Abstract
Drugs and antibodies that interrupt vascular endothelial growth factor (VEGF) signaling pathways improve outcomes in patients with a variety of cancers by inhibiting tumor angiogenesis. A major adverse effect of these treatments is hypertension, suggesting a critical role for VEGF in blood pressure (BP) regulation. However, the physiological mechanisms underlying the control of BP by VEGF are unclear. To address this question, we administered a specific antibody against the major VEGF receptor, VEGFR2, to normal mice and assessed the consequences on BP. Compared to vehicle-treated controls, administration of the anti-VEGFR2 antibody caused a rapid and sustained increase in BP of ≈10 mm Hg. This increase in BP was associated with a significant reduction in renin mRNA expression in the kidney (p=0.019) and in urinary excretion of aldosterone (p<0.05). Treatment with the anti-VEGFR2 antibody also caused marked reduction in expression of endothelial and neuronal nitric oxide synthases (eNOS and nNOS) in the kidney. To examine the role of nitric oxide (NO) in the hypertension caused by blocking VEGFR2, mice were treated with Nω-nitro-L-arginine methyl ester (L-NAME) (20 mg/kg/day), an inhibitor of NO production. L-NAME administration abolished the difference in blood pressure between the vehicle- and anti-VEGFR2-treated groups. Our data suggest that VEGF, acting via VEGFR2, plays a critical role in blood pressure control by promoting NOS expression and NO activity. Interfering with this pathway is likely to be one mechanism underlying hypertension caused by anti-angiogenic agents targeting VEGF.
Keywords: hypertension, angiogenesis, cancer, vascular endothelial growth factor, nitric oxide
Introduction
Vascular endothelial growth factor (VEGF), a 45 kDa glycoprotein, is a powerful inducer of angiogenesis, also affecting vascular permeability, endothelial cell survival and hematopoiesis1. The two major receptors for VEGF signals are the structurally related tyrosine kinases, VEGFR1 (Flt-1) and VEGFR2 (Flk-1). VEGF also interacts with neuropilins 1 and 2 (NP1 and NP2), but their roles in VEGF signaling have yet to be clearly defined. Nonetheless, there is general agreement that the angiogenic, mitogenic, and permeability-enhancing effects of VEGF1 are primarily mediated by VEGFR2. Stimulation of angiogenesis by VEGF acting through VEGFR2 is a key factor in propagation and spread of various cancers 2-4. Accordingly, antibodies and small molecules targeting VEGF and its associated signaling pathways are effective in treating a variety of human malignancies.
Along with its actions on blood vessel growth and permeability, VEGF also has acute hemodynamic effects impacting peripheral vascular resistance (PVR). For example, acute infusions of VEGF cause vasodilation and hypotension 5, 6. These vasoactive responses are likely mediated by VEGFR2 and may involve stimulation of nitric oxide (NO) and vasodilator prostanoids, such as prostaglandin I2 (PGI2)1. However, the precise molecular mechanisms underlying the effects of VEGF on PVR remain unclear. In addition to a capacity to influence acute vascular tone, a role for VEGF in chronic control of blood pressure has been suggested by clinical experiences with VEGF inhibitors. In this regard, hypertension has emerged as one of the most common side effects of these agents in patients treated for malignancies. For example, recent meta-analyses reveal substantial increases in relative risk for hypertension by 6- to 22-fold in patients treated with anti-VEGF antibody or VEGFR kinase inhibitors7-9. However, the pathophysiology of hypertension associated with anti-VEGF therapy has not been clearly delineated.
To investigate this issue, we utilized a specific monoclonal antibody to inhibit VEGFR2 in mice, and examined the consequences on blood pressure. We find that administration of the anti-VEGFR2 antibody causes robust hypertension, likely related to impaired capacity for generation of NO.
Materials and Methods
Animals
Male 129S6/SvEv mice were purchased from Taconic (Hudson, NY). Animals were maintained in the animal facility of the Durham Veterans Affairs Medical Center and studied between two and four months of age. The experimental procedures described below were approved by the respective IACUCs of the Durham VA and Duke University Medical Centers. Mice were fed a normal chow diet (0.4% NaCl; LabDiet, Richmond, IN) or low-salt diet (<0.02% NaCl; Harlan Teklad, Madison, WI), where indicated.
Blood pressure measurements
Tail cuff manometry
In the dose finding experiments, systolic blood pressures were measured in conscious mice using a computerized tail-cuff system (Hatteras Instruments, Cary, NC) after 2 weeks of daily training as described previously 10. Data were recorded at baseline for 2 weeks and then 5 days a week throughout the study period. This method has been validated previously and correlates well with direct measurements of intra-arterial pressure 11.
Radiotelemetry measurements of intra-arterial pressure
Blood pressure was measured in conscious mice by radiotelemetry using TA11PA-C10 transmitters (Data Sciences International, St. Paul, MN) as described previously12, 13. Briefly, mice were anesthetized with isoflurane and a pressure-sensing catheter was implanted into the left carotid artery, as previously described 14. The transducer unit was then inserted into a subcutaneous pouch along the right flank generated by blunt dissection inferiorly from the original neck incision. Mice were allowed to recover for 7 days after surgery to regain their normal circadian rhythms before experiments were initiated. During blood pressure measurements, mice were housed in a monitoring room in the animal facility where quiet is maintained and no other activities are permitted. Data were collected continuously with sampling every 5 minutes for 10-second intervals using Dataquest A.R.T. software (Data Sciences International).
Administration of DC101 antibody and Nω-nitro-L-arginine methyl ester (L-NAME)
DC101-expressing hybridoma cells were obtained from American Type Culture Collection (Manassas, VA; ATCC number HB-11534). These antibodies are specific for the murine tyrosine kinase receptor FLK-1 (VEGFR2) and inhibit tumor growth by suppressing tumor-induced neovascularization 15. DC101 production was performed at the Duke Cell Culture Facility. Cells were maintained in roller bottles and adapted to serum-free Hybridoma-SFM media (Invitrogen, Carlsbad, CA). Cells were placed into a hollow-fiber cartridge system and maintained in serum-free media at 37°C. DC101-containing media was recovered when glucose levels decreased 50% below baseline and new serum-free media was added to the cells. DC101-containing media was concentrated using a 30-kDa Centricon® concentrator (Amicon Corporation, Beverly, MA) and stored at -20°C until used. Protein was quantified by a modified Bradford assay (BioRad, Hercules, CA). Mice were treated twice weekly with either a low (150 μg) or high (1000 μg) dose of DC101 or vehicle (serum-free media) by intra-peritoneal injection.
L-NAME (Sigma, St. Louis, MO) was dissolved in the drinking water and administered at a dose of 20 mg/kg/day. L-NAME drinking solution was replaced every other day for the duration of the study.
Measurement of urinary prostanoid, aldosterone, and creatinine excretion
24-hour urine samples were collected by metabolic cage after four weeks of DC101 or vehicle treatment. Urine samples were centrifuged briefly to remove particulate matter, then immediately aliquoted and frozen at −80°C until assay. Stable prostanoid metabolites [13, 14-dihydro-15-keto-PGE1/PGE2 (prostaglandin E2), TxB2 (thromboxane A2), and 6-keto-PGF1a (prostaglandin I2)] and aldosterone in urine were measured using specific competitive enzyme immunoassays for each molecule (Cayman Chemical, Ann Arbor, MI). Creatinine levels in urine were assessed using an alkaline picrate assay (Creatinine Companion, Exocell, Inc., Philadelphia, PA).
Reverse transcription and real-time quantitative PCR
Total RNA was extracted (Tri-Reagent, Sigma) from kidneys of mice treated with vehicle, low-dose DC101, or high-dose DC101 for four weeks. RNA was DNase-treated using Turbo DNA-free (Ambion, Austin, TX) to remove genomic DNA contamination. RNA yield was quantified by UV spectrophotometry and integrity was verified by 1% agarose gel electrophoresis and staining with ethidium bromide. Only RNA with A260/280 > 1.7 and displaying no significant degradation was used for reverse transcription. cDNAs were synthesized from 5 μg of total RNA using random hexamers and SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). “No RT” samples lacking reverse transcriptase were prepared during each RT reaction for use as negative controls during PCR. Real-time quantitative PCR was performed using the fluorogenic 5′-exonuclease assay16. Primers and dual-labeled probe (5′-FAM, 3′-TAMRA) targeting renin were synthesized based on previously published sequences17 and primer-probe sets for NOS1 (nNOS, assay #Mm01208058_m1) and NOS3 (eNOS, assay #Mm01164908_m1) were purchased from Applied Biosystems (Foster City, CA). PCR reactions were performed in duplicate on an iCycler real-time detection system (BioRad, Hercules, CA). cDNA and negative control (no RT, water) templates (1 μl) were added to 25 μl PCR reaction mixtures consisting of 1× TaqMan Universal PCR master mix (Applied Biosystems) and either 1× human eukaryotic 18S rRNA primer-probe mix (Applied Biosystems), 2 ng/μl each of renin forward and reverse primer and 800 nM renin probe, or 1× NOS1 or NOS3 primer-probe mix. Gene expression was quantified using the two standard curve method for relative quantitation18.
Statistical analysis
All data are presented as mean ± SEM. Differences between treatment groups were analyzed by unpaired t-test or one-way ANOVA followed by Newman-Keuls multiple comparison test, as indicated. Differences within groups, before and after L-NAME treatment, were analyzed by paired t-test. A p-value of less than 0.05 was considered significant.
Results
Dose –dependent effects of anti-VEGFR2 antibody on blood pressure
To examine the capacity of VEGFR2 blockade to cause hypertension, we administered two different concentrations of anti-VEGFR2 antibody to normal 129/SvEv mice while monitoring their blood pressures by tail cuff manometry. In preliminary studies, the higher dose (1000 μg) caused maximal inhibition of tumor angiogenesis in mice, whereas the lower dose caused moderate inhibition of tumor growth (data not shown). After one week, blood pressures were significantly increased in the mice treated with the higher dose (1000 μg) of antibody (152 ± 2 mmHg) compared to controls receiving only vehicle (144±2 mmHg; p=0.006). By contrast, the lower dose of anti-VEGFR2 antibody had no effect on blood pressure (143±2 vs. 144±2 mmHg; p=ns). Thus, the dose of anti-VEGFR2 antibody that causes maximal inhibition of angiogenesis also caused a significant increase in blood pressure.
Blockade of VEGFR2 causes hypertension in mice
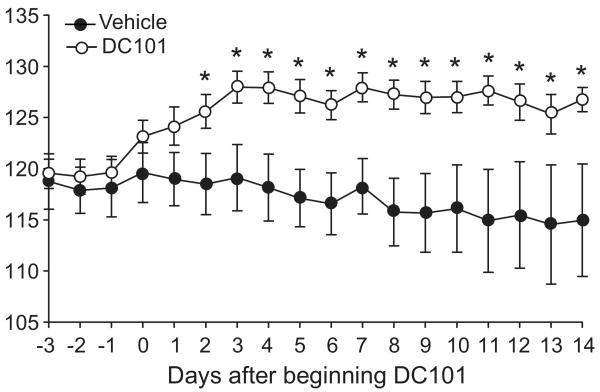
To more specifically evaluate the effects of inhibiting VEGFR2 on blood pressure, radiotelemetry units were implanted into a separate group of 129/SvEv mice to directly measure intra-arterial blood pressure. After establishing baseline blood pressures, mice were given injections of the anti-VEGFR2 antibody (DC101, 1000 μg) or vehicle every 3-4 days. As shown in Figure 1, the anti-VEGFR2 antibody caused an immediate rise in blood pressure, while blood pressures in vehicle-treated controls were unaffected. Within 2 days after beginning administration of the antibody, mean arterial pressure was significantly higher in the mice receiving DC101 compared to controls (126 ± 2 vs. 118 ± 3 mmHg, p=0.03). Moreover, this difference in blood pressure was sustained throughout the 2 weeks of antibody administration. Accordingly, average MAP during the 2-week period was significantly higher in the mice receiving the anti-VEGFR2 antibody than controls (126 ± 1 vs. 117 ± 4 mmHg; p=0.016). The magnitude of blood pressure increase (≈10 mm Hg) was very similar to that seen in the dose-finding experiments using tail cuff blood pressure measurements.
Figure 1.
Mean arterial pressure (MAP, 24 hr) in mice treated with vehicle or DC101. Blood pressure was measured continuously by radiotelemetry in conscious mice before and during twice weekly treatment with DC101 (1000 μg) or vehicle, which began on Day 0. Data are mean ± SEM for 7-8 mice per group. *p<0.05 vs. vehicle by unpaired t-test.
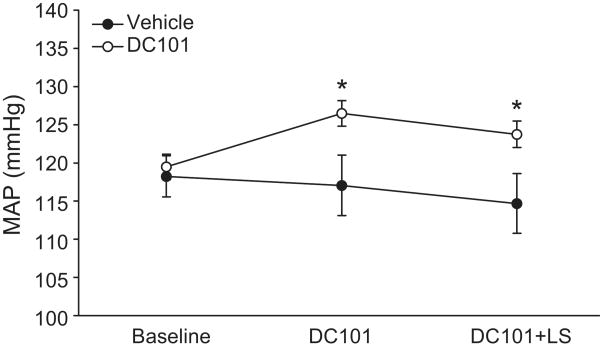
To determine whether the hypertension caused by the anti-VEGFR2 antibody could be modulated by changing dietary salt content, we fed vehicle- and DC101-treated mice a low-salt diet (<0.02% NaCl) while continuously monitoring blood pressure by radiotelemetry. We observed a slight decrease in blood pressure in both vehicle- and DC101-treated mice on low-salt diet; however, this change was not statistically significant in either group (117 ± 4 vs. 115 ± 4 mmHg vehicle, p=0.34; 126 ± 1 vs. 124 ± 1 mmHg DC101, p=0.090; Fig. 2). Furthermore, the difference in blood pressure between the experimental groups was maintained during low-salt feeding (115 ± 4 vs. 124 ± 1 mmHg, p=0.019).
Figure 2.
Mean arterial pressure (MAP, 24 hr) in DC101- and vehicle-treated mice fed a low-salt diet. Blood pressure was measured continuously by radiotelemetry while mice were fed either normal (DC101) or low-salt (DC101+LS) diet. Data are mean ± SEM for 7 days of low-salt diet, 7-8 mice per group. *p<0.05 vs. vehicle by unpaired t-test.
Anti-VEGFR2 antibody treatment suppresses the renin-angiotensin-aldosterone system
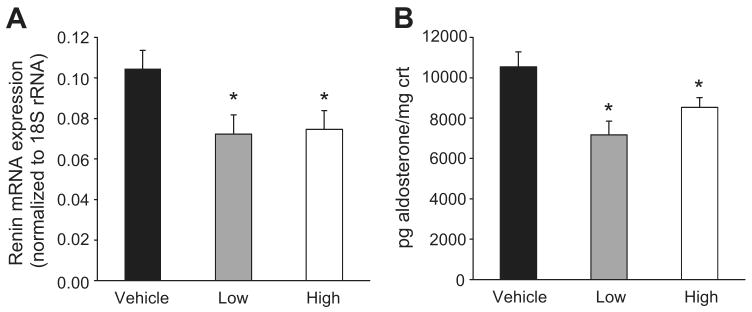
To begin to assess the pathogenesis of hypertension caused by blockade of VEGFR2, we measured kidney renin mRNA expression and urinary aldosterone excretion in mice treated with low- (150 μg) or high-doses of DC101 (1000 μg) or vehicle. Renin mRNA expression in kidneys from mice treated with either dose was reduced equivalently by ∼30% compared to mice treated with vehicle (0.072 ± 0.009 low, 0.075 ± 0.009 high vs. 0.105 ± 0.009 arbitrary units; p<0.05; Fig. 3A). Urinary aldosterone excretion was also reduced by ≈20% in the mice treated with anti-VEGFR2 antibody compared to controls (7165 ± 1920 low, 8530 ± 477 high vs. 10,534 ± 744 pg/mg creatinine; p<0.01 low, p<0.05 high; Fig. 3B). Taken together, these findings suggest that activation of the renin-angiotensin-aldosterone system is not a primary mechanism of hypertension associated with VEGFR2 inhibition.
Figure 3.
Renin mRNA expression (A) and urinary aldosterone excretion (B) in mice treated with DC101. Relative expression of renin mRNA (normalized to 18S rRNA) in whole kidneys was measured by real-time RT-PCR and aldosterone excretion by enzyme immunoassay in 24 hr urine samples from vehicle- and DC101-treated mice. Urinary aldosterone excretion was normalized to total creatinine (crt) excretion in the same sample. Mice treated with low-dose (150 μg) DC101 had blood pressures similar to vehicle-treated mice, while those treated with high-dose (1000 μg) DC101 were hypertensive. Data are mean ± SEM for 9-10 mice per group. *p<0.05 vs. vehicle by one-way ANOVA followed by Newman-Keuls multiple comparison test.
Prostanoid metabolism is unaffected by VEGFR2 blockade
Alteration of vasoactive prostanoid generation has been suggested to contribute to hypertension associated with VEGF inhibition19. Therefore, we also measured the profile of urinary prostanoid excretion in mice treated with DC101 compared to controls. Specifically, we measured the stable metabolites of three prostanoids with well-characterized actions affecting blood pressure: prostaglandin E2 (PGE2), prostaglandin I2 (PGI2), and thromboxane A2 (TxA2). We found no statistically significant differences in urinary excretion of PGE2 metabolites (432±33 vs. 447±29 pg/mg creatinine; p=NS), TxB2 (856±211 vs. 1071±149 pg/mg creatinine; p=NS), or 6-keto-PGF1α (6002±718 vs. 5164±438 pg/mg creatinine; p=NS) between DC101-and vehicle-treated mice.
Reduced expression of NOS with anti-VEGFR2 antibody treatment
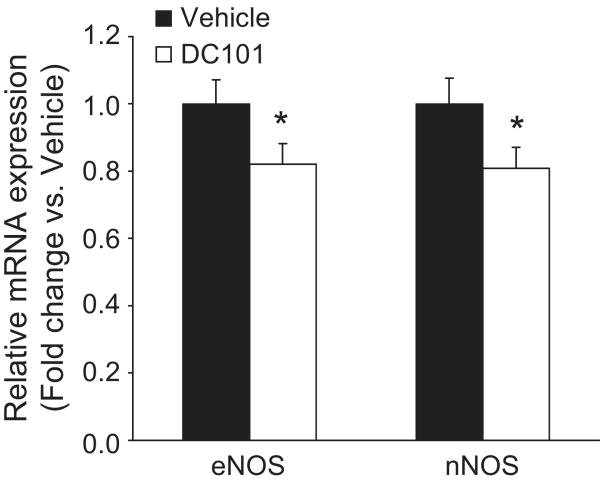
VEGF is known to modulate nitric oxide (NO) generation and VEGFR2 activation affects the activity of eNOS20. Thus, we measured expression of two NO synthase isozymes, eNOS (NOS3) and nNOS (NOS1), in the kidneys of mice from the two experimental groups by real-time quantitative RT-PCR. Levels of mRNA for both eNOS and nNOS were reduced significantly by ∼20% in kidneys from DC101-treated mice compared to controls (0.808 ± 0.062 vs. 1.000 ± 0.076-fold for eNOS, p=0.034; and 0.820 ± 0.061 vs. 1.000 ± 0.072-fold for nNOS; p=0.038; Fig. 4).
Figure 4.
Effect of VEGFR2 inhibition on nitric oxide synthase (NOS) mRNA expression in kidney. Relative expression of NOS mRNA was measured by real-time RT-PCR in whole kidneys from mice treated with vehicle or DC101 (1000 μg). Levels of endothelial (eNOS) and neuronal NOS (nNOS) were normalized to that of 18S rRNA in the same sample. Data are expressed as fold change versus vehicle for each gene. Data are mean ± SEM for 9-10 mice per group. *p<0.05 vs. vehicle by unpaired t-test.
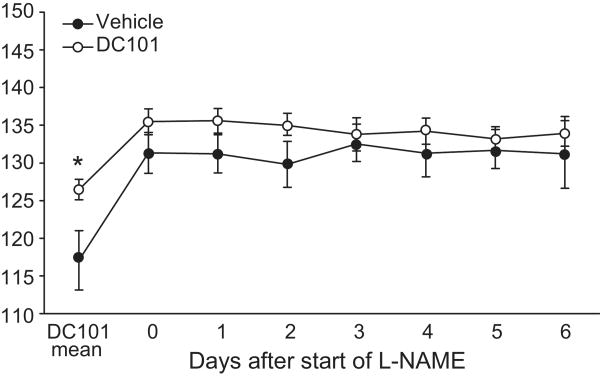
L-NAME abolishes blood pressure difference associated with VEGFR2 blockade
To determine whether the reduced expression of NOS isoforms that we observed might contribute to changes in blood pressure, we compared the effects of the NO synthesis inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME), on blood pressure in the experimental groups. L-NAME (20 mg/kg/day) was administered in drinking water to DC101- and vehicle-treated mice beginning five weeks after antibody administration was initiated. In both groups, L-NAME caused a significant increase in blood pressure (Fig. 5). Compared to controls, the effect of L-NAME to increase blood pressure was diminished in the mice receiving anti-VEGFR2 antibody, although the difference did not reach statistical significance (11±4 vs. 6±1% increase; p=0.12). However, within one day after L-NAME was started, the difference in blood pressure between the two groups of mice was abolished (132±3 vs. 136±1 mmHg, p=0.15; Fig. 5).
Figure 5.
Effect of inhibiting nitric oxide (NO) synthesis on blood pressure in vehicle- and DC101-treated mice. 24 hr MAP was measured by radiotelemetry during administration of the NO synthesis inhibitor L-NAME (20 mg/kg/day). ‘DC101 mean’ represents the average MAP of 14 days of treatment in vehicle- and DC101-treated mice. Data are mean ± SEM for 7-8 mice per group. *p<0.05 vs. vehicle by unpaired t-test.
Discussion
Angiogenesis inhibitors used as part of chemotherapy regimens improve outcomes for patients with several types of malignancies21, 22. However, hypertension has emerged as a common side effect of VEGF inhibition. These clinical observations have revealed a somewhat unexpected role for VEGF-associated signaling pathways to modulate blood pressure. However, the mechanisms underlying the control of blood pressure by VEGF have not been precisely defined. Elucidating these mechanisms will provide novel physiological insights, but could also have clinical utility for developing regimens to minimize the risk of significant hypertension, optimizing targeted anti-hypertensive drug therapies, and identifying those patients most susceptible to hypertension.
In clinical trials, hypertension has been observed following treatment with neutralizing antibodies against VEGF as well as small molecules that inhibit multiple tyrosine kinase receptors including the VEGF receptors and PDGF receptors. However, a precise linkage between specific angiogenic receptor pathways and blood pressure control has not been established. In our studies, we find that blockade of VEGFR2 is sufficient to cause an immediate and sustained increase in blood pressure. This finding suggests a tonic and non-redundant role for VEGFR2 and its associated signaling pathways to modulate blood pressure in otherwise normal mice. The effect of VEGFR2 inhibition on blood pressure is dose-dependent, as a low dose of anti-VEGFR2 antibody, one with only modest effects on tumor growth, failed to elicit a hypertensive response. The general magnitude of the blood pressure increase observed with a higher dose of anti-VEGFR2 antibody, previously shown to have potent anti-angiogenic effects in mice (≈8-10 mm Hg), is consistent with observations from clinical trials of patients receiving anti-angiogenic therapies7. Moreover, this level of blood pressure increase in humans, if sustained, would translate into a significant increase in risk for cardiovascular morbidity and mortality23. Further, it is conceivable that these effects might be amplified in patients with pre-existing hypertension, as suggested in other animal studies24. Hypertension caused by blockade of VEGFR2 in mice was not affected by a reduction in dietary sodium, suggesting that it is relatively resistant to alterations in dietary salt intake, as shown in Figure 2.
The renin-angiotensin system (RAS) is a key regulator of blood pressure and previous studies have suggested that VEGF inhibition may exaggerate the severity of angiotensin II-dependent hypertension24. Accordingly, we assessed a potential role for the RAS in hypertension caused by VEGFR2 inhibition. Since release of renin from juxtaglomerular cells in the kidney is a key rate-limiting step in the generation of angiotensin II, we compared renin mRNA expression in mice receiving the anti-VEGFR2 antibody with controls. Renin mRNA expression was reduced by ≈30% in mice treated with a dose of anti-VEGFR2 antibody (1000 μg) that was sufficient to cause hypertension. Furthermore, we saw a similar reduction of renin expression in mice treated with a lower dose of antibody (150 μg) that did not appreciably affect blood pressure, suggesting that suppression of renin may be an early compensatory response to attenuate the increase in blood pressure caused by VEGFR2 inhibition. As aldosterone production by the adrenal gland is regulated by angiotensin II, we also measured urinary aldosterone excretion as an independent assessment of RAS activity. Consistent with the reduced renin expression, we found that urinary aldosterone excretion was also significantly diminished following administration of the anti-VEGFR2 antibody. Taken together, these data indicate that stimulation of the RAS is not a primary mechanism causing hypertension in this setting.
Suppression of vasodilator prostanoids such as PGI2 relative to vasoconstrictor prostanoids such as TxA2 can cause sustained hypertension25. Since endothelial cells are the major source of PGI2 in the circulation, it has been suggested that isolated inhibition of PGI2 production by endothelial cells might contribute to hypertension associated with inhibition of VEGF19. We therefore examined the effects of VEGFR2 blockade on the general profile of prostanoid generation. Urinary excretion of 6-keto-PGF1α, the major metabolite of PGI2, was not affected by anti-VEGFR2 antibody. Likewise, excretion of TXB2, the major metabolite of the vasoconstrictor eicosanoid TXA2, was not different between DC101- and vehicle-treated mice. Urinary levels of PGE2 metabolite were also similar between the groups. Thus, alteration in the balance of vasodilator and vasoconstrictor prostanoids does not explain the development of hypertension with VEGFR2 inhibition.
Infusions of VEGF cause acute vasodilation and it has been suggested that this vasodilatory response is mediated by nitric oxide (NO)6. Moreover, VEGF is known to stimulate calcium-independent NO synthesis in vascular endothelial cells by activating the PI3-kinase/Akt pathway downstream of VEGFR2, leading to serine phosphorylation and activation of eNOS26. VEGF also upregulates eNOS mRNA and protein expression in human, bovine, and rodent endothelial cells27-29. This upregulation is mediated by VEGFR227 and appears to be the result of a post-transcriptional effect on eNOS mRNA stability28. While acute regulation of the constitutive NOS enzymes (eNOS and nNOS) occurs through post-translational mechanisms, chronic changes in NO synthesis are predominantly regulated by changes in eNOS or nNOS mRNA expression30. Therefore, we examined the effects of VEGFR2 inhibition on NOS isozyme expression in kidneys. In mice receiving the specific anti-VEGFR2 antibody, eNOS mRNA levels were significantly reduced by ≈20%. Since eNOS-deficient mice are hypertensive31, 32, such a reduction in eNOS expression might be expected to affect blood pressure homeostasis. Interestingly, we found a similar reduction in nNOS expression in mice after VEGFR2 blockade. The net contribution of this reduction of nNOS on blood pressure is less clear as blood pressure is normal in nNOS-deficient mice33. Nonetheless, recent data suggest that nNOS plays important roles in regulation of both vascular tone and renal sodium handling under certain conditions33.
Our findings of reduced expression of NOS isoforms in kidneys of mice treated with the anti-VEGFR2 antibody suggest that chronic inhibition of VEGFR2 signaling may reduce NO synthesis in the kidney and perhaps in other tissues, thereby promoting hypertension. To evaluate the role of impaired NO generation in the hypertension caused by VEGFR2 blockade, we examined responses to the NO synthesis inhibitor L-NAME. Following administration of L-NAME, the 8-10 mm Hg difference in blood pressure between the mice treated with the VEGFR2 blocker and controls was abolished. Taken together, these findings suggest that reduced levels of eNOS, and perhaps nNOS, with the coincident attenuation of NO generation are responsible for the increase in blood pressure caused by VEGFR2 blockade.
In some patients with pre-eclampsia, levels of a soluble form of VEGFR1 (sFlt1) are increased in the circulation 34. Soluble VEGFR1 specifically binds VEGF, decreasing its circulating levels causing coincident endothelial dysfunction with hypertension, proteinuria, and glomerular endotheliosis 35. Moreover, a recent report suggests that NO formation is also impaired in this setting 36. In the context of our current findings, we suggest that impaired VEGFR2 signaling may contribute to hypertension and reduced NO generation associated with circulating VEGF inhibitors in pre-eclampsia.
The mechanism of reduced NOS expression caused by VEGFR2 blockade is not clear. The rapid increase in blood pressure within days after the initial administration of the anti-VEGFR2 antibody suggests that acute interruption of signaling pathways with direct effects on NOS expression may play a role. Alternatively, the reduction in NOS expression might reflect diminution of micro-vessel density. In this regard, recent studies suggest that VEGF is required for maintenance of normal blood vessels, in particular for vessels containing fenestrated endothelia 37. In studies using mice, pharmacological inhibitors of VEGF signaling have been shown to cause vascular rarefaction, a reduction in microvessel density, in a number of tissues including the kidney37, 38, where fenestrated endothelial cells are found in glomerular and peri-tubular capillaries39.
In summary, the purpose of this study was to examine the mechanism of hypertension caused by inhibition of VEGF signaling. We demonstrate that blockade of VEGFR2 in wild-type mice is sufficient to cause robust hypertension. The increased blood pressure is not a result of renin-angiotensin system activation but rather appears to be a consequence of an impaired capacity for NO generation due to decreased NO synthase expression.
Perspectives
We demonstrate that blockade of VEGFR2 causes significant hypertension in normal mice that is likely mediated by reduced NO production. These findings indicate an important role for modulation of NO by VEGFR2 as a key signaling pathway in normal blood pressure homeostasis. Our data suggest that blocking VEGF signaling via VEGFR2 is one mechanism by which anti-VEGF therapies cause hypertension. Moreover, these studies suggest that alterations of this pathway might contribute to hypertension in other circumstances. Further work is needed to understand more completely the spatial and temporal context in which VEGFR2 signaling controls blood pressure in health and disease.
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health grant UO1-DK076136, the Medical Research Service of the Veterans Administration, and the Edna and Fred L. Mandel Center for Hypertension and Atherosclerosis Research at Duke.
Footnotes
Disclosures None
References
- 1.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrine reviews. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 2.Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest. 1995;95:1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melnyk O, Shuman MA, Kim KJ. Vascular endothelial growth factor promotes tumor dissemination by a mechanism distinct from its effect on primary tumor growth. Cancer research. 1996;56:921–924. [PubMed] [Google Scholar]
- 4.Borgstrom P, Gold DP, Hillan KJ, Ferrara N. Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer research. 1999;19:4203–4214. [PubMed] [Google Scholar]
- 5.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 6.Ku DD, Zaleski JK, Liu S, Brock TA. Vascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteries. Am J Physiol. 1993;265:H586–592. doi: 10.1152/ajpheart.1993.265.2.H586. [DOI] [PubMed] [Google Scholar]
- 7.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. The lancet oncology. 2008;9:117–123. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta oncologica (Stockholm, Sweden) 2008:1–9. doi: 10.1080/02841860802314720. [DOI] [PubMed] [Google Scholar]
- 10.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension. 2004;43:364–369. doi: 10.1161/01.HYP.0000112225.27560.24. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci U S A. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiological genomics. 2001;5:89–97. doi: 10.1152/physiolgenomics.2001.5.2.89. [DOI] [PubMed] [Google Scholar]
- 15.Prewett M, Huber J, Li Y, Santiago A, O'Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, Bohlen P, Hicklin DJ. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer research. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 16.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome research. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Lee G, John SW, Maeda N, Smithies O. Molecular phenotyping for analyzing subtle genetic effects in mice: application to an angiotensinogen gene titration. Proc Natl Acad Sci U S A. 2002;99:4602–4607. doi: 10.1073/pnas.072083799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. Journal of molecular endocrinology. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 19.Sane DC, Anton L, Brosnihan KB. Angiogenic growth factors and hypertension. Angiogenesis. 2004;7:193–201. doi: 10.1007/s10456-004-2699-3. [DOI] [PubMed] [Google Scholar]
- 20.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 21.Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: safety profile and management of adverse events. Seminars in oncology. 2006;33:S26–34. doi: 10.1053/j.seminoncol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Herbst RS. Therapeutic options to target angiogenesis in human malignancies. Expert opinion on emerging drugs. 2006;11:635–650. doi: 10.1517/14728214.11.4.635. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Circulation. Vol. 119. 2009. Heart Disease and Stroke Statistics--2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee; e21 pp.181 pp. [DOI] [PubMed] [Google Scholar]
- 24.Advani A, Kelly DJ, Advani SL, Cox AJ, Thai K, Zhang Y, White KE, Gow RM, Marshall SM, Steer BM, Marsden PA, Rakoczy PE, Gilbert RE. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc Natl Acad Sci U S A. 2007;104:14448–14453. doi: 10.1073/pnas.0703577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francois H, Athirakul K, Howell D, Dash R, Mao L, Kim HS, Rockman HA, Fitzgerald GA, Koller BH, Coffman TM. Prostacyclin protects against elevated blood pressure and cardiac fibrosis. Cell metabolism. 2005;2:201–207. doi: 10.1016/j.cmet.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovascular research. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 27.Shen BQ, Lee DY, Zioncheck TF. Vascular endothelial growth factor governs endothelial nitric-oxide synthase expression via a KDR/Flk-1 receptor and a protein kinase C signaling pathway. J Biol Chem. 1999;274:33057–33063. doi: 10.1074/jbc.274.46.33057. [DOI] [PubMed] [Google Scholar]
- 28.Bouloumie A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovascular research. 1999;41:773–780. doi: 10.1016/s0008-6363(98)00228-4. [DOI] [PubMed] [Google Scholar]
- 29.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274:H1054–1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 30.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 31.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stauss HM, Godecke A, Mrowka R, Schrader J, Persson PB. Enhanced blood pressure variability in eNOS knockout mice. Hypertension. 1999;33:1359–1363. doi: 10.1161/01.hyp.33.6.1359. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz PA, Garvin JL. Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol Regul Integr Comp Physiol. 2003;284:R628–638. doi: 10.1152/ajpregu.00401.2002. [DOI] [PubMed] [Google Scholar]
- 34.Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75:1–8. doi: 10.1016/j.mvr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandrim VC, Palei AC, Metzger IF, Gomes VA, Cavalli RC, Tanus-Santos JE. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsia. Hypertension. 2008;52:402–407. doi: 10.1161/HYPERTENSIONAHA.108.115006. [DOI] [PubMed] [Google Scholar]
- 37.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. British journal of cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 39.Pallone TL, Cao C. Renal Cortical and Medullary Microcirculations. In: Alpern RJ, Hebert SC, editors. Seldin and Giebisch's The Kidney, Physiology and Pathophysiology. Vol. 1. New York: Academic Press; 2008. pp. 627–670. [Google Scholar]