Abstract
Neural coupling of proximal and distal upper limb segments may have functional implications in the recovery of hemiparesis after stroke. The goal of the present study was to investigate whether the stretch reflex response magnitude of spastic finger flexor muscles poststroke is influenced by sensory input from the shoulder and the elbow and whether reflex coupling of muscles throughout the upper limb is altered in spastic stroke survivors. Through imposed extension of the metacarpophalangeal (MCP) joints, stretch of the relaxed finger flexors of the four fingers was imposed in 10 relaxed stroke subjects under different conditions of proximal sensory input, namely static arm posture (3 different shoulder/elbow postures) and electrical stimulation (surface stimulation of biceps brachii or triceps brachii, or none). Fast (300°/s) imposed stretch elicited stretch reflex flexion torque at the MCP joints and reflex electromyographic (EMG) activity in flexor digitorum superficialis. Both measures were greatest in an arm posture of 90° of elbow flexion and neutral shoulder position. Biceps stimulation resulted in greater MCP stretch reflex flexion torque. Fast imposed stretch also elicited reflex EMG activity in nonstretched heteronymous upper limb muscles, both proximal and distal. These results suggest that in the spastic hemiparetic upper limb poststroke, sensorimotor coupling of proximal and distal upper limb segments is involved in both the increased stretch reflex response of the finger flexors and an increased reflex coupling of heteronymous muscles. Both phenomena may be mediated through changes poststroke in the spinal reflex circuits and/or in the descending influence of supraspinal pathways.
INTRODUCTION
There is evidence for coupling of the proximal and distal segments of the upper limb, both via biomechanical and neurological mechanisms. For example, the activation of multiarticular muscles produces moments at multiple joints (Murray et al. 1995), effectively coupling joint movements in specific patterns (Graham et al. 2003). Heteronymous reflexes, such as the reflex feedback from Ia afferents of wrist muscles onto elbow muscles (Cavallari and Katz 1989; Mazevet and Pierrot-Deseilligny 1994), could also couple the activation of specific muscle groups during movement. This coupling within the upper limb may be of particular importance in the context of motor impairment of the upper limb after stroke. Specifically, it is possible that distal motor activity poststroke can be influenced by proximal sensory input. Further, the neural coupling of upper limb muscles may be altered after stroke due to neural damage or as a result of adaptation to the damage, thereby promoting the emergence of abnormal patterns of muscle activity.
The stretch reflex is commonly used as a paradigm for assessing spasticity following stroke (Kamper and Rymer 2000; Katz and Rymer 1989; Rymer and Katz 1994; Schmit et al. 1999). During these assessments, a stretch reflex perturbation, consisting of stretching a muscle, is applied to a single joint, and the response is measured at that same joint. While these measurements provide valuable information about stretch reflex activity, activation of nonstretched muscles at other joints and, conversely, the influence of proprioceptive feedback from muscles at other joints, have not been extensively studied. Heteronymous coupling of this type could have important implications in the coordination of movement poststroke. For example, Musampa et al. (2007) observed that the stretch reflex thresholds of single- and double-joint elbow flexor and extensor muscles (brachioradialis, biceps brachii, anconeus, triceps brachii) in spastic hemiparetic stroke subjects were influenced by static shoulder angle during quasi-static imposed elbow flexion and extension and during voluntary elbow flexion and extension. If the neural circuits that mediate heteronymous coupling of upper limb muscles are altered after stroke, a heightened or abnormal reflex coupling of muscles at different joints may occur. Such abnormal reflex coupling has recently been shown in the spastic upper limb poststroke (Sangani et al. 2007). Specifically, imposed extensions at the elbow elicit reflex torque responses at the shoulder that are produced through neural, rather than biomechanical, coupling. Thus it appears that the reflex response to stretch is not necessarily limited to the muscle being stretched. Abnormal reflex activity in heteronymous muscles after stroke has also been observed in the lower limb, where the activity in the uniarticular knee extensor quadriceps femoris is influenced by imposed stretch of the hip flexors (Lewek et al. 2007) and by electrical stimulation of the common peroneal nerve (Marque et al. 2001; Maupas et al. 2004).
The aim of the present study was to investigate, in spastic hemiparetic stroke subjects, the effect of sensory input from the shoulder and the elbow on the magnitude of the stretch reflex response of the relaxed finger flexor muscles and on the EMG activity in relaxed muscles throughout the upper limb in response to imposed extension of the metacarpophalangeal (MCP) joints. For this purpose, MCP extension was imposed under different conditions of static and dynamic proprioceptive input from the proximal part of the upper limb: namely, three different arm postures were tested with or without electrical stimulation applied to the skin over either the biceps brachii (BB) or the triceps brachii (TB). We postulated that modifying the sensory feedback from proximal joints would significantly change the magnitude of the stretch reflex response of the relaxed finger flexors poststroke. Further, we anticipated that heteronymous reflex coupling between the finger flexors and proximal upper limb muscles would produce, in the relaxed upper limb of stroke subjects, reflex activation of muscles that do not cross the MCP joints.
METHODS
Subjects
Ten stroke survivors (6 men and 4 women) volunteered to participate in the present study. Each subject was ≥11 mo postincident (range, 11–242 mo) and exhibited chronic unilateral motor deficits. Upper limb function was evaluated with the Fugl-Meyer Assessment of Sensorimotor Recovery After Stroke: upper extremity motor scores ranged from 12 to 52 out of a maximum score of 66. Subject ages ranged from 34 to 70 yr (mean, 56 yr). The paretic upper limb was investigated in the present study. Four of the 10 subjects had right hemiparesis and 6 had left hemiparesis (see Table 1 for demographic and clinical data).
TABLE 1.
Demographic and clinical data for the stroke subjects participating in the present study
| Initials | Sex | Age | Time after stroke, mo | Side | Fugl-Meyer |
|---|---|---|---|---|---|
| S1 | F | 53 | 232 | L | 15 |
| S2 | M | 61 | 34 | L | NA |
| S3 | F | 53 | 242 | R | 17 |
| S4 | F | 48 | 11 | L | 27 |
| S5 | M | 66 | 162 | L | 31 |
| S6 | M | 70 | 212 | R | 23 |
| S7 | M | 60 | 44 | L | 18 |
| S8 | M | 51 | 36 | R | 52 |
| S9 | F | 34 | 90 | R | 12 |
| S10 | M | 61 | 42 | L | 31 |
The time at which the experiment was conducted with respect to the occurrence of the subject's stroke (“Time after stroke”) is indicated in months. “Side” indicates whether the subject had right (“R”) or left (“L”) hemiparesis and thus which upper limb was studied. “Fugl-Meyer” indicates the subject's Fugl-Meyer upper extremity motor score, out of a maximum score of 66. The Fugl-Meyer score was not available (“NA”) for subject S2.
All subjects gave informed consent according to the Helsinki Declaration, and the experimental protocol was approved by the Institutional Review Board of Northwestern University.
Protocol
The impact of proximal sensory input on distal muscles was assessed by measuring the magnitude of the stretch reflex response to imposed extension of the MCP joints. The MCP joints of the four fingers were extended simultaneously by means of a servomotor (1.4 HP, PMI Motion Technologies, Kollmorgen, Radford, VA) fit into an experimental table next to which the subjects were seated. The fingers were coupled to the shaft of the motor such that rotation of the shaft produced equivalent rotation of the MCP joints (Kamper and Rymer 2000). A fiberglass cast was placed around the subjects' forearm and wrist to maintain the wrist in neutral position with respect to the forearm as well as to keep the thumb extended and abducted from the palm. To prevent arm translation, the cast was clamped within a testing jig that rested on the surface of the experimental table with the MCP joints aligned with the axis of the motor. Alignment was verified by the absence of wrist translation during manual rotation of the shaft. Clamping the cast within the testing jig ensured that the hand was supported and stabilized without any need for voluntary muscle activity by the subjects. The imposed extension of the MCP joints was scaled to each subject's individual passive range of motion at the MCP joints. Specifically, the maximum passive MCP flexion angle and the maximum passive MCP extension angle with the hand coupled to the shaft were determined, and the middle 75% of this passive range of motion was used by the motor for the imposed extension of the MCP joints (Kamper and Rymer 2000).
Experimental trials consisted of stretch of the finger flexor muscles by imposing constant velocity rotation of the MCP joints from the flexion limit to the extension limit of the middle 75% of the determined passive range of motion. The MCP joints were held at the extension limit for 2 s and were then rotated back to the flexion limit. The mean range of imposed extension of the MCP joints across the 10 stroke subjects was 60° (from 27° of MCP flexion to 33° of MCP extension, 0° of MCP flexion/extension corresponding to the metacarpal bones of the four fingers being aligned with the carpus). Two different constant velocities were used for the rotation of the MCP joints: 10°/s (“slow stretch”), expected not to elicit a reflex response, and 300°/s (“fast stretch”), expected to elicit a reflex response (Kamper and Rymer 2000). A single stretch was generated per experimental trial.
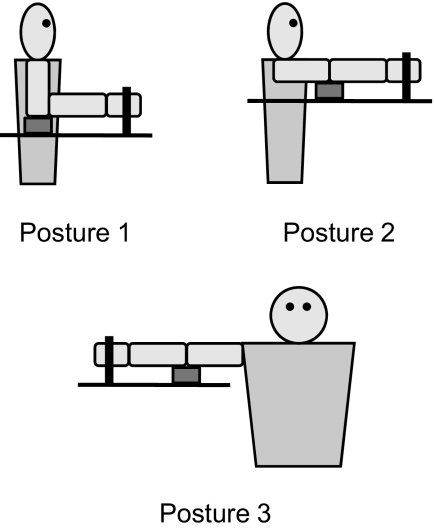
To investigate the effect of static heteronymous proprioceptive input from the shoulder and the elbow, experimental trials were performed in three different arm postures, corresponding to three different combinations of shoulder and elbow angles. The postures were as follows (Fig. 1) : for posture 1, the goal was 90° of elbow flexion, 0° of shoulder flexion, and 0° of shoulder abduction; for posture 2, the goal was full elbow extension (0° of elbow flexion) and 90° of shoulder flexion; for posture 3, the goal was full elbow extension (0° of elbow flexion) and 90° of shoulder abduction. Due to limits in passive range of motion of the shoulder or/and the elbow, not all subjects could reliably achieve these postures. The mean values of the shoulder and elbow angles across the 10 subjects were as follows: for posture 1, 75 ± 14° of elbow flexion and 32 ± 6° of shoulder abduction; for posture 2, 19 ± 9° of elbow flexion, 70 ± 8° of shoulder flexion and 30 ± 11° of horizontal shoulder abduction; for posture 3, 21 ± 7° of elbow flexion, 70 ± 10° of shoulder abduction and 77 ± 12° of horizontal shoulder abduction. In all three arm postures tested, the subjects' arm was supported by a cushioned support placed between the elbow and the surface of the experimental table (Fig. 1), such that the subjects did not need to actively support their arm. Subjects were asked about potential fatigue throughout the experiment, and none reported fatigue. Care was taken to ensure that the subjects did not feel any discomfort or pain in any of the three arm postures tested, verified by subject report to periodic questioning throughout the experiment.
FIG. 1.
Schematic representation of the 3 arm postures tested. The thick black vertical line symbolizes the U-shaped piece that was used to couple the subject's fingers to the shaft of the servomotor (Kamper and Rymer 2000). The thick black horizontal line symbolizes the surface of the experimental table next to which the subject was seated. The small gray rectangle symbolizes the cushioned support placed underneath the subject's elbow to support the subject's arm.
In addition to arm posture, the effect of heteronymous sensory input was investigated by electrically stimulating either the BB or the TB (“stimulation” trials). Thus there were three stimulation conditions, namely “no stimulation”, “BB stimulation,” and “TB stimulation.” Electrical stimulation was delivered by means of a neuromuscular stimulator (300PV, Empi, St. Paul, MN) and a pair of surface stimulating electrodes (American Imex, Irvine, CA) placed over the long head of BB or the long head of TB, respectively. Stimulation intensity was set to 120% of motor threshold where threshold was identified by palpation and visual observation. The duration of the stimulation pulse was 300 μs. Stimulation frequency was 35–40 Hz depending on comfort. Stimulation was turned on before the onset of the stretch and lasted until after the MCP joints were rotated back to the flexion limit. The electrical stimulation of a given muscle was intended to produce activation of Ia afferents, although activation of cutaneous receptors was also evidenced by subject report. Stimulation levels were perceived as nonnoxious in all experimental trials.
The aim of the present study was to investigate a distal stretch reflex response and heteronymous reflex coupling in the upper limb of spastic hemiparetic stroke subjects in the absence of voluntary motor activity. Therefore subjects were instructed to relax for the entirety of the experimental trials.
All of the subjects experienced all three stimulation conditions in all three arm postures. For each arm posture, 12 trials were performed: first 3 “slow stretch” trials were randomly mixed with 3 “fast stretch” trials under the no stimulation condition, then three “fast stretch” trials were run under each of the BB stimulation and TB stimulation conditions. Thus a total of 36 trials were conducted during the experiment. The order in which the three arm postures were tested during the experiment varied from subject to subject in random order. For all conditions, there was a short rest period of ∼30 to 60 s between successive trials.
Data collection
Throughout the experimental trials, angular position of the MCP joints (position encoder, PMI Motion Technologies), rotational velocity of the MCP joints (tachometer, PMI Motion Technologies) and torque at the MCP joints (torque transducer, Transducer Techniques, Temecula, CA) were measured. The EMG signals from nine upper limb muscles were recorded by means of pairs of active surface recording electrodes with differential amplification (Delsys, Boston, MA). Recording electrodes were lightly coated with conductive gel and positioned above the corresponding muscle belly. EMG signals were recorded from the following muscles: flexor digitorum superficialis (FDS), extensor digitorum communis (EDC), flexor carpi ulnaris (FCU), brachioradialis (B), BB, TB, pectoralis major (PM; clavicular head), latissimus dorsi (LD; 3–5 cm medial and inferior to the posterior axillary fold), deltoideus medius (DM). EMG signals were amplified (×1,000 to ×10,000) and band-pass filtered between 20 and 450 Hz (Bagnoli 8-channel EMG system, Delsys, Boston, MA). Before the experimental trials were run, subjects were instructed to perform maximum voluntary contraction (MVC) efforts for each of the nine muscles so that the EMG signals obtained for the experimental trials could be normalized by the MVC signals. The MVC signals were measured with the subjects ready to perform the experimental trials, i.e., with their fingers coupled to the servomotor and their arm in the first posture to be tested during the experiment. The motor was turned off, and the subjects were instructed to produce MVC efforts of each of the nine muscles while an experimenter provided resistance against the subjects' efforts. Furthermore, in an effort to detect potential cross talk between recording electrodes, subjects were instructed to produce targeted contractions of each of the nine muscles. For each contraction, the recorded EMG signals from the nine muscles were simultaneously displayed on a computer screen, allowing for on-line visual inspection of the signals; if crosstalk was detected, placement of the corresponding recording electrodes was changed until perceived cross talk was eliminated.
The MCP position, MCP velocity, MCP torque and EMG signals were low-pass filtered at 225 Hz (thus the EMG signals were filtered a 2nd time, after having been band-pass filtered between 20 and 450 Hz) and then sampled at 500 Hz. Using LabVIEW software (National Instruments, Austin, TX), data were displayed on a computer screen and saved for off-line analysis.
Analysis
The data recorded during the experimental trials were processed using custom MATLAB (The MathWorks, Natick, MA) scripts to quantify the magnitude of the stretch reflex response. In stroke subjects, it has been shown that the magnitude of the stretch reflex response is quantitatively related to the degree of spasticity (Katz et al. 1992; Lin and Sabbahi 1999).
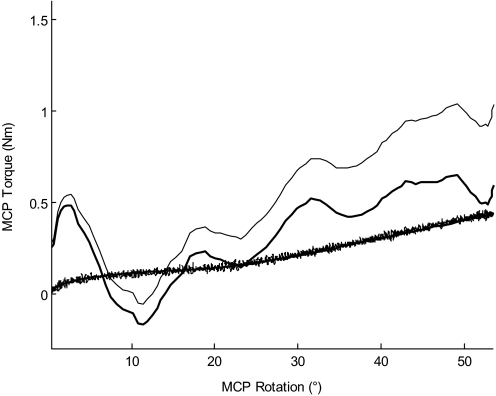
The torque data were used to quantify the magnitude of the stretch reflex response to imposed MCP extension in terms of torque produced at the MCP joints. The recorded MCP torque data from the slow stretch trials were used to estimate the passive torque generated at the MCP joints in response to the rotation by the servomotor (MCPpassive). For each of the slow stretch trials performed in a given arm posture, a polynomial of degree 5 was fit to the MCP torque versus MCP angle data, measured between the onset and the end of the stretch. The mean polynomial coefficients were then computed across the multiple “slow stretch” trials and subsequently used to estimate MCPpassive from the MCP angle data between the onset and the end of the stretch of each of the fast stretch trials in the same arm posture. For each fast stretch trial, the estimated MCPpassive was subtracted from the recorded MCP torque; the resulting torque was defined as the “MCP stretch reflex torque” (MCPreflex). The method for processing the torque data is illustrated in Fig. 2. The MCPreflex signal was then low-pass filtered at 10 Hz. Finally, the “peak MCP stretch reflex torque” (MCPreflex_max), defined as the maximum value of MCPreflex within the time window ranging from the onset of the stretch to 100 ms after the end of the stretch, was determined and used for statistical analysis.
FIG. 2.
Illustration of the method for processing the torque data. Shown are data for arm posture 1 from 1 subject. A mean polynomial coefficient was determined from the metacarpophalangeal (MCP) torque vs. MCP angle data of the multiple “slow stretch” trials (the lower thin line corresponds to 1 such slow stretch trial) in the given arm posture. This coefficient was used to estimate MCPpassive (lower thick line) during each “fast stretch” trial in that arm posture, using the MCP angle data of that trial. For the corresponding fast stretch trial, MCPpassive was subtracted from the recorded MCP torque (upper thin line) to yield MCPreflex (upper thick line). Note that the lower thin line and the lower thick line are superimposed.
The EMG data were also used to quantify the magnitude of the stretch reflex response to imposed MCP extension, in terms of activity in FDS, as well as to quantify the activity in nonstretched upper limb muscles in response to imposed MCP extension. Each recorded EMG signal was first notch filtered at 60, 120, and 180 Hz and then successively squared, low-pass filtered at 10 Hz and the square root was taken. The resulting signal was then normalized by the maximum voluntary EMG activity measured for the corresponding muscle during the MVC efforts performed prior to the experimental trials. This normalized signal (EMGnormalized) was subsequently used in the quantification of EMG activity after the stretch. Specifically, the “net EMG response” to the stretch (EMGnet) was computed for each of the nine upper limb muscles. First, the instants at which the respective normalized signals for FDS and FCU reached half of their peak values after the stretch were determined (tFDS and tFCU, respectively). Then, for each of the nine muscles, a trapezoidal integration of EMGnormalized was performed over a “poststretch time window” defined from 20 ms before tFDS or tFCU to 230 ms after tFDS or tFCU. This integration yielded the “poststretch EMG area” (EMGareapost). Whether tFDS or tFCU was used depended on which of the two muscles had the greatest peak activity value. Baseline EMG activity for each muscle was quantified by integrating EMGnormalized over a “baseline time window” of 200 ms before the onset of the stretch, yielding the “prestretch EMG area” (EMGareapre). EMGareapre was multiplied by 1.25 to account for the difference in duration of baseline time window (200 ms) and poststretch time window (250 ms). The difference in duration of the baseline and poststretch time windows was due to the fact that a duration of 200 ms proved to be the best compromise for quantifying baseline EMG activity without including contaminating artifacts in the baseline time window. EMGareapre was then subtracted from EMGareapost and the resulting value was divided by the duration of the poststretch time window (250 ms) to obtain EMGnet.
A second dependent variable, the number of occurrences of a significant EMG stretch response in a given muscle, was also computed from EMGnormalized. First, the peak value of EMGnormalized within the poststretch time window (EMGpost_max) was determined for each muscle. Significant EMG activity in response to the stretch was considered to have occurred in a given muscle when the value of EMGpost_max was greater than the mean of EMGnormalized +5 SDs of EMGnormalized, with both the SD and the mean computed over the baseline time window. Further, the onset of this significant EMG stretch response was quantified by determining the instant, with respect to the onset of the stretch, at which EMGnormalized attained a threshold value defined as the mean of EMGnormalized +3 SD of EMGnormalized, again computed over the baseline time window. The values of 5 SD of EMGnormalized and 3 SD of EMGnormalized, respectively, were chosen heuristically by visually evaluating which values yielded the best results for correctly determining occurrence and onset of a significant EMG stretch response. For each of the three arm postures, the number of occurrences of a significant EMG stretch response in a given muscle was calculated by dividing the number of trials in which a significant EMG stretch response occurred in that muscle by the total number of trials that were performed in that arm posture.
Some of the recorded EMG signals were contaminated by electrocardiographic (ECG) artifacts. In these cases, the ECG artifacts were removed prior to the quantification of the EMG signals. The spikes in the signal that were due to ECG activity were used to compute a mean ECG spike template, which was then subtracted from the signal at each location where an ECG spike occurred. EMG data from the stimulation trials were not used, due to interference from the stimulation. Additionally, some of the EMG signals from the no stimulation trials were contaminated with other artifacts, determined from spectral analysis, and were excluded from the analysis. Finally, in an effort to ensure that the upper limb of the subjects was in a relaxed state when extension of the MCP joints was imposed, EMG signals that, on visual inspection, exhibited a high level of baseline activity prior to the stretch were excluded from the analysis.
Statistical analyses were performed using SPSS software (SPSS, Chicago, IL). The potential effects of arm posture and stimulation condition on MCPreflex_max were investigated by means of a repeated-measures ANOVA, using arm posture (3 levels) and stimulation condition (3 levels) as within-subject factors. Similarly, for each of the nine EMGnet, a repeated-measures ANOVA was performed using arm posture (3 levels) as a within-subject factor. Pairwise multiple comparisons with a Bonferroni adjustment were used to investigate differences between the three levels of each of the within-subject factors arm posture and stimulation condition. A χ2 test was performed to investigate whether the number of occurrences of a significant EMG stretch response in a given muscle was the same across the three arm postures. The potential correlation of the impairment level of the subjects with MCPreflex_max and each of the nine EMGnet, respectively, was investigated by computing the Pearson correlation coefficient (r) between the Fugl-Meyer score and each of these variables. A minimum significance level of P < 0.05 was used for all tests, including for pairwise multiple comparisons after Bonferroni adjustment.
RESULTS
MCP stretch reflex torque and arm posture
Fast imposed extensions of the MCP joints elicited substantial stretch reflex flexion torques at these joints in relaxed stroke subjects. Stretch reflex responses at the MCP joints were observed in all three arm postures tested. A representative example of the recorded MCP torque profile obtained in response to a fast stretch can be seen in Fig. 2. In contrast, slow stretches did not elicit detectable stretch reflex responses (Fig. 2). The peak MCP stretch reflex flexion torque in response to a fast stretch was influenced by arm posture (ANOVA: P < 0.01). Pairwise multiple comparisons showed a significant difference in MCPreflex_max between posture 1 (90° of elbow flexion and neutral shoulder position) and posture 2 (after Bonferroni adjustment: P < 0.05) and between posture 1 and posture 3 (after Bonferroni adjustment: P < 0.05), whereas there was no significant difference between posture 2 and posture 3 (after Bonferroni adjustment: P = 1.000). When computed across the multiple trials performed under each stimulation condition by each subject in each given posture, the mean value of MCPreflex_max was greatest in posture 1 in all but 1 of the 10 subjects. When computed across all trials from all 10 subjects, the mean MCPreflex_max was 1.27 ± 0.53 (SD) Nm for posture 1, 0.94 ± 0.54 Nm for posture 2, and 0.90 ± 0.52 Nm for posture 3. MCPreflex_max was significantly negatively correlated with the Fugl-Meyer score of the subjects [Pearson: r = −0.298, P < 0.01 (2-tailed)].
MCP stretch reflex torque and electrical stimulation
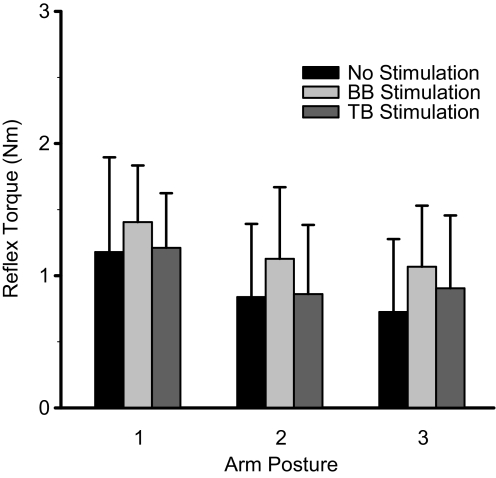
Electrical stimulation influenced the MCP stretch reflex response magnitude to fast stretch in relaxed stroke subjects. More specifically, stimulation of BB resulted in an increased peak MCP stretch reflex flexion torque. A greater mean MCPreflex_max across trials for the BB stimulation condition was observed in 7 of the 10 subjects. The repeated-measures ANOVA indicated that the effect of stimulation condition on MCPreflex_max was significant (P < 0.01). Pairwise multiple comparisons revealed a significant difference between the BB stimulation condition and the TB stimulation condition (after Bonferroni adjustment: P < 0.05) and a nonsignificant trend between the BB stimulation condition and the no stimulation condition (after Bonferroni adjustment: P = 0.063). The difference between the no stimulation and the TB stimulation conditions was not significant (after Bonferroni adjustment: P = 0.730). The mean value of MCPreflex_max computed across all trials from all 10 subjects was 1.20 ± 0.49 Nm for the BB stimulation condition, 0.92 ± 0.62 Nm for the no stimulation condition, and 0.99 ± 0.51 Nm for the TB stimulation condition. The mean MCPreflex_max across the 10 subjects was greatest for the BB stimulation condition for each of the 3 arm postures (Fig. 3). The interaction between factors arm posture and stimulation condition was not significant (ANOVA: P = 0.642).
FIG. 3.
Mean peak MCP stretch reflex torque by arm posture and by stimulation condition. Each box represents the mean value of MCPreflex_max for the corresponding arm posture and stimulation condition. Error bars represent 1 SD.
Poststretch EMG activity in FDS
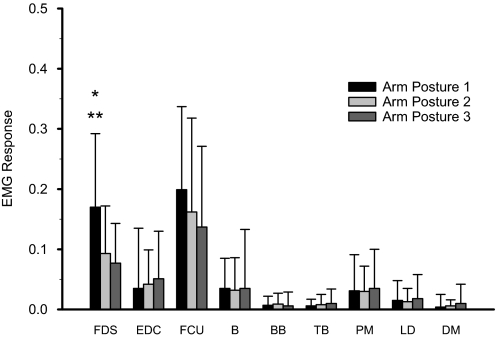
Fast imposed extensions of the MCP joints evoked EMG activity in FDS in relaxed stroke subjects in all three arm postures. An illustrative example is shown in Fig. 4. FDS activity after a fast stretch was consistently observed across subjects and across arm postures. Across all subjects, a significant EMG stretch response in FDS was observed in 93% of cases for posture 1, in 83% of cases for posture 2, and in 77% of cases for posture 3 (Table 2). Computed across the multiple trials in each posture, the mean net FDS response (FDSnet) was greater in posture 1 than in postures 2 and 3 in 8 of the 10 subjects. Seven of these eight subjects were among the nine subjects in whom there was a greater mean MCPreflex_max in posture 1 than in postures 2 and 3. The mean value of FDSnet computed across all the subjects and all the usable FDS signals (n = 10 subjects, n = 89 trials) was 0.170 ± 0.122 for posture 1 and 0.093 ± 0.079 and 0.077 ± 0.066 for postures 2 and 3, respectively (Fig. 5). The repeated-measures ANOVA was significant for the within-subject factor arm posture (P < 0.01), and pairwise multiple comparisons indicated a significant difference in FDSnet between postures 1 and 2 (after Bonferroni adjustment: P < 0.05) and between postures 1 and 3 (after Bonferroni adjustment: P < 0.01). The difference between postures 2 and 3 did not reach significance (after Bonferroni adjustment: P = 0.250). A χ2 test indicated that the number of occurrences of a significant EMG stretch response in FDS did not differ across postures (P > 0.05). FDSnet exhibited a significant negative correlation with the subjects' Fugl-Meyer score [Pearson: r = −0.410, P < 0.05 (2-tailed)].
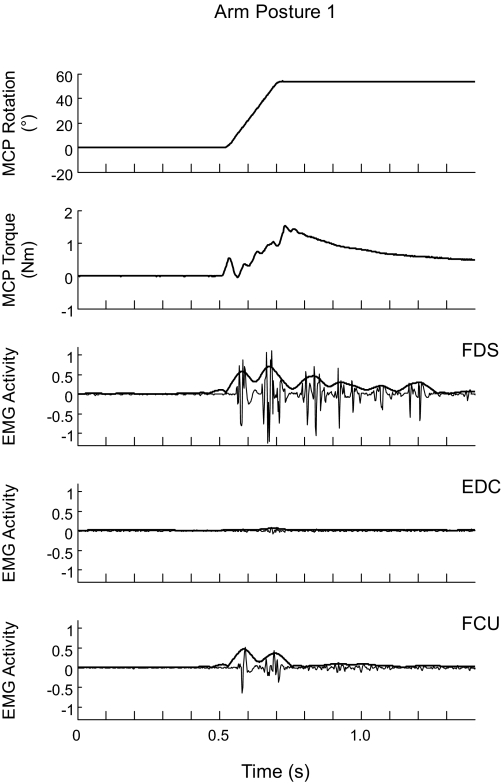
FIG. 4.
Electromyographic (EMG) activity in response to a fast stretch. The activity in flexor digitorum superficialis (FDS), extensor digitorum communis (EDC), and flexor carpi ulnaris (FCU) during a fast stretch is shown for arm posture 1. Thin lines represent the raw EMG signal, thick lines represent the normalized signal EMGnormalized. The 1st and 2nd panels from the top show the profiles of rotation of and torque at the MCP joints during the stretch, respectively. The deflection in the torque profile at the onset of the stretch is an artifact arising from the inertial torque due to the initial acceleration of the structure coupling the subject's fingers to the servomotor and of the fingers themselves, which occurs over the 1st 10% of the range of the imposed MCP extension.
TABLE 2.
Significant EMG stretch responses in the three arm postures tested
| Muscle |
Arm Posture |
|||||
|---|---|---|---|---|---|---|
|
1 |
2
|
3
|
||||
| Responses, % | Subjects | Responses, % | Subjects | Responses, % | Subjects | |
| FDS | 93 | 10 | 83 | 9 | 77 | 8 |
| EDC | 73 | 8 | 48 | 7 | 63 | 8 |
| FCU | 96 | 9 | 81 | 9 | 80 | 10 |
| B | 60 | 8 | 46 | 7 | 37 | 4 |
| BB | 29 | 6 | 24 | 4 | 17 | 3 |
| TB | 7 | 1 | 12 | 3 | 37 | 7 |
| PM | 44 | 7 | 48 | 6 | 29 | 4 |
| LD | 24 | 3 | 33 | 5 | 26 | 4 |
| DM | 3 | 1 | 18 | 4 | 10 | 2 |
The number of occurrences of a significant electromyographic (EMG) stretch response (“Responses” flexor) in a given muscle is expressed as percentage of the total number of trials. “Subjects” indicates the number of subjects in whom at least one response occurred. FDS, flexor digitorum superficialis; EDC, extensor digitorum communis; FCU, flexor carpi ulnaris; B, brachioradialis; BB, biceps brachii; TB, triceps brachi; PM, pectoralis major; LD, latissimus dorsi; DM, deltoideus medius.
FIG. 5.
Mean net EMG responses to a fast stretch in the nine upper limb muscles in the three arm postures tested. Each box represents the mean value of EMGnet for the corresponding muscle and arm posture. Error bars represent 1 SD. Astericks indicate a significant difference between posture 1 and both posture 2 (*P < 0.05, after Bonferroni adjustment) and posture 3 (**P < 0.01, after Bonferroni adjustment), as revealed by pairwise multiple comparisons with a Bonferroni adjustment.
The mean onset of the observed significant FDS stretch responses (41 ± 44 ms) was in the range of the latencies typically reported for stretch reflexes in the literature (Voerman et al. 2005).
Poststretch EMG activity in nonstretched muscles
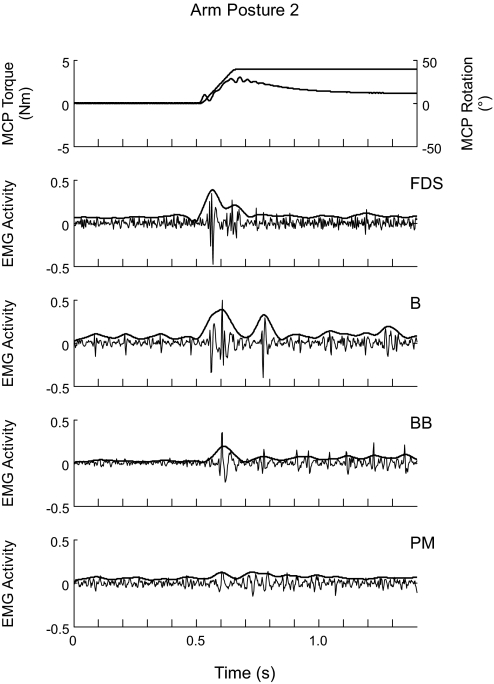
In addition to eliciting EMG activity in FDS, which was stretched when the MCP joints were extended, fast imposed MCP extensions also elicited EMG activity in muscles of the relaxed upper limb of stroke subjects that were not stretched by extension of the MCP joints. Figures 4 and 6A show examples of EMG activity evoked in nonstretched muscles. Instances of a significant EMG stretch response in a nonstretched muscle occurred in all of the eight upper limb muscles considered in the present study, although such responses were more frequent in some muscles than in others. Table 2 indicates the number of occurrences of a significant EMG stretch response in the nine upper limb muscles investigated, computed across all trials, as well as the number of subjects in whom at least one such response was observed. χ2 tests indicated no relation between arm posture and the number of occurrences of a significant EMG stretch response in any of the eight nonstretched muscles (P > 0.05), except in TB (P = 0.010). Figure 5 shows the mean net EMG responses after a fast imposed MCP extension, computed, for each given muscle, across all the subjects in whom there were usable EMG recordings for that muscle. Repeated-measures ANOVAs indicated no significant effect of arm posture on the EMGnet in any of the eight nonstretched muscles (P > 0.05). Correlation analysis revealed a significant negative correlation of the Fugl-Meyer score of the subjects with the EMGnet in several nonstretched muscles, namely EDC [Pearson: r = −0.450, P < 0.05 (2-tailed)], FCU (r = −0.424, P < 0.05), B (r = −0.486, P < 0.05), and BB (r = −0.527, P < 0.01), as well as a significant positive correlation with PMnet (r = 0.459, P < 0.05).
FIG. 6.
EMG activity in nonstretched muscles during a fast stretch. The activity in FDS, brachioradialis (B), biceps brachii (BB), and pectoralis major (PM) is shown for arm posture 2. Thin lines represent the raw EMG signal, thick lines represent the normalized signal EMGnormalized. The 1st panel from the top shows the superimposed profiles of rotation of and torque at the MCP joints during the stretch, respectively.
As for FDS, the significant EMG stretch responses observed in the eight nonstretched muscles occurred at a mean onset that was compatible with the latency of a stretch reflex (Voerman et al. 2005), ranging from 32 ± 32 to 122 ± 74 ms.
DISCUSSION
Spastic hemiparetic stroke subjects exhibited modulation of the magnitude of the stretch reflex response of the relaxed finger flexor muscles by multimodal (static posture and electrical stimulation) proximal sensory input and significant net EMG responses in relaxed nonstretched muscles to stretch of the finger flexors. Significant net EMG responses in FDS occurred at onsets that were compatible with the latencies typically observed for stretch reflexes (Voerman et al. 2005), and the onsets of significant net EMG responses in nonstretched muscles were generally below commonly described voluntary reaction times to muscle stretch (Colebatch et al. 1979; Jaeger et al. 1982) and of the order of magnitude of reflex latencies described for stretched muscles (Voerman et al. 2005). The results of the present study suggest that sensorimotor coupling of the proximal and the distal parts of the upper limb is involved in the exaggeration of both the stretch reflex response of the finger flexors and the reflex coupling of heteronymous muscles in the spastic hemiparetic upper limb poststroke. The results were overall negatively correlated with the Fugl-Meyer score of the subjects, suggesting that the effect of proximal sensory input and the heteronymous reflex coupling observed in the present study may vary with impairment severity, being more pronounced in more severely impaired individuals. However, a larger sample size would be necessary to address this point, and caution must be taken when interpreting the results of the present study given the relatively small size and wide range of impairment severity of the present sample.
Effect of arm posture and electrical stimulation
Arm posture had an effect on the magnitude of the stretch reflex response in the stroke subjects participating in the present study. We believe that this was related to heteronymous sensory feedback from the proximal muscles rather than to homonymous effects. Studies in both healthy subjects (Mirbagheri et al. 2000; Weiss et al. 1986) and spastic subjects (He 1998; Kamper et al. 2001; Li et al. 2006; Wolf et al. 1996) have shown that the reflex response of a muscle to an imposed movement is influenced by the length of the muscle, with greater muscle length resulting in an increased reflex response. However, based on a musculoskeletal model that was developed using SIMM software (MusculoGraphics, Santa Rosa, CA), elbow flexion/extension has minimal impact on the length of FDS: according to the model, the difference in FDS musculotendon length between 0 and 90° of elbow flexion, with the wrist in neutral position with respect to the forearm, is of the order of 1% of the minimum estimated FDS musculotendon length (estimated FDS musculotendon length at 0° of elbow flexion: 431 mm; estimated FDS musculotendon length at 90° of elbow flexion: 426 mm). This estimation was validated by investigating the difference in FDS length between 0 and 90° of elbow flexion using ultrasound in one healthy subject (determined difference in FDS musculotendon length: 5 mm; measured FDS musculotendon length at 0° of elbow flexion: 445 mm; measured FDS musculotendon length at 90° of elbow flexion: 430 mm). From these two results, we assume that the differences in elbow flexion/extension angle between the three arm postures tested had minimal impact on FDS muscle fiber length and thus on the force that can be generated by FDS. Furthermore, we assume that the setup of the present experiment (wrist in neutral position with respect to forearm) was such that FDS was operating in the range of optimal fiber length (Lieber et al. 1992). In this range, minimal changes in FDS muscle fiber length result in minimal changes in FDS force. Additionally, the greatest stretch reflex response magnitude, both in peak MCP stretch reflex flexion torque and in net EMG response in FDS, was observed in arm posture 1 in which the elbow was flexed at 90°, which, according to our SIMM estimation and to our ultrasound investigation, corresponds to a shorter length for FDS. In terms of FDS length, the opposite result, i.e., the smallest stretch reflex response magnitude, would be expected in posture 1.
One limitation of the present study was that not all the subjects were able to reliably achieve the intended arm postures. We propose that in all the subjects, the three arm postures tested were sufficiently close to the intended postures and sufficiently different from each other to allow comparison within subjects and that they were sufficiently similar between subjects to allow statistical tests across subjects. Furthermore, the existence of an effect of arm posture in the present study supports our conclusion that static proximal upper limb joint posture can influence a distal upper limb stretch reflex response in spastic hemiparetic stroke subjects. It cannot be ruled out, however, that deviations from the intended postures affected the results. For instance, it is possible that the differences between posture 1 and postures 2 and 3 would have been greater if the subjects had been closer to 90° of elbow flexion in posture 1 and to 0° of elbow flexion in postures 2 and 3. Moreover, differences in shoulder or/and elbow angles between subjects may have introduced variability in the results.
It has been suggested that an alteration in tonic descending synaptic input to the motoneuron may be involved in the emergence of spasticity after stroke (Katz and Rymer 1989; Powers et al. 1988). For instance, the excitability of spinal reflex circuits is under inhibitory influence from the dorsal reticulospinal tract (Hongo et al. 1969). This tract receives cortical facilitation from corticobulbar projections, and lesion of these projections due to stroke could result in reduced inhibition of spinal reflex activity (Burke et al. 1971). Furthermore, after stroke, the medial reticulospinal tract, which has an excitatory influence on spinal reflexes, could be released from cortical inhibition (Matsuyama et al. 2004), and its excitatory effect could contribute to spasticity. Such a mechanism has been proposed by Kamper et al. (2003) to account for the existence of inappropriate finger flexor activity during voluntary extension of the MCP joints in stroke subjects.
Increases in descending brain stem drive might be involved in increased synergistic coupling of targeted muscle groups after stroke. A common clinical observation in the upper limb poststroke is the existence of stereotypic movement synergy patterns involving coupling of characteristic muscle groups (Brunnström 1970): a “flexor synergy” pattern includes shoulder flexion and abduction, elbow flexion and finger flexion, and an “extensor synergy” pattern includes shoulder extension and adduction and elbow extension. It has been suggested that upregulation of brain stem pathways after stroke may contribute to a coupling of muscle groups in the upper limb (Schwerin et al. 2008). For instance, output from the medial pontomedullary reticular formation appears to facilitate flexion in the ipsilateral upper limb in the non-human primate, while simultaneously suppressing extension (Davidson and Buford 2004), suggesting a potential involvement of reticulospinal pathways in the flexor synergy pattern. The increased MCP stretch reflex flexion torque and mean net FDS response in the flexed elbow posture of posture 1 that we observed in the present study may be consistent with the flexor synergy pattern. That is, placing one element of the upper limb (the elbow) in a posture that characterizes this pattern may increase the expression of the entire pattern, including finger flexion. In the case of voluntary movements, changes in static shoulder angle have been shown to modify the abnormal shoulder adduction/elbow extension coupling in stroke subjects (Ellis et al. 2007), and it has been proposed that changing posture at the upper limb could modify the balance between descending inputs from the vestibulo- and the reticulospinal systems, the former favoring elbow extension and the latter favoring elbow flexion. If such a modulation of descending brain stem influence by arm posture exists in stroke subjects in a relaxed state, it might explain the results of the present study. Specifically, a flexed elbow posture could favor a reticulospinal influence and thus a flexed upper limb posture, including finger flexion. While the lateral vestibulospinal tract has an excitatory influence on spinal reflex activity and may be released from cortical inhibition after stroke, recent work from our laboratory (Kline et al. 2007) does not support involvement of the vestibulospinal pathways in the flexor bias characterizing the upper limb after stroke, whereas it suggests that increased excitatory influence from the reticulospinal pathways could also increase tone in the upper limb during walking poststroke.
There is evidence that, in humans, the corticospinal command to upper limb motoneurons is transmitted through propriospinal interneurons in parallel with the monosynaptic corticomotoneuronal pathway (Pierrot-Deseilligny 1996, 2002). Located rostral to the motoneurons at the cervical level, these “propriospinal premotoneurons” receive both descending corticospinal and peripheral inputs. It has been shown that both Ia afferents (Malmgren and Pierrot-Deseilligny 1988) and cutaneous afferents (Burke et al. 1992; Gracies et al. 1991) can mediate the peripheral modulation of propriospinal premotoneurons. It is therefore possible that the influence of shoulder and elbow posture on the magnitude of the stretch reflex response of the finger flexors observed in the group of subjects of the present study may occur through an effect of proximal sensory input on distal reflex activity via the propriospinal premotoneuron system.
The effect of electrical stimulation of BB on the magnitude of the stretch reflex response of the finger flexors in the stroke subjects participating in the present study could be due to either proprioceptive or/and cutaneous proximal sensory input. Although muscle spindles were targeted by the electrical stimulation, cutaneous receptors were also activated. Activation of cutaneous receptors by the electrical stimulation, rather than activation of muscle spindles, could potentially provide an explanation for an apparently contradictory aspect of our results, namely that MCP stretch reflex flexion torque was increased in a flexed elbow posture and in the presence of BB stimulation. The effects of arm posture and electrical stimulation are unlikely to both be mediated through muscle spindles, because a flexed elbow posture reduces spindle afferent input from the elbow flexors, whereas BB stimulation increases spindle afferent input. Thus a more likely explanation may be that the effect of arm posture was mediated through muscle spindles, and the effect of electrical stimulation of BB was mediated through cutaneous receptors. One potential way in which BB stimulation may have elicited an increased stretch reflex response magnitude is that although none of the subjects reported it as being painful, the stimulation may have caused anxiety on their part or/and increased their state of arousal. Indeed clinical observation suggests that stroke subjects can exhibit increased spasticity when, for instance, they are emotionally moved, upset or anxious. On the other hand, the intensity of 120% of motor threshold that we used for electrical stimulation is sufficient to directly activate muscle fibers and can therefore potentially result in muscle contraction. As a consequence, it is possible that electrical stimulation resulted in afferent input from muscle spindles and Golgi tendon organs due to contraction of BB or TB, respectively. This could, in turn, influence reflex circuits. Further study is needed to determine which mechanisms underlie the observed effect of electrical stimulation of BB on the stretch reflex response magnitude of the finger flexors, as well as why stimulation of TB did not have an effect. For example, it would be interesting to investigate how the response is influenced by cutaneous stimulation away from the muscle and by tap or/and vibration of the muscle tendon.
Reflex activity in nonstretched muscles
Reflex coupling of muscles throughout the upper limb at the spinal level could account for the significant net EMG responses observed in nonstretched muscles in response to stretch of the finger flexors in the present study. Heteronymous excitation of upper limb musculature compatible with a monosynaptic Ia circuit has been described in healthy subjects. For example, between the wrist and the elbow, both tendon tap of either flexor carpi radialis (FCR) or extensor carpi radialis (ECR) and electrical stimulation of the corresponding nerve (median nerve or radial nerve, respectively) produce facilitation of BB motoneurons (Cavallari and Katz 1989). Similarly, excitatory monosynaptic heteronymous reflexes can be elicited by tendon tap for all combinations of deltoideus posterior, PM, TB, and BB as either stimulated muscle or target muscle (McClelland et al. 2001). Heteronymous reflex coupling has been observed in shoulder muscles in response to imposed elbow extension in the relaxed upper limb of stroke subjects (Sangani et al. 2007). It is possible that the reflex activation of nonstretched muscles that we observed in relaxed stroke subjects in the present study involves spinal heteronymous reflex connections from Ia afferents of the finger flexors to nonstretched muscles.
Supraspinal reflex circuits could also contribute to the reflex EMG activity observed in nonstretched muscles in the present study. “Long-latency” components of the stretch reflex are thought to involve supraspinal, and in particular transcortical, reflex circuits (upper limb: Dick et al. 1987; Palmer and Ashby 1992; Thilmann et al. 1991). Supraspinal reflex circuits have been proposed to account for reflexes in PM evoked by stretch of flexor pollicis longus (Marsden et al. 1981) and for reflexes in trapezius and serratus anterior evoked by stimulation of either the median, ulnar or radial nerve at a distal site (Alexander and Harrison 2003). Although discriminating between short- and long-latency components was not an objective of the present study and of the EMG data analysis that was performed, the observed significant net EMG responses in the nonstretched muscles could potentially consist of long-latency components, as the time window used to quantify EMG activity after the stretch was sufficiently long (250 ms) to encompass short- and long-latency components.
It has been suggested that propriospinal premotoneurons have divergent projections onto motoneurons of multiple muscles (Mazevet and Pierrot-Deseilligny 1994). This divergence may be involved in the abnormal coupling of upper limb muscles in stroke subjects, as a result of increased divergent excitation of multiple muscles due to increased involvement of the propriospinal system after stroke (Mazevet et al. 2003; Pierrot-Deseilligny 2002). In that respect, it has been shown that the part of the corticospinal command that passes through propriospinal premotoneurons is increased in stroke subjects (Mazevet et al. 2003; Pierrot-Deseilligny 1996; Stinear and Byblow 2004). Increased involvement of the propriospinal system could be mediated through the reticulospinal pathways, which, in the cat, strongly project onto propriospinal neurons (Alstermark and Lundberg 1992; Lundberg 1999).
Some of the reflex EMG activity in nonstretched muscles observed in the present study needs to be interpreted with caution because of the possibility of cross talk between recording electrodes. Indeed, although we made an effort to avoid cross talk as much as possible when placing the electrodes, it is possible that cross talk occurred, especially between FDS and FCU and, to a lesser extent, EDC and B. In one subject, visual inspection of the EMG signals strongly suggested the presence of cross talk among FDS, FCU, and B in several trials. However, in the large majority of all trials from all 10 subjects, visual inspection indicated an absence of cross talk. We therefore feel confident that the presence of cross talk only had minor effects on the measured responses.
Conclusion
The magnitude of the stretch reflex response of the finger flexor muscles in a group of 10 spastic hemiparetic stroke subjects with a relatively wide range of impairment severity was affected by multimodal sensory input from the proximal part of the upper limb. Such heteronymous modulation of reflex excitability of the distal musculature could play an important role in the coordination of movements of the hand. As a consequence, arm posture and sensory feedback could play an important role in therapeutic interventions aimed at hand rehabilitation. Further study of the effects of sensory input from the proximal upper limb on motor control of the hand is warranted.
GRANTS
This work was supported by the National Institute of Neurological Disorders and Stroke Grant R01-NS-052509 and the National Institute on Disability and Rehabilitation Research Grant H133P040007.
Acknowledgments
We thank B. Iwamuro for help and technical assistance with the experiments and H. Chen and K. Triandafilou for assistance with data interpretation.
REFERENCES
- Alexander and Harrison 2003.Alexander CM, Harrison PJ. Reflex connections from forearm and hand afferents to shoulder girdle muscles in humans. Exp Brain Res 148: 277–282, 2003. [DOI] [PubMed] [Google Scholar]
- Alstermark and Lundberg 1992.Alstermark B, Lundberg A. The C3–C4 propriospinal system: target-reaching and food-taking. In: Muscle Afferents and Spinal Control of Movement, edited by Jami L, Pierrot-Deseilligny E, Zytnicki D. London: Pergamon, 1992, p. 327–354.
- Artieda et al. 1991.Artieda J, Quesada P, Obeso JA. Reciprocal inhibition between forearm muscles in spastic hemiplegia. Neurology 41: 286–289, 1991. [DOI] [PubMed] [Google Scholar]
- Baykousheva-Mateva and Mandaliev 1994.Baykousheva-Mateva V, Mandaliev A. Artificial feedforward as preparatory motor control in postictal hemiparesis. Electromyogr Clin Neurophysiol 34: 445–448, 1994. [PubMed] [Google Scholar]
- Brunnström 1970.Brunnström S. Movement Therapy in Hemiplegia. A Neurophysiological Approach. New York, NY: Harper and Row, 1970.
- Burke et al. 1971.Burke D, Gillies JD, Lance JW. Hamstrings stretch reflex in human spasticity. J Neurol Neurosurg Psychiatry 34: 231–235, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke et al. 1992.Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E. Convergence of descending and various peripheral inputs onto common propriospinal-like neurons in man. J Physiol 449: 655–671, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari and Katz 1989.Cavallari P, Katz R. Pattern of projections of group I afferents from forearm muscles to motoneurones supplying biceps and triceps muscles in man. Exp Brain Res 78: 465–478, 1989. [DOI] [PubMed] [Google Scholar]
- Cavallari et al. 1992.Cavallari P, Katz R, Pénicaud A. Pattern of projections of group I afferents from elbow muscles to motoneurons supplying wrist muscles in man. Exp Brain Res 91: 311–319, 1992. [DOI] [PubMed] [Google Scholar]
- Colebatch et al. 1979.Colebatch JG, Gandevia SC, McCloskey DI, Potter EK. Subject instruction and long latency reflex responses to muscle stretch. J Physiol 292: 527–534, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson and Buford 2004.Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol 92: 83–95, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick et al. 1987.Dick JPR, Rothwell JC, Day BL, Wise RJS, Benecke R, Marsden CD. Modulation of the long-latency reflex to stretch by the supplementary motor area in humans. Neurosci Lett 75: 349–354, 1987. [DOI] [PubMed] [Google Scholar]
- Ellis et al. 2007.Ellis MD, Acosta AM, Yao J, Dewald JPA. Position-dependent torque coupling and associated muscle activation in the hemiparetic upper extremity. Exp Brain Res 176: 594–602, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracies et al. 1991.Gracies JM, Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of propriospinal-like excitation to different species of human upper limb motoneurons. J Physiol 434: 151–167, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham et al. 2003.Graham KM, Moore KD, Cabel DW, Gribble PL, Cisek P, Scott SH. Kinematics and kinetics of multijoint reaching in non-human primates. J Neurophysiol 89: 2667–2677, 2003. [DOI] [PubMed] [Google Scholar]
- He 1998.He J. Stretch reflex sensitivity: effects of postural and muscle length changes. IEEE Trans Rehabil Eng 6: 182–189, 1998. [DOI] [PubMed] [Google Scholar]
- Hongo et al. 1969.Hongo T, Jankowska E, Lundberg A. The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Exp Brain Res 7: 365–391, 1969. [DOI] [PubMed] [Google Scholar]
- Jaeger et al. 1982.Jaeger RJ, Gottlieb GL, Agarwal GC. Myoelectric responses at flexors and extensors of human wrist to step torque perturbations. J Neurophysiol 48: 388–402, 1982. [DOI] [PubMed] [Google Scholar]
- Kamper and Rymer 2000.Kamper DG, Rymer WZ. Quantitative features of the stretch response of extrinsic finger muscles in hemiparetic stroke. Muscle Nerve 23: 954–961, 2000. [DOI] [PubMed] [Google Scholar]
- Kamper et al. 2003.Kamper DG, Harvey RL, Suresh S, Rymer WZ. Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle Nerve 28: 309–318, 2003. [DOI] [PubMed] [Google Scholar]
- Kamper et al. 2001.Kamper DG, Schmit BD, Rymer WZ. Effect of muscle biomechanics on the quantification of spasticity. Ann Biomed Eng 29: 1122–1134, 2001. [DOI] [PubMed] [Google Scholar]
- Katz et al. 1992.Katz RT, Rovai GP, Brait C, Rymer WZ. Objective quantification of spastic hypertonia: correlation with clinical findings. Arch Phys Med Rehabil 73: 339–347, 1992. [DOI] [PubMed] [Google Scholar]
- Katz and Rymer 1989.Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil 70: 144–155, 1989. [PubMed] [Google Scholar]
- Kline et al. 2007.Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain 130: 159–169, 2007. [DOI] [PubMed] [Google Scholar]
- Lewek et al. 2007.Lewek MD, Hornby TG, Dhaher YY, Schmit BD. Prolonged quadriceps activity following imposed hip extension: a neurophysiological mechanism for stiff-knee gait? J Neurophysiol 98: 3153–3162, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. 2006.Li S, Kamper DG, Rymer WZ. Effects of changing wrist positions on finger flexor hypertonia in stroke survivors. Muscle Nerve 33: 183–190, 2006. [DOI] [PubMed] [Google Scholar]
- Lieber et al. 1992.Lieber RL, Jacobson MD, Fazeli BM, Abrams RA, Botte MJ. Architecture of selected muscles of the arm and forearm: anatomy and implications for tendon transfer. J Hand Surg 17: 787–798, 1992. [DOI] [PubMed] [Google Scholar]
- Lin and Sabbahi 1999.Lin FM, Sabbahi M. Correlation of spasticity with hyperactive stretch reflexes and motor dysfunction in hemiplegia. Arch Phys Med Rehabil 80: 526–530, 1999. [PubMed] [Google Scholar]
- Lundberg 1999.Lundberg A. Descending control of forelimb movements in the cat. Brain Res Bull 50: 323–324, 1999. [DOI] [PubMed] [Google Scholar]
- Malmgren and Pierrot-Deseilligny 1988.Malmgren K, Pierrot-Deseilligny E. Evidence for non-monosynaptic Ia excitation of human wrist flexor motoneurons, possibly via propriospinal neurons. J Physiol 405: 747–764, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marque et al. 2001.Marque P, Simonetta-Moreau M, Maupas E, Roques CF. Facilitation of transmission in heteronymous group II pathways in spastic hemiplegic patients. J Neurol Neurosurg Psychiatry 70: 36–42, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden et al. 1981.Marsden CD, Merton PA, Morton HB. Human postural responses. Brain 104: 513–534, 1981. [DOI] [PubMed] [Google Scholar]
- Matsuyama et al. 2004.Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Mori S. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog Brain Res 143: 239–249, 2004. [DOI] [PubMed] [Google Scholar]
- Maupas et al. 2004.Maupas E, Marque P, Roques CF, Simonetta-Moreau M. Modulation of the transmission in group II heteronymous pathways by tizanidine in spastic hemiplegic patients. J Neurol Neurosurg Psychiatry 75: 130–135, 2004. [PMC free article] [PubMed] [Google Scholar]
- Mazevet et al. 2003.Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain 126: 988–1000, 2003. [DOI] [PubMed] [Google Scholar]
- Mazevet and Pierrot-Deseilligny 1994.Mazevet D, Pierrot-Deseilligny E. Pattern of descending excitation of presumed propriospinal neurones at the onset of voluntary movement in humans. Acta Physiol Scand 150: 27–38, 1994. [DOI] [PubMed] [Google Scholar]
- McClelland et al. 2001.McClelland VM, Miller S, Eyre JA. Short latency heteronymous excitatory and inhibitory reflexes between antagonist and heteronymous muscles of the human shoulder and upper limb. Brain Res 899: 82–93, 2001. [DOI] [PubMed] [Google Scholar]
- Mirbagheri et al. 2000.Mirbagheri MM, Barbeau H, Kearney RE. Intrinsic and reflex contributions to human ankle stiffness: variation with activation level and position. Exp Brain Res 135: 423–436, 2000. [DOI] [PubMed] [Google Scholar]
- Murray et al. 1995.Murray WM, Delp SL, Buchanan TS. Variation of muscle moment arms with elbow and forearm position. J Biomech 28: 513–525, 1995. [DOI] [PubMed] [Google Scholar]
- Musampa et al. 2007.Musampa NK, Mathieu PA, Levin MF. Relationship between stretch reflex thresholds and voluntary arm muscle activation in patients with spasticity. Exp Brain Res 181: 579–593, 2007. [DOI] [PubMed] [Google Scholar]
- Palmer and Ashby 1992.Palmer E, Ashby P. Evidence that a long latency stretch reflex in humans is transcortical. J Physiol 449: 429–440, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny 1996.Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Prog Neurobiol 48: 489–517, 1996. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny 2002.Pierrot-Deseilligny E. Propriospinal transmission of part of the corticospinal excitation in humans. Muscle Nerve 26: 155–172, 2002. [DOI] [PubMed] [Google Scholar]
- Powers et al. 1988.Powers RK, Marder-Meyer J, Rymer WZ. Quantitative relations between hypertonia and stretch reflex threshold in spastic hemiparesis. Ann Neurol 23: 115–124, 1988. [DOI] [PubMed] [Google Scholar]
- Rymer and Katz 1994.Rymer WZ, Katz RT. Mechanical quantification of spastic hypertonia. In: Physical Medicine and Rehabilitation: State of the Art Reviews, edited by Katz RT. Philadelphia, PA: Hanley and Belfus, 1994, p. 455–463.
- Sangani et al. 2007.Sangani SG, Starsky AJ, McGuire JR, Schmit BD. Multijoint reflexes of the stroke arm: neural coupling of the elbow and shoulder. Muscle Nerve 36: 694–703, 2007. [DOI] [PubMed] [Google Scholar]
- Schmit et al. 1999.Schmit BD, Dhaher Y, Dewald JPA, Rymer WZ. Reflex torque response to movement of the spastic elbow: theoretical analyses and implications for quantification of spasticity. Ann Biomed Eng 27: 815–829, 1999. [DOI] [PubMed] [Google Scholar]
- Schwerin et al. 2008.Schwerin S, Dewald JPA, Haztl M, Jovanovich S, Nickeas M, MacKinnon C. Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp Brain Res 185: 509–519, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear and Byblow 2004.Stinear JW, Byblow WD. The contribution of cervical propriospinal premotoneurons in recovering hemiparetic stroke patients. J Clin Neurophysiol 21: 426–434, 2004. [DOI] [PubMed] [Google Scholar]
- Thilmann et al. 1991.Thilmann AF, Schwarz M, Töpper R, Fellows SJ, Noth J. Different mechanisms underlie the long-latency stretch reflex response of active human muscle at different joints. J Physiol 444: 631–643, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voerman et al. 2005.Voerman GE, Gregorič M, Hermens HJ. Neurophysiological methods for the assessment of spasticity: the Hoffmann reflex, the tendon reflex, and the stretch reflex. Disabil Rehabil 27: 33–68, 2005. [DOI] [PubMed] [Google Scholar]
- Weiss et al. 1986.Weiss PL, Kearney RE, Hunter IW. Position dependence of stretch reflex dynamics at the human ankle. Exp Brain Res 63: 49–59, 1986. [DOI] [PubMed] [Google Scholar]
- Wolf et al. 1996.Wolf SL, Segal RL, Catlin PA, Tschorn J, Raleigh T, Kontos H, Pate P. Determining consistency of elbow joint threshold angle in elbow flexor muscles with spastic hypertonia. Phys Ther 76: 586–600, 1996. [DOI] [PubMed] [Google Scholar]