Abstract
Monoamines are strong modulators and/or activators of spinal locomotor networks. Thus monoaminergic fibers likely contact neurons involved in generating locomotion. The aim of the present study was to investigate the serotonergic innervation of locomotor-activated neurons within the thoraco-lumbar spinal cord following induction of hindlimb locomotion. This was determined by immunohistochemical co-localization of serotonin (5-HT) fibers or 5-HT7/5-HT2A/5-HT1A receptors with cells expressing the activity-dependent marker c-fos. Experiments were performed on paralyzed, decerebrate cats in which locomotion was induced by electrical stimulation of the mesencephalic locomotor region. Abundant c-fos immunoreactive cells were observed in laminae VII and VIII throughout the thoraco-lumbar segments of locomotor animals. Control sections from the same segments showed significantly fewer labeled neurons, mostly within the dorsal horn. Multiple serotonergic boutons were found in close apposition to the majority (80–100%) of locomotor cells, which were most abundant in lumbar segments L3–7. 5-HT7 receptor immunoreactivity was observed on cells across the thoraco-lumbar segments (T7–L7), in a dorsoventral gradient. Most locomotor-activated cells co-localized with 5-HT7, 5-HT2A, and 5-HT1A receptors, with largest numbers in laminae VII and VIII. Co-localization of c-fos and 5-HT7 receptor was highest in the L5–L7 segments (>90%) and decreased rostrally (to ∼50%) due to the absence of receptors on cells within the intermediolateral nucleus. In contrast, 60–80 and 35–80% of c-fos immunoreactive cells stained positive for 5-HT2A and 5-HT1A receptors, respectively, with no rostrocaudal gradient. These results indicate that serotonergic modulation of locomotion likely involves 5-HT7/5-HT2A/5-HT1A receptors located on the soma and proximal dendrites of serotonergic-innervated locomotor-activated neurons within laminae VII and VIII of thoraco-lumbar segments.
INTRODUCTION
A substantial amount of information exists concerning the innervation of the spinal cord by noradrenergic and serotonergic fibers. Recent studies have focused on the synaptic relations of these monoaminergic fibers to identified spinal neurons (e.g., Alvarez et al. 1998; Jankowska et al. 1995, 1997; Pearson et al. 2000) and on the distribution of monoamine receptor subtypes within the spinal cord (e.g., Doly et al. 2004, 2005; Giroux et al. 1999; Helton et al. 1994; Thor et al. 1993). However, the distribution of monoamine receptors and terminals of monoaminergic fibers on spinal neurons that are activated during locomotion is poorly understood. Because monoamines may induce or modulate ongoing locomotor activity (e.g., Barbeau and Rossignol 1990, 1991; Brustein and Rossignol 1999; Chau et al. 1998; Feraboli-Lohnherr et al. 1999; Rossignol et al. 1986), it is expected that terminals of monoaminergic fibers make contact onto neurons that are involved in the generation of locomotion and, subsequently, affect their activity. While it can be expected that monoaminergic terminals make synaptic contact on locomotor neurons, a number of studies have also documented a high percentage of nonsynaptic monoaminergic terminations in spinal tissue (Marlier et al. 1991a; Maxwell et al. 1983; Rajaofetra et al. 1992; Ridet et al. 1993, 1994) including those observed on identified spinal neuronal soma (Jankowska et al. 1997). Indeed, recent studies have demonstrated substantial increases in extracellular levels of 5-HT (and norepinephrine) within the spinal cord following stimulation of the nucleus raphe magnus/locus ceruleus (Hentall et al. 2003, 2006) and the mesencephalic locomotor region (Noga et al. 1999, 2006, 2007) indicating that the control of spinal neurons by descending monoaminergic pathways is, in part, mediated by extrasynaptic or volume transmission (Agnati et al. 1995; Zoli et al. 1998). Calculations by Bunin and Wightman (1998) for 5-HT release within the substantia nigra reticulata indicate that even for brief stimulation, 5-HT diffuses on average 20 μm in 200 ms. In the striatum, dopamine diffuses a mean of 10 μm from release sites within 50 ms (Wightman and Zimmerman 1990) and norepinephrine diffuses 10 μm in the locus ceruleus, interacting subsequently with autoreceptors and transporters (Callado and Stamford 2000). Thus the spatial requirements of monoaminergic terminals in relation to target neurons are not as strict for nonsynaptic neurotransmission as they are for synaptic transmission.
This study examines locomotor-activated neurons within the thoraco-lumbar spinal cord in the cat. It documents the distribution of locomotor-activated neurons with soma and proximal dendrites that are innervated by or in close proximity to serotonergic terminals and whether or not they possess 5-HT7, 5-HT2A, or 5-HT1A receptors. These three major 5-HT receptor subtypes have been previously implicated in the production of locomotion (Antri et al. 2003; Beato and Nistri 1998; Hochman et al. 2001; Landry et al. 2006; Liu and Jordan 2005; Liu et al. 2009; Madriaga et al. 2004; Pearlstein et al. 2005; Ung et al. 2008). We chose to utilize the fictive locomotion preparation (Noga et al. 2003) to investigate cells activated by centrally generated signals rather than those that are activated by sensory feedback from the moving limb to gain a perspective on cells responsible for generating locomotor movements (Dai et al. 2005). For the present study, the middle-lower lumbar segments were chosen for immunohistochemical processing because they are known to contain neurons believed to be involved in the production of hindlimb locomotion (Dai et al. 2005; Huang et al. 2000; Noga et al. 1995). The lower thoracic/upper lumbar segments were also taken for comparison because this area is known to play a major role in the generation of locomotor activity in the rat (Bertrand and Cazalets 2002; Cazalets et al. 1995; Kjaerulff and Kiehn 1996; Magnuson et al. 1999). We used fluorescent c-fos immunohistochemistry (Herdegen and Leah 1998) as an activity-dependent marker following mesencephalic locomotor region (MLR) induced fictive locomotion (Dai et al. 2005; Huang et al. 2000). Cells were inspected for co-localization with either 5-HT-immunoreactive axons/boutons (Jankowska et al. 1997) or 5-HT7, 5-HT2A, and 5-HT1A receptors (Cornea-Hébert et al. 1999; Neumaier et al. 2001). The results demonstrate that the majority of locomotor-activated neurons in lumbar segments are innervated by or are in close proximity to serotonergic boutons and possess 5-HT7, 5-HT2A, and 5-HT1A receptors. The data reveal an anatomical basis for serotonergic modulation and activation of locomotor activity (e.g., Antri et al. 2002, 2003; Barbeau and Rossignol 1990, 1991; Brustein and Rossignol 1999; Cazalets et al. 1992; Cowley and Schmidt 1994; Feraboli-Lohnherr et al. 1999; Kiehn and Kjærulff 1996) and supports the concept that MLR evoked locomotion is mediated by the parallel activation of descending reticulospinal (Noga et al. 2003) and monoaminergic pathways (Noga et al. 1999, 2006, 2007). Furthermore, they indicate that laminae VII and VIII are likely sites for serotonergic modulation (via 5-HT7, 5-HT2A, and 5-HT1A receptors) of the centrally activated locomotor network. Preliminary data has been presented previously (Johnson et al. 2002).
METHODS
Animal preparation
Experiments were carried out on adult cats (Felis silvestris catus) in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 80–23; revised 1996) and following protocol approval by the University of Miami's Institutional Animal Care and Use Committee. The number of animals used, and their pain and distress, were minimized. Animals weighing between 3.25 and 4.3 kg were anesthetized with halothane in a mixture of 70% nitrous oxide and 30% oxygen. Anesthetic was initially delivered through a face mask until the trachea could be intubated for direct administration. The left common carotid was cannulated and connected to a pressure transducer for blood pressure monitoring. The right common carotid artery was loosely tied for temporary occlusion to prevent excess loss of blood during the decerebration. The right external jugular vein was cannulated for the administration of fluids and drugs. A bicarbonate solution (100 mM NaHCO3 with 5% glucose) was infused at 5 ml/h throughout the experiment to replace fluid loss and help maintain a normal pH balance. Lactated Ringer solution was also administered to replace fluid and electrolyte loss and to maintain blood pressure ≥80 mmHg throughout the experiment. Body temperature was maintained at ∼37°C using feedback-controlled heating lamps. Nerves to muscles supplying the hindlimb and forelimb were dissected, bilaterally, and mounted in tunnel electrodes. These included nerves to quadriceps, sartorius, (and anterior biceps in locomotor cat 2 only), triceps brachii, and biceps brachii. The head of each animal was placed in a Transvertex headframe and the body suspended with all four limbs pendant using a vertebral clamp placed on a low thoracic vertebral spinous process and hip-pins attached to the iliac crests. Following a craniotomy, the anesthetic delivery was terminated and while the animal was still anesthetized, a precollicular-postmammillary decerebration was rapidly performed. Blood volume lost during the decerebration was replaced with intravenous injection of saline or lactated Ringers solution. Each animal was given 2–4 mg dexamethasone (Hexadrol phosphate, Organon) intravenously to prevent tissue swelling. After a brief recovery period, the animals were paralyzed with pancuronium bromide (Astra, Westborough, MA: 0.1–0.2 mg/kg as needed—usually every 1–2 h) and artificially ventilated. Expired CO2, O2, and tissue oxygenation (SpO2) was monitored throughout the experiment using a Datex Oscaroxy multigas monitor and pulse oximeter and the end tidal CO2 maintained between 3.5 and 4.5%. Blood gas determinations were done periodically throughout the experiments (every 1–2 h) to ensure pCO2, pO2, bicarbonate and pH levels were within normal ranges.
Stimulation and data collection
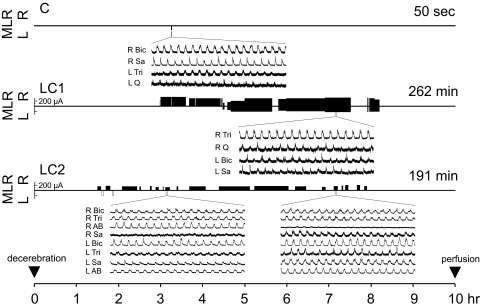
Following a recovery period of 1.5-3 h after decerebration, four-limbed locomotion was evoked by electrical stimulation of the MLR (1.0-ms square-wave pulses, 15–20 Hz, 110–325 μA) using a monopolar stimulating electrode (SNE-300; David Kopf Instruments) as previously described (Noga et al. 2003). In the absence of movement, the rhythmic activity of the peripheral nerves (electroneurograms) was used as a monitor of “fictive” locomotion (Fig. 1). Representative electroneurogram activity was obtained from the bouts of locomotion throughout the stimulation period. The electroneurograms were amplified, rectified and low-pass filtered (10- or 20-ms time constant) and subsequently digitized at 4 kHz for later analysis. The strength of stimulation was adjusted to a level that was suitable to maintain locomotion for prolonged periods. Rest periods were allowed when locomotor activity dwindled and increasing the stimulation strength failed to improve it. In a few instances (2 times in each locomotor cat), slight movements of the limbs were noted as the animals came out of paralysis. When this occurred, MLR stimulation was ceased immediately and only resumed once paralysis was re-established. Only one control animal was used because the number of cells with c-fos labeling in control animals in previous studies (n = 6, Dai et al. 2005; n = 3, Huang et al. 2000) was highly consistent and significantly lower than animals performing the locomotor task. The control cat received identical treatment as the locomotion test animals, except that it was not subject to the locomotor task once it was determined that it was capable of producing locomotion with MLR stimulation (Fig. 1). This was done to ensure that the health or viability of brain stem and spinal cord was comparable to locomotor animals.
FIG. 1.
Experimental history of control (C) and locomotor cats 1 and 2 (LC1, LC2) over the 10-h period between decerebration and perfusion. The side, intensity, and duration of mesencephalic locomotor region (MLR) stimulation throughout each experiment is indicated along the timeline according to the scale at left. Locomotion was successfully generated where stimulation episodes were filled (black). Explorative (nonlocomotor generating) stimulation is indicated by empty (white) stimulation periods. All animals were capable of generating locomotion with stimulation, indicating comparable health of the brain stem and spinal cord, but the MLR of the control animal was only briefly stimulated. Total locomotor time is indicated to the right above each timeline. Fictive locomotor activity was monitored by electroneurographic recordings from peripheral nerves from all limbs and is illustrated for representative nerves from ≥1 nerve/limb. R, right; L, left; Q, quadriceps; Sa, sartorius; AB, anterior biceps (LC2 only); Tri, triceps brachii; and, Bic, biceps brachii.
In all of the experiments reported here, there was a 10-h interval between decerebration and perfusion to minimize c-fos expression from surgery. Animals were re-anesthetized with halothane and transcardially perfused with normal saline prefixative (0.3 ml/g of animal weight) containing 0.1% NaNO2 and 100 unit/ml heparin, followed by 4% paraformaldehyde, 0.2% picric acid in 0.1 M phosphate-buffered saline (4°C), pH 7.4 (1 ml/g of animal weight). The spinal cord and brain stem were removed, postfixed in the same fixative solution for 5 h, and cryoprotected by washing in 0.1 phosphate buffer containing 25% sucrose, 10% glycerol, and 0.001% sodium azide in 50 mM phosphate buffer at 4°C for several days. The total length of the spinal cord and each segment was measured before dividing the segments for sectioning.
Immunohistochemistry
Frozen tissue sections of 30 μm thickness were sectioned coronally with a sliding microtome and collected in 0.1 M phosphate-buffered saline (PBS). To optimize immunohistochemical procedures, a small group of sections were randomly collected from the rostral half of each lumbar segment or from cervical segments and a primary antibody dilution series performed. In addition, for the preadsorption control, cat tissue sections were incubated only with preimmuno serum without the primary antibodies. Immunoreactivity was totally absent after omission of all primary antibodies. A group of equally spaced sections was processed with immunohistochemical procedures to label c-fos and serotonergic fibers (19–20 sections each for a total of 570–600 μm). To visualize c-fos, the sections were prewashed in 0.1 M PBS, rinsed in 0.3% H2O2 and PBS, then blocked in PBS containing 0.3% Triton X-100 and 10% normal goat serum (NGS) albumin (S-1000: Vector Laboratories, Burlingame, CA) at room temperature. They were then incubated for 48 h in rabbit polyclonal anti-c-fos protein IgG (PC38-100U: Oncogene Research Products/Calbiochem, San Diego, CA: generated against synthetic peptide sequence corresponding to amino acids 4–17 of human c-fos and recognizing the ∼55 kDa c-fos and ∼62 kDa v-fos proteins) 1:2,500 in 0.2 M PBS containing 0.3% Triton X-100 and 5% NGS albumin at 4°C. To label serotonergic fibers with fluorescent immunohistochemical techniques, sections were incubated overnight with rat monoclonal anti-5-HT IgG (MAB352: Chemicon International, Temecula, CA: generated against 5-HT conjugated to bovine serum albumin) 1:100 in 0.3% Triton X-100 and 5% NGS at 4°C. Each secondary antibody was conjugated to a different fluorophore (Molecular Probes-Invitrogen, Carlsbad, CA): Alexa 488 (green) for c-fos (1:500; goat anti-rabbit; A-11008) and Alexa 594 (red) for 5-HT (1:200; goat anti-rat; A-11007). Incubations were for 1.5 h in 0.1 M PBS with 0.3% Triton X-100 and 3% NGS. Incubations with each secondary antibody were performed following each primary (c-fos protocol first). Some sections were also incubated for 15 min in NeuroTrace 640 Nissl (blue) in PBS (1:100) at room temperature to increase visibility of the soma. For 5-HT7 and 5-HT2A receptor immunocytochemistry, sections reacted with mouse monoclonal anti-c-fos IgG (sc-8047: Santa Cruz Biotechnology, Santa Cruz, CA) 1:300 in 5% NGS were incubated with either rabbit polyclonal anti-5-HT7 (No. 24430; Immunostar: generated against synthetic peptide sequence corresponding to amino acids 8–23 of rat 5-HT7 receptor) 1:100 in 5% NGS (overnight at 4°C) or immunoaffinity purified rabbit polyclonal anti 5-HT2A IgG (PC176: Oncogene Research Products/Calbiochem: generated against the ∼53 kDa synthetic peptide corresponding to amino acids 22–41 of the rat 5-HT2A receptor) 1:200 in 5% NGS. For 5-HT1A receptors, sections reacted with mouse monoclonal anti-c-fos IgG (sc-8047: Santa Cruz Biotechnology) 1:300 in 5% normal donkey serum (NDS) albumin (S30: Chemicon International) were incubated with goat polyclonal anti 5-HT1A IgG (sc-1459: Santa Cruz Biotechnology: generated against a peptide mapping at the C-terminus of SR-1A of human origin, 55/65 kDa) 1:100 in 5% NDS (overnight at 4°C). Secondary antibodies were conjugated to Alexa 488 (green) for c-fos (1:500, goat anti-mouse; A-11011; 3% NGS; or 1:500, donkey anti-mouse, A-21202, 3% NDS) and Alexa 594 (red) for 5-HT receptors (1:200; goat anti-rabbit, A-11012; 3–5% NGS for 5-HT7 or 5-HT2A receptors and 1:200, donkey anti-goat, A-11058; 3% NDS for 5-HT1A receptors). Sections were mounted on gelatin coated glass slides, dried for 1 h at room temperature, coated with Vectashield (Vector Laboratories), then coverslipped and stored at 4°C.
Sections of the midbrain were also processed (Noga et al. 2008) to determine the location of the MLR stimulation sites (determined from depth measurements taken from the surface of the inferior colliculus of the electrode along the reconstructed electrode tracks and also from a small electrolytic lesion made in locomotor cat 2).
To establish antigen specificity for the 5-HT7 primary antibody, an additional Western blot control experiment was run. The 5-HT7 antibody was characterized against human cloned 5-HT7A receptors (gift from Guthrie cDNA Resource Center, Guthrie Research Institute, Sayre, PA) and rat, cat, and human tissues. Western blots demonstrated that the 5-HT7 antibody recognizes specific epitopes (49 kDa) in rat (frontal cortex and hippocampus), cat (spinal cord) and human brain (spinal cord and hippocampus) tissues and for 5-HT7A and 5-HT7B receptor from stably expressing cell lines.
Data analysis and interpretation
Sections were examined with Zeiss Axioline microscopes using fluorescence microscopy. Cells were mapped using Neurolucida software. Cell counts were done using stereologic cell counting methods (Stereo Investigator 5.0, Microbrightfield Bioscience, Williston, VT) giving estimates of cell number/segment in different laminae. Confocal microscopy (Zeiss LSM510, with Ar multiline and HeN1 564) was used to examine the three-dimensional structure of the contacts in selected cells. Selected cells were scanned in a series of optical sections and three-dimensional reconstructions of the images of the cells and terminals photographed.
“Contacts,” used synonymously with “close appositions,” were defined (Pearson et al. 2000) as: the presynaptic element must be a swelling of the axon; the postsynaptic element must contain a c-fos-positive nucleus surrounded by a clearly observed soma; and, the presynaptic and postsynaptic elements should be juxtaposed with little or no intervening space between them; i.e., no gap discernible at high magnification when both are in the same focal plane, or for profiles lying on top of or underneath each other, the structures should be resolvable by minimal focusing adjustments (see also Alvarez et al. 1998; Jankowska et al. 1995; Pilowski et al. 1990; Voss et al. 1990).
Differences in the number of 5-HT-innervated cells per section among the three animals [control, locomotor cats 1 and 2, (LC1 and LC2)] were analyzed using a one-way ANOVA followed by Bonferroni corrected post hoc test. For each segment of each animal (i.e., C, LC1, and LC2), cells were counted for 19 sections. All statistical tests were two-tailed with significance level of P ≤ 0.05 using Systat statistical software, version 12.0 (Point Richmond, CA).
RESULTS
Four-limbed locomotion was evoked by stimulation of the MLR in both experimental animals, for a total of ∼3.25-4.5 h over a period of ∼5–6.5 h (Fig. 1). In LC1, bilateral stimulation was necessary midway through the experiment to maintain locomotion. The stimulation strength was reduced whenever possible to ∼50–75% of the maintenance strength required for unilateral stimulation in previous trials. In LC2, one-sided stimulation was sufficient to maintain four-limbed locomotion for prolonged periods of time. The control cat was capable of four-limbed locomotion but was stimulated only briefly (∼1 min), 7 h before perfusion. Thus the brain stem and spinal cord viability was comparable in control and locomotor cats. Tips of brain stem stimulating electrodes from all animals were located in the caudal pole of the cuneiform nucleus.
5-HT/c-fos immunohistochemistry
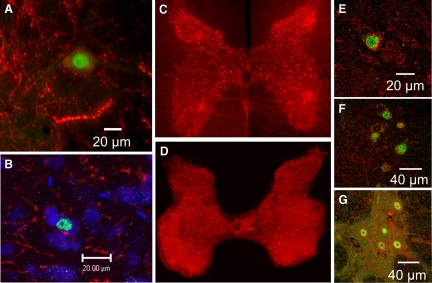
C-fos labeled nuclei were observed in numerous spinal neurons in the locomotor cats (Fig. 2, A and B). In most cases, the labeled nuclei were observed juxtaposed to fibers and varicosities stained positive for 5-HT. Serotonergic axons were seen to course across the soma with two to four boutons/fiber in close apposition to the surface. Two or more of these fibers were often observed, although this was not quantified. The proximity of 5-HT fibers and neurons staining positive for c-fos was examined in multiple spinal sections to produce complete maps (T12–L7) of the serotonergic innervation of c-fos-labeled neurons from control and locomotor animals.
FIG. 2.
Representative immunocytochemical identification of locomotor-activated neurons by c-fos nuclear labeling (green), juxtaposed to fibers and varicosities (red) stained positive for 5-HT (A and B). The cells in B have an additionally Nissl-stained cytoplasm (blue). Cells located in lamina VII of the 6th lumbar (L6) segment of LC1. Photomontages of 5-HT7 receptor stained neurons (red) from single sections of the L1 and L5 lumbar segments of LC1 are illustrated in C and D, respectively. Higher power confocal images showing the co-localization of 5-HT7, 5-HT2A, and 5-HT1A receptors (red) and c-fos (green) is illustrated for locomotor-activated neurons in E–G, respectively. E: lamina VII, L5 segment, LC2, 4 optical slices. F: medial lamina VII, L5 segment, LC1, single optical slice. G: lateral lamina VII, L4 segment, LC2, single optical slice.
CONTROL EXPERIMENT.
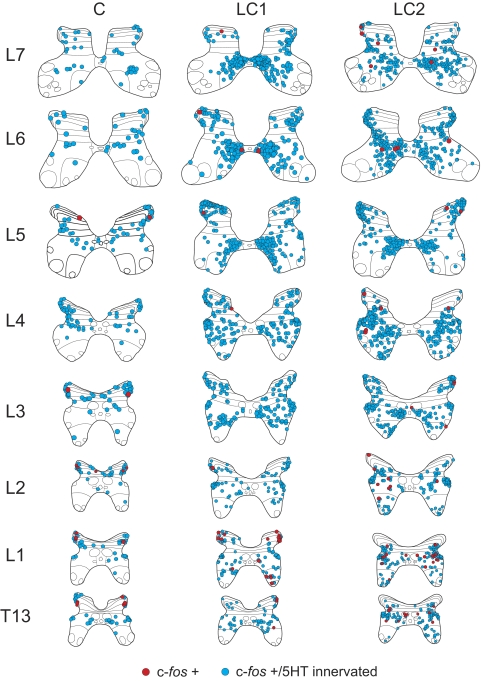
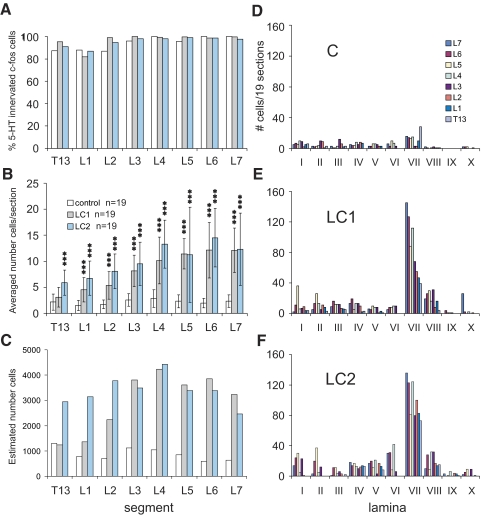
Maps constructed from spinal segments in the control animal showed small numbers of neurons containing c-fos-labeled nuclei (Fig. 3). The percentage of c-fos-positive cells contacted by serotonergic fibers was between 85 and 100% (Fig. 4A) with upper segments (T13–L2) showing a slightly lower percentage overall. Most contacted cells were found scattered in the dorsal horn with additional cells in intermediate zone areas and few within the ventral horn (Fig. 4D). The average number of c-fos-labeled cells contacted by serotonergic fibers per section averaged <3 (Fig. 4B). Taking the number of sections/segment into consideration (which varied according to the length of each segment), the overall number of c-fos cells with serotonergic contacts within the control cord was estimated to be between 500 and 1,200/segment with no obvious rostrocaudal trends (Fig. 4C).
FIG. 3.
Maps showing the distribution of c-fos immunoreactive neurons with or without serotonergic contacts in the T12–L7 thoraco-lumbar spinal segments of control (C) and locomotor cats (LC1, LC2). C-fos-positive cells with serotonergic contacts are indicated with blue circles. Those without are indicated with red circles (see legend). Each diagram includes all labeled cells from 19 sections of that segment. Each dot represents one labeled cell in this and other figures. In the control animal, labeled cells were lightly scattered in the dorsal horn and intermediate zone with the majority of them showing serotonergic contacts. Substantially more cells were seen in animals undergoing fictive locomotion than in the control. Greatest numbers of contacted cells were observed in lamina VII with a tendency for these cells to cluster in medial areas in lower lumbar segments. The spinal laminae illustrated for each segment in this and other figures are identified according to the classification of Rexed (1954).
FIG. 4.
Percentage and distribution (segmental and laminar) of serotonergic innervated c-fos-positive neurons observed within the T13–L7 thoraco-lumbar segments of control and locomotor animals. The percentage of serotonergic innervated c-fos-labeled cells observed in 19 sections from each segment of LC1, LC2, and C cats is compared in A. Note that 82–100% of the cells showed serotonergic contacts. The total number of cells/section (mean ± SD) for each segment is illustrated in B. Middle and caudal lumbar segments showed the largest number of labeled cells/section. The total number of innervated cells per section were significantly greater in cats undergoing locomotion (Asterisks, P < 0.001) than in control animals, with the exception of the T13 segment for LC1 (see results). The number of innervated locomotor-activated neurons estimated for each segment (taking into account the length of each segment) is plotted in C. Note that the number of serotonergic contacted cells is estimated to peak in the L4 segment of both locomotor animals. The laminar distribution of serotonergic innervated neurons (from 19 sections) within the different segments of locomotor and control animals is shown in D–F. A dramatic increase in the number of labeled neurons was observed in lamina VII of locomotor animals with largest numbers found in more caudal segments.
LOCOMOTION EXPERIMENTS.
The locomotor task produced a clear increase in c-fos-labeled cells in the intermediate zone and ventral horns (laminae VII and VIII; Fig. 3). Groupings of neurons were apparent in medial areas of gray matter of lower (L5–7) lumbar segments. At more rostral locations, cells in the intermediate zone were spread out, blending into lamina VIII of the ventral horn. As described before (Dai et al. 2005), labeling of the motor nuclei was rare, indicating that most motoneurons do not express c-fos in response to repetitive activation. Serotonergic immunoreactive boutons were found in close apposition to the soma (Fig. 2A) of the large majority (82–100%) of c-fos-positive spinal neurons from the thoraco-lumbar segments (Fig. 4A) with a tendency for rostral segments (T13–L2) to show a slightly lower percentage. The average number of c-fos-labeled cells/section contacted by serotonergic fibers (calculated from 19 sections from each segment) increased with locomotor activity (Fig. 4B). The total number of cells/section was largest in L4–7 segments (10–15, peaking in L6 for both locomotor cats) and decreased rostrally. Expressed as a percentage increase over that observed in control sections, the increase was largest in L6 (524 and 646% for LC1 and LC2, respectively). Percentage increases above control for the T13–L7 segments from locomotor animals were, respectively: LC1, 44, 197, 209, 216, 257, 393, 524, and 411%; LC2 173, 341, 367, 269, 367, 386, 646, and 420%. The ANOVA demonstrated that the number of innervated cells per section was significantly different between control and locomotor cats. The F-values for the ANOVA ranged between 16.6 for L5 and 71.7 for L6 and were all highly significant (P < 0.000). The subsequent Bonferroni post hoc analyses revealed that the number of innervated cells per section was significantly greater in locomotor animals than in the control animals in all but one segment (T13 of LC1; Fig. 4B). Taking the length of each individual segment into consideration (7–15 mm), the average number of innervated cells/section and a section thickness of 30 μm we estimated the overall number of contacted c-fos cells within the T13–L7 segments of locomotor animals to range between 1,200 and 4,500 neuron/segment (Fig. 4C) with a peak at the L4 segment in both animals.
The laminar distributions of serotonergic innervated neurons within the different segments from both locomotor and control animals are plotted in Fig. 4, D–F. In locomotor animals, the largest number of labeled cells with serotonergic contacts was observed in lamina VII. In general, the number of contacted cells in lamina VII was higher in more caudal lumbar segments, decreasing as one progressed to more rostral segments. Increased numbers of contacted c-fos-labeled neurons were also found in other laminae of locomotor animals compared with control. Of this group, the largest and most consistent change (increase) was that seen in lamina VIII for both locomotor animals. This was especially the case for mid-lumbar segments. The labeling of dorsal horn neurons appeared to be weak, however, and could have been related to the slight movements observed during MLR stimulation in both locomotor animals when the paralytic temporarily wore off (see Stimulation and data collection).
5-HT c-fos immunohistochemistry
Sections taken from control and locomotor animals were processed for 5-HT7 immunoreactivity. The overall pattern of immunolabeling observed in LC1 is shown in Figs. 2, C and D (photomontages of L1 and L5 segments), and in 5 (schematics of various thoraco-lumbar segments). Labeled neurons were seen in all segments and all laminae examined. The numbers and locations of labeled cells were very similar in both control (not shown) and locomotor animals. Highest numbers were found in lower lumbar sections as section area increased. Overall, the staining pattern was similar for thoracic and lumbar sections: staining was predominant in small cells of superficial dorsal horn (laminae I–III), diminishing progressively toward deeper laminae. Laminae VI and VII also showed substantial numbers of 5-HT7-positive neurons, especially in middle and lower lumbar segments and aggregations could be observed in medial areas, where locomotor-activated c-fos-positive neurons (medial lamina VII) were present (Fig. 5 : L4–7). 5-HT7 immunoreactive neurons were also present in lamina VIII. A few motoneurons were also stained positive (large cells in Fig. 2D) although this was not always evident from lower lumbar sections (Fig. 5).
FIG. 5.
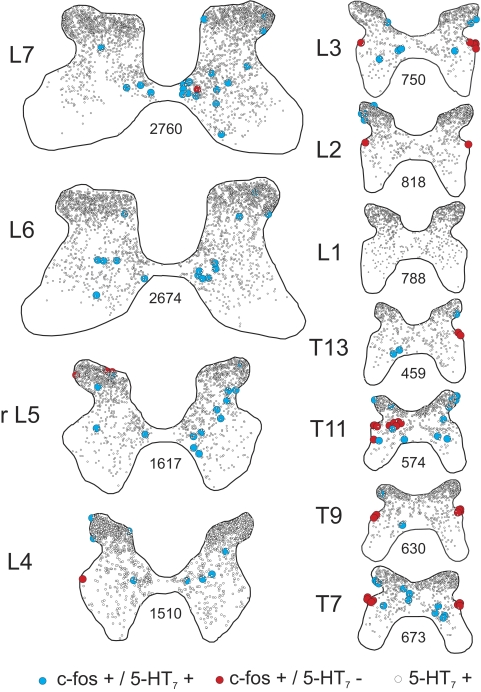
5-HT7/c-fos immunohistochemistry of single spinal cord sections taken from the thoracolumbar (T7–L7) segments of locomotor cat LC1. 5-HT7 receptor positive cells and c-fos-positive cells labeled with or without 5-HT7 receptors are indicated with open, blue, and red circles, respectively (see legend). The number of 5-HT7 receptor immunoreactive neurons from each section is indicated. rL5: rostral L5.
5-HT7 receptors were co-localized with neurons staining positive for c-fos as illustrated in the higher power confocal image in Fig. 2E. The occurrence and location of this co-localization observed in single sections (Fig. 5) was documented for multiple sections from the various segments of locomotor cats (19 sections for LC1; 12 sections for LC2) to produce maps of 5-HT7/c-fos-labeled neurons (Figs. 6 and 7). In locomotor animals (as described before: see Fig. 3), increased numbers of c-fos-labeled cells in laminae VII and VIII were observed in both lumbar and thoracic segments (Figs. 6 and 7). The majority of these c-fos-positive neurons were immunoreactive for 5-HT7 receptors. Interestingly, most cells in the intermediolateral cell column (T7–L4) did not stain for 5-HT7 receptors even though cells in this area of the T13–L4 segments were innervated by serotonergic fibers (Fig. 3). Analysis of spinal segments of the control animal showed small numbers of c-fos-positive cells in lumbar segments (as described before; see Fig. 3), but the majority of these cells were also immunoreactive for 5-HT7 receptors. In thoracic segments, most of these doubly labeled cells were found in the dorsal horn and were few in the intermediate zone.
FIG. 6.
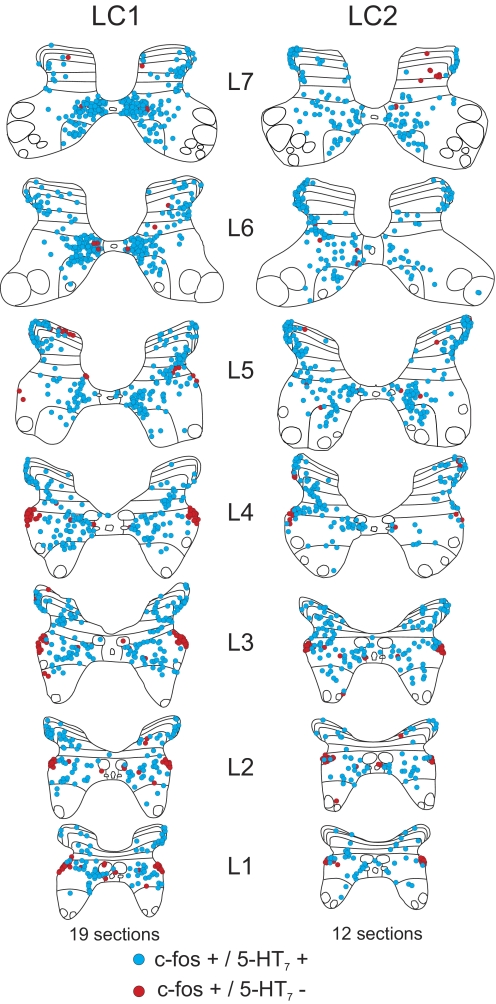
Maps of c-fos immunoreactive neurons co-localized with or without 5-HT7 receptors from the lumbar segments (L1-7) of locomotor cats LC1 and LC2. C-fos-positive cells labeled with or without 5-HT7 receptors are indicated with blue and red circles, respectively. Each diagram includes all labeled cells from 19 (LC1) or 12 (LC2) sections of that segment.
FIG. 7.
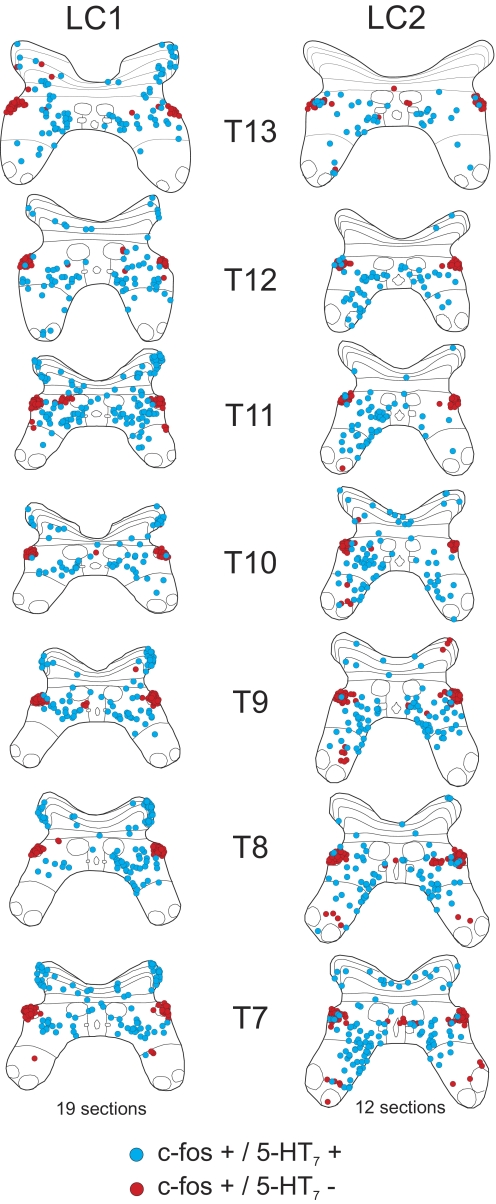
Maps of c-fos immunoreactive neurons co-localized with or without 5-HT7 receptors from the thoracic segments (T7–13) of locomotor cats LC1 and LC2. C-fos-positive cells labeled with or without 5-HT7 receptors are indicated with blue and red circles, respectively. Each diagram includes all labeled cells from 19 (LC1) or 12 (LC2) sections of that segment.
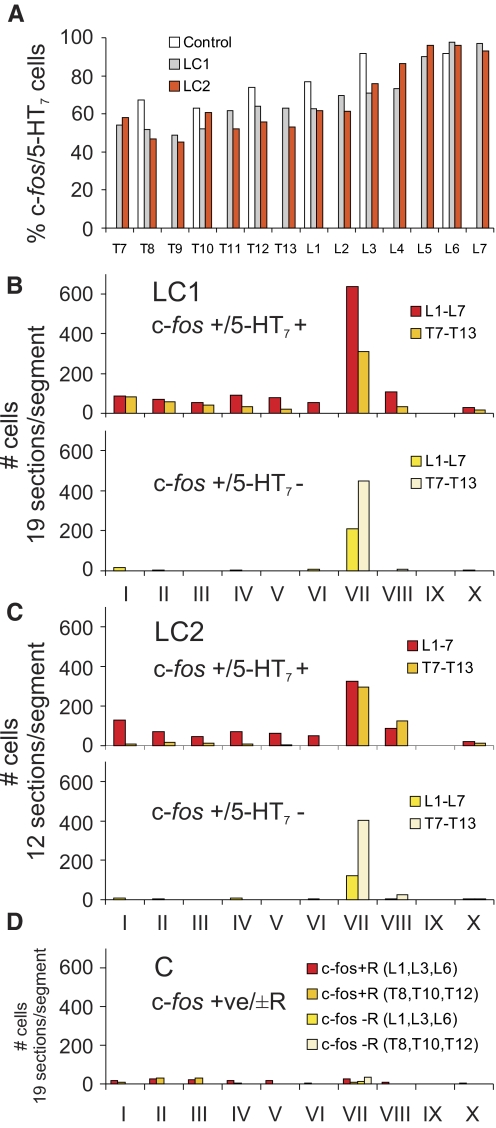
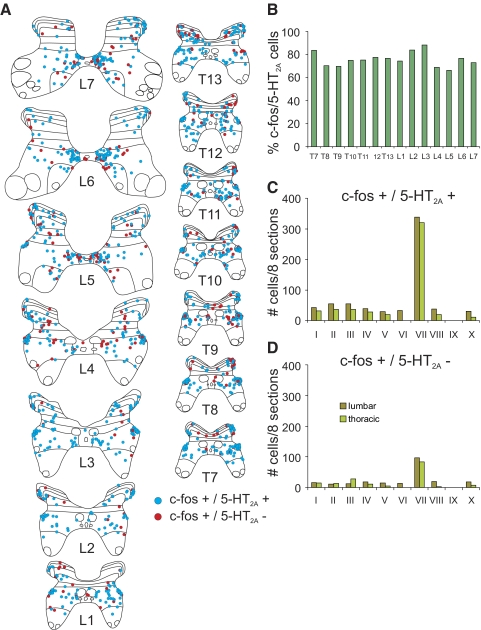
The relative proportion of 5-HT7 immunoreactive c-fos-positive spinal neurons from the thoraco-lumbar segments is illustrated in Fig. 8A. In locomotor animals, the greatest percentage of co-localization was found in lower lumbar segments (∼90–100%). The degree of co-localization decreased rostrally (∼60–70% in upper lumbar segments; ∼40–60% in thoracic segments). A similar rostrocaudal gradient was observed in the control. Examination of the maps in Figs. 6 and 7 indicates that the rostrocaudal gradient was due, in part, to the appearance of a group of c-fos-positive cells in the intermediolateral cell column (lateral lamina VII) that did not stain for the 5-HT7 receptor. The majority of other c-fos-labeled cells were immunoreactive for 5-HT7 receptors. The laminar distribution of 5-HT7 immunoreactive (and nonimmunoreactive) c-fos staining neurons within each thoraco-lumbar segment from locomotor and control animals is illustrated in Fig. 8, B–D. In locomotor animals, the largest number of c-fos immunoreactive cells stained positive for 5-HT7 receptor in lumbar and thoracic segments was found in lamina VII (as described before for serotonergic innervated cells—see Fig. 3). However, relatively larger numbers of cells were found in the lumbar segments compared with the thoracic segments in LC1 than that seen in cat LC2. Cells staining positively for c-fos but not for 5-HT7 receptors were also found primarily in lamina VII of both locomotor cats. More cells of this type, however, were found in thoracic segments (intermediolateral cell column). In the control animal, 5-HT7 immunoreactive c-fos-positive spinal neurons were commonly found in dorsal horn as well as lamina VII, although they were at about equal amounts in thoracic and lumbar areas. Cells staining positively for c-fos but not for the 5-HT7 receptor were found primarily in lamina VII.
FIG. 8.
Segmental and laminar distribution of co-localized c-fos/5-HT7 receptor positive neurons within the T7–L7 thoaco-lumbar segments of control and locomotor animals. The percentage of c-fos-labeled cells immunoreactive for 5-HT7 receptors observed within 12 or 19 sections taken from each segment of LC1, LC2, and C cats is plotted in A. Note that the percent co-localization increases caudally to peak in the lower lumbar segments. The laminar distribution of c-fos-labeled cells with (top) or without (bottom) co-localized 5-HT7 receptors (R) within the different segments from LC1, LC2, and C animals is illustrated in B–D, respectively. Data are categorized into lumbar or thoracic regions with an equal number of sections and segments tabulated for each category within each animal. In locomotor animals, largest numbers of cells of both types were found in lamina VII of thoracic and lumbar regions. In the control, c-fos cells stained positive for 5-HT7 receptor were relatively more abundant in the dorsal horn.
5-HT2A/c-fos immunohistochemistry and co-localization
Sections from thoraco-lumbar segments (T7–L7) of LC1 were processed for c-fos and 5-HT2A receptor immunoreactivity (Fig. 2F). The majority of c-fos immunoreactive cells were also immunoreactive for the receptor as can be seen for maps constructed from thoraco-lumbar segments (Fig. 9A). Approximately 70–90% of the neurons showed co-localization with no obvious trends in the rostrocaudal distribution (Fig. 9B). Co-localized cells were found mostly in lamina VII and included cells in the intermediolateral cell column (Fig. 9, A, C, and D), in contrast to cells co-localizing with 5-HT7 receptors. Lamina VII neurons showed a tendency to cluster in the medial gray areas of L5–7 lumbar segments and then spread out at more rostral locations. Cells staining positively for c-fos but not for 5-HT2A receptors were also most numerous in lamina VII.
FIG. 9.
Segmental and laminar distribution of colocalized c-fos/5-HT2A-positive neurons. Maps of c-fos immunoreactive neurons co-localized with or without 5-HT2A receptors from the T7–L7 thoraco-lumbar segments of LC1 are shown in A. C-fos-positive cells labeled with or without 5-HT2A receptors are indicated with blue and red circles, respectively. Each diagram includes all labeled cells from 8 sections of that segment. The percentage of c-fos-labeled cells immunoreactive for 5-HT2A receptors observed within these sections for the different segments is plotted in B. The percentage varied between ∼70 and 90% without any apparent rostrocaudal distribution. The laminar distribution of c-fos-labeled cells with or without co-localized 5-HT2A receptors within the thoraco-lumbar segments is illustrated in C and D, respectively. Data are categorized into lumbar and thoracic regions with an equal number of sections and segments for each category. Note that the largest numbers of cells of both types were found in lamina VII of thoracic and lumbar regions.
5-HT1A/c-fos immunohistochemistry and co-localization
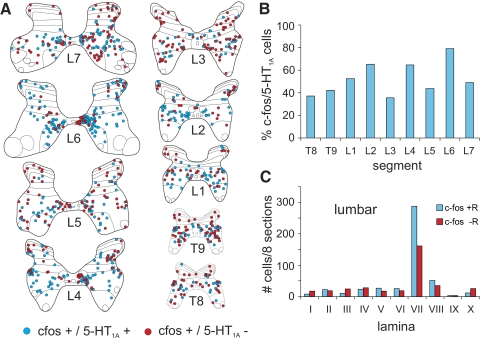
Sections from thoracolumbar segments (T8, T9, L1–L7) of LC1 were processed for c-fos and 5-HT1A receptor immunoreactivity (Fig. 2G). Maps illustrated in Fig. 10A. The proportion of c-fos immunoreactive neurons showing co-localization with the receptor was variable, ranging between ∼35 and 80% with no obvious rostrocaudal distribution (Fig. 10B). Double labeled neurons were mostly observed in lamina VII, including the intermediolateral cell column (Figs. 2G and 11, A and C). Cells staining positively for c-fos but not for 5-HT1A receptors were also most commonly found in lamina VII (Fig. 10, A and C).
FIG. 10.
Segmental and laminar distribution of colocalized c-fos/5-HT1A-positive neurons. The segmental and laminar distribution of co-localized c-fos/5-HT1A receptor positive neurons within the thoraco-lumbar T8, T9, L1–L7 segments of LC1 is shown in A. C-fos-positive cells labeled with or without 5-HT1A receptors are indicated with blue and red circles, respectively. Each diagram includes all labeled cells from 8 sections of that segment. The percentage of c-fos immunoreactive cells co-localizing 5-HT1A receptors for the different segments is plotted in B. The percentage varied between ∼35 and 80% without any apparent rostro-caudal distribution. The laminar distribution of c-fos-labeled cells with or without co-localized 5-HT1A receptors within the L1–L7 lumbar segments is illustrated in C. The largest numbers of cells of both types were found in lamina VII.
DISCUSSION
The present study demonstrates that the majority of fictive locomotor-activated neurons in the thoraco-lumbar spinal cord of the cat are innervated by serotonergic fibers: terminals of serotonergic fibers were found in close proximity to and appeared to make contact with these cells and the majority of these neurons were immunoreactive for three of the major serotonergic receptors implicated in the control of locomotion, namely 5-HT7, 5-HT2A, and 5-HT1A receptors, although the proportion of neurons staining for each class of receptor varied. Serotonergic innervation of the spinal cord arises from the raphe nuclei and parapyramidal region of the brain stem (e.g., Jones and Light 1992; Kwiat and Basbaum 1992; Törk 1990), the cells of which receive afferent projections from the MLR or its anatomical equivalent (Behbehani and Zemlan 1986; Edwards 1975; Sotnichenko 1986; Steeves and Jordan 1984). Serotonergic neurons in the medullary raphe nuclei, which increase their activity and which may be rhythmically active during locomotion (Jacobs and Fornal 1995; Veasey et al. 1995), are a likely source of this spinal innervation. Taken together with our preliminary observations that stimulation of the MLR increases activity-dependent labeling in brain stem serotonergic neurons (Noga et al. 2008) and results in the spinal release of 5-HT (Noga et al. 1999, 2006, 2007), the results of the present study provide the anatomical basis for the central control of locomotor activity by 5-HT in the absence of peripheral afferent feedback from moving limbs. Thus in addition to reticulospinal command neurons (Noga et al. 1988, 1991, 2003), serotonergic neurons comprise a major component of the central descending control of spinal locomotor neurons. The central role of 5-HT in the activation/modulation of locomotion by stimulation of the MLR is underscored by the numerous studies demonstrating the effectiveness of 5-HT and various serotonergic drugs in modulating or inducing locomotor-like activity in acute or chronically spinalized cats (Barbeau and Rossignol 1990, 1991; Miller at al. 1975; Rossignol et al. 1986), rabbits (Viala and Buser 1969, 1971), adult rats (Antri et al. 2002, 2003, 2005; Feraboli-Lohnherr et al. 1997, 1999), and the isolated neonatal rat's spinal cord (Cazalets et al. 1992, 1995; Cowley and Schmidt 1994; Kiehn and Kjærulff 1996; MacLean et al. 1998; McEwen et al. 1997).
Rostrocaudal distribution of serotonergic innervated locomotor-activated neurons
The majority (82–100%) of c-fos-positive neurons from the thoraco-lumbar (T13–L7) segments were contacted by serotonergic immunoreactive boutons (Figs. 3 and 4A), matching the ubiquitous nature of serotonergic innervation of the spinal cord. The distribution of serotonergic innervated locomotor-activated neurons, therefore closely matches the distribution of locomotor-activated neurons per se. The majority of innervated cells were found in laminae VII and VIII with the highest average number of cells/section in the caudal lumbar segments (peak L6). Taking into consideration the length (and therefore the number of sections) of each segment, we estimated that the total number of innervated cells/segment would peak in L4. The number and distribution of innervated neurons was thus similar to that reported previously in the L3–L7 lumbar segments of the cat for animals undergoing MLR-evoked or spontaneous fictive locomotion (Dai et al. 2005; Huang et al. 2000). Only in nonparalyzed animals was additional, extensive labeling observed elsewhere (especially laminae III and IV) (see also Ichiyama et al. 2008a) due to afferent feedback from the moving limb (Dai et al. 2005). The similar distribution of c-fos immunoreactive neurons with greatest numbers of activated neurons in laminae VII and VIII of middle/caudal lumbar segments is a common finding, reaffirming the idea that cells in this area are important for the central generation of locomotion in the cat (Dai et al. 2005; Noga et al. 1995). This conclusion is supported by the observations that MLR stimulation evokes the largest field potentials within laminae VII and VIII of the L4–L6 segments (Noga et al. 1995); the ability to initiate locomotion in spinal animals is lost after spinal transection below caudal L5 (Grillner and Zangger 1979) or with lesions in L3–L4 (Langlet et al. 2005; Marcoux and Rossignol 2000); injections of noradrenergic drugs into the L3–L4 or the L5–L6 segments are sufficient to induce or block walking (Delivet-Mongrain et al. 2008; Marcoux and Rossignol 2000); and the L3–L5 segments are the “leading” ones for the production of rhythmic oscillations in the lumbar spinal cord (Deliagina et al. 1983).
Although the largest numbers of c-fos-positive/5-HT innervated cells are found in middle-to-lower lumbar segments in the cat, significant numbers are additionally observed at rostral lumbar levels. Furthermore, there are indications that neurons in rostral segments may participate in the generation of locomotion in cats (Delivet-Mongrain et al. 2008) and the ability of 5-HT to initiate locomotion in spinal cats increases when the level of spinalization is extended rostrally and the caudal stump includes the T12 and/or T13 segments (Edgerton et al. 1997; see discussion by Schmidt and Jordan 2000). Locomotor-activated neurons are also distributed in the spinal cord of the rat although the distribution varies depending on the method of locomotor induction. For example, an increasing rostral-to-caudal gradient of c-fos neurons is observed in intact (Ahn et al. 2006) and spinal (Ichiyama et al. 2008a) adult rats following treadmill walking. In contrast, for drug (N-methyl-d-aspartate and/or 5-HT)-induced locomotion in the neonatal rat, locomotor-activated neurons are equally distributed over the L1–L5 (Cina and Hochman 2000) or L1–L4 lumbar segments (Kjaerulff et al. 1994). In contrast, a variety of pharmacological and lesion studies have shown that there exists a rostral-to-caudal gradient of rhythmogenic potential, acting on the dominant component of the locomotor network in the intermediate zone (Cazalets et al. 1995; Cowley and Schmidt 1997; Kjaerulff and Kiehn 1996; Kremer and Lev-Tov 1997). The locomotor network in the rostral segments of the rat spinal cord is particularly sensitive to 5-HT (Cowley and Schmidt 1997; Gimenez y Ribotta et al. 2000; Kiehn and Kjærulff 1996), the actions of which are likely mediated by 5-HT7 receptors (Lui and Jordan 2005; see relationship of 5-HT7 receptors to locomotor-activated neurons). To examine this issue further in the cat, we decided to extend our investigation of 5-HT receptor co-localization to include thoracic segments as high as T7 (Figs. 7, 9, and 10). Analysis of thoracic segments in the control animal revealed relatively few c-fos immunoreactive neurons (Fig. 8D) as before. This indicates that increased numbers of labeled neurons observed in experimental animals were due to locomotor activity. In these animals, many neurons, particularly in laminae VII and sometimes VIII showed co-localization with 5-HT7 (Figs. 7 and 8, B and C), 5-HT2A (Fig. 9), and 5-HT1A receptors (Fig. 10; see in the following text for each category of receptor). The similarity to lumbar segments is further affirmation of the importance of these laminae in locomotor processes.
Nature of serotonergic contacts on locomotor-activated neurons
In the present study, two or more serotonergic fibers (each with 2–4 boutons) were typically observed apposing the membrane of c-fos-labeled neurons. Whether these presumed contacts are synaptic or nonsynaptic was not determined because our goal was to examine the general relationship between serotonergic pathways and locomotor-activated neurons across many segments. In addition to synaptic contacts, a high percentage of nonsynaptic monoaminergic terminations are known to exist in spinal tissue (Marlier et al. 1991a; Maxwell et al. 1983; Rajaofetra et al. 1992; Ridet et al. 1993, 1994) including those observed on identified spinal neuronal soma (Jankowska et al. 1997). Because 5-HT typically diffuses 20 μm in 200 ms (Bunin and Wightman 1998) following stimulation of afferent pathways in the substantia nigra, spatial requirements of serotonergic terminals in relation to target neurons are not as strict for nonsynaptic versus synaptic neurotransmission. Thus the proximity of contacts observed in the present study is sufficient, at least, for an influence of 5-HT on neuronal function via nonsynaptic or volume neurotransmission (see role of 5-ht in the control of spinal locomotor activity via volume neurotransmission). Serotonergic contacts on unidentified (Pretel and Piekut 1991) and identified spinal neurons (Hammar and Maxwell 2002; Jankowska et al. 1995, 1996; Maxwell and Jankowska 1996; Maxwell et al. 2000) have been examined previously and the numbers observed on the soma of some cell types are comparable (Maxwell et al. 2000) to that observed here. Cells with serotonergic contacts on their soma invariably show contacts on their dendrites, although the number of contacts per 100 μm2 (the packing density) on the soma may be more (Hammar and Maxwell 2002) or less (Maxwell et al. 2000) than that observed for dendrites. Thus the influence of 5-HT on the neuronal somata of locomotor-activated neurons inferred in the present study will likely be augmented by the action of 5-HT at the dendritic level.
Spinal 5-HT7 receptors and locomotor-activated neurons
DISTRIBUTION of 5-HT7 RECEPTORS in the CAT SPINAL CORD.
We have found 5-HT7 receptor immunoreactivity at all examined thoraco-lumbar segments (T7–L7) of the cat spinal cord. Staining is predominant in small cells of superficial dorsal horn (laminae I–III) and diminishes progressively in deeper laminae (Figs. 2, C and D, and 5). These findings are consistent with our previous analysis of feline 5-HT7 receptor distribution using quantitative receptor autoradiography (Noga et al. 2002). In the rat, 5-HT7 receptors have also been reported in cells of the superficial dorsal horn (laminae I and II) of the upper L2–3 segments (Meuser et al. 2002) and in small cells of laminae I–III of the lower (L4 and L6) lumbar segments (Doly et al. 2005). This predominant dorsal horn localization is consistent with the idea that this receptor is involved sensory information processing (Garraway and Hochman 2001; Meuser et al. 2002).
RELATIONSHIP of 5-HT7 RECEPTORS to LOCOMOTOR-ACTIVATED NEURONS.
The extensive anatomical co-localization of 5-HT7 receptors with locomotor-activated neurons (Figs. 2E, 6, and 7) substantiates pharmacological studies demonstrating a role for this receptor in the generation of locomotion (Hochman et al. 2001; Landry et al. 2006; Liu and Jordan 2005; Liu et al. 2009; Madriaga et al. 2004; Pearlstein et al. 2005). The majority (80–100%; Fig. 8A) of locomotor-activated cells (especially within laminae VII and VIII) of the middle and caudal lumbar segments (Fig. 4, B and C) are labeled for 5-HT7 receptors. This indicates that cells in this area are likely important for 5-HT7 receptor-mediated effects on locomotor activity possibly via actions (Liu et al. 2009) on pattern formation neurons of the locomotor pattern generator (Lafreniere-Roula and McCrea 2005). The degree of co-localization decreases from a high in caudal segments to a low in thoracic segments (∼40–60%). This decrease, is due to the appearance of a group of c-fos immunoreactive cells in the intermediolateral nucleus (beginning about L3 and extending to T7) that does not stain for the 5-HT7 receptor (Figs. 6 and 7; see Functional implications), which decrease the overall percentage of labeling. In contrast, most other locomotor-activated cells within laminae VII/VIII of these segments showed double labeling (Figs. 6 and 7). Some preliminary observations of the co-localization of 5-HT7 receptors with locomotor-activated neurons have been reported for the rat. Over 80% of the c-fos-labeled cells observed following treadmill locomotion in the thoracic T10 segment of the adult rat are labeled for 5-HT7 receptor (Jordan and Schmidt 2002). In contrast, 37% of sulforhodamine-labeled cells in the T10–L2 thoraco-lumbar segments of the neonatal rat observed following 5-HT-induced fictive locomotion are labeled for the receptor (Hochman et al. 2001). The degree of co-localization decreases to 9% in more caudal L3–S1 segments (Hochman et al. 2001). This rostral-to-caudal decrease in percentage of 5-HT7-labeled cells observed in 5-HT-induced locomotion may reflect the fact that 5-HT (Cowley and Schmidt 1997; Gimenez y Ribotta et al. 2000; Kiehn and Kjærulff 1996) acts on rhythmogenic neurons in the rostral segments of the rat spinal cord (Kjaerulff and Kiehn 1996), in part, via 5-HT7 receptors (Liu and Jordan 2005). Whether a similar rostral 5-HT7 -sensitive network exists in cats is unknown, although there is preliminary evidence (Schmidt and Jordan 2000) to indicate a role for 5-HT7 receptors in MLR-evoked locomotion, which is blocked by intrathecal clozapine applied over the caudal thoracic and lumbar cord. More work is needed to determine whether disparities between cat and rat are related to other aspects such as stage of development, the method of locomotion induction and/or the immunohistochemical method.
Spinal 5-HT2A receptors and locomotor-activated neurons
Our results show that ∼70–90% of c-fos immunoreactive neurons co-localize the 5-HT2A receptor with no obvious rostrocaudal gradient across the T7–L7 thoraco-lumbar cord (Fig. 9B). Thus the distribution of these neurons follows that described for c-fos-positive/5-HT innervated cells with most double-labeled neurons localized to laminae VII and VIII, including the intermediolateral nucleus (Fig. 2G) and showing a tendency to cluster in the medial gray areas of L5–7 lumbar segments and then spread out at more rostral locations (Fig. 9, A, C, and D). Spinal 5-HT2A receptors have previously been observed on dendrites and cell bodies of neurons in dorsal horn, intermediate zone, and ventral horn with predominance to ventral locations (Cornea-Hébert et al. 1999; Doly et al. 2004; Helton et al. 1994; Marlier et al. 1991b) and labeling of many (but not all) types of motoneurons (Basura et al. 2001; Doly et al. 2004; Thor et al. 1993). Strong staining of neurons in the intermediolateral nucleus, where serotonergic innervated (Krukoff et al. 1985) sympathetic preganglionic neurons are found, has also been described (Cornea-Hébert et al. 1999; Doly et al. 2004; Maeshima et al. 1998). This is consistent with our observations and the report that the activity of sympathetic nerves increases with locomotor activity (Schomburg et al. 2003) and underscores the importance of the control of autonomic (e.g., cardiovascular and respiratory) functions during locomotion and other forms of exercise (Waldrop et al. 1996).
The co-localization of c-fos/5-HT2A receptor immunoreactivity in the present study is in keeping with pharmacological studies that indicate a role for these receptors in the generation of locomotor activity. Agonists acting primarily on the 5-HT2A receptor, such as quipazine, are well known to elicit and/or modulate locomotor-like patterns in immature reduced preparations (Beato and Nistri 1998; Gordon and Whelan 2006; Madriaga et al. 2004; Norreel et al. 2003; Pearlstein et al. 2005), spinalized adult rodents (Antri et al. 2003; Fong et al. 2005; Ichiyama et al. 2008b; Landry and Guertin 2004; Landry et al. 2006; Ung et al. 2008) and spinal-cord-injured cats (Brustein and Rossignol 1999; Edgerton et al. 1997). Quipazine may also synergistically enhance locomotor-like movements induced by administration of L-DOPA (Guertin 2004; Landry and Guertin 2004; McEwen et al. 1997) or epidural stimulation (Ichiyama et al. 2008b). On the other hand, 5-HT2A antagonists block locomotor-like activity (Bracci et al. 1998; Cazalets et al. 1992; Liu and Jordan 2005; Madriaga et al. 2004; Pearlstein et al. 2005). According to results reported by Liu and Jordan (2005), drugs acting on 5-HT2A receptors in caudal compartments of the spinal cord of neonatal rats reduce the amplitude of ventral root activity rather than cycle duration, suggesting that this receptor has an action on patterning components (premotor interneurons and/or motoneurons) rather than on rhythm-generating locomotor neurons located rostrally, which may be more sensitive to 5-HT7 drugs (see however, Liu et al. 2009). Their observation that 5-HT2A receptor antagonists do not affect locomotor activity when applied to rostral segments (above L3) is in contrast to results obtained by Ung et al. (2008) that show that quipazine is capable of inducing locomotor activity in spinalized mice. This effect was attributed to action at the 5-HT2A receptor in the lateral intermediate zone where significant upregulation of 5-HT2A mRNA was observed. While there is correspondence of this data with our observation of 5-HT2A receptor/c-fos colocalization in the intermediolateral cell column, we favor the involvement of other thoraco-lumbar lamina VII interneurons in mediating the locomotor inducing effect of quipazine.
Spinal 5-HT1A receptors and locomotor-activated neurons
The results of the present study indicate that ∼35–80% of c-fos immunoreactive neurons within the thoraco-lumbar (T8, T9, L1–7) segments co-localize the 5-HT1A receptor with no obvious rostrocaudal gradient (Fig. 10B). Most of the double-labeled neurons were localized to laminae VII and included some cells in the intermediolateral nucleus (Fig. 10, A and C). A substantial number of c-fos immunoreactive neurons without receptor were also found in lamina VII. Quantitative ligand binding autoradiography studies have localized 5-HT1A receptors to the dorsal horn and the area of the central canal in both cat and rodent, with lower levels of binding in the intermediolateral nucleus and other areas of the cord (Giroux et al. 1999; Lanoir et al. 2006; Laporte et al. 1995; Marlier et al. 1991b; Thor et al. 1993). A similar distribution has been observed in other immunohistochemical studies with additional labeling found on motorneurons within the cervical segments (Kheck et al. 1995; Kia et al. 1996). The low levels of binding in areas of the spinal cord where locomotor-activated neurons are located may explain the relatively lower level of co-localization of c-fos immunoreactive neurons with the 5-HT1A receptor compared with the 5-HT7 and 5-HT2A receptors. Although a rostrocaudal gradient of 5-HT1A labeling was seen in the rat (Marlier et al. 1991b; Thor et al. 1993), this gradient was not observed in the cat (Giroux et al. 1999), consistent with the present study. At the cellular level, 5-HT1A receptors have been localized to the axon hillock (Kheck et al. 1995), the soma and dendrites (Kia et al. 1996) as seen in the present study (Fig. 2G). Preembedding immunogold labeling procedures have also shown that 5-HT1A receptors are primarily neuronal, somatodendritic, membrane bound and extrasynaptic in rat brain, and therefore likely to mediate diffuse serotonergic neurotransmission (Riad et al. 2000).
The co-localization of c-fos/5-HT1A receptor immunoreactivity in the present study is consistent with pharmacological studies that indicate a role for these receptors in the modulation of locomotor activity (Cazalets et al. 1992). 5-HT1A receptors have been reported to be inhibitory to locomotion induced by N-methyl-d-aspartate (NMDA) by contributing to the slowing of the rhythm by 5-HT (Beato and Nistri 1998; Schmidt and Jordan 2000). In contrast, injections of 8-hydroxy-2-(di-N-propylamino)-tetralin (8-OH-DPAT), a 5-HT1A/7 receptor agonist, facilitates recovery of locomotion following spinal cord injury in the rodent (Antri et al. 2003, 2005; Lapointe and Guertin 2008), probably by action at both receptor subtypes (Landry et al. 2006).
Functional implications
THE MULTIPLICITY OF 5-HT RECEPTOR SUBTYPES ON LOCOMOTOR-ACTIVATED NEURONS IN THE SPINAL CORD.
The fact that a high percentage of c-fos immunoreactive neurons co-localize with either 5-HT7, 5-HT2A, or 5-HT1A receptors indicates that locomotor-activated neurons possess multiple 5-HT receptor subtypes. For example, 80–100% of c-fos immunoreactive neurons co-localize 5-HT7 receptors. The majority of these neurons should also co-localize with 5-HT2A and/or 5-HT1A receptors, which are found in 60–80 and 35–80%, respectively, of c-fos immunoreactive neurons. Whether 5-HT2A and 5-HT1A receptors co-localize with c-fos immunoreactive neurons is also likely, albeit less so. Interestingly, 5-HT-mediated motor responses have been suggested to require co-activation of multiple receptor subtypes (including some if not all of those observed in the present study), possibly on the same neuron, which would therefore imply co-localization of the receptors (Backus et al. 1990; Darmani et al. 1990; Glennon et al. 1991). Such an interaction has been suggested for spinal neurons mediating locomotion (Antri et al. 2003, 2005) although the operative relationships of the different receptors are not understood (Beato and Nistri 1998; Cazalets et al. 1992). The overall response of these neurons to 5-HT will thus depend on the type and numbers of bound receptors, the types of G-proteins expressed in the target cells, etc. (Raymond et al. 2001). Receptor transduction mechanisms for these receptor subtypes are multiple, often differ and may be opposing or complementary to each other (Raymond et al. 2001). The relative location of the receptors to their targets (which may include other receptors compartmentalized into macrodomains according to the location of afferent inputs which utilize them), to the signaling components (which may be compartmentalized into microdomains), and to the sites of release (synaptic vs. nonsynaptic transmission) (Vergé and Calas 2000) is also important.
ROLE OF 5-HT IN THE CONTROL OF SPINAL LOCOMOTOR ACTIVITY VIA VOLUME NEUROTRANSMISSION.
Extrasynaptic or volume transmission has been proposed to be important in monoaminergic systems (Agnati et al. 1995; Zoli et al. 1998). This is especially true for the dorsal horn where serotonergic terminals exhibit little synaptic specialization (Jankowska et al. 1997; Marlier et al. 1991a; Maxwell et al. 1983; Ridet et al. 1993, 1994) and where a significant proportion of 5-HT receptors are located remotely from release sites (Doly et al. 2004; Ridet et al. 1994). In contrast, synaptic contacts are predominant for serotonergic terminals in the intermediolateral cell column (Poulat et al. 1992) and the ventral horn (Ridet et al. 1994), where volume transmission may be less important (see, however, Doly et al. 2004). In support of these conclusions, relatively high extracellular concentrations of monoamines are observed in well-defined loci of the spinal cord during steady-state conditions (Noga et al. 2004). In addition, electrical stimulation of the nucleus raphe magnus results in a large extrasynaptic overflow of 5-HT in many laminae including those with locomotor-activated neurons (Hentall et al. 2006). These data indicate that the raphe-spinal system, like the locus ceruleus (Hentall et al. 2003), uses volume neurotransmission as a means of communication. This overflow, which is typically 30–40 nM (with a maximum of 287 nM) is well above the Km of most 5-HT receptor subtypes with the exception of the 5-HT2A receptor with an EC50 of 13 μmol (Hochman et al. 2001). However, the localization of the 5-HT2A receptor to the undifferentiated plasma membranes of cell somata and dendrites covering a large proportion of the cell surface has led to the suggestion that this receptor, like the 5-HT1A receptor (Riad et al. 2000), may mediate a paracrine (nonsynaptic) action of 5-HT in both dorsal and ventral horn areas (Doly et al. 2004). In contrast to the spinal 5-HT levels observed with raphe nuclei stimulation mentioned in the preceding text, measurements of 5-HT release during MLR-evoked fictive locomotion show maximal increases in the extracellular 5-HT in the low micromolar range within the spinal cord of the cat (Noga et al. 2006, 2007). While this difference may be related to species differences (in relative numbers of uptake transporters, for example), other explanations are considered likely. For example, the MLR is known to project to midline raphe nuclei (Steeves and Jordan 1984) and activate serotonergic neurons within these nuclei as well as the parapyramidal region of the medulla (Noga et al. 2008). Stimulation of the MLR is therefore likely to activate a larger number of descending serotonergic neurons than is likely with direct microstimulation of the raphe. Providing that serotonergic terminals are relatively close by and that the intensity of firing is substantially high, it is possible that 5-HT2A receptors could mediate serotonergic extrasynaptic neurotransmission.
Considering the different affinities of 5-HT for its receptors (Hochman et al. 2001), the response of locomotor-activated neurons to 5-HT will depend, in part, on the ratio of synaptic and nonsynaptic receptors because transmitter concentrations in synaptic clefts and the extracellular space can be fundamentally different (Vizi et al. 2004). Our recent finding that spinal monoamine release, and therefore the concentration of 5-HT in the extracellular space (and by implication within the synaptic cleft), is dynamically regulated on a time scale spanning seconds (locomotion) or minutes (at rest) underscores the complex nature of this signaling pathway (Noga et al. 2006, 2007; see also Hentall et al. 2006). Small changes in transmitter concentration could have a profound effect on those receptors that bind readily (low EC50) to 5-HT, such as 5-HT1A and 5-HT7, and have less effect on receptors with a high EC50 such as the 5-HT2A receptor. Thus the effect of 5-HT on neurons may vary temporally throughout a bout of locomotion depending on the assortment of receptors they possess. Whether the dynamic variation in transmitter levels can modulate neuronal responsiveness to other neuronal inputs at these timescales is not clear, considering that the signaling transduction pathways for the receptors investigated are mediated by second messenger systems. It is likely, however, that signaling components will require physical or functional compartmentalization into “microdomains” to achieve such temporal modulation (Raymond et al. 2001).
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-46404, the State of Florida, and The Miami Project to Cure Paralysis.
Acknowledgments
We thank B. Frydel for assistance in the use of StereoInvestigator and Neurolucida, Dr. Eva Widerström-Noga for statistical analysis, A. Blythe and F. Sanchez for graphics assistance, and Dr. Ian D. Hentall for comments on the manuscript.
Present addresses: D.M.G. Johnson, National Institute of Mental Health, 10 Center Dr., Bethesda, MD 20892; A Pinzon, Dept. of Neurology, Albert Einstein College of Medicine of Yeshiva University, Bronx, NY 10461.
REFERENCES
- Agnati et al. 1995.Agnati LF, Zoli M, Strömberg I, Fuxe K. Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69: 711–726, 1995. [DOI] [PubMed] [Google Scholar]
- Ahn et al. 2006.Ahn SN, Guu JJ, Tobin AJ, Edgerton VR, Tillakaratne NJK. Use of c-fos to identify activity-dependent spinal neurons after stepping in intact adult rats. Spinal Cord 44: 547–559, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez et al. 1998.Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe REW. Distribution of 5-hydroxytryptamine-immunoreactive boutons on α-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol 393: 69–83, 1998. [PubMed] [Google Scholar]
- Antri et al. 2005.Antri M, Barthe J-Y, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett 384: 162–167, 2005. [DOI] [PubMed] [Google Scholar]
- Antri et al. 2003.Antri M, Mouffle C, Orsal D, Barthe J-Y. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur J Neurosci 18: 1963–1972, 2003. [DOI] [PubMed] [Google Scholar]
- Antri et al. 2002.Antri M, Orsal D, Barthe J-Y. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur J Neurosci 16: 467–476, 2002. [DOI] [PubMed] [Google Scholar]
- Backus et al. 1990.Backus LI, Sharp T, Grahame-Smith DG. Behavioral evidence for a functional interaction between central 5-HT2 and 5-HT1A receptors. Brit J Pharmacol 100: 793–799, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau and Rossignol 1990.Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res 514: 55–67, 1990. [DOI] [PubMed] [Google Scholar]
- Barbeau and Rossignol 1991.Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res 546: 250–260, 1991. [DOI] [PubMed] [Google Scholar]
- Basura et al. 2001.Basura GJ, Zhou S-Y, Walker PD, Goshgarian HG. Distribution of serotonin 2A and 2C receptor mRNA expression in the cervical ventral horn and phrenic motoneurons following spinal cord hemisection. Exp Neurol 169: 255–263, 2001. [DOI] [PubMed] [Google Scholar]
- Beato and Nistri 1998.Beato M, Nistri A. Serotonin-induced inhibition of locomotor rhythm of the rat isolated spinal cord is mediated by the 5-HT1 receptor class. Proc R Soc Lond B Biol Sci 265: 2073–2080, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani and Zemlan 1986.Behbehani MM, Zemlan FP. Response of nucleus raphe magnus neurons to electrical stimulation of nucleus cuneiformis: role of acetylcholine. Brain Res 369: 110–118, 1986. [DOI] [PubMed] [Google Scholar]
- Bertrand and Cazalets 2002.Bertrand S, Cazalets JR. The respective contribution of lumbar segments to the generation of locomotion in the isolated spinal cord of newborn rat. Eur J Neurosci 16: 1741–50, 2002. [DOI] [PubMed] [Google Scholar]
- Bracci et al. 1998.Bracci E, Beato M, Nistri A. Extracellular K+ induces locomotor-like patterns in the rat spinal cord in vitro: comparison with NMDA or 5-HT induced activity. J Neurophysiol 79: 2643–2652, 1998. [DOI] [PubMed] [Google Scholar]
- Brustein and Rossignol 1999.Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. II. Effects of noradrenergic and serotonergic drugs. J Neurophysiol 81: 1513–1530, 1999. [DOI] [PubMed] [Google Scholar]
- Bunin and Wightman 1998.Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci 18: 4854–4860, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callado and Stamford 2000.Callado LF, Stamford JA. Spatiotemporal interaction of α2 autoreceptors and noradrenaline transporters in the rat locus coeruleus: implications for volume transmission. J Neurochem 74: 2350–2358, 2000. [DOI] [PubMed] [Google Scholar]
- Cazalets et al. 1995.Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci 15: 4943–4951, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets et al. 1992.Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol 455: 187–204, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau et al. 1998.Chau C, Barbeau H, Rossignol S. Effects of intrathecal α1- and α2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. J Neurophysiol 79: 2941–2963, 1998. [DOI] [PubMed] [Google Scholar]
- Cina and Hochman 2000.Cina C, Hochman S. Diffuse distribution of sulforhodamine-labeled neurons during serotonin-evoked locomotion in the neonatal rat thoracolumbar spinal cord. J Comp Neurol 423: 590–602, 2000. [DOI] [PubMed] [Google Scholar]
- Cornea-Hébert et al. 1999.Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol 409: 187–209, 1999. [DOI] [PubMed] [Google Scholar]
- Cowley and Schmidt 1994.Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-d-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett 171: 147–150, 1994. [DOI] [PubMed] [Google Scholar]
- Cowley and Schmidt 1997.Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern generating network in the neonatal rat spinal cord. J Neurophysiol 77: 247–259, 1997. [DOI] [PubMed] [Google Scholar]
- Dai et al. 2005.Dai X, Noga BR, Douglas JR, Jordan LM. Localization of spinal neurons activated during locomotion using the c-fos immunohistochemical method. J Neurophysiol 93: 3442–3452, 2005. [DOI] [PubMed] [Google Scholar]
- Darmani et al. 1990.Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav 36: 901–906, 1990. [DOI] [PubMed] [Google Scholar]
- Deliagina et al. 1983.Deliagina TG, Orlovsky GN, Pavlova GA. The capacity for generation of rhythmic oscillations is distributed in the lumbosacral spinal cord of the cat. Exp Brain Res 53: 81–90, 1983. [DOI] [PubMed] [Google Scholar]
- Delivet-Mongrain et al. 2008.Delivet-Mongrain H, Leblond H, Rossignol S. Effects of localized intraspinal injections of a noradrenergic blocker on locomotion of high decerebrate cats. J Neurophysiol 100: 907–921, 2008. [DOI] [PubMed] [Google Scholar]
- Doly et al. 2005.Doly S, Fischer J, Brisorgueil M-J, Vergé D, Conrath M. Pre- and postsynaptic localization of the 5-HT7 receptor in rat dorsal spinal cord: immunocytochemical evidence. J Comp Neurol 490: 256–269, 2005. [DOI] [PubMed] [Google Scholar]
- Doly et al. 2004.Doly S, Madeira A, Fischer J, Brisorgueil MJ, Daval G, Bernard R, Verge D, Conrath M. The 5-HT2A receptor is widely distributed in the rat spinal cord and mainly localized at the plasma membrane of postsynaptic neurons. J Comp Neurol 472: 496–511, 2004. [DOI] [PubMed] [Google Scholar]
- Edgerton et al. 1997.Edgerton VR, de Leon RD, Tillakaratne N, Recktenwald MR, Hodgson JA, Roy RR. Use-dependent plasticity in spinal stepping and standing. Adv Neurol 72: 233–247, 1997. [PubMed] [Google Scholar]
- Edwards 1975.Edwards SB. Autoradiographic studies of the projections of the midbrain reticular formation: descending projections of nucleus cuneiformis. J Comp Neurol 161: 341–358, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraboli-Lohnherr et al. 1999.Feraboli-Lohnherr D, Barthe JY, Orsal D. Serotonin-induced activation of the network for locomotion in adult spinal rats. J Neurosci Res 55: 87–98, 1999. [DOI] [PubMed] [Google Scholar]
- Feraboli-Lohnherr et al. 1997.Feraboli-Lohnherr D, Orsal D, Yakovleff A, Giménez y Ribotta M, Privat A. Recovery of locomotor activity in the adult chronic spinal rat after sublesional transplantation of embryonic nervous cells: specific role of serotonergic neurons. Exp Brain Res 113: 443–454, 1997. [DOI] [PubMed] [Google Scholar]
- Fong et al. 2005.Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci 25: 11738–11747, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway and Hochman 2001.Garraway SM, Hochman S. Pharmacological characterization of serotonin receptor subtypes modulating primary afferent input to deep dorsal horn neurons in the neonatal rat. Br J Pharmacol 132: 1789–1798, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez y Ribotta et al. 2000.Gimenez y Ribotta M, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci 20: 5144–5152, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux et al. 1999.Giroux N, Rossignol S, Reader TA. Autoradiographic study of 1,2-noradrenergic and serotonin 1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol 406: 402–14, 1999. [PubMed] [Google Scholar]
- Glennon et al. 1991.Glennon RA, Darmani NA, Martin BR. Multiple populations of serotonin receptors may modulate the behavioral effects of serotonergic agents. Life Sci 48: 2493–2498, 1991. [DOI] [PubMed] [Google Scholar]
- Gordon and Whelan 2006.Gordon IT, Whelan PJ. Monoaminergic control of cauda-equina-evoked locomotion in the neonatal mouse spinal cord. J Neurophysiol 96: 3122–3129, 2006. [DOI] [PubMed] [Google Scholar]
- Grillner and Zangger 1979.Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res 34: 241–261, 1979. [DOI] [PubMed] [Google Scholar]
- Guertin 2004.Guertin PA. Synergistic activation of the central pattern generator for locomotion by L-beta-3,4-dihydroxyphenylalanine and quipazine in adult paraplegic mice. Neurosci Lett 358: 71–74, 2004. [DOI] [PubMed] [Google Scholar]
- Hammar et al. 2004.Hammar I, Bannatyne BA, Maxwell DJ, Edgley SA, Jankowska E. The actions of monoamines and distribution of noradrenergic and serotonergic contacts on different subpopulations of commissural interneurons in the cat spinal cord. Eur J Neurosci 19: 1305–1316, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar and Maxwell 2002.Hammar I, Maxwell DJ. Serotonergic and noradrenergic axons make contacts with neurons of the ventral spinocerebellar tract in the cat. J Comp Neurol 443: 310–319, 2002. [DOI] [PubMed] [Google Scholar]
- Helton et al. 1994.Helton LA, Thor KB, Baez M. 5-Hydroxytryptamine2A, 5-hydroxytryptamine2B, and 5-hydroxytryptamine2C receptor mRNA expression in the spinal cord of rat, cat, monkey and human. Neuroreport 5: 2617–2620, 1994. [DOI] [PubMed] [Google Scholar]
- Hentall et al. 2003.Hentall ID, Mesigil R, Pinzon A, Noga BR. Temporal and spatial profiles of pontine-evoked monoamine release in the rat's spinal cord. J Neurophysiol 89: 2943–2951, 2003. [DOI] [PubMed] [Google Scholar]
- Hentall et al. 2006.Hentall ID, Pinzon A, Noga BR. Spatial and temporal patterns of serotonin release in the rat's lumbar spinal cord following electrical stimulation of the nucleus raphe magnus. Neuroscience 142: 893–903, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdegen and Leah 1998.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos, and Krox, and CREB/ATF proteins. Brain Res Rev 28: 370–490, 1998. [DOI] [PubMed] [Google Scholar]
- Hochman et al. 2001.Hochman S, Garraway SM, Machace DW, Shay BL. 5-HT receptors and the neuromodulatory control of spinal cord function. In: Motor Neurobiology of the Spinal Cord: Methods and New Frontiers in Neuroscience, edited by Cope TC. Boca Raton: CRC, 2001, p. 47–87.
- Huang et al. 2000.Huang A, Noga BR, Carr PA, Fedirchuk B, Jordan LM. Spinal cholinergic neurons activated during locomotion: localization and electrophysiological characterization. J Neurophysiol 83: 3537–3547, 2000. [DOI] [PubMed] [Google Scholar]
- Ichiyama et al. 2008a.Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci 28: 7370–7375, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama et al. 2008b.Ichiyama RM, Gerasimenko Y, Jindrich DL, Zhong H, Roy RR, Edgerton VR. Dose dependence of the 5-HT agonist quipazine in facilitating spinal stepping in the rat with epidural stimulation. Neurosci Lett 438: 281–285, 2008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs and Fornal 1995.Jacobs BL, Fornal CA. Activation of 5-HT neuronal activity during motor behavior. Semin Neurosci 7: 401–408, 1995. [Google Scholar]
- Jankowska et al. 1997.Jankowska E, Maxwell DJ, Dolk S, Dahlström A. A confocal and electron microscopic study of contacts between 5-HT fibres and dorsal horn interneurons in pathways from muscle afferents. J Comp Neurol 387: 430–438, 1997. [PubMed] [Google Scholar]
- Jankowska et al. 1995.Jankowska E, Maxwell DJ, Dolk S, Krutki P, Belichenko PV, Dahlström A. Contacts between serotonergic fibres and dorsal horn spinocerebellar tract neurons in the cat and rat: a confocal microscopic study. Neuroscience 67: 477–487, 1995. [DOI] [PubMed] [Google Scholar]
- Johnson et al. 2002.Johnson DMG, Riesgo MI, Pinzon A, Noga BR. Monoaminergic innervation of locomotor activated cells in cat spinal cord. Soc Neurosci Abstr 28: 65.15, 2002. [Google Scholar]
- Jones and Light 1992.Jones SL, Light AR. Serotonergic medullary raphespinal projection to the lumbar spinal cord in the rat: a retrograde immunohistochemical study. J Comp Neurol 322: 599–610, 1992. [DOI] [PubMed] [Google Scholar]
- Jordan and Schmidt 2002.Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Prog Brain Res 137: 125–40, 2002. [DOI] [PubMed] [Google Scholar]
- Kheck et al. 1995.Kheck NM, Gannon PJ, Azmitia EC. 5-HT1A receptor localization on the axon hillock of cervical spinal motoneurons in primates. J Comp Neurol 355: 211–220, 1995. [DOI] [PubMed] [Google Scholar]
- Kia et al. 1996.Kia HK, Miquel M-C, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Vergé D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol 365: 289–305, 1996. [DOI] [PubMed] [Google Scholar]
- Kiehn and Kjærulff 1996.Kiehn O, Kjærulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol 75: 1472–1482, 1996. [DOI] [PubMed] [Google Scholar]
- Kjærulff et al. 1994.Kjærulff O, Barajon I, Kiehn O. Sulphorhodamine-labelled cells in the neonatal rat spinal cord following chemically induced locomotor activity in vitro. J Physiol 478: 265–273, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff and Kiehn 1996.Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer and Lev-Tov 1997.Kremer E, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol 77: 1155–1170, 1997. [DOI] [PubMed] [Google Scholar]
- Krukoff et al. 1985.Krukoff TL, Ciriello J, Calaresu FR. Segmental distribution of peptide- and 5HT-like immunoreactivity in nerve terminals and fibers of the thoracolumbar sympathetic nuclei of the cat. J Comp Neurol 240: 103–116, 1985. [DOI] [PubMed] [Google Scholar]
- Kwiat and Basbaum 1992.Kwiat GC, Basbaum AI. The origin of brainstem noradrenergic and serotonergic projections to the spinal cord dorsal horn in the rat. Somatosens Mot Res 9: 157–173, 1992. [DOI] [PubMed] [Google Scholar]
- Lafreniere-Roula and McCrea 2005.Lafreniere-Roula M, McCrea D. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol 94: 1120–1132, 2005. [DOI] [PubMed] [Google Scholar]
- Landry and Guertin 2004.Landry ES, Guertin PA. Differential effects of 5-HT1 and 5-HT2 receptor agonists on hindlimb movements in paraplegic mice. Prog Neuropsychopharmacol Biol Psychiatry 28: 1053–1060, 2004. [DOI] [PubMed] [Google Scholar]
- Landry et al. 2006.Landry ES, Lapointe NP, Rouillard C, Levesque D, Hedlund PB, Guertin PA. Contribution of spinal 5-HT1A and 5-HT7 receptors to locomotor-like movement induced by 8-OH-DPAT in spinal cord-transected mice. Eur J Neurosci 24: 535–546, 2006. [DOI] [PubMed] [Google Scholar]
- Langlet et al. 2005.Langlet C, Leblond H, Rossignol S. Mid-lumbar segments are needed for the expression of locomotion in chronic spinal cats. J Neurophysiol 93: 2474–2488, 2005. [DOI] [PubMed] [Google Scholar]
- Lanoir et al. 2006.Lanoir J, Hilaire G, Seif I. Reduced density of functional 5-HT1A receptors in the brain, medulla and spinal cord of monoamine oxidase-A knockout mouse neonates. J Comp Neurol 495: 607–623, 2006. [DOI] [PubMed] [Google Scholar]
- Lapointe and Guertin 2008.Lapointe NP, Guertin PA. Synergistic effects of D1/5 and 5-HT1A/7 receptor agonists on locomotor movement induction in complete spinal cord-transected mice. J Neurophysiol 100: 160–168, 2008. [DOI] [PubMed] [Google Scholar]
- Laporte et al. 1995.Laporte AM, Fattaccini CM, Lombard MC, Chauveau J, Hamon M. Effects of dorsal rhizotomy and selective lesion of serotonergic and noradrenergic systems on 5-HT1A, 5-HT1B and 5-HT3 receptors in the rat spinal cord. J Neural Transm 100: 207–223, 1995. [DOI] [PubMed] [Google Scholar]
- Liu et al. 2009.Liu J, Akay T, Hedlund PB, Pearson KG, Jordan LM. Spinal 5-HT7 receptors are critical for alternating activity during locomotion: in vitro neonatal and in vivo adult studies using 5-HT7 receptor knockout mice. J Neurophysiol 102: 337–348, 2009. [DOI] [PubMed] [Google Scholar]
- Liu and Jordan 2005.Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat bran stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol 94: 1392–1404, 2005. [DOI] [PubMed] [Google Scholar]
- MacLean et al. 1998.MacLean JN, Cowley KC, Schmidt BJ. NMDA receptor-mediated oscillatory activity in the neonatal rat spinal cord is serotonin dependent. J Neurophysiol 79: 2804–2808, 1998. [DOI] [PubMed] [Google Scholar]
- Madriaga et al. 2004.Madriaga MA, McPhee LC, Chersa T, Christie KJ, Whelan PJ. Modulation of locomotor activity by multiple 5-HT and dopaminergic receptor subtypes in the neonatal mouse spinal cord. J Neurophysiol 92: 1566–1576, 2004. [DOI] [PubMed] [Google Scholar]
- Maeshima et al. 1998.Maeshima T, Ito R, Hamada S, Senzaki K, Hamaguchi-Hamada K, Shutoh F, Okado N. The cellular localization of 5-HT2A receptors in the spinal cord and spinal ganglia of the adult rat. Brain Res 797: 118–124, 1998. [DOI] [PubMed] [Google Scholar]
- Magnuson et al. 1999.Magnuson DSK, Trinder TC, Zhang YP, Burke D, Morassutti DJ, Shields CB. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol 156: 191–204, 1999. [DOI] [PubMed] [Google Scholar]
- Marcoux and Rossignol 2000.Marcoux J, Rossignol S. Initiating or blocking locomotion in spinal cats by applying noradrenergic drugs to restricted lumbar spinal segments. J Neurosci 20: 8577–8585, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlier et al. 1991a.Marlier L, Sandillon F, Poulat P, Rajaofetra N, Geffard M, Privat A. Serotonergic innervation of the dorsal horn of rat spinal cord: light and electron microscopic immunocytochemical study. J Neurocytol 20: 310–322, 1991a. [DOI] [PubMed] [Google Scholar]
- Marlier et al. 1991b.Marlier L, Teilhac J-R, Cerruti C, Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res 550: 15–23, 1991b. [DOI] [PubMed] [Google Scholar]
- Maxwell and Jankowska 1996.Maxwell DJ, Jankowska E. Synaptic relationships between serotonin-immunoreactive axons and dorsal horn spinocerebellar tract cells in the cat spinal cord. Neuroscience 70: 247–253, 1996. [DOI] [PubMed] [Google Scholar]
- Maxwell et al. 1983.Maxwell DJ, Leranth C, Verhofstad AAJ. Fine structure of serotonin-containing axons in the marginal zone of the rat spinal cord. Brain Res 266: 253–259, 1983. [DOI] [PubMed] [Google Scholar]
- Maxwell et al. 2000.Maxwell DJ, Riddell JS, Jankowska E. Serotonergic and noradrenergic axonal contacts associated with premotor interneurons in spinal pathways from group II muscle afferents. Eur J Neurosci 12: 1271–1280, 2000. [DOI] [PubMed] [Google Scholar]
- McEwen et al. 1997.McEwen ML, Van Hartesveldt C, Stehouwer DJ. L-DOPA and quipazine elicit air-stepping in neonatal rats with spinal cord transections. Behav Neurosci 111: 825–833, 1997. [DOI] [PubMed] [Google Scholar]
- Meuser et al. 2002.Meuser T, Pietruck C, Gabriel A, Xie GX, Lim KJ, Pierce Palmer P. 5-HT7 receptors are involved in mediating 5-HT-induced activation of rat primary afferent neurons. Life Sci 71: 2279–89, 2002. [DOI] [PubMed] [Google Scholar]
- Miller et al. 1975.Miller S, Van Der Burg J, Van Der Meche FGA. Locomotion in the cat: basic programms of movement. Brain Res 91: 239–253, 1975. [DOI] [PubMed] [Google Scholar]
- Neumaier et al. 2001.Neumaier JF, Sexton TJ, Yracheta J, Diaz AM, Brownfield M. Localization of 5-HT7 receptors in rat brain by immunocytochemistry, in situ hybridization, and agonist stimulated cFos expression. J Chem Neuroanat 21: 63–73, 2001. [DOI] [PubMed] [Google Scholar]
- Noga et al. 2006.Noga BR, Blythe AD, Brumley MR, Hentall ID, Kadam BH, Sanchez FJ. Relation between monoamine levels and parameters of fictive locomotion. Soc Neurosci Abstr 32: 252.23, 2006. [Google Scholar]
- Noga et al. 2007.Noga BR, Blythe AD, Brumley MR, Hentall ID, Sanchez FJ, Taberner AM. Modulation of spinal serotonin and norepinephrine levels during locomotion and at rest: real-time measurements with multi-electrode cyclic voltammetry. Soc Neurosci Abstr 33: 924.13, 2007. [Google Scholar]
- Noga et al. 1995.Noga BR, Fortier PA, Kriellaars DJ, Dai X, Detillieux GR, Jordan LM. Field potential mapping of neurons in the lumbar spinal cord activated following stimulation of the mesencephalic locomotor region. J Neurosci 15: 2203–2217, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga et al. 2002.Noga BR, Johnson DMG, Pinzon A, Riesgo M, Basile M, Mash DC. Changes in 5-HT7 receptor distribution in cat spinal cord following chronic transection. Soc Neurosci Abstr 28: 853.1, 2002. [Google Scholar]
- Noga et al. 1988.Noga BR, Kettler J, Jordan LM. Locomotion produced in mesencephalic cats by injections of putative transmitter substances and antagonists into the medial reticular formation and the pontomedullary locomotor strip. J Neurosci 8: 2074–2086, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga et al. 2003.Noga BR, Kriellaars DJ, Brownstone RM, Jordan LM. Mechanism for activation of locomotor centers in the spinal cord by stimulation of the mesencephalic locomotor region. J Neurophysiol 90: 1464–1478, 2003. [DOI] [PubMed] [Google Scholar]
- Noga et al. 1991.Noga BR, Kriellaars DJ, Jordan LM. The effect of selective brainstem or spinal cord lesions on treadmill locomotion evoked by stimulation of the mesencephalic or pontomedullary locomotor regions. J Neurosci 11: 1691–1700, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga et al. 1999.Noga BR, Mesigil R, Hentall ID, Hesse D. Real-time measurement of monoamine release in the cat lumbar spinal cord during brain-stem-evoked fictive locomotion. Soc Neurosci Abstr 25: 467.7, 1999. [Google Scholar]
- Noga et al. 2004.Noga BR, Pinzon A, Mesigil RP, Hentall ID. Steady-state levels of monoamines in the rat lumbar spinal cord—spatial mapping and the effect of acute spinal cord injury. J Neurophysiol 92: 567–577, 2004. [DOI] [PubMed] [Google Scholar]
- Noga et al. 2008.Noga BR, Xie S, Sanchez FJ, Blythe AD. Activity dependent labeling of brainstem monoaminergic and non-monoaminergic neurons during mesencephalic locomotor region evoked fictive locomotion in the cat. Soc Neurosci Abstr 34: 576.4, 2008. [Google Scholar]
- Norreel et al. 2003.Norreel J-C, Pflieger J-F, Pearlstein E, Simeoni-Alias J, Clarac F, Vinay L. Reversible disorganization of the locomotor pattern after neonatal spinal cord transection in the rat. J Neurosci 23: 1924–1932, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson et al. 2000.Pearson JC, Sedivec MJ, Dewey DE, Fyffe RE. Light microscopic observations on the relationships between 5-hydroxytryptamine-immunoreactive axons and dorsal spinocerebellar tract cells in Clarke's column in the cat. Exp Brain Res 130: 320–327, 2000. [DOI] [PubMed] [Google Scholar]
- Pearlstein et al. 2005.Pearlstein E, Mabrouk FB, Pflieger JF, Vinay L. Serotonin refines the locomotor-related alternations in the in vitro neonatal rat spinal cord. Eur J Neurosci 21: 1338–1346, 2005. [DOI] [PubMed] [Google Scholar]
- Pilowsky et al. 1990.Pilowsky PM, de Castro D, Llewellyn-Smith I, Lipski J, Voss MD. Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci 10: 1091–1098, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulat et al. 1992.Poulat P, Marlier L, Rajaofetra N, Privat A. 5-Hydroxytryptamine, substance P and thyrotropin-releasing hormone synapses in the intermediolateral cell column of the rat thoracic spinal cord. Neurosci Lett 136: 19–22, 1992. [DOI] [PubMed] [Google Scholar]
- Pretel and Piekut 1991.Pretel S, Piekut DT. Enkephalin, Substance P, and serotonin axonal input to c-fos-like immunoreactive neurons of the rat spinal cord. Peptides 12: 1243–1250, 1991. [DOI] [PubMed] [Google Scholar]
- Raymond et al. 2001.Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Thera 92: 179–212, 2001. [DOI] [PubMed] [Google Scholar]
- Rasmussen et al. 1986.Rasmussen K, Morilak DA, Jacobs BL. Single unit activity of locus coeruleus neurons in the freely moving cat. I. During naturalistic behaviors and in response to simple and complex stimuli. Brain Res 371: 324–334, 1986. [DOI] [PubMed] [Google Scholar]
- Rajaofetra et al. 1992.Rajaofetra N, Ridet J-L, Poulat P, Marlier L, Sandillon F, Geffard M, Privat A. Immunocytochemical mapping of noradrenergic projections to the rat spinal cord with an antiserum against noradrenaline. J Neurocytol 21: 481–494, 1992. [DOI] [PubMed] [Google Scholar]
- Rexed 1954.Rexed B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100: 297–379, 1954. [DOI] [PubMed] [Google Scholar]
- Riad et al. 2000.Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, El Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol 417: 181–194, 2000. [PubMed] [Google Scholar]
- Ridet et al. 1993.Ridet J-L, Rajaofetra N, Teilhac JR, Geffard M, Privat A. Evidence for non-synaptic serotonergic and noradrenergic innervation of the rat dorsal horn and possible involvement of neuron-glia interactions. Neuroscience 52: 143–157, 1993. [DOI] [PubMed] [Google Scholar]
- Ridet et al. 1994.Ridet J-L, Tamir H, Privat A. Direct immunocytochemical localization of 5-hydroxytryptamine receptors in the adult rat spinal cord: a light and electron microscopic study using an anti-idiotypic antiserum. J Neurosci Res 38: 109–121, 1994. [DOI] [PubMed] [Google Scholar]
- Rossignol et al. 1986.Rossignol S, Barbeau H, Julien C. Locomotion of the adult chronic spinal cat and its modification by monoaminergic agonists and antagonists. In: Development and Plasticity of the Mammalian Spinal Cord, edited by Goldberger M, Gorio A, Murray M. Pradova, Italy: Liviana, 1986, p. 323–346.
- Schmidt and Jordan 2000.Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53: 689–710, 2000. [DOI] [PubMed] [Google Scholar]
- Schomburg et al. 2003.Schomburg ED, Steffens H, Dembowsky K. Rhythmic phrenic, intercostal and sympathetic activity in relation to limb and trunk motor activity in spinal cats. Neurosci Res 46: 229–240, 2003. [DOI] [PubMed] [Google Scholar]
- Sotnichenko 1986.Sotnichenko TS. Differentiation of efferent projections of the medial (cuneiform nucleus) and lateral regions of the reticular formation in cat midbrain. Neurophysiol 17: 466–471, 1986. [PubMed] [Google Scholar]
- Steeves and Jordan 1984.Steeves JD, Jordan LM. Autoradiographic demonstration of the projections from the mesencephalic locomotor region. Brain Res 307: 263–276, 1984. [DOI] [PubMed] [Google Scholar]
- Thor et al. 1993.Thor KB, Nickolaus S, Helke CJ. Autoradiographic localization of 5-hydroxytryptamine1A, 5-hydroxytryptamine1B and 5-hydroxytryptamine1C/2 binding sites in the rat spinal cord. Neuroscience 55: 235–252, 1993. [DOI] [PubMed] [Google Scholar]
- Törk 1990.Törk I. Anatomy of the serotonergic system. Ann NY Acad Sci 600: 9–34, 1990. [DOI] [PubMed] [Google Scholar]
- Ung et al. 2008.Ung R-V, Landry ES, Rouleau P, NP Lapointe, Rouillard C, Guertin PA. Role of spinal 5-HT2 receptor subtypes in quipazine-induced hindlimb movements after a low-thoracic spinal cord transection. Eur J Neurosci 28: 2231–2242, 2008. [DOI] [PubMed] [Google Scholar]
- Veasey et al. 1995.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15: 5346–5359, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergé and Calas 2000.Vergé D, Calas A. Serotonergic neurons and serotonin receptors: gains from cytochemical approaches. J Chem Neuroanat 18: 41–56, 2000. [DOI] [PubMed] [Google Scholar]
- Viala and Buser 1969.Viala D, Buser P. The effects of DOPA and 5-HTP on rhythmic efferent discharges in hind limb nerves in the rabbit. Brain Res 12: 437–443, 1969. [DOI] [PubMed] [Google Scholar]
- Viala and Buser 1971.Viala D, Buser P. Modalites d'obtention de rythmes locomoteurs cez le lapin spinal par traitements phamacologiques (DOPA, 5-HTP, D-amphetamine). Brain Res 35: 151–165, 1971. [DOI] [PubMed] [Google Scholar]
- Vizi et al. 2004.Vizi ES, Kiss JP, Lendvai B. Nonsynaptic communication in the central nervous system. Neurochem Int 45: 443–451, 2004. [DOI] [PubMed] [Google Scholar]
- Voss et al. 1990.Voss MD, de Castro D, Lipski J, Pilowski PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol 295: 208–218, 1990. [DOI] [PubMed] [Google Scholar]
- Waldrop et al. 1996.Waldrop TG, Eldridge FL, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems, edited by Rowell LB, Shepherd JT. New York: Oxford, 1996, sect, 12, p. 333–380.
- Wightman and Zimmerman 1990.Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Rev 15: 135–144, 1990. [DOI] [PubMed] [Google Scholar]
- Zoli et al. 1998.Zoli M, Torri C, Ferrari R, Jansson A, Zini I, Fuxe K, Agnati LF. The emergence of the volume transmission concept. Brain Res Rev 26: 136–147, 1998. [DOI] [PubMed] [Google Scholar]