Summary
Toxoplasma gondii is a universally-distributed pathogen that infects over 1 billion people worldwide. Host resistance to this protozoan parasite depends on the potent production of IL-12 and IFN-γ. Although Toll-like receptor 11 (TLR11) plays a major role in controlling Th1 immunity to this pathogen in mice, this innate immune receptor is not functional in humans, and the mechanisms of TLR11-independent sensing of T. gondii remain elusive. Here, we show that both mucosal innate and adaptive immune responses to T. gondii rely on the indirect stimulation of dendritic cells by normal gut microflora. We identify a novel function for commensal bacteria in which they provide an immunostimulatory effect on dendritic cells during parasitic infection. Our results reveal that indirect immunostimulation by gut commensals provides protection against T. gondii in the absence of TLR11.
Introduction
Toxoplasma gondii is a ubiquitous parasite of the Phylum Apicomplexa, the largest and most important group of obligate parasites, which also includes the human pathogens Plasmodium (the cause of malaria) and Cryptosporidium spp (Sibley, 2004; Soldati et al., 2004). Humans and other warm-blooded animals are often infected with T. gondii through the ingestion of contaminated food and water (Black and Boothroyd, 2000). Once ingested, T. gondii parasites penetrate the intestine and rapidly disseminate through all organs of the body. Infection with this protozoan stimulates rapid production of IL-12 and IFN-γ by immune cells (Suzuki et al., 1988; Gazzinelli et al., 1993). These cytokines play a key role in host resistance to T. gondii by promoting strong Th1 responses (Hunter et al., 1994; Scharton-Kersten et al., 1996). However, both IL-12 and IFN-γ can also be detrimental to the host when overproduced in response to the parasite (Vossenkämper et al., 2004; Gazzinelli et al., 1996; Liesenfeld et al., 1996). Thus, while these cytokines play a major function in parasite control, identification of the molecular and cellular mechanisms responsible for their regulation is crucial to understanding the immunopathology associated with T. gondii infection.
Signaling through the Toll-like receptor (TLR) adaptor protein MyD88 is indispensible for activating early innate immune responses and inducing IL-12 (Scanga et al., 2002; Del Rio et al., 2004; Debierre-Grockiego et al., 2007; Yarovinsky, 2008). It has also been shown that TLR11 plays a dominant role in sensing T. gondii (Yarovinsky et al., 2005), and in the absence of this receptor, there is impaired DC activation, IL-12 secretion, and Th1 polarization after parasite infection (Yarovinsky et al., 2006). TLR11-dependent sensing of the parasite is driven by the recognition of T. gondii profilin (Plattner et al., 2008). However, human TLR11 is a non-functional pseudogene (Roach et al., 2005), and the mechanism of human T. gondii recognition is currently unknown.
Here, we demonstrate that in the absence of TLR11, both innate and adaptive immunity to T. gondii depends on the stimulation of DCs by gut bacteria. This finding indicates that intestinal commensal bacteria function as a molecular adjuvant during parasitic infections, providing TLR-dependent immunostimulatory signals to DCs. We also reveal the benefits of indirect stimulation of adaptive immunity to T. gondii via TLR11-independent pathways versus direct stimulation of DCs by the parasite. We establish that while TLR11-dependent immune responses are associated with inflammatory reactions, commensal bacteria provide immunostimulatory signals without the occurrence of immunopathology in response to the protozoan parasite.
Results
TLR11-dependent and -independent mechanisms of DC activation during mucosal immune responses to T. gondii
To investigate the mechanisms of TLR11-independent, IL-12-dependent IFN-γ responses to T. gondii, we investigated host resistance in TLR11-/- animals during natural peroral-route infection, which reflects the major route of infection in humans. TLR11-/- animals that were orally infected with T. gondii parasites (ME49 strain) exhibited diminished, but not abolished, production of IL-12 in response to infection (Figure 1A). In agreement with our previous observations (Yarovinsky et al., 2005) but in contrast to those made following oral infection, IL-12 responses to systemic infection with the same dose of the parasite were completely dependent upon TLR11 (Figure 1A). These results suggest that the mucosal immune system can initiate an immune response against T. gondii in the absence of TLR11. This hypothesis is also supported by the observed enhanced survival of TLR11-/- mice infected orally with T. gondii relative to systemic infection with the same dose of the parasite (Figure S1).
Figure 1.
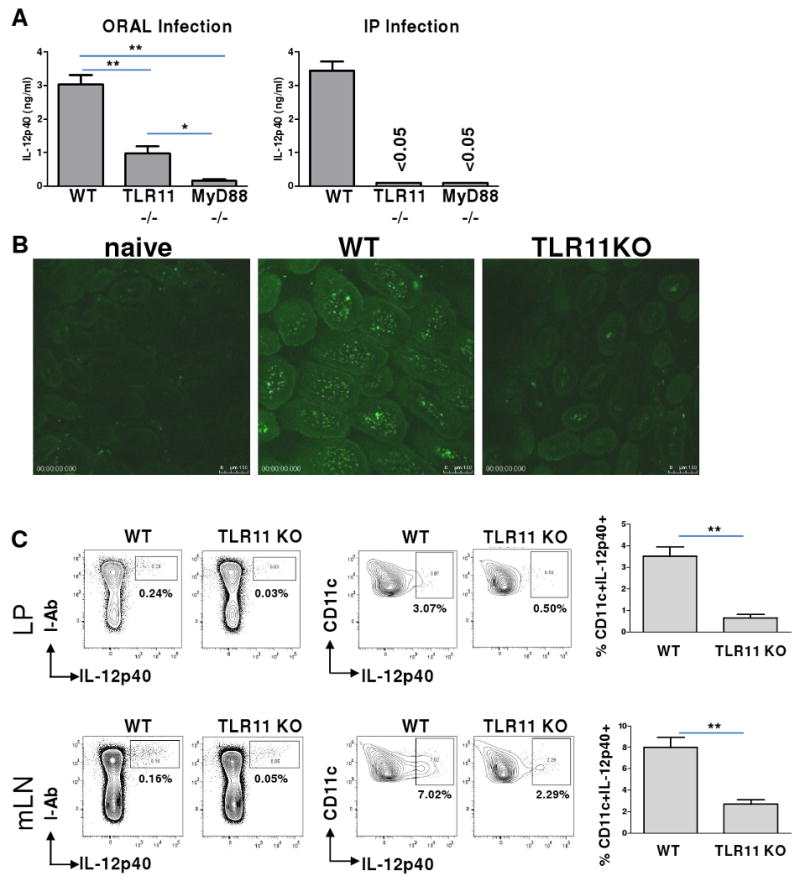
TLR11 is essential for in vivo DC-IL-12 responses to T. gondii during systemic infection, but is dispensable for mucosal immune responses to the parasite.
(A) WT, TLR11-/-, and MyD88–/– mice (five animals per group) were infected orally or intraperitoneally (IP) with an average of 20 T. gondii ME49 strain cysts per mouse. Serum IL-12p40 responses were measured 5 days later by ELISA. The data shown are the mean ± SD; the results are representative of four independent experiments. (B) Visualization of IL-12p40-producing cells during oral infection with T. gondii. WT IL-12p40-YFP reporter animals (Yet40) or TLR11-/-×Yet40 mice were infected orally with T. gondii, as described above. Small intestines were removed for microscopic analysis on day 5 post-infection to visualize IL-12p40-producing cells. Images were acquired with a Leica SPE with a 63× objective. The experiment shown is representative of the twelve performed. (C) DCs are the major IL-12-producing cells in WT and TLR11-/- mice. The different mucosal cell populations in the lamina propria (LP) of the small intestine and in the mLNs were examined for their ability to produce IL-12 in response to T. gondii. WT Yet40 and TLR11-/-×Yet40 mice were infected orally with T. gondii, as described above. On day 5 post-infection, small intestine and mLN single cell suspensions were prepared, stained with antibodies to I-Ab, CD11c, CD11b, and CD8α cell surface markers, and analyzed using flow cytometry. The data shown are the mean ± SD; the results are representative of four independent experiments, each involving four or five animals per group. * P< 0.05; ** P< 0.01.
To investigate the molecular mechanisms of the mucosal-specific immune response to the parasite, we generated animals expressing yellow fluorescent protein (YFP) reporter in the place of IL-12p40 by crossing TLR11-/- with IL-12p40-YFP mice (Yet40) (Im et al., 2005; Reinhardt et al., 2006). Innate immune cells that are capable of recognizing T. gondii and producing IL-12 in the absence of TLR11 can be identified using this model. Visualization of YFP-positive cells revealed that T. gondii infection resulted in the rapid appearance of IL-12p40-producing cells within 3-5 days of infection, predominantly in the small intestine (Figure 1B). In agreement with ELISA data (Figure 1A), TLR11 deficiency resulted in a reduced, but not abolished, appearance of YFP+ cells during experimental toxoplasmosis (Figure 1B). To investigate which cells accounted for the IL-12 responses to T. gondii, we isolated cells from lamina propria (LP), Peyer's patches, mesenteric lymph nodes (mLNs), and spleen and examined the phenotype of the YFP+ cells. Flow cytometric analysis revealed that all YFP+ (IL-12p40) cells appeared to be DCs (Figures 1C, S2-S4). In congruence with serum IL-12 levels (Figure 1A), the frequency of YFP+ DCs in the small intestine was reduced from 3-4% in WT animals to approximately 1% in TLR11-deficient mice (Figure 1C). The appearance of IL-12p40+ cells in WT and TLR11-/- mice was also detected in the mLNs and spleens of infected animals on days 3-5 after oral infection (Figure 1C, S2, and S4). It is interesting to note that, while systemic (intraperitoneal) infection resulted in the TLR11-dependent activation of the CD8+ DC subset (Reis e Sousa et al., 1997; Yarovinsky et al., 2005), oral infection led to IL-12 production by CD8- DCs in both WT and TLR11-deficient animals (Figure S2).
These results indicate that the mechanisms responsible for the initiation of host responses to T. gondii are distinct during oral and systemic infections. Since IL-12p40 production in response to the parasite was completely abrogated in MyD88-/- mice infected systemically or orally (Figure 1A), our results suggest that mucosal DCs utilize a TLR11-independent, MyD88-dependent mechanism for sensing parasitic infection.
TLR11-independent mechanism of Th1 induction during peroral route of the T. gondii infection
Previous studies have established that the IL-12p40 subunit can associate with either IL-12p35 or p19 to form biologically active IL-12 (IL-12p70) or IL-23, respectively (Trinchieri et al., 2003). In several instances, IL-12p40 can also function as a homodimer with chemoattractant functions (Khader et al., 2006). In the case of host resistance to intracellular parasites including T. gondii, IL-12 mediated induction of IFN-γ plays a major role in establishing protective immune responses (Lieberman et al., 2004). As the amounts of biologically active IL-12 triggered by T. gondii in sera of infected animals were relatively low and were barely detected by IL-12p70-specific ELISA (data not shown), we investigated if T. gondii could initiate IFN-γ production by CD4+ T cells in the absence of TLR11. We observed that TLR11 deficiency has a minor effect on IFN-γ responses to T. gondii during oral infection (Figure 2A). Furthermore, the amount of IFN-γ produced by CD4+ T cells was only slightly reduced when compared with their WT counterparts (Figures 2A and 2B). These results are distinct from those observed during experimental peritoneal infection with the parasites in which TLR11 was essential for Th1 responses to T. gondii (Figure 2B). Based on these observations, we considered two possible models. First, it was possible that mucosal DCs directly recognize T. gondii through a TLR11-independent mechanism that governs IL-12 production. A second possibility was that the mucosal microbial environment is responsible for the indirect stimulation of anti-parasitic responses, providing immunostimulatory (adjuvant) effects against T. gondii infection. To distinguish between these two models, we first tested whether purified mucosal DCs could initiate TLR11-independent cytokine responses to T. gondii in vitro. DCs were isolated from the small intestines of WT, TLR11-/-, and MyD88-/- mice and incubated for 24 h with live parasites. As a control, these cells were also stimulated with purified recombinant T. gondii profilin (TLR11 ligand). We observed that TLR11 deficiency prevented T. gondii sensing by mucosal DCs (Figure 3A). These results were obtained not only with the TLR11-deficient DCs, but also with WT DCs stimulated with parasites lacking the TLR11 agonist profilin (Figure 3B). Taken together, these results establish that TLR11 is essential for IL-12 responses by mucosal DCs in vitro. Furthermore, all other tested TLR-deficient mucosal DCs demonstrated normal IL-12 production in response to T. gondii in vitro (Figure 3C).
Figure 2.
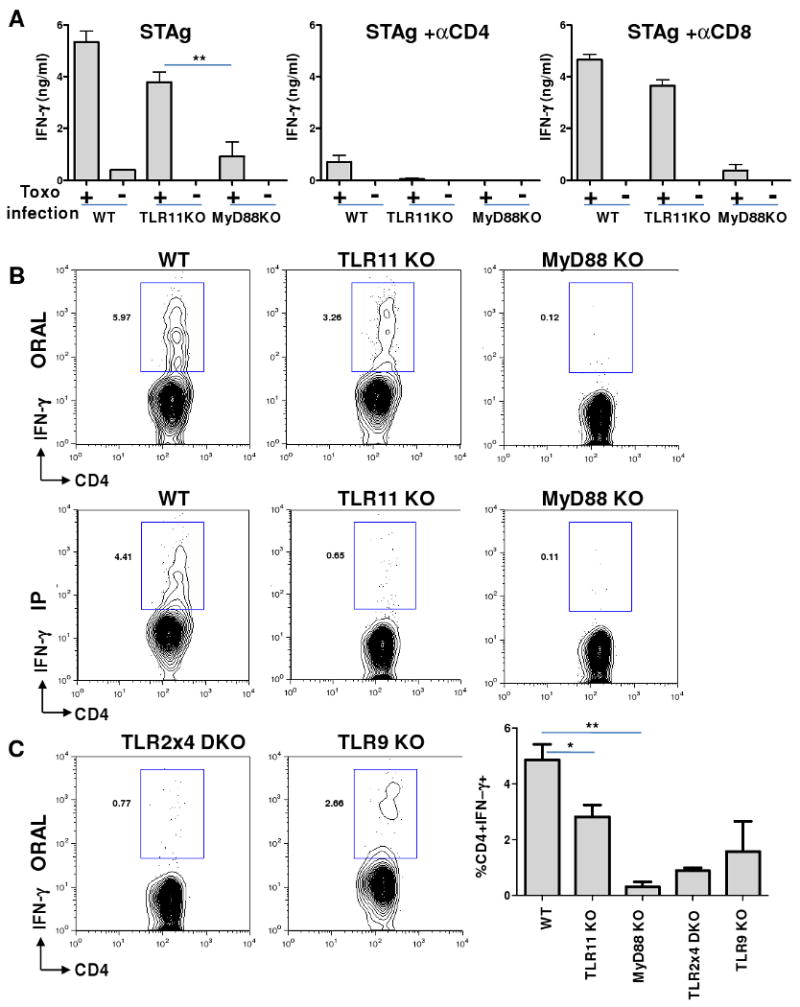
Natural peroral route infection by T. gondii results in TLR11-independent Th1 responses to the parasite.
(A) Recall response to STAg in WT, TLR11-/-, and MyD88-/- mice during oral infection with T. gondii. mLNs were harvested from WT, TLR11-/-, and MyD88-/- mice on day 7 post infection and restimulated with STAg (10 ug/ml) alone or in the presence of blocking anti-CD4 mAb or anti-CD8 mAb (20 ug/ml) for 48 hours. IFN-γ levels were quantified by ELISA. (B) Single-cell analysis of IFN-γ secretion by CD4+ T lymphocytes revealed that TLR11 regulates the Th1 response to T. gondii during systemic, but not oral, infection. WT, TLR11-/-, and MyD88–/– mice were infected orally or intraperitoneally (IP) with an average of 20 T. gondii ME49 strain cysts per mouse, and IFN-γ production by CD4+ T cells was analyzed on day 7 post-infection. (C) TLR2, -4, and -9 play important roles in regulating IFN-γ responses during T. gondii peroral infection. The indicated animals were infected as described above and the percentage of IFN-γ+ cells was analyzed and quantified using flow cytometry. The data shown are the mean ± SD; the results are representative of four independent experiments performed, each involving three animals per group. * P< 0.05; ** P< 0.01.
Figure 3.
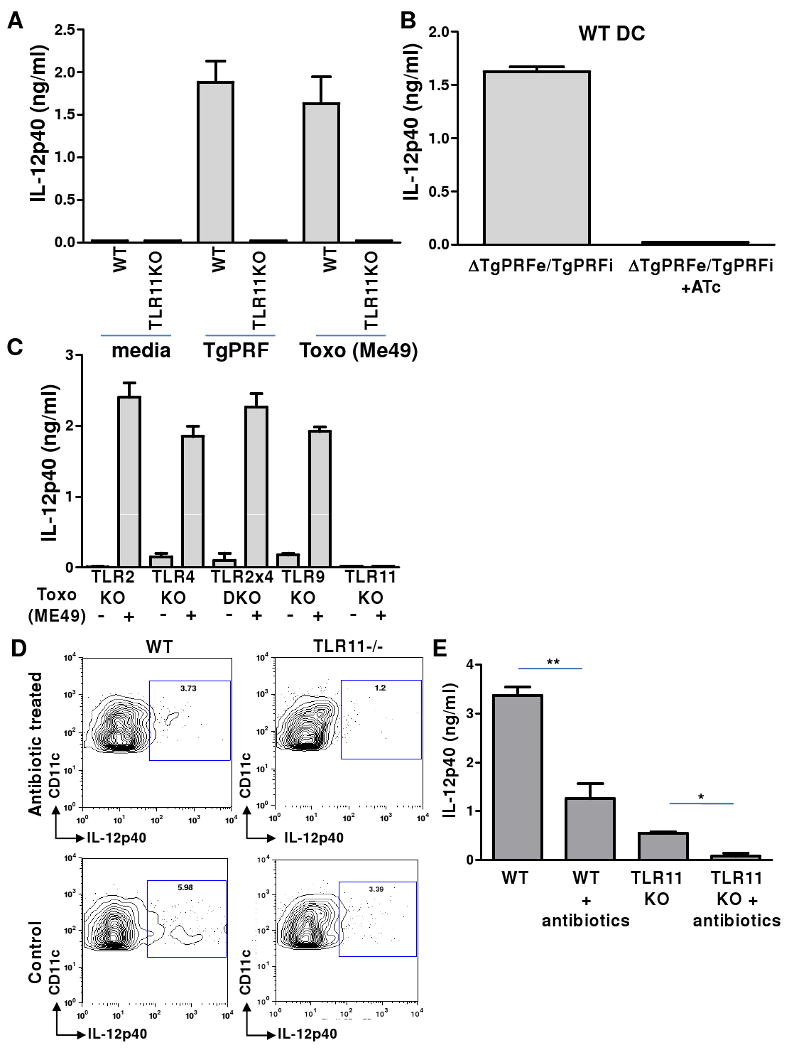
Commensal bacteria activate IL-12 production by DCs following oral T. gondii infection of TLR11-deficient mice.
(A) Sensing of T. gondii in vitro by lamina propria DCs depends upon TLR11. WT and TLR11-/- LP DCs were prepared from small intestines and incubated with media alone, T. gondii profilin (TgPRF, 1 ug/ml), or live parasites (ME49 strain, 1:1 ratio). After 20 hr, cell culture supernatants were harvested and IL-12 production was analyzed by ELISA. (B) WT DCs were stimulated with conditional T. gondii null mutants for profilin (ΔTgPRFe/TgPRFi +ATc) for 20 hr and IL-12 responses were analyzed, as described above. (C) The DC IL-12 response to T. gondii in vitro does not require TLR2, TLR4, or TLR9. LP DCs were prepared from TLR2, TLR4, TLR2×4, TLR9, and TLR11–deficient animals and stimulated with live T. gondii (ME49) for 20 hr. Analysis of cell culture supernatants revealed that only TLR11 is required for DC IL-12 responses to T. gondii in vitro. (D) Commensal bacteria are responsible for TLR11-independent IL-12 production by DCs in vivo during T. gondii infection. Gut commensal microflora in WT and TLR11-/- mice were depleted by antibiotic treatment prior to T. gondii infection. DC IL-12 responses from control and antibiotic-treated mice were analyzed by intracellular staining on day 3 post-T. gondii infection. (E) IL-12 secretion from WT and TLR11-/- mLN cultures on day 3 after oral infection with the parasite demonstrated that antibiotic treatment reduced the production of this cytokine in WT animals and abolished IL-12 secretion in gut commensal microflora-depleted TLR11-/- mice. The data shown are the mean ± SD. The results are representative of three experiments performed, each involving at least four animals per group. * P< 0.05; ** P< 0.01.
Commensal bacteria activate IL-12 production by DCs following oral T. gondii infection
We next examined the possibility that commensal gut bacteria may activate MyD88-dependent IL-12 production following T. gondii infection. Given the impact of MyD88 on IL-12 responses during oral infection with the parasite, we tested if TLRs that are normally involved in sensing bacteria may play a role in regulating Th1 immunity during T. gondii infection. When infected orally, mice lacking both TLR2 and TLR4 demonstrated profound deficiencies in their abilities to mount IFN-γ responses to the parasite (Figure 2C). Remarkably, when these animals were infected systemically with the same dose of the parasite, we observed no detectable effects of these TLRs on the regulation of Th1 responses to the protozoan (Figure S5). Similarly, TLR9 played a role during oral, but not systemic, immune responses (Figures 2C and S5). These results demonstrate that TLR2, TLR4, and TLR9, which normally play no role in the regulation of IL-12 production in response to T. gondii (Figure 3C), are indispensible regulators of IFN-γ responses during peroral infection. To directly examine the possibility that commensal bacteria are responsible for the activation of these innate immune receptors, WT and TLR11-/- animals were treated with broad-spectrum antibiotics for 3-4 weeks to deplete gut microflora (Figure S6) and then infected with T. gondii. WT animals treated with antibiotics exhibited reduced, but not abolished, IL-12 induction on days 3-5 post infection, whereas TLR11-/- mice depleted of commensals were not able to mount IL-12 responses against the parasite (Figures 3D, 3E, and S7). Furthermore, the ability of TLR11-/- DCs to produce IL-12 during T. gondii infection could be fully rescued by oral treatment with LPS, a bacterial TLR4 agonist (Figure S8). At the same time, antibiotic treatment had little effect on IL-12 responses during systemic infection with the parasite (Figure S7). These results demonstrate that intestinal cell damage triggered by parasitic infection allows commensal bacteria to initiate MyD88-dependent signals in DCs that are essential for protective IL-12 immune responses to T. gondii (Figure 1).
We next directly examined effects of commensals on Th1 polarization during systemic and oral infection with the parasite. As expected from the analysis of IL-12 production, depletion of commensal bacteria decreased CD4+ T cell IFN-γ production during oral infection of TLR11-/- mice (Figure S9). Furthermore, we observed that commensals play an important role in the regulation of Th1 polarization during oral, but not systemic, infection with the parasite even in WT animals (Figure S9). The ability of commensals to function as bacterial adjuvants during initiation of the parasite-specific Th1 response was confirmed in CD4+ T cell recall assay with STAg-fed DC (Figure S10). These results were further supported by an observed impairment in Th1 polarization in T. gondii-infected WT germ-free animals that are intrinsically deficient in commensals-induced innate stimuli (Figure S11). It is interesting to note that, while oral administration of LPS in GF or antibiotic-treated conventional animals during T. gondii infection enhanced the IFN-γ response to the parasite (Figure S12), infection of GF animals with T. gondii and simultaneous re-colonization with Bacteroides thetaiotaomicron (B. theta), a normal component of microflora, had a relatively minor effect on the induction of Th1 responses (Figure S11). These observations suggest that complex microflora signals are essential for potent adjuvant effects. This hypothesis was further confirmed in experiments with GF or antibiotic-treated WT and TLR11-/- mice re-colonized with conventional microflora during T. gondii infection (Figure S12). In addition to MyD88, non-TLRs, especially NOD2, have been shown to be involved in the regulation of mucosal inflammation (Kobayashi et al., 2005; Maeda et al., 2005; Watanabe et al., 2006). However, mice lacking NOD2 or caspase-1 exhibited normal Th1 immunity to T. gondii (data not shown), confirming a major role for TLRs in directing adjuvant effects during oral infection with this protozoan parasite.
Commensal bacteria provide immunostimulatory signals without inducing immunopathology in response to T. gondii
Because TLR11 is encoded in the human genome as a pseudogene (Roach et al., 2005), we next examined whether TLR11-independent activation of anti-T. gondii immune responses might be advantageous relative to TLR11-dependent immune stimulation. Infection of WT (TLR11-sufficient) animals with T. gondii induced an acute inflammatory response in the small intestine (acute ileitis) and dramatic, visible pathological changes in the liver (Figure 4). In contrast, TLR11-/- mice were free of detectable pathology in the small intestine and other tissues examined (Figure 4). These results suggest that TLR11 is a major immune receptor that is responsible for immunopathological responses during toxoplasmosis. Furthermore, when animals were infected with increasing doses of T. gondii, significant mortality was observed in WT, but not TLR11-/- mice, even when challenged with the highest tested dose of the parasite (Figure S13). A more complex picture was revealed in mice lacking MyD88 or the TLRs involved in bacterial recognition. The absence of TLR2, TLR4, or TLR9 diminished the inflammatory reaction in the small intestine but resulted in liver necrosis in infected animals (Figure 4). The degree of pathology correlated with the appearance of both anaerobic and aerobic bacterial colonies during T. gondii infection (Figures 4, S14). The role of commensal bacteria in liver pathology was also confirmed by the absence of immunopathology in TLR-deficient mice that were treated with broad-spectrum antibiotics (Figure S15). At the same time, NOD2 played no apparent role in the regulation of Th1 responses or immunopathology during T. gondii infection (Figure S16). These results establish that commensal bacteria provide MyD88-dependent immunostimulatory signals sufficient for development of Th1 immune responses without the occurrence of immunopathology in the absence of TLR11, while TLR11-dependent immune responses are associated with both protective immunity and inflammatory reactions (Figure S17).
Figure 4.
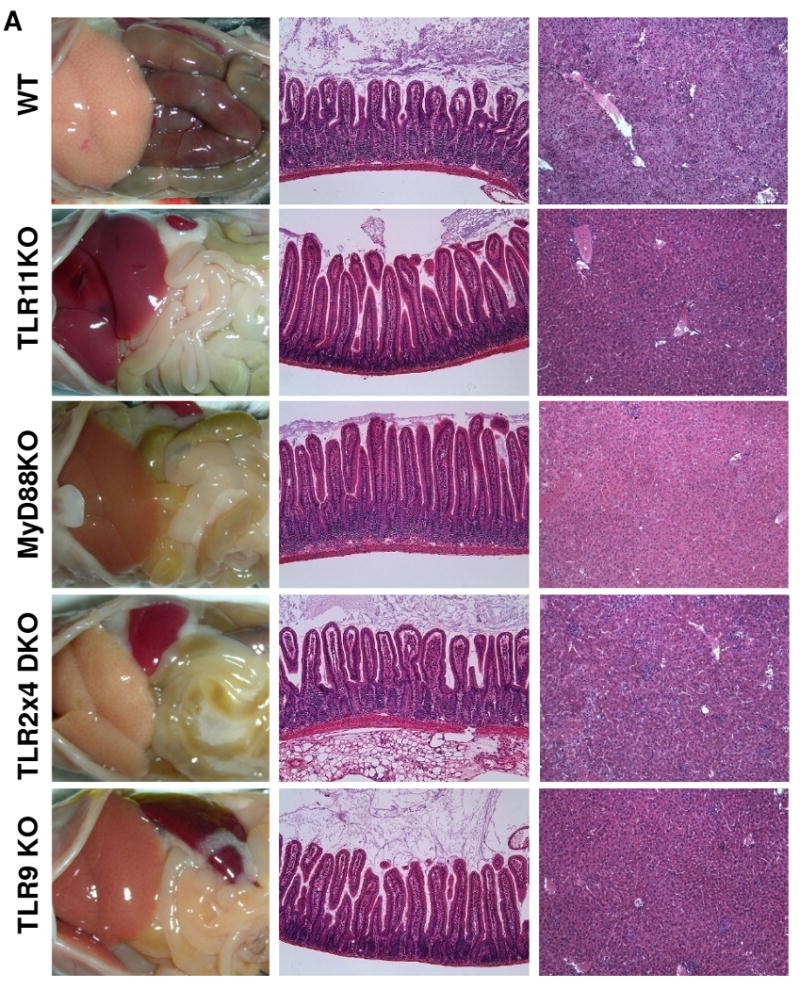
Lack of TLR11 prevents the development of T. gondii-initiated acute ileitis and liver damage.
(A) WT, TLR11-/-, MyD88-/-, TLR2×4-/-, and TLR9-/- mice (five animals per group) were infected orally with an average of 20 T. gondii ME49 strain cysts per mouse, and the immunopathology of the small intestines and liver tissues was analyzed on day 7 post-infection. The results are representative of five experiments performed, each involving at least four animals per group
Discussion
Beneficial relationships between commensal microorganisms and the host immune system are increasingly evident (Hooper et al., 2001; Rakoff-Nahoum et al., 2004; Backhed et al., 2005; Artis, 2008; Brandl et al., 2008; Mazmanian et al., 2008). Studies using gnotobiotic (germ-free) animals have established that the immunostimulatory signals induced by microbial components stimulate the development of both gut-associated lymphoid tissues and the microarchitecture of the peripheral lymphoid organs, including Peyer's patches and spleen (Macpherson and Harris, 2004). The lack of commensal bacteria results in multiple defects in CD4+ T cell subsets, including the impaired development of T cells with regulatory functions (Coombes and Powrie, 2008; Cario and Podolsky, 2006). In the normal gut, commensal bacteria also protect the host from a variety of enteric pathogens (Stecher and Hardt, 2008). Normal flora not only compete with pathogens for adhesion receptors and available nutrition factors, but also stimulate the production of mucin and antimicrobial peptides (Hooper et al., 2001; Brandl et al., 2008; Stecher and Hardt, 2008; Cash et al., 2006). Conserved structural elements present in commensal bacteria provide direct stimulation of innate immune receptors, including TLRs (Rakoff-Nahoum et al., 2004; Cario and Podolsky, 2006). Studies with mice deficient in factors that negatively regulate TLR signaling, such as SIGIRR and A20, have established that gut microflora constitutively activate the TLR adaptor protein MyD88 (Xiao et al., 2007; Turer et al., 2008). Epithelial cells, bone marrow-derived DCs, and macrophages are involved in a pivotal immunosensory role in the gut (Colonna et al., 2006; Denning et al., 2007; Artis, 2008; Coombes and Powrie, 2008). TLR activation on epithelial cells results in the induction of anti-microbial peptides and maintenance of epithelial homeostasis (Brandl et al., 2008; Vaishnava et al., 2008; Rakoff-Nahoum et al., 2004). The effects of TLR activation on bone marrow-derived immune cells are more complex. Lamina propria DCs continuously sample the luminal contents and initiate MyD88-dependent and TLR-independent activation programs that result in the production of tightly regulated amounts of both pro- and anti-inflammatory cytokines (Kelsall and Rescigno, 2004; Iwasaki, 2007). Mucosal DCs and macrophages also play a pivotal role in the regulation of adaptive immune responses, including the induction of both highly polarized effector and immunoregulatory CD4+ T cells (Izcue et al., 2006; Denning et al., 2007; Coombes and Powrie, 2008). At present, the mechanisms behind the balanced responsiveness of intestinal DCs and macrophages to bacterial TLR ligands are not completely understood. Furthermore, it is not clear how DCs and macrophages can discriminate between pathogenic and enteric bacteria since both types of microorganisms share multiple common elements that are responsible for the activation of innate and adaptive immune responses.
Intestinal parasites represent another numerous and widespread group of pathogens of major medical importance. Both helminthes and protozoa initiate a variety of immune responses, and among them, T. gondii is a ubiquitous pathogen that is associated with potent induction of Th1 immunity (Black and Boothroyd, 2000; Lieberman and Hunter, 2002). We recently demonstrated that the T. gondii protein profilin directly activates DCs via TLR11 and that this sensing mechanism plays a major role in the regulation of IL-12-dependent host responses to the parasite (Yarovinsky et al., 2005; Yarovinsky et al., 2006). Experimental data obtained with TLR11-/- mice are completely mirrored with WT animals infected with profilin-deficient T. gondii (Plattner et al., 2008). In both studies, the lack of TLR11 activation resulted in abolished IL-12 production by DCs during systemic infection with the parasite. Furthermore, TLR11-deficient animals demonstrate severe defects in the activation of CD4+ T cells (Yarovinsky et al., 2006). Despite some progress in the understanding of TLR11-dependent mechanisms of Th1 immunity, TLR11 is a non-functional pseudogene in humans and many other species (Roach et al., 2005); it is not clear how the parasite initiates IL-12 production by DCs in the absence of TLR11. It has been suggested that other TLRs, in particular TLR2, TLR4, and TLR9, may substitute for TLR11 in the direct sensing of T. gondii (Mun et al., 2003; Minns et al., 2006; Debierre-Grockiego et al., 2007). However, inactivation of these TLRs did not affect DC IL-12 responses during systemic infection with the parasite (Scanga et al., 2002; Yarovinsky, 2008, and Figure 3C). Furthermore, our results indicate that only TLR11 and MyD88 have significant effects on the establishment of a Th1 phenotype during experimental toxoplasmosis. In contrast, oral infection with T. gondii revealed an important role for bacterial-sensing TLRs in controlling IL-12 and IFN-γ responses to the parasite. Our results are consistent with recent reports that examined immune responses to T. gondii in TLR9-/- mice or in germ-free animals (Heimesaat et al., 2006; Minns et al., 2006). In these studies, TLR9 deficiency resulted in a reduced induction of IFN-γ responses to the parasite. Since T. gondii genomic DNA cannot itself activate TLR9, it is likely that bacterial DNA provides important immunostimulatory signals. The presence of gram-positive and gram-negative bacteria in the lumen of the gut stimulates TLR2 and TLR4, respectively. In all cases, the MyD88 adaptor protein seems to play a central role in controlling host resistance during both oral and systemic infection with T. gondii (Scanga et al., 2002; Minns et al., 2006; Sukhumavasi et al., 2008). Intriguingly, a report from Sukhumavasi and colleagues suggests that in addition to TLR-dependent regulation of Th1 responses, synergistic interactions between commensals and T. gondii may result in transient MyD88-independent induction of IFN-γ that, nevertheless, is not sufficient for host resistance to the parasite (Sukhumavasi et al., 2008).
While most studies of the immune response aim to understand the ways in which infectious pathogens are directly sensed by specific receptors, we suggest that in the absence of TLR11, immune responses to T. gondii depend on the indirect stimulation of DCs provided by commensal microflora. Gut bacteria, but not T. gondii, activate MyD88 via TLR2, TLR4, and TLR9. This is an essential step for the IL-12 response to the parasite. Our results establish a novel role for commensal bacteria as a molecular adjuvant during parasitic infection. It is likely that the direct activation of DCs by both bacteria and epithelial cell-derived cytokines plays a major role in the regulation of the innate and adaptive immune responses to intestinal pathogens. Importantly, the coexistence of the host mucosal immune system and intestinal microflora assure balanced immune responses that do not result in intestinal immunopathology, but are fully sufficient for initiating protective Th1 immunity against accidental invaders.
Experimental Procedures
Reagents
Recombinant T. gondii profilin (TgPRF) was purified to homogeneity (as judged by SDS-PAGE) using a combination of anion exchange (HiPrep 16/10 Q) and Sepahacryl S-100 chromatography, as described previously (Yarovinsky et al., 2005). STAg (soluble tachyzoite antigen) was prepared from tissue culture-derived tachyzoites of the ME49 strain, as previously described (Yarovinsky et al., 2005). Fluorescein isothiocyanate (FITC)-labeled CD8α, CD11b, B220, phycoerythrin (PE)-labeled CD11c, anti-IL-12p40, anti-IFN-γ, PE-Cy5-Gr1, and allophycocyanin (APC)-CD11c monoclonal antibodies were purchased from BD Biosciences (San Diego, CA). Anhydrotetracycline (ATc), ampicillin, vancomycin, neomycin sulfate, and metronidazole were purchased from Sigma. Fetal bovine serum was obtained from HyClone. All other cell culture reagents were purchased from Invitrogen.
Animals
C57BL/6 were obtained from the University of Texas Southwestern (UTSW) Medical Center Mouse Breeding Core Facility. TLR2-/-, TLR4-/-, TLR9-/-, MyD88-/-, TLR11-/-, and Caspase-1 -/- mice were generously provided by Drs. S. Akira, S. Ghosh, and R. Flavell. NOD2-/- and IL-12p40-YFP reporter (Yet40) mice were obtained from Jackson Laboratories (Bar Harbor, ME). All of the above animals were maintained at an American Association of Laboratory Animal Care-accredited animal facility at UTSW Medical Center. Germ-free C57BL/6 mice were maintained in plastic gnotobiotic isolators as previously described (Vaishnava et al., 2008). All animals used were age and sex matched and maintained in the same animal room.
Toxoplasma gondii infections and histopathology
All mice were infected orally or intraperitoneally (IP) with an average of 20 T. gondii cysts (ME49 strain). In some experiments, animals were infected with 50, 100, or 200 cysts of the same ME49 T. gondii strain. At days 3, 5, and 7 post-infection, animals were bled and necropsied; portions of the small intestines and livers were fixed in Bouin's fixative, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. Conditional T. gondii null mutants for profilin (ΔTgPRFe/TgPRFi) were grown for 72 hr ± ATc, as described previously (Plattner et al., 2008).
Ex vivo imaging of IL-12p40 producing cells in small intestine
Portions of the small intestines were kept at 37°C in complete RPMI 1640 medium supplemented with 10% fetal bovine serum, L-glutamine, penicillin, streptomycin, sodium pyruvate, nonessential amino acids, and 10 mM HEPES. For imaging, tissues were placed in a glass-bottom microwell dish (MatTek Corporation). The bath temperature was maintained at 37°C with a stage heater (PeCon) and an objective heater. Time-lapse images were acquired with a Leica SPE system fitted with a Leica 63× objective NA 1.4. The data sets were processed with Leica Advanced Fluorescence software (Leica).
Dendritic cell cytokine response assays
For purification of DCs from WT, MyD88-/-, and TLR2, -4, -2×4, -9, -11–deficient mice, small intestines were removed and cleaned of their mesentery. Peyer's patches were excised and the intestines were opened longitudinally and washed of fecal contents. Small intestinal segments were treated with PBS containing 10% FCS, 20 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 10 mM EDTA for 30 min with continuous stirring at 37 °C. Cell suspensions were passed through a strainer and the remaining tissues were washed with PBS and digested with 0.4 mg/ml collagenase D (Roche) and 10 ug/ml DNase I (Roche) for 45 min. Cell suspensions were collected and passed through a strainer and then pelleted by centrifugation at 300 × g. In some experiments, cells were spun through a 15.5% Accudenz (Accurate Chemical & Scientific) solution to enrich for DCs. Cell suspensions were enriched for CD11c+ cells by positive selection with CD11c microbeads (Miltenyi Biotec). In some experiments, intestinal samples enriched for CD11c+ cells were stained with PE-conjugated CD11c and allophycocyanin-conjugated anti-I-Ab antibodies (BD Biosciences) and were sorted on a MoFlo flow cytometer (Dako Cytomation) at the UTSW Flow Cytometry Core Facility. DCs were inoculated with freshly egressed T. gondii parasites and incubated for 20 hr. IL-12p40 in culture supernatants was then measured by ELISA using a commercially-available kit (R&D Systems).
Ex Vivo Measurement of Antigen-Specific CD4+ T-Cell Responses
To assay the response of animals infected with T. gondii, mLNs were harvested from mice on day 7 post-infection. Single cell suspensions were prepared and restimulated with STAg (10 ug/ml) for 48 hr. After restimulation with microbial antigens, cell culture supernatants were removed for cytokine assays. Where indicated, 20 μg/ml blocking anti-CD4 mAb (GK1.5, rat IgG2b) or 20 μg/ml blocking anti-CD8 mAb (2.43, rat IgG2b) was added to the cultures. In some experiments, the CD4+ populations were purified by cell sorting, and aliquots (105 cells each) were mixed with irradiated WT splenocytes (as a source of APC) in 96-well plates. After STAg addition, the cultures were incubated for 48 hr, and cell culture supernatants were removed for cytokine assays.
In some experiments, 105 purified CD4+ T cells were co-cultured with equal numbers of STAg (10 ug/ml) or parasite (1:1) exposed DCs in 96-well plates for 5 hr in the presence of GolgiPlug (Brefeldin A, BD Biosciences). After in vitro restimulation (5 hr), cells were washed, stained with FITC-labeled anti-CD4 mAb, and fixed for 40 min in BD Cytofix/Cytoperm. Cells were then stained using fluorochrome-conjugated antibodies according to the manufacturer's protocol (BD Biosciences).
Intracellular Cytokine Staining
Analyses of intracellular cytokine expression were performed on mLN cultures restimulated with 1 ug/ml αCD3 (BD Biosciences) for 5 hr in the presence of GolgiPlug (Brefeldin A, BD Biosciences). After in vitro restimulation, cells were washed once in RPMI, stained with FITC-labeled anti-CD4 mAb, and fixed for 40 min in BD Cytofix/Cytoperm. Cells were then stained using fluorochrome-conjugated antibodies according to the manufacturer's protocol (BD Biosciences). In short, after incubation in the permeabilization buffer supplemented with anti-FcγRII/III mAb (2.4G2; 5 μg/ml) at 4°C, cells were stained for 30 min with PE anti-IFN-γ (XMG1.2), washed three times, and resuspended in PBS + 1% FBS. Cell fluorescence was measured using a FACSCalibur or LSRII flow cytometer, and data were analyzed using FlowJo software (Tree Star).
For the analysis of IL-12-producing cells, mLN cultures were isolated on days 3 and 5 post-infection and incubated in vitro for 6 hr in the presence of GolgiPlug (BD Biosciences). Cells were stained with FITC-CD11b, PE-Cy5-Gr1, and APC-CD11c, then fixed and permeabilized as described above and incubated for 30 min with PE-conjugated anti-IL-12p40 (BD Biosciences). Cell fluorescence measurements and analyses were performed as described above. The specificity of the cytokine staining was confirmed in initial experiments in which preincubation with corresponding unlabeled mAb completely inhibited the signal obtained with labeled anti-cytokine mAb.
Bacterial Cultures and LPS Administration Studies
For the determination of anaerobic and aerobic bacterial colonies in liver tissues during T. gondii infection, livers were removed on day 7 post-infection, placed into 15 ml conical tubes with PBS, and homogenized. Aerobic bacteria were grown on LB agar or blood agar. Anaerobic bacteria were grown on blood agar using an anaerobic vented jar (BD Biosciences). Culturable bacteria counts were obtained by plating serial dilutions of bacteria on the corresponding media for 48 hr (aerobes) and 72 hr (anaerobes).
To deplete gut commensal microflora, WT and TLR11-/- animals were provided ampicillin (1 g/L), vancomycin (500 mg/L), neomycin sulfate (1 g/L), and metronidazole (1 g/L) in drinking water for four weeks, as described previously (S3). The efficiency of commensal bacteria depletion was verified by bacteriological analysis of feces by cultivating aerobic and anaerobic bacteria on blood agar. Because many intestinal bacteria are unculturable, the efficiency of antibiotic treatments in some experiments was confirmed using RT-PCR with a set of universal primers described by Barman et al., 2008.
For B. thetaiotaomicron colonization experiments, age-matched germ-free C57BL/6 mice were orally gavaged with 107 CFU of stationary phase bacterial culture (Bacteroides thetaiotaomicron strain VPI-5482). Intestinal colonization levels at the time of sacrifice were measured by dilution plating of luminal contents. In some experiments germ-free C57BL/6 mice were conventionalized by mixing bedding from conventional mice one or three days prior T. gondii infection. The efficiency of colonization was verified by bacteriological analysis of feces by the cultivation of aerobic and anaerobic bacteria on blood agar.
For LPS administration experiments, antibiotic-treated or germ-free mice were orally gavaged with 500 ug of LPS from E. coli serotype 0111:B4 (Sigma) during T. gondii infection and on days 3 and 5 post infection with the parasite.
Statistical analysis
All data were analyzed with Prism (Version 5; GraphPad). Data were considered statistically significant for P values less than 0.05 that were obtained with a two-tailed t-test.
Supplementary Material
Acknowledgments
The authors are grateful to Shipra Vaishnava and Cynthia A. Leifer for critically reading and discussing the manuscript. We also thank Daniel L. Barber for his input on T. gondii-specific CD4+ T cell recall responses and John Shelton for help with histology. This work was supported by the UT Southwestern Medical Center Endowed Scholars Program, by Award Number R21AI079371 (to F.Y.) from the National Institute of Allergy and Infectious Diseases (NIAID), by NIH R01 DK070855 to L.V.H., and by the Howard Hughes Medical Institute (L.V.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607–623. doi: 10.1128/mmbr.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, Dematteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008 doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario E, Podolsky DK. Toll-like receptor signaling and its relevance to intestinal inflammation. Ann N Y Acad Sci. 2006;1072:332–338. doi: 10.1196/annals.1326.006. [DOI] [PubMed] [Google Scholar]
- Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Pulendran B, Iwasaki A. Dendritic cells at the host-pathogen interface. Nat Immunol. 2006;7:117–120. doi: 10.1038/ni0206-117. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debierre-Grockiego F, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, Mansur DS, Weingart R, Schmidt RR, Golenbock DT, Gazzinelli RT, Schwarz RT. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- Del Rio L, Butcher BA, Bennouna S, Hieny S, Sher A, Denkers EY. Toxoplasma gondii triggers myeloid differentiation factor 88-dependent IL-12 and chemokine ligand 2 (monocyte chemoattractant protein 1) responses using distinct parasite molecules and host receptors. J Immunol. 2004;172:6954–6960. doi: 10.4049/jimmunol.172.11.6954. [DOI] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Gobel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- Hunter CA, Subauste CS, Van Cleave VH, Remington JS. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im W, Kim H, Yun D, Seo SY, Park SH, Locksley RM, Hong S. Cytokine reporter mouse system for screening novel IL12/23 p40-inducing compounds. Mol Cells. 2005;20:288–296. [PubMed] [Google Scholar]
- Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- Kelsall BL, Rescigno M. Mucosal dendritic cells in immunity and inflammation. Nat Immunol. 2004;5:1091–1095. doi: 10.1038/ni1104-1091. [DOI] [PubMed] [Google Scholar]
- Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, Ghilardi N, Desauvage FJ, Lund FE, Cooper AM. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- Lieberman LA, Cardillo F, Owyang AM, Rennick DM, Cua DJ, Kastelein RA, Hunter CA. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol. 2004;173:1887–1893. doi: 10.4049/jimmunol.173.3.1887. [DOI] [PubMed] [Google Scholar]
- Lieberman LA, Hunter CA. The role of cytokines and their signaling pathways in the regulation of immunity to Toxoplasma gondii. Int Rev Immunol. 2002;21:373–403. doi: 10.1080/08830180213281. [DOI] [PubMed] [Google Scholar]
- Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Minns LA, Menard LC, Foureau DM, Darche S, Ronet C, Mielcarz DW, Buzoni-Gatel D, Kasper LH. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J Immunol. 2006;176:7589–7597. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- Mun HS, Aosai F, Norose K, Chen M, Piao LX, Takeuchi O, Akira S, Ishikura H, Yano A. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int Immunol. 2003;15:1081–1087. doi: 10.1093/intimm/dxg108. [DOI] [PubMed] [Google Scholar]
- Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, Soldati-Favre D. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008;3:77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Reinhardt RL, Hong S, Kang SJ, Wang ZE, Locksley RM. Visualization of IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation. J Immunol. 2006;177:1618–1627. doi: 10.4049/jimmunol.177.3.1618. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Hieny S, Gazzinelli RT, Sher A. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- Sibley LD. Intracellular parasite invasion strategies. Science. 2004;304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- Soldati D, Foth BJ, Cowman AF. Molecular and functional aspects of parasite invasion. Trends Parasitol. 2004;20:567–574. doi: 10.1016/j.pt.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Stecher B, Hardt WD. The role of microbiota in infectious disease. Trends Microbiol. 2008;16:107–114. doi: 10.1016/j.tim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Sukhumavasi W, Egan CE, Warren AL, Taylor GA, Fox BA, Bzik DJ, Denkers EY. TLR adaptor MyD88 is essential for pathogen control during oral toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J Immunol. 2008;181:3464–3473. doi: 10.4049/jimmunol.181.5.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- Turer EE, Tavares RM, Mortier E, Hitotsumatsu O, Advincula R, Lee B, Shifrin N, Malynn BA, Ma A. Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20. J Exp Med. 2008;205:451–464. doi: 10.1084/jem.20071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenkamper A, Struck D, Alvarado-Esquivel C, Went T, Takeda K, Akira S, Pfeffer K, Alber G, Lochner M, Forster I, Liesenfeld O. Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur J Immunol. 2004;34:3197–3207. doi: 10.1002/eji.200424993. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25:473–485. doi: 10.1016/j.immuni.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H, Tuohy VK, Fairchild RL, de la MC, Cua D, Vallance BA, Li X. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Yarovinsky F. Toll-like receptors and their role in host resistance to Toxoplasma gondii. Immunol Lett. 2008;119:17–21. doi: 10.1016/j.imlet.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Yarovinsky F, Kanzler H, Hieny S, Coffman RL, Sher A. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+ T cell response. Immunity. 2006;25:655–664. doi: 10.1016/j.immuni.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


