LC and calcium-dependent processes can be blocked by adding cobalt to the perfusate (Filosa and Putnam 2003). Cobalt treatment after TTX treatment reversed even the small residual hypercapnic depolarization that persisted in the presence of hypercapnia and TTX (Fig. 4D). Neurons within the LC were slightly depolarized during treatment with hypercapnia, TTX, and cobalt (0.11 ± 0.4 mV, P > 0.05; n = 59); when the hypercapnia was removed, however, the membrane potential fell and was significantly hyperpolarized compared with the aCSF control condition (−1.5 ± 0.4 mV; P < 0.001). Thus some residual CO2-sensitive process remained after inhibition of sodium and calcium channels and, during normocapnia, a cobalt-sensitive process seemed to depolarize Vm (since Vm was hyperpolarized when this process was blocked). This pattern of responses to hypercapnia, TTX, and cobalt measured with di-8-ANEPPS is virtually identical to the pattern of responses measured in LC neurons using perforated or whole cell patch recordings (Filosa and Putnam 2003).
FIG. 4.
A: sample trace of the ratio of di-8-ANEPPS fluorescence from individual LC neuron in response to normocapnic and hypercapnic aCSF. Note the depolarization during hypercapnia and the return to control levels when the hypercapnic solution was removed. B: sample trace of fluorescence from a single cell treated with tetrodotoxin (TTX) with and without hypercapnia. Note that the hypercapnic response was greatly reduced by synaptic blockade with TTX. Calcium channels were blocked by treatment with cobalt, which caused a further slight fall in Vm. C: the average effects of hypercapnia on Vm in cells in the LC: hypercapnia alone, white bar; hypercapnia plus synaptic blockade with high Mg2+/low Ca2+, gray bar; and hypercapnia plus synaptic and action potential (AP) blockade with TTX, black bar. Hypercapnia depolarized LC neurons; the depolarization persisted during synaptic blockade, indicating that there are intrinsically CO2 sensitive neurons in the LC; and TTX blocked the intrinsic, AP-dependent activity, but hypercapnia had a slightly depolarizing effect that persisted even in the absence of AP-related activity. D: the results during hypercapnia with TTX have been replicated from C for ease of comparison, white bar; the average effect of hypercapnia plus TTX plus cobalt treatment is shown by the gray bar; and the effect of normocapnic aCSF plus TTX plus cobalt is shown by the black bar. Treatment with TTX and cobalt leaves little or no residual depolarization, but when the hypercapnic stimulus is removed, a hyperpolarizing effect of cobalt plus TTX is revealed. “*” in both C and D indicates that the value is significantly less than the value during hypercapnia alone (see text for P values). All the treatments except hypercapnia plus TTX plus cobalt changed the membrane potential significantly from the control normocapnic value (P < 0.05 in all cases).
Responses in dorsal chemosensory nuclei: the LC and NTS
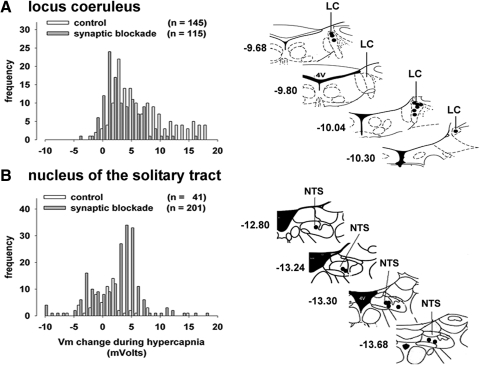
In the second half of this study, we examined the hypercapnic sensitivity of neurons from identified chemosensory nuclei within the pons and medulla and from two putative nonchemosensory medullary regions before and after synaptic blockade. Histograms of the change in membrane potential that occurred during hypercapnia and during hypercapnia plus synaptic blockade are shown in Fig. 5. The anatomical sites from which data were taken are shown in the same figure on schematic cross sections of the brain stem. We defined a chemosensory response as any membrane potential change greater than ±3 mV from 0. A ±3-mV change is >3SDs greater than the spontaneous variation in membrane potential when we simply observed di-8-ANEPPS fluorescence over time. During hypercapnia, 74% of the cells were excited (Vm change >3 mV) and 29% of the LC cells did not respond to CO2. In the cells exposed to hypercapnia and synaptic blockade, only 38% of the cells were excited, membrane potential was unchanged in 61% of the cells, and one cell was hyperpolarized (Vm < −3). Thus there are many intrinsically CO2 sensitive cells in the LC, but approximately half of the cells activated by hypercapnia were not intrinsically CO2 sensitive, but were excited by synaptic events. The estimated number of intrinsically CO2 sensitive cells may be high since synaptic blockade may not disrupt gap junctions, which are present in the LC (Dean et al. 2001; Hartzler et al. 2007).
FIG. 5.
Changes in membrane potential of neurons in dorsal chemosensory nuclei: the LC and nucleus of the solitary tract (NTS). A: histogram of the membrane potential changes (in mV) of LC neurons after hypercapnic treatment. The anatomical sites from which the data in A were taken are shown to the right. B: histogram of the membrane potential changes of NTS neurons during hypercapnic treatment, and the anatomical sites studied are shown on the right. All dimensions on the anatomical figures are measured from the bregma.
In addition to comparing the fraction of cells activated or inhibited by hypercapnia, the response to CO2 may be quantitated by studying the magnitude of depolarization during any given hypercapnic condition. The chemosensitivity measured in this way is an index of the chemosensitivity of individual cells, but this measure does not capture the magnitude of the neural activity arising from a given nucleus. To estimate the excitatory and inhibitory chemosensory drive originating within each nucleus, we calculated the average chemosensory index (CSI) for cells depolarized or hyperpolarized by >3 mV (mV change/5% CO2 change) and then divided that number by the fraction of the total cells studied in each nucleus that were either depolarized or hyperpolarized, to obtain a normalized excitatory or inhibitory chemosensory drive (CD) for each nucleus. These values are summarized for each nucleus in Table 1. The CSI that we calculated is not comparable with that of other studies, but is useful to compare sensitivity among nuclei in our study. The average membrane potential change among the LC neurons excited by hypercapnia was 8.6 ± 0.4 mV during hypercapnia and 5.8 ± 0.4 mV during hypercapnia plus synaptic blockade. Synaptic blockade significantly reduced the response to CO2 (P < 0.001). A large fraction of cells within the LC were excited by hypercapnia; therefore the chemosensory drive is also substantial (6.1). However, this was reduced during synaptic blockade, reflecting the modest reduction in CO2 sensitivity of individual cells, but also the large reduction in the number of cells that were actually excited by CO2 during synaptic blockade. Thus the LC contains intrinsically CO2 sensitive cells, but even within the brain slice, synaptic connections significantly enhance the total CO2-dependent excitatory activity in the LC. There were few cells inhibited by hypercapnia in the LC.
TABLE 1.
Summary of chemosensory index (CSI) and excitatory or inhibitory chemosensory drive (CD) values for each nucleus
| Nucleus | Synaptic Blockade | Activated | Unchanged | Inhibited | eCSI | eCD | iCSI | iCD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Chemosensory nuclei | ||||||||||||||||
| Locus coeruleus | − | 103 ± 71% | 42 ± 29% | 0 | 8.6 ± 0.4 | 6.1 | — | — | ||||||||
| + | 44 ± 38% | 70 ± 61% | 1 ± 1% | 5.8 ± 0.4 | 2.2 | — | — | |||||||||
| Solitary tract | − | 8 ± 14% | 44 ± 77% | 5 ± 9% | 7.0 ± 1.3 | 1.0 | −4.7 ± 0.4 | −0.5 | ||||||||
| + | 95 ± 47% | 91 ± 45% | 15 ± 7% | 5.2 ± 0.3 | 2.4 | −5.4 ± 0.5 | −0.8 | |||||||||
| Retrotrapezoid | − | 18 ± 21% | 53 ± 62% | 14 ± 16% | 8.4 ± 1.2 | 1.8 | −7.6 ± 1.0 | −1.3 | ||||||||
| + | 25 ± 23% | 64 ± 59% | 19 ± 18% | 4.3 ± 0.2 | 1.0 | −6.3 ± 0.4 | −1.3 | |||||||||
| Lateral paragiganto- | − | 7 ± 11% | 54 ± 86% | 2 ± 3% | 3.6 ± 0.2 | 0.4 | −4.1 ± 0.4 | −0.2 | ||||||||
| cellular | + | 11 ± 16% | 57 ± 84% | 0 | 4.0 ± 0.2 | 0.6 | — | — | ||||||||
| Raphé obscurus | − | 25 ± 16% | 125 ± 78% | 11 ± 7% | 4.4 ± 0.2 | 0.7 | −4.0 ± 0.1 | −0.6 | ||||||||
| + | 12 ± 20% | 14 ± 23% | 34 ± 56% | 9.8 ± 2.6 | 2.1 | −11.4 ± 0.8 | −6.4 | |||||||||
| Raphé pallidus | − | 0 | 82 ± 100% | 0 | — | — | — | — | ||||||||
| + | 24 ± 18% | 108 ± 81% | 2 ± 1% | 5.7 ± 0.5 | 1.0 | −3.9 ± 0.5 | −0.3 | |||||||||
| B. Nonchemosensory nuclei | ||||||||||||||||
| Giganto-cellular | − | 34 ± 41% | 38 ± 46% | 10 ± 12% | 7.7 ± 0.6 | 3.2 | −5.2 ± 0.5 | −0.8 | ||||||||
| reticular | + | 0 | 29 ± 66% | 15 ± 34% | — | — | −5.5 ± 0.4 | −2.4 | ||||||||
| Spinal trigeminal | − | 48 ± 53% | 34 ± 38% | 8 ± 9% | 8.6 ± 0.5 | 4.6 | −8.7 ± 0.3 | −1.0 | ||||||||
| + | 9 ± 7% | 109 ± 91% | 2 ± 2% | 3.8 ± 0.3 | 0.3 | −3.7 ± 0.2 | −0.2 | |||||||||
A similar analysis of cells within the NTS is shown in Fig. 5B. During hypercapnia alone, 14% of the cells were excited (Vm change >3 mV); 77% of the NTS cells did not respond to CO2; and 1% of the cells were inhibited during hypercapnia. In the cells exposed to hypercapnia and synaptic blockade, a greater fraction of the cells were excited by hypercapnia during synaptic blockade (42%) than by hypercapnia alone. Membrane potential was unchanged in 45% of the cells and 7% of the cells were hyperpolarized. Thus there are intrinsically CO2 sensitive cells in the NTS, but the activity of many of these intrinsically CO2 sensitive cells may be inhibited by synaptic inputs arising from within the brain slice (Conrad et al. 2009; Nichols et al. 2009). The excitatory CSI was 7.0 ± 1.3 during hypercapnia (Table 1) and 5.2 ± 0.3 during hypercapnia plus synaptic blockade. These values were not significantly different. However, relatively few cells within the NTS were excited, so the excitatory CD was only 1.0 during hypercapnia and 2.4 during hypercapnia plus synaptic blockade. The NTS contains cells with intrinsic CO2 sensitivity that is comparable to that of the LC, but it has fewer of these cells and thus may contribute less to the overall CO2-dependent chemosensory drive. The excitatory CD rose after synaptic blockade, which indicates that synaptic-dependent inhibition within the NTS may actually reduce the contribution of the intrinsically CO2 sensitive cells to the overall CO2-dependent chemosensory drive (Leiter 2009). The hyperpolarization present in some cells during hypercapnia was also sizable, but the inhibitory CSI was not affected by the presence of synaptic blockade. However, there were relatively few cells hyperpolarized by hypercapnia and, as a consequence, the inhibitory CDs before and after synaptic blockade were relatively modest (−0.5 and −0.8, respectively).
Responses in rostroventrolateral chemosensory nuclei: the RTN and PGL
Histograms of the change in membrane potential that occurred during hypercapnia and during hypercapnia plus synaptic blockade in the RTN and PGL are shown in Fig. 6. During hypercapnia, 21% of the cells were excited (Vm change >3 mV); 62% of the cells did not respond to CO2; and 16% of the cells were hyperpolarized during the hypercapnic exposure. In the cells exposed to hypercapnia and synaptic blockade, 23% of the cells were still excited; membrane potential was unchanged in 59% of the cells; and 18% cells were hyperpolarized. Thus there were intrinsically CO2 sensitive cells in the RTN, but this sensitivity to hypercapnia was manifest as inhibition of activity in a significant fraction of the cells studied. The neurons in the RTN, like the LC, had a relatively high excitatory CSI (excitatory CSI = 8.4 ± 1.2), which was, however, reduced significantly (4.3 ± 0.2; P < 0.01) by synaptic blockade. The excitatory CD was rather low (1.8) since few of the total cell population were excited by hypercapnia and the excitatory drive dropped further after synaptic blockade (1.0). A small fraction of the RTN cells were strongly inhibited by hypercapnia before (inhibitory CSI = −7.6 ± 1.0) and after synaptic blockade (inhibitory CSI = −6.3 ± 0.4). The number of cells involved, however, was small and the inhibitory CD was low before and after synaptic blockade (−1.3 in both cases).
FIG. 6.
Changes in membrane potential of neurons in ventrolateral chemosensory nuclei during hypercapnia: the retrotrapezoid nucleus (RTN) and lateral paragigantocellularis nucleus (PGL). The figure conventions are identical to those in Fig. 5.
During hypercapnia alone, 11% of the cells in the PGCL were excited (Vm change >3 mV); 86% of the cells did not respond to CO2; and 3% of the cells were inhibited during hypercapnia. In the cells exposed to hypercapnia and synaptic blockade, 16% of the cells were excited; membrane potential was unchanged in 84% of the cells; and none of the cells was hyperpolarized. The cells in the PGCL were modestly CO2 sensitive (excitatory CSI = 3.6 ± 0.2), which was not changed significantly by synaptic blockade (4.0 ± 0.2). The excitatory CD was quite low during hypercapnia (0.4), since few cells were excited by hypercapnia, and was not affected by synaptic blockade (excitatory CD = 0.6). Few cells in the PGCL were inhibited by hypercapnia and there were no cells inhibited by hypercapnia during synaptic blockade. As a consequence, there was little or no inhibitory CD originating in the PGCL.
Responses in caudal raphé nuclei
No cells were depolarized by hypercapnia alone in the raphé pallidus (Fig. 7) and 100% of the cells were unresponsive to hypercapnia by our definition (Vm did not change by >3 mV during hypercapnia). In contrast, 18% of the cells responded to hypercapnia in the presence of synaptic blockade; 81% of the cells were unresponsive to hypercapnia; and 1% of the cells were hyperpolarized. Synaptic blockade revealed that intrinsically CO2 sensitive cells exist in the raphé pallidus that are modestly excited (excitatory CSI = 5.7 ± 0.5) and inhibited (inhibitory CSI = −3.9 ± 0.5). The chemosensory drive is low, however, since there simply are not that many CO2-sensitive cells in this nucleus.
FIG. 7.
Changes in membrane potential of neurons in raphé chemosensory nuclei during hypercapnia: the raphé pallidus and raphé obscurus. The figure conventions are identical to those in Fig. 5.
In the raphé obscurus, 16% of the cells were excited by hypercapnia alone; 78% of the cells did not respond to CO2; and 7% of the cells were inhibited during hypercapnia. In the cells exposed to hypercapnia and synaptic blockade, 20% of the cells were excited; membrane potential was unchanged in 23% of the cells; but 56% cell were hyperpolarized by hypercapnia. The neurons in the raphé obscurus demonstrated a modest excitatory CSI during hypercapnia (excitatory CSI = 4.4 ± 0.2), which was further increased by synaptic blockade (9.8 ± 2.6; P < 0.054). However, the excitatory CD was low since there were so few cells excited by hypercapnia. Some raphé obscurus cells were inhibited by hypercapnia (inhibitory CSI = −4.0 ± 0.1) and the hypercapnic inhibition was significantly greater after synaptic blockade (inhibitory CSI = −11.4 ± 0.8; P < 0.001). A peculiar situation exists in the raphé obscures, in which the intrinsic CO2 sensitivity of excited and inhibited cells is quite high. However, the overall drive originating in the raphé obscurus is probably small since synaptic inputs within the slice seem to reduce both excitatory and inhibitory chemosensitivity and the majority of cells in the raphé obscurus are not sensitive to hypercapnia except during synaptic blockade. Thus the excitatory CD was 0.7 without synaptic blockade, but 2.1 during synaptic blockade, and the inhibitory CD was −0.6 before synaptic blockade, but −6.4 after synaptic blockade.
Responses in putative nonchemosensory nuclei: the GCR and the STN
In the gigantocellularis reticular nucleus (GCR) during hypercapnia, 41% of the cells were excited; 46% of the cells did not respond to CO2; and 12% of the cells were hyperpolarized (Fig. 8). In the cells exposed to hypercapnia and synaptic blockade, none of the cells was excited, membrane potential was unchanged in 66% of the cells, and 34% of the cells were hyperpolarized. Thus the excitatory CSI was high during hypercapnia alone (7.7 ± 0.6) and there was a modest excitatory chemosensory drive (3.2) from the gigantocellular reticular nucleus, although this was entirely dependent on synaptic inputs. The inhibitory CSI was modest before synaptic blockade (−5.5 ± 0.4) and after (−5.2 ± 0.5). The inhibitory CD was small during hypercapnia, but increased after synaptic blockade.
FIG. 8.
Changes in membrane potential of neurons in nonchemosensory nuclei during hypercapnia: the gigantocellularis reticular nucleus (GCR) and the spinotrigeminal nucleus (STN). The figure conventions are identical to those in Fig. 5.
In a similar analysis of cells within the spinotrigeminal nucleus (STN), 53% of the cells were excited during hypercapnia alone; 38% of the cells did not respond to CO2; and 9% of the cells were inhibited during hypercapnia. In the cells exposed to hypercapnia and synaptic blockade, 7% of the cells were excited; membrane potential was unchanged in 91% of the cells; and 2% of the cells were hyperpolarized. The excitatory CSI was high during hypercapnia alone (8.6 ± 0.5) and the excitatory CD was 4.6, but this was largely dependent on synaptic inputs since the excitatory CSI dropped significantly to 3.8 ± 0.3 (P < 0.01), and the excitatory CD was reduced to 0.3 after synaptic blockade. The inhibitory CSI was high (−8.7 ± 0.3), but the chemosensory drive was small during hypercapnia (−1.0; not that many cells were involved). The inhibitory CSI was reduced after synaptic blockade (−3.7 ± 0.2; P = 0.02) and the inhibitory CD was further reduced since even fewer cells were inhibited by CO2 during hypercapnia and synaptic blockade. In general, there were many cells excited by hypercapnia in these nonchemosensory medullary nuclei, but few of these cells were intrinsically CO2 sensitive.
DISCUSSION
We used a voltage-sensitive dye to assess the responses to hypercapnia of cells located in a variety of putative chemosensory and nonchemosensory nuclei in the medulla and pons. Despite arguments about the primacy of one site or the other in central CO2 chemoreception (Feldman et al. 2003; Guyenet et al. 2008; Putnam et al. 2004; Severson et al. 2003), the sites were more similar in terms of the distribution of CO2-sensitive cells than they were different. All of the sites contained intrinsically CO2 sensitive cells (cells that were either excited or inhibited by hypercapnia during synaptic blockade). The distributions of excitatory and inhibitory responses differed slightly among regions, but the distribution of these responses was strikingly dependent on both excitatory and inhibitory synaptic inputs even within the limited synaptic circuitry present in the brain slice. Thus the apparent chemosensory drive arising from each nucleus depended significantly on whether synaptic blockade was present. Which state with respect to synaptic blockade better represents the chemosensitivity of a nucleus depends on the question being asked (Leiter 2009).
Performance characteristics and limitations of di-8-ANEPPS
Optical techniques using potentiometric dyes have been used to study the electrophysiological responses of single cells, which requires very fast image acquisition speeds and higher concentrations of the voltage-sensitive dyes (Djurisic et al. 2008; Obaid et al. 2004; Salzberg et al. 1973) or to describe macroscopic patterns of electrical activity across the surface of the brain stem, which does not require fast image acquisition speeds and uses dye concentrations that can be loaded into the cell membrane by diffusion from the bath, but also has low spatial and temporal resolution (Ito et al. 2004). We used conventional epifluorescence microscopy coupled with a cooled CCD camera and di-8-ANEPPS at microscopic spatial resolution to assess individual electrophysiological responses of large numbers of neurons within single brain slices and to determine the distribution of CO2-sensitive cells in regions of the brain involved in central chemosensitivity. Di-8-ANEPPS is a fast voltage-sensitive dye that intercalates into the membrane and produces a 10% change in fluorescence for a 100-mV change in membrane potential (Rohr and Salzberg 1994). Di-8-ANEPPS is not fluorescent in aqueous solution, so background fluorescence is negligible (Montana et al. 1989). The dye is suitable for ratiometric measurements (Beach et al. 1996), which reduces problems associated with photobleaching and compensates for uneven dye loading, but also reduces the temporal resolution of the measurements (two images are required for each estimate of membrane voltage). The ratio emission method (RFL) is noninvasive compared with standard electrophysiological techniques, can be normalized for differences in dye loading, and compared with single emission dyes, decreases optical noise, permits photobleaching correction (see Fig. 2), exhibits increased voltage sensitivity, and is readily calibrated (see Fig. 3) (Bullen and Saggau 1999); moreover, pharmacological/ion substitution experiments can be used to enhance the electrophysiological measurements (see Fig. 4). There are also disadvantages. The spectral and structural properties of these dyes are subject to environmental influence and an absolute value for membrane potential is difficult to obtain (Zhang et al. 1998). Therefore data are reported as the change in fluorescence from some baseline condition. Second, one is recording the average Vm over time. The effect of individual APs is lost in the optical output and rapid nonsustained fluctuations in Vm, whether intrinsic to the cell or synaptically driven, are not detected. Moreover, any pH effects on ion channels or receptors that do not lead to steady depolarization or hyperpolarization are not detected. One cannot differentiate between neurons and astrocytes using di-8-ANEPPS at low temporal resolution. Last, fluorescent optical dyes can be toxic. Toxicity appears to be limited when using di-8-ANEPPS, probably because the fluorescence is restricted to lipid membranes. Studies using voltage-sensitive dyes fall halfway between electrophysiological studies of single cells and studies of fos expression in large collections of cells. Optical methods are good at identifying large populations of CO2-sensitive cells in brain slices, irrespective of cell size, and one can differentiate inhibitory from excitatory responses and identify the effect of synaptic activity, both inhibitory and excitatory, on CO2 responses.
Central chemosensory responses in multiple brain stem nuclei
All of the chemosensory nuclei that we studied have also been examined using whole animal methods with lesions or focal stimulation, microelectrode electrophysiology, or fos immunohistochemistry. In general, the results from these multiple methods are concordant.
Previous studies in intact animals demonstrated that cell-specific lesions in the region of the LC reduced the ventilatory response to CO2 during wakefulness and sleep (Biancardi et al. 2008; Li and Nattie 2006) and focal injections of acetazolamide, which acidify the extracellular space, increased ventilation (Coates et al. 1993). There are intrinsically CO2 chemosensitive neurons in the LC (Filosa and Putnam 2003; Filosa et al. 2002) and there was prominent expression of fos in LC neurons after inhalation of hypercapnic gases (Haxhiu et al. 1996; Teppema et al. 1997). In addition, studies using di-4-ANEPPS, a voltage-sensitive congener of di-8-ANEPPS, indicated that the LC was depolarized on average by elevated concentrations of CO2 (the response of the nucleus as a whole, rather than individual cells, was studied) and this hypercapnic depolarization persisted after synaptic blockade (Ito et al. 2004). Thus the increased activation of a relatively large fraction of cells within the LC that we observed is consistent with previous studies. A smaller fraction of cells activated by hypercapnia were intrinsically CO2 sensitive in our studies (Table 1) and in the studies of others (Filosa and Putnam 2003; Filosa et al. 2002; Nichols et al. 2008). Many of the CO2-sensitive cells activated during hypercapnia without synaptic blockade were probably activated by excitatory synaptic mechanisms present within the brain slice.
Similar studies indicate that the NTS is a CO2-chemosensitive nucleus and contains intrinsically CO2 sensitive cells. Lesions made acutely in the NTS decreased the ventilatory response to CO2 (Berger and Cooney 1982; Parisian et al. 2004) and acid stimulation increased ventilation (Nattie and Li 2002a; Parisian et al. 2004). Intrinsically CO2 sensitive neurons have been identified in the NTS (Dean et al. 1989, 1990) and hypercapnia leads to extensive expression of fos in the NTS (Belegu et al. 1999; Ruggiero et al. 1999; Sica et al. 1999; Teppema et al. 1997). Our findings using the voltage-sensitive dye are consistent with these previous studies, but we found a greater fraction of intrinsically CO2 sensitive cells in the NTS during hypercapnia plus synaptic blockade than during hypercapnia alone. This indicates the presence of significant inhibitory synaptic activity within the brain slice (Conrad et al. 2009; Leiter 2009; Nichols et al. 2009) and there are abundant GABAergic cells scattered throughout the NTS (Bailey et al. 2008; Okada et al. 2008).
A significant number of CO2-sensitive neurons are also present in the RTN. The PGCL is, to some extent, an extension of the RTN, and some investigators have actually studied them as if they were a single site (Nattie and Li 2002b). We agree with this designation: there are fewer CO2-sensitive cells in the parapyramidal region (Table 1) (Richerson 1995) and the neuronal populations are not identical (Stornetta et al. 2006), but the distribution of CO2 sensitivity and the response to synaptic blockade were functionally quite similar to those of cells in the RTN and parapyramidal regions. For these reasons, we have considered these cell populations together. Lesions in the RTN reduced the ventilatory response to CO2 (Akilesh et al. 1997) and focal acidification of the RTN increased ventilation (Hewitt et al. 2004; Li and Nattie 2002). There are CO2-sensitive cells in electrophysiological studies (Mulkey et al. 2004) that may be intrinsically CO2 sensitive (Guyenet et al. 2005a; Onimaru et al. 2008). Moreover, hypercapnia increases fos expression in the RTN and PGCL (Belegu et al. 1999; Sica et al. 1999; Teppema et al. 1997). We found evidence of CO2-sensitive cells in brain slices in the RTN and PGCL and evidence of many intrinsically CO2 sensitive cells, but synaptic blockade did not change the distribution of excited, unaffected, and inhibited cells during hypercapnia. This finding is slightly at odds with a previous study (Guyenet et al. 2005a), but the neuroanatomical source of inhibitory inputs that may reduce CO2 sensitivity in the RTN was excluded from the brain slices we studied, which may explain why we did not see evidence of inhibition (or excitation) of chemosensitivity within the RTN.
We found many CO2-sensitive cells in the raphé obscurus and many of these were intrinsically CO2 sensitive. However, the intrinsic response of a large fraction of the cells was inhibitory. Therefore there seem to be significant excitatory synaptic inputs sustaining hypercapnic activation of cells within the raphé obscurus. The raphé pallidus contained intrinsically CO2 sensitive cells, but these were apparent only after synaptic blockade. Inhibitory synaptic activity seemed to be more important in the raphé pallidus than that in the raphé obscurus and other putative chemosensory nuclei. The mechanism whereby the caudal raphé nuclei contribute to the ventilatory response to CO2 is the subject of debate. In electrophysiological studies, there are not many CO2-sensitive cells in these caudal raphé regions (Richerson 1995; Veasey et al. 1995; Wang and Richerson 1999). Similarly, fos staining, although present in the medullary raphé, is less frequent than in the more rostral and dorsal chemosensitive areas discussed earlier, especially in adult animals (Belegu et al. 1999; Johnson et al. 2005; Teppema et al. 1997). On the other hand, lesions in the more rostral medullary raphé reduce the ventilatory response to CO2 (Nattie et al. 2004) and focal acidic stimulation of the rostral medullary raphé increases ventilation (Nattie and Li 2001). However, the effect of simultaneous focal hypercapnic stimulation of the caudal raphé nuclei and RTN and pharmacological inhibition of serotonergic neurons within the caudal raphé indicated that the caudal raphé may modulate the ventilatory response to CO2 by an indirect effect through other chemosensory nuclei (Dias et al. 2008; Li et al. 2006). Thus the caudal raphé may participate in the ventilatory response to CO2 by modulating the chemosensitivity of other nuclei rather than by virtue of direct activation of respiratory centers by the relatively small number of CO2-sensitive cells in this region. Such a hypothesis is consistent with previous work demonstrating the modulatory function of serotonergic neurons in raphé nuclei with respect to autonomic and somatomotor activity (Lovick 1997).
The putative nonchemosensitive nuclei have fewer CO2-sensitive neurons than most of the putative chemosensory nuclei, but there are clearly cells that respond to CO2 in the spinotrigemial nucleus and the gigantocellularis reticular nucleus, whether they are intrinsically CO2 sensitive or CO2 sensitive by virtue of synaptic inputs. Whether using fos expression or electrophysiological studies, both of our “control” nonchemosensitive nuclei contain CO2-sensitive cells (Belegu et al. 1999; Haxhiu et al. 1996; Miles 1983; Teppema et al. 1997). The proof that CO2-sensitive cells within these nuclei are not chemosensitive is negative in that lesions or focal stimulation in these areas, to the extent they have been studied, do not change the ventilatory response to CO2. The distribution of CO2 sensitivity of individual cells within nuclei does not actually give one any useful information about whether a nucleus is a central chemosensory site; intrinsically CO2 sensitive cells and synaptically driven CO2 sensitivity cells are present throughout the brain stem (Belegu et al. 1999; Haxhiu et al. 1996; Miles 1983; Teppema et al. 1997). The definition of CO2-chemosensitive nuclei depends on tests in intact animals studied awake and asleep (Coates et al. 1993; Putnam et al. 2004) and probably reflects the density of CO2-sensitive cells within a nucleus and the pattern of synaptic connectivity with other elements of the respiratory control system rather than the simple presence or absence of CO2-sensitive cells.
The relative sensitivity of the chemosensory responses that we calculated, although numerically different, is similar to previous rankings of sensitivity among these nuclei (Putnam et al. 2004). However, the relative rankings and one's assessment of the relative chemosensory contribution of particular nuclei depend on which calculations one uses. The rankings of excitatory responses during measurements of intrinsic chemosensitivity in the presence of synaptic blockade yield the following ranking: raphé obscurus > LC ≈ raphé pallidus ≈ NTS > RTN ≈ LPGC > STN > GCR. This ranking conforms to our expectations—the nominally chemosensory nuclei have high rankings and the putative nonchemosensory nuclei have low rankings. However, intrinsic chemosensitivity is not really a measure of the chemosensory source of the behavioral response to CO2 (Leiter 2009) and the rankings of apparent excitatory chemosensitivity are different, even in these highly reduced slice preparations, when synaptic activity is present: LC = STN ≈ RTN > GCR > NTS > raphé obscurus > LPGC ≫ raphé pallidus. This calculation makes the STN appear to be highly CO2 sensitive. Neither of the foregoing estimates of chemosensory activity takes into account the density of chemosensory cells and the final ranking, which orders chemosensory excitatory output at the nuclear level, leads to one additional, completely different ranking: LC > STN > GCR > RTN > NTS > raphé obscurus > LPGC > raphé pallidus. This later ranking is really an estimate of the excitatory input of each nucleus to the chemosensory drive (assuming the outputs from each cell are additive, which they may not be). Similar rankings can be made for the CO2-dependent inhibitory inputs from these nuclei with similarly divergent sets of rankings. These calculations do not resolve how chemosensitivity should be ranked, but they do indicate that measures of intrinsic chemosensitivity may bear little relationship to the importance of particular nuclei in the chemosensory response to CO2 in intact animals (Leiter 2009).
Defining central CO2 chemosensitivity
The definition of a chemosensory neuron usually requires that the cell be intrinsically CO2 sensitive. However, establishing that a neuron is intrinsically CO2 sensitive is not straightforward (Richerson et al. 2005). Synaptic blockade does not preclude neurotransmitter release by nonvesicular neurotransmitter mechanisms and gap junctions are not blocked by low Ca2+/high Mg2+ solutions. Gap junctions are important in central CO2 chemosensitivity. Putative chemosensory cells in the LC (Filosa et al. 2002) and NTS (Dean et al. 2002) are coupled by gap junctions and disruption of gap junctions by focal administration of carbenoxolone in the RTN or NTS alters the ventilatory response to CO2 (Hewitt et al. 2004; Parisian et al. 2004). Nevertheless, the results obtained with di-8-ANEPPS indicate that even within the relatively small network of connectivity maintained within a brain slice, CO2 chemosensitivity may be dynamically determined. It is clear from previous studies of cells excited by hypercapnia that only a fraction of these cells will remain CO2 sensitive when synaptic activity is blocked. What is less obvious is that cells that have no response to CO2 during hypercapnia alone may, nonetheless, possess intrinsic CO2 sensitivity when synaptic blockade is added to hypercapnia. The changes in the distributions of hypercapnic CO2 sensitivity before and after synaptic blockade raise the possibility that synaptic inputs in intact animals may have a larger role determining which cells respond to CO2, even in those cells that are intrinsically CO2 sensitive. Thus intrinsic CO2 sensitivity defined in vitro is diminished in importance in defining the behavioral/ventilatory response to CO2 (Leiter 2009).
There is controversy whether respiratory chemoreceptors are widely distributed or restricted to a small number of dedicated cells—the specialized chemoreceptor theory (Guyenet et al. 2005b). The specialized chemoreceptor theory posits a restricted population of CO2-sensitive cells (and implied in this is that the chemoreceptor cells are intrinsically CO2 sensitive) provide the dominant CO2-dependent input to the network of neurons controlling the respiratory apparatus (and the neurons in this network are not intrinsically CO2 sensitive) (Guyenet et al. 2005b). There are neurons in the ganglia of pulmonate snails that fulfill these criteria: the subesophageal ganglia contain neurons that are intrinsically CO2 sensitive; the activity of these neurons is tightly coupled to respiratory activity; and these cells were not respiratory motor neurons or part of the respiratory pattern generator (Erlichman and Leiter 1997). Putative chemoreceptor cells in mammalian preparations do not meet these criteria: the contribution of any single cell in mammalian preparations is insufficient to modify the respiratory output in any way that can be unequivocally associated with a particular neuron and the definition of intrinsic CO2 sensitivity in a slice or culture precludes obtaining evidence that the putative chemosensory cell is actually part of the respiratory control system.
The criteria established by Guyenet (2005b) are difficult—if not impossible—to fulfill in mammalian preparations. One can only say that any particular cell or site behaves in such a way that it is a likely candidate to contribute to chemosensory responses. Among the many sites identified as containing likely chemoreceptive cells, there is evidence that all of them have activity that is associated with or can be correlated with the respiratory response to CO2. Lesion or stimulation studies, fos expression studies, electrophysiological studies, and the di-8-ANEPPS data indicate that many putative chemosensory nuclei and nonchemosensory nuclei contain intrinsically CO2 sensitive cells and many cells are activated by CO2 through synaptic mechanisms or activated as part of the behavioral response to CO2. None of the methods used to date has defined a unique chemoreceptor mechanism among all these sites—the stimulus and the putative pH sensitive channels and receptors are widely distributed among both chemosensory and nonchemosensory regions of the brain, including regions outside the brain stem (Williams et al. 2007). A parsimonious interpretation of these data is that any of the intrinsically CO2 sensitive cells may participate in chemoreceptivity, regardless of location, and any of the cells activated during hypercapnia, whether intrinsically CO2 sensitive or activated by synaptic mechanisms and whether in chemosensory or nonchemosensory nuclei, may be important elements in the behavioral response to CO2. Whether they participate in any given setting will depend on the particular web of connectivity present and the state of the animal. Thus the bulk of the evidence seems consistent with the hypothesis that chemosensitivity is a distributed process (Nattie and Li 2009).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-71001 and HL-56683 and National Science Foundation Grant IOB-0517698.
REFERENCES
- Akilesh et al. 1997.Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesion of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol 82: 469–479, 1997. [DOI] [PubMed] [Google Scholar]
- Albowitz and Kuhnt 1993.Albowitz B, Kuhnt U. Evoked changes of membrane potential in guinea pig sensory neocortical slices: an analysis with voltage-sensitive dyes and a fast optical recording method. Exp Brain Res 93: 213–225, 1993. [DOI] [PubMed] [Google Scholar]
- Bailey et al. 2008.Bailey TW, Appleyard SM, Jin Y-H, Andresen MC. Organization and properties of GABAergic neurons in the solitary tract nucleus (NTS). J Neurophysiol 99: 1712–1722, 2008. [DOI] [PubMed] [Google Scholar]
- Beach et al. 1996.Beach JM, McGahren ED, Xia J, Duling BR. Ratiometric measurement of endothelial depolarization in arterioles with a potential-sensitive dye. Am J Physiol Heart Circ Physiol 270: H2216–H2227, 1996. [DOI] [PubMed] [Google Scholar]
- Belegu et al. 1999.Belegu R, Hadžiefendic S, Dreshaj IA, Haxhiu MA, Martin RJ. CO2-induced c-fos expression in medullary neurons during early development. Respir Physiol 117: 13–28, 1999. [DOI] [PubMed] [Google Scholar]
- Berger and Cooney 1982.Berger AJ, Cooney KA. Ventilatory effects of kainic acid injection of the ventrolateral solitary nucleus. J Appl Physiol 52: 131–140, 1982. [DOI] [PubMed] [Google Scholar]
- Biancardi et al. 2008.Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Eur J Physiol 455: 1119–1128, 2008. [DOI] [PubMed] [Google Scholar]
- Bullen and Saggau 1999.Bullen A, Saggau P. High-speed, random-access fluorescence microscopy: II. Fast quantitative measurements with voltage-sensitive dyes. Biophys J 76: 2272–2287, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates et al. 1993.Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol 75: 5–14, 1993. [DOI] [PubMed] [Google Scholar]
- Conrad et al. 2009.Conrad SC, Nichols NL, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitivity in neurons from the nucleus tractus solitarius (NTS) of neonatal rats. Respir Physiol Neurobiol 166: 4–12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean et al. 2002.Dean JB, Ballantyne D, Cardone DL, Erlichman JS, Solomon IC. Role of gap junctions in CO2 chemoreception and respiratory control. Am J Physiol Lung Cell Mol Physiol 283: L665–L670, 2002. [DOI] [PubMed] [Google Scholar]
- Dean et al. 1990.Dean JB, Bayliss PA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience 36: 207–216, 1990. [DOI] [PubMed] [Google Scholar]
- Dean et al. 2001.Dean JB, Kinkade EA, Putnam RW. Cell–cell coupling in CO2/H+-excited neurons in brainstem slices. Respir Physiol 129: 83–100, 2001. [DOI] [PubMed] [Google Scholar]
- Dean et al. 1989.Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes the nucleus tractus solitarii. Exp Brain Res 76: 656–661, 1989. [DOI] [PubMed] [Google Scholar]
- Dias et al. 2008.Dias MB, Li A, Nattie EE. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol 105: 83–90, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djurisic et al. 2008.Djurisic M, Popovic M, Carnevale NT, Zecevic D. Functional structure of the mitral cell dendritic tuft in the rat olfactory bulb. J Neurosci 28: 4057–4068, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas et al. 2001.Douglas RM, Trouth CO, James SD, Sexcius LM, KC P, Dehkordi O, Valladares ER, McKenzie JC. Decreased CSF pH at ventral brain stem induces widespread c-FOS immunoreactivity in rat brain neurons. J Appl Physiol 90: 475–485, 2001. [DOI] [PubMed] [Google Scholar]
- Erlichman et al. 2004.Erlichman JS, Cook A, Schwab MC, Budd TW, Leiter JC. Heterogeneous patterns of pH regulation in glial cells in the dorsal and ventral medulla. Am J Physiol Regul Integr Comp Physiol 286: R289–R302, 2004. [DOI] [PubMed] [Google Scholar]
- Erlichman and Leiter 1997.Erlichman JS, Leiter JC. Identification of CO2 chemoreceptors in Helix pomatia. Am Zool 37: 54–64, 1997. [Google Scholar]
- Feldman et al. 2003.Feldman JL, Mitchell C, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa et al. 2002.Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol 541: 493–509, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa and Putnam 2003.Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol Cell Physiol 284: C145–C155, 2003. [DOI] [PubMed] [Google Scholar]
- Guyenet et al. 2005a.Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25: 8923–8947, 2005a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet et al. 2008.Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol 586: 2043–2048, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet et al. 2005b.Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol 90: 247–257, 2005b. [DOI] [PubMed] [Google Scholar]
- Hartzler et al. 2007.Hartzler LK, Dean JB, Putnam RW. Developmental changes in the chemosensitive response in locus coeruleus neurons from neonatal rats. Soc Neurosci Abstr 297.8, 2007.
- Haxhiu et al. 1996.Haxhiu MA, Yung K, Erokwu B, Cherniack NS. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Respir Physiol 105: 35–45, 1996. [DOI] [PubMed] [Google Scholar]
- Hewitt et al. 2004.Hewitt A, Barrie R, Graham M, Bogus K, Leiter JC, Erlichman JS. Ventilatory effects of gap junctions in the RTN in awake rats. Am J Physiol Regul Integr Comp Physiol 287: R1407–R1418, 2004. [DOI] [PubMed] [Google Scholar]
- Ito et al. 2004.Ito Y, Oyamada Y, Okada Y, Hakuno H, Aoyama R, Yamaguchi K. Optical mapping of pontine chemosensitive regions of neonatal rat. Neurosci Lett 366: 103–106, 2004. [DOI] [PubMed] [Google Scholar]
- Johnson et al. 2005.Johnson PL, Hollis JH, Mortatalla R, Lightman SL, Lowry CA. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol 19: 327–341, 2005. [DOI] [PubMed] [Google Scholar]
- Kao et al. 2001.Kao WY, Davis CE, Kim YI, Beach JM. Fluorescence emission spectral shift measurements of membrane potential in single cells. Biophys J 81: 1163–1170, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter 2009.Leiter JC. Intrinsic chemosensitivity: how is it measured; what does it mean; and how does it help us understand the ventilatory response to CO2? Respir Physiol Neurobiol 166: 13–15, 2009. [DOI] [PubMed] [Google Scholar]
- Li and Nattie 2002.Li A, Nattie EE. CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol 133: 11–22, 2002. [DOI] [PubMed] [Google Scholar]
- Li and Nattie 2006.Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol 570: 385–396, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. 2006.Li A, Zhou S-S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreased breathing and the CO2 response in conscious rats. J Physiol 577: 307–318, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeschcke 1973.Loeschcke HH. Respiratory chemosensitivity in the medulla oblongata. Acta Neurobiol 33: 97–112, 1973. [PubMed] [Google Scholar]
- Loew 1996.Loew LM. Potentiometric dyes: imaging electrical activity of cell membranes. Pure Appl Chem 68: 1405–1409, 1996. [Google Scholar]
- Lovick 1997.Lovick TA. The medullary raphe nuclei: a system for integration and gain control in autonomic and somatomotor responsiveness? Exp Physiol 82: 31–41, 1997. [DOI] [PubMed] [Google Scholar]
- Miles 1983.Miles R. Does low pH stimulate central chemoreceptors located near the ventral medullary surface? Brain Res 271: 349–353, 1983. [DOI] [PubMed] [Google Scholar]
- Mulkey et al. 2004.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004. [DOI] [PubMed] [Google Scholar]
- Nakagami et al. 1997.Nakagami Y, Saito H, Matsuki N. Optical recording of trisynaptic pathway in rat hippocampal slices with a voltage-sensitive dye. Neuroscience 81: 1–8, 1997. [DOI] [PubMed] [Google Scholar]
- Nattie and Li 2001.Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol 90: 1247–1257, 2001. [DOI] [PubMed] [Google Scholar]
- Nattie and Li 2002a.Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol 92: 2119–2130, 2002a. [DOI] [PubMed] [Google Scholar]
- Nattie and Li 2002b.Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol 544: 603–616, 2002b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie and Li 2009.Nattie EE, Li A. Central chemoreception is a complex system function that involves multiple brainstem sites. J Appl Physiol 106: 1464–1466, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie et al. 2004.Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol 556: 235–253, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols et al. 2008.Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Med Biol 605: 348–352, 2008. [DOI] [PubMed] [Google Scholar]
- Nichols et al. 2009.Nichols NL, Mulkey DK, Wilkinson KA, Powell FL, Dean JB, Putnam RW. Characterization of the chemosensitive response of individual solitary complex neurons from adult rats. Am J Physiol Regul Integr Comp Physiol 296: R7630–R7773, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid et al. 2004.Obaid AL, Loew LM, Wuskell JP, Salzberg BM. Novel naphthylstyryl-pyridium potentiometric dyes offer advantages for neural network analysis. J Neurosci Methods 134: 179–190, 2004. [DOI] [PubMed] [Google Scholar]
- Okada et al. 2008.Okada T, Tashiro Y, Kato F, Yanagawa Y, Obata K, Kawai Y. Quantitative and immunohistochemical analysis of neuronal types in the mouse caudal nucleus tractus solitarius: focus on GABAergic neurons. J Chem Neuroanat 35: 275–284, 2008. [DOI] [PubMed] [Google Scholar]
- Okada et al. 2002.Okada Y, Chen Z, Jiang W, Kuwana S-I, Eldridge FL. Anatomical arrangement of hypercapnia-activated cells in the superficial ventral medulla of rats. J Appl Physiol 93: 427–439, 2002. [DOI] [PubMed] [Google Scholar]
- Onimaru et al. 2008.Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express phox2b in the neonatal rat. J Neurosci 28: 12845–12850, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyamada et al. 1998.Oyamada Y, Ballantyne D, Mückenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. J Physiol 513: 381–398, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisian et al. 2004.Parisian K, Wages P, Hewitt A, Leiter JC, Erlichman JS. Ventilatory effects of gap junction blockade in the NTS in awake rats. Respir Physiol Neurobiol 142: 127–143, 2004. [DOI] [PubMed] [Google Scholar]
- Pineda and Aghajanian 1997.Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier current. Neuroscience 77: 723–743, 1997. [DOI] [PubMed] [Google Scholar]
- Prechtl et al. 1997.Prechtl JC, Cohen LB, Pesaran B, Mitra PP, Kleinfeld D. Visual stimuli induce waves of electrical activity in turtle cortex. Proc Natl Acad Sci USA 94: 7621–7626, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam et al. 2004.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid sensing in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004. [DOI] [PubMed] [Google Scholar]
- Richerson 1995.Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol 73: 933–944, 1995. [DOI] [PubMed] [Google Scholar]
- Richerson et al. 2005.Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol 90: 259–269, 2005. [DOI] [PubMed] [Google Scholar]
- Ritucci et al. 2005.Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential (Vm) and intracellular pH (pHi) to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus (RTN). Am J Physiol Regul Integr Comp Physiol 289: R851–R861, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr and Salzberg 1994.Rohr S, Salzberg BM. Multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures: assessing electrical behavior, with microsecond resolution, on a cellular and subcellular scale. Biophys J 67: 1301–1315, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero et al. 1999.Ruggiero DA, Gootman PM, Ingenito S, Wong C, Gootman N, Sica AL. The area postrema of newborn swine is activated by hypercapnia: relevance to sudden infant death syndrome. J Auton Nerv Syst 76: 167–175, 1999. [DOI] [PubMed] [Google Scholar]
- Salzberg et al. 1973.Salzberg BM, Davila HV, Cohen LB. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature 246: 508–509, 1973. [DOI] [PubMed] [Google Scholar]
- Salzberg et al. 1977.Salzberg BM, Grinvald A, Cohen LB, Davila HV, Ross WN. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol 40: 1281–1291, 1977. [DOI] [PubMed] [Google Scholar]
- Severson et al. 2003.Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci 6: 1139–1140, 2003. [DOI] [PubMed] [Google Scholar]
- Sica et al. 1999.Sica AL, Gootman PM, Ruggiero DA. CO2-induced expression of c-fos in the nucleus of the solitary tract and the area postrema of developing swine. Brain Res 837: 106–116, 1999. [DOI] [PubMed] [Google Scholar]
- Solomon 2003.Solomon IC. Focal CO2/H+ alters phrenic motor output response to chemical stimulation of cat pre-Bötzinger complex in vivo. J Appl Physiol 94: 2151–2157, 2003. [DOI] [PubMed] [Google Scholar]
- Stornetta et al. 2006.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema et al. 1997.Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol 388: 169–190, 1997. [DOI] [PubMed] [Google Scholar]
- Tominaga et al. 2000.Tominaga T, Tominaga Y, Yamada H, Matsumoto G, Ichikawa M. Quantification of optical signals with electrophysiological signals in neural activities of di-4-ANEPPS stained rat hippocampal slices. J Neurosci Methods 102: 11–23, 2000. [DOI] [PubMed] [Google Scholar]
- Veasey et al. 1995.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15: 5346–5359, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang and Richerson 1999.Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience 90: 1001–1011, 1999. [DOI] [PubMed] [Google Scholar]
- Wickström et al. 2002.Wickström R, Hökfelt T, Lagercrantz H. Development of CO2-response in the early newborn period in rat. Respir Physiol Neurobiol 132: 145–158, 2002. [DOI] [PubMed] [Google Scholar]
- Williams et al. 2007.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA 104: 10685–10690, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. 2001.Xu F, Zhang Z, Frazier DT. Microinjection of acetazolamide into the fastigial nucleus augments respiratory output in the rat. J Appl Physiol 91: 2342–2350, 2001. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 1998.Zhang J, Davidson RM, Wei M-d, Loew LM. Membrane electric properties by combined patch clamp fluorescence ratio imaging in single neurons. Biophys J 74: 48–53, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochowski et al. 2000.Zochowski M, Wachowiak M, Falk CX, Cohen LB, Lam Y-W, Antic S, Zecevic D. Imaging membrane potential with voltage-sensitive dyes. Biol Bull 198: 1–21, 2000. [DOI] [PubMed] [Google Scholar]