Abstract
Decreased pH increases extracellular adenosine in CNS regions as diverse as hippocampus and ventral medulla. However, thus far there is no clear consensus whether the critical pH change is a decrease in intracellular and/or extracellular pH. Previously we showed that a decrease in extracellular pH is necessary and a decrease in intracellular pH alone is not sufficient, to increase extracellular adenosine in an acute hippocampal slice preparation. Here we explored further the role of intracellular pH under different synaptic conditions in the hippocampal slice. When synaptic excitability was increased, either during γ-aminobutyric acid type A receptor blockade in CA1 or after the induction of persistent bursting in CA3, a decrease in intracellular pH alone was now sufficient to: 1) elevate extracellular adenosine concentration, 2) activate adenosine A1 receptors, 3) decrease excitatory synaptic transmission (CA1), and 4) attenuate burst frequency in an in vitro seizure model (CA3). Hippocampal slices obtained from adenosine A1 receptor knockout mice did not exhibit these pH-mediated effects on synaptic transmission, further confirming the role of adenosine acting at the adenosine A1 receptor. Taken together, these data strengthen and add significantly to the evidence outlining a change in pH as an important stimulus influencing extracellular adenosine. In addition, we identify conditions under which intracellular pH plays a dominant role in regulating extracellular adenosine concentrations.
INTRODUCTION
Adenosine—the purinergic core of adenosine triphosphate (ATP)—is a potent inhibitory neuromodulator with anticonvulsant and neuroprotective properties. Under normal conditions, ATP is released from glia into the extracellular space, where it is rapidly dephosphoylated into adenosine and provides tonic inhibition of neuronal excitability in hippocampus (Dunwiddie et al. 1997; Pascual et al. 2005). During times of metabolic stress, such as stroke, seizure, hypoxia, and ischemia, ATP is consumed rapidly (Dunwiddie and Masino 2001). When ATP consumption outstrips ATP production, adenosine levels rise and adenosine enters the extracellular space (Lloyd et al. 1993; Meghji et al. 1989), although the mechanism by which adenosine exits the cell is not universally accepted (Frenguelli et al. 2007).
Once adenosine reaches the extracellular space, it can activate adenosine receptors, including adenosine A1 receptors (A1Rs), the functionally predominant form of adenosine receptors in the hippocampus (Johansson et al. 2001). When activated, A1Rs mediate the inhibitory effects of adenosine, including inhibition of presynaptic calcium channels (decreased neurotransmitter release) and activation of postsynaptic potassium channels (postsynaptic hyperpolarization) (Dunwiddie and Fredholm 1989). These effects are responsible for adenosine's powerful neuroprotective and anticonvulsant properties (Dunwiddie 1999; Etherington and Frenguelli 2004; Fedele et al. 2006; Huber et al. 2001). Many attempts at harnessing adenosine's potential have been made, with varying degrees of success (Jacobson and Gao 2006; Manole and Saladino 2007; Muller and Ferre 2007; Yu et al. 2008). To exploit these properties therapeutically, the mechanisms that control the release of adenosine must be more completely understood.
Previous studies indicated that changes in pH and/or carbon dioxide (CO2) modulate extracellular adenosine levels (Dale 2006; Dulla et al. 2005; Gourine et al. 2005; Otsuguro et al. 2006) and it is well known that changes in pH affect neuronal excitability. In general, acidic environments depress excitability and alkaline environments enhance excitability (Chesler 2003; Xiang and Bergold 2000). Previous work by our group showed that increases in CO2 partial pressure (Pco2) and decreases in extracellular pH (pHe) increased the extracellular production of adenosine from ATP via ecto-ATPase, an extracellular nucleotidase, and depressed synaptic and seizure activity. Conversely, decreased Pco2 reduced extracellular adenosine and promoted neuronal excitability (Dulla et al. 2005). In these experiments we found that a decrease in intracellular pH (pHi) alone of similar magnitude was not sufficient to alter field excitatory postsynaptic potentials (fEPSPs) (Dulla et al. 2005). Although this study showed that a decrease in pHe, or a combination of decreased pHe and decreased pHi, can alter adenosine levels, it did not eliminate the possibility that adenosine levels may also be modulated by changes in pHi alone under different synaptic conditions.
Several previous studies point to a correlation between changes in pHi and adenosine release. For example, N-methyl-d-aspartate (NMDA) receptor activation causes adenosine release in hippocampal slices (Manzoni et al. 1994) and decreases pHi but not pHe in cultured hippocampal neurons (Irwin et al. 1994). Furthermore, the onset and termination of epileptiform activity in the dentate gyrus of the hippocampus correlates closely with crossing certain pHi thresholds (Xiong et al. 2000). Based on previous work and preliminary studies, we hypothesized that during periods of increased neuronal excitability, changes in pHi may be necessary or sufficient to cause adenosine release.
Here we examined the effects of intracellular acidification in models of hyperexcitability in the hippocampal slice. The hippocampal slice provides several useful synaptic settings to investigate the role of adenosine in controlling neurotransmission. One of these is the well-characterized Schaffer collateral to CA1 synapse, a prototypical glutamatergic synapse in an area rich in A1Rs. Another synapse of interest is the CA3/CA3 recurrent collateral pathway. The CA3 region of the hippocampus is commonly used to study synchronized epileptiform neuronal discharge. CA3 pyramidal cells send recurrent glutamatergic axons onto the dendrites of neighboring pyramidal cells (MacVicar and Dudek 1982), creating an interconnected network of CA3 pyramidal cells (Stasheff et al. 1985). When potentiated by brief high-frequency electrical stimulation, the recurrent collateral pathway is capable of initiating synchronized, spontaneous action potentials known as bursts (Stasheff et al. 1985). The frequency of the bursts is extremely sensitive to the strength of the recurrent collateral synapses, thus making it an excellent model to study how changes in excitability affect network events (Bains et al. 1999; Behrens et al. 2005; Staley et al. 1998).
Using these two model pathways we found that when neuronal excitability is increased, by either blockade of γ-aminobutyric acid type A (GABAA) receptors or induction of persistent CA3 epileptiform bursting, decreasing pHi caused adenosine release, adenosine-dependent inhibition of neuronal excitability, and adenosine-dependent reductions in epileptiform activity. In addition, we examined the effects of decreased pHi under conditions of enhanced excitability in hippocampal slices obtained from A1R knockout mice and their wild-type littermates. The wild-type slices showed responses similar to those of normal rat hippocampal slices, but the effects of a decrease in pHi during enhanced excitability were absent in slices obtained from A1R knockout animals. Taken together, these experiments 1) point to a key role for intracellular pH in regulating and augmenting adenosine release during conditions of increased excitability and 2) elucidate further details regarding pH-mediated adenosine regulation as a general physiological principle in different brain regions.
METHODS
Slice preparation
Transverse hippocampal slices were obtained from 6- to 8-week-old Sprague–Dawley rats and adenosine A1 receptor knockout mice (Johansson et al. 2001) by using standard procedures (Dunwiddie and Lynch 1978). After decapitation into ice-cold artificial cerebrospinal fluid (aCSF; see composition in the following text) 400-μm slices were made on a Sorvall TC-2 tissue chopper and were incubated completely submerged at 32.5°C. The aCSF used for dissection, incubation, and submerged, perfused recordings contained (in mM): NaCl 126.0, KCl 3.0, MgCl2 1.5, d-glucose 11, CaCl2 2.4, NaH2PO4 1.2, and NaHCO3 25.9, bubbled continuously with a 95% O2-5% CO2 mixture, unless otherwise noted. Slices were incubated undisturbed for 60 min before electrophysiological recording.
Electrophysiological recording
Slices were placed on a nylon net in the recording chamber completely submerged in aCSF, superfused continuously (2.0 ml/min) with aCSF bubbled with 95% O2-5% CO2, and kept at 32.5°C throughout all manipulations. Control aCSF contained 5% CO2 and 26 mM NaHCO3 with an equilibrium pH of 7.4. Extracellular fEPSPs were recorded from the CA1 region of the stratum radiatum by using glass micropipettes (10–15 MΩ) filled with 3 M NaCl. A twisted bipolar insulated tungsten stimulating electrode (0.05-mm wire diameter) was placed to stimulate the Schaffer collaterals in s. radiatum every 10 s. Each pulse consisted of a 1-ms constant-voltage stimulation. Stimulation intensity was adjusted such that the fEPSP peak was between 0.5 and 1.5 mV. Data were recorded via an AC amplifier (World Precision Instruments, Sarasota, FL) filtered between 1 Hz and 3 kHz, digitized at a rate of 3 kHz, and stored in the computer for later analysis. All solutions were continuously oxygenated and perfused to avoid degassing in the perfusion lines. All time courses of fEPSPs are moving averages of five data points that provide further filtering of all noise ≳1 kHz. fEPSPs were quantified by measuring the change in amplitude over time for all experiments, except for those in which GABAA receptors were blocked. In these experiments fEPSPs were quantified by measuring the change in initial slope of the fEPSP over time.
Burst induction
Bursting in CA3 was induced by tetanic stimulation of the CA3 pyramidal cell layer. Here the recording electrode was placed in the cell layer of area CA3 and the stimulating electrode was placed in close proximity, thus allowing for orthodromic stimulation of the recurrent collateral inputs of CA3 pyramidal cells. Tetanic stimulation (100-Hz, 1-s train) of sufficient amplitude to elicit a population spike was used to induce CA3 bursting. If a single tetanus did not induce bursting, it was repeated after a 10-min interval. During CA3 bursting experiments extracellular solutions were modified to: 1.3 mM Ca2+, 0.9 mM Mg2+, and 3.3 mM K+ (Stasheff et al. 1985). Any change in burst frequency was computed by dividing the change in burst frequency by the average baseline burst frequency before that manipulation and multiplying by 100. Time courses of bursting frequencies are moving averages of five data points.
Statistical significance for each manipulation was determined by performing paired t-tests on the burst frequency before and after each treatment. Statistical significance between manipulations was performed using ANOVA and Fisher's protected least-significant difference (PLSD) tests. Time courses of bursting frequency are moving averages of five data points.
pH Imaging
Slices were placed into an airtight 1.5-ml dark glass tube and loaded with 2′,7′-bis(2-carboxyethyl)-5,6-carboxyfluorescein acetoxymethyl ester (BCECF-AM). Slices were incubated in 5–20 μM dye in oxygenated aCSF plus 0.0133% anhydrous dimethyl sulfoxide (DMSO), 6.0 × 10−5 % pluronic acid, and 5 mM probenecid. Slices were incubated in dye solution at 32.5°C for ≥30 min. Slices were then placed in a recording chamber and perfused as in electrophysiological experiments. Slices were imaged using a Zeiss LSM 510 laser scanning confocal microscope. A Coherent Mira Ti:sapphire tunable infrared laser was used for two-photon excitation at the pH-sensitive wavelength (795 nM) and fluorescence was detected at the emission wavelength of BCECF-AM (535 nM). BCECF-AM preferentially loaded CA1 pyramidal cells (for unknown reasons), which enabled specific imaging of pH changes occurring in neuronal cell bodies. Data were analyzed using Zeiss software to quantify the amount of fluorescence change in a CA1 cell body. Baseline fluorescence was taken for ≥1.5 min prior to manipulations and all data were subtracted from a baseline bleaching curve extrapolated from the 1.5-min baseline. The average percentage change in fluorescence was computed by dividing the average of five steady-state peak fluorescence measurements 2–4 min after changing the pH level by the average of five fluorescence measurements directly preceding the pH change. For all fluorescence experiments, autofluorescence was analyzed by monitoring an unloaded slice during all experimental manipulations. No significant changes in autofluorescence were seen for any manipulation tested. Extracellular pH was taken to be equivalent to aCSF buffer pH (Lee et al. 1996).
Adenosine sensor
An enzymatic sensor was used to measure adenosine release in rat hippocampal slices (Dale et al. 2000). The sensor (Sycopel International, Tyne and Wear, UK) has an overall width of about 500 μm and is comprised of two identical parallel semipermeable barrels. Each barrel contains a 50-μm platinum wire polarized to +650 mV using a potentiostat and the two barrels are linked via a master/slave box (Sycopel International). One barrel is filled with adenosine deaminase, nucleoside phosphorylase, and xanthine oxidase—an enzyme combination that results in the sequential breakdown of adenosine to inosine to hypoxanthine/xanthine and then to uric acid and hydrogen peroxide. The hydrogen peroxide is oxidized on the platinum wire to yield a current proportional to the concentration of adenosine. In the second barrel, the enzyme combination lacks adenosine deaminase, will not convert adenosine, and is the reference barrel. The two barrels are calibrated before each experiment with 2 μM adenosine and 2 μM inosine. A differential potential recorded between the two barrels yields a current that is linearly related to the concentration of adenosine. Sensor-differential recordings were created by recording each barrel's current (nA/V) separately (×8 amplification using custom-built differential amplifiers, sampled at 200 Hz, with no filtering) and then subtracting one recording from the other based on calibrations using custom-created software.
Drugs and chemicals
Adenosine, inosine, 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), xanthine oxidase, nucleoside phosphorylase, adenosine deaminase, DMSO, picrotoxin, propionic acid, and probenecid were all obtained from Sigma (St. Louis, MO) and dissolved in water, unless noted in the following text. All salts were also obtained from Sigma. Anhydrous DMSO was obtained from Pierce (Rockford, IL). BCECF-AM and pluronic acid were obtained from Molecular Probes (Eugene, OR). DPCPX and picrotoxin were dissolved in DMSO and then diluted 1:10 to make a final DMSO concentration of 10% (100×, 0.1% final concentration DMSO). Probenecid was dissolved in 2 ml 1 N NaOH, titrated back to pH 7.4 by addition of 800 μl 1 N HCl, and then diluted to a total of 20 ml in water. The concentration of all drugs used was based on accepted concentrations found in the literature and used previously in the laboratory to completely block the specified receptor but minimize any effects at other sites.
RESULTS
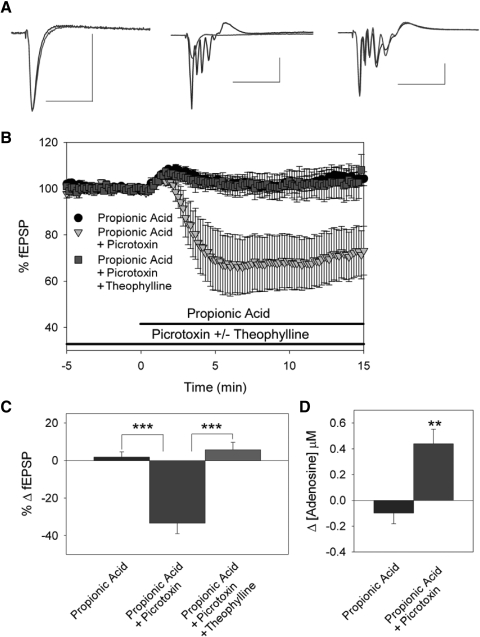
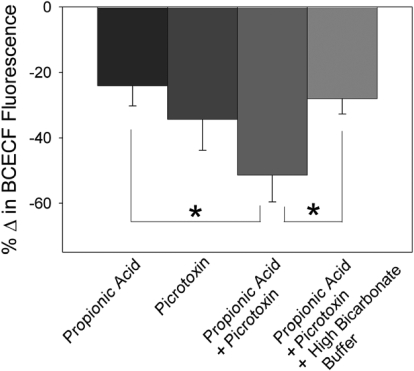
We first assessed the influence of intracellular acidification on synaptic transmission. Baseline fEPSPs were established and measured from rat hippocampal slices in CA1 stratum radiatum. Under normal recording conditions, applying 20 mM propionic acid, a weak organic acid, did not alter extracellular pH nor did it affect fEPSP amplitude (Fig. 1, A, left, B, and C, n = 12) or cause adenosine release (Fig. 1D, n = 5). Using two-photon imaging of hippocampal CA1 pyramidal cells loaded with BCECF, a pH-sensitive dye, we previously reported that under control conditions intracellular pH is 7.4 ± 0.1 (Dulla et al. 2005). Exposure to propionic acid did elicit an intracellular acidification of hippocampal CA1 pyramidal somas, as shown by a decrease in BCECF fluorescence (Fig. 2, n = 6).
FIG. 1.
Intracellular acidification releases adenosine and inhibits synaptic transmission. A: field excitatory postsynaptic potentials (fEPSPs) in CA1 stratum radiatum. Left: averaged fEPSP traces during control (black) and 20 mM propionic acid exposure (gray). Center: averaged fEPSP traces during 100 μM picrotoxin (black) and picrotoxin + propionic acid exposure (gray). Right: averaged fEPSP traces during picrotoxin + 250 μM theophylline (black) and propionic acid exposure (gray). Scale bars = 50 ms and 0.5 mV. B: time course of propionic acid alone (black circles, n = 12), propionic acid in picrotoxin pretreatment (gray triangles, n = 23), propionic acid in picrotoxin, and theophylline pretreatment (gray squares, n = 12). C: average change in fEPSP slope (***P < 0.001). D: change in adenosine levels when propionic acid was added under control conditions (n = 5) and when propionic acid was added after γ-aminobutyric acid type A (GABAA) receptors were blocked with picrotoxin (n = 7, **P < 0.01, Student's t-test).
FIG. 2.
Changes in BCECF [2′,7′-bis(2-carboxyethyl)-5,6-carboxyfluorescein] fluorescence in CA1 pyramidal cells. Percentage change in BCECF fluorescence was measured during exposure to propionic acid (n = 6), picrotoxin (n = 7), picrotoxin + propionic acid (n = 8), and high buffering power buffer + picrotoxin + propionic acid (n = 6). *P < 0.05 compared with the change in BCECF fluorescence during picrotoxin + propionic acid exposure( ANOVA, Fisher's protected least-significant difference test).
In contrast, during enhanced excitability in area CA1, intracellular acidification caused adenosine release and adenosine-mediated synaptic inhibition. When GABAA receptors were blocked with 100 μM picrotoxin, fEPSPs increased by 73.9 ± 16.7% due to loss of GABAergic inhibition (representative response shown in Fig. 1A, center, n = 44). Interestingly, blockade of GABAA receptors alone significantly decreased intracellular pH based on two-photon imaging studies (Fig. 2, n = 7) and increased extracellular adenosine levels by 0.25 ± 0.09 μM (data not shown, n = 5). With GABAA receptors blocked, we again examined the effects of intracellular acidification. Application of 20 mM propionic acid during GABAA receptor blockade decreased the fEPSP slope by 33.6 ± 5.6% (Fig. 1, A, B, and C, n = 23; P < 0.001, compared with propionic acid without picrotoxin, Student's t-test) and increased extracellular adenosine levels by 0.44 ± 0.11 μM (Fig. 1D, n = 7; P < 0.01, compared with propionic acid without picrotoxin, Student's t-test). Propionic acid also caused a much larger change in intracellular pH when applied during GABAA receptor blockade compared with propionic acid alone (Fig. 2, n = 8; P < 0.05, ANOVA, Fisher's PLSD).
To address the role of adenosine in mediating propionic acid–induced inhibition we repeated the previous experiments in the presence of the nonselective adenosine receptor antagonist theophylline. When adenosine receptors were blocked with 250 μM theophylline, the decrease in excitability caused by propionic acid was abolished (Fig. 1, A, B, and C, 5.7 ± 6.2% increase in fEPSP amplitude, n = 14; P < 0.001, compared with and without theophylline, Student's t-test). This indicated that when excitability was increased by blockade of GABAA receptors, intracellular acidification inhibited neuronal excitability by increasing extracellular adenosine levels and activating adenosine receptors.
Adenosine release and synaptic inhibition are due to decreased intracellular pH
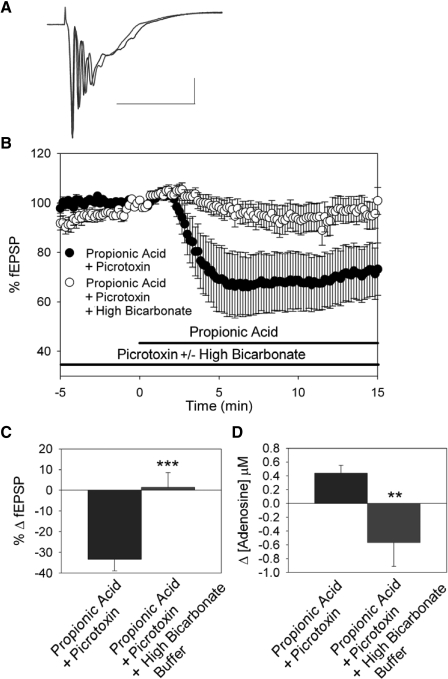
To test the pH dependence of adenosine release and inhibition of fEPSPs caused by intracellular acidification during GABAA receptor blockade, we used a highly buffered aCSF to block the intracellular acidification caused by propionic acid. This buffer contained 52 mM bicarbonate (control = 26 mM bicarbonate), was equilibrated with 10% CO2 (control = 5% CO2), and had a control pH of 7.4 ± 0.1 (n = 5). This buffer alone did not affect fEPSP amplitude (4.1 ± 1.7% decrease in fEPSP amplitude, n = 7; not significant, Student's t-test) and caused no significant change in extracellular adenosine concentration (0.07 ± 0.06 μM decrease, n = 7; not significant, Student's t-test). In the presence of this high-buffering power aCSF and a GABAA receptor blocker (picrotoxin, 100 μM), application of 20 mM propionic acid caused a significantly smaller intracellular acidification compared with that in control aCSF (Fig. 2, n = 6; P < 0.05, ANOVA, Fisher's PLSD). This high-bicarbonate buffer also significantly decreased the inhibition of synaptic activity caused by propionic acid when GABAA receptors were blocked (Fig. 3, A, B, and C, n = 14; P < 0.001, Student's t-test; compare Fig. 3 with Fig. 1). When propionic acid was applied in highly buffered aCSF the increase in extracellular adenosine was also attenuated (Fig. 3D, n = 3; P < 0.01, Student's t-test). A trend toward decreased extracellular adenosine was seen under these conditions but was not significantly different from baseline (prepropionic acid treatment).
FIG. 3.
Reducing intracellular acidification attenuates the effects of propionic acid during GABAA receptor blockade. A: fEPSPs in CA1 stratum radiatum. Averaged fEPSP traces during 100 μM picrotoxin (black) and picrotoxin + propionic acid exposure in high buffering power buffer (gray). Scale bars = 50 ms and 0.5 mV. B: time course of propionic acid effects. Propionic acid in picrotoxin pretreatment (black circles, n = 23; also shown in Fig. 1), propionic acid in picrotoxin, and high buffering power buffer pretreatment (open circles, n = 7). C: average change in fEPSP slope was appreciably attenuated in high bicarbonate buffer (***P < 0.001, compared with inhibition of fEPSP slope caused by 20 mM propionic acid in the presence of picrotoxin alone; propionate and picrotoxin data also shown in Fig. 1C). D: propionic acid treatment caused adenosine release when GABAA receptors were blocked with picrotoxin (n = 7), but did not if a high buffering power buffer was used (n = 3, **P < 0.01, Student's t-test).
Intracellular acidification during GABAA receptor blockade does not alter neuronal excitability in A1R knockout mice
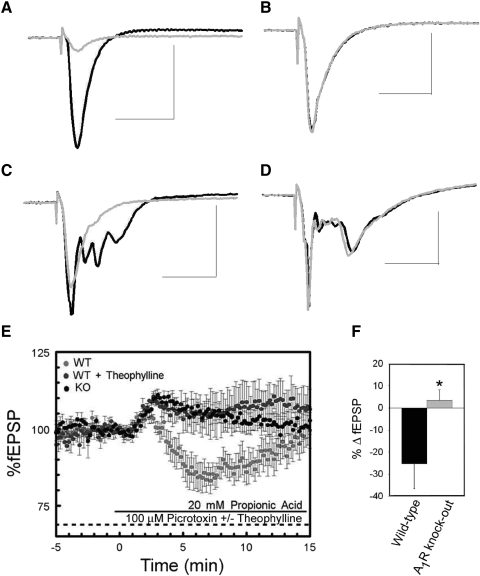
Genetically modified mice (Johansson et al. 2001) were used to confirm the role of A1Rs in mediating the effects of intracellular acidification during GABAA receptor blockade. These mice lack all binding and functional effects of A1Rs and changes in their learning and memory, anxiety, and locomotion have been described previously (Gimenez-Llort et al. 2005; Johansson et al. 2001). To confirm the genetic identity of each hippocampal slice, we first applied 50 μM adenosine to all slices during baseline recordings in CA1. As previously shown (Johansson et al. 2001), the fEPSP slope was significantly inhibited in wild-type mice (Fig. 4 A) but was not substantially altered in hippocampal slices obtained from A1R knockout mice (Fig. 4B).
FIG. 4.
Adenosine A1 receptor (A1R) knockout genotype eliminates the effects of intracellular acidification on fEPSP slope during GABAA receptor blockade. fEPSPs from CA1 stratum radiatum in hippocampal slices obtained from wild-type mice (A) and A1R knockout mice (B) during control conditions (black) and during exposure to 100 μM adenosine (gray). fEPSPs from wild-type mice (C) and A1R knockout mice (D) during exposure to picrotoxin (black) and propionic acid and picrotoxin (gray). Scale bars = 50 ms and 0.5 mV. E: time course of propionic acid effect on fEPSP in wild-type mice (light-gray), wild-type mice in the presence of theophylline (dark gray), and A1R knockout mice (black). F: in wild-type mice propionic acid decreased fEPSP slope when GABAA receptors were blocked with picrotoxin (n = 10). In A1R knockout mice this effect was abolished (n = 10, *P < 0.05, Student's t-test).
After electrophysiologically verifying the wild-type or A1R knockout genotype we performed experiments equivalent to those performed in rats. In wild-type mice propionic acid exposure during GABAA receptor blockade inhibited the fEPSP slope by 25.4 ± 11.5% (Fig. 4, C, E, and F, n = 10), similar to the magnitude of the inhibition observed in rat hippocampal slices. In contrast, the fEPSP was not inhibited by application of 20 mM propionic acid in hippocampal slices from A1R knockout mice and the effects on synaptic transmission were significantly different from those in wild-type slices (Fig. 4, D, E, and F, n = 10, 2.4 ± 4.5%; P < 0.05, Student's t-test). Combined with our other physiological and pharmacological evidence, these experiments using a genetic mutant mouse confirm that A1Rs play a major role in mediating the inhibitory effects of intracellular acidification during GABAA receptor blockade in area CA1.
Intracellular acidification causes adenosine-dependent reduction of area CA3 epileptiform bursting
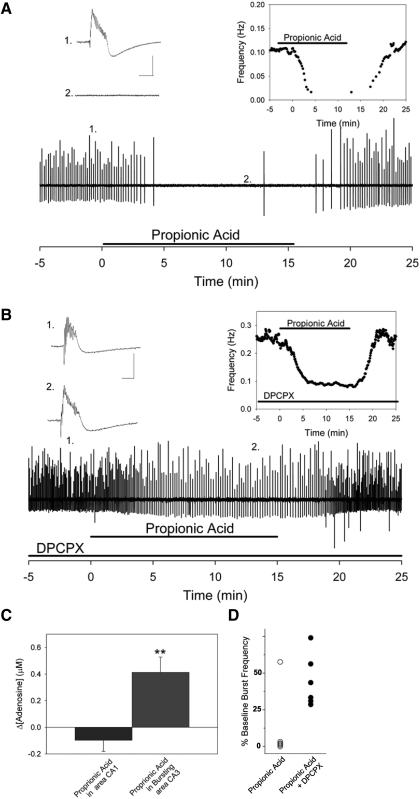
We also examined the effects of intracellular acidification on neuronal excitability and adenosine release during a more physiological form of increased neuronal activity—epileptiform bursting in hippocampal area CA3 (Fig. 5). Persistent bursting was induced using a tetanic stimulation and, under control conditions, burst frequency was 0.10 ± 0.01 Hz (Fig. 5A, inset). When 20 mM propionic acid was applied to the bursting CA3 network, bursting frequency was slowed significantly and dramatically (Fig. 5A, inset, n = 6, frequency = 0.006 ± 0.001 Hz; P < 0.001, Student's t-test). Within 3–5 min of propionic acid exposure, bursting was abolished in five of six slices (92.6 ± 1.8% decrease in burst frequency). This effect reversed on removing propionic acid. We simultaneously monitored changes in adenosine levels in area CA3 and found that extracellular adenosine levels rose by 0.41 ± 0.11 μM (Fig. 5C, n = 4) during propionic acid exposure. This increase in adenosine elicited by propionic acid in bursting CA3 was significantly larger than that elicited in area CA1 under nonbursting control conditions (P < 0.01, Student's t-test).
FIG. 5.
Propionic acid attenuates epileptiform activity in area CA3 via A1Rs. Extracellular recordings of synchronized bursting in area CA3. A: propionic acid exposure reversibly attenuates bursting. Inset, left, 1: example of a single burst recorded during control conditions (scale bars 1 mV, 50 ms for all). 2: example of extracellular recording during propionic acid exposure, with no bursts present. (Note: exact locations of samples indicated below.) Inset, right: burst frequency during control and propionic acid exposure. B: when A1Rs were blocked with 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), burst frequency was increased compared with A, and subsequent application of propionic acid slowed ongoing bursting activity. Unlike A, where propionic acid caused a complete cessation of bursting in 5/6 slices, bursting activity slowed significantly but never ceased in the presence of DPCPX. Inset, left, 1: example of a single burst recorded during control conditions with application of DPCPX. 2: example of a single burst recorded during propionic acid with application of DPCPX, with bursts still present. Inset, right: burst frequency during control/DPCPX and propionic acid/DPCPX conditions. C: during synchronized network activity in area CA3 propionic acid causes a significant release of adenosine (n = 4, **P < 0.01), whereas under control recording conditions in area CA1 propionic acid does not cause a change in adenosine release (n = 5, P = not significant). D: propionic acid reduced burst frequency in all slices. Propionic acid alone abolished bursting in 5 of 6 slices (left column, open symbol, n = 6). In the presence of DPCPX, bursting frequency decreased but was never abolished in any slice (right column, solid symbol, n = 6).
In the CA3 bursting model we tested the role of A1Rs in mediating the inhibition caused by intracellular acidification by blocking these receptors with 100 nM DPCPX, an A1R-selective antagonist. DPCPX was applied after stable bursting was established to ensure that it did not influence burst initiation (Thummler and Dunwiddie 2000). When A1Rs were blocked with DPCPX, burst frequency rose to 0.19 ± 0.02 Hz (Fig. 5B, inset) attributed to the loss of endogenous adenosine-mediated inhibition (Thummler and Dunwiddie 2000). In the presence of DPCPX, propionic acid caused a significantly smaller decrease in burst frequency (0.08 ± 0.02 Hz, 53.5 ± 4.4% decrease in burst frequency, n = 7; P < 0.001, Student's t-test), compared with the effects of propionic acid with A1Rs active. It should be noted that although burst frequency slowed, propionic acid was never able to stop bursting in the presence of DPCPX. In contrast, propionic acid alone completely abolished bursting in five of six slices (Fig. 5D). Although there are clearly strong effects of intracellular acidification that are not mediated by adenosine, these results indicate that during epileptiform bursting, intracellular acidification is sufficient to cause adenosine-dependent attenuation and even cessation of bursting. These results also further demonstrate the powerful anticonvulsant properties of A1Rs.
DISCUSSION
Adenosine is a powerful inhibitory neuromodulator in the CNS. Based on previous studies we hypothesized that adenosine levels increase during acidification (Fujiwara et al. 1992; Irwin et al. 1994; Masino and Dunwiddie 1999) and recent studies confirm pH and/or CO2-mediated release of ATP and/or adenosine in a variety of brain regions (Dulla et al. 2005; Gourine et al. 2005; Otsuguro et al. 2006) Thus far, however, the critical role of intracellular versus extracellular pH had not been examined under a variety of synaptic conditions. Here we show pH-mediated adenosine release under conditions of increased synaptic excitability in hippocampal slices. Specifically, intracellular acidification causes adenosine release and adenosine-dependent inhibition of neurotransmission in area CA1 when excitability is increased via blockade of inhibitory GABAA receptors or via recurrent bursting in CA3. The effects of intracellular acidification in area CA1 were specifically due to changes in pH because highly buffered aCSF attenuated intracellular acidification, adenosine release, and inhibition of neuronal excitability. These effects were shown to be mediated by A1Rs as specific A1R antagonists attenuated the inhibition of fEPSPs and the synaptic inhibition was lost entirely in A1R knockout mice. Similarly, in area CA3 intracellular acidification attenuated spontaneous network burst activity and caused adenosine release; intracellular acidification abolished network bursting in most slices. However, the effects of intracellular acidification in area CA3 may not be mediated completely by A1Rs—bursting was still present but slowed by intracellular acidification in the presence of an A1R antagonist.
The major question raised by our current findings is: Why can intracellular acidification cause adenosine release during states of heightened neuronal excitability but does not appear to do so during normal neurotransmission (Dulla et al. 2005)? One possibility is that the magnitude of pHi change seen under control conditions is insufficient to cause adenosine release. Evidence supporting this includes the finding that increased excitability combined with an acid challenge results in a much larger pHi decrease. Our two-photon pH imaging studies show that 20 mM propionic acid causes a significantly larger intracellular acidification (Fig. 2; decreased pHi indicated by a decrease in BCECF fluorescence) when GABAA receptors are blocked. This may explain the differential effects of intracellular acidification during different excitability states. Sodium and calcium influx coupled with Na+/Ca2+ and Na+/H+ exchange, the loss of the bicarbonate conductance mediated by GABAA receptors (Tasker and Dudek 1991), and a reduced availability of ATP during times of heightened excitability (Budd and Nicholls 1996) may all contribute to larger pH changes induced by an acid challenge during times of heightened activity.
During times of increased neuronal activity intracellular calcium levels are known to rise (Skrede and Malthe-Sorenssen 1981; van der Linden et al. 1993). A second possibility is that changes in pHi are insufficient to cause adenosine release under control conditions, but are sufficient when coupled with increased intracellular calcium concentration. Based on our studies we cannot exclude the possibility that calcium signaling pathways converge with intracellular molecules mediating the functional effects of intracellular acidification. Indeed we find this possibility quite likely: NMDA activation causes both increased intracellular calcium levels (Alford et al. 1993) and intracellular acidification (Irwin et al. 1994) and is a robust adenosine-release–evoking stimulus (Manzoni et al. 1994). Future studies using calcium imaging could directly address these questions. If calcium and proton signaling pathways do converge to mediate adenosine release, it would suggest an even more refined and integrated control of purinergic neuromodulation than previously proposed.
An intriguing additional possibility is increased pH sensitivity of glial release of purines during heightened excitability. A rapidly evolving field is the astrocyte-mediated release of purines, particularly ATP (Pascual et al. 2005). Glia are now recognized to play an integral role in both synaptic physiology and pathophysiology (Pascual et al. 2005) and are a major source of enzymatic regulation of adenosine via adenosine kinase (Boison 2007). Glial metabolism is coupled tightly to and regulated by neuronal metabolism and activity and changes in astrocytic release of purines during heightened excitability could contribute significantly to adenosine's long-recognized role as a “retaliatory metabolite” (Newby 1984). This possibility opens a new conceptual framework for the feedback among neural metabolism, regulation of adenosine, and its ongoing influence on synaptic transmission. Although they add to our growing knowledge of the physiological regulation of adenosine, our studies do not resolve the longstanding debate regarding the specific source of adenosine.
Blockade of GABAA receptors with picrotoxin caused an increase in extracellular adenosine. This is most likely due to increased ATP consumption resulting from the heightened metabolic need associated with increased neuronal activity. When energy demand is high, ATP is consumed rapidly and intracellular adenosine levels rise. When this occurs adenosine can exit the cell and add to the tonic levels of extracellular adenosine. We would expect that the adenosine transporter would be critical to mediating these effects because intracellular adenosine increases are translated to the extracellular space via the nucleoside transporter. Addressing this question is complex, however, because pharmacologically blocking the nucleoside transporter results in increased extracellular adenosine inhibiting neuronal excitability and thus subverts manipulations of heightened excitability. Furthermore, it is not clear that nucleoside transporters constitute the only mechanism for the direct release of adenosine, particularly during ischemic conditions (Frenguelli et al. 2007), and the relationship between astrocytes and neurons in regulating extracellular purines may also play a critical role.
Another interesting aspect of this study is that propionic acid does not cause adenosine release under control conditions in CA1 (Dulla et al. 2005) but it does in the bursting CA3 network. One interpretation is that during bursting activity, intracellular changes occur that deplete intracellular ATP, increase calcium, or otherwise confer added pH sensitivity to one or more adenosine-metabolizing enzymes or adenosine-transporting molecules. Changes in phosphorylation state, cellular localization, or enzyme posttranslational modification could all result in increased pH sensitivity. Additionally, pHi oscillations have been noted during bursting (Xiong and Stringer 2000) and perhaps the pH decrease during the burst is of sufficient magnitude, similar to that observed with picrotoxin in CA1. It must be noted, however, that the effects of pH-dependent adenosine release in area CA3 may not be solely A1R mediated, as can be seen from the decrease in bursting frequency that still occurs after exposure to propionic acid in the presence of DPCPX. A further unexplored possibility is that protons may act directly on neurotransmitter receptors, signal transduction molecules, or metabolic processes and may thus affect excitability in an adenosine-independent manner.
The results presented here complement those of our previous study, demonstrating that under physiological conditions, changes in neuronal excitability caused by CO2 were mediated by pHe-dependent changes in adenosine and ATP signaling (Dulla et al. 2005). Although in our previous work we found that a decrease in pHi was not sufficient to increase extracellular adenosine under control conditions, we did not determine whether coordination between pHe and pHi might be essential. In general we do not exclude the possibility that synergy between pHe and pHi is relevant or required under certain circumstances to potentiate adenosine release. However, here we show that changes in pHi alone can cause adenosine release in the hippocampus, although pHi is sufficient only during heightened neuronal excitability.
A recent study in rat spinal cord suggested that intracellular acidification decreases the activity of adenosine kinase, an adenosine metabolizing enzyme, resulting in increased extracellular adenosine concentration (Otsuguro et al. 2006). Although these studies do not directly address the specific mechanisms that mediate changes in extracellular adenosine levels, they do confirm that intracellular acidification is an adenosine-releasing stimulus. In general, evidence is accumulating regarding the role of pH in regulating adenosine; these studies further highlight the complexity of pH-mediated purinergic signaling in diverse types of neuronal tissue (Dale 2006). The implications of the influence of pH on adenosine release and the specific experiments presented here on hyperexcitable states orient this basic research in a clear clinical direction. Acute neuropathological conditions, such as head injury, cerebral ischemia, and epileptic seizures, are associated with a decrease in pH (Clausen et al. 2005; Dunwiddie and Masino 2001) and a dramatic release of adenosine into the extracellular space (Boison 2007; Cunha 2005; Gourine et al. 2005). Using changes in intracellular pH may help harness the anticonvulsant properties of adenosine and may provide clues to more specific molecules or strategies with similar therapeutic potential.
GRANTS
This work was supported by an American Epilepsy Foundation predoctoral fellowship to C. G. Dulla; Epilepsy Research UK grant to B. G. Frenguelli; National Institute of Neurological Disorders and Stroke Grants NS-34360-12 to K. J. Staley; NS-29173, and NS-61290, National Science Foundation, and a Howard Hughes institutional award to Trinity College to S. A. Masino.
REFERENCES
- Alford 1993.Alford S, Frenguelli BG, Schofield JG, Collingridge GL. Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. J Physiol 469: 693–716, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains 1999.Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci 2: 720–726, 1999. [DOI] [PubMed] [Google Scholar]
- Behrens 2005.Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci 8: 1560–1567, 2005. [DOI] [PubMed] [Google Scholar]
- Boison 2007.Boison D. Adenosine as a modulator of brain activity. Drug News Perspect 20: 607–611, 2007. [DOI] [PubMed] [Google Scholar]
- Budd 1996.Budd SL, Nicholls DG. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem 67: 2282–2291, 1996. [DOI] [PubMed] [Google Scholar]
- Chesler 2003.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003. [DOI] [PubMed] [Google Scholar]
- Clausen 2005.Clausen T, Khaldi A, Zauner A, Reinert M, Doppenberg E, Menzel M, Soukup J, Alves OL, Bullock MR. Cerebral acid–base homeostasis after severe traumatic brain injury. J Neurosurg 103: 597–607, 2005. [DOI] [PubMed] [Google Scholar]
- Cunha 2005.Cunha RA. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A2A receptor blockade. Purinergic Signal 1: 111–134, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale 2006.Dale N. The acid nature of CO2-evoked adenosine release in the CNS (Abstract). J Physiol 574: 633, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale 2000.Dale N, Pearson T, Frenguelli BG. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J Physiol 526: 143–155, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulla 2005.Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link Pco2 to cortical excitability via pH. Neuron 48: 1011–1023, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie 1999.Dunwiddie TV. Adenosine and suppression of seizures. Adv Neurol 79: 1001–1010, 1999. [PubMed] [Google Scholar]
- Dunwiddie 1997.Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci 17: 7673–7682, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie 1989.Dunwiddie TV, Fredholm BB. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J Pharmacol Exp Ther 249: 31–37, 1989. [PubMed] [Google Scholar]
- Dunwiddie 1978.Dunwiddie TV, Lynch GS. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J Physiol 276: 353–367, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie 2001.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24: 31–55, 2001. [DOI] [PubMed] [Google Scholar]
- Etherington 2004.Etherington LA, Frenguelli BG. Endogenous adenosine modulates epileptiform activity in rat hippocampus in a receptor subtype-dependent manner. Eur J Neurosci 19: 2539–2550, 2004. [DOI] [PubMed] [Google Scholar]
- Fedele 2006.Fedele DE, Li T, Lan JQ, Fredholm BB, Boison D. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp Neurol 200: 184–190, 2006. [DOI] [PubMed] [Google Scholar]
- Frenguelli 2007.Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem 101: 1400–1413, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara 1992.Fujiwara N, Abe T, Endoh H, Warashina A, Shimoji K. Changes in intracellular pH of mouse hippocampal slices responding to hypoxia and/or glucose depletion. Brain Res 572: 335–339, 1992. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort 2005.Gimenez-Llort L, Masino SA, Diao L, Fernandez-Teruel A, Tobena A, Halldner L, Fredholm BB. Mice lacking the adenosine A1 receptor have normal spatial learning and plasticity in the CA1 region of the hippocampus, but they habituate more slowly. Synapse 57: 8–16, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine 2005.Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436: 108–111, 2005. [DOI] [PubMed] [Google Scholar]
- Huber 2001.Huber A, Padrun V, Deglon N, Aebischer P, Mohler H, Boison D. Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc Natl Acad Sci USA 98: 7611–7616, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin 1994.Irwin RP, Lin SZ, Long RT, Paul SM. N-Methyl-d-aspartate induces a rapid, reversible, and calcium-dependent intracellular acidosis in cultured fetal rat hippocampal neurons. J Neurosci 14: 1352–1357, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson 2006.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5: 247–264, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson 2001.Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hardemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA 98: 9407–9412, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee 1996.Lee J, Taira T, Pihlaja P, Ransom BR, Kaila K. Effects of CO2 on excitatory transmission apparently caused by changes in intracellular pH in the rat hippocampal slice. Brain Res 706: 210–216, 1996. [DOI] [PubMed] [Google Scholar]
- Lloyd 1993.Lloyd HGE, Lindström K, Fredholm BB. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem Int 23: 173–185, 1993. [DOI] [PubMed] [Google Scholar]
- MacVicar 1982.MacVicar BA, Dudek FE. Local circuit interactions in rat hippocampus: interactions between pyramidal cells. Brain Res 242: 341–344, 1982. [DOI] [PubMed] [Google Scholar]
- Manole 2007.Manole MD, Saladino RA. Emergency department management of the pediatric patient with supraventricular tachycardia. Pediatr Emerg Care 23: 176–185, 2007. [DOI] [PubMed] [Google Scholar]
- Manzoni 1994.Manzoni OJ, Manabe T, Nicoll RA. Release of adenosine by activation of NMDA receptors in the hippocampus. Science 265: 2098–2101, 1994. [DOI] [PubMed] [Google Scholar]
- Masino 1999.Masino SA, Dunwiddie TV. Temperature-dependent modulation of excitatory transmission in hippocampal slices is mediated by extracellular adenosine. J Neurosci 19: 1932–1939, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghji 1989.Meghji P, Tuttle JB, Rubio R. Adenosine formation and release by embryonic chick neurons and glia in cell culture. J Neurochem 53: 1852–1860, 1989. [DOI] [PubMed] [Google Scholar]
- Muller 2007.Muller CE, Ferre S. Blocking striatal adenosine A2A receptors: a new strategy for basal ganglia disorders. Recent Patents CNS Drug Discov 2: 1–21, 2007. [DOI] [PubMed] [Google Scholar]
- Newby 1984.Newby AC. Adenosine and the concept of “retaliatory metabolites.” Trends Biol Sci 9: 42–44, 1984. [Google Scholar]
- Otsuguro 2006.Otsuguro K, Yamaji Y, Ban M, Ohta T, Ito S. Involvement of adenosine in depression of synaptic transmission during hypercapnia in isolated spinal cord of neonatal rats. J Physiol 574: 835–847, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual 2005.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science 310: 113–116, 2005. [DOI] [PubMed] [Google Scholar]
- Skrede 1981.Skrede KK, Malthe-Sorenssen D. Increased resting and evoked release of transmitter following repetitive electrical tetanization in hippocampus: a biochemical correlate to long-lasting synaptic potentiation. Brain Res 208: 436–441, 1981. [DOI] [PubMed] [Google Scholar]
- Staley 1998.Staley KJ, Longacher M, Bains JS, Yee A. Presynaptic modulation of CA3 network activity. Nat Neurosci 1: 201–209, 1998. [DOI] [PubMed] [Google Scholar]
- Stasheff 1985.Stasheff SF, Bragdon AC, Wilson WA. Induction of epileptiform activity in hippocampal slices by trains of electrical stimuli. Brain Res 344: 296–302, 1985. [DOI] [PubMed] [Google Scholar]
- Tasker 1991.Tasker JG, Dudek FE. Electrophysiology of GABA-mediated synaptic transmission and possible roles in epilepsy. Neurochem Res 16: 251–262, 1991. [DOI] [PubMed] [Google Scholar]
- Thummler 2000.Thummler S, Dunwiddie TV. Adenosine receptor antagonists induce persistent bursting in the rat hippocampal CA3 region via an NMDA receptor-dependent mechanism. J Neurophysiol 83: 1787–1795, 2000. [DOI] [PubMed] [Google Scholar]
- van der Linden 1993.van der Linden JA, Joels M, Karst H, Juta AJ, Wadman WJ. Bicuculline increases the intracellular calcium response of CA1 hippocampal neurons to synaptic stimulation. Neurosci Lett 155: 230–233, 1993. [DOI] [PubMed] [Google Scholar]
- Xiang 2000.Xiang Z, Bergold P. Synaptic depression and neuronal loss in transiently acidic hippocampal slice. Brain Res 881: 77–87, 2000. [DOI] [PubMed] [Google Scholar]
- Xiong 2000.Xiong Z, Saggau P, Stringer J. Activity-dependent intracellular acidification correlates with the duration of seizure activity. J Neurosci 20: 1290–1296, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong 2000.Xiong ZQ, Stringer JL. Extracellular pH responses in CA1 and the dentate gyrus during electrical stimulation, seizure discharges, and spreading depression. J Neurophysiol 83: 3519–3524, 2000. [DOI] [PubMed] [Google Scholar]
- Yu 2008.Yu L, Shen HY, Coelho JE, Araujo IM, Huang QY, Day YJ, Rebola N, Canas PM, Rapp EK, Ferrara J, Taylor D, Muller CE, Linden J, Cunha RA, Chen JF. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol 63: 338–346, 2008. [DOI] [PubMed] [Google Scholar]