Abstract
Objective
To determine whether there is a survival benefit associated with cardiac transplantation in Germany.
Design
Prospective observational cohort study.
Setting
All 889 adult patients listed for a first heart transplant in Germany in 1997.
Main outcome measure
Mortality, stratified by heart failure severity.
Results
Within 1 year after listing, patients with a predicted high risk had the highest global death rate (51% v 32% and 29% for medium and low risk patients respectively; P<0.0001), had the highest risk of dying on the waiting list (32% v 20% and 20%; P=0.0003), and were more likely to receive a transplant (48% v 45% and 41%; P=0.01). Differences between the risk groups in outcome after transplantation did not reach significance (P=0.2). Transplantation was not associated with a reduction in mortality risk for the total cohort, but it did provide a survival benefit for the high risk group.
Conclusion
Cardiac transplantation in Germany is currently associated with a survival benefit only in patients with a predicted high risk of dying on the waiting list. Patients with a predicted low or medium risk have no reduction in mortality risk associated with transplantation; they should be managed with organ saving approaches rather than transplantation.
Introduction
The survival benefit of cardiac transplantation as compared with conventional treatment in advanced heart failure has not been tested in a prospective randomised trial. The main reason has probably been the assumption that, since the introduction of cyclosporin (ciclosporin) in 1980, the benefit of cardiac transplantation over conventional treatment is clinically evident.1 Meanwhile, opposing developments have occurred. On the one hand, scientific advances have improved medical and surgical treatment for advanced heart failure.2–4 On the other hand, listing of more critically ill patients and use of so called marginal donor hearts, driven by the increasing scarcity of donors, have been associated with a lack of improvement of outcomes after cardiac transplantation,5,6 even with better transplant management. As a result, the survival benefit of cardiac transplantation over other treatment options is less clear than it seemed 10-20 years ago. Consequently, the presently liberal practice of listing patients with heart failure for transplantation must be questioned until clear evidence of the benefits is available. This reasoning is in line with the current reassessment of treatment options for patients with end stage liver, lung, and renal disease.7–9 
Since a randomised trial to test the survival benefit of cardiac transplantation would be hampered by ethical concerns,10 we performed a prospective observational study. Such a method requires the assessment of clinical and prognostic profiles in order to analyse the effect of the intention to treat by transplantation. In advanced heart failure only one validated prognostic tool is currently available—the heart failure survival score,11 which is an index for predicting mortality in stable patients awaiting a heart transplant. Much as the Child-Pugh and the Mayo scores are used to guide clinical decisions at the time of listing for liver transplantation,12 we applied the heart failure survival score as an index of disease severity to our unselected cohort of patients with advanced heart failure in order to assess differences in outcomes. Specifically, we analysed global mortality, waiting list mortality, and mortality after transplantation as well as the effect of cardiac transplantation on survival for the first time in a complete national cohort of candidates for heart transplantation stratified by disease severity at the time of listing.
Patients and methods
Patients, parameters, and data acquisition
The study originated in a consensus decision of the Heart Committee of the German Transplantation Society and the Eurotransplant International Foundation (Eurotransplant). Eurotransplant is the organ exchange organisation in which transplant centres in Austria, Belgium, Luxembourg, Germany, and the Netherlands collaborate with their donor hospitals and tissue typing laboratories. The goal was to create a continuous database to monitor quality of care in compliance with the new German transplant law, which was enacted in November 1997. The project was named the “comparative outcomes and clinical profiles in transplantation (COCPIT)” study.
All adults aged 16 or over who were consecutively listed for cardiac transplantation between 1 January and 31 December 1997 in any one of the centres then performing cardiac transplantation in Germany were included (see complete list of centres in the Appendix). Data were transmitted to Eurotransplant by every centre and entered into a central database. Allocation of heart transplants was in agreement with official Eurotransplant allocation rules at the time. In brief, the rules combine medical urgency, geography, matching of donor and recipient body length, matching of ABO blood group, and cumulative waiting time. Besides the category of general urgency, centres were allowed special urgency requests to Eurotransplant for up to 15% of their transplantations for patients considered very sick. They were also allowed high urgency requests to Eurotransplant for patients with acute graft failure and, consequently, in urgent need of retransplantation.
Heart failure survival score
The original heart failure survival score was derived and validated between 1986 and 1995 in two US cohorts of stable outpatient candidates for a cardiac transplant.11 It is the weighted sum of seven non-invasive clinical parameters, the weighting reflecting the strength and direction of the association between each parameter and outcome. Outcome was defined as composite end points of death on the waiting list and urgent transplantation. These parameters were the presence of coronary artery disease (impact of aetiology); the presence of intraventricular conduction delay (degree of cardiac damage); left ventricular ejection fraction (extent of impairment of left ventricular function); heart rate and serum sodium concentration (measures of activation of the sympathetic nervous system and renin-angiotensin system); and mean arterial pressure and peak oxygen uptake (reflections of the systemic impact of chronic heart failure).
The heart failure survival score was calculated as the absolute value of the sum of the products of the parameters and their computed coefficients:
Score = ‖[1(if coronary artery disease) and 0 (if not)] × 0.6931 + [1 (if intraventricular conduction delay) and 0 (if not)] × 0.6083 + (left ventricular ejection fraction (%)) × −0.0464 + (heart rate) × 0.0216 + (sodium concentration) × −0.0470 + (mean arterial pressure) × −0.0255 + (peak oxygen uptake) × −0.0546‖.
By means of multivariate modelling and definition of arbitrary cut-off points, three groups of disease severity were defined in the derivation cohort for the heart failure survival score (1986-91) and thereafter confirmed in the validation cohort (1993-5). The low risk, medium risk, and high risk patients had a one year event-free survival of 93%, 72%, and 43% respectively in the derivation cohort and 88%, 60%, and 35% in the validation cohort.
For each patient in our study we calculated a heart failure survival score using the same methodology. We used the mean values of the derivation cohort for missing covariates in our COCPIT cohort (see table). We chose this approach in order to use the same cut-off values for the definition of the three risk groups.
Statistical methods
Patients were followed up to 1 January 2000, giving a minimum follow up of two years after registration. Waiting list outcome consisted of one of the following: transplantation, death on the waiting list, removal from the list because of worsening condition, and removal because of clinical improvement. We used the competing risk method to calculate the probability of each waiting list outcome13,14 and the Kaplan-Meier method to analyse the survival rates. To analyse global mortality we ignored whether patients had received a transplant.
Throughout the analysis we tested the null hypothesis of an absence of a difference among the three risk groups versus the alternative hypothesis of a group effect. For outcomes after listing we used the likelihood ratio test. To assess global mortality and differences between groups in outcome after transplantation we used the log rank test.
We applied a time dependent, non-proportional hazard model to cope with the problem of comparing mortality for transplanted patients against that for patients on the waiting list.8 This type of modelling is required because the interpretation of this comparison is hampered by a so called “time to event” bias. Thus, if high risk patients were to receive a transplant within days of listing, they would not have time to die on the waiting list, and so a low waiting list mortality would be obtained for this group. If, however, such high risk patients were to receive a transplant after a longer waiting time, they would have had more opportunity to die on the waiting list and would be, by selection, a less sick group at the time of transplantation than the group initially classified as high risk. Consequently, they would have a better outcome after transplantation. Hence, an analysis of patient mortality by treatment modality in which the modality is not fixed in time for all patients in the study needs to take into account the time of switching from the waiting list group to the transplantation group.
Furthermore, this approach allows for transient modelling of the mortality risk after transplantation, which is initially high and then decreases. We reported the transplantation effect as relative risk, which is the ratio of death after transplantation versus the risk of death while on the waiting list for the same period of time. If the relative risk falls below one the risk of dying after transplantation is lower than the risk of dying on the waiting list, which implies that patients benefit from transplantation. If the relative risk remains above one, survival after transplantation is not superior to waiting list survival.
Results
Patients' baseline characteristics
The table shows the patients' characteristics. The range and distribution of the heart failure survival score was similar in the score's original derivation cohort and in the COCPIT cohort. In our study, 12%, 41%, and 47% of patients were high risk, medium risk, and low risk respectively, compared with 21%, 35%, and 44%, in the original derivation cohort.
Outcome after listing
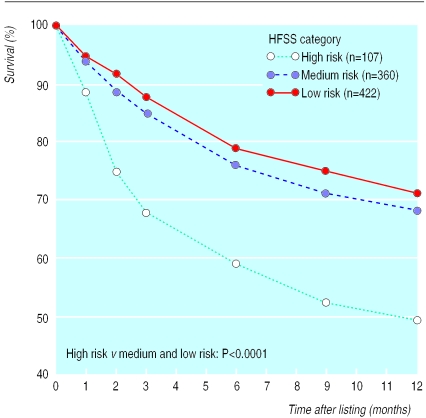
One year after listing, 33% of our total cohort had died either before or after transplantation. Those patients who were high risk according to heart failure survival score had a significantly higher chance of dying than patients at medium and low risk (51% v 32% and 29% respectively; P<0.0001) (fig 1).
Figure 1.
Survival of patients after listing for cardiac transplantation, stratified by heart failure survival score (HFSS)
Waiting list outcome
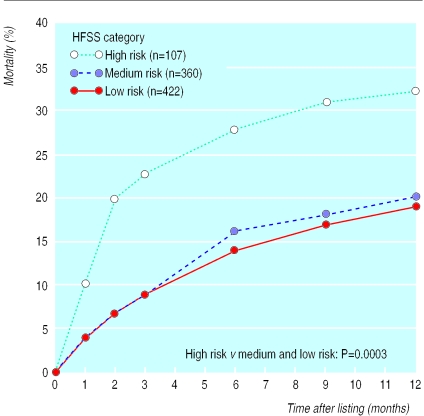
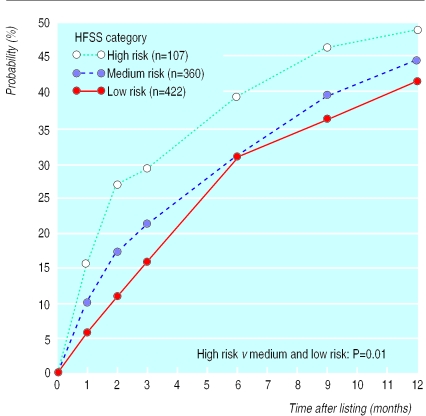
One year after registration, 424 patients (48%) had undergone cardiac transplantation (358 in the general urgency category, 62 in the special urgency category, and four in the high urgency category); 196 patients (22%) had been removed from the list because of death (n=182) or deterioration (n=14); 79 patients (9%) had been removed from the list because of improvement in their condition; and 190 (21%) were still on the waiting list. Figure 2 shows the mortality of patients on the waiting list without transplantation, stratified by heart failure survival score. Twenty per cent of the high risk patients died within two months of listing, and this proportion gradually increased to 32% at one year after listing. In the medium and low risk groups the probability of dying while waiting was significantly lower (P=0.0003) and without the initial high mortality seen in the high risk group. The likelihood of transplantation was also significantly higher in the high risk group than in the medium and low risk groups (P=0.01): by two months and 12 months of listing respectively, 27% and 48% of high risk patients, 17% and 44% of medium risk patients, and 11% and 41% of the low risk patients had received transplants (fig 3).
Figure 2.
Mortality of patients on waiting list for cardiac transplantation, stratified by heart failure survival score (HFSS)
Figure 3.
Probability of receiving a transplant for patients after listing for cardiac transplantation, stratified by heart failure survival score (HFSS)
Outcome after transplantation
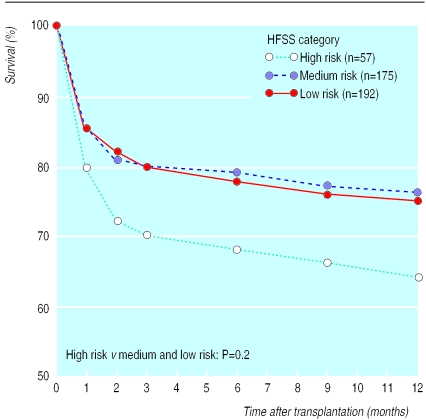
Survival at one year after transplantation for all patients was 71% (95% confidence interval 68% to 74%). Survival at one year for the high risk group was 64%, not significantly different from the 76% and 75% for the medium and low risk groups respectively (P=0.2) (fig 4).
Figure 4.
Survival after receiving a cardiac transplant for patients stratified by heart failure survival score (HFSS)
Transplantation effect
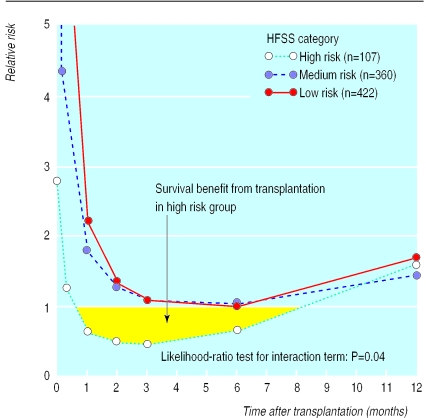
For the total cohort there was no survival benefit from transplantation (data not shown). For high risk patients, however, a mortality risk reduction was observed within two weeks of transplantation (relative risk<1.0). This benefit disappeared after eight months (fig 5). On the other hand, the relative risk did not fall below one for the medium and low risk patients at any time after transplantation.
Figure 5.
Relative risk of death for patients after receiving a cardiac transplant, stratified by heart failure survival score (HFSS)
Discussion
In this complete national cohort of adult patients consecutively listed for cardiac transplantation in Germany in 1997 no survival benefit accrued from transplantation for the group as a whole. Yet, transplantation had a beneficial effect in the high risk group as early as two weeks after transplantation. Use of the heart failure survival score allowed us to detect differences between risk groups not only with regard to the transplant effect but also with regard to mortality while on the waiting list and global mortality.
Rationale for study
Since the introduction of cyclosporin in 1980 cardiac transplantation has been considered superior to conventional treatment for heart failure, though the survival benefit of transplantation has never been examined in depth. The Registry of the International Society for Heart and Lung Transplantation reported a one year survival of 78% in 1999 that had remained constant over the previous five years,6 but the Henry Mondor Hospital in Paris, a large and experienced cardiac transplant centre, reported a much lower survival rate at one year after transplantation of 62%.5 An examination of the survival benefit from transplantation is therefore warranted. A randomised trial has been viewed as ethically unacceptable because one of the principles of such trials is that there is genuine uncertainty about the comparative therapeutic merits of each arm in a clinical trial. When the medical community has judged one proposed treatment to be better than another, randomised trials cannot be truly free of bias and may be considered unethical.10,15,16 Furthermore, the application of results from randomised trials is restricted to patients with characteristics similar to those of the cohort examined in the trials.17 Therefore, we considered an outcome analysis based on a prospective observational study to be a reasonable approach to test our hypothesis. On the basis of our results, the need for a randomised trial in cardiac transplantation should be debated.
To assess the effect of cardiac transplantation on outcome, a validated scoring system for assessing the clinical severity and associated risk of advanced heart failure is mandatory since any judgment on outcome should take into account the severity of disease. We therefore used the previously validated heart failure survival score to assess risk. Our results confirm the predictive power of the score for risk stratification in this German waiting list cohort. Most importantly, the survival advantage conferred by transplantation on the different risk groups as defined by the score could be assessed with a time dependent, proportional hazards model.
Implications of study
The high risk patients in our cohort had a global mortality of 51% at one year after listing. This rate reflects the cumulative mortality of patients on the waiting list and after transplantation. To our knowledge, no other studies have used the same combined end point because patients are usually censored at the time of transplantation. The high global death rate warrants a closer clinical surveillance at the time of listing of high risk patients.
The transient nature of the survival benefit in the high risk group is due to the changing composition of the cohorts who have received a transplant and who are still on the waiting list over time. Once patients have been on the waiting list for several months they become, by selection, a more stable population. All the frail patients have already left the cohort, as a result of either death or transplantation. This observation that survival benefit vanishes as the waiting time lengthens was described in 1991.18 Since disease severity is not constant over time, the frequency of re-evaluation of all listed patients should match the current risk of death. It should be higher in high risk states and lower in low risk states.
Our results also imply that the relative importance of medical urgency and waiting time in the listing of patients and allocation to transplantation should be discussed. A policy may opt to allow high risk patients only on the waiting list; this tends to balance organ availability and organ need. Alternatively, all potential candidates can be accepted on the waiting list and subsequently prioritised according to medical urgency, thereby decreasing the impact of waiting time in the allocation algorithm for cardiac transplantation. This change has been suggested by the German Transplantation Society19 and the US Department of Health and has been reinforced by the Institute of Medicine of the US National Institutes of Health.20
Limitations of study
Our study cohort had a 71% survival at one year after transplantation, compared with the 78% reported by the Registry of the International Society for Heart and Lung Transplantation and the 62% reported by the Henry Mondor Hospital. Possible explanations for these differences include variations in selection of recipients and donors for heart transplantation, interdisciplinary cooperation, and management protocols as well as considerable variability between centres and regions. The German Transplantation Society has initiated an audit to investigate the influence of these factors.19 Whether our results are specific to the German situation or may be generalised to other countries needs to be investigated.
What is already known on this topic
Heart transplantation is considered the treatment of choice for end stage heart failure because it is assumed to improve survival and quality of life of recipients
This assumption has not been tested in randomised trials or observational cohort studies
What this study adds
In this complete national German cohort of patients listed for cardiac transplantation during 1997 a survival benefit from transplantation was found only in the subgroup at high risk of dying without transplantation, whereas the medium risk and low risk groups did not derive a survival benefit
These findings challenge the current role of cardiac transplantation in heart failure management and may support the initiative for a randomised trial
This study did not address the question of whether quality of life was enhanced or cost of treatment reduced by cardiac transplantation. These issues are clearly important and need to be addressed in future studies. Specifically, high risk, medium risk, and low risk patients may benefit to different degrees from cardiac transplantation with regard to quality of life and resource consumption.
Conclusions
In this complete national cohort of German candidates for cardiac transplantation we found a survival benefit from transplantation only for patients with a predicted high risk of dying on the waiting list. This observation suggests that heart transplantation listing in Germany should currently be limited to the sickest patients. Patients with a predicted low or medium risk had no mortality risk reduction from transplantation; they should instead be managed with organ saving treatments.
Table.
Patients' characteristics in the derivation cohort for heart failure survival score (HFSS)11 and in the present cohort (COCPIT study). Values are means (SD) unless stated otherwise
| Characteristic | HFSS derivation cohort (n=268) | COCPIT cohort (n=889) |
|---|---|---|
| Age (years) | 50 (11) | 52 (11) |
| % male patients | 80 | 85 |
| New York Heart Association class | 2.8 (0.9) | 3.3 (0.5) |
| HFSS parameters: | ||
| Left ventricular ejection fraction (%) | 20 (8) | 22 (8) |
| Peak oxygen uptake (ml/min/kg) | 14.6 (5.4) | 15.8 (19.2)* |
| Heart rate (beats/min) | 87 (15) | 85 (17) |
| Mean arterial pressure (mm Hg) | 86 (13) | 82 (12) |
| Serum sodium concentration (mmol/l) | 137 (4) | 138 (5) |
| % of patients with intraventricular conduction delay | 27 | NK† |
| % of patients with ischaemic cardiomyopathy | 45 | 41 |
| Range of HFSS variability | 5.8-10.5 | 5.6-10.5 |
| Severity of heart failure (No (%) of patients): | ||
| High risk (HFSS⩽7.19) | 56 (21) | 107 (12) |
| Medium risk (HFSS=7.20-8.09) | 94 (35) | 360 (41) |
| Low risk (HFSS⩾8.10) | 118 (44) | 422 (47) |
HFSS=heart failure survival score.
COCPIT=comparative outcome and clinical profile in transplantation study.
NK=not known (since COCPIT study started before publication of the heart failure survival score, intraventricular conduction delay was not collected in our cohort).
Incomplete data (139/889).
Acknowledgments
We thank Professor G Breithardt for critical comments on the manuscript and Mrs Lilian Streeder for her work in data entry and management. We thank all the participating centres for their enthusiasm and continued support in this national consensus project.
Participating centres in comparative outcome and clinical profiles in transplantation (COCPIT) study (including chairmen of the cardiothoracic surgery departments at start of study)
Aachen University (Professor B Messmer), Heart Center Bad Krozingen (Dr H Tollenaere), Kerckhoff Center Bad Nauheim (Professor W P Kloevekorn), Heart Center Nordrhein-Westfalia Bad Oeynhausen (Professor R Koerfer), German Heart Center Berlin (Professor R Hetzer), Charite University Berlin (Professor W Konertz), Bochum University (Professor A Laczkovics), Heart Center Dresden (Professor S Schueler), Düsseldorf University (Professor E Gams), Essen University (Professor J C Reidemeister), Frankfurt University (Professor A Moritz), Freiburg University (Professor F Beyersdorf), Heart Center Fulda (Professor T Stegmann), Gießen University (Professor F W Hehrlein), Göttingen University (Professor H Dalichau), Halle University (Professor H R Zerkowski), Hamburg University (Professor R Kalmar), Hannover Medical School (Professor A Haverich), Heidelberg University (Professor S Hagl), Homburg University (Professor H J Schaefers), Heart Center Kaiserslautern (Professor W Seyboldt-Epting), Heart Center Karlsruhe (Dr A Posival), Kiel University (Professor D Regensburger), Köln University (Professor E R De Vivie), Leipzig University (Professor F W Mohr), Mainz University (Professor H Oelert), German Heart Center München (Professor H Meisner), München University (Professor B Reichart), Münster University (Professor H H Scheld), Regensburg University (Professor D Birnbaum), Tübingen University (Professor G Ziemer), Würzburg University (Professor R E Silber).
Editorial by Hunt
Footnotes
Funding: Eurotransplant International Foundation.
Competing interests: None declared.
References
- 1.Hunt SA. 24th Bethesda conference: cardiac transplantation. J Am Coll Cardiol. 1993;22:1–64. [PubMed] [Google Scholar]
- 2.Swedberg K for Consensus Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 3.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. for the US Carvedilol Heart Failure Study Group. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure N Engl J Med 19963341349–1355. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998;339:1522–1533. doi: 10.1056/NEJM199811193392107. [DOI] [PubMed] [Google Scholar]
- 5.Kirsch M, Baufreton C, Naftel DC, Benvenuti C, Loisance DY. Pretransplantation risk factors for death after transplantation: the Henry Mondor experience. J Heart Lung Transplant. 1999;17:268–277. [PubMed] [Google Scholar]
- 6.Hosenpud JD, Bennett LE, Keck BM, Fiol B, Boucek MM, Novick RJ. The Registry of the International Society for Heart and Lung Transplantation: sixteenth official report—1999. J Heart Lung Transplant. 1999;18:611–626. doi: 10.1016/s1053-2498(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 7.Edwards EB, Bennett LE, Daily OP, Detre K. Risk of mortality for UNOS status 3 liver recipients: a comparison of the risk posttransplant to the risk on the waiting list. Transplant Proc. 1997;29:459–460. doi: 10.1016/s0041-1345(96)00201-1. [DOI] [PubMed] [Google Scholar]
- 8.Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351:24–27. doi: 10.1016/S0140-6736(97)06405-2. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LYC, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 10.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–145. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 11.Aaronson KD, Schwartz JS, Chen TMC, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 12.Lucey MR, Brown KA, Everson GT, Fung JJ, Gish R, Keefe EB, et al. Minimal criteria for placement of adults on the liver transplant waiting list. Transplantation. 1998;66:956–962. doi: 10.1097/00007890-199810150-00034. [DOI] [PubMed] [Google Scholar]
- 13.Smits JMA, van Houwelingen HC, de Meester JMJ, Persijn GG, Claas FHJ. Analysis of the renal transplant waiting list. Application of a parametric competing risk method. Transplantation. 1998;66:1146–1153. doi: 10.1097/00007890-199811150-00006. [DOI] [PubMed] [Google Scholar]
- 14.De Meester J, Smits JMA, Persijn GG, Haverich A. Lung transplant waiting list: differential outcome of type of end-stage lung disease, one year after registration. J Heart Lung Transplant. 1999;18:563–571. doi: 10.1016/s1053-2498(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 15.Byar DP, Simon RM, Friedewald WT, Schlesselman J, DeMets DL, Ellenberg JH, et al. Randomized clinical trials. N Engl J Med. 1976;295:74–80. doi: 10.1056/NEJM197607082950204. [DOI] [PubMed] [Google Scholar]
- 16.Jadad AR, Rennie D. The randomized controlled trial gets a middle-aged checkup. JAMA. 1998;279:319–320. doi: 10.1001/jama.279.4.319. [DOI] [PubMed] [Google Scholar]
- 17.McGiffin DC, Naftel DC, Kirklin JK, Bourge RC. Advancing knowledge in cardiac transplantation and the place of outcome analysis. Curr Opin Organ Transplant. 1998;3:44–50. [Google Scholar]
- 18.Stevenson LW, Hamilton MA, Tillisch IH, Moriguchi JD, Kobashigawa J, Creaser JA, et al. Decreasing survival benefit from cardiac transplantation for outpatients as the waiting list lengthens. J Am Coll Cardiol. 1991;18:919–925. doi: 10.1016/0735-1097(91)90747-w. [DOI] [PubMed] [Google Scholar]
- 19.Deng MC, DeMeester J, Scheld HH. Development of cardiac transplant policy in Germany. Thorac Cardiovasc Surg. 2000;48:183–185. doi: 10.1055/s-2000-9637. [DOI] [PubMed] [Google Scholar]
- 20.Gibbons RD, Meltzer D, Duan N other members of the Institute of Medicine Committee on Organ Procurement and Transplantation. Waiting for organ transplantation. Science. 2000;287:237–238. doi: 10.1126/science.287.5451.237. [DOI] [PubMed] [Google Scholar]