Abstract
Two distinct mechanisms mediate potentiating effects of depolarization on synaptic transmission. Recently there has been renewed interest in a type of plasticity in which a neuron's somatic membrane potential influences synaptic transmission. We study mechanisms that mediate this type of control at a synapse between a mechanoafferent, B21, and B8, a motor neuron that receives chemical synaptic input. Previously we demonstrated that the somatic membrane potential determines spike propagation within B21. Namely, B21 must be centrally depolarized if spikes are to propagate to an output process. We now demonstrate that this will occur with central depolarizations that are only a few millivolts. Depolarizations of this magnitude are not, however, sufficient to induce synaptic transmission to B8. B21-induced postsynaptic potentials (PSPs) are only observed if B21 is centrally depolarized by ≥10 mV. Larger depolarizations have a second impact on B21. They induce graded changes in the baseline intracellular calcium concentration that are virtually essential for the induction of chemical synaptic transmission. During motor programs, subthreshold depolarizations that increase calcium concentrations are observed during one of the two antagonistic phases of rhythmic activity. Chemical synaptic transmission from B21 to B8 is, therefore, likely to occur in a phase-dependent manner. Other neurons that receive mechanoafferent input are electrically coupled to B21. Differential control of spike propagation and chemical synaptic transmission may, therefore, represent a mechanism that permits selective control of afferent transmission to different types of neurons contacted by B21. Afferent transmission to neurons receiving chemical synaptic input will be phase specific, whereas transmission to electrically coupled followers will be phase independent.
INTRODUCTION
The selection of sensory inputs is an essential function within the CNS. It is, therefore, not surprising that a variety of regulatory and gating mechanisms have been described, operating at multiple loci and by multiple means (e.g., Büschges and El Manira 1998; Rossignol et al. 2006; Rudomin and Schmidt 1999). We study the regulation of sensorimotor transmission in an experimentally advantageous model system, the mollusk Aplysia californica. More specifically, these experiments focus on synaptic transmission between a feeding mechanoafferent, B21 (Rosen et al. 2000b), and motor neurons that receive chemical excitatory input, the B8 neurons (Rosen et al. 2000b).
In previous work, we characterized a form of gating that is membrane potential dependent. We demonstrated that active spike propagation in B21 is dynamically regulated during motor programs by centrally induced changes in membrane potential (Evans et al. 2003). Specifically, B21 is a bipolar neuron with a centrally located soma and two major processes referred to as medial and lateral (Fig. 1A). The medial process innervates the periphery, whereas the lateral process is the primary point of contact with the B8 neurons (Rosen et al. 2000b). If peripherally generated spikes are to propagate from the medial process to the lateral process, impulses must be conducted through the relatively inexcitable somatic region (Evans et al. 2007). This does not occur when B21 is at its resting membrane potential. However, it does occur when B21 is centrally depolarized (Evans et al. 2003). Regulation of spike propagation is not unique to B21, i.e., it has been reported in several other systems (e.g., Monsivais et al. 2005; Verdier et al. 2003).
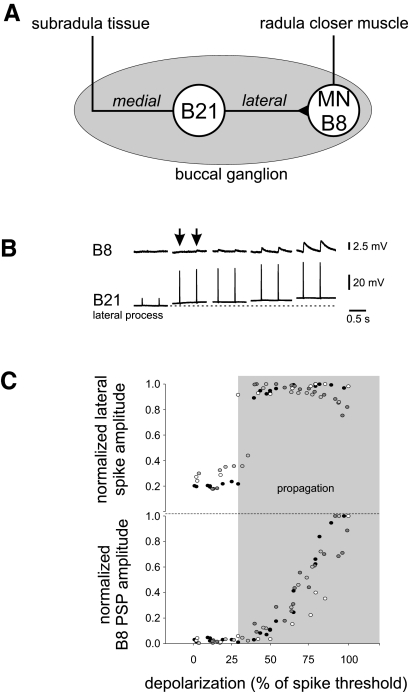
FIG. 1.
A: schematic of the preparation. Mechanical stimulation of the subradula tissue evokes spikes, which travel along the medial process, across the central soma, and into the lateral branch where the main contacts between B21 and follower motor neuron B8 are located. B: effects of depolarization on B21–B8 transmission. Simultaneous recordings from B21's lateral process and B8 during peripheral activation of B21. The dashed line under the lateral process trace indicates the resting membrane potential. The 1st 2 responses were evoked at resting membrane potential. Thereafter B21 was progressively depolarized by current injection into the soma (trace not shown). In each case, 2 responses are shown at each membrane potential. When B21 was peripherally activated at resting potential, spikes did not actively propagate to the lateral process, and PSPs in B8 were virtually nonexistent. As B21 was depolarized, the spike propagation failure was relieved, but initially PSPs in B8 were virtually undetectable (arrows). With further depolarization, PSP amplitude progressively increased. C: summary of data for the experiment shown in B from 4 animals, each plotted with a different symbol. y axis data have been normalized to maximum spike height or peak PSP amplitude. X axis data have been normalized to the amount of depolarization that was needed to evoke spikes centrally. The shaded region indicates the approximate point at which spike propagation to the lateral process begins. Note that the B8 PSPs are still virtually undetectable at this point. Also note the graded, progressive increase in PSP amplitude as the holding potential was progressively increased.
In the current study, we characterize a second potentiating effect of central depolarization on afferent transmission to B8. We demonstrate that changes in membrane potential produce graded increases in the background (i.e., resting) calcium concentration in B21. These types of increases have been described in other systems and generally potentiate spike-mediated synaptic transmission (e.g., Awatramani et al. 2005; Connor et al. 1986; Ivanov and Calabrese 2003; Kemenes et al. 2006). The B21–B8 synapse is, however, unusual in that background increases in the intracellular calcium concentration are virtually essential for synaptic transmission. When they are pharmacologically blocked, and B21 is depolarized so that spikes propagate, postsynaptic potentials (PSPs) in the B8 neurons are not recorded. In other systems, synaptic transmission generally occurs when spikes are evoked relatively close to resting membrane potential. Taken together, previous data and these results indicate that our preparation is unique in that central depolarization exerts two effects that are both essential for synaptic transmission. First it ensures that spikes actively propagate to the lateral process. Second, it ensures that propagating spikes effectively induce transmitter release.
What is the unique functional significance of the two mechanisms that regulate B21 mechanoafferent transmission? To approach this issue, we sought to determine whether they will necessarily be simultaneously invoked, i.e., whether they are induced in the same voltage range. We show that this is not the case. Depolarizations of only a few millivolts alter spike propagation, whereas central depolarizations that exceed ∼10 mV are necessary to increase calcium concentrations and induce chemical synaptic transmission. Although both effects necessarily go hand in hand when afferent transmission to B8 is regulated, this is not likely to be the case for some of the other neurons that receive afferent input from B21, i.e., cells that are electrically coupled to B21 (Borovikov et al. 2000; Rosen et al. 2000a,b). B21-induced coupling potentials are apparent in these neurons at membrane potentials where chemical synaptic transmission to the B8 neurons is not induced. We suggest that differential control of spike propagation and chemical synaptic transmission may represent a mechanism that permits selective control of afferent transmission to different types of neurons contacted by B21.
METHODS
Preparation
Experiments were conducted with Aplysia californica (200–400 g) obtained from Marinus Scientific (Garden Grove, CA) and held in tanks at 14–16°C for several days. Animals were anesthetized by injection of ∼100 ml isotonic MgCl2. Depending on the experiment, the isolated buccal ganglion, the buccal and cerebral ganglia, or the buccal ganglion with attached radula nerve and subradula tissue (SRT) were removed from the animal, and pinned out in a silicone elastomer (Sylgard, Dow Corning, Midland, MI)-lined dish. Ganglia were desheathed to facilitate intracellular recordings. During most experiments, the preparation was continuously superfused with artificial seawater (in mM: 460 NaCl, 10 KCl, 11 CaCl2, 55 MgCl2, 10 HEPES, pH = 7.6) at 14–16°C. In one set of experiments, the preparation was superfused with high-Ca2+/low-Mg2+ artificial seawater (in mM: 495 NaCl, 10 KCl, 33 CaCl2, 10 MgCl2, 10 HEPES, pH = 7.6) at 16°C. A capital “N” indicates the number of preparations, whereas a lowercase “n” indicates the number of observations.
In some experiments, we bath applied 10 μM nifedipine (No. 1075, Tocris, Ellisville, MO). In other experiments, ethylene glycol tetraacetic acid, E-4378 (EGTA, Sigma Chemical, St. Louis, MO) was intracellularly injected into the somatic region of B21 via pipettes filled with a 250 mM solution.
Electrophysiology
Electrophysiological recordings were obtained with sharp glass electrodes filled with 3 M KAc and 30 mM KCl, or 3 M KCl (typical impedance 7 MΩ). Signals were amplified with a SEC-10 LX amplifier (×10 headstage, npi electronic GmbH, Tamm, Germany) in discontinuous current clamp or bridge mode or an AxoClamp 2B amplifier (Molecular Devices, Sunnyvale, CA) in bridge mode. Signals were then filtered (LP 1 kHz) with a Model 410 instrumentation amplifier (Brownlee Precision, San Jose, CA) and sampled at 3 kHz with a Power 1401 A/D converter (CED, Cambridge, UK) using Spike II software (version 6.01–6.09, CED). In the experiments to determine B21 depolarization during motor activity and experiments with recordings from B21's lateral process, signals were amplified with an AxoClamp 2B or Getting 5A amplifier (Getting Instruments, Iowa City, IA), filtered (LP 1 kHz) with a model AM502 differential amplifier (Tektronix, Richardson, TX), and sampled with a Digidata 1322A (Molecular Devices) A/D converter using pClamp (version 9, Molecular Devices) software. Data were analyzed with Spike II. Excel (version 2002, Microsoft, Redmond, WA) was used for statistical tests and Excel and CorelDraw (version 13, Corel, Mountain View, CA) to plot the data and prepare the figures.
Motor activity was triggered in the isolated nervous system by either intracellular stimulation of the cerebral buccal interneuron 2 (CBI-2) or extracellular stimulation of the esophageal nerve (EN) of the buccal ganglion. For extracellular stimulation, the nerve was cut, drawn into a suction electrode, and stimulated using the stimulus input of a model 1700 amplifier (A-M Systems, Carlsborg, WA), a Grass SIU5 stimulus isolation unit, and a Grass S48 stimulator (both Astro-Med, West Warwick, RI). Stimulus artifacts were graphically removed in the figure shown.
Imaging
For imaging experiments, B21 was filled with Calcium Orange C-3013 (Invitrogen, Carlsbad, CA) by electrophoretic injection. The preparation was then transferred to a FN 1 fixed stage upright microscope (Nikon Instruments, Melville, NY) equipped with Nikon 10X/0.30w Plan Fluor and 40X/0.80w NIR Apo objectives and ×0.5 camera adapter. The light from an X-Cite 120 PC metal halide lamp (EXFO, Mississauga, Ontario, Canada) was filtered using an ET-CY3 filter set (Chroma Technology, Rockingham, VT). Fluorescence was detected with a CoolSNAP HQ2 CCD camera (Photometrics, Tucson, AZ). The image-acquisition rate depended on the resolution of the image, with 30 image/s being typical for a 200 × 900 pixel area. The fluorescence of the Ca2+-sensitive dye was measured in defined regions of the image using NIS Elements AR (versions 2.10 and 2.30, Nikon Instruments), normalized, background subtracted, and exported to Spike II using custom-written software that synchronized electrophysiological and optical data. The relative change in fluorescence was calculated as dF/F0 = (F − F0)/F0 where F0 denotes the background subtracted prestimulus fluorescence level (Yuste and Konnerth 2005). Tests of the sensitivity and dynamic range of the Ca2+ imaging setup were done with calcium calibration buffer kit 2 (C-3009, Invitrogen).
1 fixed stage upright microscope (Nikon Instruments, Melville, NY) equipped with Nikon 10X/0.30w Plan Fluor and 40X/0.80w NIR Apo objectives and ×0.5 camera adapter. The light from an X-Cite 120 PC metal halide lamp (EXFO, Mississauga, Ontario, Canada) was filtered using an ET-CY3 filter set (Chroma Technology, Rockingham, VT). Fluorescence was detected with a CoolSNAP HQ2 CCD camera (Photometrics, Tucson, AZ). The image-acquisition rate depended on the resolution of the image, with 30 image/s being typical for a 200 × 900 pixel area. The fluorescence of the Ca2+-sensitive dye was measured in defined regions of the image using NIS Elements AR (versions 2.10 and 2.30, Nikon Instruments), normalized, background subtracted, and exported to Spike II using custom-written software that synchronized electrophysiological and optical data. The relative change in fluorescence was calculated as dF/F0 = (F − F0)/F0 where F0 denotes the background subtracted prestimulus fluorescence level (Yuste and Konnerth 2005). Tests of the sensitivity and dynamic range of the Ca2+ imaging setup were done with calcium calibration buffer kit 2 (C-3009, Invitrogen).
Mechanical stimulation
Some experiments required mechanical stimulation of the SRT to evoke action potentials in the sensory neuron B21. To accomplish this, a nonconductive tip was attached to a small loudspeaker which was driven by a Grass SIU 5 stimulus isolation unit attached to a Grass S48 stimulator (Astro-Med, West Warwick, RI). The tip was positioned to lightly touch the SRT, which resulted in individual action potentials being triggered in B21 by a 3-ms square pulse to the loudspeaker (see also Cropper et al. 1996).
RESULTS
Effect of B21 depolarization on spike propagation and synaptic transmission
When B21 is peripherally activated at its resting potential, PSPs are not induced in the B8 neurons. In contrast, when B21 is centrally depolarized and then peripherally activated, PSPs are observed (Rosen et al. 2000a). The effect of membrane potential is graded, i.e., as the holding potential is progressively increased, PSP amplitude progressively increases (Evans et al. 2003) (also see Fig. 1, B and C, bottom). Previously we characterized one effect of depolarization on afferent transmission (Evans et al. 2003, 2007). We demonstrated that somatic depolarization modifies spike propagation in B21's processes. In the current study, we sought to determine whether other phenomena are additionally invoked. In particular, we sought to determine whether we could identify a mechanism that could account for the graded nature of the effect of membrane potential.
To determine whether central depolarization impacts synaptic transmission beyond the point where spike propagation is modified, we simultaneously monitored both spike propagation in B21 and synaptic transmission to B8. These experiments were performed without the dye usually injected in B21 to facilitate the impalement of the lateral process because dye injection potentially impacts synaptic transmission (van Hooft 2002). As expected, when B21 was peripherally activated at its resting membrane potential, spikes did not actively propagate to the lateral process and PSPs were absent in B8 (Fig. 1, B and C). To induce spike propagation to the lateral process, we centrally depolarized B21 by injecting current into the soma (trace not shown) and then peripherally activated it. Interestingly, PSPs in B8 were still virtually absent (Fig. 1, B, arrows, and C, bottom). To verify the integrity of the B21–B8 synaptic connection in these preparations, we increased the depolarization in B21. PSPs did in fact become apparent (Fig. 1, B and C). We measured B8 PSP amplitude for different amounts of depolarization in B21 and, as previously reported, potentiating effects of membrane potential were graded (Fig. 1C, bottom) (Evans et al. 2003). These results suggest that signal transmission in B21 is not solely determined by the control of spike propagation. An additional mechanism is invoked at membrane potentials that are more depolarized than those that alter propagation to the lateral process.
Do subthreshold changes in membrane potential alter intracellular calcium concentrations in B21?
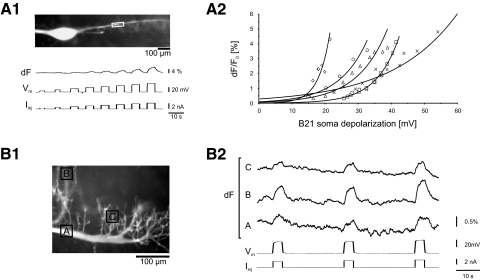
A number of well-characterized mechanisms for the graded presynaptic regulation of transmitter release involve alterations in calcium currents. For example, relatively steady-state, low-threshold calcium currents can be induced and can modify the strength of spike-induced synaptic transmission (e.g., Connor et al. 1986; Ivanov and Calabrese 2003). To determine whether subthreshold depolarizations alter intracellular calcium concentrations in B21, we electrophoretically injected it with Calcium Orange, a Ca2+ indicator dye (Fig. 2A1, top). We then centrally depolarized it via somatic injection of current pulses (Fig. 2A1, bottom 2 traces). Depolarization resulted in a graded increase in fluorescence (dF/F0) in the primary lateral process (Fig. 2A, 1, top trace, and 2). For example, when the soma was depolarized by 20 mV, we observed an average dF/F0 increase of 1.3 ± 0.3% (N = 5) in the lateral process. When the soma was depolarized by 30 mV, we observed a 2.7 ± 0.8% increase in dF/F0 (N = 5; Fig. 2A2). To determine whether changes in calcium fluorescence are also observed in secondary and tertiary branches of the lateral process, we performed a second set of experiments in which we imaged B21 using a ×40 objective (Fig. 2B1). Again we observed increases in dF/F0 when subthreshold current pulses were injected into the soma of B21 (Fig. 2B2; N = 4).
FIG. 2.
Subthreshold depolarizations increase the intracellular calcium concentration in B21. A1, photo: low-power (×10) image of a neuron B21 after injection of the Ca2+ indicator dye Calcium Orange. The box on the lateral process marks where optical measurements were made in the recordings shown below. Traces: subthreshold current pulses of progressively increasing amplitudes were injected into the soma of B21 (Iinj). These pulses induced changes in membrane potential (Vm) and graded increases in calcium fluorescence (dF). A2: group data and fitted exponential functions from 5 experiments like the 1 shown in A1. Note the graded increase in dF as pulse amplitude was increased. B1: higher (×40)-power image of a B21 neuron injected with Calcium Orange. The boxes mark the regions imaged in B2. Subthreshold current pulses were injected into the soma of B21 (Iinj), which induced changes in membrane potential (Vm) and increases in calcium fluorescence (dF).
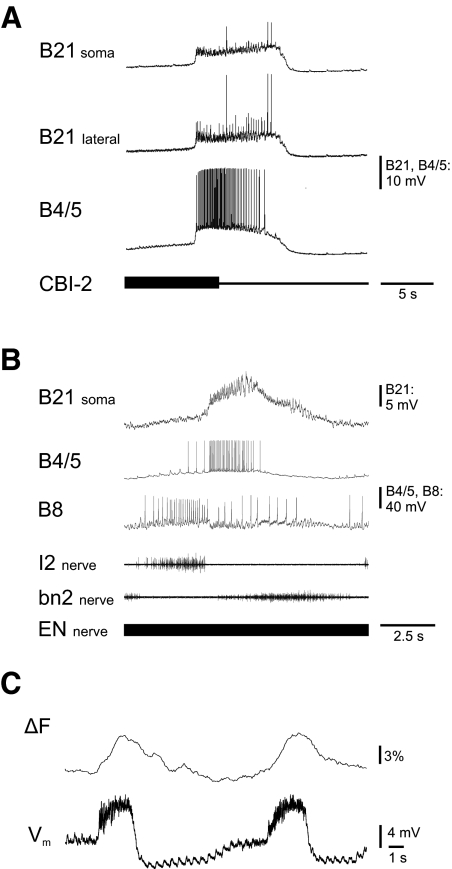
During feeding motor programs B21 is depolarized within the CNS via electrical coupling with pattern-generating interneurons (Rosen et al. 2000a,b). To determine whether these depolarizations are likely to be sufficient for inducing changes in calcium fluorescence, we performed two types of experiments. In one type of experiment, we induced rhythmic activity by stimulating cerebral buccal interneuron 2 (CBI-2; Fig. 3A) to obtain primarily ingestive motor programs (Jing and Weiss 2001). This type of feeding motor program has been most extensively utilized for studies of the regulation of B21 mechanoafferent transmission. During CBI-2-evoked activity, we were not technically able to directly monitor changes in calcium fluorescence in the lateral process (due to problems impaling CBI-2 while using our imaging setup). We were, however, able to record from the lateral process and measure the magnitude of the centrally generated depolarization (so that it could be related to the depolarization that was used to induce increases in calcium fluorescence in imaging experiments such as the one shown in Fig. 2A1). We found that the mean lateral process depolarization was 13.6 ± 0.9 mV (N = 6, n = 12), which was not significantly different from the mean depolarization in the soma of 15.7 ± 1.1 mV (N = 9, n = 20, t-test P > 0.05 dF: 30; Fig. 3A, 1 and 2).
FIG. 3.
Changes in membrane potential and calcium fluorescence in B21 during motor programs. A: experiment conducted in the isolated nervous system in which a cycle of a motor program was induced by stimulating the command-like neuron CBI-2. Note that centrally induced depolarizations were observed in both the soma and lateral process of B21. B: experiment conducted in the isolated nervous system in which a cycle of a motor program was induced by stimulating a gut afferent, the esophageal nerve (EN). Note that B21 was centrally depolarized. C: 2 cycles of an EN-evoked motor program in an experiment in which we monitored both the somatic membrane potential of B21 and the change in calcium fluorescence in the lateral process. Note the phasic increases in the 2 signals.
To relate the change in membrane potential in the lateral process observed during the motor program to the experimental depolarizations in imaging experiments, we determined the lateral process length constant. These experiments were necessary because the changes in membrane potential monitored during imaging were somatic. The lateral process length constant was, therefore, needed to determine how a somatic change in membrane potential would impact the lateral process. To measure the length constant, we used animals that were similar in size to those used in the previously described experiments and inserted recording electrodes in the soma and lateral process of B21, as well as a third electrode in the soma for current injection. We measured the distance between soma and lateral process recording sites and calculated λ = −d/ln (Va/Vb), with d being the distance between recording sites, and Va and Vb being the voltage changes resulting from current injection in the soma and lateral process, respectively. We found λ to be ∼390 ± 59 μm (N = 6, n = 14). This calculation indicates that it is necessary to depolarize the soma by ∼30 mV to induce a 14 mV depolarization 300 μm into the lateral process. This is roughly at the midpoint of the lateral process because the total average length is 622 ± 20 μm (N = 4). As noted in the preceding text, this change in somatic membrane potential produces a significant increase in fluorescence (see Fig. 2B).
In the second type of motor program experiment, activity was induced via stimulation of the esophageal nerve (EN) (Fig. 3, B and C). These types of motor programs are primarily egestive (Proekt et al. 2004) but are advantageous in that we were able to directly image changes in fluorescence in the lateral process. We compared somatic depolarizations during the two types of motor programs, and found that they were similar. During cycles of EN-evoked activity, they were on average 12.5 ± 2.6 mV (N = 6), which is not significantly different from the 15.7 mV observed during cycles of CBI-2-evoked programs (t-test P > 0.05, dF: 42). In calcium imaging experiments, we did, in fact, observe changes in fluorescence in the lateral process that corresponded to changes in the somatic membrane potential of B21 (Fig. 3C). Taken together these data suggest that motor program induced alterations in membrane potential are sufficient to induce changes in the intracellular calcium concentration of the lateral process of B21.
Is the effect of B21 depolarization on PSP size a result of an increase in intracellular calcium?
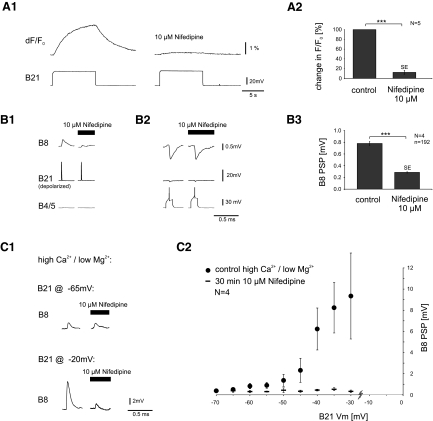
The determine whether B21–B8 synaptic transmission is impacted by changes in baseline calcium concentrations, we took advantage of previous work that has demonstrated that these types of increases can be blocked in Aplysia by nifedipine (Edmonds et al. 1990). Importantly, effects of nifedipine have been shown to be selective, i.e., calcium currents that contribute to synaptic plasticity are modified, whereas calcium currents that contribute to exocytosis are not (Edmonds et al. 1990). To verify its effectiveness in our preparation, we bath applied 10 μM nifedipine and imaged Calcium Orange signals induced by somatic depolarization of B21 (Fig. 4A1). In the presence of nifedipine, somatic depolarizations produced fluorescence increases that were on average only 12.3 ± 3.9% of control values (N = 5, t-test: P < 0.001; Fig. 4A2).
FIG. 4.
Inhibitory effects of nifedipine on calcium fluorescence in B21, and on B21–B8 synaptic transmission. A1: changes in calcium fluorescence induced by subthreshold depolarizing pulses were significantly reduced by nifedipine (10 μM, bath applied). A2: group data from 5 animals for the type of experiment shown in A1. The data are normalized to the maximum response. B1: B21 was peripherally activated and centrally depolarized so that PSPs were induced in B8 (left). Nifedipine (10 μM, bath applied) significantly reduced PSP amplitude (right). B2: in the same preparation, B8 PSPs induced by stimulating a B4/5 neuron were unaffected by nifedipine. B3: group data from 4 animals for the type of experiment shown in B1. C1: the experiment shown in B1 was repeated in high-calcium/low-magnesium saline. Under these conditions, B8 PSPs were detectable without central depolarization of B21. This did not change when 10 μM nifedipine was bath applied (top). In the same preparation, central depolarization increased PSP amplitude in the absence of nifedipine but not in its presence (bottom). C2: averaged data from 4 experiments as shown in C1. Dot symbols plot B8 PSP size under control conditions; dash symbols plot PSP size after 30-min bath application of 10 μM nifedipine. Note that nifedipine only affects B8 PSP size when B21 is depolarized.
To study effects of nifedipine on synaptic transmission, we peripherally activated B21 (to induce spiking) and centrally depolarized it so that synaptic transmission was maximally potentiated (Fig. 4B1, left). We then bath applied 10 μM nifedipine (Fig. 4B1, right). The nifedipine significantly reduced B21–B8 PSP peak amplitude (N = 4, t-test: P < 0.001; Fig. 4B3). PSPs became very small and almost indistinguishable from noise (as they are when spikes initially propagate and synaptic transmission has not been potentiated by further depolarization; e.g., Fig. 4B, 1, right, vs. 1B).
Because PSPs are so small in the presence of nifedipine, we performed two additional manipulations to verify the selectivity of nifedipine in our preparation, i.e., to determine whether the concentration of nifedipine used blocked the relatively high-voltage-activated calcium channels that contribute to exocytosis (as well as the relatively low-voltage channels that we hypothesize contribute to plasticity). In experiments such as the one shown in Fig. 4B1 we additionally monitored synaptic transmission at another synapse (Fig. 4B2). Nifedipine did not significantly effect synaptic transmission. Second, we conducted experiments in a modified saline solution. We increased the calcium concentration (to 33 mM, which is 3X) and decreased the magnesium concentration (to 10 mM), to increase PSP amplitude and excitability. In this type of saline, B8 PSPs are detectable when B21 is peripherally activated at its resting membrane potential (as well as when it is centrally depolarized; Fig. 4C1). B8 PSPs continued to be detectable in the presence of 10 μM nifedipine (Fig. 4C, 1, top, and 2). As expected, when B21 was depolarized, nifedipine significantly decreased PSP amplitude (Fig. 4C, 1, bottom, and 2). These data indicate that synaptic transmission can in fact occur in the presence of nifedipine in our preparation.
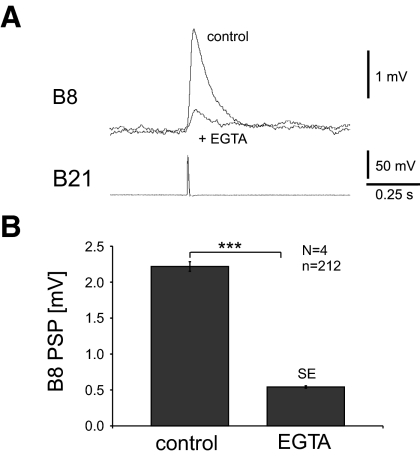
In a final set of experiments, we reduced the intracellular Ca2+ concentration in B21 by injecting EGTA (ethylene glycol tetra acetic acid) (Deitmer et al. 1986). EGTA is a chelating agent with a high affinity for Ca2+. While reducing the background calcium level, it is thought to be too slow to have a direct effect on the calcium currents mediating transmitter release (Haugland 2005). Again, B21 was peripherally activated to evoke spikes and centrally depolarized so that PSPs were maximally potentiated. EGTA was then intracellularly injected into B21's soma. Approximately 10 minutes later, PSP amplitude was significantly decreased (N = 4, t-test: P < 0.001; Fig. 5, A and B). Taken together the nifedipine and EGTA data indicate that B21 to B8 synaptic transmission is impacted by increases in baseline calcium concentrations.
FIG. 5.
Effects of EGTA on B21-B8 synaptic transmission. A: B21 was peripherally activated and centrally depolarized so that a PSP was evoked in B8 (control). Ten minutes after injection of EGTA into B21 via a glass capillary filled with 250 mM EGTA, the amplitude of the B8 PSP size was dramatically reduced. B: group data for the type of experiment shown in A. Note that EGTA produced a significant decrease in PSP amplitude.
DISCUSSION
Previously we demonstrated that spike propagation in neuron B21 is regulated so that spikes propagate to the lateral process (an output region) when B21 is centrally depolarized but do not propagate when B21 is close to its resting membrane potential (Evans et al. 2003). In neurons where spike propagation is regulated, one might expect an all or none aspect to sensorimotor control (Segev and Schneidman 1999). This is, however, not what we found in previous experiments that studied synaptic transmission from B21 to its postsynaptic follower, B8. Instead we found effects of membrane potential to be graded (Evans et al. 2003). This study sought to determine how the graded regulation is mediated. We demonstrate that subthreshold changes in membrane potential produce changes in the intracellular-free calcium concentration in B21. Our results suggest that these increases are virtually essential for synaptic transmission. For example, nifedipine effectively blocks both increases in calcium fluorescence induced by subthreshold depolarizations, and B21 induced PSPs in B8. Additionally, B21–B8 synaptic transmission is depressed when the calcium chelator EGTA is injected into B21. Increases in the intracellular calcium are a graded function of membrane potential, and they are likely to account (at least in part) for the graded effect of holding potential on B21 to B8 synaptic transmission.
Graded effects of membrane potential on synaptic transmission are observed in other systems
Graded effects of membrane potential on synaptic transmission have been described in a number of preparations (Marder 2006). In the retina and some invertebrate neurons, the threshold for neurotransmitter release is very close to resting potential, and release occurs in the absence of action potentials (Heidelberger 2007). At the B21–B8 synapse, there is no evidence for this form of graded transmission. Depolarizations are not observed in B8 if spikes are not evoked in B21. Thus changes in membrane potential do not induce significant synchronous transmitter release in B21. Instead they modify spike propagation and spike-mediated synaptic transmission.
Although graded effects of membrane potential on spike-mediated synaptic transmission were originally described >30 years ago (e.g., Graubard 1978; Nicholls and Wallace 1978; Shimara and Tauc 1975; Tauc 1962; Waziri 1977), interest in this phenomenon has recently been renewed. In part this is due to the fact that although the phenomenon was originally identified in invertebrates, it has become apparent that it is also likely to play a significant role in synaptic transmission in vertebrates, e.g., in local cortical networks (Alle and Geiger 2006; Clark and Hausser 2006; Marder 2006). Most of the synapses in the mammalian cortex are formed by nonmyelinated axons that are likely to be a millimeter or less from somatic regions (Douglas et al. 1995). Recent measurements of axonal length constants have indicated that they can be surprisingly long, and that somatic depolarizations can impact spike-mediated synaptic transmission in hippocampal mossy fibers and neocortical pyramidal axons (Alle and Geiger 2006; Shu et al. 2006).
Additionally, recent experiments in invertebrates have identified a new function for plasticity that is based on persistent changes of membrane potential. Studies of single-trial classical conditioning have demonstrated that persistent subthreshold depolarizations may be important for encoding a type of long-term memory (Frost 2006; Fusi 2008; Kemenes et al. 2006).
Mechanisms underlying graded effects of membrane potential on synaptic transmission
In some systems where changes in membrane potential potentiate synaptic transmission, mechanisms are largely calcium independent, i.e., subthreshold presynaptic depolarizations have no effect on resting or evoked calcium signaling (e.g., Scott et al. 2008). Other systems are similar to the B21–B8 synapse in that elevations in background (or resting) calcium levels are clearly important (Awatramani et al. 2005; Connor et al. 1986; Ivanov and Calabrese 2003; Kemenes et al. 2006). In systems where calcium-dependent effects have been demonstrated, subthreshold depolarizations open relatively low-voltage-activated calcium channels (Awatramani et al. 2005; Ivanov and Calabrese 2003). These channels are presumably distinct from the channels that directly trigger exocytosis. It has been hypothesized that background calcium interacts with a high-affinity sensor that is distinct from the exocytosis calcium sensor (Ivanov and Calabrese 2003, 2006a,b). The net result is a potentiation of synaptic transmission (Awatramani et al. 2005; Ivanov and Calabrese 2003; Shapiro et al. 1980).
Our data support the idea that a similar mechanism operates at the B21–B8 synapse in that nifedipine effectively reduced potentiating effects of depolarization on synaptic transmission. In Aplysia, nifedipine blocks a calcium current that does not contribute to synchronous synaptic transmission that is evoked at “resting” potential, i.e., without a baseline depolarization (Edmonds et al. 1990). At resting potential, spike-induced transmitter release is mediated by a second type of current that is rapidly inactivating and dihydropyridine insensitive (Edmonds et al. 1990; Eliot et al. 1993). B21 differs from other Aplysia cells in the extent to which the two types of currents impact synaptic transmission. In other cells, when spikes are triggered at resting potential, PSPs are observed in follower neurons (Shapiro et al. 1980). In contrast, when B21 is depolarized to the point where spikes propagate but where there is very little induction of the dihydropyridine-sensitive current, PSPs are virtually absent in B8. While PSPs can be observed under high extracellular calcium conditions, the probability of release in B21 under normal conditions is likely to be low relative to other cells that have been studied. Our imaging technique did not allow us to directly detect the dihydropyridine insensitive current, which is likely to be spatially highly restricted (Ivanov and Calabrese 2003).
Previous experiments that characterized the dihydropyridine-sensitive current in Aplysia were limited in that they were conducted under conditions that did not permit an evaluation of its functional significance. For example, the current was induced via stimuli that were not designed to mimic a physiological input. With a somatic voltage clamp, the current did not become apparent until approximately −30 mV (Edmonds et al. 1990) (a membrane potential that is relatively high for the induction of a “background” current). Our data indicate that when depolarization is induced in a more physiologically relevant manner (i.e., via distributed synaptic input within the CNS), increases in intracellular calcium concentrations are observed a little over 10 mV from B21's resting membrane potential (which is generally approximately −65 mV). Thus our data indicate that the dihydropyridine-sensitive current in Aplysia can play a significant role in determining the strength of synaptic transmission under physiologically relevant conditions.
Possible functional significance of two mechanisms for the regulation of B21–B8 synaptic transmission
B21 differs from other cells that have been studied in that depolarizations that modify intracellular calcium levels also modify spike propagation. Thus there are two distinct mechanisms that regulate B21 mechanoafferent transmission. A possible functional consequence of this arrangement is that it enables selective control of afferent input to different types of follower neurons. This report focuses on one type of follower motor neuron, the B8 cells, which receive chemical synaptic input. Other neurons that receive mechanoafferent input are electrically coupled to B21 (Rosen et al. 2000a,b). Our data suggest that B21 mechanoafferent transmission to electrically coupled follower neurons may occur under conditions where afferent transmission to chemical follower neurons does not. We show that when B21 is peripherally activated and centrally depolarized by only a few millivolts, spikes can propagate to the lateral process. Under these conditions, coupling potentials will be generated in electrically coupled “follower” neurons. We also show that relatively small depolarizations that modify spike propagation do not necessarily induce chemical synaptic transmission to the B8 neurons. PSPs are only induced in the B8 cells under conditions where there is a larger central depolarization. Our data, therefore, suggest that B21 may provide afferent input to electrically coupled motor neurons under conditions where it does not induce PSPs in the B8 neurons.
Under physiological conditions there are two potential sources of central depolarization in B21. During motor programs, B21 is depolarized via phasic input it receives from feeding interneurons (Rosen et al. 2000a,b). In this report, we demonstrate that these depolarizations are sufficient to both modify spike propagation and induce chemical synaptic transmission. Consequently, postsynaptic follower neurons (the B8 neurons) will presumably receive afferent input during the radula retraction phase of motor programs. Second, B21 is centrally depolarized in the absence of rhythmic activity when peripherally applied stimuli generate bursts of spikes in B21, and coactivate other neurons of the radula mechanoafferent cluster (e.g., Miller et al. 1994). Baseline changes in membrane potential are relatively small under these conditions but are within the range where we demonstrate spike propagation in this study (i.e., ∼3 mV from resting potential). Interestingly, B21 is activated in this manner during the radula protraction phase of motor programs (Borovikov et al. 2000). During protraction, B21 is, therefore, likely to provide afferent input to electrically coupled followers despite the fact that chemical synaptic transmission to B8 does not occur.
GRANTS
This work was supported by National Institute of Mental Health Grant MH-51393. Some of the Aplysia used in this study were provided by the National Resource for Aplysia of the University of Miami under Grant RR-10294 from the National Center for Research Resources.
Acknowledgments
We thank F. S. Vilim for expert advice on technical aspects of the experiments and K. R. Weiss for valuable comments on an earlier version of this manuscript.
REFERENCES
- Alle and Geiger 2006.Alle H, Geiger JRP. Combined analog and action potential coding in hippocampal mossy fibers. Science 311: 1290–1293, 2006. [DOI] [PubMed] [Google Scholar]
- Awatramani et al. 2005.Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron 48: 109–121, 2005. [DOI] [PubMed] [Google Scholar]
- Borovikov et al. 2000.Borovikov D, Evans CG, Jing J, Rosen SC, Cropper EC. A proprioceptive role for an exteroceptive mechanoafferent neuron in Aplysia. J Neurosci 20: 1990–2002, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büschges and El Manira 1998.Büschges A, El Manira A. Sensory pathways and their modulation in the control of locomotion. Curr Opin Neurobiol 8: 733–739, 1998. [DOI] [PubMed] [Google Scholar]
- Clark and Häusser 2006.Clark B, Häusser M. Neural coding: hybrid analog and digital signalling in axons. Curr Biol 16: R585–588, 2006. [DOI] [PubMed] [Google Scholar]
- Connor et al. 1986.Connor JA, Kretz R, Shapiro E. Calcium levels measured in a presynaptic neurone of Aplysia under conditions that modulate transmitter release. J Physiol 375: 625–642, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper et al. 1996.Cropper EC, Evans CG, Rosen SC. Multiple mechanisms for peripheral activation of the peptide-containing radula mechanoafferent neurons B21 and B22 of Aplysia. J Neurophysiol 76: 1344–1351, 1996. [DOI] [PubMed] [Google Scholar]
- Deitmer et al. 1986.Deitmer JW, Eckert R, Schlue WR. Changes in the intracellular free calcium concentration of Aplysia and leech neurons measured with calcium-sensitive microelectrodes. Can J Physiol Pharmacol 65: 934–939, 1986. [DOI] [PubMed] [Google Scholar]
- Douglas et al. 1995.Douglas RJ, Koch C, Mahowald M, Martin KAC, Suarez HH. Recurrent excitation in neocortical circuits. Science 269: 981–985, 1995. [DOI] [PubMed] [Google Scholar]
- Edmonds et al. 1990.Edmonds B, Klein M, Dale N, Kandel ER. Contribution of two types of calcium channels to synaptic transmission and plasticity. Science 250: 1142–1147, 1990. [DOI] [PubMed] [Google Scholar]
- Eliot et al. 1993.Eliot LS, Kandel ER, Siegelbaum SA, Blumenfeld H. Imaging terminals of Aplysia sensory neurons demonstrates role of enhanced Ca2+ influx in presynaptic facilitation. Nature 361: 634–637, 1993. [DOI] [PubMed] [Google Scholar]
- Evans et al. 2003.Evans CG, Jing J, Rosen SC, Cropper EC. Regulation of spike initiation and propagation in an Aplysia sensory neuron: gating-in via central depolarization. J Neurosci 23: 2920–2931, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans et al. 2008.Evans CG, Kang T, Cropper EC. Selective proagation in the central processes of an invertebrate neuron. J Neurophysiol 100: 2940–2947, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans et al. 2007.Evans CG, Ludwar BCh, Cropper EC. Mechanoafferent neuron with an inexcitable somatic region: consequences for the regulation of spike propagation and afferent transmission. J Neurophysiol 97: 3126–3130, 2007. [DOI] [PubMed] [Google Scholar]
- Frost 2006.Frost W. Memory traces: snails reveal a novel storage mechanism. Curr Biol 16: R640–641, 2006. [DOI] [PubMed] [Google Scholar]
- Fusi 2008.Fusi S. A quiescent working memory. Science 319: 1495–1496, 2008. [DOI] [PubMed] [Google Scholar]
- Graubard 1978.Graubard K. Synaptic transmission without action potentials: input-output properties of a nonspiking presynaptic neuron. J Neurophysiol 41: 1014–1025, 1978. [DOI] [PubMed] [Google Scholar]
- Haugland 2005.Haugland RP. The Handbook (10th ed.). Carlsbad, CA: Invitrogen CA, 2005.
- Heidelberger 2007.Heidelberger R. Mechanims of tonic, graded release: lessons from the vertebrate photoreceptor. J Physiol 585: 663–667, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov and Calabrese 2000.Ivanov AI, Calabrese RL. Intracellular Ca2+ dynamics during spontaneous and evoked activity of leech heart interneurons: low-threshold Ca currents and graded synaptic transmission. J Neurosci 23: 1206–1218, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov and Calabrese 2003.Ivanov AI, Calabrese RL. Modulation of spike-mediated synaptic transmission by presynaptic background Ca2+ in leech heart interneurons. J Neurosci 23(4): 1206–1218, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov and Calabrese 2006a.Ivanov AI, Calabrese RL. Graded inhibitory synaptic transmission between leech interneurons: assesing the roles of two kinetically distinct low-threshold Ca currents. J Neurophysiol 96: 218–234, 2006a. [DOI] [PubMed] [Google Scholar]
- Ivanov and Calabrese 2006b.Ivanov AI, Calabrese RL. Spike-mediated and graded inhibitory synaptic transmission between leech interneurons: evidence for shared release sites. J Neurophysiol 96: 235–251, 2006b. [DOI] [PubMed] [Google Scholar]
- Jing and Weiss 2001.Jing J, Weiss KR. Neural mechanisms of motor program switching in Aplysia. J Neurosci 21: 7349–7362, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenes et al. 2006.Kemenes I, Straub VA, Nikitin ES, Staras K, O'Shea M, Kemenes G, Benjamin PR. Role of delayed nonsynaptic neuronal plasticity in long-term associative memory. Curr Biol 16: 1269–1279, 2006. [DOI] [PubMed] [Google Scholar]
- Marder 2006.Marder E. Neurobiology: extending influence. Nature 441: 702–703, 2006. [DOI] [PubMed] [Google Scholar]
- Miller et al. 1994.Miller MW, Rosen SC, Schissel SL, Cropper EC, Kupfermann I, Weiss KR. A population of SCP-containing neurons in the buccal ganglion of Aplysia are radula mechanoafferents and receive excitation of central origin. J Neurosci 14: 7008–7023, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsivais et al. 2005.Monsivais P, Clark BA, Roth A, Häusser M. Determinants of action potential propagation in cerebellar Purkinje cell axons. J Neurosci 25: 464–472, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls and Wallace 1978.Nicholls J, Wallace BG. Modulation of transmission at an inhibitory synapse in the central nervous system of the leech. J Physiol 241: 157–170, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt et al. 2004.Proekt A, Brezina V, Weiss KR. Dynamical basis of intentions and expectations in a simple neuronal network. Proc Natl Acad Sci USA 101: 9447–9452, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen et al. 2000a.Rosen SC, Miller MW, Cropper EC, Kupfermann I. Outputs of radula mechanoafferent neurons in Aplysia are modulated by motor neurons, interneurons, and sensory neurons. J Neurophysiol 83: 1621–1636, 2000a. [DOI] [PubMed] [Google Scholar]
- Rosen et al. 2000b.Rosen SC, Miller MW, Evans CG, Cropper EC, Kupfermann I. Diverse synaptic connections between peptidergic radula mechanoafferent neurons and neurons in the feeding system of Aplysia. J Neurophysiol 83: 1605–1620, 2000b. [DOI] [PubMed] [Google Scholar]
- Rossignol et al. 2006.Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. [DOI] [PubMed] [Google Scholar]
- Rudomin and Schmidt 1999.Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal chord revisited. Exp Brain Res 129: 1–37, 1999. [DOI] [PubMed] [Google Scholar]
- Scott et al. 2008.Scott R, Ruiz A, Henneberger C, Kullmann DM, Rusakov DA. Analog modulation of mossy fiber transmission is uncoupled from changes in presynaptic Ca2+. J Neurosci 28: 7765–7773, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev 1990.Segev I. Computer study of presynaptic inhibition controlling the spread of action potentials into axonal terminals. J Neurophysiol 63: 987–998, 1990. [DOI] [PubMed] [Google Scholar]
- Segev and Schneidman 1999.Segev I, Schneidman E. Axons as computing devices: basic insights gained from models. J Physiol 93: 263–270, 1999. [DOI] [PubMed] [Google Scholar]
- Shapiro et al. 1980.Shapiro E, Castellucci VF, Kandel ER. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc Natl Acad Sci USA 77: 629–633, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimara and Tauc 1975.Shimara T, Tauc L. Multiple interneuronal afferents to the giant cells in Aplysia. J Physiol 247: 299–319, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu et al. 2006.Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature 441: 761–765, 2006. [DOI] [PubMed] [Google Scholar]
- Tauc 1963.Tauc L. Site of origin and propagation of spike in the giant neuron of Aplysia. J Gen Physiol 45: 1077–97, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooft 2002.Hooft JA. Fast Green FCF (Food Green 3) inhibits synaptic activity in rat hippocampal interneurons. Neurosci Lett 318: 163–165, 2002. [DOI] [PubMed] [Google Scholar]
- Verdier et al. 2003.Verdier D, Lund JP, Kolta A. GABAergic control of action potential propagation along axonal branches of mammalian sensory neurons. J Neurosci 23: 2002–2007, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waziri 1977.Waziri R. Presynaptic electrical coupling in Aplysia: effects on postsynaptic chemical transmission. Science 195: 790–792, 1977. [DOI] [PubMed] [Google Scholar]
- Yuste and Konnerth 2005.Yuste R, Konnerth A. Imaging in Neuroscience and Development. Cold Spring Harbor, NY: Cold Spring Harbor Press, 2005.