Abstract
Objective
JAM-C is an adhesion molecule that has multiple roles in inflammation and vascular biology but many aspects of its functions under pathological conditions are unknown. Here we investigated the role of JAM-C in leukocyte migration in response to ischemia reperfusion (I/R) injury.
Methods and Results
Pre-treatment of mice with soluble JAM-C (sJAM-C), used as a pharmacological blocker of JAM-C-mediated reactions, significantly suppressed leukocyte migration in models of kidney and cremaster muscle I/R injury (39 and 51% inhibition, respectively). Furthermore, in the cremaster muscle model (studied by intravital microscopy), both leukocyte adhesion and transmigration were suppressed in JAM-C deficient mice (JAM-C−/−) and enhanced in mice over-expressing JAM-C in their endothelial cells (ECs). Analysis of JAM-C subcellular expression by immunoelectron microscopy indicated that in I/R-injured tissues, EC JAM-C was redistributed from cytoplasmic vesicles and EC junctional sites to non-junctional plasma membranes, a response that may account for the role of JAM-C in both leukocyte adhesion and transmigration under conditions of I/R injury.
Conclusions
The findings demonstrate a role for EC JAM-C in mediating leukocyte adhesion and transmigration in response to I/R injury and indicate the existence of a novel regulatory mechanism for redistribution and hence function of EC JAM-C in vivo.
Keywords: JAM-C, Ischemia reperfusion injury, Leukocyte transmigration, Inflammation, Adhesion molecules
Leukocyte migration into inflamed tissues is a characteristic feature of inflammatory disorders including numerous cardiovascular conditions such as atherosclerosis, myocardial infarction and stroke. This response involves a cascade of cellular and molecular events that have culminated in the paradigm of the leukocyte adhesion cascade.1 The final step in this process involves leukocyte migration through endothelial cells (ECs) which can occur via both para- or transcellular routes, and the subsequent breaching of the basement membrane underlying ECs and embedding pericytes.1 Leukocyte transendothelial cell migration involves a number of adhesion molecules the expression of which is highly concentrated at junctions between adjacent ECs. These molecules include PECAM-1, ICAM-2, CD99, ESAM and members of the junctional adhesion molecule (JAM) family.1-3 Our understanding of the roles, mechanisms of action and potential interactions of these molecules has significantly enhanced in recent years but many aspects of their functions in particular under in vivo pathological conditions remains unclear.
JAMs are members of an immunoglobulin subfamily, currently composed of JAM-A, -B, -C, JAM-4, ESAM (EC-selective adhesion molecule) and CAR (coxsackie virus and adenovirus receptor) that localize to cell-cell contacts and are specifically enriched at tight junctions with some being directly implicated in leukocyte transendothelial cell migration.3 Amongst these molecules JAM-C is unique in terms of its broad expression and functional profile. Specifically, JAM-C expression has been reported on ECs, spermatids, intestinal epithelial cells, smooth muscle cells, fibroblasts, and has recently been detected on Schwann cells in the peripheral nervous system.4-11 Furthermore, in humans, JAM-C is expressed on platelets and lymphocytes, whereas murine haematopoietic cells only express JAM-C during early development.4, 12-16 Due to this wide expression pattern, JAM-C has been implicated in numerous events such as leukocyte trafficking, regulation of cell polarity, vascular permeability, angiogenesis and appears to be critical in maintaining the integrity of the myelin sheath and the function of peripheral nerves.3, 5, 6, 10, 17-19 A number of ligands have been reported for JAM-C, namely JAM-C, JAM-B and Mac-1,3, 17 although their contributions in the diverse functions of JAM-C remains unclear.
The functional role of JAM-C has largely been investigated using in vitro models of cell/cell interactions8, 11, 13, 20 but more recently a growing body of in vivo studies have demonstrated a significant role for this molecule in inflammatory and vascular events.7, 12, 18, 21-23 Despite this however, many aspects of the role(s) of JAM-C remain unknown, in particular its role in different stages of the leukocyte adhesion cascade and regulation of expression under in vivo pathological conditions. In the present study we have investigated the functional role of JAM-C in leukocyte migration in two murine models of I/R injury, namely I/R injury in the kidney and the cremaster muscle, the latter being investigated by intravital microscopy (IVM). The role of JAM-C was investigated in these models using both a pharmacological blocker of JAM-C (soluble JAM-C; sJAM-C) and genetically modified mice deficient in JAM-C or selectively over-expressing JAM-C in their ECs.6, 12 Collectively, the findings demonstrate a role for JAM-C in leukocyte infiltration as elicited by I/R injury and indicate that JAM-C can support this response by mediating both leukocyte adhesion and transmigration, two distinct phases of the leukocyte adhesion cascade. Furthermore, analysis of venules by immunoelectron microscopy (IEM) detected for the first time the expression of JAM-C in EC intracellular vesicles in vivo and indicated that I/R injury can lead to re-distribution of JAM-C within ECs, most notably from EC junctions and intracellular vesicles to EC non-junctional membrane sites. The findings provide novel insights into the role and mechanism of action of JAM-C and highlight a potentially novel mechanism through which regulated expression of JAM-C may mediate different phases of leukocyte/vessel wall interactions under pathological inflammatory conditions.
METHODS
Mouse strains used were C57BL/6 (WT), JAM-C−/− and mice over-expressing JAM-C in their ECs (EC JAM-C transgenics).6, 12 Analysis of JAM-C and PECAM-1 expression in murine tissues was performed by immunofluorescence staining and confocal microscopy. Mice pre-treated with flag-tagged sJAM-C (3mg/kg, i.v.) or a control molecule (flag-tag peptide or soluble fibronectin) were subjected to I/R injury. In the renal I/R injury model (30min/24h), leukocyte infiltration into the kidneys was quantified by immunofluorescence and immunohistochemistry. Leukocyte adhesion and transmigration responses in mouse cremasteric venules as elicited by I/R injury (30min/2h) was studied by IVM using WT mice (pre-treated with a control molecule or sJAM-C), JAM-C−/− and EC JAM-C transgenic mice, as compared to relevant controls. The expression level of different adhesion molecules was investigated in blood cells from JAM-C−/− and WT mice by flow cytometry. Cell transfer experiments were performed between WT and JAM-C−/− and the response of fluorescently-labelled leukocytes in recipient mice was analyzed by fluorescent IVM in the cremasteric vasculature. Subcellular localization and redistribution of JAM-C by I/R injury was investigated by IEM. (Please see www.ahajournals.org).
RESULTS
JAM-C is expressed in the vasculature of multiple organs in mice
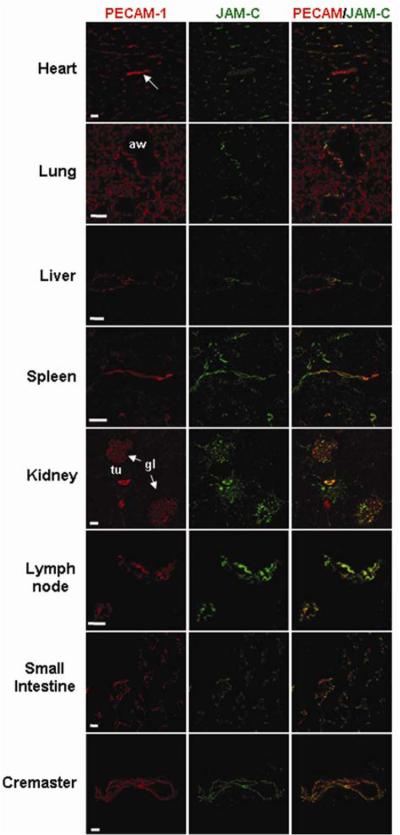
As JAM-C protein expression has not been investigated in a systematic manner in murine tissues, initial studies aimed to address this point by immunofluorescence staining and confocal microscopy. In all tissues studied (heart, lung, liver, spleen, kidney, lymph nodes, small intestine and cremaster muscle), JAM-C expression was closely associated with the EC marker PECAM-1, although the extent of co-localisation varied between different organs (Figure 1). Please see www.ahajournals.org for more details. As strong vascular expression of JAM-C was noted in kidneys and the cremaster muscle, these organs were analysed for the functional role of JAM-C under conditions of I/R injury.
Figure 1. Investigation of the expression profile of JAM-C as compared to that of PECAM-1 in multiple murine organs by immunofluorescence staining and confocal microscopy.
Soluble JAM-C inhibits leukocyte infiltration in models of kidney and cremaster muscle I/R injury
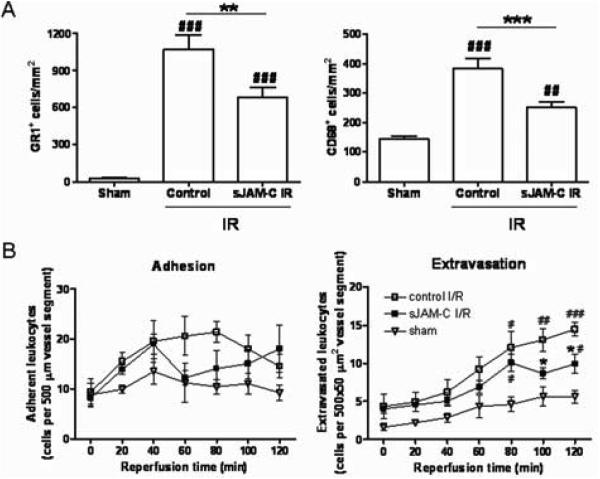
The role of JAM-C in leukocyte migration in two models of I/R injury, kidney and cremaster muscle, was investigated using sJAM-C as a pharmacological blocker of JAM-C-mediated responses.12, 23 In the kidney model, a significant leukocyte infiltration was noted in mice subjected to I/R injury as compared to sham-operated mice. In this model, the infiltrating leukocytes consisted of both neutrophils and monocytes/macrophages, though the latter appeared to form a minority population (~1:4 ratio of CD68+ cells [monocyte/macrophage] to GR1+ cells [neutrophils and GR1+ inflammatory monocytes]) (Figure 2A and supplemental Figure I). Pre-treatment of mice with sJAM-C (3 mg/kg, i.v.), but not a flag control peptide, significantly suppressed the infiltration of both GR1+ and CD68+ cells into inflamed kidneys (37% and 55% inhibition, respectively), suggesting a role for JAM-C in both neutrophil and monocyte migration in this model. To investigate the stage in the leukocyte adhesion cascade mediated by JAM-C under conditions of I/R injury, the role of JAM-C in leukocyte migration was also investigated in real time by IVM24 in the mouse cremaster muscle.
Figure 2. sJAM-C decreases leukocyte migration in kidney and cremaster muscle models of I/R injury.
A) GR1+ (infiltrated inflammatory leukocytes) and CD68+ (monocytes/macrophages) cells quantified in kidney sections. B) The number of adherent and extravasated leukocytes was quantified in cremasteric venules by IVM. (Please see www.ahajournals.org).
In the I/R injury of the cremaster muscle, following a 30min ischemia period, leukocyte/vessel wall interactions were observed by IVM during a 2h reperfusion period. In this model, leukocyte adhesion to and extravasation through venular walls increased in a time-dependent manner over the 2h reperfusion period, as compared to sham-operated animals. In mice pre-treated with sJAM-C a marked reduction in leukocyte adhesion was noted (Figure 2B left) which was associated with a significant suppression of leukocyte transmigration, as compared to mice receiving a flag-control peptide (51% inhibition at 120mins reperfusion time; Figure 2B right). Fibronectin, used as a control soluble protein had no significant effect on leukocyte adhesion or transmigration as compared to responses obtained in flag-control peptide-treated mice (not shown). These results demonstrate that sJAM-C suppresses leukocyte migration in the kidney and cremaster muscle models of I/R injury, indicating a role for JAM-C in this inflammatory scenario.
JAM-C−/− mice exhibit reduced leukocyte adhesion and transmigration in cremasteric venules as induced by I/R injury
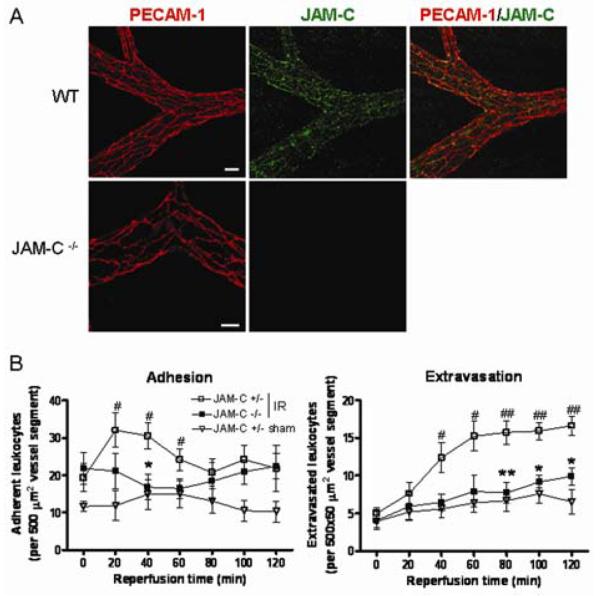
To further investigate the role of JAM-C in regulating leukocyte/vessel wall interaction, the response of JAM-C−/−mice to I/R injury was studied by IVM in the cremaster muscle. Initially, some characterisation of these mice was performed. Figure 3A shows the EC junctional co-localisation of JAM-C with the EC marker PECAM-1 in WT cremasteric venules, and its lack of expression in JAM-C−/− mice. The expression of PECAM-1 and other EC adhesion molecules such as VE-Cadherin and ICAM-1 appeared normal in JAM-C−/− mice (Figure 3A and not shown). In addition, flow cytometry analysis revealed that the expression of key leukocyte adhesion molecules (PECAM-1, ICAM-2, Mac-1, L-selectin and α4 integrins) on blood neutrophils, monocytes (inflammatory and non-inflammatory) and lymphocytes was normal in JAM-C−/− mice (data not shown). Finally, as this is the first reported IVM study on JAM-C−/− mice, some haematological parameters were also quantified (supplemental Table I), which showed elevated circulating leukocyte number in JAM-C−/− mice in line with previous reports,7 whereas no differences in mean arterial blood pressure was noted between WT, JAM-C−/−, JAM-C+/− and EC JAM-C transgenic mice.
Figure 3. JAM-C−/− exhibit reduced leukocyte adhesion and transmigration in cremasteric venules in response to I/R injury.
A) Confocal images of cremasteric venules. Scale bar:20μm. B) The number of adherent and extravasated leukocytes was quantified in cremasteric venules by IVM. (Please see www.ahajournals.org).
In the cremaster muscle I/R injury model, JAM-C−/− mice exhibited a significantly reduced leukocyte adhesion (88% inhibition at 40min reperfusion) and extravasation (66% inhibition at 120min reperfusion) response as compared to their littermate JAM-C+/− control mice (Figure 3B), findings that are in line with the results obtained under conditions of pharmacological blockade of JAM-C with sJAM-C (Figure 2B). Of relevance, no differences in leukocyte responses were noted between JAM-C+/− mice and WT animals (not shown). Since the inhibitory effect noted in the JAM-C−/− may have been due to defects in either leukocyte or EC functions, preliminary cell transfer experiments were performed to address this issue. Briefly, calcein-labelled JAM-C−/− leukocytes injected into WT recipients responded in the same manner as WT leukocytes whereas fluorescently-labelled control leukocytes (from WT or JAM-C+/− mice) injected into JAM-C−/− recipients exhibited a reduced transmigration response (81% inhibition at 120min reperfusion; not shown). Collectively these data provide the first indication that EC JAM-C plays a key role in mediating leukocyte adhesion and extravasation in vivo. To further address this point, I/R injury was assessed in mice over-expressing JAM-C in their ECs.
Transgenic mice over-expressing JAM-C in ECs exhibit enhanced leukocyte adhesion and transmigration in response to I/R injury
To extend the findings above, the cremaster muscle model was used to study I/R injury in mice over-expressing JAM-C in their ECs (EC JAM-C transgenic).12 Confocal microscopy studies revealed that these mice exhibited an overall enhanced expression of JAM-C in ECs (not shown) as previously reported.12 Interestingly, in a small number of tissue samples (~<1%), some ECs (in both venules and arterioles) exhibited a “patchy” expression profile of JAM-C where the distribution of the molecule was very different to that seen in WT mice (compare Figures 3A and 4A). Specifically, whilst in WT tissues JAM-C expression appeared to be largely localised at EC junctions, in some vessels of the transgenic mice JAM-C was detected both at EC junctions and also showing a strong apical/cytoplasmic expression. Of relevance the expression of other molecules under the control of this promoter has been reported to be unevenly distributed and/or patchy.25
Figure 4. Transgenic mice over-expressing JAM-C in their ECs exhibit enhanced leukocyte adhesion and transmigration in cremasteric venules in response to I/R injury.
A) Cremasteric venules from JAM-C transgenic mice. Bar:20μm. B) Adherent and extravasated leukocytes were quantified in cremasteric venules by IVM. (Please see www.ahajournals.org).
The EC JAM-C transgenic mice exhibited a consistently higher level of leukocyte adhesion and extravasation in cremasteric venules (e.g. 62% increase at 120min reperfusion; Figure 4B) as compared to WT littermate controls in response to I/R injury. These results together with the findings of studies using sJAM-C and JAM-C−/− mice demonstrate that in this inflammatory scenario, EC JAM-C can support both leukocyte adhesion and transmigration. To gain a better understanding of the expression and regulation of expression of EC JAM-C under conditions of I/R injury, JAM-C subcellular localization was studied in cremaster muscle tissues by IEM.
I/R injury promotes JAM-C redistribution in ECs in vivo
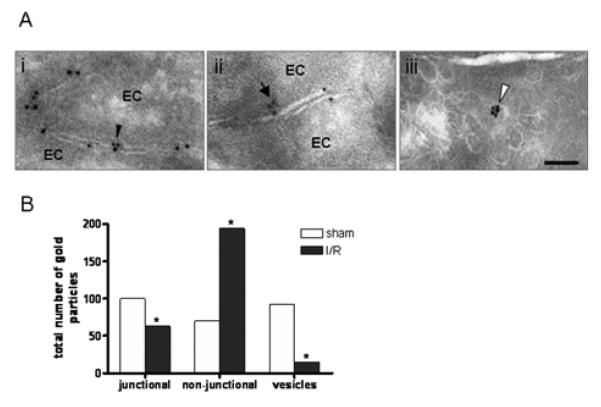
Our immunofluorescent and confocal microscopy studies in WT animals indicated the EC JAM-C expression to be predominantly junctional (Figures 1 and 3A), in line with previous reports.5 More recent studies have also reported on the intracellular expression of JAM-C in cultured microvascular (HDMECs) and macrovascular (HUVECs) ECs.19, 26 To investigate the expression profile of EC JAM-C in vivo and more importantly to assess how this may be regulated under conditions of I/R injury, JAM-C expression was studied by IEM,10, 27 an approach that allows in situ localization of JAM-C at the subcellular level. Using this technique, in control sham operated mice, JAM-C expression was noted both in ECs and in the myelin sheath of nearby nerves (not shown) as previously reported.10 In ECs, the expression of JAM-C was mostly at junctions between adjacent cells (3.6±0.4 particles per junction, n=28 ECs; Figure 5Ai) with a significantly lower level of immunolabelled JAM-C being found along the non-junctional plasma membrane of ECs (1.4±0.1 particles/field, n=49 ECs; Figure 5Aii). Interestingly, JAM-C was also localized in small single membrane-bound cytoplasmic vesicles (56±1.8 nm diameter vesicles expressing 2.2±0.2 particles/vesicle, n=41; Figure 5Aiii). These vesicles represented <1% of all EC vesicles and were found throughout the cytoplasm.
Figure 5. JAM-C localizes in different compartments within ECs and redistributes after I/R injury.
A) Localization (A) and quantification (B) of JAM-C at EC junctional sites (i), at non-junctional domains of the EC membrane (ii), and within cytoplasmic vesicles (iii). Scale bar:100nm. (Please see www.ahajournals.org).
In cremaster muscles subjected to I/R injury, JAM-C was detected in the same cellular compartments but there was a clear shift in the distribution of JAM-C (p<0.0001 by the chi square test). Specifically, the number of particles per field of non-junctional membrane was higher and the number of cytosolic vesicles and of junctional membrane domains immunolabeled for JAM-C was lower as compared to sham-operated tissue samples (Figure 5B). Hence, under conditions of I/R injury JAM-C expression in cytoplasmic vesicles was reduced by ~7 fold while increasing in the non-junctional membrane by ~2.5 fold. This in vivo re-distribution of JAM-C was associated with an unusually frequent presence of leukocytes in the lumen of the vessels (not shown). Collectively, the present results provide the first in vivo indication for redistribution of JAM-C from EC junctional sites and intracellular compartments towards the plasma membrane under inflammatory conditions, demonstrating the existence of a novel regulatory mechanism for relocalization and hence function of EC JAM-C in vivo.
DISCUSSION
JAM-C is a relatively new addition to the growing number of EC junctional adhesion molecules implicated in leukocyte trafficking3, 12, 17, 20, 23 and as such many aspects of its mechanism of action, in particular within in vivo pathological scenarios, remain unknown. Here we provide evidence for the involvement of JAM-C in leukocyte infiltration as elicited by I/R injury and demonstrate that in mediating this response, JAM-C can support both leukocyte adhesion and transmigration, two distinct phases of the leukocyte adhesion cascade. Furthermore, the study reports on the in vivo existence of EC vesicular stores of JAM-C that are re-distributed to the plasma membrane in response to I/R, providing a novel regulatory mechanism for EC JAM-C function in vivo.
JAM-C is expressed by multiple cell types such as ECs, leukocytes, Schwann cells, spermatids, and intestinal epithelial cells4-7, 9-11 and is expressed in the vasculature of most organs investigated at mRNA level28 and at protein level, as demonstrated in the present study. Specifically, in murine tissues, JAM-C was detected at a high expression level in HEVs of lumbar lymph nodes, in line with previous reports,5, 28 and was also detected in the vasculature of the heart, lungs, liver, spleen, kidneys, the small intestine and cremaster muscle in close association with that of PECAM-1. The broad cellular and tissue distribution of JAM-C suggests a role for this molecule in multiple essential biological functions, as illustrated by the diverse and severe defects (e.g. immune-deficiency, growth retardation and neurological and reproductive defects), exhibited by JAM-C deficient mice.6, 7, 10 JAM-C has also been implicated in the pathogenesis of numerous inflammatory and cardiovascular conditions such as arthritis, acute pancreatitis, peritonitis, pulmonary inflammation and atherosclerosis as largely investigated using murine disease models.7, 8, 21, 23, 29 The aim of the present studies was to further investigate the role of JAM-C under pathological cardiovascular conditions by investigating its involvement in leukocyte infiltration as induced by I/R injury. For this purpose, two murine models were employed, namely I/R injury in the kidney and in the cremaster muscle, the latter being studied in real time by IVM. The functional role of JAM-C was investigated here using both a pharmacological blocker of JAM-C, sJAM-C, and genetically modified mice lacking JAM-C (JAM-C−/−) or over-expressing JAM-C in their ECs (EC JAM-C transgenic).5, 6, 12, 27 Treatment of mice with i.v. sJAM-C resulted in a significant suppression of leukocyte infiltration (both neutrophils and monocytes) into kidneys subjected to I/R injury. Analysis of leukocyte/vessel wall interactions in the cremaster muscle model by IVM showed that sJAM-C could suppress leukocyte adhesion and extravasation in response to I/R injury. These results were in line with findings in JAM-C−/− mice where a significant suppression of leukocyte adhesion and extravasation was observed under conditions of I/R injury. Overall the findings suggest that the noted suppression of leukocyte extravasation under conditions of pharmacological or genetic deletion of JAM-C may be at least partly due to reduced leukocyte adhesion. As murine circulating leukocytes are reported not to express JAM-C,12, 16 the results are likely to be caused by loss of function of endothelial JAM-C. In agreement with this hypothesis, studies performed using a cell transfer technique demonstrated that vascular (but not leukocyte) JAM-C deficiency lead to suppression of leukocyte/vessel wall interactions. In addition, EC JAM-C transgenic mice exhibit consistently higher levels of leukocyte adhesion and a significantly enhanced leukocyte extravasation response as induced by I/R injury.
Since JAM-C is largely expressed at EC junctions and has to date been heavily implicated in leukocyte transendothelial cell migration, identifying a role for JAM-C in leukocyte adhesion in vivo was of interest. We hypothesized that JAM-C may mediate leukocyte adhesion, an earlier step in the leukocyte adhesion cascade that is prerequisite to leukocyte transendothelial cell migration, under conditions where JAM-C is enhanced on the luminal surface of the endothelium. In this context, a number of in vitro studies have demonstrated enhanced expression of EC junctional molecules on non-junctional regions. Specifically, non-junctional expression of PECAM-1 and JAM-A on cultured ECs has been shown to be enhanced by certain cytokine combinations.30, 31 More recently, Keiper et al found that cultured ECs stimulated with oxidized LDL can support enhanced monocyte adhesion in a manner that is partly JAM-C-dependent and appears to be associated with enhanced expression of JAM-C on ECs at non-junctional sites.8 A similar phenomenon was also observed under conditions of blocking JAM-B/JAM-C interactions.27 To explore the potential mechanisms that may account for JAM-C-mediated leukocyte adhesion, the subcellular expression and localization of EC JAM-C was studied in cremaster muscles by IEM. The findings identified JAM-C in three distinct EC regions, junctional membrane, non-junctional membrane and an intracellular vesicular store of JAM-C. Whilst representing a small proportion of EC vesicles, the latter novel finding may provide mechanistic insights to regulation of expression and function of JAM-C under different inflammatory conditions. Indeed, comparison of tissues from control and I/R injured mice showed a clear re-distribution of JAM-C from junctional and vesicular domains to non-junctional regions, a finding that could well account for JAM-C-mediated leukocyte adhesion under conditions of I/R injury. Such a re-distribution may provide a means of enhancing leukocyte/vessel wall interaction within the vascular lumen, possibly via JAM-C/Mac-1 interactions.27 In addition, increased luminal expression of JAM-C may promote migration of leukocytes to EC junctions through increased intravascular crawling, a response that appears to support efficient leukocyte transmigration and is reportedly Mac-1-dependent,32 as well as by creating an adhesive haptotactic gradient that guides luminal leukocytes to EC junctions. Of relevance, in humans, vascular expression of JAM-C appears to be enhanced under certain inflammatory disease conditions such as atherosclerosis and rheumatoid arthritis8, 29 though the associated mechanisms are unknown highlighting the need for a better understanding of the molecular events that regulate JAM-C expression under different disease conditions.
Collectively, the present results show a role for JAM-C in leukocyte adhesion and transmigration in response to I/R injury and demonstrate the redistribution of EC JAM-C during this vascular insult in a manner that could potentially determine the functional role of JAM-C.
ACKNOWLEDGEMENTS
The authors thank P. Hammel and D. Caille for excellent technical assistance.
SOURCES OF FUNDING
This work was supported by funds from The Wellcome Trust, UK and The British Heart Foundation to S.N. (grants 081172/Z/06/Z and PG/03/123/16102, respectively). Other authors were funded as follows: N.S.A.P by the WHR foundation and Kidney Research UK (PDF4/2009); P.M. by the Swiss National Science Foundation (310000-122430), Juvenile Diabetes Research Foundation International (1-2007-158), the EU (FP-7 BETAIMAGE 222980) and Novo Nordisk; M.A-L. by the Swiss National Foundation (310000-112551) and Avenir Program, Inserm; B.A.I. by Oncosuisse (OCS-01812-12-2005) and the Swiss National Science Foundation (310000-120184).
Footnotes
Disclosures None
REFERENCES
- 1.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 3.Bradfield PF, Nourshargh S, Aurrand-Lions M, Imhof BA. JAM family and related proteins in leukocyte migration. Arterioscler Thromb Vasc Biol. 2007;27:2104–2112. doi: 10.1161/ATVBAHA.107.147694. [DOI] [PubMed] [Google Scholar]
- 4.Arrate MP, Rodriguez JM, Tran TM, Brock TA, Cunningham SA. Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J Biol Chem. 2001;276:45826–45832. doi: 10.1074/jbc.M105972200. [DOI] [PubMed] [Google Scholar]
- 5.Aurrand-Lions M, Duncan L, Ballestrem C, Imhof BA. JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J Biol Chem. 2001;276:2733–2741. doi: 10.1074/jbc.M005458200. [DOI] [PubMed] [Google Scholar]
- 6.Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 7.Imhof BA, Zimmerli C, Gliki G, Ducrest-Gay D, Juillard P, Hammel P, Adams R, Aurrand-Lions M. Pulmonary dysfunction and impaired granulocyte homeostasis result in poor survival of Jam-C-deficient mice. J Pathol. 2007;212:198–208. doi: 10.1002/path.2163. [DOI] [PubMed] [Google Scholar]
- 8.Keiper T, Al-Fakhri N, Chavakis E, Athanasopoulos AN, Isermann B, Herzog S, Saffrich R, Hersemeyer K, Bohle RM, Haendeler J, Preissner KT, Santoso S, Chavakis T. The role of junctional adhesion molecule-C (JAM-C) in oxidized LDL-mediated leukocyte recruitment. Faseb J. 2005;19:2078–2080. doi: 10.1096/fj.05-4196fje. [DOI] [PubMed] [Google Scholar]
- 9.Morris AP, Tawil A, Berkova Z, Wible L, Smith CW, Cunningham SA. Junctional adhesion molecules (JAMs) are differentially expressed in fibroblasts and co-localize with ZO-1 to adherens-like junctions. Cell Commun Adhes. 2006;13:233–247. doi: 10.1080/15419060600877978. [DOI] [PubMed] [Google Scholar]
- 10.Scheiermann C, Meda P, Aurrand-Lions M, Madani R, Yiangou Y, Coffey P, Salt TE, Ducrest-Gay D, Caille D, Howell O, Reynolds R, Lobrinus A, Adams RH, Yu AS, Anand P, Imhof BA, Nourshargh S. Expression and function of junctional adhesion molecule-C in myelinated peripheral nerves. Science. 2007;318:1472–1475. doi: 10.1126/science.1149276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zen K, Babbin BA, Liu Y, Whelan JB, Nusrat A, Parkos CA. JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol Biol Cell. 2004;15:3926–3937. doi: 10.1091/mbc.E04-04-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aurrand-Lions M, Lamagna C, Dangerfield JP, Wang S, Herrera P, Nourshargh S, Imhof BA. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J Immunol. 2005;174:6406–6415. doi: 10.4049/jimmunol.174.10.6406. [DOI] [PubMed] [Google Scholar]
- 13.Santoso S, Sachs UJ, Kroll H, Linder M, Ruf A, Preissner KT, Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ody C, Jungblut-Ruault S, Cossali D, Barnet M, Aurrand-Lions M, Imhof BA, Matthes T. Junctional adhesion molecule C (JAM-C) distinguishes CD27+ germinal center B lymphocytes from non-germinal center cells and constitutes a new diagnostic tool for B-cell malignancies. Leukemia. 2007;21:1285–1293. doi: 10.1038/sj.leu.2404689. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham SA, Rodriguez JM, Arrate MP, Tran TM, Brock TA. JAM2 interacts with alpha4beta1. Facilitation by JAM3. J Biol Chem. 2002;277:27589–27592. doi: 10.1074/jbc.C200331200. [DOI] [PubMed] [Google Scholar]
- 16.Praetor A, McBride JM, Chiu H, Rangell L, Cabote L, Lee WP, Cupp J, Danilenko DM, Fong S. Genetic deletion of JAM-C reveals a role in myeloid progenitor generation. Blood. 2009;113:1919–1928. doi: 10.1182/blood-2008-06-159574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 18.Lamagna C, Hodivala-Dilke KM, Imhof BA, Aurrand-Lions M. Antibody against junctional adhesion molecule-C inhibits angiogenesis and tumor growth. Cancer Res. 2005;65:5703–5710. doi: 10.1158/0008-5472.CAN-04-4012. [DOI] [PubMed] [Google Scholar]
- 19.Orlova VV, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J Exp Med. 2006;203:2703–2714. doi: 10.1084/jem.20051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson-Leger CA, Aurrand-Lions M, Beltraminelli N, Fasel N, Imhof BA. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood. 2002;100:2479–2486. doi: 10.1182/blood-2001-11-0098. [DOI] [PubMed] [Google Scholar]
- 21.Vonlaufen A, Aurrand-Lions M, Pastor CM, Lamagna C, Hadengue A, Imhof BA, Frossard JL. The role of junctional adhesion molecule C (JAM-C) in acute pancreatitis. J Pathol. 2006;209:540–548. doi: 10.1002/path.2007. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig RJ, Zollner TM, Santoso S, Hardt K, Gille J, Baatz H, Johann PS, Pfeffer J, Radeke HH, Schon MP, Kaufmann R, Boehncke WH, Podda M. Junctional adhesion molecules (JAM)-B and -C contribute to leukocyte extravasation to the skin and mediate cutaneous inflammation. J Invest Dermatol. 2005;125:969–976. doi: 10.1111/j.0022-202X.2005.23912.x. [DOI] [PubMed] [Google Scholar]
- 23.Chavakis T, Keiper T, Matz-Westphal R, Hersemeyer K, Sachs UJ, Nawroth PP, Preissner KT, Santoso S. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J Biol Chem. 2004;279:55602–55608. doi: 10.1074/jbc.M404676200. [DOI] [PubMed] [Google Scholar]
- 24.Woodfin A, Reichel CA, Khandoga A, Corada M, Voisin MB, Scheiermann C, Haskard DO, Dejana E, Krombach F, Nourshargh S. JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood. 2007;110:1848–1856. doi: 10.1182/blood-2006-09-047431. [DOI] [PubMed] [Google Scholar]
- 25.Anghelina M, Moldovan L, Moldovan NI. Preferential activity of Tie2 promoter in arteriolar endothelium. J Cell Mol Med. 2005;9:113–121. doi: 10.1111/j.1582-4934.2005.tb00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sircar M, Bradfield PF, Aurrand-Lions M, Fish RJ, Alcaide P, Yang L, Newton G, Lamont D, Sehrawat S, Mayadas T, Liang TW, Parkos CA, Imhof BA, Luscinskas FW. Neutrophil transmigration under shear flow conditions in vitro is junctional adhesion molecule-C independent. J Immunol. 2007;178:5879–5887. doi: 10.4049/jimmunol.178.9.5879. [DOI] [PubMed] [Google Scholar]
- 27.Lamagna C, Meda P, Mandicourt G, Brown J, Gilbert RJ, Jones EY, Kiefer F, Ruga P, Imhof BA, Aurrand-Lions M. Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion. Mol Biol Cell. 2005;16:4992–5003. doi: 10.1091/mbc.E05-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98:3699–3707. doi: 10.1182/blood.v98.13.3699. [DOI] [PubMed] [Google Scholar]
- 29.Palmer G, Busso N, Aurrand-Lions M, Talabot-Ayer D, Chobaz-Peclat V, Zimmerli C, Hammel P, Imhof BA, Gabay C. Expression and function of junctional adhesion molecule-C in human and experimental arthritis. Arthritis Res Ther. 2007;9:R65. doi: 10.1186/ar2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozaki H, Ishii K, Horiuchi H, Arai H, Kawamoto T, Okawa K, Iwamatsu A, Kita T. Cutting edge: combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol. 1999;163:553–557. [PubMed] [Google Scholar]
- 31.Romer LH, McLean NV, Yan HC, Daise M, Sun J, DeLisser HM. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J Immunol. 1995;154:6582–6592. [PubMed] [Google Scholar]
- 32.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]