Abstract
The barn owl's midbrain and forebrain contain neurons tuned to sound direction. The spatial receptive fields of these neurons result from sensitivity to combinations of interaural time (ITD) and level (ILD) differences over a broad frequency range. While a map of auditory space has been described in the midbrain, no similar topographic representation has been found in the forebrain. The first nuclei that belong exclusively to the forebrain and midbrain pathways are the thalamic nucleus ovoidalis (Ov) and the external nucleus of the inferior colliculus (ICx), respectively. The midbrain projects to the auditory thalamus before sharp spatial receptive fields emerge; although Ov and ICx receive projections from the same midbrain nuclei, they are not directly connected. We compared the spatial tuning in Ov and ICx. Thalamic neurons respond to a broader frequency range and their ITD and ILD tuning varied more across frequency. However, neurons in Ov showed spatial receptive fields as selective as neurons in ICx. Thalamic spatial receptive fields were tuned to frontal and contralateral space and correlated with their tuning to ITD and ILD. Our results indicate that spatial tuning emerges in both pathways by similar combination selectivity to ITD and ILD. However, the midbrain and the thalamus do not appear to repeat exactly the same processing, as indicated by the difference in frequency range and the broader tuning to binaural cues. The differences observed at the initial stages of these sound-localization pathways may reflect diverse functions and coding schemes of midbrain and forebrain.
INTRODUCTION
In barn owls, a midbrain map of auditory space (Knudsen and Konishi 1978) contains neurons tuned to sound direction. These space-specific neurons are selective to binaural spatial cues: the interaural time (ITD) and level (ILD) difference (Moiseff and Konishi 1981). For the owl, ITD and ILD encode the horizontal and vertical coordinates of sound direction, respectively (Moiseff 1989). Two independent brain stem pathways process ITD and ILD and converge in the midbrain (Moiseff and Konishi 1983; Takahashi et al. 1984, 1989), where combination selectivity to these cues underlies the emergence of spatial receptive fields (Moiseff and Konishi 1983). In the central nucleus of the inferior colliculus, the processing of auditory space bifurcates into a midbrain (also called tectal) and a forebrain pathway (Fig. 1) (Arthur 2005; Cohen et al. 1998; Knudsen and Knudsen 1983). The forebrain pathway ascends from the midbrain to the forebrain via the thalamus. Activation of the midbrain pathway is sufficient to produce head saccades (du Lac and Knudsen 1990; Masino and Knudsen 1990). The forebrain auditory pathway can mediate a recovery of the head-orienting behavior when the midbrain map is inactivated (Knudsen et al. 1993; Wagner 1993).
FIG. 1.
Sound localization pathways in the barn owl. In the owl's brain stem, time and intensity are processed in parallel pathways that originate in a bifurcation of the auditory nerve (8th n) and converge in the inferior colliculus (IC). The main nuclei are shown, on the top for the interaural time difference (ITD) pathway (NM, nucleus magnocellularis; NL, nucleus laminaris) and on the bottom for the interaural level difference (ILD) pathway (NA, nucleus angularis; LLDp, posterior part of the dorsal nucleus of the lateral lemniscus). IC projects to the forebrain and midbrain sound localization pathways. ICx, external nucleus of the inferior colliculus; OT, optic tectum; Ov, nucleus ovoidalis; FL, field L.
Up to the midbrain, ITD and ILD are topographically represented in the owl's sound localization pathway (Carr and Konishi 1990; Knudsen and Konishi 1978; Manley et al. 1988). However, no topographic representation of auditory space has been found in the forebrain (Cohen and Knudsen 1999; Knudsen et al. 1977; Proctor and Konishi 1997). Despite the lack of a map, the owl's forebrain contains neurons tuned to sound direction (Cohen and Knudsen 1999; Cohen et al. 1998; Knudsen and Knudsen 1996a,b; Knudsen et al. 1977, 1993; Miller and Knudsen 2001, 2003; Reches and Gutfreund 2008). These neurons do not receive projections from the midbrain map (Arthur 2005; Knudsen and Knudsen 1983). However, the forebrain and midbrain share inputs that originate in the same brain stem areas (Arthur 2004; Moiseff and Konishi 1983; Proctor and Konishi 1997; Takahashi and Konishi 1988; Takahashi et al. 1984).
The first nuclei that belong exclusively to the midbrain and forebrain sound localization pathways are, respectively, the external subdivision of the inferior colliculus (ICx) and the thalamic nucleus ovoidalis (Ov). While the processing leading to the emergence of a map in ICx has been extensively studied (Konishi 2003), it is still not clear how auditory spatial cues are integrated in the forebrain and whether the forebrain encodes spatial information as in the midbrain. In this paper, we examine the extent to which the processing for sound direction in the midbrain and forebrain is redundant. We recorded Ov neurons in anesthetized barn owls and studied their tuning to frequency, ITD, and ILD. For the same neurons, we measured the tuning to binaural cues with earphones and the tuning in real space with an array of 144 speakers. To compare these results with the processing in the midbrain pathway, we performed the same measurements in space-specific neurons of ICx.
METHODS
Surgery
Adult barn owls (Tyto alba) of both sexes were anesthetized with intramuscular injections of ketamine hydrochloride (20 mg/kg; Ketaset) and xylazine (4 mg/kg; Anased). At the beginning of each session, prophylactic antibiotics (oxytetracycline; 20 mg/kg; Phoenix Pharmaceuticals) and lactate Ringer (10 ml sc) were administered. An adequate level of anesthesia was maintained with supplemental injections of ketamine and xylazine during the experiment. A heating pad (American Medical Systems, Cincinnati, OH) was used to maintain body temperature. The wings were restrained with a soft leather jacket. In an initial surgical procedure, owls were anesthetized and placed in a custom-built stereotaxic device that held the head such that the ventral surface of the palatine ridge was at 30° from the horizontal plane. A stainless-steel plate and a reference pin were implanted in the skull to subsequently reproduce the stereotaxic position. Recording sessions began a week after this initial surgery. We then opened a small hole on the area of the skull overlying the stereotaxic coordinates for Ov (2 mm anterior to the interaural line and 2 mm lateral to the medial plane) and cut a slit in the duramater for electrode insertion. The same method was followed for recording units in ICx (0 mm anterior to the interaural line and 5 mm lateral to the medial plane). At the end of the experiment, we covered the craniotomy with gelfoam and dental cement and closed the skin incision. Owls were returned to their individual cages and monitored for recovery. Depending on the owl's weight and recovery conditions, experiments were repeated every 7–10 days for a period of several weeks. These procedures comply with guidelines set forth by the National Institutes of Health and Albert Einstein College of Medicine's Institute of Animal Studies guidelines.
Acoustic stimuli
All recordings were performed in a double-walled sound-attenuating chamber (Industrial Acoustics) covered with acoustic foam (Sonex, Illbruck).
Dichotic stimulation
Custom software, written in Matlab and C, was used to generate sound stimuli and to collect and analyze the data.
Each earphone consisted of a speaker (Knowles 1914) and a microphone (Knowles 1319) contained in a custom-made metal case that fits the owl's ear canal. Previous calibration of the Knowles microphones with a Bruel and Kjaer microphone made it possible to translate the voltage output of the Knowles into sound intensity in dB SPL. The Knowles microphones were used to calibrate the earphone assemblies at the beginning of each experiment. The calibration data contained the amplitudes and phase angles measured in frequency steps of 100 Hz. The stimulus generation software then used these calibration data to automatically correct irregularities in the amplitude and phase response of each earphone from 0.5 to 12 kHz.
Auditory stimuli delivered through the earphones consisted of 10 repetitions of 50-ms duration broadband signals (0.5−10 kHz) or tones, with a 5-ms linear rise/fall time and amplitude of 40 dB SPL. Stimuli were presented once per second, changing the ITD and ILD values in a randomized order.
Free-field stimulation
We measured the free-field spatial tuning of the neurons using stimuli delivered through a custom-made hemispherical array of 144 speakers (Sennheiser, 3P127A). The array was constructed inside the sound-attenuating chamber that was also used for the dichotic experiments. The area of space mapped covered ±110° in azimuth and ±80° in elevation around the frontal, or zero, position (0° azimuth, 0° elevation). The angular separation between speakers varied from 10 to 30° with the density of speakers being greatest at angles between ±40° from zero. During recordings, the owl was placed in a stereotaxic frame at the center of the speaker array such that all speakers were equidistant from and oriented toward the owl's head.
At the beginning of the recording sessions, each speaker in the array was calibrated using a 1/2-in Bruel and Kjaer microphone (model 4190), preamplifier (model 2669), and conditioning amplifier (Nexus model 2690). The microphone was mounted in a custom-built pan-tilt robot located at the center of the array, and the robot was used to orient the microphone toward the speaker being calibrated. A laser beam and camera mounted on the robot allowed the user to accurately orient (± 3°) the microphone under visual guidance. Each speaker's transfer function was then measured using a Golay code technique (Zhou et al. 1992), after which an output RMS voltage versus stimulus-intensity (dB SPL) curve was measured and stored on computer. All signals played through the speakers and recorded from the calibration microphone were digitized at 48-kHz, 24-bit resolution using a TDT RX-8 module. The frequency and phase response curves were then used to adjust the stimuli for deviations from flat frequency and phase response, and the voltage versus dB SPL curve was used to set the output RMS voltage level to deliver a specified stimulus level. To confirm the accuracy of the calibration measurement, calibrated broadband test stimuli (0.3- to 12-kHz bandwidth, 40 dB SPL intensity) and calibrated tones (0.3–12 kHz, 40 dB SPL) were synthesized and played through the speaker. Responses were recorded by the calibration microphone, after which the frequency and phase spectra were examined and the power of the measured response was calculated. All speakers used in the array had frequency spectra that were within ± 2 SD (σ = 3.7 dB magnitude, 7.85 μs phase) from the overall mean and had linear voltage versus dB SPL curves. Speakers that did not meet these criteria were replaced.
Auditory stimuli used to measure free-field spatial receptive fields consisted of 10 repetitions of 50-ms duration broadband signals (0.5−0 kHz) with a 5-ms linear rise/fall time and amplitude of 40 dB SPL. Stimuli were presented once per second, randomizing speaker locations. In some cases, the spatial receptive fields were also separately measured using narrowband signals of bandwidths from 1 to 4 kHz and from 4 to 7 kHz.
Data collection
Single units were recorded in Ov and ICx using tungsten electrodes (1 MΩ, 0.005 in, A-M Systems). Action potentials were amplified and filtered (Warner Instrument, DP-301) and converted to transistor-transistor logic (TTL) pulses with a spike discriminator (SD1, Tucker Davis Technologies). The data were stored in a computer via a time converter (ET1, Tucker Davis Technologies) and an A/D converter (DD1, Tucker Davis Technologies) with a sampling rate of 48 kHz and 16-bit resolution.
Ov was located using stereotaxic coordinates and by first identifying its characteristic tonotopically organized region. Ov contains a tonotopic area (Fig. 2; Ovc) with high frequencies represented dorsally and low frequencies ventrally (Proctor 1993; Proctor and Konishi 1997). In the auditory midbrain, however, the tonotopy is reversed (Knudsen 1983; Takahashi and Konishi 1988; Wagner et al. 1987). The recordings performed in this tonotopic region were used to identify the thalamic nucleus; lateral to the tonotopic area of Ov, neurons are tuned to the same best frequency, between 4.5 and 5.0 kHz (Proctor 1993). Looking for broadly tuned neurons, we aimed our electrodes to this lateral region (Fig. 2; Ovl) (Durand et al. 1992). This study focused on thalamic neurons broadly tuned to frequency because given the owl's ability to encode phase at remarkably high frequencies (Koppl 1997), integration across frequency is necessary for unambiguous neural (Mazer 1998) and behavioral (Saberi et al. 1999) spatial detection. ICx was located stereotaxically and by the unambiguous response to ITD and ILD with broadband-sound stimulation and the broad frequency tuning (Brainard and Knudsen 1993; Mazer 1998; Takahashi and Konishi 1986; Wagner et al. 2007). The electrodes were advanced with a microdrive (Motion Controller, Model ESP300, Newport) in steps of 100 μm until the nucleus was reached. The size of the steps was then reduced to 2–4 μm to search and isolate single units.
FIG. 2.
Frequency tuning in Ov subdivisions. A: coronal section with three recording sites marked by tracer injections in Ov's core (Ovc). On the right, frequency tuning curves recorded in 2 of these injection sites (1 and 2). B: coronal section with an electrode track, produced by electrolytic lesion, reaching the lateral region of Ov (Ovl). A frequency tuning curve obtained at the end of the track is shown on the right (3). Ov subdivisions (Durand et al. 1992) are indicated by dashed lines. The midline is located on the right in both figures. Note the narrower frequency tuning and the decrease in best frequency with depth in Ovc. C: half-height width of the frequency-tuning curves plotted against lateral position. Ovm: Ov's medial region, TOv: tractus nuclei ovoidalis. Scale bar: 1 mm. Error bars in C represent SE.
The number of impulses obtained for specific values of stimulus parameters such as frequency, ITD, ILD, azimuth, and elevation constitutes the raw data in this study.
For each Ov and ICx neuron, we first studied the ITD, ILD and frequency tuning during dichotic stimulation. We computed the mean firing rate as a function of 1) ITD (rate-ITD curves), varied in 30-μs steps within a range from −500 to 500 μs (negative ITDs indicate ipsilateral ear leading); 2) ILD (rate-ILD curves), varied in steps of 5 dB within a range from −30 to 30 dB (negative ILDs mean left ear louder); and 3) frequency varied in 100-Hz steps at a sound intensity of 30–40 dB above threshold (iso-intensity frequency tuning curve, FTC). Each tuning curve was obtained by averaging the neural response over ≥10 repetitions of each stimulus. Stimulus parameters were randomly varied during the recording of neuronal responses.
We considered that an Ov neuron was “sensitive” to ITD and ILD if there was a significant change of firing rate as a function of each of these. We estimated the ITD and ILD that elicited the maximum response of the neuron using the ITD and ILD curves. In the case of ITD curves with multiple peaks of similar amplitudes, we used the peak closest to 0 μs. For “sigmoidal” ILD curves, we considered the mean of the range of ILDs that evoked the maximal response as the best ILD. These best ITD and ILD values where then used during the recording to determine the neuron's frequency tuning curve.
For each neuron, 5–10 frequencies that elicited a similarly strong response and spaced 0.5–1 kHz were chosen to evaluate the ITD and ILD tuning across frequency. The frequencies tested were chosen by observing each neuron's iso-intensity frequency tuning curve and selecting those that elicited a significant (above half height) firing rate.
After measuring the ITD, ILD, and frequency tuning using dichotic stimulation, the earphones were carefully removed to study the spatial tuning with free-field stimulation. Special attention was paid to the amplitude of the spikes and the spontaneous firing before and after removing the earphones. If the recording changed, it was discontinued at this point.
We measured spatial receptive fields by collecting the response to ten repetitions of spectrally identical broadband noise presented randomly in 144 different locations in space.
Free-field stimuli were synthesized and experiments were controlled using custom software written in Matlab (Mathworks) and RPVD software (TDT). Stimuli were converted to analog signals using a 24-channel D/A interface (TDT RX-8, 48 kHz, 24 bit) and routed to the speakers via a 144-channel analog multiplexer (GS3, Caltech Biology Electronics Shop). The experimental computer controlled the GS3 multiplexer via a USB serial interface.
For a subset of Ov neurons broadly tuned to frequency, we collected frequency tuning curves not only using the earphones but also with free-field stimulation, using a speaker located in the center of the neuron's receptive field. We measured the spatial receptive fields separately for low and high frequencies with narrowband signals.
Once the data with free-field stimulation was collected, the earphones were replaced and recalibrated and we searched for a new unit.
Data analysis
Frequency tuning
The widths of the iso-intensity frequency-tuning curves (FTC) were measured at 50% of the distance between the minimum and maximum response levels (“half-height width,” W50). The midpoint of the frequency range at W50 was the center frequency (F50). The spontaneous firing rate was removed in performing these calculations.
ITD tuning
We measured the ITD tuning for broadband and tonal stimulation. We used the peak response of the rate-ITD curve obtained with broadband noise as an estimate of the best ITD. The width of the main peak was measured at half the amplitude between the maximum and minimum response during stimulation with broadband noise.
We examined ITD tuning across frequency by first converting ITD to interaural phase difference (IPD) when tonal stimulation was used. Rate-IPD curves for tones in Ov and ICx do not show the quasi-sinusoidal shape of lower brain stem neurons that allows fitting them to cosine functions (Peña et al. 1996; Viete et al. 1997). We then fitted the curves to Gaussian functions using a least-square method (Pérez and Peña 2006). Only fits that passed the χ2 test were used (P > 0.05). The center of the Gaussian fit was our estimate of the mean interaural phase (MIP) that elicited the maximum response at each frequency. Only neurons that passed the χ2 test in at least four rate-IPD curves were used to study ITD tuning across frequency. We then plotted MIP as a function of frequency and computed linear regression on them. The deviation from linearity was measured by weighting the residuals between each point and the regression line by the mean firing rate, the strength of ITD tuning, and the number of frequencies tested, using a method similar to Kuwada et al. (1987). Briefly, each data point was weighted by the product of the synchronization coefficient and the mean firing rate. The synchronization coefficient was computed using a vector-averaging method (Kuwada et al. 1987; Yin and Kuwada 1983). We then calculated a weighted mean square error as the weighted sum of squared residuals, multiplied by the number of data points and divided by the number of degrees of freedom (Kuwada et al. 1987).
ILD tuning
We calculated the best ILD as the weighted average of rate-ILD curves measured with broadband noise. The best ILD = Σ (ILDi * Respi)/Σ (Respi), where Respi is the spike count for the ith measurement (Olsen et al. 1989). The width of the ILD curves for broadband noise was measured at half the amplitude between the maximum and minimum response. We only considered peaked ILD curves for measuring the width. In other words, only curves that reached half-height on both sides around the peak were considered for calculating the ILD width.
The variation in ILD tuning across frequency was quantified by computing the largest difference between best ILDs measured at different frequencies (Arthur 2004). We also compared the SD of best ILDs across frequency in each nucleus.
Spatial tuning
The speaker azimuth and elevation angles, measured in degrees, were converted to angular distance in radians to produce thee-dimensional plots of the response as a function of space (Knudsen 1982). Negative azimuths and elevations refer to angular deviations to the left and below the frontal position, respectively. We used Matlab to quantify the area and dimensions of the spatial receptive fields. Contour lines at half-height between the minimum and maximum responses were computed. The area of the contours that contained the maximum response was considered the estimate of the receptive field area (Fig. 7, top right). We did not include secondary areas that did not reach the maximum response in this analysis. The contours that contained the maxima were always the largest contours of the plot. Only units presenting spatial receptive fields with a unique contour containing the maximum response were included in the sample. The width of the receptive field in azimuth and elevation was measured, respectively, over the horizontal and vertical lines that passed through the maximum. If two adjacent speakers elicited the maximum response, the one closest to the frontal point was selected as the reference. The distance between the speaker that elicited the response maximum and the frontal point was the angular distance between these points in radians. We also measured the azimuth and elevation of the peak of maximum response.
We calculated a spatialization index (SI) to determine the compactness of the neurons spatial receptive fields (SRFs) (Euston and Takahashi 2002). The SI was defined as the sum across all speaker locations of the average number of spikes per stimulus multiplied by the distance (in radians) between each speaker and the SFR's peak. This quantity is inversely correlated with the spatial selectivity of the neuron.
Histology
In 10 of 12 owls, the recording sites were marked in the last experiment by electrolytic lesions or tracer injection. The previously recorded sites were reconstructed by keeping records of the stereotaxic coordinates in each experiment. A week after marking the recording area, owls were overdosed with sodium pentobarbital (Nembutal, Abbot Laboratories) and perfused with saline followed by 2% paraformaldehyde (Fisher Scientific). Brains were blocked in the plane of the electrode penetration, removed from the skull and placed in 30% sucrose until they sank. They were then cut in 60 μm sections, mounted on slides, and visually examined to verify the location of the recording site.
RESULTS
Included in the sample are 157 Ov and 71 ICx neurons. Ov units are a subset of a dataset consisting of 289 neurons tuned to ITD and ILD. Units were selected if they showed ITD and ILD tuning, and were broadly tuned to frequency. These units were recorded in the lateral region, which is known for its absence of tonotopy (Proctor 1993).
Frequency tuning
ICx neurons showed broad frequency tuning, as has been previously reported (Konishi 2003). The center frequencies in ICx ranged from 2.6 to 7.5 kHz (Fig. 3,  ). The median half-height width (W50) of iso-intensity tuning curves was 1.92 kHz, and no correlation was observed between the half-height width and center frequency in ICx.
). The median half-height width (W50) of iso-intensity tuning curves was 1.92 kHz, and no correlation was observed between the half-height width and center frequency in ICx.
FIG. 3.
Frequency tuning in ICx and Ov. Frequency range measured at half-maximal response level in ICx ( ) and Ov (▪) units. Neurons are arranged on the y axis according to the sequence in which they were recorded.
) and Ov (▪) units. Neurons are arranged on the y axis according to the sequence in which they were recorded.
In Ov, neurons were narrowly tuned to frequency and arranged tonotopically in the core and broadly tuned to frequency more laterally (Fig. 2 ). Ov neurons were classified as broadly tuned to frequency when the W50 of the iso-intensity frequency tuning curve was equal to or larger than the median W50 of the sample of ICx neurons (1.92 kHz). Center frequencies (F50) in Ov ranged from 1.7 to 8.1 kHz (Fig. 3, ▪). No correlation was observed between W50 and center frequency in Ov broadly tuned neurons. W50 in Ov was noticeably larger than in ICx (t-test, P < 0.0001). Fifty five of 157 Ov neurons (35.0%) showed FTCs that at half-height extended into frequencies <3.0 kHz. This is in contrast with the sample of ICx neurons in which only 4 of 71 did (5.6%). Similarly, fifty-nine Ov neurons (37.6%) and only 6 (8.4%) in ICx responded to frequencies >7 kHz (Fig. 3).
ITD and ILD tuning
Ov neurons were considered ITD and ILD tuned when their response to broadband sound varied significantly as a function of the corresponding parameter (Kruskal-Wallis test, P < 0.01) (Bremen et al. 2007). All ICx neurons were tuned to ITD and ILD, which is consistent with the extensive literature on response properties of ICx neurons (see Konishi 2003 for a review).
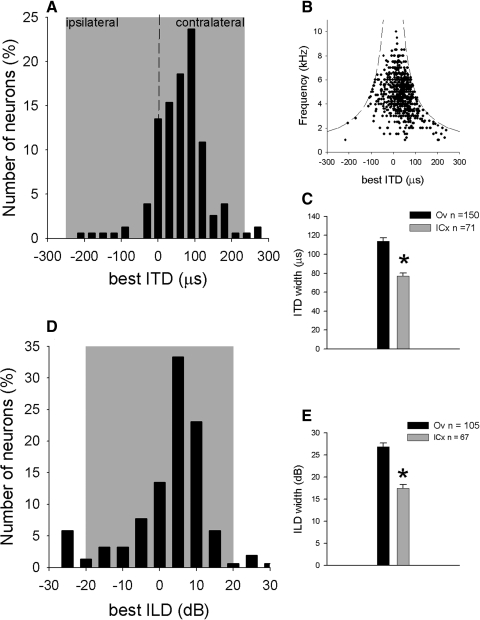
The ITD and ILD tuning was measured for broadband sound and for tones. The best ITDs in Ov, measured with broadband noise, fell within the owl's physiological range (Fig. 4 A; n = 157) of approximately ±250 μs (Keller et al. 1998; Poganiatz et al. 2001; von Campenhausen and Wagner 2006). In 74 Ov neurons, rate-ITD curves were collected for tones at different frequencies. In this sample, we measured the mean interaural phase (MIP) of each tonal rate-ITD curve (see methods). The MIP was then used to compute the smallest ITD that elicited a response peak. For low frequencies (<3 kHz), peaks could exceed the physiological range. However, even at low frequencies, peaks were distributed uniformly over the physiological range of ITD for the owl (Fig. 4B).
FIG. 4.
ITD and ILD tuning in ICx and Ov. A: distribution of best ITDs in Ov. B: ITD tuning for different stimulating frequencies. The dashed lines indicate the ITD at ±0.5 cycles. C: half-height width of rate-ITD curves in ICx ( ) and Ov (▪) measured using broadband-noise. D: distribution of best ILDs in Ov (positive ILDs represent right-side louder). E: half-height width of rate-ILD curves in ICx (
) and Ov (▪) measured using broadband-noise. D: distribution of best ILDs in Ov (positive ILDs represent right-side louder). E: half-height width of rate-ILD curves in ICx ( ) and Ov (▪) using broadband-noise. The shaded areas in A and D indicate the owl's approximate physiological range for ITD and ILD, respectively. Error bars represent SE. *, statistically significant differences (t-test, P < 0.0001).
) and Ov (▪) using broadband-noise. The shaded areas in A and D indicate the owl's approximate physiological range for ITD and ILD, respectively. Error bars represent SE. *, statistically significant differences (t-test, P < 0.0001).
The half-height width of the main peak of rate-ITD curves obtained with broadband noise was significantly broader (t-test, P < 0.0001) in Ov (113.9 ± 43.4 μs, n = 157) than in ICx (77.0 ± 26.0 μs, n = 71; Fig. 4C).
Best ILDs in Ov measured with broadband noise (Fig. 4D) also fell within the owl's physiological range (Moiseff 1989). The half-height width of rate-ILD curves obtained with broadband noise was also significantly larger in Ov (26.7 ± 9.1 dB, n = 105) than in ICx (18.3 ± 7.6 dB, n = 71; t-test, P < 0.0001; Fig. 4E).
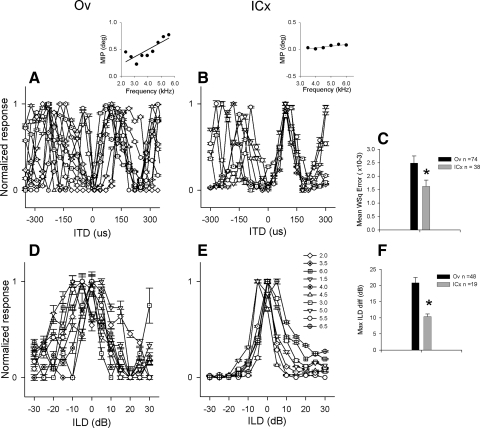
To compare the ITD tuning across frequency in Ov and ICx, we studied the relationship between MIP and stimulus frequency in both areas. We obtained rate-ITD curves with tonal stimulation and determined the MIP elicited by each stimulating frequency. Overlaying rate-ITD curves at different frequencies (Fig. 5, A and B) is used to estimate if there is an ITD to which neurons respond with the same relative firing rate (characteristic delay, CD) of the neuron. If MIP and the stimulus frequency are linearly related, the slope plotting MIP as a function of stimulating frequency offers an accurate means to determine the neuron's CD (Fig. 5, A and B, insets) (Rose et al. 1966; Yin and Kuwada 1983). A previous study showed that this relationship is less linear in Ov (Pérez and Peña 2006). Here we expanded the analysis computing a weighted mean squared residual (see methods) (Kuwada et al. 1987) between the data points and the regression line in a larger sample. This value was significantly larger in Ov (0.0025 ± 0.0022, n = 74) than in ICx (0.0016 ± 0.0014, n = 38, t-test, P < 0.05; Fig. 5C). Thus the relationship between MIP and stimulating frequency is less linear in Ov than in ICx.
FIG. 5.
ITD and ILD tuning across frequency in ICx and Ov. Rate-ITD curves obtained at different frequencies in an Ov (A) and an ICx (B) neuron. Insets: plots of mean interaural phase (MIP) as a function of stimulating frequency with corresponding linear regressions (—). Note the less linear relationship in Ov than in ICx. C: weighted mean square residuals in Ov (▪) and in ICx ( ), t-test, P = 0.01. Rate-ILD curves for tones in Ov (D) and in ICx (E). Each frequency was represented by a different symbol (shown in E in kHz). F: maximal difference (Max ILD diff) between best ILDs across frequency in Ov (▪) and in ICx (
), t-test, P = 0.01. Rate-ILD curves for tones in Ov (D) and in ICx (E). Each frequency was represented by a different symbol (shown in E in kHz). F: maximal difference (Max ILD diff) between best ILDs across frequency in Ov (▪) and in ICx ( s), t-test, P = 0.0002. Error bars in E and F represent SE.
s), t-test, P = 0.0002. Error bars in E and F represent SE.
We studied the ILD tuning across frequency by obtaining rate-ILD curves with tonal stimulation. The difference between best ILDs at multiple frequencies was significantly larger in Ov (mean: 20.8 ± 11.1 dB, n = 48) than in ICx (10.4 ± 3.6 dB, n = 19; t-test, P = 0.0002; Fig. 5, D–F). The values obtained for ICx neurons are consistent with similar measurements reported by other studies (Arthur 2004). The SD of best ILDs across frequency was also significantly larger in Ov (11.15 ± 1.6, n = 48) than in ICx (3.56 ± 0.8, n = 19; t-test, P < 0.001).
Spatial tuning
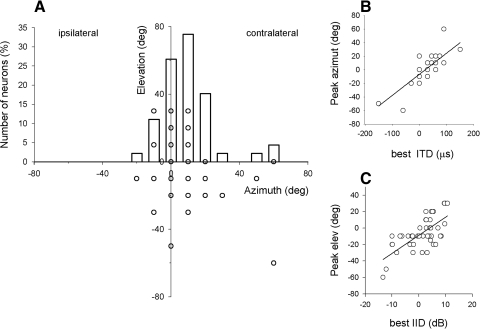
We measured the spatial tuning in 45 Ov and 19 ICx neurons using free-field stimulation. In Ov, the peak firing rate in the SRFs occurred within the frontal and contralateral space, relative to the recording side (Fig. 6A). The horizontal coordinate of the SRFs' peaks was correlated with the ITD tuning (r = 0.76, P < 0.0001) and the vertical coordinate with the ILD tuning (r = 0.69, P < 0.0001; Fig. 6, B and C). These results are consistent with previous recordings in ICx (Moiseff and Konishi 1981, 1983) and optic tectum (Olsen et al. 1989) and reflect the barn owls' use of ITD to localize in azimuth and ILD in elevation (Moiseff 1989).
FIG. 6.
Tuning to frontal and contralateral auditory space in Ov. A: azimuth and elevation of spatial receptive fields' (SRFs') peaks in Ov overlapped with a histogram of peaks' azimuths (n = 45). Due to discrete sampling of space, some data points of the scatter plot overlap. B: horizontal coordinate of the SRF's peak as a function of best ITD. C: vertical coordinate of the SRF's peak as a function of best ILD.
As has been shown in several studies (Bala et al. 2007; Euston and Takahashi 2002; Keller et al. 1998; Knudsen and Konishi 1978; Takahashi et al. 2003), the spatial tuning of ICx neurons was narrower in azimuth (mean width in azimuth = 0.6 ± 0.2 radians) than in elevation (mean width in elevation = 1.7 ± 0.5 radians). The same trend was observed in Ov neurons (mean width in azimuth: 0.8 ± 0.4 radians; mean width in elevation: 1.9 ± 0.5 radians; see examples in Fig. 7, Supplementary Table S11 ). However, the size of the SRFs (Fig. 7, top right) was, overall, larger and more variable in Ov (mean: 1.6 ± 1.3 squared radians, n = 45) than in ICx (mean: 0.7 ± 0.3 squared radians, n = 19; t-test, P = 0.003; Supplementary Table S1).
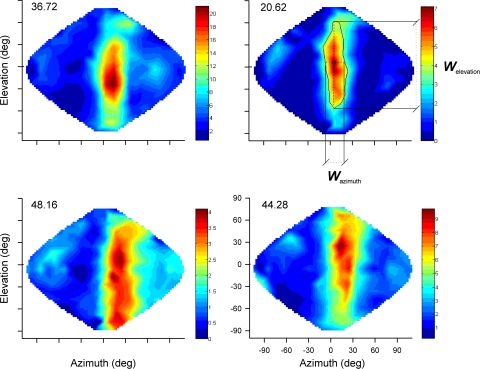
FIG. 7.
Spatial tuning in Ov. SRFs of 4 representative Ov neurons. The spatialization indices (SIs) are indicated for each neuron on the top left corner of each plot. The color-code bars represent firing rate (spike/s). An example of the half-height contour used to measure the SRF area is shown on the top right plot (corresponding to neuron 25 in Table S1 of the supplementary data). The widths in azimuth (Wazimuth) and in elevation (Welevation) are indicated by dotted lines passing through the point of maximum response (circle). The 2 examples shown on top are Ov neurons with SIs equal or smaller than the maximum SI found in ICx.
We assessed the spatial specificity of the neurons by computing a spatialization index (SI, see methods), previously used in ICx for stimulation in virtual space (Euston and Takahashi 2002). The SI was significantly larger in Ov (mean SI: 48.9 ± 20.8) than in ICx (mean SI: 24.2 ± 7.1, t-test P ≪ 0.001; Supplementary Table S1) indicating that, for the whole sample of Ov neurons, the spatial tuning is less restricted than in ICx. We found no correlation in either nucleus between the SI and the width and center frequency of the frequency tuning curves, nor did we find significant correlation between the width of the frequency tuning curves and the SRF width in azimuth and elevation.
For the purpose of comparing populations of neurons with similar spatial tuning, we selected Ov neurons with SIs equal or smaller than the maximum SI found in ICx (SI: 37.3; Fig. 7, top row; n = 15, Supplementary Table S1). The width of the rate-ITD and rate-ILD curves, was still significantly broader in this subset of Ov units (mean ITD width: 93.8 ± 26.3 μs, mean ILD width: 28.7 ± 9.3 dB) than in ICx (mean ITD width: 69.2 ± 18.1 μs, mean ILD width: 21.0 ± 5.5 dB, t-test P = 0.003 for ITD and P = 0.005 for ILD). In 9 of these 15 Ov neurons, we measured the ITD tuning across frequency to quantify the linearity of the MIP as a function of stimulating frequency. The weighted mean square error was again significantly larger in Ov (0.0035 ± 0.0020) than in ICx (0.0016 ± 0.0014, t-test, P = 0.001). For 10 of these 15 Ov neurons, we measured the best ILDs at different frequencies. The mean difference was significantly larger in Ov (17.0 ± 1.7 dB) than in ICx (10.4 ± 3.6 dB; t-test, P = 0.0004). These results indicate that the differences in tuning to binaural cues observed for the whole sample are also present in Ov neurons with similar spatial tuning as in ICx.
We studied the effect of the frequency range contained in the sound on the spatial tuning of nine Ov neurons by measuring SRFs with narrowband signals restricted to low (1–4 kHz) and high (4–7 kHz) frequencies. Neither the width in azimuth nor the location of the SRF's peaks varied between low and high frequencies (data not shown). However, the SRF's width in elevation was significantly larger for low (mean: 2.2 ± 0.3 rad) than for high frequencies (1.8 ± 0.4 rad, paired t-test P < 0.01, see example in Fig. 8). Thus neurons are more broadly tuned to elevation at low frequencies.
FIG. 8.
Spatial tuning in Ov measured with sounds of high and low frequency. A: SRF of an Ov neuron measured with narrowband stimulation at high frequency (HF). B: SRF measured with low-frequency narrowband stimulation (LF). Note the expansion in elevation of the receptive field during LF stimulation. C: SRFs' horizontal width for high and low frequencies. D: SRFs' vertical width for high and low frequencies.
Given that the spectrum of the sound that reaches the tympanic membrane is a function of the filtering properties of the ear canal and facial ruff, i.e., the head-related transfer function (HRTF) (Keller et al. 1998), it is possible that the neurons' frequency tuning differs under dichotic and free-field stimulation. To address this issue, we compared FTCs in Ov measured during dichotic stimulation and using speakers located in the center of the neurons SRF. The half-height width of the FTCs did not significantly differ in either stimulation type (data not shown; n = 9, paired t-test, P = 0.6). These results suggest that the differences in frequency tuning between Ov and ICx may also be present in natural sound stimulation.
DISCUSSION
We measured the spatial tuning of the owl's thalamic neurons using real-space stimulation and found that some thalamic neurons are as sharply tuned to space as ICx's space-specific neurons (Knudsen and Konishi 1978; Takahashi and Konishi 1986). This was unexpected because the auditory thalamus does not receive direct input from ICx (Arthur 2004; Knudsen and Knudsen 1983). Because midbrain space-specific neurons have only been reported in ICx, the spatial tuning in the thalamus is likely computed within the thalamus itself. In addition, ICx neurons do not respond to frequencies as low and as high as those found in Ov and the forebrain (Pérez and Peña 2006; Vonderschen and Wagner 2009). This indicates that frequency convergence already differs from the midbrain at the entryway to the forebrain.
Ov receives projections from the ipsilateral lateral shell (ICcls) and core (ICcc) subdivisions of the central nucleus of the inferior colliculus as well as from the part posterior of the dorsal nucleus of the lateral lemniscus (LLDp), among other brain stem nuclei (Durand et al. 1992; Karten 1967: Proctor and Konishi 1997; Wild 1987). Previous studies have suggested that ICcc projects mainly to Ov's core (Proctor and Konishi 1997). A noteworthy difference between ICcls and ICcc is the spatial tuning of their neurons. Whereas ICcls neurons are tuned to frontal and contralateral space (Takahashi et al. 1989), ICcc neurons are tuned to frontal and ipsilateral space (Takahashi et al. 1989; Wagner et al. 1987, 2007). Our data show that the lateral region of Ov contains neurons tuned to the frontal and contralateral space. Thus this region of Ov's shell must receive input from ICcls.
The larger variability of ITD and ILD tuning across frequency (Fig. 5) could explain why, when broadband signals are used, ITD and ILD tuning curves in Ov are broader (Fig. 4, C and E). For Ov being the first stage in the forebrain, it is possible that downstream forebrain neurons became more sharply tuned to ITD and ILD. However, ITD and ILD tuning curves in other regions of the forebrain are also broader than in ICx (Cohen and Knudsen 1994, 1995; Vonderschen and Wagner 2009). It thus seems that there is a difference in the forebrain processing of binaural cues that begins in the thalamus and is carried to higher-order nuclei. Neither our nor previous studies have used HRTFs (Keller et al. 1998) to measure spatial tuning in the owl's forebrain. ITD and ILD tuning could become narrower when variations induced by the filtering effect of head and feathers are taken into consideration. This possibility remains to be tested.
The broader frequency range over which ITD and ILD information is delivered to the forebrain compared with ICx and the optic tectum, raises the question of why this difference exists. One reason might be that the tectal pathway excludes frequencies that cause significant variability of binaural spatial cues under natural conditions. Keller et al. (1998) measured HRTFs of owls and computed the frequency dependence of the ITD and ILD cues present in the ear canals. At nearly all frequencies from 1 to 8 kHz, the ITD cues were robust; that is, they consistently varied with sound source azimuth. However, ILD cues only showed consistent variation with elevation for frequencies >3 kHz; below this value, ILD varied more with azimuth than with elevation. Thus the best correlation between ILD and elevation occurs >3 kHz. This is consistent with our observation that SRFs measured with low frequencies become significantly broader in elevation. On the other hand, the barn owl's auditory system is able to encode sound phase—and therefore ITD—at ≤8 kHz (Carr and Konishi 1990; Köppl 1997; Sullivan and Konishi 1984). Thus the midbrain pathway works over a frequency range within which ITD and ILD encode orthogonal dimensions reliably.
How ITD is encoded at high and low frequencies could also explain the different frequency tuning ranges of Ov and ICx neurons. Theoretical studies suggest that firing rate could more accurately encode ITD at low sound frequencies, while maxima of neural activity distributed over the entire physiological range could be used for coding ITD at high frequencies (Harper and McAlpine 2004). Because a topographic representation of space requires the latter scheme, eliminating low frequencies may be necessary for the midbrain to produce the map of auditory space found in ICx. In the thalamus, however, neurons respond to a broader range of frequencies, most notably lower frequencies (Pérez and Pena 2006). This expansion into low frequencies did not seem to affect the spatial tuning significantly, as neurons were still tuned to frontal and contralateral space. Downstream forebrain nuclei appear to conserve the low-frequency information to represent auditory space (Vonderschen and Wagner 2009), although the role of low frequencies on the actual spatial tuning remains to be elucidated in these areas.
Do our results indicate that the coding of auditory space in the midbrain and the thalamus are radically different? Although there is no evidence of a map of auditory space in Ov and no clear correlation between frequency tuning and spatial tuning, the SRFs in Ov and ICx are similar in that they are both distributed over the frontal and contralateral space and they are both correlated with the tuning to binaural cues. This argues against models of rate coding with neurons tuned to ranges of binaural cues outside the physiological range. Rate coding such as that proposed for mammals with small head size (Harper and McAlpine 2004) rely on spatial receptive fields with maxima away from frontal space. For the owl's head size, Harper and McAlpine (2004) predict that coding schemes should begin to change below 3 kHz. This is unlikely to be an issue for ICx neurons, as they respond to high frequencies, but it could be in Ov, where neurons respond to frequencies <3 kHz. Recent reports show differences between the tuning to binaural cues in ICx and the owl's auditory archistriatum (AAr). Vonderschen and Wagner (2009) found that AAr neurons show, on average, a small shift in ITD tuning away from 0 μs (Vonderschen and Wagner 2009) and that ITD tuning becomes frequency dependent at low frequencies. Based on this evidence, Vonderschen and Wagner (2009) suggested that a change toward rate coding takes place in the owl's forebrain. Our results are partially consistent with theirs. Although a slight shift in the ITD tuning away from frontal space can also be observed in our data (Fig. 4A), it is impossible to rule out that this is due to sampling bias. In addition, we found no overrepresentation of large ITDs at low frequencies (Fig. 4B). Overall, best ITDs in Ov lie largely within the owl's physiological range of approximately ±250 μs (Keller et al. 1998; Poganiatz et al. 2001; von Campenhausen and Wagner 2006) and SRFs are distributed over the frontal and contralateral space. Thus spatial tuning in Ov still appears to be consistent with maxima of neural activity distributed over the entire space.
In summary, spatially tuned thalamic neurons differ from midbrain ones in two features: first, the frequency range of Ov neurons consistently expands into lower and higher frequencies; second, the ITD and ILD tunings in Ov are broader and more variable across frequency compared with midbrain neurons. Yet Ov neurons display SRFs that can be, as far as our data show, as well tuned to space as in ICx. The question of why these differences exist is intriguing. The answer may lie in the frequency range necessary for each pathway to serve its function. The tectal pathway may represent a fast system dedicated to orienting behavior, equivalent to the superior collicular system in mammals (Freedman and Sparks 1997; King and Hutchings 1987; Middlebrooks and Knudsen 1984; Withington et al. 1994), which discards spectral information for speed and efficiency. The forebrain pathway, however, may be involved in functions that require processing information over a broader frequency range.
GRANTS
This work was funded by National Institute of Deafness and Other Communications Disorders Grant DC-007690.
Supplementary Material
Acknowledgments
We thank L. Steinberg, M. Rosen, K. Keller, T. Takahashi, and the Takahashi lab for comments on this paper. A. Strong assisted with the design of the free-field speaker array. This work is part of M. L. Perez's doctoral project for Programa de Desarrollo de las Ciencias Básicas (PEDECIBA)-Uruguay.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Arthur 2004.Arthur BJ. Sensitivity to spectral interaual intensity difference cues in space-specific neurons of the barn owl. J Comp Physiol [A] 190: 91–104, 2004. [DOI] [PubMed] [Google Scholar]
- Arthur 2005.Arthur BJ. Distribution within the barn owl's inferior colliculus of neurons projecting to the optic tectum and thalamus. J Comp Neurol 492: 110–121, 2005. [DOI] [PubMed] [Google Scholar]
- Bala et al. 2007.Bala AD, Spitzer MW, Takahashi TT. Auditory spatial acuity approximates the resolving power of space-specific neurons. PLoS ONE 2: e675, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard and Knudsen 1993.Brainard MS, Knudsen EI. Experience-dependent plasticity in the inferior colliculus: a site for visual calibration of the neural representation of auditory space in the barn owl. J Neurosci 13: 4589–4608, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremen et al. 2007.Bremen P, Poganiatz I, von Campenhausen M, Wagner H. Sensitivity to interaural time difference and representation of azimuth in central nucleus of inferior colliculus in the barn owl. J Comp Physiol [A] 193: 99–112, 2007. [DOI] [PubMed] [Google Scholar]
- Carr and Konishi 1990.Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci 10: 3227–3246, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen and Knudsen 1994.Cohen YE, Knudsen EI. Auditory tuning for spatial cues in the barn owl basal ganglia. J Neurophysiol 72: 285–298, 1994. [DOI] [PubMed] [Google Scholar]
- Cohen and Knudsen 1995.Cohen YE, Knudsen EI. Binaural tuning of auditory units in the forebrain archistriatal gaze fields of the barn owl: local organization but no space map. J Neurophysiol 15: 5152–5168, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen and Knudsen 1999.Cohen YE, Knudsen EI. Map versus clusters: different representations of auditory space in the midbrain and forebrain. Trends Neurosci 22: 128–135, 1999. [DOI] [PubMed] [Google Scholar]
- Cohen et al. 1998.Cohen YE, Miller GL, Knudsen EI. Forebrain pathway for auditory space processing in the barn owl. J Neurophysiol 79: 891–902, 1998. [DOI] [PubMed] [Google Scholar]
- Durand et al. 1992.Durand SE, Tepper JM, Cheng M-F. The shell region of the nucleus ovoidalis: a subdivision of the avian audtory thalamus. J Comp Neurol 323: 495–518, 1992. [DOI] [PubMed] [Google Scholar]
- du Lac and Knudsen 1990.du Lac S, Knudsen EI. Neural maps of head movement vector and speed in the optic tectum of the barn owl. J Neurophysiol 63: 131–146, 1990. [DOI] [PubMed] [Google Scholar]
- Euston and Takahashi 2002.Euston DR, Takahashi TT. From spectrum to space: the contribution of level difference cues to spatial receptive fields in the barn owl inferior colliculus. J Neurosci 22: 284–293, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman and Sparks 1997.Freedman EG, Sparks DL. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J Neurophysiol 78: 1669–1690, 1997. [DOI] [PubMed] [Google Scholar]
- Harper and McAlpine 2004.Harper NS, McAlpine D. Optimal population coding of an auditory spatial cue. Nature 430: 682–686, 2004. [DOI] [PubMed] [Google Scholar]
- Karten 1967.Karten HJ. The organization of the ascending auditory pathway in the pigeon (Columba livia). I. Diencephalic projections of the inferior colliculus (nucleus mesencephali lateralis, pars dorsalis). Brain Res 6: 409–427, 1967. [DOI] [PubMed] [Google Scholar]
- Keller et al. 1998.Keller CH, Hartung K, Takahashi T. Head-related transfer functions of the barn owl: measurement and neural responses. Hear Res 118: 13–34, 1998. [DOI] [PubMed] [Google Scholar]
- King and Hutchings 1987.King AJ, Hutchings ME. Spatial response properties of acoustically responsive neurons in the superior colliculus of the ferret: a map of auditory space. J Neurophysiol 57: 596–625, 1987. [DOI] [PubMed] [Google Scholar]
- Knudsen 1982.Knudsen EI. Auditory and visual maps of space in the optic tectum of the owl. J Neurosci 2: 1177–1194, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen 1983.Knudsen EI. Subdivisions of the inferior colliculus in the barn owl (Tyto alba). J Comp Neurol 218: 174–186, 1983. [DOI] [PubMed] [Google Scholar]
- Knudsen and Knudsen 1983.Knudsen EI, Knudsen PF. Space-mapped auditory projections from the inferior colliculus to the optic tectum in the barn owl (Tyto alba). J Comp Neurol 218: 187–196, 1983. [DOI] [PubMed] [Google Scholar]
- Knudsen and Knudsen 1996a.Knudsen EI, Knudsen PF. Contribution of the forebrain archistriatal gaze field to auditory orienting behavior in the barn owl. Exp Brain Res 108: 23–32, 1996a. [DOI] [PubMed] [Google Scholar]
- Knudsen and Knudsen 1996b.Knudsen EI, Knudsen PF. Disruption of auditory spatial working memory by inactivation of the forebrain archistriatum in barn owl. Nature 383: 428–431, 1996b. [DOI] [PubMed] [Google Scholar]
- Knudsen and Konishi 1978.Knudsen EI, Konishi M. A neural map of auditory space in the owl. Science 200: 795–797, 1978. [DOI] [PubMed] [Google Scholar]
- Knudsen et al. 1977.Knudsen EI, Konishi M, Pettigrew JD. Receptive fields of auditory neurons in the owl. Science 198: 1278–1280, 1977. [DOI] [PubMed] [Google Scholar]
- Knudsen et al. 1993.Knudsen EI, Knudsen PF, Masino T. Parallel pathways mediating both sound localization and gaze control in the forebrain and midbrain of the barn owl. J Neurosci 13: 2837–2852, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi 2003.Konishi M. Coding of auditory space. Annu Rev Neurosci 26: 31–55, 2003. [DOI] [PubMed] [Google Scholar]
- Köppl 1997.Köppl C. Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl, Tyto alba. J Neurosci 17: 3312–3321, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada et al. 1987.Kuwada S, Stanford TR, Batra R. Interaural phase-sensitive units in the inferior colliculus of the unanesthetized rabbit: effects of changing frequency. J Neurophysiol 57: 1338–1360, 1987. [DOI] [PubMed] [Google Scholar]
- Manley et al. 1988.Manley GA, Köppl C, Konishi M. A neural map of interaural intensity differences in the brain stem of the barn owl. J Neurosci 8: 2665–2676, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino and Knudsen 1990.Masino T, Knudsen E. Horizontal and vertical components of head movement are controlled by distinc neuralcircuits in the barn owl. Nature 345: 434–437, 1990. [DOI] [PubMed] [Google Scholar]
- Mazer 1998.Mazer JA. How the owl resolves auditory coding ambiguity. Proc Natl Acad Sci USA 95: 10932–10937, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks and Knudsen 1984.Middlebrooks JC, Knudsen EI. A neural code for auditory space in the cat's superior colliculus. J Neurosci 4: 2621–2634, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller and Knudsen 2001.Miller GL, Knudsen EI. Early auditory experience induces frequency-specific, adaptive plasticity in the forebrain gaze fields of the barn owl. J Neurophysiol 85: 2184–2194, 2001. [DOI] [PubMed] [Google Scholar]
- Miller and Knudsen 2003.Miller GL, Knudsen EI. Adaptive plasticity in the auditory thalamus of juvenile barn owl. J Neurosci 23: 1059–1065, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseff 1989.Moiseff A. Bi-coordinate sound localization by the barn owl. J Comp Physiol 164: 637–644, 1989. [DOI] [PubMed] [Google Scholar]
- Moiseff and Konishi 1981.Moiseff A, Konishi M. Neuronal and behavioral sensitivity to binaural time differences in the owl. J Neurosci 1: 40–48, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseff and Konishi 1983.Moiseff A, Konishi M. Binaural characteristics of units in the owl's brain stem auditory pathway: precursors of restricted spatial receptive fields. J Neurosci 3: 2553–2562, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen et al. 1989.Olsen JF, Knudsen EI, Esterly SD. Neural maps of interaural time and intensity differences in the optic tectum of the barn owl. J Neurosci 9: 2591–2605, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña et al. 1996.Peña JL, Viete S, Albeck Y, Konishi M. Tolerance to sound intensity of binaural coincidence detection in the nucleus laminaris of the owl. J Neurosci 16: 7046–7054, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez and Peña 2006.Pérez ML, Peña JL. Comparison of midbrain and thalamic space-specific neurons in barn owls. J Neurophysiol 95: 783–790, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poganiatz et al. 2001.Poganiatz I, Nelken I, Wagner H. Sound-localization experiments with barn owls in virtual space: influence of interaural time difference on head-turning behavior. J Assoc Res Otolaryngol 2: 1–21, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor 1993.Proctor L. Characterization of the Auditory Thalamic Nucleus of the Barn Owl. (PhD thesis). California Institute of Technology, Pasadena, CA, 1993.
- Proctor and Konishi 1997.Proctor L, Konishi M. Representation of sound localization cues in the auditory thalamus of the barn owl. Proc Natl Acad Sci USA 94: 10421–10425, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reches and Gutfreund 2008.Reches A, Gutfreund Y. Stimulus-specific adaptations in the gaze control system of the barn owl. J Neurosci 28: 1523–1533, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose et al. 1966.Rose JE, Gross NB, Geisler CD, Hindi JE. Some neural mechanism in the inferior colliculus of the cat which may be relevant to localization of a sound source. J Neurophysiol 29: 288–314, 1966. [DOI] [PubMed] [Google Scholar]
- Saberi et al. 1999.Saberi K, Takahashi Y, Farahbod H, Konishi M. Neural bases of an auditory illusion and its elimination in owls. Nat Neurosci 2: 656–659, 1999. [DOI] [PubMed] [Google Scholar]
- Sullivan and Konishi 1984.Sullivan WE, Konishi M. Segregation of stimulus phase and intensity coding in the cochlear nucleus of the owl. J Neurosci 4: 1787–1799, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi et al. 2003.Takahashi TT, Bala AD, Spitzer MW, Euston DR, Spezio ML, Keller CH. The synthesis and use of the owl's auditory space map. Biol Cybern 89: 378–387, 2003. [DOI] [PubMed] [Google Scholar]
- Takahashi and Konishi 1986.Takahashi T, Konishi M. Selectivity for interaural time difference in the owl's midbrain. J Neurosci 6: 3413–3422, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi and Konishi 1988.Takahashi T, Konishi M. Projections of the cochlear nuclei and nucleus laminaris to the inferior colliculus of the barn owl. J Comp Neurol 274: 190–211, 1988. [DOI] [PubMed] [Google Scholar]
- Takahashi et al. 1984.Takahashi T, Moiseff A, Konishi M. Time and intensity cues are processed independently in the auditory system of the owl. J Neurosci 4: 1781–1786, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi et al. 1989.Takahashi T, Wagner H, Konishi M. Role of commissural projections in the representation of bilateral auditory space in the barn owl's inferior colliculus. J Comp Neurol 281: 545–554, 1989. [DOI] [PubMed] [Google Scholar]
- Viete et al. 1997.Viete S, Peña JL, Konishi M. Effects of interaural intensity difference on the processing of interaural time difference in the owl's nucleus laminaris. J Neurosci 17: 1815–1824, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Campenhausen and Wagner 2006.von Campenhausen M, Wagner H. Influence of the facial ruff on the sound-receiving characteristics of the barn owl's ears. J Comp Physiol [A] 192: 1073–1082, 2006. [DOI] [PubMed] [Google Scholar]
- Vonderschen and Wagner 2009.Vonderschen K, Wagner H. Tuning to interaural time difference and frequency differs between the auditory arcopallium and the external nucleus of the inferior colliculus. J Neurophysiol 101: 2348–2361, 2009. [DOI] [PubMed] [Google Scholar]
- Wagner 1993.Wagner H. Sound-localization deficits induced by lesions in the barn owl's auditory space map. J Neurosci 13: 371–386, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner et al. 2007.Wagner H, Asadollahi A, Bremen P, Endler F, Vonderschen K, von Campenhausen M. Distribution of interaural time difference in the barn owl's inferior colliculus in the low- and high-frequency ranges. J Neurosci 11: 4191–4200, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner et al. 1987.Wagner H, Takahashi T, Konishi M. Representation of interaural time difference in the central nucleus of the barn owl's inferior colliculus. J Neurosci 7: 3105–3116, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild 1987.Wild JM. Nuclei of the lateral lemniscus project directly to the thalamic auditory nuclei in the pigeon. Brain Res 408: 303–307, 1987. [DOI] [PubMed] [Google Scholar]
- Withington et al. 1994.Withington DJ, Binns KE, Ingham NJ, Thorton SK. Plasticity in the superior collicular auditory space map of adult guinea pigs. Exp Physiol 79: 319–25, 1994. [DOI] [PubMed] [Google Scholar]
- Yin and Kuwada 1983.Yin TCT, Kuwada S. Binaural interaction in low-frequency neurons in the inferior colliculus in the cat. III. Effects of changing frequency. J Neurophysiol 50: 1020–1042, 1983. [DOI] [PubMed] [Google Scholar]
- Zhou et al. 1992.Zhou B, Green DM, Middlebrooks JC. Characterization of external ear impulse response using Golay codes. J Acoust Soc Am 92: 1169–1171, 1992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.