Abstract
Electrocorticogram (ECoG) recordings of the 6-hydroxydopamine (6-OHDA)–lesioned parkinsonian rat have shown an increase in the power of cortical β-band (15–30 Hz) oscillations ipsilateral to the lesion. The power of these oscillations is decreased with dopamine agonist administration. Here, we demonstrate that stimulation of an electrode implanted in the subthalamic nucleus alters the power of cortical β and γ oscillations in 6-OHDA–lesioned animals. These alterations are dependent on stimulation frequency, charge, and amplitude/pulse width. Oscillations were significantly reduced during 200- and 350-Hz stimulation. A minimum charge of 4 nC was required to elicit a reduction in oscillation power. A number of amplitude and pulse width combinations that reached 4 nC were tested; it was found that only the combinations of 33 μA/120 μs and 65 μA/60 μs significantly reduced cortical oscillations. The reduction in β/γ oscillation power due to deep brain stimulation (DBS) was consistent with a significant reduction in the animals' rotational behavior, a typical symptom of parkinsonism in the rat. A significant shift from high β to low γ was observed in the peak frequencies of ECoG recordings while animals were at rest versus walking on a treadmill. However, DBS exhibited no differential effect on oscillations between these two states. EEG recordings from rodent models of DBS may provide surrogate information about the neural signatures of Parkinson's disease relative to the efficacy of DBS.
INTRODUCTION
Deep brain stimulation (DBS) is now being used clinically as a treatment for Parkinson's disease (PD). The subthalamic nucleus (STN) is a frequent target of high-frequency stimulation (HFS) to moderate the symptoms of PD for patients both on and off medication (Obeso et al. 2001). Abnormal oscillations manifest in PD near the β band (∼13–30 Hz) in humans (Brown and Williams 2005; Brown et al. 2001; Cassidy et al. 2002; Hammond et al. 2007; Levy et al. 2002) and rodent models of PD (Mallet et al. 2008; Sharott et al. 2005). Previous work has correlated cortico-cortical electroencephalographic (EEG) synchrony of β oscillations in humans with the severity of their parkinsonism (Silberstein et al. 2005), including synchronization and coherence of EEG with the STN field potentials (Williams et al. 2002). The severity of PD symptoms, however, was found to have no correlation with the percentage of STN neuronal β oscillations but, instead, correlated with the magnitude of preoperative motor response to dopaminergic medication (Weinberger et al. 2006). Indeed, administration of levodopa, a precursor of dopamine, has a profound effect on the motor symptoms of PD (Kühn et al. 2006) and has been shown to diminish the abnormal β oscillations (Alonso-Frech et al. 2006; Brown et al. 2001; Doyle et al. 2005; Levy et al. 2002; Priori et al. 2004; Williams et al. 2002). However, the degree to which β oscillations correlate to the severity of parkinsonism is not clear because dopaminergic therapy has been shown to increase cortico-cortical coupling in patients in the early stages of therapy who experience marginal motor improvement (Stoffers et al. 2008).
HFS of the STN has also been shown to reduce STN β oscillations (Wingeier et al. 2006), thus corresponding to reduced severity of parkinsonian symptoms, particularly related to akinesia (Kühn et al. 2008), rigidity, and bradykinesia (Kühn et al. 2009). Yet, during HFS, changes in lower-frequency STN oscillations (1–7 Hz) have been observed without changes in β oscillations during HFS (Rossi et al. 2008), with β oscillations having no correlation to motor symptom improvement (Foffani et al. 2006). This observation challenges the relevance of β oscillations as an indicator for therapeutic stimulation. Motor improvements brought about by STN DBS, however, have been correlated to reduced coherence between multiple EEG electrodes in the 10- to 35-Hz range in patients with advanced PD, reflecting a disruption of synchronous β-band oscillations (Silberstein et al. 2005). The DBS-induced reduction in β-oscillation power tends to last longer following longer periods of DBS (Bronte-Stewart et al. 2009). The regions of the STN that exhibit the greatest abnormal β-band oscillations occur at the dorsal STN border (Kühn et al. 2005; Trottenberg et al. 2007; Weinberger et al. 2006), which corresponds well with models of patient-specific activation patterns that provide maximal clinical response to DBS (Butson et al. 2007). Further, it has been shown in studies combining low-frequency DBS (<10 Hz) with EEG recordings that motor areas are highly activated during the stimulation (MacKinnon et al. 2005). These studies suggest that the EEG from motor frontal cortex contains a PD signature that is sensitive to STN DBS therapy.
The objective of this study was to investigate the change in cortical β/γ-band power as a function of STN DBS parameters in a rodent surrogate of DBS for alleviating the symptoms of PD. Frequency of stimulation, total charge (a covariation of current amplitude and pulse width), and behavioral state (resting and walking) were manipulated while continuously recording the electrocorticogram (ECoG). ECoG recordings over the motor cortex of rats have been shown to change in response to a chemical lesion induced by selective destruction of dopamine neurons by 6-hydroxydopamine (6-OHDA) (Vorobyov et al. 2003), a standard model for PD (Cadet et al. 1992; Perese et al. 1989). The changes in ECoG were, specifically, an increase in the power of β/γ-band frequency (20–45 Hz) that could be seen differentially in the same animal (Vorobyov et al. 2003), in that the chemical lesion was created only unilaterally (Schwarting and Huston 1996). Previous attempts to reduce the β-frequency power on the lesioned side of 6-OHDA–treated rats have been made using pharmacotherapy, such as dopamine agonists (Sharott et al. 2005). Here we test the hypothesis that abnormal cortical oscillations can be reduced with STN DBS in the hemiparkinsonian rat and that reducing the power of these oscillations corresponds to improved motor symptoms. We demonstrate that β/γ-band power in the ECoG of parkinsonian rats can be significantly reduced with HFS and that these alterations in the ECoG power may be indicative of the therapeutic effects of STN DBS.
METHODS
Experiments were carried out on 14 male Sprague–Dawley rats (3 normal and 11 lesioned, as described in the following text). All animals were housed in a temperature- and humidity-controlled room with a 12-h light/12-h dark cycle. All procedures were conducted in accordance with protocols approved by the University of Michigan University Committee on Use and Care of Animals.
Unilateral dopamine lesion
Unilateral 6-OHDA lesions were carried out on 11 rats. One hour prior to lesioning, animals were given intraperitoneal (ip) injections of 1) pargyline (50 mg/kg), to amplify the effects on dopaminergic neurons and to help preserve noradrenergic neurons; and 2) desipramine (25 mg/kg), to prevent toxicity to the noradrenergic neurons (Schwarting and Huston 1996). The rats were anesthetized either with inhaled isoflurane or an ip injection of a mixture of ketamine (25 mg/kg), xylazine (1.25 mg/kg), and acepromazine (0.1 mg/kg). They were then placed in a stereotactic frame (MyNeuroLab, St. Louis, MO). A 2-mm craniotomy was made. 6-Hydroxydopamine hydrobromide (6-OHDA, 10 μg; Sigma Chemical, St. Louis, MO), stabilized in 0.01% ascorbic acid with 0.9% normal saline (2 μl total volume), was infused at a rate of 0.5 μl/min through a 26-gauge, 10-μl syringe in the right medial forebrain bundle (MFB) site: 4.4 mm posterior and 1.2 mm lateral to the bregma and 7.5 mm ventral to the dura mater. All coordinates were obtained from the atlas of Paxinos and Watson (1998). After the injection of 6-OHDA, the cannula was left in place for 5 min before being slowly retracted.
Lesion testing
Each rat was tested anywhere from 2 to 4 wk after the 6-OHDA injection. Each test began with an injection of amphetamine (5 mg/kg, ip) followed by placement in a rotometer (Ungerstedt and Arbuthnott 1970). One week later animals were injected with apomorphine (0.025 mg/kg, administered subcutaneously) and again placed in the rotometer. A lesion was considered successful if the rat managed at least six rotations/min in the clockwise direction in the time period between 30 and 90 min after initial injection during amphetamine challenge and at least two rotations/min in the counterclockwise direction between 30 and 60 min after apomorphine challenge; of the 11 animals initially injected with 6-OHDA, only 7 (64%) met these criteria and were considered for electrode implantation.
Electrode implantation
After demonstrating symptomatic evidence of 6-OHDA lesion, each rat was anesthetized and placed in a stereotactic frame as described earlier. Two ECoG screws were located at anterior–posterior (AP) 1.7 mm, medial–lateral (ML) 2.5 mm bilaterally to bregma (Supplemental Fig. S1).1 Two additional screws serving as ECoG reference and ground were placed over the parietal lobe and cerebellum. Two types of electrodes were placed in the STN. Initially two animals, one normal and one lesioned, were implanted with a monopolar, stainless steel microelectrode with a 100-μm tip diameter (FHC Instruments, Bowdoin, ME). However, stereotactic implantation of this electrode proved difficult without a guide cannula and histological analysis of the electrode location showed consistent misplacement of these electrodes. All subsequent animals (two normal, six lesioned) received a stainless steel microelectrode with a 100-μm tip diameter surrounded by a concentric guide cannula (MS308; Plastics1). STN coordinates used were AP −3.6, ML 2.5 to bregma. During surgery, we recorded extracellular action potentials from the stimulating electrode as it was advanced. The electrode was attached via amplifier and audio output (Tucker-Davis Technologies [TDT], Alachua, FL). At various points along the tract, electrode advancement was halted and potentials were monitored to determine optimal depth for STN recording and stimulation. Ideal positioning of the electrode would require that it not be retreated after initial advancement, to minimize damage to surrounding tissue. The paradigm developed consisted of advancing the electrode to 1–2 mm proximal of the intended stereotactic depth of dorsal–ventral (DV) 7.6 mm. Potentials were monitored for spike activity. When activity decreased, the electrode was assumed to be in the relatively silent zona incerta. Advancing by 50-μm increments, we encountered an increase in activity that was presumed to be the STN and the electrode was fixed at this location.
Electrophysiological recording and electrical stimulation
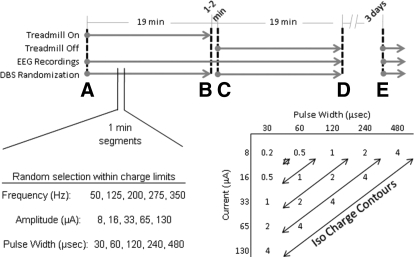
Each animal was placed in a Faraday cage on a pneumatic treadmill while its ECoG was measured and DBS delivered. Left and right ECoG signals were routed through a commutator before being differentially amplified (×10,000) with respect to a reference bone screw over the parietal lobe, filtered 0.3–300 Hz, 6 dB/octave rolloff (SR560; Stanford Research Systems, Sunnyvale, CA), and digitized at 1 kHz along with DBS timing-event markers (Multichannel Neuron Acquisition Processor; Plexon, Dallas, TX). This configuration allowed recording of robust noise- and artifact-free cortical signals that were stable across days and the behavioral state of the animal. Electrical stimulation was controlled through Matlab (release 7; The MathWorks, Natick, MA) running ActiveX controls that modulated the parameters of the stimulation pulses generated in RPvds software and RP2.1 hardware (TDT). Stimuli were then delivered to the animal through an optically isolated, constant-current stimulator (Model 2200; A-M Systems, Carlsborg, WA). Stimulus pulses consisted of square-wave biphasic, charge-balanced, cathodic-first, constant-current pulse pairs delivered at <50 μC/cm2 charge density, the maximum charge deliverable through stainless steel (Merrell 2005). This configuration ensured that 1) the amount of charge entering the tissue could be precisely controlled during each phase of pulsing; and 2) a net charge, as close to zero as possible, was delivered during all stimulation pulses, to prevent excessive charge buildup that could lead to destruction of the electrode and/or tissue. Additional safety measures were implemented by continuously monitoring the voltage at the electrode such that the voltage did not exceed the water window of stainless steel (i.e., the voltage at which hydrolysis of water into hydrogen and oxygen occurs, which is approximately ±1 V). Trains of pulses were delivered at frequencies of 50, 125, 200, 275, and 350 Hz (Frequency), whereas current amplitude and pulse width covaried between 130, 65, 33, 16, and 8 μA (Amplitude) and 30, 60, 120, 240, and 480 μs (Pulse Width), respectively, to achieve a maximum charge of ≤3.9 nC/phase (Charge, Fig. 1). DBS polarity was essentially monopolar due to the much larger surface area (∼ ×600) of the guide cannula that served as the current sink. ECoG measurements during stimulation were achieved by coupling the stimulating mechanism with the blanking input on the ECoG amplifiers that would reduce the amplifier gain to zero during the brief periods of stimulation, to prevent amplifier saturation.
FIG. 1.
Electrocorticogram (ECoG) signals were recorded in two 76-min sessions per animal. Each session consisted of alternating 19-min segments of the animal at rest or walking on the treadmill. A total of 75 possible stimulation combinations of frequency, current amplitude, and pulse width (not including no-stimulation trials) were delivered to each animal at random without replacement. Animals were continuously stimulated for 1 min for each stimulation parameter set during each 19-min segment. Current amplitude and pulse width combinations are presented as charge (nanocoulombs [nC]).
ECoG signals were recorded in two 76-min sessions per animal. Each session consisted of alternating 19-min segments of the animal at rest or walking on the treadmill (State) at about 14 ft/min. The two sessions were separated by ≥3 days. A total of 75 possible stimulation combinations of frequency, current amplitude, and pulse width (not including no-stimulation trials) were delivered to each animal at random without replacement. Animals were continuously stimulated for 1 min for each stimulation parameter set during each 19-min segment. The animals were not stimulated for 1–2 min to engage or disengage the treadmill at the end of each 19-min segment.
Behavioral testing
Animals were placed in a rotometer built in-house that allowed optical encoding of the number of rotations an animal makes in 22.5° increments (Grayhill, LaGrange, IL), while delivering DBS to the STN through a two-channel mercury commutator (Mercotac, Carlsbad, CA). Optical encoder information was digitized (TDT) and total rotations in clockwise and counterclockwise directions per minute were plotted in Matlab. All electrical stimulation was delivered as described previously. Animals were allowed to rotate freely in either direction while the number of rotations was recorded over a period of 8 h either in the presence or the absence of continuous DBS.
Data analysis
Fast Fourier transforms (FFTs) were made to analyze ECoG data in the frequency domain from 0 to 100 Hz. Power spectral densities (PSDs) were estimated from the FFT using 256 Hann-window segments based on the Welch method (NeuroExplorer, Littleton, MA) and normalized by 10 log10 (PSD). Particular attention was focused on data between 20 and 45 Hz (high β-band, low γ-band). The mean power of the ECoG PSD over this range was subtracted from the maximum power over this range to compare the effects of DBS across animals. This operation accounts for variations in signal power from animal to animal and focuses on absolute changes from mean power within each measurement. A one-sample Kolmogorov–Smirnov test (K-S, P < 0.05 to reject) was used to test the null hypothesis that the normalized ECoG power for each comparison could be drawn from a normal distribution. In instances in which data passed the K-S test (i.e., assumption of normality), paired-sample Student's t-tests were used to assess differences in mean power (P < 0.05 to reject). Likewise, one-way ANOVAs on related samples were used with Bonferroni-corrected post hoc tests for repeated measures (P < 0.05 to reject). In instances where the distribution of normalized ECoG power did not pass the K-S test, paired-sample Wilcoxon nonparametric tests were used to test rank values (P < 0.05 to reject). Likewise, Friedman nonparametric ANOVAs on related samples were used with Tukey–Kramer post hoc tests (P < 0.05 to reject). Statistical analyses were made with SPSS (release 17) and/or Matlab using the statistics toolbox. Spectrograms (time-indexed power spectra) were calculated using parameters similar to those of PSD calculations in 10-s bins with 5-s overlap (Chronux shareware running Matlab, http://chronux.org/). All statistical results can be found in Supplemental Tables S1 and S2.
Histology
At the end of the study, each animal was deeply anesthetized with urethane (1,400 mg/kg, ip) and perfused intracardially with 4% paraformaldehyde. After postfixation for 24 h, brains were cryoprotected in a series of graded sucrose washes, cryoembedded, and sectioned at 20-μm thickness. Sections containing the stimulating electrode were stained with cresyl violet (Nissl) and imaged with standard light microscopy to verify electrode location. Sections posterior to the STN containing the substantia nigra (SNc/SNr) were processed for antityrosine hydroxylase (TH) immunohistochemistry. Briefly, sections were hydrated, blocked in 10% normal goat serum (NGS), and incubated in a primary antibody mixture of TH and NeuN (neuronal nuclei; Chemicon, Billerica, MA) overnight at 4°C. Slides were rinsed, incubated in fluorescent secondary antibodies (Invitrogen, Carlsbad, CA) for 1 h at room temperature, and counterstained with Hoescht to label nuclei. Slides were rinsed a final time and coverslipped with ProLong AntiFade reagent. Antibodies were diluted into PBS containing 5% NGS and 0.3% Triton X-100 at 1:100, 1:100, and 1:200 ratios for TH, NeuN, and secondaries, respectively. These slides were then imaged with an Olympus (model BX-51) epifluorescent microscope to visualize unilateral destruction of dopaminergic neurons in the SNc.
Verification of electrode location and 6-OHDA lesion
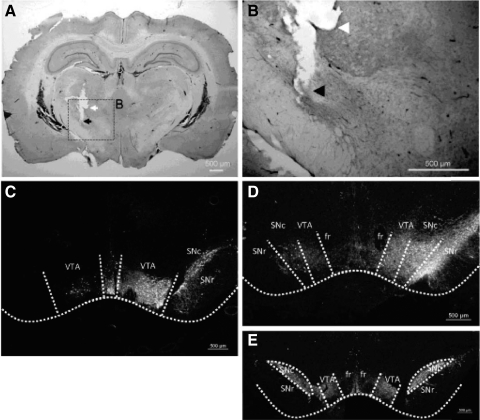
Histological analysis verified that the DBS electrode was located in the STN in seven of nine animals (two normal, five lesioned; Fig. 2A). Only data from these animals were included for subsequent analyses. All lesioned animals that passed the amphetamine/apomorphine challenge (n = 7, including those with misplaced electrodes) showed depletion of TH immunoreactive neurons in the SNc on the animals' right side (ipsilateral, lesioned) with respect to the left (contralateral, nonlesioned), an example of which can be seen in Fig. 2, C and D, demonstrating the success of the 6-OHDA lesion. Control animals that did not receive the 6-OHDA lesion did not demonstrate this deficit (Fig. 2E). In agreement with the immunofluoresence, all lesioned animals passed the amphetamine challenge in the rotometer (Supplemental Fig. S2). Animals rotated ipsilaterally (clockwise) on average per animal ranging from 6 to 12.5 times/min over the course of 90 min.
FIG. 2.
Verification of deep brain stimulation (DBS) electrode location and destruction of dopaminergic neurons. A: DBS electrode relative to the subthalamic nucleus (STN). Coronal section, 50 μm thick, shows the location of the tip of the DBS electrode (cathode, black arrow) relative to the surrounding guide cannula (anode, white arrow) positioned in the STN (STN 8). B: magnified image at left. C and D: immunofluorescence of antityrosine hydroxylase (TH)–reactive neurons from lesioned animals. An absence of TH-reactive neurons can be seen in these 20-μm-thick sections of different locations in the substantia nigra on the 6-OHDA–lesioned side (animal's right) relative to the intact (nonlesioned) contralateral side (C, STN 2, approximately −5.8 bregma; D, STN 6, approximately −4.8 bregma). E: immunofluorescence of TH-reactive neurons from a control (nonlesioned) animal (STN 9, approximately −5.3 bregma). VTA, ventral tegmental area; fr, fasciculus retroflexus; SNc, substantia nigra compact; SNr, substantia nigra reticular.
RESULTS
Electrocorticogram recordings
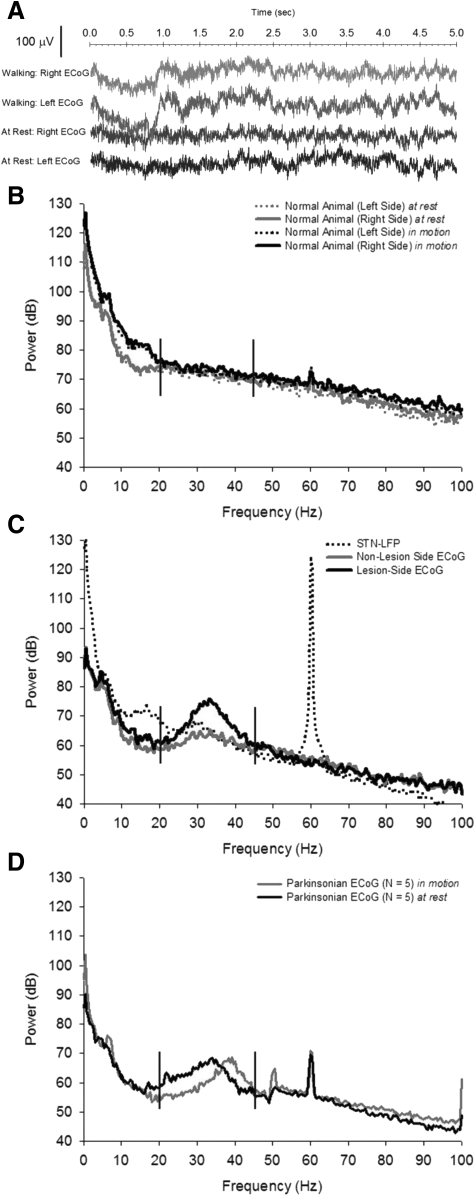
After recovery, animals were placed in a semianechoic chamber that allowed simultaneous recording of ECoG signals and DBS of the STN through a multichannel commutator. Animals were allowed to freely explore their environment during all recording and stimulation sessions. In all subsequent results, the left and right frontal ECoG signals were referenced to the bone screw located over the parietal cortex. The raw ECoG signal from the left and right hemispheres of a single parkinsonian animal can be seen while the animal was walking on a treadmill in Fig. 3A (top two traces) compared with at rest (bottom two traces). The power spectra of the left and right ECoG signals recorded over 5 min were then calculated in the normal animal (Fig. 3B) and the hemiparkinsonian animal (Fig. 3C). As can be seen in Fig. 3C, the right-side (6-OHDA lesioned) ECoG exhibits significantly increased power in the β/γ-band frequencies compared with the contralateral (left-side, nonlesioned) ECoG. The distribution of the maximum power (mean subtracted) between 20 and 45 Hz across all hemiparkinsonian animals, regardless of animal State, exhibited significantly higher power on the 6-OHDA–lesioned ECoG compared with the nonlesioned-side ECoG during sham stimulation (paired t-test, t = −3.34, P = 0.009). The local field potential (LFP) of the STN was recorded through the stimulating electrode and compared with the ECoG over the right-side motor cortex (Fig. 3C). The power in the β-band frequencies of the STN LFP was less than that recorded from the cortical surface and did not exhibit the decrease in power between 10 and 20 Hz observed in the ECoG.
FIG. 3.
Raw ECoG in the temporal and frequency domains. A: sample ECoG recorded from the left (normal, nonlesioned) and right (6-OHDA–lesioned) hemispheres of a single animal while walking on a treadmill (top 2 traces) or at rest (bottom 2 traces). All signals were referenced to a bone screw over the left parietal area and recorded during high-frequency, 50-Hz amplifier blanking (STN 5). B: power spectral density (PSD) from a single normal (nonlesioned) animal. The 2 light traces are the ECoG power while the animal is at rest. The 2 dark traces are the ECoG power while the animal is walking on a treadmill. C: power spectrum from a single hemiparkinsonian animal at rest only. The 6-OHDA lesion was made in the right medial forebrain bundle. Extensive power is seen in the high-β, low-γ bands (demarcated by vertical bars) that is absent in the power spectrum of normal animals. Some abnormal power can be seen in the left (nonlesioned) side recordings. Additionally, the STN local field potential (LFP), recorded through the DBS electrode, is shown. Note that less power is present in these frequencies in the LFP than in the ECoG. All data are representative of 5-min recordings in the awake, unrestrained animal (STN 1, B; STN 6, C). D: a clear, significant shift in parkinsonian ECoG power is seen during locomotion. The mean ECoG power is shown for all 5 parkinsonian animals while at rest (dark trace) and walking on a treadmill (light trace), both during 0-μA, 50-Hz DBS (sham stimulus). A shift is observed in the parkinsonian power from the β band at rest to the low γ band while walking. The slight increase in power at 50 Hz is due to the amplifier “blanking” artifact while sham stimulation was delivered to the animal. The artifact at 60 Hz is attributed to electrical noise.
The abnormal power recorded through the ECoG of parkinsonian animals shifted from the high-β band to the low-γ band as the animals started walking on the treadmill, as can be seen in the power spectra of a single animal (Fig. 3D). The distribution of frequencies that exhibit maximum power in the power spectrum (peak frequencies) while the animal was at rest was normally distributed (n = 380, K-S Z = 1.00, P = 0.268), however, was skewed toward higher frequencies while the animal was walking (n = 380, K-S Z = 3.25, P < 0.001). These distributions were significantly different (Wilcoxon Z = −13.9, P < 0.001; Supplemental Fig. S3). This shift is consistent over time, as can seen in a typical parkinsonian animal's spectrogram (Supplemental Fig. S4).
Deep brain stimulation
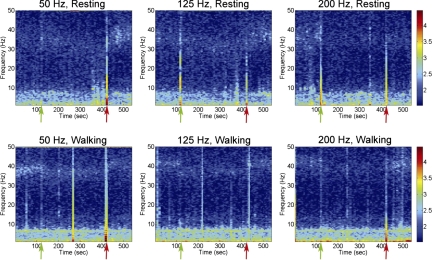
Electrical stimulation parameters were chosen based on rodent studies that reduced or eliminated amphetamine-induced rotations (Maesawa et al. 2004) but were within safe levels of charge (Temel et al. 2005). The change in the β power of the ECoG signal was abrupt following the onset and offset of DBS of the STN, as seen in the spectrograms of Fig. 4. In this figure, the ECoG was recorded for 5 min without electrical stimulation, followed by 5 min of DBS, followed by no stimulation. During the stimulation, β-band power was clearly decreased and depended on the frequency of the DBS, as described in the following text. Once the stimulation was removed, the ECoG power reverted to its prestimulation, high-β (animal at rest) or low-γ (animal walking) power signature.
FIG. 4.
DBS effects parkinsonian power in the ECoG with frequency dependence. Spectrograms of a sample animal were calculated from ECoG recordings made for 2 min prior to 5 min of DBS delivered at 50 μC/cm2, 50 pulses/s (left column), 125 pulses/s (center column), or 200 pulses/s (right column), followed by 2 min of no stimulation while the animal was at rest (top row) or walking on a treadmill (bottom row). Green arrows indicate the start of DBS and red arrows indicate the end of DBS. DBS delivered at 200 Hz caused a greater decrease in abnormal parkinsonian power in the ECoG than 50-Hz DBS for when the animal was at rest and walking. Note that the effect of the DBS on reduction in the parkinsonian power is nearly instantaneous. Data are representative of STN 5, 33 μA/120 μs pulse width. Units are in decibels.
Stimulation parameter sets were varied randomly and the ECoG was recorded for 1 min per set. The power spectrum during each minute of recording was calculated and the mean power of the PSD in frequencies from 20 to 45 Hz (high-β band, low-γ band) for each stimulation parameter was subtracted from the maximum power of the PSD over this range. Data recorded while DBS was turned on in parkinsonian animals were not normally distributed (on: n = 750, K-S Z = 2.91, P < 0.001; off: n = 10, K-S Z = 0.81, P = 0.536). A comparison of ECoG data between DBS on and off, regardless of parameter (e.g., charge, frequency, state, and amplitude), revealed no significant difference (Wilcoxon Z = −1.89, P = 0.059). However, when DBS delivered at maximum charge (i.e., 4 nC) was tested against no DBS, data were significant (on: n = 250, K-S Z = 2.02, P = 0.001; off: n = 10, K-S Z = 0.81, P = 0.536; Wilcoxon Z = −2.70, P = 0.007), in that DBS reduced normalized ECoG oscillation power between 20 and 45 Hz. Data recorded while DBS was turned on in normal animals were likewise not normally distributed (on: n = 300, K-S Z = 1.49, P = 0.024; off: n = 4, K-S Z = 0.4, P = 0.998). A comparison of ECoG data while DBS was turned off with DBS on in normal animals, regardless of parameter (e.g., charge, frequency, state, and amplitude), revealed no significant difference (Wilcoxon Z = −0.730, P = 0.465). To confirm that DBS did not cause an effect on normalized ECoG power in normal animals, data were divided by maximum charge. DBS delivered to normal animals at maximum charge were normally distributed (on: n = 100, K-S Z = 1.21, P = 0.108, off: n = 4, K-S Z = 0.4, P = 0.998) and no significant difference was found (Student's t-test, t = −1.24, P = 0.305). No further testing was performed on normal animal ECoG. ECoG data during DBS in parkinsonian animals at maximum charge were then divided by State (i.e., resting, walking). There was no significant difference in ECoG power while DBS was turned on at maximum charge or turned off while animals were at rest or walking (resting, on: n = 125, K-S Z = 1.04, P = 0.234; off: n = 5, K-S Z = 0.84, P = 0.474; Student's t-test, t = 2.64, P = 0.058; walking, on: n = 125, K-S Z = 1.92, P = 0.001; off: n = 5, K-S Z = 0.45, P = 0.989; Wilcoxon Z = −1.48, P = 0.138). In subsequent analyses, data from both states were combined.
Frequency of stimulation
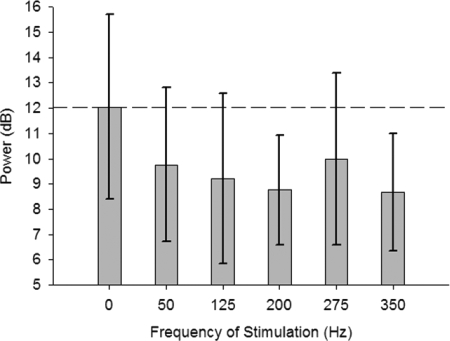
Normalized ECoG data were then divided by the frequency of stimulation (i.e., 0, 50, 125, 200, 275, and 350 Hz) delivered at maximum charge (regardless of State or Amplitude) to assess the effects of Frequency of DBS on cortical oscillations. These data passed the K-S test (see Supplemental Table S1) and a one-way repeated-measures ANOVA was significant [F(5,259) = 3.31, P = 0.007]. Pairwise post hoc comparison of the frequency of stimulation revealed significant decreases from 0 Hz (sham) stimulation in normalized ECoG power for DBS at frequencies of 200 Hz (P = 0.021) and 350 Hz (P = 0.015) after Bonferroni corrections for multiple comparisons (Supplemental Table S2). These results suggest that 200- and 350-Hz DBS resulted in the largest decrease in cortical β/γ oscillations (Figs. 4 and 5).
FIG. 5.
Parkinsonian ECoG power is modulated by the frequency of DBS. Data are the max ECoG power between 20 and 45 Hz (mean power removed) of all animals (mean, SD) regardless of State at maximum charge (i.e., 4 nC). A stimulation frequency of 0 Hz indicates no (sham) stimulation was delivered. Stimulation at 200 and 350 Hz significantly reduced cortical oscillation power in parkinsonian animals.
Charge response
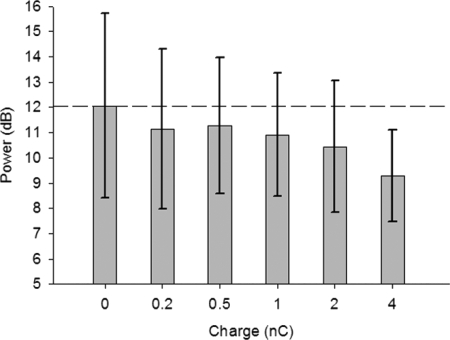
DBS at each frequency was delivered with current amplitude and pulse width combinations that limited the charge per phase to 3.9 nC (50 μC/cm2). Current and pulse width combinations that make less charge were also delivered (i.e., 0.2, 0.5, 1.0, and 2.0 nC). According to Fig. 1, there are more amplitude and pulse width combinations that make approximately 4 nC than there are for 2, 1, 0.5, and 0.2 nC, which can result in very large unequal sample sizes. Unequal sample sizes have implications for the use of the harmonic mean in post hoc tests. Therefore one charge was taken from each animal for each Frequency of DBS and State, resulting in 50 measurements for each charge (one per animal per frequency per state). In cases in which more than one charge of the same value exist (due to amplitude/pulse width combinations), these charges were averaged (i.e., 0 nC, 0 averages; 0.2 nC, 0 averages; 0.5 nC, average of two samples; 1.0 nC, average of three samples; 2.0 nC, average of four samples; and 4.0 nC, average of five samples). These data passed the K-S test (see Supplemental Table S1) and a one-way repeated-measures ANOVA for Charge (i.e., 0, 0.2, 0.5, 1.0, 2.0, and 4.0 nC) was significant [F(5,259) = 4.34, P = 0.001]. Pairwise post hoc comparison of charge of stimulation revealed significant decreases from 0 nC (sham) stimulation in normalized ECoG power only for DBS delivered at a charge of 4 nC (P = 0.039) after Bonferroni corrections for multiple comparisons (Supplemental Table S2). Likewise, the normalized ECoG was significantly reduced by 4 nC DBS compared with 0.2 nC (P = 0.007), 0.5 nC (P = 0.003), and 1.0 nC (P = 0.032) but not for 2.0 nC (P = 0.422). Figure 6 demonstrates the effects of increasing the amount of charge delivered to a maximum of 3.9 nC (regardless of State, Frequency, or Amplitude) on reducing the abnormal power in the ECoG signal. Results of this analysis suggest that 3.9 nC was the least amount of charge in these experiments required to reduce abnormal oscillations.
FIG. 6.
DBS dose response. Data are the maximum power in the ECoG between 20 and 45 Hz (mean power removed) as a function of charge delivered (mean, SD) from all 5 parkinsonian animals, regardless of Frequency and State. For each of the 5 charges shown, the actual charge delivered (current amplitude × pulse width) was rounded to the nearest nanocoulomb and grouped according to Fig. 1. Only 4-nC charge produced significant decreases in cortical oscillation power, suggesting that this is the minimum amount of charge needed to observe this effect in this study.
Charge versus amplitude
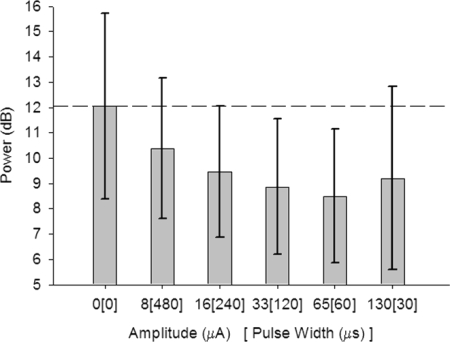
There are a number of current amplitude and pulse width combinations that deliver 3.9 nC charge (50 μC/cm2). We then determined whether it was the current or the maximum charge that was important for reducing abnormal parkinsonian oscillation power by testing current amplitude at maximum charge (i.e., 3.9 nC: 0 μA, 8 μA/480 μs, 16 μA/240 μs, 33 μA/120 μs, 65 μA/60 μs, and 130 μA/30 μs). Normalized ECoG data divided by Amplitude at maximum charge were not normally distributed for the combination of 33 μA/120 μs (n = 50, K-S Z = 1.41, P = 0.039). Therefore the Friedman nonparametric ANOVA on related samples was created and found to be significant (χ2 = 13.83, P = 0.017). Tukey–Kramer pairwise post hoc comparison of the amplitude of stimulation revealed significant decreases from 0 μA (sham) stimulation in normalized ECoG power for DBS delivered at 33 μA/120 μs (P = 0.021) and 65 μA/60 μs (P = 0.007) (Supplemental Table S2). Likewise, the normalized ECoG was significantly reduced by 65 μA/60 μs DBS compared with 8 μA/480 μs (P = 0.017). Figure 7 shows the parkinsonian data separated by Amplitude at maximum charge (i.e., 3.9 nC) regardless of Frequency or State. The results of this analysis suggest that DBS at 33 μA/120 μs or 65 μA/60 μs is more effective at reducing abnormal cortical oscillations rather than by the charge alone.
FIG. 7.
DBS current amplitude vs. maximum Charge. Six different DBS current amplitude and pulse width combinations (including 0 μA, sham stimulation) were delivered to the animals to limit the charge at the electrode to 3.9 nC/phase (50 μC/cm2). Data are the maximum power in the ECoG between 20 and 45 Hz (mean power removed) as a function of current amplitude/pulse width delivered (mean, SD) from all 5 parkinsonian animals regardless of Frequency and State. These results suggest that DBS at either 33 μA/120 μs or 65 μA/60 μs is more effective at reducing abnormal cortical oscillations rather than by the charge alone.
Behavior
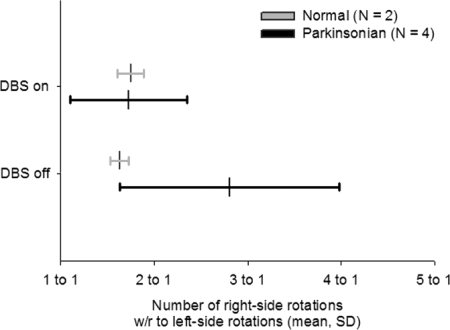
Animals that received 6-OHDA lesions tend to favor ambulation ipsilateral to the lesion in the absence of amphetamine or apomorphine. Animals were placed in the rotometer for 8-h sessions in which DBS was continuously delivered either at 200 Hz, 130 μA, 30 μs/phase, or 350 Hz, 65 μA, 60 μs/phase. No stimulation sessions were recorded as well (Fig. 8). The rotational behavior of animals was normally distributed (Supplemental Table S1). However, the sample size was very small for the behavioral data set, which has implications for the validity of applying Student's t-test on these data. Therefore Wilcoxon nonparametric tests on related paired samples were performed for all behavioral data. DBS delivered to normal control animals had no effect on the number of rotations the animals made in the rotometer compared with that for no stimulation (Wilcoxon Z = −0.69, P = 0.533). Unstimulated parkinsonian animals rotated on average close to a ratio of 3:1 ipsilateral to contralateral rotations. Stimulated parkinsonian animals made fewer ipsilateral rotations than nonstimulated parkinsonian animals (Wilcoxon Z = 1.95, P = 0.049); this was a significant reduction in ipsilateral rotations from no stimulation. Only four of five parkinsonian animals were tested because one animal (STN8) died prior to testing. Whereas the P values of nonparametric tests tend to have low power for small sample sizes, these results suggest that DBS at 3.9 nC (50 μC/cm2) is sufficient to reduce ipsilateral turning, a behavioral symptom of parkinsonism in the hemiparkinsonian rat.
FIG. 8.
Rotational behavior is reduced with DBS. Data represent the ratio of the number of rotations to the lesion side (right side) with respect to the nonlesion side (left side) rotations over the course of one 8-h period for each animal (mean, SD). DBS did not have an effect on normal control animals (light gray, n = 2). DBS significantly decreased the ipsilateral turning behavior in parkinsonian animals (dark gray, n = 4; one lesion animal, STN 8, died before rotational behavior could be collected). These data suggest that DBS that maximally reduces abnormal cortical oscillations also reduced the rotational behavioral symptoms induced by the 6-OHDA lesion.
DISCUSSION
Abnormal parkinsonian oscillations
The concept of measuring β oscillation activity as an indirect indicator of the neural parkinsonian symptoms has been previously proposed (Sharott et al. 2005). It has been demonstrated that the administration of apomorphine, a classic dopamine agonist that has been used as a clinical treatment for PD, can reduce the β power measured in the EcoG over the affected side (Vorobyov et al. 2003). Despite reduction in β-oscillation power, rats given apomorphine would continue to rotate away from the lesioned side. In the current study, we applied DBS to the STN in an effort to mimic the effects of dopamine agonist administration on β oscillations and animal behavior. In response to DBS, the rats did exhibit a significant decrease in β-oscillation activity similar to an administration of apomorphine. There were some important differences, however. First, DBS stimulation seemed to consistently extinguish the β-power peak within a few seconds of activation, whereas apomorphine took longer and lasted for variable extended periods. Second, the β-power peak returned shortly after turning off the stimulator. This abrupt change in the ECoG power is similar to quick changes (milliseconds to minutes) observed in human parkinsonian tremor symptoms following the activation and adjustment of STN DBS (Rodriguez-Oroz et al. 2001). In humans, tremor has been correlated with an increase in γ oscillations (Weinberger et al. 2009), but not β oscillations (Kühn et al. 2009). It is not clear how PD tremor symptoms in humans translate to the parkinsonian rat. Likewise, it is unknown how the changes in oscillatory power are affected by stimulation over longer periods of time (i.e., hours, days, etc.) that affect other parkinsonian symptoms such as akinesia, bradykinesia, and rigidity. Indeed the return of physical parkinsonian symptoms such as akinesia, bradykinesia, and rigidity following long-term DBS has been shown to take hours (Temperli et al. 2003).
Recently, magnetoencephalography in humans has demonstrated increased cortico-cortical coupling of β-band activity in patients who demonstrate ≲40% improvement following dopaminergic replacement therapy (Stoffers et al. 2008). Certainly, in rat models of PD, 6-OHDA lesion-related β oscillations have been observed in the STN in single units, ensembles, and LFPs that manifest over the course of about 4 days (Mallet et al. 2008). Furthermore, these oscillations are profoundly affected by dopaminergic therapy (Sharott et al. 2005). Perhaps then, the 6-OHDA–lesioned rat model of PD could be a surrogate representative of late-stage Parkinsonism or, possibly, represents models of PD symptoms responsive to dopaminergic therapy. Indeed, a predictive indicator of the responsiveness of STN DBS in humans is their response to dopaminergic therapy (Pinter et al. 1999; Welter et al. 2002). Despite mounting evidence that links cortico-cortical and cortico-pallidal oscillations to the severity of Parkinson's, the extent to which the power of parkinsonian oscillations reflects underlying parkinsonian symptoms remains to be seen (Brown 2003, 2006).
Deep brain stimulation of the subthalamic nucleus in the hemiparkinsonian rat
Previous studies that examined the behavioral effects of DBS of the STN in the rat model of PD found that stimulation at high amplitudes either reduced or stopped ipsilateral rotations during amphetamine (Maesawa et al. 2004) or apomorphine (Darbaky et al. 2003) challenge or caused contralateral rotations (Meissner et al. 2002). STN DBS in animals acutely treated with a dopamine receptor antagonist to induce catalepsy improved the level of activity and significantly improved akinesia (Dejean et al. 2009). When stimulating through microelectrodes that approach the size of the STN of rodents, it is crucial to control the amount of charge delivered, given the surface area, type of metal, and impedance of the electrode so that stimulating potentials are within the “water window” (i.e., the voltage at which hydrolysis of water into hydrogen and oxygen occurs), thereby preventing irreversible faradaic reactions at the electrode–tissue interface (Merrill et al. 2005) and/or charge–charge densities that exceed safe levels of electrical stimulation (Shannon 1992). Irreversible faradaic reactions at the electrode–tissue interface have been shown to have devastating effects on the electrode material and tissue (Harnack et al. 2004; McCreery et al. 1990; Temel et al. 2004). The absence of our observation of contralateral rotations during DBS could stem from the relatively low charge and high charge density used in this study. In this report, both charge and charge density were kept within the charge capacity of stainless steel and the theoretical limits of safe charge injection. Stimulation that elicited dyskinesia was not performed. Additional safety measures were in place to limit the voltage at the electrode to stay within ±1 V, the approximate water window for stainless steel.
Neuromodulation of β oscillations and motor symptoms with deep brain stimulation
There is much evidence to suggest that STN DBS affects β oscillations. Low-frequency STN stimulation (<10 Hz) in humans has demonstrated evoked potentials recorded from the scalp surface (MacKinnon et al. 2005). Likewise, high-frequency stimulation of the rat STN elicits antidromic activation of cortex (Li et al. 2007). Low-frequency stimulation (20 Hz) in the range of β oscillations has been shown to impair motor performance in humans, suggesting that excessive synchronization between the basal ganglia and cortex contributes to bradykinesia (Chen et al. 2007). Additionally, 20-Hz stimulation increases abnormal synchronization that is reduced with higher-frequency stimulation (Brown et al. 2004). Yet, there is evidence in humans from a study that used simultaneous LFP recordings through unused electrodes during STN DBS that showed little change in β power during clinically effective DBS (Rossi et al. 2007), challenging the relevance of β oscillations with respect to improvements in motor symptoms observed with efficacious DBS. The animal data presented here demonstrate robust high-power oscillations between 20 and 45 Hz present in the ECoG recorded over motor cortex. Furthermore, the power of these oscillations can be modulated by HFS of the STN that corresponds to a decrease in parkinsonian rotational behavior. The hemiparkinsonian rat model of STN DBS may provide insight into the relationship between abnormal oscillations and parkinsonian symptoms.
Current amplitude versus charge and charge density
The total charge in the extracellular environment delivered through electrical stimulation has been described as being interrelated between charge per phase (interpreted as the total volume within which neurons are excited) and the charge density (interpreted as the proportion of neurons close to an electrode that are excited) (McCreery et al. 1990). Therefore it would be desirable to maximize charge density (within the limits of the charge capacity of the electrode material) for a given charge. The limitation of the DBS electrodes in these experiments is the charge capacity of stainless steel relative to the geometric surface area of the electrode (50 μC/cm2 based on ∼7,850 μm2). Although 3.9 nC was enough charge to reduce the abnormal β oscillations, these parameters (current amplitude and pulse width) limited the amount of charge to 3.9 nC/phase and do not demonstrate the limitations of the amount of charge that can be delivered. Indeed, Dejean and colleagues (2009) demonstrated that the threshold for eliciting an evoked response in frontal cortex of rat during STN DBS was about 82 μA (at a chronaxie of 40 to 75 μs). Although the charge delivered in this study is within the range of the Dejean study, it is possible that delivering >3.9 nC would improve on the reduction of the oscillations. The results presented here could be further analyzed with improvements in electrode technologies, to identify how increasing amounts of charge delivered to the STN affects parkinsonian oscillations and animal behavior. Furthermore, there are a number of current amplitude and pulse width combinations that deliver any given amount of charge. Our data demonstrate that current amplitude itself, as proposed in other studies (Desbonnet et al. 2004; Temel et al. 2005), is not a determining factor in the stimulation-evoked decrease in the power of β oscillations; rather, this effect can be attributed to an interrelationship with current amplitude and pulse width.
Oscillations at rest versus during locomotion
In resting animals, the peak power in the abnormal parkinsonian oscillations was observed in the high-β band; this power peak shifted to a narrow range of frequencies in the lower-γ band when the animals were walking at a constant rate, as previously demonstrated (Sharott et al. 2005). This shift in the power may be indicative of a need to alter the parameters of DBS depending on the state of the animal. Cortical EEG and local field potentials recorded from the STN in humans have demonstrated coherence between other frequency bands such as θ (3–7 Hz), α (8–13 Hz), and low β (14–20 Hz), suggesting that several functional loops are affected by PD (Fogelson et al. 2006). Additionally, γ-band energy (65–90 Hz), although significantly lower powered than β-band energy (14–35 Hz), was found to be significantly increased from rest during a finger-tapping task in both LFP recordings from STN and mesial EEG, whereas no change in power was observed in the β-band energy (Lalo et al. 2008). Our data did not identify a significant effect between animal states. This may be a limitation of the rat model of DBS for parkinsonism, possibly underlining the differences between human and rodent parkinsonian symptoms. These data did identify a significant effect of 200- and 350-Hz DBS for reducing the abnormal cortical oscillations, demonstrating the potential utility of the model. Indeed, there is strong indication that interregional EEG coherence changes in the β range during high-frequency DBS are indicative of the severity of parkinsonian symptoms in human subjects at rest (Silberstein et al. 2005) and that LFP recordings through the stimulating contact in the STN that produced the best clinical effect also had the highest coherence with midline EEG oscillations (Marsden et al. 2001). DBS in the hemiparkinsonian rat model may provide the means to rapidly assess new stimulation therapies to inform DBS treatment of the symptoms of human PD.
In conclusion, the hemiparkinsonian rat model of STN DBS may prove to be a valuable surrogate for human manifestations of PD. Although there are clear differences between rodent parkinsonism and human symptoms of PD, the rat model could be useful for determining the neural mechanisms of parkinsonism and possibly help to inform future treatment of human PD with DBS. Future research will be needed to determine the stability, dynamic range, and long-term effects of DBS on cortical oscillations.
GRANTS
This work was supported in part by National Institutes of Health Grants F32 DC-7797 to M. J. Lehmkuhle and P41 EB-002030 to D. R. Kipke. Special thanks to Henry Ford Hospital, Department of Neurosurgery, Detroit for providing support to S. S. Bhangoo.
Supplementary Material
Acknowledgments
We thank A. R. Harris, T. C. Marzullo, H. Parikh, and N. Langhals for assistance with the mechanics of the experiments and E. K. Purcell for help with the immunohistochemistry.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Alonso-Frech et al. 2006.Alonso-Frech F, Zamarbide I, Alegre M, Rodriguez-Oroz MC, Guridi J, Manrique M, Valencia M, Artieda J, Obeso JA. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson's disease. Brain 129: 1748–1757, 2006. [DOI] [PubMed] [Google Scholar]
- Bronte-Stewart et al. 2009.Bronte-Stewart H, Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN beta-band profile in Parkinson's disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol 215: 20–28, 2009. [DOI] [PubMed] [Google Scholar]
- Brown et al. 2001.Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci 21: 1033–1038, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown and Williams 2005.Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol 116: 2510–2519, 2005. [DOI] [PubMed] [Google Scholar]
- Butson et al. 2007.Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. NeuroImage 34: 661–670, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet et al. 1992.Cadet JL, Zhu SM, Angulo JA. Quantitative in situ hybridization evidence for differential regulation of proenkephalin and dopamine D2 receptor mRNA levels in the rat striatum: effects of unilateral intrastriatal injections of 6-hydroxydopamine. Brain Res Mol Brain Res 12: 59–67, 1992. [DOI] [PubMed] [Google Scholar]
- Cassidy et al. 2002.Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain 125: 1235–1246, 2002. [DOI] [PubMed] [Google Scholar]
- Darbaky et al. 2003.Darbaky Y, Forni C, Amalric M, Baunez C. High frequency stimulation of the subthalamic nucleus has beneficial antiparkinsonian effects on motor functions in rats, but less efficiency in a choice reaction time task. Eur J Neurosci 18: 951–956, 2003. [DOI] [PubMed] [Google Scholar]
- Dejean et al. 2009.Dejean C, Hyland B, Arbuthnott G. Cortical effects of subthalamic stimulation correlate with behavioral recovery from dopamine antagonist induced akinesia. Cereb Cortex 19: 1055–1063, 2009. [DOI] [PubMed] [Google Scholar]
- Desbonnet et al. 2004.Desbonnet L, Temel Y, Visser-Vandewalle V, Blokland A, Hornikx V, Steinbusch HW. Premature responding following bilateral stimulation of the rat subthalamic nucleus is amplitude and frequency dependent. Brain Res 1008: 198–204, 2004. [DOI] [PubMed] [Google Scholar]
- Doyle et al. 2005.Doyle LM, Kühn AA, Hariz M, Kupsch A, Schneider GH, Brown P. Levodopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson's disease. Eur J Neurosci 21: 1403–1412, 2005. [DOI] [PubMed] [Google Scholar]
- Foffani et al. 2006.Foffani G, Ardolino G, Egidi M, Caputo E, Bossi B, Priori A. Subthalamic oscillatory activities at beta or higher frequency do not change after high-frequency DBS in Parkinson's disease. Brain Res Bull 69: 123–130, 2006. [DOI] [PubMed] [Google Scholar]
- Fogelson et al. 2006.Fogelson N, Williams D, Tijssen M, van Bruggen G, Speelman H, Brown P. Different functional loops between cerebral cortex and the subthalmic area in Parkinson's disease. Cereb Cortex 16: 64–75, 2006. [DOI] [PubMed] [Google Scholar]
- Hammond et al. 2007.Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci 30: 357–364, 2007. [DOI] [PubMed] [Google Scholar]
- Harnack et al. 2004.Harnack D, Winter C, Meissner W, Reum T, Kupsch A, Morgenstern R. The effects of electrode material, charge density and stimulation duration on the safety of high-frequency stimulation of the subthalamic nucleus in rats. J Neurosci Methods 138: 207–216, 2004. [DOI] [PubMed] [Google Scholar]
- Kühn et al. 2008.Kühn AA, Kempf F, Brücke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, Vandenberghe W, Nuttin B, Brown P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci 28: 6165–6173, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn et al. 2006.Kühn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur J Neurosci 23: 1956–1960, 2006. [DOI] [PubMed] [Google Scholar]
- Kühn et al. 2005.Kühn AA, Trottenberg T, Kivi A, Kupsch A, Schneider GH, Brown P. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson's disease. Exp Neurol 194: 212–220, 2005. [DOI] [PubMed] [Google Scholar]
- Kühn et al. 2009.Kühn AA, Tsui A, Aziz T, Ray N, Brücke C, Kupsch A, Schneider GH, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp Neurol 215: 380–387, 2009. [DOI] [PubMed] [Google Scholar]
- Lalo et al. 2008.Lalo E, Thobois S, Sharott A, Polo G, Mertens P, Pogosyan A, Brown P. Patterns of bidirectional communication between cortex and basal ganglia during movement in patients with Parkinson disease. J Neurosci 28: 3008–3016, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy et al. 2002.Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain 125: 1196–1209, 2002. [DOI] [PubMed] [Google Scholar]
- MacKinnon et al. 2005.MacKinnon CD, Webb RM, Silberstein P, Tisch S, Asselman P, Limousin P, Rothwell JC. Stimulation through electrodes implanted near the subthalamic nucleus activates projections to motor areas of cerebral cortex in patients with Parkinson's disease. Eur J Neurosci 21: 1394–1402, 2005. [DOI] [PubMed] [Google Scholar]
- Maesawa et al. 2004.Maesawa S, Kaneoke Y, Kajita Y, Usui N, Misawa N, Nakayama A, Yoshida J. Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. J Neurosurg 100: 679–687, 2004. [DOI] [PubMed] [Google Scholar]
- Mallet et al. 2008.Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci 28: 4795–4806, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden et al. 2001.Marsden JF, Limousin-Dowsey P, Ashby P, Pollak P, Brown P. Subthalamic nucleus, sensorimotor cortex and muscle interrelationships in Parkinson's disease. Brain 124: 378–388, 2001. [DOI] [PubMed] [Google Scholar]
- McCreery et al. 1990.McCreery DB, Agnew WF, Yuen TG, Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans Biomed Eng 37: 996–1001, 1990. [DOI] [PubMed] [Google Scholar]
- Meissner et al. 2002.Meissner W, Harnack D, Paul G, Reum T, Sohr R, Morgenstern R, Kupsch A. Deep brain stimulation of subthalamic neurons increases striatal dopamine metabolism and induces contralateral circling in freely moving 6-hydroxydopamine-lesioned rats. Neurosci Lett 328: 105–108, 2002. [DOI] [PubMed] [Google Scholar]
- Merrill et al. 2005.Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods 141: 171–198, 2005. [DOI] [PubMed] [Google Scholar]
- Obeso et al. 2001.Obeso JA, Olanow CW, Rodriguez-Oroz MC, Krack P, Kumar R, Lang AE. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. New Engl J Med 345: 956–963, 2001. [DOI] [PubMed] [Google Scholar]
- Paxinos and Watson 1998.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, 1998.
- Perese et al. 1989.Perese DA, Ulman J, Viola J, Ewing SE, Bankiewicz KS. A 6-hydroxydopamine-induced selective parkinsonian rat model. Brain Res 494: 285–293, 1989. [DOI] [PubMed] [Google Scholar]
- Pinter et al. 1999.Pinter MM, Alesch F, Murg M, Helscher RJ, Binder H. Apomorphine test: a predictor for motor responsiveness to deep brain stimulation of the subthalamic nucleus. J Neurol 246: 907–913, 1999. [DOI] [PubMed] [Google Scholar]
- Priori et al. 2004.Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, Locatelli M, Moxon KA, Villani RM. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson's disease. Exp Neurol 189: 369–379, 2004. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz et al. 2001.Rodriguez-Oroz MC, Rodriguez M, Guridi J, Mewes K, Chockkman V, Vitek J, DeLong MR, Obeso JA. The subthalamic nucleus in Parkinson's disease: somatotopic organization and physiological characteristics. Brain 124: 1777–1790, 2001. [DOI] [PubMed] [Google Scholar]
- Rossi et al. 2008.Rossi L, Marceglia S, Foffani G, Cogiamanian F, Tamma F, Rampini P, Barbieri S, Bracchi F, Priori A. Subthalamic local field potential oscillations during ongoing deep brain stimulation in Parkinson's disease. Brain Res Bull 76: 512–521, 2008. [DOI] [PubMed] [Google Scholar]
- Schwarting and Huston 1996.Schwarting RK, Huston JP. Unilateral 6-hydroxydopamine lesions of meso-striatal dopamine neurons and their physiological sequelae. Prog Neurobiol 49: 215–266, 1996. [DOI] [PubMed] [Google Scholar]
- Shannon 1992.Shannon RV. A model of safe levels for electrical stimulation. IEEE Trans Biomed Eng 39: 424–426, 1992. [DOI] [PubMed] [Google Scholar]
- Sharott et al. 2005.Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci 21: 1413–1422, 2005. [DOI] [PubMed] [Google Scholar]
- Silberstein et al. 2005.Silberstein P, Pogosyan A, Kühn AA, Hotton G, Tisch S, Kupsch A, Dowsey-Limousin P, Hariz MI, Brown P. Cortico-cortical coupling in Parkinson's disease and its modulation by therapy. Brain 128: 1277–1291, 2005. [DOI] [PubMed] [Google Scholar]
- Stoffers et al. 2008.Stoffers D, Bosboom JL, Wolters E, Stam CJ, Berendse HW. Dopaminergic modulation of cortico-cortical functional connectivity in Parkinson's disease: an MEG study. Exp Neurol 213: 191–195, 2008. [DOI] [PubMed] [Google Scholar]
- Temel et al. 2005.Temel Y, Visser-Vandewalle V, Aendekerk B, Rutten B, Tan S, Scholtissen B, Schmitz C, Blokland A, Steinbusch HW. Acute and separate modulation of motor and cognitive performance in parkinsonian rats by bilateral stimulation of the subthalamic nucleus. Exp Neurol 193: 43–52, 2005. [DOI] [PubMed] [Google Scholar]
- Temel et al. 2004.Temel Y, Visser-Vandewalle V, van der Wolf M, Spincemaille GH, Desbonnet L, Hoogland G, Steinbusch HW. Monopolar versus bipolar high frequency stimulation in the rat subthalamic nucleus: differences in histological damage. Neurosci Lett 367: 92–96, 2004. [DOI] [PubMed] [Google Scholar]
- Temperli et al. 2003.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 60: 78–81, 2003. [DOI] [PubMed] [Google Scholar]
- Trottenberg et al. 2007.Trottenberg T, Kupsch A, Schneider GH, Brown P, Kühn AA. Frequency-dependent distribution of local field potential activity within the subthalamic nucleus in Parkinson's disease. Exp Neurol 205: 287–291, 2007. [DOI] [PubMed] [Google Scholar]
- Ungerstedt and Arbuthnott 1970.Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res 24: 485–493, 1970. [DOI] [PubMed] [Google Scholar]
- Vorobyov et al. 2003.Vorobyov VV, Schibaev NV, Morelli M, Carta AR. EEG modifications in the cortex and striatum after dopaminergic priming in the 6-hydroxydopamine rat model of Parkinson's disease. Brain Res 972: 177–185, 2003. [DOI] [PubMed] [Google Scholar]
- Weinberger et al. 2009.Weinberger M, Hutchison WD, Lozano AM, Hodaie M, Dostrovsky JO. Increased gamma oscillatory activity in the subthalamic nucleus during tremor in Parkinson's disease patients. J Neurophysiol 101: 789–802, 2009. [DOI] [PubMed] [Google Scholar]
- Weinberger et al. 2006.Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J Neurophysiol 96: 3248–3256, 2006. [DOI] [PubMed] [Google Scholar]
- Welter et al. 2002.Welter ML, Houeto JL, Tezenas du Montcel S, Mesnage V, Bonnet AM, Pillon B, Arnulf I, Pidoux B, Dormont D, Cornu P, Agid Y. Clinical predictive factors of subthalamic stimulation in Parkinson's disease. Brain 125: 575–583, 2002. [DOI] [PubMed] [Google Scholar]
- Williams et al. 2002.Williams D, Tijssen M, Van Bruggen G, Bosch A, Insola A, Di Lazzaro V, Mazzone P, Oliviero A, Quartarone A, Speelman H, Brown P. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain 125: 1558–1569, 2002. [DOI] [PubMed] [Google Scholar]
- Wingeier et al. 2006.Wingeier B, Tcheng T, Koop MM, Hill BC, Heit G, Bronte-Stewart HM. Intra-operative STN DBS attenuates the prominent beta rhythm in the STN in Parkinson's disease. Exp Neurol 197: 244–251, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.