Abstract
Background/aims
Impaired inhibition of the alternative complement pathway by complement factor H (CFH) is linked to age-related macular degeneration (AMD) based on the strong association between CFH variant and AMD. Chlamydia pneumoniae (C pneumoniae) infection can trigger the alternative pathway, but the evidence for an association between C pneumoniae and AMD is contradictory. This study investigated whether C pneumoniae infection is associated with AMD and whether the presence of C pneumonia modulates AMD risk conferred by CFH variants.
Methods
Genomic DNA extracted from peripheral blood of 148 advanced AMD patients and 162 controls was subjected to Taqman and PCR-RFLP for the CFH polymorphism and PCR for the C pneumoniae gene. Genomic DNA was also examined from microdissected macular cells from 59 AMD and 16 age-matched non-AMD archived slides. χ2 testing was performed for case-control analysis.
Results
C pneumoniae infection was associated with increased risk of AMD (OR = 2.17, p<0.017). A CFH variant was also linked to increased risk of AMD (OR = 1.98, p<0.0001). However, no relationship was found between risk-conferring CFH variant and C pneumoniae (OR = 1.81, p = 0.08).
Conclusion
There is a possible association between AMD and C pneumoniae infection, although CFH may not be directly involved in the pathogenesis of C pneumoniae infection-mediated AMD.
Age-related macular degeneration (AMD) is the leading cause of blindness among older people in the world.1 The prevalence of AMD increases dramatically with age, resulting in significant medical, social and economic costs to patients and a considerable burden on society. Its prevalence is expected to increase with the overall ageing of the population.
While the pathophysiological basis of AMD remains to be elucidated, the current paradigm supports a role for environmental triggers amidst a genetically predisposed backdrop. Indeed, studies at several loci have suggested a strong genetic component to AMD.2 3 The most commonly documented genetic association is between the complement factor H (CFH) Y402H polymorphism (SNP) and AMD,4–7 and meta-analyses have demonstrated a significant role for the CFH Y402H SNP in Caucasian AMD patients at the population level.8–10 CFH is an inhibitor of the alternative complement pathway, and functional studies of the Y402H CFH SNP suggest that it possesses impaired complement inhibitory activities. These studies are in accordance with the proposed role in AMD of inflammation in general and complement overactivity in particular, both of which can lead to tissue damage if not properly controlled.11
Chronic Chlamydia pneumoniae (C pneumoniae) infection has been linked to AMD in a limited number of small case-control studies.12–14 Serum anti-C pneumoniae antibodies are reported to be higher in AMD patients compared with controls, and an increased titre of serum antibody against C. pneumonia has been linked to rapid progression of AMD.14 Using immunohistochemistry and polymerase chain reaction (PCR) techniques, Kalayoglu et al have demonstrated the presence of C pneumoniae in AMD neovascular membranes.15 In contrast, two studies by Robman et al and Kessler et al demonstrated no significant association between C pneumoniae and AMD or AMD CNV, respectively.16 17 The conflicting data regarding the role for C pneumoniae in AMD pathogenesis warrant further studies to evaluate the link, if any, between chronic C pneumoniae infection and AMD risk.
C pneumoniae activates the alternative complement pathway or induces a chronic inflammatory state, which might contribute to the pathogenesis of AMD.11 18 Furthermore, it is possible that in patients with the risk-conferring CFH variant, C pneumoniae infection represents the trigger for the alternative pathway, which thereby runs uninhibited due to impaired CFH activity in these patients. In this study, we investigate whether C pneumoniae infection is associated with AMD and whether there is any relationship among chronic C pneumoniae infection, the CFH SNP, and risk of AMD using our previously reported cohort of individuals with and without AMD.19–23
MATERIALS AND METHODS
Subjects
The description of the eligibility criteria and recruitment of participants into this study has been previously described.21 23 In brief, all participants were recruited from a National Eye Institute (NEI) cohort, which was approved by NEI Institutional Review Boards, and each participant signed the informed consent at enrolment. All participants were Caucasians residing in the greater Washington, DC area. Only patients with advanced AMD and controls with normal retina were recruited. Clinical diagnosis of advanced AMD was based upon fundus photographs graded as geographic atrophy involving the centre of the fovea and/or choroid neovascularisation associated with large drusen in at least one eye. The controls have no or few small drusen. A total of 148 patients with advanced AMD and 162 non-AMD controls were enrolled. Their peripheral blood cells were collected for genomic DNA extraction using a QIAamp DNA Blood Maxi kit (Qiagen, Valencia, California).
Archived paraffin-embedded ocular slides from 59 pathologically diagnosed advanced geographic or neovascular AMD and 16 age-matched cases with normal retina were also examined. The macular cells on these slides were manually microdissected, and their genomic DNA was extracted as described previously.21 24 25
Analysis of CFH SNP genotyping
The SNP genotyping was performed by Taqman SNP Genotyping Assay (Applied Biosystems, Foster City, California), assay ID# C_2530286_20 for CFH intron (rs380390).
Detection of C pneumonia DNA
DNA isolated from peripheral blood cells was subjected to PCR amplification of the C pneumoniae 16S rRNA gene using with a primer pair of CPN90/CPN91, for which the sequences are 5′-GGT CTC AAC CCC ATC CGT GTC GG-3′ and 5′-TGC GGA AAG CTG TAT TTC TAC AGT T-3′.26 DNA isolated from microdissected cells was first incubated with 20 μl of TE buffer containing 0.5 mg/ml proteinase K at 37°C for overnight. After incubation at 90°C, 5 μl of DNA solution was subjected to PCR with 32P-labelled primer pair of CPN90/CPN91. A total volume of 25 μl containing 10 ng of blood DNA or 5 μl of DNA from microdissection, 1X PCR buffer, 0.4 mM dNTP, 2.0 mM MgCl2 and 2 units of AmpliTag Gold (Applied Biosystems) was amplified for 7 min at 95°C, followed by 60 cycles of denaturation at 94°C for 45 s, annealing beginning at 65°C and ending at 52°C for 45 s, and extension at 72°C for 1 min. The annealing temperature was lowered by 1°C every four cycles until it reached 52°C, and this annealing temperature was maintained until the end of the cycling process. The 197 bp products of the PCR amplification were subjected for polyacrylamide gel electrophoresis and visualised by ethidium bromide staining or autoradiography.
Statistical analysis
Data for C pneumoniae status, CFH alleles, and AMD versus control status were entered into contingency tables using the GraphPad Prism software (GraphPad Software, San Diego, California). χ2 analyses were performed to test for differences in these categorical variables between two or more groups. Specifically, χ2 tables were used to assess for associations between CFH variants and AMD in all patients and in patients with the C pneumoniae risk factor, C pneumoniae infection and AMD in all patients and in patients with the risk-conferring CFH allele. Statistical significance was established when p<0.05 for all analyses.
Odds ratios and relative risk for AMD were also calculated for the presence of one risk factor (risk-conferring CFH allele or C pneumoniae positivity) and for the presence of both risk factors.
RESULTS
The demographic information from the two data samples (peripheral blood and archived slides) is summarised in table 1.
Table 1.
Demographic data of the participants
| NEI cohort |
Archived slide |
|||
|---|---|---|---|---|
| Group | AMD | Control | AMD | Control |
| No | 148 | 162 | 59 | 16 |
| Age, mean (SD) | 79 (8) | 66 (11) | 83 (9) | 72 (8) |
| Female | 78 | 92 | 25 | 5 |
| Male | 70 | 70 | 17 | 7 |
| Unknown gender | 0 | 0 | 17 | 4 |
AMD, age-related macular degeneration; NEI, National Eye Institute.
CFH intron SNP was closely associated with AMD risk
The CFH intron G/C SNP (rs380390) and CFH-Y402H are in complete linkage disequilibrium (LD), and both the C allele at rs380390 and the Y402H SNPs have been shown to be significantly associated with AMD.4 21 Our study similarly found an association between the C allele at rs380390 and risk of AMD. Among the 148 AMD patients, the numbers of G/G, G/C and C/C allele were 51, 53 and 44, respectively. Among the 162 control subjects, the numbers of G/G, G/C and C/C allele were 76, 70 and 16, respectively. χ2 analysis demonstrated a statistically significant association between presence of the C allele at this position and risk of AMD (p<0.0001).
C pneumoniae infection associated with increased risk of AMD
C pneumoniae DNA was identified in the peripheral blood and macular cells of both advanced AMD patients and controls using PCR and PAGE (fig 1). As delineated in table 2, 20.3% of AMD patients exhibited chronic C pneumoniae infection, while only 10.5% of controls did, suggesting an association between C pneumoniae infection and AMD risk (p<0.017).
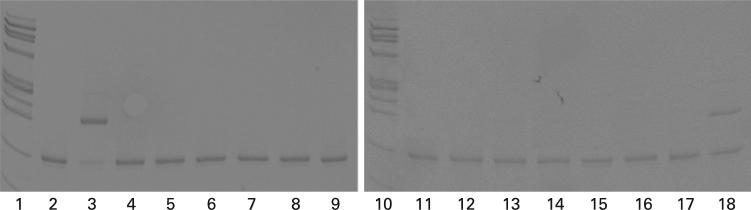
Figure 1.
Amplification of Chlamydia pneumoniae in peripheral blood DNA. Lanes 1 and 10, ϕx 174 DNA/Hae III fragments. Lanes 2–9, DNA from AMD patients. Lanes 11–18, DNA from non-AMD controls.
Table 2.
Detection of Chlamydia pneumoniae DNA signature in the participants
| Age-related macular degeneration |
Control |
|||
|---|---|---|---|---|
| Specimen | Total cases | C pneu+ (%) | Total cases | C pneu+ (%) |
| Archived eye DNA | 59 | 2 (3.3%) | 16 | 1 (6.2%) |
| Blood DNA | 148 | 30 (20.3%) | 162 | 17 (10.5%) |
C pneu+, positive C pneumoniae DNA.
However, no difference was found in the archived samples, which most likely resulted from the small sample size and sample material, as well as a relatively low incidence of C pneumoniae infection in this population (table 2).
CFH variant was not involved in the pathogenesis of C pneumoniae-associated AMD
χ2 analysis indicated that the presence of C pneumoniae infection did not affect the risk of AMD in patients with the risk-conferring CFH variant. Similarly, the CFH SNP did not affect the risk of AMD in patients with chronic C pneumoniae infection (table 3). Interestingly, a higher frequency of C pneumoniae infection (positive C pneumoniae DNA) seemed to relate to the risk-conferring CFH variant (C allele) in the dry AMD patients. Since this study only included 35 dry AMD patients, the data would have even less impact.
Table 3.
Distribution of complement factor H SNP and C pneumoniae DNA among the participants
| AMD |
Control |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Complement factor H intron | Wet | C pneu+ in wet | Dry | C pneu+ in dry | W or D unknown | C pneu+ in unknown | Total | C pneu+ in total | Total | C pneu+ |
| G/G | 38 | 8 (21.0%) | 10 | 3 (30.0%) | 3 | 1 | 51 | 12 (23.5%) | 76 | 3 (4.0%) |
| G/C | 32 | 4 (12.5%) | 14 | 5 (35.7%) | 7 | 0 | 53 | 9 (17.0%) | 70 | 11 (15.7%) |
| C/C | 28 | 5 (17.9%) | 11 | 4 (36.4%) | 5 | 0 | 44 | 9 (20.5%) | 16 | 3 (18.8%) |
| Total | 98 | 17 (17.3%) | 35 | 12 (34.2%) | 15 | 1 | 148 | 30 (20.3%) | 162 | 17 (10.5%) |
AMD, age-related macular degeneration; C pneu+, positive C pneumoniae DNA; Dry, dry AMD; Unknown, unknown AMD subtype; Wet, wet AMD.
The presence of the C allele of CFH (risk-variant) led to a relative risk (RR) = 1.411 (95% CI 1.202 to 1.658) and odds ratio (OR) = 1.980 (95% CI 1.427 to 2.746) of AMD, which represented a statistically significant association (p<0.0001). The presence of chronic C pneumoniae infection led to an RR = 1.423 (95% CI 1.189 to 1.702) and OR = 2.168 (95% CI 1.376 to 3.416), which was also significant (p = 0.0008). In contrast, the presence of both factors (C pneumoniae infection and C allele of CFH) did not increase the RR or OR over that seen with one factor alone, with RR = 1.314 (95% CI 1.023 to 1.688) and OR = 1.813 (0.967 to 3.399) (p = 0.0840).
DISCUSSION
AMD development and progression have been associated with increasing antibody titre to C pneumoniae.14 Moreover, CNV specimens from AMD patients have been reported to be significantly more likely to show evidence of C pneumoniae by immunohistochemistry, when compared with non-AMD CNV and non-AMD eyes.15 Macrophages are shown to produce the proangiogenic vascular endothelial growth factor (VEGF) after infection with C pneumoniae, providing one mechanism by which C pneumoniae infection may predispose to CNV development. In contrast to these reports of positive associations between AMD and C pneumoniae infection, there are also negative findings in the literature.16 17
This study confirms the potential association between AMD and C pneumoniae infection, with a significantly higher incidence of the C pneumoniae's genetic signature in blood from 148 AMD patients than in blood from 162 control patients. Our finding of C pneumoniae DNA in macular cells from the pathological slides also suggests a possible role for C pneumoniae in AMD.
C pneumoniae is an obligate intracellular pathogen responsible for 6–20% of cases of community-acquired pneumonia.27 Most infections are mild or asymptomatic. The micro-organism is disseminated through blood circulation and has a particular attraction for vascular tissues, where it may result in chronic infection.28 Circulating C pneumoniae elementary bodies and/or C pneumoniae reticulate bodies in macrophages and other cells in the AMD lesions serve as infective triggers for AMD.29 Recently C pneumoniae has been posited as a potentially important factor for several systemic diseases such as asthma,30 multiple sclerosis31 and cardiovascular diseases.32
As a microbe, Chlamydia can also activate the alternative pathway of complement, which is strongly implicated in AMD pathogenesis.4 5 18 33 34 Indeed, chronic inflammation from C pneumoniae might be one of the triggers that activates the alternative complement pathway and ultimately leads to complement overactivity in patients with the risk variant of CFH, which possesses impaired complement inhibitory function. If so, we would expect that the presence of both the risk-conferring variant of CFH and C pneumoniae positivity would result in an increased risk of AMD, as compared with the presence of either the risk-conferring CFH variant or C pneumoniae positivity alone. Our findings demonstrate that individually each factor—CFH risk allele and C pneumoniae gene—is associated with AMD risk, but that the presence of the second factor does not significantly increase this risk. However, it is possible that such gene–gene interaction may not be detected in a study of relatively small sample size in our study population.
The link between C pneumoniae and AMD is still controversial, which may be due to difficulties in verifying laboratory techniques for the detection of the micro-organism in the chronic infection stage.35 36 Our data resulting from a relatively small study certainly support a positive association between chronic C pneumoniae infection and AMD. Our study fails to find any additive or a combined effect of C pneumoniae and the risk-conferring CFH variants on AMD. While the association between C pneumoniae and AMD may not be directly linked to an impaired complement alternative pathway, it may be mediated by other inflammatory mechanisms such as macrophages, inflammatory cytokines and/or chemokines. For example, it is possible that macrophages infected with C pneumoniae may upregulate adhesion molecules, promote recruitment of inflammatory cellular migration through the blood–brain/retina barrier and/or facilitate homing of inflammatory cells into the macula.37 Further investigations are warranted to parse out the underlying mechanisms by which C pneumoniae might promote AMD development and progression.
Footnotes
Funding: The study is funded by the National Eye Institute Intramural Research Program.
Competing interests: None.
Ethics approval: Ethics approval was provided by the National Eye Institute Review Boards.
Patient consent: Obtained.
REFERENCES
- 1.Klein R, Peto T, Bird A, et al. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–95. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 2.Tuo J, Bojanowski CM, Chan CC. Genetic factors of age-related macular degeneration. Prog Retin Eye Res. 2004;23:229–49. doi: 10.1016/j.preteyeres.2004.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad S, Chen CA, Santangelo SL, et al. The genetics of age-related macular degeneration: a review of progress to date. Surv Ophthalmol. 2006;51:316–63. doi: 10.1016/j.survophthal.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards AO, Ritter R, 3rd, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 6.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 7.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher SA, Abecasis GR, Yashar BM, et al. Meta-analysis of genome scans of age-related macular degeneration. Hum Mol Genet. 2005;14:2257–64. doi: 10.1093/hmg/ddi230. [DOI] [PubMed] [Google Scholar]
- 9.Thakkinstian A, Han P, McEvoy M, et al. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum Mol Genet. 2006;15:2784–90. doi: 10.1093/hmg/ddl220. [DOI] [PubMed] [Google Scholar]
- 10.Swaroop A, Branham KE, Chen W, et al. Genetic susceptibility to age-related macular degeneration: a paradigm for dissecting complex disease traits. Hum Mol Genet. 2007;16:174–82R. doi: 10.1093/hmg/ddm212. (Spec No 2) [DOI] [PubMed] [Google Scholar]
- 11.Patel M, Chan CC. Immunopathological aspects of age-related macular degeneration. Semin Immunopathol. 2008;30:97–110. doi: 10.1007/s00281-008-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalayoglu MV, Galvan C, Mahdi OS, et al. Serological association between Chlamydia pneumoniae infection and age-related macular degeneration. Arch Ophthalmol. 2003;121:478–82. doi: 10.1001/archopht.121.4.478. [DOI] [PubMed] [Google Scholar]
- 13.Ishida O, Oku H, Ikeda T, et al. Is Chlamydia pneumoniae infection a risk factor for age related macular degeneration? Br J Ophthalmol. 2003;87:523–4. doi: 10.1136/bjo.87.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robman L, Mahdi O, McCarty C, et al. Exposure to Chlamydia pneumoniae infection and progression of age-related macular degeneration. Am J Epidemiol. 2005;161:1013–19. doi: 10.1093/aje/kwi130. [DOI] [PubMed] [Google Scholar]
- 15.Kalayoglu MV, Bula D, Arroyo J, et al. Identification of Chlamydia pneumoniae within human choroidal neovascular membranes secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2005;243:1080–90. doi: 10.1007/s00417-005-1169-y. [DOI] [PubMed] [Google Scholar]
- 16.Kessler W, Jantos CA, Dreier J, et al. Chlamydia pneumoniae is not detectable in subretinal neovascular membranes in the exudative stage of age-related macular degeneration. Acta Ophthalmol Scand. 2006;84:333–7. doi: 10.1111/j.1600-0420.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 17.Robman L, Mahdi OS, Wang JJ, et al. Exposure to Chlamydia pneumoniae infection and age-related macular degeneration: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 2007;48:4007–11. doi: 10.1167/iovs.06-1434. [DOI] [PubMed] [Google Scholar]
- 18.Baird PN, Robman LD, Richardson AJ, et al. Gene–environment interaction in progression of AMD: the CFH gene, smoking and exposure to chronic infection. Hum Mol Genet. 2008;17:1299–305. doi: 10.1093/hmg/ddn018. [DOI] [PubMed] [Google Scholar]
- 19.Tuo J, Smith BC, Bojanowski CM, et al. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:1297–9. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bojanowski CM, Tuo J, Chew EY, et al. Analysis of Hemicentin-1, hOgg1, and E-selectin single nucleotide polymorphisms in age-related macular degeneration. Trans Am Ophthalmol Soc. 2005;103:37–44. [PMC free article] [PubMed] [Google Scholar]
- 21.Tuo J, Ning B, Bojanowski CM, et al. Synergic effect of polymorphisms in ERCC6 59 flanking region and complement factor H on age-related macular degeneration predisposition. Proc Natl Acad Sci U S A. 2006;103:9256–61. doi: 10.1073/pnas.0603485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bojanowski CM, Shen D, Chew EY, et al. An apolipoprotein E variant may protect against age-related macular degeneration through cytokine regulation. Environ Mol Mutagen. 2006;47:594–602. doi: 10.1002/em.20233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross RJ, Bojanowski CM, Wang JJ, et al. The LOC387715 polymorphism and age-related macular degeneration: replication in three case-control samples. Invest Ophthalmol Vis Sci. 2007;48:1128–32. doi: 10.1167/iovs.06-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan CC, Tuo J, Bojanowski CM, et al. Detection of CX3CR1 single nucleotide polymorphism and expression on archived eyes with age-related macular degeneration. Histol Histopathol. 2005;20:857–63. doi: 10.14670/hh-20.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan CC, Shen D, Zhou M, et al. Human HtrA1 in the archived eyes with age-related macular degeneration. Trans Am Ophthalmol Soc. 2007;105:92–7. discussion 7–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Madico G, Quinn TC, Boman J, et al. Touchdown enzyme time release-PCR for detection and identification of Chlamydia trachomatis, C pneumoniae, and C. psittaci using the 16S and 16S–23S spacer rRNA genes. J Clin Microbiol. 2000;38:1085–93. doi: 10.1128/jcm.38.3.1085-1093.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo CC, Jackson LA, Campbell LA, et al. Chlamydia pneumoniae (TWAR). Clin Microbiol Rev. 1995;8:451–61. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blasi F. Atypical pathogens and respiratory tract infections. Eur Respir J. 2004;24:171–81. doi: 10.1183/09031936.04.00135703. [DOI] [PubMed] [Google Scholar]
- 29.Abdelrahman YM, Belland RJ. The chlamydial developmental cycle. FEMS Microbiol Rev. 2005;29:949–59. doi: 10.1016/j.femsre.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland ER, Martin RJ. Asthma and atypical bacterial infection. Chest. 2007;132:1962–6. doi: 10.1378/chest.06-2415. [DOI] [PubMed] [Google Scholar]
- 31.Fainardi E, Castellazzi M, Seraceni S, et al. Under the microscope: focus on Chlamydia pneumoniae infection and multiple sclerosis. Curr Neurovasc Res. 2008;5:60–70. doi: 10.2174/156720208783565609. [DOI] [PubMed] [Google Scholar]
- 32.Watson C, Alp NJ. Role of Chlamydia pneumoniae in atherosclerosis. Clin Sci (Lond) 2008;114:509–31. doi: 10.1042/CS20070298. [DOI] [PubMed] [Google Scholar]
- 33.Haines JL, Pericak-Vance MA. Rapid dissection of the genetic risk of age-related macular degeneration: achieving the promise of the genomic era. JAMA. 2007;297:401–2. doi: 10.1001/jama.297.4.401. [DOI] [PubMed] [Google Scholar]
- 34.Ross RJ, Verma V, Rosenberg KI, et al. Genetic markers and biomarkers for age-related macular degeneration. Expert Rev Ophthalmol. 2007;2:443–57. doi: 10.1586/17469899.2.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammerschlag MR, Apfalter P, Boman J, et al. The role of Chlamydia pneumoniae in multiple sclerosis: real or fictitious? J Infect Dis. 2005;192:1305–7. doi: 10.1086/466533. author reply 7. [DOI] [PubMed] [Google Scholar]
- 36.Guymer R, Robman L. Chlamydia pneumoniae and age-related macular degeneration: a role in pathogenesis or merely a chance association? Clin Exp Ophthalmol. 2007;35:89–93. doi: 10.1111/j.1442-9071.2006.01392.x. [DOI] [PubMed] [Google Scholar]
- 37.MacIntyre A, Abramov R, Hammond CJ, et al. Chlamydia pneumoniae infection promotes the transmigration of monocytes. J Neurosci Res. 2003;71:740–50. doi: 10.1002/jnr.10519. [DOI] [PubMed] [Google Scholar]