Abstract
A growing body of evidence suggests that the barrel and septal regions in layer IV of rat primary somatosensory (SI) cortex may represent separate processing channels. To assess this view, pairs of barrel and septal neurons were recorded simultaneously in the anesthetized rat while a 4 × 4 array of 16 whiskers was mechanically stimulated at 4, 8, 12, and 16 Hz. Compared with barrel neurons, regular-spiking septal neurons displayed greater increases in response latencies as the frequency of whisker stimulation increased. Cross-correlation analysis indicated that the incidence and strength of neuronal coordination varied with the relative spatial configuration (within vs. across rows) and compartmental location (barrel vs. septa) of the recorded neurons. Barrel and septal neurons were strongly coordinated if both neurons were in close proximity and resided in the same row. Some barrel neurons were weakly coordinated, but only if they resided in the same row. By contrast, the strength of coordination among pairs of septal neurons did not vary with their spatial proximity or their spatial configuration within the arcs and rows of the barrel field. These differential responses provide further support for the view that the barrel and septal regions represent the cortical gateway for processing streams that encode specific aspects of the sensorimotor information associated with whisking behavior.
INTRODUCTION
In rats, layer IV of the primary somatosensory cortex (SI) contains an isomorphic map of the contralateral whiskers in which discrete neuronal aggregates called “barrels” are separated from each other by septa (Land and Simons 1985; Woolsey and van der Loos 1970). In accordance with this spatial topography, barrel neurons respond maximally to deflections of the corresponding or “principal” whisker (Chapin and Lin 1984; Simons 1978; Simons and Woolsey 1979; Welker 1976). Neurons in the septa, by contrast, respond equally well to several whiskers (Armstrong-James and Fox 1987; Brecht and Sakmann 2002; Brumberg et al. 1999). These distinctions in receptive fields are consistent with a growing body of evidence indicating that the barrels and septa represent the cortical input stages for whisker-related processing streams (Alloway 2008).
Much of the evidence for processing streams within the barrel field comes from studies showing differences in the afferent connections of the barrels and septa. Whereas barrels receive lemniscal inputs from “core” barreloid regions in the ventroposteromedial (VPM) nucleus (Chmielowska et al. 1989; Killackey 1973; Killackey and Leshin 1975; Koralek et al. 1988; Lu and Lin 1993), the septa are innervated by other thalamic regions, including the medial part of the posterior (POm) complex (Lu and Lin 1993), the ventral lateral “tail” of the VPM barreloids (Pierret et al. 2000), and the dorsal “head” region of the VPM (Furuta et al. 2009). In contrast to barrels and their barreloid inputs, neurons in the septa-related parts of the thalamus have multi-whisker receptive fields (Bokor et al. 2008; Chiaia et al. 1991; Diamond et al. 1992; Landisman and Connors 2007; Pierret et al. 2000; Urbain and Deschenes 2007).
The barrels and septa are also differentiated by their efferent connections. Barrels project to supragranular sites located above the barrel and its adjacent neighbors in the same row (Kim and Ebner 1999; Lubke et al. 2000; Petersen et al. 2003), and the septa project to supragranular neurons located above other septa (Kim and Ebner 1999). This vertical specificity extends to interlaminar connections with the infragranular layers (Kim and Ebner 1999). Collectively, these interlaminar connections suggest the presence of functional columns of barrel and septa-related circuits that process different types of information. This view is supported by the fact that SI projections to the whisker region in primary motor (MI) cortex originate almost exclusively from extragranular neurons aligned with the layer IV septa (Alloway et al. 2004; Chakrabarti and Alloway 2006). Furthermore, MI neuronal responses to peripheral whisker stimulation are temporally correlated with neuronal discharges in the septa but not in the barrels (Chakrabarti et al. 2008).
If the barrels and septa represent processing streams, they should exhibit differences in the temporal patterns of their responses to whisker stimulation. Although barrel and septal neurons display similar response latencies to the initial onset of whisker deflections (Brumberg et al. 1999), we recently reported that increases in the frequency of whisker stimulation from 5 to 8 Hz lead to increases in the response latencies of neurons in the septa but not in the barrels (Chakrabarti et al. 2008). This is consistent with responses recorded from POm and their postsynaptic targets in layer Va of barrel cortex (Ahisssar et al. 2001; Sosnik et al. 2001), but some controversy has arisen because not all studies have observed frequency-dependent changes in the response latencies of POm neurons (Masri et al. 2008).
In this study, we re-examined the temporal patterns of neuronal responses in the barrels and septa. In contrast to our previous work (Chakrabarti et al. 2008), which varied the frequency of whisker movements from 2 to 8 Hz, this study extended the range of whisker movement frequencies ≤16 Hz. Furthermore, we recorded separate sites in the barrel field simultaneously, thereby enabling cross-correlation analysis of the relative timing of neuronal discharges in the barrels and septa. We hypothesize that neurons in different processing streams should discharge independently, whereas neurons in the same processing stream should be coordinated in ways that reflect their underlying circuit connections. Although neighboring barrels in the same row have some interconnections (Bernardo et al. 1990a,b), physiologic evidence indicates that barrels are largely independent (Laaris and Keller 2002; Petersen and Sakmann 2001). By contrast, divergent thalamocortical projections to the septa (Furuta et al. 2009), as well as dense collections of axons coursing horizontally through the layer IV septa (Chapin et al. 1987; Hoeflinger et al. 1995), might coordinate the discharge times of septal neurons.
Compared with regular-spiking neurons (RSUs) in the barrels, our results confirm that RSUs in the septa show greater increases in response latency as the frequency of whisker stimulation increases. Furthermore, cross-correlation analysis indicated that the incidence and strength of neuronal coordination varied with spatial configuration (within vs. across rows) and compartmental location (barrel vs. septa). Barrel neurons are weakly coordinated, but only if they reside in barrels of the same row. By comparison, the strength of coordination among septal neurons did not depend on whether the neurons were located in the same or different septal rows.
METHODS
Experiments were conducted on 25 male Sprague-Dawley rats ranging from 300 to 600 g. All procedures complied with guidelines issued by the National Institutes of Health and were approved by our institutional animal care and welfare committee. Each animal was subjected to surgery, mechanical whisker stimulation, and extracellular neuronal recording.
Surgery
Each rat was initially anesthetized with an intramuscular injection of ketamine (20 mg/kg) and xylazine (6 mg/kg). Atropine sulfate (0.05 mg/kg, im) and dexamethasone (0.5 mg/kg, im) were administered to reduce bronchial secretions and prevent tissue inflammation. Following intubation through the oral cavity, the rat was placed in a stereotaxic frame and artificially ventilated with a 2:1 mixture of nitrous oxide and oxygen. Ophthalmic ointment was applied to prevent corneal drying. After exposing the cranium, the wound margins were infiltrated with 2% lidocaine. As ketamine and xylazine wore off, the rat was ventilated with concentrations of isoflurane (0.75–2%) that prevented corneal and muscular reflexes. Body temperature was maintained at 37°C with a heated water pad (under the trunk) and a homeothermic heating blanket (over the trunk). To ensure a stable plane of anesthesia, we continuously monitored heart rate, blood oxygenation level, and end-tidal CO2.
When each recording session was completed, a 6- to 8-μA DC current was passed through each electrode for 8–10 s to create microlesions that would show the electrode location within layer IV. Following this, the rat received a lethal dose of pentobarbital sodium (100 mg/kg, ip) and was transcardially perfused with 4% paraformaldehyde followed by 4% paraformaldehyde with 10% sucrose. After removing the brain, the cortex was dissected from the underlying hemisphere and flattened between glass slides and stored overnight in 4% paraformaldehyde with 30% sucrose. The next day, the cortical slab was placed on the microtome stage (pial surface down) and cut tangentially into 60-μm sections. Each section was processed for cytochrome oxidase to visualize the layer IV barrels and septa (Chakrabarti et al. 2008; Land and Simons 1985; Wong-Riley 1979). The sections were inspected with a light microscope (Olympus BH-2) at ×10 and ×20, and photomicrographs of the microlesions were obtained with a Cool Snap HQ CCD digital camera (Roper Scientific, Tucson, AZ).
Mechanical whisker stimulation
As in our previous studies (Chakrabarti et al. 2008; Zhang and Alloway 2004), a square piece of plastic window screen attached to a Galvanometer was used to deflect a 4 × 4 array of mystacial vibrissae. The screen was positioned 8–12 mm away from the face so that 16 whiskers (rows B–E, arcs 1–4), which were trimmed to a length of 10–15 mm, protruded through the screen (Chakrabarti et al. 2008; Zhang and Alloway 2004). The protruding whiskers contacted the rostral edge of the holes in the screen so that initial movements of the screen, which were always in the caudal direction, produced immediate deflections of the whiskers. Screen movements were produced by 50-ms pulses emitted by a waveform generator (model LW 420, LeCroy, Chestnut Ridge, NY) whose output was amplified by a DC-coupled amplifier (Techron LVC608, AE Techron, Elkhart, IN). Each 50-ms pulse was equivalent to a half-cycle of a 10-Hz sine wave. Amplifier gain was adjusted to produce movements 1 mm in amplitude in the caudal direction for the first 25 ms of the wave, thereby producing a peak velocity of 40 mm/s, which is equivalent to 160°/s.
Each trial consisted of four blocks of eight 50-ms pulses administered at 4, 8, 12, and 16 Hz. A 2,000-ms prestimulus period was followed by the four frequency blocks, which were separated from each other by an interblock interval of 1,000 ms. Each trial was 10 s long, and consecutive trials were separated by an intertrial interval of 0.5 s. Neuronal discharges were recorded continuously during the prestimulus period, the four frequency blocks, and the interblock intervals, but not during the intertrial interval. Neuronal responses to screen movements were recorded for 300 or 400 trials.
Extracellular recording
A craniotomy was made 1–3 mm caudal and 4–6 mm lateral to bregma, thus exposing the SI barrel cortex. The dura was resected, and the brain surface was covered with saline. A low impedance (0.2–0.5 MΩ) carbon fiber electrode was lowered 500 μm below the pial surface, and the whiskers were deflected by a hand-held dowel stick while monitoring neuronal discharges on an acoustic speaker. Because the septa are narrow and occupy a smaller area than the barrels, the probability of recording pairs of septal neurons by chance is relatively low. Therefore in most experiments, we tried to locate sites that were likely to be in the septa. For this purpose, we carefully mapped separate parts of the barrel field until we found at least one site in which the neurons responded equally to multiple whiskers. These sites were tentatively identified as representing the layer IV septa. After mapping was completed, the low impedance electrode was withdrawn, and higher-impedance (1–3 MΩ) carbon fiber electrodes were inserted for single neuron recording. In most rats (n = 13), two electrodes were lowered into layer IV (450–800 μm) at an angle of 25° from the sagittal plane. In these cases, each electrode was independently controlled by a different micromanipulator (David Kopf Instruments, Tujunga, CA). In the remaining rats (n = 12), however, each of two micromanipulators held a pair of the high-impedance carbon fiber electrodes so that a total of four electrodes were used to record four SI sites simultaneously.
Each electrode usually recorded isolated waveforms at two or, occasionally, at three depths within layer IV (450–800 μm) before an electrolytic microlesion was made at the last recording site in each penetration. Receptive fields (RFs) were evaluated for each neuronal recording site at the start and end of each set of trials to ensure that electrode position had not changed during the stimulation protocol. Using hand-held probes, barrel neurons displayed strong, single-whisker responses, whereas septal neurons displayed weaker multi-whisker RFs (Brecht and Sakmann 2002; Chakrabarti et al. 2008). Classification of each neuron as being in a barrel or the septa was ultimately determined by visualizing the electrode tracks and microlesions with respect to the CO-labeled barrels in layer IV. During recordings, the craniotomy was covered with agar to dampen brain movements produced by ventilation or cardiovascular activity.
Extracellular neuronal discharges were amplified by a Lynx-8 amplifier (Neuralynx, Tucson, AZ) and displayed on a digital phosphor oscilloscope (Tektronix DPO 4000). For each electrode channel, neuronal waveforms with a signal-to-noise ratio ≥3 were sampled at 36 kHz (Data Translation 2839, Marlboro, MA), time-stamped to a resolution of 0.1 ms, and displayed on-line as a peri-stimulus timed histogram (PSTH) (Datawave Technologies, Broomfield, CO). Two waveforms were occasionally recorded from a single electrode, and these were sorted off-line according to spike amplitude, spike width, peak time, and valley time (Autocut 3.0, Datawave Technologies). Once sorted, the mean waveform was constructed and its duration was measured as in Fig. 1. Sorted waveforms were used to generate PSTHs and cross-correlograms (CCGs) using commercial (Neuroexplorer 3.0, Nex Technologies, Littleton, MA) or custom software.
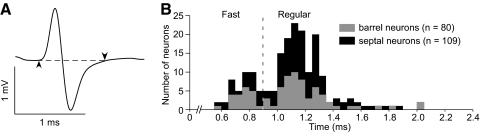
FIG. 1.
Classification of neurons as fast or regular spiking units (FSUs and RSUs, respectively) based on extracellular waveform durations. A: representative waveform showing how duration was measured. B: distribution of waveform durations recorded from layer IV. Vertical dashed line at 0.9 ms shows demarcation between FSUs and RSUs.
Neuronal responses were analyzed according to statistical criteria. For PSTHs that displayed neuronal activity during all 32 stimuli (8 stimuli administered at 4 frequencies), the 99% confidence limits were calculated from the number of spontaneous discharges in the 2,000-ms prestimulus period. For frequency-specific PSTHs, which display the mean neuronal response to the last seven stimuli in each frequency block, the 99% CIs were calculated from the sum of all discharges in the 20-ms intervals preceding each of the seven stimuli. Neuronal responses were considered significant only if they exceeded the 99% confidence limits (i.e., above spontaneous activity by 2.58 SD).
To determine response latencies at each stimulus frequency, responses to the first cycle in each frequency block were considered separately from responses to the next seven cycles. We adopted this procedure because the first stimulus cycle in each block was preceded by intervals that were longer than those occurring within the frequency block. Thus the 4-Hz stimulus block was preceded by an intertrial interval of 2.5 s, whereas the other stimulus blocks were separated by intervals of 1.0 s. Hence, the first cycle in each stimulus block represented an effective stimulation frequency of 1 Hz or less. The latency was defined as the first bin in which neuronal activity exceeded the 99% confidence limits in two contiguous bins of the frequency-specific PSTHs, which were constructed using 1-ms bin widths.
Cross-correlation analysis
Pairs of neurons recorded simultaneously from separate electrodes were used to construct cross-correlation histograms (CCGs) that displayed temporal variations in the probability of a “target” neuron discharge given that the other reference neuron discharged at time 0. In the absence of sensory stimulation, cross-correlation analysis may identify neurons whose spontaneous discharges are temporally correlated because they are embedded in an interconnected circuit. When sensory stimulation is used to activate neuronal activity, however, the correlations that appear in the raw CCG might be caused by coordination exerted by the stimulus across segregated pathways. Therefore to distinguish these possibilities, we removed potential instances of stimulus-induced coordination by subtracting a shift predictor from the raw CCG to yield a shift-corrected CCG (Alloway et al. 1993; Chakrabarti et al. 2008; Gerstein and Perkel 1972; Johnson and Alloway 1996; Roy and Alloway 1999; Zhang and Alloway 2004).
The shift predictor was constructed by shifting one spike train with respect to the other by a single trial. In this procedure, the discharge times of one neuron on a specific trial are compared with the discharge times of the other neuron on the subsequent trial. Because the discharges are compared across different trials, any correlations reflect neural events that are time-locked to the stimulus. Subtraction of the shift predictor from the raw CCG assumes that all events time-locked to the stimulus are caused by stimulus coordination even if the neurons are interconnected. Hence, shift-corrected correlations that exceed the expectation density represent a very conservative estimate of the coordination produced by neural connectivity. The shift predictor was used to calculate 99% CIs. This was done by multiplying the square root of the shift predictor (for each bin) by 2.58 (Aersten et al. 1989). Small peaks that barely crossed the CIs were often seen and therefore the shift-corrected CCGs were subjected to a three-point averaging procedure. Only those peaks that exceeded the 99% CIs in both the smoothed (i.e., 3 bin average) and unsmoothed shift-corrected CCGs were considered significant (Alloway et al. 2002; Chakrabarti et al. 2008; Zhang and Alloway 2004).
Shift-corrected CCGs were constructed from stimulus-induced discharges that occurred 10–60 ms after each whisker deflection. This time segment was chosen because stimulus movements lasted 50 ms and we consistently found that SI neuronal responses to slow stimulus velocities (i.e., 40 mm/s or less) have latencies of 10 ms or more (Chakrabarti et al. 2008; Zhang and Alloway 2004). Although neuronal discharges may occur within 10 ms of stimulus onset, we never observed responses that exceeded the 99% CIs in the first 10-ms interval. Spontaneous coordination was analyzed by constructing CCGs from all discharges that occurred during the prestimulus period. A shift procedure was also used to produce shift-corrected CCGs for spontaneous activity.
Correlation coefficients, P(τ), were calculated as described previously (Eggermont 1992). Briefly
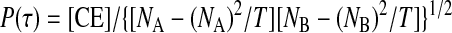 |
where CE is the number of correlated events in the tallest 2-ms period of a peak in the shift-corrected CCG; T represents the time interval over which the CCG was calculated; and NA and NB represent the number of discharges for neurons A and B, respectively, over time T. Both raw and shift-corrected CCGs were binned at 1 ms.
To determine whether neuronal coordination was altered by stimulation frequency, we constructed normalized population CCGs. This procedure was necessary because the smaller number of spikes produced at each frequency was usually inadequate for producing reliable, consistent peaks that exceeded the 99% confidence limits. Therefore the shift-corrected CCG produced at each frequency was divided by the total number of stimuli (7 stimuli × 400 trials or 7 stimuli × 300 trials) to produce a CCG in which each bin expressed the number of correlated events per stimulus. Each bin in the resulting CCG was multiplied by 100 to express the number of correlated events produced by 100 stimuli at each frequency. The frequency-specific CCGs for several neuron pairs were summed and divided by the total number of neuron pairs to yield the normalized population CCG. For barrel-septal pairs, the barrel neuron was the reference neuron. For the other two groups, the reference neuron was chosen so that the CCGs for each neuron pair had the tallest bin on the right side of time 0.
RESULTS
We recorded pairs of neurons simultaneously in layer IV of SI barrel cortex using either two (n = 13 rats) or four (n = 12 rats) carbon-fiber electrodes. In some instances, the compartmental location (septa vs. barrel) of a recorded neuron was ambiguous because the electrolytic microlesion was not recovered or was so large it could not be assigned to one compartment. After discarding these cases, a total of 189 well-isolated single units were recorded from the septal (n = 109) and barrel (n = 80) compartments. Based on responses to manual stimulation, every barrel neuron was associated with a well-defined principal whisker. By contrast, septal neurons responded similarly to inputs from multiple whiskers.
Neurons were classified as fast (FSUs) or regular spiking units (RSUs) according to their waveform durations (Bruno and Simons 2002; Simons 1978; Zhang and Alloway 2004). As shown by Fig. 1, the duration of each waveform was defined by its departure from the baseline potential. Based on the bimodal distributions of the waveform durations (Fig. 1B), 21 barrel neurons (26.25%) and 21 septal neurons (19.27%) had durations <0.9 ms and were classified as FSUs. Previous studies indicated that most FSUs in barrel cortex are local inhibitory neurons (Amitai and Connors 1995; Gibson et al. 1999; Kawaguchi and Kubota 1993; McCormick et al. 1985; Porter et al. 2001).
As shown in Table 1, nearly one half (n = 94) of the neurons recorded in the barrel field responded when the whiskers were deflected at 1 or 4 Hz but did not respond to 8 Hz and higher. Among the remaining 95 neurons, the majority (n = 52) responded to all of the tested frequencies (i.e., 1, 4, 8, 12, and 16 Hz). The remaining neurons responded over a range of frequencies extending from 1 to 8 Hz (n = 20) or from 1 to 12 Hz (n = 23).
TABLE 1.
Incidence of neuronal responsiveness in each frequency range
| Compartment | Phenotype | Sample | ≤1 Hz | 1–4 Hz | 1–8 Hz | 1–12 Hz | 1–16 Hz |
|---|---|---|---|---|---|---|---|
| Barrel | RSU | 59 | 18 | 11 | 7 | 8 | 15 |
| FSU | 21 | 0 | 0 | 7 | 3 | 11 | |
| Septal | RSU | 88 | 35 | 20 | 5 | 9 | 19 |
| FSU | 21 | 8 | 2 | 1 | 3 | 7 | |
| Total | 189 | 52 | 42 | 20 | 23 | 52 |
RSU, regular spiking unit; FSU, fast spiking unit.
Effect of stimulus frequency on response latency
Several studies have reported frequency-dependent differences in thalamic nuclei (i.e., VPM and POm) that project to barrel cortex (Ahissar and Zacksenhouse 2001; Ahissar et al. 2000; Sosnik et al. 2001). To assess whether barrel and septal neurons possess similar distinctions in their response properties, we compared their response latencies, response magnitudes (i.e., spikes per stimulus), and firing rates (i.e., spikes per second) during stimulation of the whiskers at each frequency. This analysis was conducted on the barrel (n = 51) and septal (n = 44) neurons that displayed responses at 8 Hz or higher. Neurons that responded only during 4 Hz or lower were not analyzed because this small response range prevented a systematic assessment of the relationship between the frequency of whisker deflections and neuronal responsiveness.
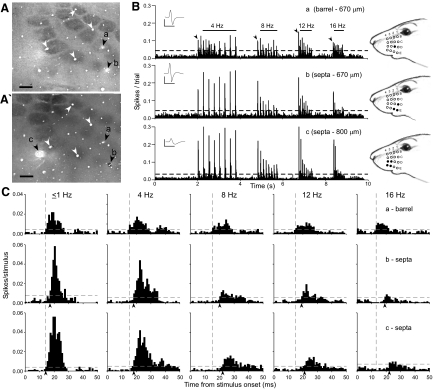
Representative examples of barrel and septal neuronal responses during different frequencies of whisker stimulation are shown in Fig. 2. As indicated, a barrel neuron and two septal neurons were recorded simultaneously from three electrodes (neuronal responses were not isolated on the fourth electrode). The receptive field of the barrel neuron was characterized by a principal whisker, whereas both septal neurons were equally activated by multiple whiskers. As stimulation frequency increased from 1 to 16 Hz, the response onset for barrel neuron a ranged from 13 to 15 ms (Fig. 2C). By comparison, the response latency of septal neuron b shifted from 17 ms at 1 Hz to 20 ms at 8 Hz, but decreased to 19 ms during 12- or 16-Hz stimulation. Septal neuron c displayed a larger latency increase, having an onset at 14 ms in response to 1-Hz stimulation and responding at latencies of 21 or 22 ms during stimulation at 8, 12, or 16 Hz (Fig. 2C).
FIG. 2.
Neuronal responses to whisker stimulation at 4, 8, 12, and 16 Hz (SC48). A: photomicrographs of layer IV tangential sections, 120 μm apart, processed for cytochrome oxidase (CO). Black arrowheads show electrolytic lesions of electrode positions; white arrowheads show blood vessels. Scale: 400 μm. B: peristimulus timed histograms (PSTHs) showing responses to 4, 8, 12, and 16 Hz for 3 neurons recorded simultaneously. Binwidth, 10 ms; insets show mean waveforms. Scale 1 mV, 1 ms. Schematics show receptive fields (RFs) for each neuron. C: frequency-specific PSTHs show changes in stimulus-induced responses. Responses to the 1st stimulus in each frequency block (arrowheads in B) were combined to yield responses at ≤1 Hz. Binwidth, 1 ms. Vertical dashed lines indicate the latency for the barrel neuron; arrowheads mark response latencies for neurons b and c. Horizontal dashed lines mark 99% CIs.
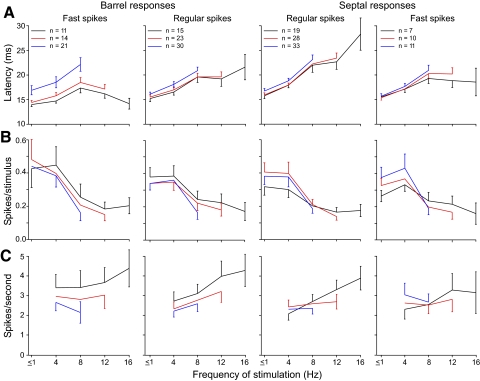
Figure 3 A shows the effects of stimulus frequency on the mean latencies of all barrel and septal neurons that responded up to 8 Hz or higher. Regardless of whether the analysis was conducted on responses to a subset of frequencies (e.g., 1–8 Hz) or on neurons that responded to all of the tested frequencies (i.e., 1–16 Hz), the changes in response latencies were similar within each class of neurons. Perhaps the most striking result concerns the distinction between RSUs and FSUs, which are likely to represent projection neurons and local interneurons, respectively. Whereas the RSUs showed increased response latencies with higher stimulation frequencies, the FSUs showed latency increases as stimulation increased from 1 to 8 Hz but a decrease in latency when stimulus frequency was increased from 8 to 16 Hz. This pattern was most apparent among the barrel FSUs (Fig. 3A, left). For FSUs that responded to all frequencies, one-way ANOVAs indicated that stimulus frequency produced a significant effect on the mean latencies of the barrel FSUs (F = 3.09; P < 0.05) but not on the septal FSUs (F = 0.98; P > 0.4).
FIG. 3.
Mean responses of barrel and septal neurons grouped according to their waveform phenotypes. A: response latencies. B: response magnitudes expressed as the number of spikes evoked by each 50-ms stimulus. C: firing rates, which are expressed as spikes per second, were based on the number of discharges that occurred over a continuous time period across the last 7 cycles of each stimulus frequency. The 1st stimulus in each frequency block was used to compute responses at <1 Hz. Each graph presents responses of all neurons that responded to all frequencies (black lines), ≤12 Hz (red lines), or ≤8 Hz (blue lines). Error bars indicate SE; for clarity, overlapping error bars are not presented.
The septal RSUs represented the only class of neurons that displayed an increase in the mean response latency for each successive increase in stimulation frequency. For those septal RSUs that responded to all stimulus frequencies, a one-way ANOVA indicated that this increase in latency is statistically significant (F = 7.29; P < 0.0001). By comparison, among the barrel RSUs that responded to all stimulus frequencies, changes in the rate of stimulation produced a smaller effect on response latency that reached statistical significance (F = 2.99; P < 0.05). Direct comparisons between the output responses of the barrel and septal compartments indicated that septal RSUs had longer latencies than barrel RSUs at 12 Hz (t = 2.52, P < 0.05) but not at 8 or 16 Hz (t ≤1.79; P ≤ 0.15).
On occasion, we recorded neurons in a barrel column that were located >800 μm below the pial surface. In these cases, we observed clear shifts in response latencies as a function of stimulus frequency. Barrel neurons in layer IV showed small changes in response latency as the frequency of stimulation increased, but neurons recorded by the same electrode showed a large increase in response latency as a function of stimulus frequency when the electrode reached a depth of 800–900 μm (data not shown). Such responses are consistent with the view that these responses were recorded from neurons in layer Va (Ahissar et al. 2001), and we discarded them from the analysis. These findings were produced in the early part of our study, and subsequently we recorded neuronal responses and made microlesions within 800 μm of the cortical surface.
Effects of stimulus frequency on response magnitudes and firing rates
We also measured the effect of stimulus frequency on the mean response magnitudes (spikes per stimulus) and firing rates (spikes per second) of the 95 neurons that responded to stimulus frequencies of 8 Hz or higher (Fig. 3, B and C). As this figure indicates, an increase in the frequency of whisker stimulation >4 Hz usually produced a noticeable decline in mean responsiveness within each neuronal class (Fig. 3B). Among RSUs that responded to all stimulus frequencies, one-way ANOVAs indicated that stimulus frequency had a significant effect on mean response magnitude regardless of whether the neurons were located in the barrels (F = 3.03, P < 0.05) or the septa (F = 3.22, P < 0.05). By comparison, the mean responsiveness of FSUs that responded to all of the tested frequencies was not as closely related to stimulus frequency. Although septal FSUs showed an overall decrease in mean response magnitudes as stimulus frequency increased, these changes were not significant (barrel FSUs: F = 2.20, P > 0.05; septal FSUs: F = 1.72; P > 0.1).
When firing rates for each group were plotted, both barrel and septal RSUs displayed increases in mean firing rate as the rate of whisker deflections increased from 4 to 16 Hz (Fig. 3C). In this analysis, the firing rate produced by 1 Hz was unknown because this stimulus parameter was derived from the first stimulus in each frequency block; hence, the response rate to consecutive stimuli over a continuous time period could not be calculated for 1 Hz. Nonetheless, among the neurons that responded to all of the tested frequencies, a one-way ANOVA indicated that the increase in stimulus frequency from 4 to 16 Hz produced a significant increase in the mean firing rates of the septal RSUs (F = 2.99; P < 0.05). By comparison, the changes in mean firing rates over the same range of stimulus frequencies were not significant for the other neuronal groups (barrel RSUs: F = 1.24, P > 0.25; barrel FSUs: F = 0.32, P > 0.80; septal FSUs: F = 0.36, P > 0.75). Because many neurons did not respond during stimulation at 12 or 16 Hz, we also examined the effects of varying whisker movements across more limited frequency ranges, including 4–12 and 4–8 Hz. When all of the neurons that responded to these stimulus frequencies were examined, the change in stimulus frequency did not have a significant effect on mean firing rates for any of the neuronal groups.
Cross-correlation analysis
We analyzed spontaneous and stimulus-induced coordination among pairs of neurons recorded simultaneously in the barrels and/or septa. The 189 neurons yielded a total of 248 neuron pairs in which each neuron in the pair was recorded by a different electrode. From this sample, we conducted cross-correlation analysis on 159 neuron pairs in which each neuron in the pair discharged ≥1,000 times in response to the 50-ms stimulus pulses administered on 300 or 400 trials. This analysis indicated that 57 of the neuron pairs displayed significant amounts of stimulus-induced correlated activity in the shift-corrected CCGs. Table 2 indicates the incidence of coordinated neuron pairs according to the spatial configuration (within-row or across-row) and compartmental locations (barrel-barrel, barrel-septal, or septal-septal) of the constituent neurons.
TABLE 2.
Incidence of neuronal coordination
| Spatial Configuration | Compartments | Pairs | Separation, μm | Coordinated pairs |
|---|---|---|---|---|
| Within row | Barrel-barrel | 20 | 843.5 ± 58.5 | 11 (55.0%) |
| Within row | Barrel-septal | 34 | 841.2 ± 65.7 | 18 (52.9%) |
| Within row | Septal-septal | 25 | 869.9 ± 62.9 | 9 (36.0%) |
| Across row | Barrel-barrel | 5 | 779.4 ± 102.6 | 0 (0%) |
| Across row | Barrel-septal | 32 | 904.5 ± 86.1 | 7 (21.8%) |
| Across row | Septal-septal | 43 | 1090.9 ± 72.8 | 12 (27.9%) |
| Total | 159 | 888.24 ± 33.05 | 57 (35.8%) |
Values are mean ± SE or n (%).
The incidence of significant correlations in the barrel field was much higher for pairs of neurons in the same row than for pairs of neurons in different rows. Nearly one half (48.1%) of the 79 neuron pairs in the same row exhibited significant amounts of correlated activity during multi-whisker deflections, but less than one fourth (23.7%) of the 80 neuron pairs located across rows were coordinated. A Pearson test of association confirmed that the incidence of coordination was significantly greater for neurons in the same row (χ2 = 10.24, P < 0.005).
As shown by Table 2, a majority of barrel-barrel (55%) and barrel-septal (52.94%) neuron pairs in the same barrel row showed significant coordination, but only 36% of the septal-septal neuron pairs in the same septal row exhibited significant correlations. By comparison, a minority of neuron pairs located across different rows were coordinated. Only 21.9% of the barrel-septal group and 27.9% of the septal-septal group displayed significant levels of correlated activity. We recorded five barrel-barrel neuron pairs that extended across different rows, but none of them showed significant coordination; the probability of this because of chance is only 3%.
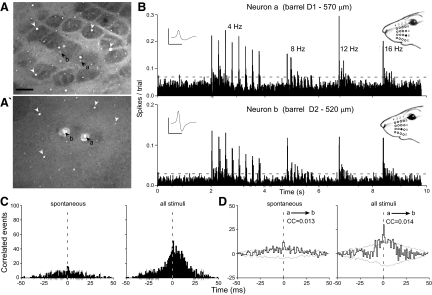
Neuronal coordination in the same barrel field row
Even though more than one half of the barrel-barrel pairs in the same row displayed significant amounts of correlated activity, the strength of these correlations was relatively weak compared with the barrel-septa and septa-septa neuron pairs. Furthermore, 73% (8 of 11) of the barrel-barrel neuron pairs that displayed significant correlations involved pairs of neurons in adjacent barrels of the same row. For example, Fig. 4 shows weak correlations between a pair of neurons in barrels D1 and D2 that were separated by a distance of only 602 μm. The shift-corrected CCGs showed peaks exceeding the 99% CIs (Fig. 4D), but the correlation coefficients were only 0.013 and 0.014 for spontaneous and stimulus-induced activity, respectively.
FIG. 4.
Weak coordination among barrel neurons in the same row (SC69). A: tangential sections, 180 μm apart, processed for cytochrome oxidase (CO). Black arrowheads show electrolytic lesions for electrode sites; white arrowheads show blood vessels. Scale: 500 μm. B: PSTHs of neuronal responses; binwidth, 10 ms. Insets: mean waveforms; scale: 500 mV, 1 ms. RFs shown at right. C: raw cross-correlation histograms (CCGs) showing spontaneous and stimulus-induced coordination. D: shift-corrected CCGs showing spontaneous and stimulus-induced coordination; neuron a is reference neuron. Binwidth, 1 ms. Horizontal dashed lines indicate 99% CIs. CC, correlation coefficient.
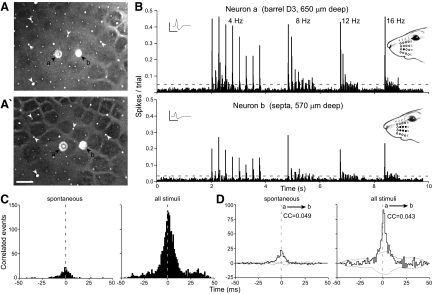
Pairs of barrel and septal neurons in the same row showed the strongest levels of coordination. As shown by Fig. 5, a neuron located in barrel D3 was strongly synchronized with a neuron located 529 μm away in the septa between rows C and D. The stimulus-induced CCG was characterized by a tall, narrow peak just after time 0 (Fig. 5D). This indicates a high level of temporal precision in the relative discharge patterns of these two neurons, and this is consistent with the correlation coefficient (0.043) obtained from the shift-correlated stimulus-induced CCG. The correlation coefficient calculated from spontaneous activity was larger (0.049), but the number of correlated events during the prestimulus period was much smaller.
FIG. 5.
Coordination among barrel and septal neurons in the same whisker barrel row (SC85). All data shown as in Fig. 4. A: tangential sections, 60 μm apart, processed for CO. Scale: 500 μm. B: PSTHs show responses to 4, 8, 12, and 16 Hz. Insets: mean waveforms; scale: 1 mV, 1 ms. C: raw CCGs show spontaneous and stimulus-induced coordination. D: shift-corrected CCGs show spontaneous and stimulus-induced coordination.
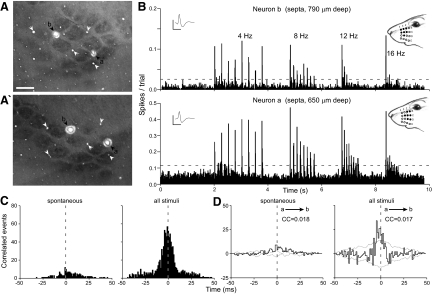
Only 36% of the neuron pairs in the same septal row displayed significant synchronization. To be considered in the same septal row, both neurons had to reside in the same septal strip between two barrel rows or, if one neuron was between two barrels in the same row, the other neuron had to be in one of the septa adjoining that barrel row. In about a third of these coordinated neuron pairs, correlations were detected even though the neurons were separated by >1 mm. Figure 6, for example, shows responses from a pair of neurons in the same septal row that were separated by 1,165 μm, which is about twice the neuronal separations shown in Figs. 4 and 5. Despite this wider spatial separation, whisker stimulation produced prominent peaks in the raw and shift-corrected CCGs, and the correlation coefficient was 0.017. Although the correlation coefficient was slightly larger for spontaneous activity (0.018), the number of correlated events during the prestimulus period was relatively small.
FIG. 6.
Coordination among septal neurons in the same whisker row (SC63). Data shown as in Figs. 4 and 5. A: tangential sections, 120 μm apart, processed for CO. Scale: 500 μm. B: PSTHs show responses to 4, 8, 12, and 16 Hz. Insets: mean waveforms; scale: 1 mV, 1 ms. C: raw CCGs show spontaneous and stimulus-induced coordination. D: shift-corrected CCGs show spontaneous and stimulus-induced coordination.
Neuronal coordination across different rows
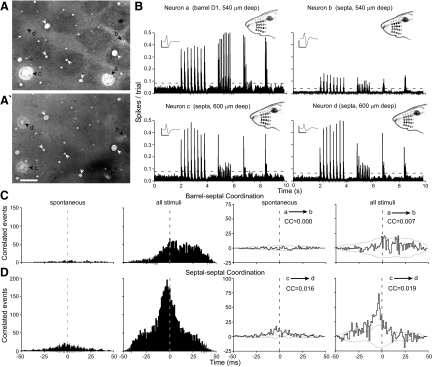
Among neurons located across different rows, the incidence of coordinated activity was greatest for pairs of neurons located in different septa (Table 2). As shown in Fig. 7, in which a barrel neuron was recorded simultaneously with three septal neurons, across-row coordination was also stronger for septa-septa than for barrel-septa neuron pairs. In this experiment, in which electrode a was in barrel D1 and the other three electrodes (b, c, and d) were in the septa, significant amounts of correlated activity were detected among all four across-row neuron pairs, (a-b, b-c, b-d, c-d). The shift-corrected CCG for the barrel-septa (a-b) pair, which spanned across a distance of 595 μm, contained a small peak that had a correlation coefficient of only 0.007. By comparison, a pair of septal neurons (c-d) separated by a similar distance (604 μm) had a much larger CCG peak that was associated with a correlation coefficient of 0.019. The two other septal neuron pairs (b-c and b-d), each of which spanned nearly 1,500 μm of cortex, had correlation coefficients (0.012 and 0.017, data not shown) that were larger than the barrel-septa pair.
FIG. 7.
Relative coordination among barrel-septal (a-b) and septal-septal (c-d) pairs in different rows (SC54). Data shown as in Figs. 4–6. A: tangential sections, 60 μm apart, processed for CO. Scale: 250 μm. B: PSTHs show responses of all 4 neurons. Insets: mean waveforms; scale: 1 mV, 1 ms. C: raw and shift-corrected CCGs show spontaneous and stimulus-induced coordination in the barrel-septal neuron pair. D: raw and shift-corrected CCGs show spontaneous and stimulus-induced coordination in the septal-septal neuron pair.
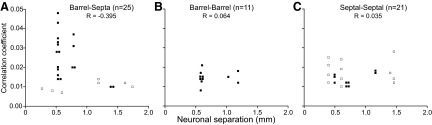
For those across-row neuron pairs that displayed significant peaks in their shift-corrected CCGs, analysis of the correlation coefficients indicated that neurons in different septal rows are more strongly coordinated than barrel-septal pairs that span different rows. As seen in Fig. 8, A and C, pairs of barrel and septal neurons (n = 7) that resided in different rows had mean correlation coefficients (0.010 ± 0.0009; means ± SE) that were significantly weaker than those (0.017 ± 0.001) measured from pairs of septa-septa neurons (n = 12) located in different rows (t = 3.18; P < 0.01). However, the distance separating the barrel and septal (1,005 ± 218 μm) neurons was similar for the septa-septa (819 ± 139 μm) neuron pairs (t = 0.75; P > 0.40).
FIG. 8.
Relationship between shift-corrected correlation coefficients and neuronal proximity for (A) barrel-septa, (B) barrel-barrel, and (C) septa-septa neuron pairs that displayed significant interactions in the shift-corrected CCGs. Filled symbols indicate neuron pairs in the same whisker barrel row; open symbols indicate pairs of neurons in different rows. The correlation between spatial separation and the strength of coordination is given by the R values.
Neuronal coordination and neuronal proximity
Neuronal coordination in the layer IV barrel field was more likely for neurons located in close proximity to each other. Although 46.6% (42 of 90) of neuron pairs with separations <1.0 mm were coordinated, only 21.7% (15 of 69) of the neuron pairs located across a distance of 1.0 mm or more displayed significant amounts of correlated activity. A Pearson test of association confirmed that the incidence of coordination was significantly larger for pairs of neurons separated by <1.0 mm (χ2 = 10.5, P < 0.005). Further analysis showed a parallel trend for neuron pairs in each combination of compartmental locations. Thus as neuronal separations increased from <1.0 to >1.0 mm, the incidence of neuronal coordination declined for barrel-septa (46 to 24%), barrel-barrel (53 to 30%), and septa-septa (44 to 18%) neuron pairs.
Among the 57 neuron pairs that displayed significant amounts of correlated activity, the relationship between neuronal proximity and coordination strength depended on the spatial and compartmental configurations of the neuron pairs. As shown in Fig. 8A, barrel and septal neurons in close proximity (<1.0 mm) had correlation coefficients >0.02, but only if both neurons were in the same row. For barrel and septal neurons separated by >1.0 mm, the strength of coordination was lower and did not matter whether the neurons were in the same row. This inverse relationship is indicated by a negative correlation (R = −0.395; P = 0.05079). When the analysis was restricted to barrel-septa pairs residing in the same row, the inverse correlation between neuronal coordination and neuronal proximity was even stronger (R = −0.48; P < 0.05).
For barrel-barrel pairs, the effect of neuronal proximity on the strength of neuronal coordination was not apparent. More importantly, all instances of barrel-barrel coordination involved neurons that resided in the same row, and the majority (8 of 11) of these were in neighboring barrels. Barrel neurons in different rows were never correlated with each other, even if they were separated by as little as 500 μm.
Neuronal proximity had no apparent effect on the strength of coordination among the septa-septa neuron pairs that displayed significant amounts of correlated activity. Furthermore, regardless of whether the neurons were in the same or in different septal rows, correlation coefficients were similar across separations extending up to 1.5 mm (Fig. 8C). In contrast to neurons in the barrels, this indicates that septal coordination is equally strong in all directions.
Strength of coordination among different neuronal groups
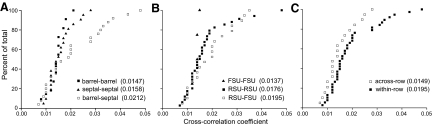
To assess the main factors that account for neuronal coordination in the barrel field, the 57 neuron pairs that showed significant levels of synchronization were grouped according to their compartmental locations (barrel-barrel, barrel-septal, or septal-septal), their phenotypes (RSU-RSU, RSU-FSU, or FSU-FSU), and their spatial configurations (within vs. across rows). Cumulative frequency distributions for these different groups of neuron pairs are shown in Fig. 9.
FIG. 9.
Cumulative frequency distributions of the shift-corrected correlation coefficients for the neuron pairs (n = 57) that displayed significant amounts of correlated activity during whisker stimulation. Each panel displays 57 neuron pairs sorted according to their compartmental locations (A), neuronal phenotypes (B), or spatial configurations (C). Numbers in parentheses indicate mean correlation coefficients for each group.
For neuron pairs grouped according to compartmental locations (Fig. 9A), the barrel-barrel neuron pairs had the lowest mean correlation coefficients even though all of these pairs involved neurons within the same barrel row. The barrel-septa neuron pairs had stronger cross-correlation coefficients that far exceeded those of the two other groups, whereas the septa-septa neuron pairs possessed intermediate cross-correlation coefficients. A one-way ANOVA detected significant differences in the correlation coefficients of barrel-barrel, barrel-septal, and septal-septal neuron pairs (F = 3.18; P < 0.05).
When the same 57 neuron pairs were grouped according to their waveform phenotypes, the RSU-RSU and RSU-FSU neuron pairs had correlation coefficients that were noticeably larger than the FSU-FSU neuron pairs. In our sample, however, only 4 of 19 FSU-FSU neuron pairs displayed significant amounts of coordination. Consistent with the fact that the cumulative distributions for the other two groups were similar (Fig. 9B), a one-way ANOVA failed to show a significant effect of neuronal phenotype on the strength of correlation (F = 0.75, P > 0.40).
When the correlation coefficients were grouped according to their spatial configuration, the cumulative distributions of the within-row and across-row neuron pairs did not overlap (Fig. 9C). Statistical analysis of these cumulative distributions with the nonparametric Wilcoxon rank sum test showed significant differences between these groups (z = −2.048, P < 0.05). This is consistent with our previous work indicating that neurons in the same row of barrel cortex have stronger levels of coordination than neurons in different rows (Zhang and Alloway 2004).
Comparison of stimulus-induced and spontaneous coordination
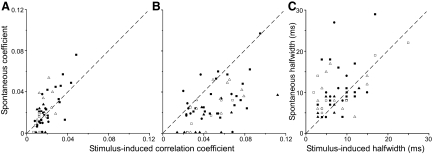
Most of the neurons coordinated during whisker stimulation were also coordinated when sensory inputs were absent. Among the 57 neuron pairs that were coordinated during whisker stimulation, 47 also displayed significant amounts of correlated activity during the prestimulus period, which represents spontaneous activity. Furthermore, among the 102 neuron pairs that were not coordinated during whisker stimulation, 94 of them failed to show coordination of spontaneous activity. Only eight neuron pairs that displayed spontaneous coordination were not coordinated during whisker stimulation.
The relationship between the strength of spontaneous and stimulus-induced coordination is shown in Fig. 10. For neuron pairs that displayed both spontaneous and stimulus-induced correlations (n = 47), the correlation coefficients derived from the prestimulus periods (0.0248 ± 0.002) were larger than those derived from the stimulus-induced responses (0.0191 ± 0.001). This suggests that spontaneous activity might be more strongly coordinated than stimulus-induced interactions, but subtraction of the shift predictor assumes that all of the discharges that are time-locked to the stimulus must represent the effects of stimulus coordination. Given that these 47 neuron pairs displayed significant levels of coordination in the shift-corrected CCGs, their correlated discharges are probably caused by functional connections. Hence, it is plausible that many of the discharges time-locked to stimuli on successive trials could represent the effects of common inputs or other functional connections among the recorded neurons. This would mean that subtraction of the shift predictor could underestimate the strength of the connections that are coordinating the recorded neurons. Therefore to determine the actual incidence of correlated events during spontaneous and stimulus-induced activity, we also calculated the correlation coefficients from the raw CCGs. As shown in Fig. 10B, the correlation coefficients from the raw CCGs indicated stronger coordination during whisker stimulation (0.052 ± 0.003) than during the prestimulus period (0.032 ± 0.002; paired t = 6.85; P < 0.00001).
FIG. 10.
Comparison of spontaneous and stimulus-induced coordination among barrel-barrel (circles), barrel-septa (squares), and septal-septa (triangles) neuron pairs located in the same (filled symbols) or different (open symbols) rows. A: scatterplot of correlation coefficients from the shift-correlated CCGs (n = 57); neuron pairs that did not show spontaneous coordination (n = 10) were assigned correlation coefficients of 0. B: scatterplot of correlation coefficients from the raw CCGs for the same neuron pairs appearing in A. C: scatterplots of the half-widths for the shift-corrected CCG peaks of neuron pairs that showed both spontaneous and stimulus-induced coordination (n = 47).
We also analyzed the half-widths of the peaks in the shift-corrected CCGs for the same neuron pairs (Fig. 10C). For 70% of the neuron pairs, spontaneous coordination produced CCG peaks that were broader than those produced during whisker stimulation. Thus when compared with spontaneous coordination, stimulus-induced coordination was characterized by less variability in the relative timing of the correlated discharges. These results suggest that whisker stimulation enhances the temporal coupling of neurons that are functionally connected.
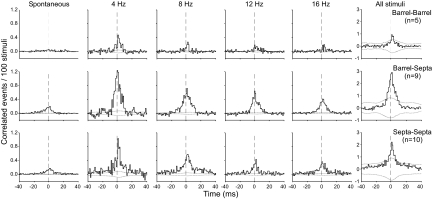
Neuronal coordination and stimulus frequency
The relationship between stimulus frequency and neuronal coordination was examined by constructing population CCGs for barrel-barrel, barrel-septal, and septal-septal neuron pairs. Normalized population CCGs (see methods) were constructed from the neuron pairs whose correlation coefficients were in the top half of their cumulative distributions because these responses are the most likely to reflect underlying circuit connections. Furthermore, population CCGs for the barrel-barrel and barrel-septa pairs were constructed only from those neuron pairs residing in the same row because little coordination was detected among these neuron types when the neurons were in different rows. For the septal-septal pairs, however, both within-row and across-row pairs were used to construct the population CCGs.
Normalized population CCGs for each compartmental configuration are shown in Fig. 11. In this figure, each panel portrays the mean coordination produced in a pair of neurons in response to 100 stimuli administered at a specific frequency. For each compartmental group, the CCG peaks were largest at 4 Hz and gradually declined in size as the frequency of whisker stimulation increased. Consistent with the results described earlier, barrel-septa and septa-septa neuron pairs displayed much larger CCG peaks than the barrel-barrel pairs. Furthermore, the population CCGs derived from spontaneous activity also showed clear coordination among the barrel-septa and septa-septa neuron pairs but not for the barrel-barrel pairs.
FIG. 11.
Normalized shift-corrected population CCGs showing effects of stimulus frequency on the coordination of barrel-barrel, barrel-septal, and septal-septal neuron pairs. Each panel represents the mean shift-corrected response for 100 stimuli. Barrel-septal and barrel-barrel CCGs were constructed only for pairs in which both neurons were in the same barrel row. For barrel-septal CCGs, the barrel neuron was always the reference neuron. Dotted lines indicate 99% CIs.
DISCUSSION
We measured the response latencies, response magnitudes (spikes/stimulus), and firing rates (spikes/s) of barrel and septal neurons during mechanical stimulation of multiple whiskers at rates of 4, 8, 12, and 16 Hz. In contrast to FSUs, which do not display a monotonic relationship between response latency and stimulus frequency, RSUs in the barrels and septa showed significant increases in response latency as stimulus frequency increased. Although septal and barrel RSUs showed similar response latencies during low rates of whisker stimulation, at 12 Hz, the responses of the septal RSUs were significantly delayed with respect to the responses of the barrel RSUs. When response magnitudes were compared, both septal and barrel RSUs showed similar declines in responsiveness as the frequency of whisker stimulation increased. When the responses were expressed as firing rates, septal RSUs showed increases in discharge rates as a function of stimulation frequency, but only for those neurons that responded to all of the tested frequencies (4–16 Hz). When we analyzed all RSUs that responded at 8 or 12 Hz, there was no difference in firing rates when septal RSUs were compared with barrel RSUs.
We also analyzed the effects of compartmental location, spatial configuration, and spatial proximity on the incidence and strength of neuronal coordination in the layer IV barrel field. Some barrel neurons were weakly correlated, but only if they occupied barrels in the same row. The strongest correlations occurred among barrel and septal neurons in close proximity in the same row. By comparison, pairs of septal neurons showed intermediate amounts of correlated activity across all distances and spatial configurations that we tested. Whereas barrel-barrel and barrel-septal pairs showed decreases in the incidence and strength of coordination across different barrel rows, this spatial parameter had little influence on the coordination of septal neurons. This indicates an important distinction in the functional connectivity of the barrel and septal circuits.
Technical considerations
We initially classified barrel and septal neurons by the presence or absence of a principal whisker in their receptive fields when the whiskers were manually stimulated. This classification was subsequently verified by locating the electrode tracks and electrolytic microlesions with respect to the CO-labeled barrels. Despite these efforts, we cannot exclude the possibility that we may have recorded neurons outside the area marked by the lesion. Furthermore, neurons at the edges of barrels are sensitive to manipulations of the adjacent whiskers and, therefore, we may have misclassified the compartmental location of some neurons. To minimize such errors, cases where lesions extended across the barrel boundaries were discarded from our analysis.
Our experimental paradigm used pulse-like movements of multiple whiskers to evoke discharges in the SI barrel field. Such movements, however, do not resemble the continuous back-and-forth movements of the whiskers during normal exploratory behavior. Although stimulus pulses are useful for measuring response latencies, the presence of stationary intervals between successive pulse-like movements is not ideal for measuring firing rates because some fraction of the overall firing rate is caused by activity that occurs during the brief intervals between successive stimuli within each frequency block. To detect potential differences in the firing rates of the barrel and septal circuits, it would be better to use continuous sinusoidal movements over a time duration that encompasses several oscillatory cycles.
Pulse-like stimuli may also interfere with cross-correlation analysis of neural coordination if they reduce the variation in neuronal response times across successive stimuli. When discharges are precisely time-locked to the stimulus, it is difficult to determine whether correlated events are caused by neural connectivity or stimulus coordination. To minimize this problem, we used a stimulus in which the peak velocity of the whisker movements was only 40 mm/s. This rate of whisker movement is relatively slow and therefore more likely to produce greater variability in the discharge times across successive stimuli. The tradeoff with this approach, however, is that slower moving stimuli produce longer response latencies. Although latencies of 10 ms or less have been observed in the barrel field (Brumberg et al. 1999), the shortest response latencies in this study were between 10 and 15 ms.
The final technical consideration concerns the effects of anesthesia on neuronal responsiveness. Many neurons responded to whisker stimulation at low frequencies but not at 8 Hz or higher. In addition, virtually all neurons displayed a decline in response magnitude as stimulus frequency increased. Although isoflurane enables a relatively constant plane of anesthesia over the course of each experimental session, there is little doubt that this anesthetic reduces neuronal responsiveness. Neurons that failed to respond at 8 Hz or higher might have responded to these frequencies in the unanesthetized state.
Role of septal circuits in processing stimulus frequency
Several studies suggest that the paralemniscal and lemniscal pathways respond differentially to increases in the rate of whisker stimulation. This view is based on studies in which POm neurons and their targets in layer Va of barrel cortex displayed latency shifts as a function of increases in the frequency of whisker movements (Ahissar 1998; Ahissar and Kleinfeld 2003; Ahissar and Zacksenhouse 2001; Ahissar et al. 2000, 2001; Sosnik et al. 2001; Zacksenhouse and Ahissar 2006). Despite controversy about the response properties of POm neurons (Masri et al. 2008), it is conceivable that increases in response latency that are coupled with a fixed response offset could encode the frequency of whisker movements (Ahissar and Kleinfeld 2003; Kleinfeld et al. 2006).
Our data indicate that septal RSUs are more consistent than barrel RSUs in showing latency shifts in response to progressive increases in the frequency of whisker stimulation. Thus septal RSUs showed a reliable frequency-dependent increase in response latency that was larger than the latency shift seen among barrel RSUs. The distinction between septal and barrel RSUs was ambiguous, however, when the data were expressed as mean firing rates (spikes/s). Among RSUs that responded to all of the tested frequencies (4–16 Hz), an increase in stimulus frequency produced a significant increase in mean firing rate among septal RSUs but not for barrel RSUs. The difference between septal and barrel neurons was not present, however, when the analysis included additional RSUs that responded to 8 or 12 Hz.
A recent study reported that repetitive air-puff stimulation of the whiskers in awake rats produces an increase in the discharge rates of septal neurons as frequencies increased up to 18 Hz, but the barrel neurons increased their firing rates only up to 9 Hz (Melzer et al. 2006). Although we observed a similar trend among septal RSUs that responded to 16 Hz, we also saw increased firing rates, albeit smaller, among barrel RSUs. The reasons for the apparent discrepancies between the two studies are unclear, but could be related to the use of isoflurane anesthesia in our study or to differences in mechanical stimulation of the whiskers.
Septa as a functional unit
Several studies indicate that neurons in SI barrel cortex are organized into functional columns aligned with either the barrels or the septa. Although barrel neurons have connections with the surrounding septa in layer IV, substantial projections from the barrels terminate in the supragranular sites above the barrel and its adjacent neighbors in the same row (Kim and Ebner 1999). In turn, the supragranular neurons within a barrel column project mainly to other barrel columns in the same row (Feldmeyer et al. 2006; Kim and Ebner 1999; Petersen et al. 2003). Neurons in the septal columns have parallel sets of local projections in which the neurons located above or below the septa project mainly to other extragranular sites that are aligned with the septa (Kim and Ebner 1999; Shepherd and Svoboda 2005). Further evidence for a distinction between septal and barrel columns comes from our work showing that SI projections to the MI whisker region originate primarily from extragranular neurons aligned with the septa (Alloway et al. 2004; Chakrabarti and Alloway 2006). Consistent with these data, MI neurons are strongly coordinated with septal neurons but not with the barrel neurons (Chakrabarti et al. 2008). These columnar distinctions, as well as the horizontal connections within the septa (Chapin et al. 1987; Hoeflinger et al. 1995), suggest that neurons in the septa may represent a distributed neural network that processes information needed to regulate whisking behavior (Alloway 2008).
This study supports this view by showing distinctions in the coordination patterns of barrel and septal neurons. Consistent with the local connections between barrel neurons and nearby septal neurons in the same row (Bernardo et al. 1990a,b; Hoeflinger et al. 1995; Kim and Ebner 1999), this study corroborates a recent report that observed significant correlations among barrel and septal neurons in close proximity (Ghoshal et al. 2009). We also found weak correlations among barrel neurons residing in the same row, which is congruent with the few projections that interconnect neighboring barrels in the same row (Bernardo et al. 1990a,b). By contrast, the strength of coordination among pairs of septal neurons did not vary systematically across the distances that we tested, nor did it depend on their relative locations within different septal rows or arcs. These findings suggest that distributed groups of septal neurons might cooperate with each other as a “functional unit.” This prompts us to hypothesize that distributed populations of septal neurons are synchronized by whisker movements to activate their extragranular targets in SI and, ultimately, their multisynaptic targets in the MI whisker region.
Several mechanisms could account for the coordinated responses that we observed across widespread septal sites. Correlated neuronal activity throughout POm or the other septa-related thalamic regions could simultaneously activate large parts of the septa. In addition, divergent projections of individual thalamocortical neurons could also synchronize different parts of the septa. Support for this mechanism comes from a recent study showing that individual neurons in the “head” region of VPM have collateral axonal arbors that terminate in separate septal sites, including different septal rows (Furuta et al. 2009). Furthermore, local interconnections within the septa could also contribute to septal coordination. Previous anatomical studies have revealed dense horizontal projections within the layer IV septa (Chapin et al. 1987; Hoeflinger et al. 1995; Kim and Ebner 1999), but whether these horizontal processes represent connections between pairs of septal neurons remains unclear.
Neural basis for correlated activity in the barrel field
Our comparisons of spontaneous and stimulus-induced interactions provide additional support for the view that the coordination observed in this study is based on specific sets of anatomical connections. In general, neurons that displayed correlations in their spontaneous activity were also correlated during whisker stimulation. Likewise, neurons that were uncorrelated during the prestimulus period remained uncorrelated during subsequent whisker stimulation. Indeed, among 159 neuron pairs subjected to cross-correlation analysis, only 11% (n = 18) switched timing patterns (correlated or uncorrelated) in response to the transition between prestimulus and stimulus periods. Furthermore, we frequently recorded three or four neurons simultaneously that displayed similar PSTH responses. Although some neurons had correlations characterized by tall CCG peaks, the other simultaneously recorded neurons were uncorrelated and had CCGs that were essentially flat. These findings effectively rule out the possibility that the presence or absence of correlated activity is state dependent.
Instead, our findings suggest that neurons embedded within an interconnected circuit will display correlated events during both spontaneous and stimulus-induced activity. Indeed, we have previously shown that thalamocortical and corticocortical interactions are present during both spontaneous and stimulus-induced activity (Chakrabarti et al. 2008; Johnson and Alloway 1996; Zhang and Alloway 2004). Even though the measured strength of functional connections may vary from moment to moment (Aertsen et al. 1989), neural connectivity revealed by the relative timing of stimulus-induced responses is usually confirmed by the relative timing of the spontaneous discharges.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-37532 and NS-052689.
Acknowledgments
Present address of S. Chakrabarti: Sensorimotor Research Group, Cognitive Neuroscience Laboratory, German Primate Center, Kellnerweg 4, Göttingen 37077, Germany.
REFERENCES
- Aertsen et al. 1989.Aertsen AMHJ, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity.” J Neurophysiol 61: 900–917, 1989. [DOI] [PubMed] [Google Scholar]
- Ahissar 1998.Ahissar E. Temporal-code to rate-code conversion by neuronal phase-locked loops. Neural Comput 10: 597–650, 1998. [DOI] [PubMed] [Google Scholar]
- Ahissar and Kleinfeld 2003.Ahissar E, Kleinfeld D. Closed-loop neuronal computations: focus on vibrissa somatosensation in rat. Cereb Cortex 13: 53–62, 2003. [DOI] [PubMed] [Google Scholar]
- Ahissar et al. 2001.Ahissar E, Sosnik R, Bagdasarian K, Haidarliu S. Temporal frequency of whisker movement. II. Laminar organization of cortical representations. J Neurophysiol 86: 354–367, 2001. [DOI] [PubMed] [Google Scholar]
- Ahissar et al. 2000.Ahissar E, Sosnik R, Haidarliu S. Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature 406: 302–306, 2000. [DOI] [PubMed] [Google Scholar]
- Ahissar and Zacksenhouse 2001.Ahissar E, Zacksenhouse M. Temporal and spatial coding in the rat vibrissal system. Prog Brain Res 130: 75–87, 2001. [DOI] [PubMed] [Google Scholar]
- Alloway 2008.Alloway KD. Information processing streams in rodent barrel cortex: the differential functions of barrel and septal circuits. Cereb Cortex 18: 979–989, 2008. [DOI] [PubMed] [Google Scholar]
- Alloway et al. 1993.Alloway KD, Johnson MJ, Wallace MB. Thalamocortical interactions in the somatosensory system: interpretations of latency and cross-correlation analyses. J Neurophysiol 70: 892–908, 1993. [DOI] [PubMed] [Google Scholar]
- Alloway et al. 2004.Alloway KD, Zhang M, Chakrabarti S. Septal columns in rodent barrel cortex: functional circuits for modulating whisking behavior. J Comp Neurol 480: 299–309, 2004. [DOI] [PubMed] [Google Scholar]
- Alloway et al. 2002.Alloway KD, Zhang M, Dick SH, Roy SA. Pervasive synchronization of local neural networks in the secondary somatosensory cortex of cats during focal cutaneous stimulation. Exp Brain Res 147: 227–242, 2002. [DOI] [PubMed] [Google Scholar]
- Amitai and Connors 1995.Amitai Y, Connors BW. Intrinsic physiology and morphology of single neurons in neocortex. In: Cerebral Cortex: The Barrel Cortex of Rodents, edited by Jones EG, Diamond IT. New York: Plenum 1995, p. 299–331.
- Armstrong-James and Fox 1987.Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol 263: 265–281, 1987. [DOI] [PubMed] [Google Scholar]
- Bernardo et al. 1990a.Bernardo KL, McCasland JS, Woolsey TA. Local axonal trajectories in mouse barrel cortex. Exp Brain Res 82: 247–253, 1990a. [DOI] [PubMed] [Google Scholar]
- Bernardo et al. 1990b.Bernardo KL, McCasland JS, Woolsey TA, Strominger RN. Local intra- and interlaminar connections in mouse barrel cortex. J Comp Neurol 291: 231–255, 1990b. [DOI] [PubMed] [Google Scholar]
- Bokor et al. 2008.Bokor H, Acsady L, Deschenes M. Vibrissal responses of thalamic cells that project to the septal columns of the barrel cortex and to the second somatosensory area. J Neurosci 28: 5169–5177, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht and Sakmann 2002.Brecht M, Sakmann B. Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex. J Physiol 543: 49–70, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg et al. 1999.Brumberg JC, Pinto DJ, Simons DJ. Cortical columnar processing in the rat whisker-to-barrel system. J Neurophysiol 82: 1808–1817, 1999. [DOI] [PubMed] [Google Scholar]
- Bruno and Simons 2002.Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci 22: 10966–10975, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti and Alloway 2006.Chakrabarti S, Alloway KD. Differential origin of projections from SI barrel cortex to the whisker representations in SII and MI. J Comp Neurol 198: 624–636, 2006. [DOI] [PubMed] [Google Scholar]
- Chakrabarti et al. 2008.Chakrabarti S, Zhang M, Alloway KD. MI neuronal responses to peripheral whisker stimulation: relationship to neuronal activity in SI barrels and septa. J Neurophysiol 100: 50–63, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin and Lin 1984.Chapin JK, Lin CS. Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol 229: 199–213, 1984. [DOI] [PubMed] [Google Scholar]
- Chapin et al. 1987.Chapin JK, Sadeq M, Guise JLU. Corticocortical connections within the primary somatosensory cortex of the rat. J Comp Neurol 263: 326–346, 1987. [DOI] [PubMed] [Google Scholar]
- Chiaia et al. 1991.Chiaia NL, Rhoades RW, Fish SE, Killackey HP. Thalamic processing of vibrissal information in the rat. II. Morphological and functional properties of medial ventral posterior nucleus and posterior nucleus neurons. J Comp Neurol 314: 217–236, 1991. [DOI] [PubMed] [Google Scholar]
- Chmielowska et al. 1989.Chmielowska J, Carvell G, Simons DJ. Spatial organization of thalamocortical and corticothalamic projection system in the rat SmI barrel cortex. J Comp Neurol 285: 325–338, 1989. [DOI] [PubMed] [Google Scholar]
- Diamond et al. 1992.Diamond ME, Armstrong-James M, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol 318: 462–476, 1992. [DOI] [PubMed] [Google Scholar]
- Eggermont 1992.Eggermont JJ. Neural interaction in cat primary auditory cortex. Dependence on recording depth, electrode separation, and age. J Neurophysiol 68: 1216–1228, 1992. [DOI] [PubMed] [Google Scholar]
- Feldmeyer et al. 2006.Feldmeyer D, Lubke J, Sakmann B. Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. J Physiol 575: 583–602, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta et al. 2009.Furuta T, Kaneko T, Deschenes M. Septal neurons in barrel cortex derive their receptive field input from the lemniscal pathway. J Neurosci 29: 4089–4095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein and Perkel 1972.Gerstein GL, Perkel DH. Mutual temporal relationships among neuronal spike trains. Statistical techniques for display and analysis. Biophys J 5: 453–473, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal et al. 2009.Ghoshal A, Pouget P, Popescu M, Ebner F. Early bilateral sensory deprivation blocks the development of coincident discharges in rat barrel cortex. J Neurosci 29: 2384–2393, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson et al. 1999.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999. [DOI] [PubMed] [Google Scholar]
- Hoeflinger et al. 1995.Hoeflinger BF, Bennett-Clarke CA, Chiaia NL, Killackey HP, Rhoades RW. Patterning of local intracortical projection within the vibrissae representation of rat primary somatosensory cortex. J Comp Neurol 354: 551–563, 1995. [DOI] [PubMed] [Google Scholar]
- Johnson and Alloway 1996.Johnson MJ, Alloway KD. Cross-correlation analysis reveals laminar differences in thalamo-cortical interactions in the somatosensory system. J Neurophysiol 75: 1444–1457, 1996. [DOI] [PubMed] [Google Scholar]
- Kawaguchi and Kubota 1993.Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindin-D28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol 70: 387–396, 1993. [DOI] [PubMed] [Google Scholar]
- Killackey 1973.Killackey HP. Anatomical evidence for cortical subdivisions based on vertically discrete thalamic projections from the ventral posterior nucleus to cortical barrels in the rat. Brain Res 51: 326–331, 1973. [DOI] [PubMed] [Google Scholar]
- Killackey and Leshin 1975.Killackey HP, Leshin S. The organization of specific thalamocortical projections to the posteromedial barrel subfield of the rat somatic sensory cortex. Brain Res 86: 469–472, 1975. [DOI] [PubMed] [Google Scholar]
- Kim and Ebner 1999.Kim U, Ebner FF. Barrels and septa: separate circuits in rat barrel field cortex. J Comp Neurol 408: 489–505, 1999. [PubMed] [Google Scholar]
- Kleinfeld et al. 2006.Kleinfeld D, Ahissar E, Diamond ME. Active sensations: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol 16: 1–10, 2006. [DOI] [PubMed] [Google Scholar]
- Koralek et al. 1988.Koralek KA, Jensen KF, Killackey HP. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res 463: 346–351, 1988. [DOI] [PubMed] [Google Scholar]
- Laaris and Keller 2002.Laaris N, Keller A. Functional independence of layer IV barrels. J Neurophysiol 87: 1028–1034, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land and Simons 1985.Land PW, Simons DJ. Cytochrome oxidase staining in the rat SmI barrel cortex. J Comp Neurol 238: 225–235, 1985. [DOI] [PubMed] [Google Scholar]
- Landisman and Connors 2007.Landisman CE, Connors BW. VPM and POm nuclei of the rat somatosensory thalamus: intrinsic neuronal properties and corticothalamic feedback. Cereb Cortex 17: 2853–2865, 2007. [DOI] [PubMed] [Google Scholar]
- Lu and Lin 1993.Lu SM, Lin RC. Thalamic afferents of the rat barrel cortex: a light- and electronmicroscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res 10: 1–16, 1993. [DOI] [PubMed] [Google Scholar]
- Lubke et al. 2000.Lubke J, Egger V, Sakmann B, Feldmeyer D. Columnar organization of dendrites and axons of single and synaptically coupled excitatory spiny neurons in layer 4 of the rat barrel cortex. J Neurosci 20: 5300–5311, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri et al. 2008.Masri R, Bezdudnaya T, Trageser JC, Keller A. Encoding of stimulus frequency and sensor motion in the posterior medial thalamic nucleus. J Neurophysiol 100: 681–689, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick et al. 1985.McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54: 782–806, 1985. [DOI] [PubMed] [Google Scholar]
- Melzer et al. 2006.Melzer P, Sachdev RNS, Jenkinson N, Ebner FF. Stimulus-frequency processing in awake rat barrel cortex. J Neurosci 26: 12198–12205, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen et al. 2003.Petersen CCH, Grinvald A, Sakmann B. Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci 23: 1298–1309, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen and Sakmann 2001.Petersen CCH, Sakmann B. Functionally independent columns of rat somatosensory barrel cortex revealed with voltage-sensitive dye imaging. J Neurosci 21: 8435–8446, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierret et al. 2000.Pierret T, Lavallee P, Deschenes M. Parallel streams for the relay of vibrissal information through the thalamic barreloids. J Neurosci 20: 7455–7462, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter et al. 2001.Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci 21: 2699–2710, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy and Alloway 1999.Roy SA, Alloway KD. Synchronization of local neural networks in the somatosensory cortex: a comparison of stationary and moving stimuli. J Neurophysiol 81: 999–1013, 1999. [DOI] [PubMed] [Google Scholar]
- Shepherd and Svoboda 2005.Shepherd GM, Svoboda K. Laminar and columnar organization of ascending excitatory projections to layer 2/3 pyramidal neurons in rat barrel cortex. J Neurosci 25: 5670–5679, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons 1978.Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol 41: 798–820, 1978. [DOI] [PubMed] [Google Scholar]
- Simons and Woolsey 1979.Simons DJ, Woolsey TA. Functional organization in mouse barrel cortex. Brain Res 165: 327–332, 1979. [DOI] [PubMed] [Google Scholar]
- Sosnik et al. 2001.Sosnik R, Haidarliu S, Ahissar E. Temporal frequency of whisker movement. I. Representations in brain stem and thalamus. J Neurophysiol 86: 339–353, 2001. [DOI] [PubMed] [Google Scholar]
- Urbain and Deschenes 2007.Urbain N, Deschenes M. A new pathway of vibrissal information processing modulated by motor cortex. J Neurosci 27: 12407–12412, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der and Woolsey 1973.Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science 179: 395–398, 1973. [DOI] [PubMed] [Google Scholar]
- Welker 1976.Welker C. Receptive fields of barrels in the somatosensory neocortex of the rat. J Comp Neurol 166: 173–189, 1976. [DOI] [PubMed] [Google Scholar]
- Wong-Riley 1979.Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res 171: 11–28, 1979. [DOI] [PubMed] [Google Scholar]
- Woolsey and van der Loos 1970.Woolsey TA, van der Loos H. The structural organization of layer IV in the somatosensory region, SI, of mouse cerebral cortex. Brain Res 17: 205–242, 1970. [DOI] [PubMed] [Google Scholar]
- Zacksenhouse and Ahissar 2006.Zacksenhouse M, Ahissar E. Temporal decoding by phase-locked loops: unique features of circuit-level implementations and their significance for vibrissal information processing. Neural Comput 18: 1611–1636, 2006. [DOI] [PubMed] [Google Scholar]
- Zhang and Alloway 2004.Zhang M, Alloway KD. Stimulus-induced intercolumnar synchronization of neuronal activity in rat barrel cortex: a laminar analysis. J Neurophysiol 92: 1464–1478, 2004. [DOI] [PubMed] [Google Scholar]