Abstract
Event-related brain potentials were examined in 32 adolescents (50% female) from a high-risk sample, who were exposed to cocaine and other drugs prenatally. Adolescents were selected for extreme high- or low-risk behavior on the Balloon Analog Risk Task, a measure of real-world risk-taking propensity. The feedback error-related negativity (fERN), an event-related potential (ERP) that occurs when an expected reward does not occur, was examined in a game in which choices lead to monetary gains and losses with feedback delayed 1 or 2 s. The fERN was clearly visible in the fronto-central scalp region in this adolescent sample. Feedback type, feedback delay, risk status, and sex were all associated with fERN variability. Monetary feedback also elicited a P300-like component, moderated by delay and sex. Delaying reward feedback may provide a means for studying complementary functioning of dopamine and norepinephrine systems.
Keywords: Adolescence, Risk-taking, Event-related potential, Feedback monitoring, Reward
Introduction
Adolescence is a developmental period of increased risk-taking and novelty-seeking behavior, as well as a phase of heightened biological vulnerability to addictive substances [Arnett, 1992; Chambers et al., 2003; Silveri et al., 2004; Spear, 2000]. Neuroimaging work supports the view of adolescence as a time of heightened reward sensitivity, with adolescents showing greater nucleus accumbens activity during reward processing than children or adults [Ernst et al., 2005; Galvan et al., 2006]. Not all adolescents show risky behaviors or develop addictions; however, individual differences in risk-taking propensity can be tracked by brain activity differences in the reward-related neural circuitry [Galvan et al., 2007]. In this study, we examined adolescent risk-taking and response to reward- related feedback among youths who were assessed using event-related potentials (ERP). Furthermore, we rely on a sample of people who themselves are high-risk because of prenatal drug exposure (including cocaine, alcohol, and/or tobacco). Children with such a history may be conceived of as generally ‘at risk’ because of the range of co-occurring factors associated with illicit drug use and adverse pregnancy outcomes, including poor prenatal care, multi-substance use during pregnancy, poverty, poor nutrition, physical abuse, maternal depression, stress, and lack of social support [Bauer et al., 2002; Bendersky et al., 1996, 2006; Curry, 1998; Streissguth et al., 1991]. As such, understanding of the link between reward processing and adolescent risk-taking within this group of at-risk youths has significant public health implications.
The frontostriatal circuitry, implicated in behavioral regulation, continues to develop both structurally and functionally during adolescence [Casey et al., 2008]. A growing body of evidence suggests that mesencephalic dopamine neurons and their target structures play a key role in reward processing and risky behaviors [Blum et al., 2000]. Dopamine neurons in particular are thought to modulate synaptic plasticity and to promote feedback-based learning by transmitting reward prediction error signals reflecting the difference between expected and actual outcomes [Schultz and Dickinson, 2000; Schultz et al., 2000]. One of the prevailing models of brain ERP contends that signaling of these reward prediction errors is evident at the level of the scalp [Holroyd and Coles, 2002]. In this regard, ERP studies of environmental feedback monitoring and reward processing find that when individuals make errors on simple cognitive tasks, there occurs a negative deflection in the ERP, referred to as the error-related negativity (ERN) [Falkenstein, 2004; Gehring et al., 1993; Holroyd and Coles, 2002]. The response-locked ERN, localized to frontocentral sites, is maximal at approximately 50–120 ms following error commission. A related ERP, the feedback ERN (fERN), is observed when feedback (monetary loss, wrong response) indicates that performance is worse than expected. Presenting with a similar scalp topography to the ERN, the medial frontal negativity or fERN occurs approximately 250 ms after feedback. Source localization studies, estimating the location of these ERP neural generators, consistently point to the anterior cingulate cortex as the neural generator of the ERN and the fERN [Gehring and Willoughby, 2002; Luu et al., 2003].
From one perspective, the ERN/fERN is thought to reflect functioning in a general error processing system, which influences reinforcement learning. The ERN and fERN are thought to be generated when transient dips in mesencephalic dopamine signal disinhibitory neurons in the anterior cingulate cortex [Holroyd and Coles, 2002]. In turn, the anterior cingulate cortex uses the error signal to modify ongoing performance. From another view, the ERN and fERN reflect activity in a conflict monitoring system after an error is committed [Botvinick et al., 2001]. The conflict monitoring system works with cognitive control centers, guiding attention, and thus behavior, to avoid negative outcomes. From both perspectives, the ERN/fERN reflect brain responses to negative outcomes that serve to guide future decisions.
As probes of errors and feedback monitoring, the ERN and fERN are responsive to individual differences in personality and developmental level. The ERN tends to be larger in amplitude for adults who report more trait worry and obsessive compulsive disorder-like symptoms [Gehring et al., 2000; Hajcak et al., 2003; Hajcak and Simons, 2002]. The fERN as a correlate of personality has not been as extensively studied, but recent work suggests that individuals who have a bias to learn from negative information show larger fERN to negative feedback [Frank et al., 2005, 2007;]. There are to date no developmental studies examining the fERN across different ages, but there are several studies now examining the ERN from middle childhood and adolescence through adult-hood [Davies et al., 2004; Ladouceur et al., 2007]. For instance, Davies et al. [2004] examined the ERN in a normative sample of children, adolescents, and adults from 7 to 25 years of age. They observed smaller ERN in children, which did not reach adult levels until mid to late adolescence (approx. 17–18 years). Similarly, Ladouceur et al. [2007] observed that ERN were smaller for an early-adolescence group (mean age 12.3 years) versus a late-adolescence group (mean age 16.5 years) and an adult group (mean age 28.7 years). Given that the period of adolescence is marked by increased risky behavior and maturational changes in the reward-related neural circuitry [Ernst et al., 2005; Galvan et al., 2006], including the anterior cingulate cortex [Casey et al., 1997], ERP studies that bring together risk, reward, and the adolescent developmental period are needed.
To understand the processes that underlie adolescent risk-taking behavior, it is important to utilize risk strategies best suited to this goal. Adolescent risk-taking behavior has been most often studied with self-report instruments [DiClemente et al., 1996; Gullone and Moore, 2000; Jessor and Jessor, 1977]. These types of assessments, while useful, have limitations including response biases, socially desirable responding, and method variance issues. Lejuez et al., [2002] approached these methodological problems by developing a behavioral risk task, the Balloon Analog Risk Task (BART). In this task, the individual is presented with an empty balloon, which may be inflated incrementally for money. At any time, the individual has the opportunity to stop pumping and collect the money accumulated to that point in a temporary bank or to continue pumping. However, if the balloon pops, all the money accrued in the temporary bank on that specific balloon is lost and another balloon appears. Each pump is a risky decision, as the probability that a balloon will pop increases with each pump while the relative gain for each pump diminishes compared to that accumulated in the temporary bank.
In adult samples, BART performance has been associated with self-report measures of risk behaviors, i.e. drug use, gambling, stealing, and unprotected sexual inter-course [Lejuez et al., 2002]. Likewise, the BART has shown itself to be a valid marker for real-life risk behavior in adolescence above and beyond traditional rating instruments, and after controlling for demographic variables and risk-related personality constructs [Aklin et al., 2005; Lejuez et al., 2003, 2007]. Recently, the BART was used to distinguish between adolescents with a risk-prone clinical profile (patients with conduct disorder and substance use disorder symptoms) and controls [Crowley et al. 2006]. With the accumulating evidence of the validity of the BART as an index of risk-taking behavior, investigations with concurrent physiology are beginning to emerge. Fein and Chang [2008] collected a low-density EEG while adult treatment-naïve alcoholics played an adaptation of the BART. They observed a fERN for popped balloons over the frontocentral region. Moreover, among this select group, smaller fERNs were associated with a greater family history density of alcohol problems. Taken together, findings highlight the utility of the BART as an assessment of real-world risky behavior in adolescents and adults, which has now been linked to a relevant bio-signal – the fERN in adults. We are unaware of any studies examining the BART in the context of the fERN in adolescence. Although there would be merit in beginning such research in a community sample, the high-risk status for prenatally exposed adolescents adds additional public health importance.
In this study, we took a different approach from assessing ERP feedback monitoring directly with the BART. Instead, we used the BART as a screening tool to identify extremes of high and low risk-taking among male and female adolescents. We chose a sample of adolescents with histories of early exposures to a range of adverse conditions in whom there is a greater likelihood to find high-risk takers. We then examined ERP responses in a simple ERP reward-feedback decision task that led to gains and losses, modeled after Holroyd et al. [2003]. We hypothesized that those evidencing a greater propensity to take risks, indexed by their riskiness on the BART, would show reduced sensitivity to negative feedback as compared to those evidencing lower risk-taking propensity, which would be associated with the opposite pattern. In addition to probing this individual difference effect, we assessed 1 parametric aspect of feedback. Nieuwenhuis et al. [2005b] suggested that delaying feedback might decrease its motivational significance. Moreover, prediction error responses are sensitive to the time of the reward as well as its occurrence [Hollerman and Schultz, 1998]. Thus, to examine the effect of differential feedback delay, we provided feedback (win or loss) that was delayed either 1 or 2 s in the same task. We know of no study that has directly examined the effect of feedback delay on the fERN. We hypothesize that increasing feedback delay will diminish the fERN.
Subjects and Methods
Participants
The initial sample consisted of 32 children (16 females and 16 males) screened for behavioral risk-taking from a larger sample of 89 children (44 female) who were all exposed to cocaine and other drugs prenatally, as well as considerable adversity postnatally (e.g. severe poverty, maternal stress and continued maternal drug use) [Mayes et al., 1996]. One female opted not to participate and the data of 1 male were not useable due to data artifact (less than 60% useable trials). Children were fluent in English with no evidence of serious mental illness (e.g. psychosis). The mean age of the children was 15.05 years (SD = 0.55). Handedness was evaluated using the Edinburgh Handedness Inventory [Oldfield, 1971], yielding a mean handedness quotient of 0.75 (SD = 0.29, range, −0.17 to 1.00). A score of −0.40 or lower is considered to indicate left-handedness. None of the children in the sample were left-handed.
The 32 children participating in this study were drawn from a cohort of children participating in a 15-year longitudinal study of fetal cocaine exposure effects on physical, cognitive, and social-emotional development. Prenatal cocaine exposure was determined by a combination of maternal report, urine toxicology in the prenatal or postpartum period and meconium toxicology. The sample was recruited at birth with children and families seen biannually thereafter. Families were initially recruited when they sought prenatal care at the Yale-New Haven Hospital or when they were admitted to the postpartum ward in the case of no prenatal care. The larger group, from which the 32 children in this study were recruited, consisted of 89 cocaine-exposed children seen at 15 years of age with assessment of their risk behavior using the BART. The 32 elected children comprised 81.3% African-American, 3.1% Hispanic, 3.1% Asian, and 12.5% Caucasian. For the present study, the mothers were identified as users of cocaine and other drugs (including alcohol and tobacco) since the beginning of their pregnancy and who continued their use at least through the months immediately after delivery. The procedures for ascertaining prenatal exposure status are detailed elsewhere [Mayes et al., 2005]. Mothers also uniformly reported considerable stress in their parenting role, and were all living in extreme poverty.
Prenatal drug-exposure status was ascertained at the time of recruitment into the longitudinal follow-up study (either prenatally or at the time of delivery). After obtaining verbal consent for an interview, all women were questioned about substance use in a detailed interview that covered lifetime use of cocaine, tobacco, alcohol, marijuana, and other drugs (e.g. sedatives, opiates), and frequency and amount of use of these agents during the preceding 30 days. For all women regardless of drug use history, a urine sample was obtained for toxicology. Standard urine screening for drug level or metabolites of cocaine (e.g. benzoylecognine), opioids, benzodiazepines, and tetrahydrocannabinol was performed using the Abbott TDx system and the recommended cutoff levels [Poklis, 1987].
The Balloon Analogue Risk Task
The BART [Lejuez et al., 2002] was used to assess risk-taking propensity separately for male and female children. Initially a computer screen displayed 4 items: a small balloon, balloon pump, a reset button labeled ‘Collect $ $ $,’ a ‘Total Earned’ display, and a second display labeled ‘Last Balloon’ listing the money earned on the last balloon. Each mouse click on the pump inflated the balloon incrementally (about 0.3 cm in all directions). With each pump, money was accumulated in a temporary bank. This program feature allowed for money to accumulate at a rate of 2 cents per pump. A permanent bank appeared on screen for participants to view, which consisted of a square box with a dollar figure (beginning with $0.00). Each balloon had a predetermined explosion point. If a balloon was pumped past its individual explosion point, the computer generated a ‘pop’ sound effect. For exploded balloons, all money in the temporary bank was lost, and no money was transferred to the permanent bank. A participant could stop pumping the balloon and click the ‘Collect $ $ $’ button at any point during each balloon trial. Choosing this button transferred all money from the temporary bank to the permanent bank, and each time the total earned would be incrementally updated, coinciding with a slot machine payoff sound. A new balloon appeared on the screen after each balloon explosion or money collection until a total of 30 balloons (i.e. trials) were completed. The probability that a balloon would explode was fixed at 1/128 for the first pump. If the balloon did not explode after the first pump, the probability that the balloon would explode was 1/127 on the second pump, 1/126 on the third pump, and so on until the 128th pump the probability of an explosion was 1/1 or a certainty. According to this algorithm, the average breakpoint was 64 pumps. Detailed instructions provided to the participant were based on those provided by Lejuez et al. [2002].
We relied on an adjusted number of pumps across balloons to select participants (i.e. BART score) from the total group of 89. This adjusted value, defined as the average number of pumps on balloons that did not explode, is preferable to the unadjusted average because the number of pumps is necessarily constrained on balloons that exploded, thereby limiting between-participant variability in the unadjusted averages [Lejuez et al., 2002]. In this study, we selected participants from a sample of 45 male and 44 female adolescent children. We selected participants at the top 20% and the bottom 20% of the distribution, separately for males and females (see table 1 for high- and low-risk group means).
Table 1.
Age and BART scores (mean adjusted pumps) for high- and low-risk male and female adolescents
| n | Age, years | Mean score | Minimum score | Maximum score | |
|---|---|---|---|---|---|
| Males | |||||
| High BART (top 20%) | 7 | 15.07 ± 0.45 | 46.70 ± 6.23 | 38.25 | 58.94 |
| Low BART (bottom 20%) | 7 | 15.41 ± 0.61 | 13.49 ± 3.15 | 7.76 | 16.23 |
| Male sample | 45 | 31.21 ± 17.83 | 7.76 | 58.94 | |
| Females | |||||
| High BART (top 20%) | 6 | 14.91 ± 0.60 | 41.79 ± 5.97 | 35.52 | 50.11 |
| Low BART (bottom 20%) | 9 | 14.86 ± 0.61 | 7.07 ± 3.89 | 1.80 | 13.00 |
| Female sample | 44 | 20.96 ± 18.45 | 1.80 | 54.94 |
Data are presented as means ± SD.
ERP Reward-Feedback Task
A gambling task modeled after Holroyd et al. [2003] presented the individual with 4 balloons of different colors (red, green, orange, and blue) that randomly appeared in different positions along a row. Although there were 4 options (balloons) on a given trial, feedback was rigged to have a reward: punishment probability of 1/2 (gain/lose 25 cents) across the task, and feedback was random. The task was divided into 4 blocks. At the outset of each block, the participant was allowed to gain on 10–12 consecutive trials. These trials were included to ensure that on average the subject would have a winning balance. Subsequent to balloon selection, feedback was delayed either 1 or 2 s. Each winning trial is worth 25 cents. A total of 288 trials (72 per condition) were administered for the purpose of computing ERP. Feedback lasted 800 ms, with a 700-ms inter-trial interval before the balloons reappeared. Participants made balloon choices at their own pace.
Procedures
After obtaining parental permission and child assent, each child was seated 1 m in front of a 15-inch Dell computer monitor. Each child’s head circumference was measured to determine the appropriate net size and to mark Cz as the juncture of the half-way point between nasion to inion and left to right preauricular notches. Next, a high-density array of 128 Ag/AgCl electrodes arranged into a net (Geodesic Sensor Net, Electrical Geodesics) was placed on the child’s head using standard procedures. Before this, the net was soaked in warm potassium chloride solution that served as the electrolyte. The potassium chloride solution enabled electroencephalogram (EEG) collection even through hair and without the need for abrading the participant’s scalp. The filters were set at 0.1–30 Hz. Brain wave δ were recorded through the Nestation version 4.0 software package (Electrical Geodesics) and high impedance amplifiers (Electrical Geodesics), sampling at 250 Hz. All electrodes were referenced to Cz for recording and then re-referenced offline for data analysis. All impedances remained at or under 40 kilo-Ohms as indicated by impedance measures made immediately before and after the test session. EGIS version 4.2.1 (Electrical Geodesics) and E-prime version 1.2 (Psychological Software Tools) software packages controlled the stimulus presentation. Each child’s EEG and behavior were continuously monitored across the session, so that stimulus presentation occurred only when the child was sitting still and looking at the monitor.
Each ERP epoch included a 100-ms prestimulus baseline and a 600-ms poststimulus interval. After artifact rejection, the single trial data were re-referenced from the vertex (Cz) to an average reference of all electrodes because the latter was thought to be a better representation of a true zero [Junghöfer et al., 1999]. Trial by trial data were then averaged separately for each of the 128 electrode sites and each of the 2 stimulus conditions.
Results
Preliminary Analyses
Child age data were submitted to a sex × risk group ANOVA. The main effect for sex was not significant, F(1, 26) = 2.87, nor was the main effect for risk group, F(1, 26) = 0.61, or the sex × risk interaction, F(1, 26) = 1.08. For purposes of comparison with our ERP data, we tested for BART score sex differences within each risk group with independent t tests. For participants designated as low risk, boys selected by distributional cutoffs were significantly less risky than girls, t(14) = −3.55, p < 0.01 (low-risk boys vs. low-risk girls; mean difference, −6.42). The high-risk female and male groups were not significantly different on the BART, t(11) = −1.18. Means and standard deviations (age and mean adjusted number of pumps on the BART) are displayed by risk group in table 1.
Visual ERP data (in response to feedback) for all participants were segmented into epochs including a 100-ms prestimulus baseline and a 600-ms poststimulus interval. All data were re-referenced offline after data collection from the vertex (Cz) to the average of all electrodes [Junghöfer et al., 1999]. Next, artifact rejection was carried out to eliminate ERP contaminated by movement and eye artifacts from further analysis. Rejection rates were comparable across stimulus conditions. The segmented data were averaged individually for each participant. Data from electrodes identified as bad (poor signal quality on 10% or more of the trials) were replaced using spherical spline interpolation. For data to be included in the analyses, a total of no more than 12 channels could be considered bad. Averaged data were baseline-corrected by subtracting the average microvolt value across the 100-ms prestimulus interval from the poststimulus segment.
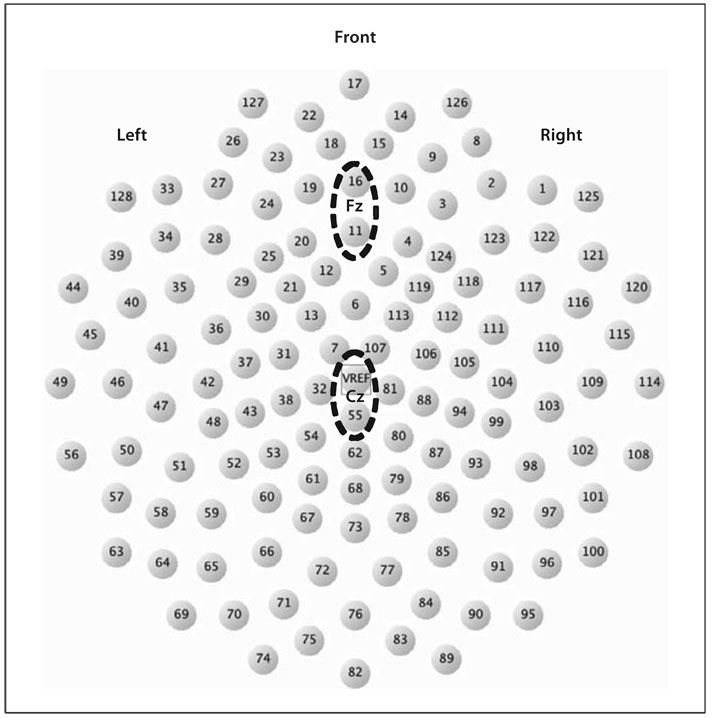
Past work on feedback negativity has localized the fERN to the medial frontal region along the midline at site Fz (10-10 system). We relied on the average signal of 2 electrodes over the midline in this region, 11 and 16, that correspond to Fz and A Fz, respectively (fig. 1). For purposes of comparison, we examined a second pair of electrodes, also on the midline but more posterior, at Cz and Cpz (electrodes 129, 55). We expected this posterior site to favor the error positivity, which tends to be more posterior to the fERN.
Fig. 1.
Electrical Geodesics 128-electrode dense array. Sites Fz, Cz, and corresponding channels circled.
ERP Data
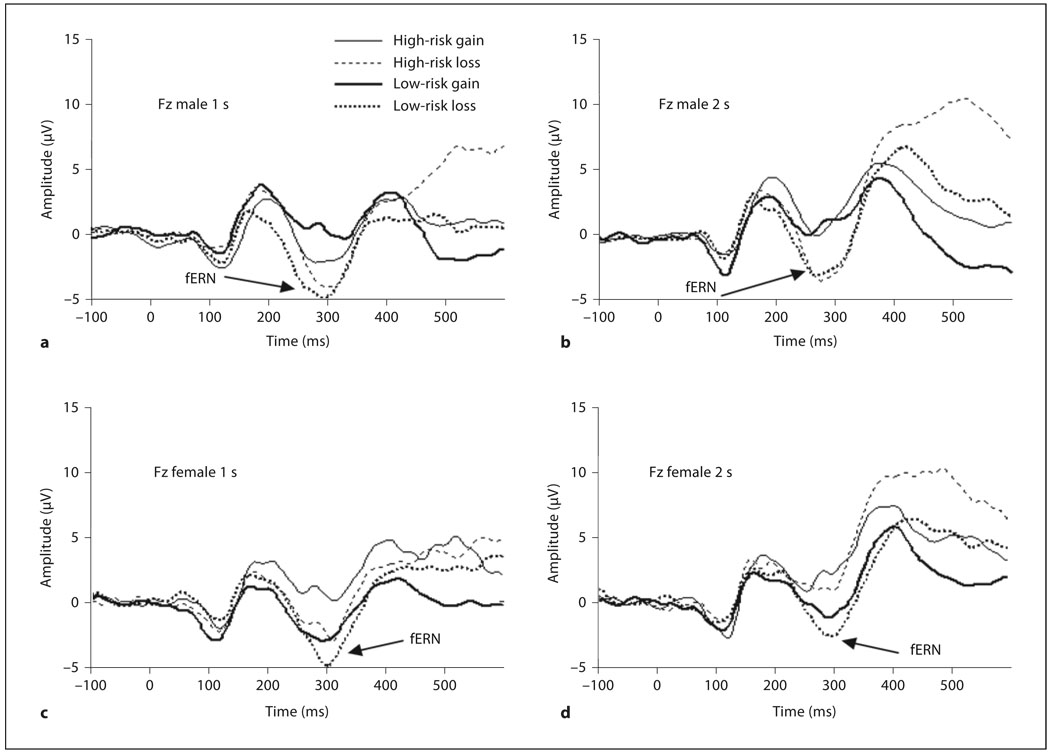
Data were analyzed with repeated-measures ANOVA and all F tests reported with the Greenhouse-Geisser correction [Greenhouse and Geisser, 1959]. Repeated measures ANOVAs consisted of feedback (gain/reward vs. loss/punishment) and time (1 vs. 2 s) as within-subjects factors, and sex (male vs. female) and risk (low vs. high) as between-subjects factors. For post hoc comparisons throughout, if the mean voltage difference (or t value) was positive, then the first level of the factor produced a relatively more positive (less negative) voltage than the second level; a negative mean voltage difference (or t value) indicates the second level of the factor produced a relatively more positive (less negative) voltage than the first level. Results are illustrated by average waveforms following gain and loss by sex and risk group for sites Fz and Cz (fig. 1). Because the fERN occurred later at Fz than at Cz (fig. 2), we relied on different windows in our peak analysis.
Fig. 2.
Grand average ERP at Fz for high- and low-risk groups, gain and loss. a Males (delay: 1 s). b Males (delay: 2 s). c Females (delay: 1 s). d Females (delay: 2 s).
Feedback Negativity
At site Fz the fERN is clearly visible at approximately 300 ms after feedback (fig. 2). We used an interval of 250–350 ms to capture the fERN, computing the mean amplitude in this time window. We observed a main effect for feedback, F(1, 26) = 35.08, p < 0.001, η2 = 0.57 (mean difference = 2.01), with loss producing more negative voltages than gain at Fz. An effect of time was also significant, F(1, 26) = 45.83, p < 0.001, η2 = 0.64, indicating that ERP to feedback were more negative at the 1-second delay than at 2-second delay. The feedback × sex interaction was significant, F(1, 26) = 5.97, p < 0.05, η2 = 0.19. Paired-sample t tests indicated that although loss was more negative than gain for females, t(14) = 2.74, p < 0.05, (mean difference = 1.15), the effect was about twice as large for males, t(14) = 5.42, p < 0.001 (see t values for equal sample sizes). Testing the same effect by an independent sample test, the difference was significantly larger for males than for females, t(28) = 2.49, p < 0.05 (male mean = 2.80, female mean = 1.15, mean difference = 1.66). The main finding with respect to the fERN was a significant feedback × time × sex × risk interaction, F(1, 26) = 6.61, p < 0.05, η2 = 0.20, β = 0.70. We decomposed the interaction by examining feedback and risk separately for males and females at 1- and 2- second delays. For males at the 1-second delay, the feedback × risk interaction was significant, F(1, 13) = 7.60, p < 0.05, η2 = 0.37, but the 2-second delay was not significant, F(1, 13) = 0.10. A post hoc analysis examining the difference of gain and loss, 1-second delay, was significant, t(13) = 2.76, p < 0.05 (mean difference = 2.72), suggesting that low-risk males were more responsive to the difference between gain and loss feedback than the high-risk males at the 1-second delay. The feedback × risk interaction for females did not reach significance at the 1-second delay, F(1, 13) = 1.94, or 2-second delay, F(1, 13) = 0.38.
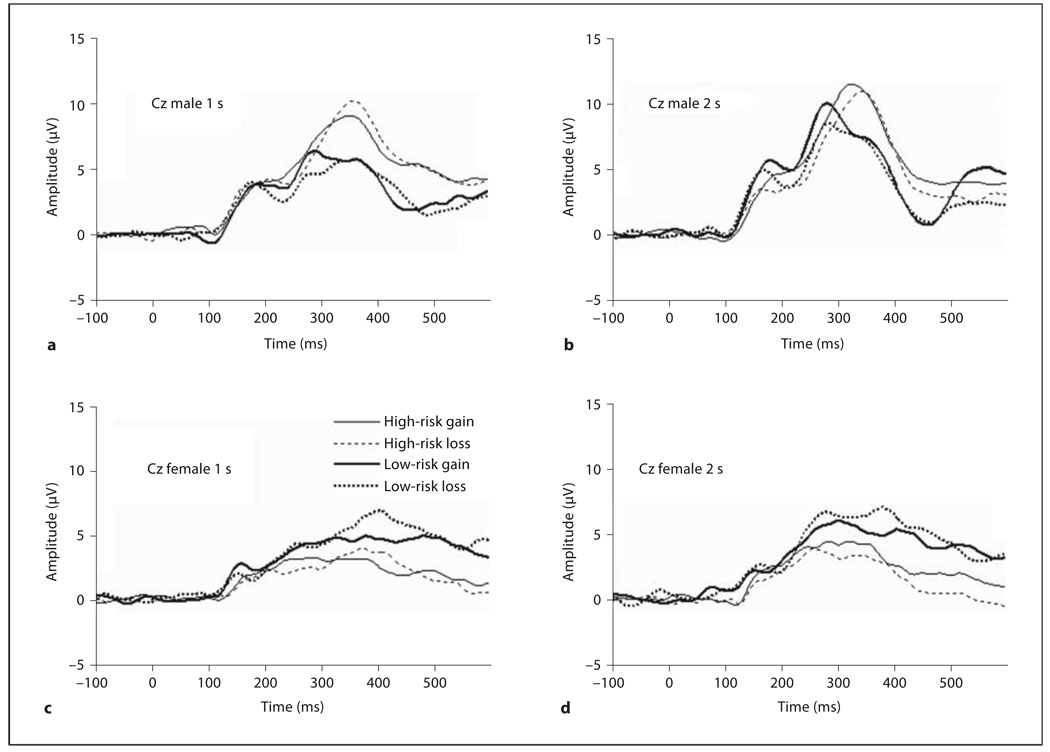
At site Cz, we used an interval of 180–280 ms to capture the fERN, computing the mean amplitude in this time window. The fERN appeared earlier and was clearly more pronounced in males than females (approx. 230 ms; fig. 2). We observed a significant effect of feedback, F(1, 26) = 12.29, p < 0.01, η2 = 0.32, mean difference = 0.74, with loss more negative than gain in the 180 to 280-ms window at Cz. The time effect was also significant, F(1, 26) = 17.71, p < 0.001, η2 = 0.41, with the 1-second delay more negative than the 2-second delay (mean difference = −1.25). An effect of sex was significant, (1, 26) = 4.56, p < 0.05, η2 = 0.15, (mean difference = 1.76). This effect was qualified by a significant feedback × sex interaction, F(1, 26) = 4.79, p < 0.05, η2 = 0.16. Post hoc analyses examined feedback effects separately by sex. For males, loss was more negative than gain, t(14) = 4.72, p < 0.001 (mean difference = 1.21). Females were not significantly different in their responses to positive versus negative feedback in this window, t(14) = 0.70. Comparing the gain-loss difference, males had better separation for feedback than females: t(28) = 2.41, p < 0.05, mean difference = 0.98 (male mean = 1.21, female mean = 0.22).
P300
At site Fz we relied on a window of 350–600 ms to capture the P300, computing the mean amplitude in this time window. The P300 is clearly visible for males and females, high and low risk, occurring at approximately 400 ms (fig. 3). We observed a main effect for feedback, F(1, 26) = 14.37, p < 0.001, η2 = 0.36 (mean difference = −2.42) with loss producing more positive voltages than gain. A main effect of time was also present, F(1, 26) = 22.34, p < 0.001, η2 = 0.46 (mean difference = −2.77) with the 2-second delay being more positive than the 1-second delay. The feedback × time interaction was also significant, F(1, 26) = 7.99, p < 0.01, η2 = 0.24. Separate post hoc comparisons for the 1- and 2-second delays indicated that there were no differences between gain and loss at 1 s, t(29) = −1.95, but at 2 s (Fz 350–600 ms), loss was more positive than gain, t(29) = −4.42, p < 0.001, mean difference = −3.58. Thus, looking across the fERN and the P300 at Fz, we saw that negative feedback with a 1-second delay produced a larger fERN than at 2 s, whereas the 2-second delay produced a larger P300 for negative feedback.
Fig. 3.
Grand average ERP at Cz for high- and low-risk groups, gain and loss. a Males (delay: 1 s). b Males (delay: 2 s). c Females (delay: 1 s). d Females (delay: 2 s).
At site Cz, we relied on an earlier window of 300–400 ms window to capture the P300. We observed a prominent P300 that was modulated by time, F(1, 26) = 15.72, p < 0.001, η2 = 0.38, with the 2-second delay more positive than the 1-second delay, mean difference = −0.41. A main effect of sex, F(1, 26) = 7.32, p < 0.05, η2 = 0.22 (mean difference = 3.33) revealed male was more positive, qualified by a sex × risk interaction, F(1, 26) = 4.42, p < 0.05, η2 = 0.15. Independent post hoc t tests first compared high- and low-risk groups separately by sex. Neither high- and low-risk males, t(13) = −1.65, nor high- and low-risk females differed significantly, t(13) = 1.32. Comparing males and females within each risk group, low-risk males and females were not significantly different, t(14) = 0.43. However, voltages in the 300–400 time window were significantly greater and more positive for high-risk males, t(12) = 3.41, p < 0.01, mean difference = 5.91, than for high-risk females.
Discussion
This study examined ERP responses to reward (gain) and punishment (loss) feedback among male and female adolescents grouped specifically as low- and high-risk takers using a well-validated behavioral risk assessment tool (the BART). Comparing these low- and high-risk behavior groups, the present study sought to determine whether risk status on the BART was associated with fERN differences. Consistent with previous work, a focal ERP negativity was present in the fronto-central region approximately 300 ms after feedback for loss compared to gain trials. This effect suggests that overall the task worked in this adolescent sample to elicit a fERN response when expected rewards did not occur. While published studies with adolescents have examined the ERN [Davies et al., 2004; Ladouceur et al., 2007], this is the first report we know of to examine the fERN in an adolescent sample. Feedback type, risk status, sex, and feedback delay were all associated with variability in the fERN in our sample. Also, as others have found, we observed a prominent feedback-related P300 component across sites Fz and Cz, somewhat more robust at site Cz.
We hypothesized that the fERN would differentiate adolescents classified as high and low risk takers on the BART. Partial support for this prediction was found, qualified by sex and feedback delay variables. Only at the 1-second delay were low-risk males more responsive to loss versus gain feedback when compared to high-risk males. One possible interpretation of this finding is that the medial frontal system responsible for feedback monitoring may be differentially active across the male risk groups. A number of studies have examined individual difference variables that relate to the ERN [Gehring et al., 2000; Hajcak et al., 2003; Hajcak and Simons, 2002]. Far fewer studies examine individual difference variables that relate to the fERN [e.g. Fein and Chang, 2008]. Because the BART is a well-validated index of real life risk-taking in adolescence, we might expect that the fERN difference we observed would also translate to individual differences in risk-taking proclivity among adolescent males, with smaller fERN amplitudes indicative of more risk proclivity. We saw no effect for high- versus low-risk females in terms of fERN. While males in the population tend to generally show more risk-taking than females [Byrnes et al., 1999], our high- and low-risk females were at least as extreme as our males were on the BART, suggesting that low- versus high-risk status does not account for a lack of difference in terms of the fERN in girls. One intriguing possibility is that the lack of fERN separation for high- and low-risk females reflects a sexual dimorphism across males and females in terms of reward responsivity in the task. Our findings are consistent with Hoeft et al. [2008], who found greater brain activation and functional connectivity of mesocorticolimbic circuitry among males compared to females in terms of computer game play in a rewarding task. Other possibilities could account for the lack of a female risk status effect, including developmental differences among males and females in the middle adolescent period we assessed, or that adolescent females are more homogeneous in terms of the brain processes that underlie feed-back monitoring. Post hoc examination of the female high- and low-risk groups alone for feedback suggests that power was not an issue, F(1, 13) = 0.14, η2 = 0.06, β = 0.06; there was no effect of risk group for females. At the same time, multiple brain processes are involved in risk-taking and decision making, and we focused on just one.
Given the overall pattern of results for females versus males, females may have been less motivationally engaged in our ERP feedback task than males, which may have accounted for the reduced variability in their fERN responses. Two findings in our data support this interpretation. First, irrespective of risk status, we observed a sex × feedback interaction indicating that males differentiated more between the gain and loss conditions than females. The effect of differential response to feedback (loss vs. gain) was over twice as large for males. Second, the P300 thought to possibly reflect the motivational significance of feedback [Overbeek et al., 2005] was significantly more pronounced for males than females at site Cz, regardless of the 1- or 2-second feedback delay.
The second main finding of our study was that feedback delay modulated the fERN. Nieuwenhuis et al. [2005b] suggested that delaying feedback might decrease its motivational significance. In our study, the 1-second delay produced a more robust feedback response than the 2-second delay, consistent with this interpretation. Possibly increases in time between selection and feedback lead to reduced expectation of reward. However, the fERN was still clearly visible at a 2-second delay (fig. 2 b, d). Interestingly, as we describe below, increased delay had the opposite effect on the P300 component, which was larger for the 2-second delay.
A number of studies report that monetary feedback stimuli elicit a P300-like component, which increases in amplitude with the amount of money either won or lost [Johnston, 1979; Sato et al., 2005; Sutton et al., 1978; Yeung and Sanfey, 2004]. Two recent studies reported a dissociation whereby regardless of feedback type (gain or loss) the P300 was sensitive to magnitude (large or small), whereas the fERN is sensitive to gain or loss [Sato et al., 2005; Yeung and Sanfey, 2004]. We observed a positive component consistent with the P300 at site Fz and at Cz. Across both sites (Fz and Cz) the 2-second delay induced a more pronounced effect than a 1-second delay. At Fz, a feedback × time effect indicated that loss produced a more pronounced P300 than gain, but only for a 2-second delay. This finding is at odds with previous studies, but may reflect the inclusion of the 1- and 2-second delays in feedback in the same assessment. At site Cz, we also observed a significant effect of sex, with females showing smaller responses than males for the 2-second delay. Given that the P300 is associated with novelty and thought to reflect phasic norepinephrine modulation [Nieuwenhuis et al., 2005a], it may be that females were less engaged in the task or habituated to the 1- versus 2-second delay more than males.
Study Limitations
Our findings should be considered in light of our sample. All adolescents reported on here are from a high-risk group with their primary risk being prenatal exposure to potential neurotoxins, primarily cocaine. Thus, findings can only be generalized to adolescents with similar exposure status. Because we did not include a comparison group of non-exposed adolescents, we can-not draw direct conclusions about exposure status or the impact of exposure on the fERN. At the same time, the risk of prenatal drug exposure continues to be a problem for society [Kuczkowski, 2007] and children with such exposures are also often growing up in considerable stress and adversity which increases the likelihood of their own maladaptive risk-taking, including substance use, by adolescence. Our findings can be productively viewed against the backdrop of recent animal work which found that gestational cocaine exposure results in permanent alterations of the structure and function of brain reward systems [Estelles et al., 2006] including the anterior cingulate cortex [Stanwood et al., 2001], a putative neural generator of the medial frontal negativity effect. We do not know if or how cocaine exposure affects the fERN or the development of medial prefrontal brain regions and the reward-related neural circuitry in adolescence. Given that our behaviorally comparable (high/low BART risk) male and female subjects diverged significantly in terms of the fERN (not moderated by risk status at 1-second delay in females) and the P300 (generally reduced in females at 2-second delay compared to males), we may have tapped into a sexually dimorphic effect of cocaine exposure. Interestingly, in a study with Long-Evans rats, Gendle et al. [2003] reported a sexually dimorphic effect of cocaine exposure that resembles our findings for females. In their study of sustained attention, cocaine exposure was associated with a heightened reactivity to errors for both sexes. While males remained engaged in the task, females appeared to disengage, refusing to participate when their capacity to sustain attention was exceeded.
Another study limitation bearing on this point is the type of task we used. It is possible that our ERP balloon task was less appealing to females than to males in terms of its graphics and the rewards offered. There is a small body of literature on gender differences in video game dependence suggesting that males tend to be more dependent on video games [Griffiths et al., 2004]. Perhaps a task designed to use rewards that involve personal relationships would more strongly engage females. Lastly, our study relies on a relatively small sample of subjects. Replication in a larger sample is warranted.
In summary, our work has several important implications. First, the BART, as a real-world assessment of risk-taking, is associated with individual differences in the fERN for male adolescents. Second, sex emerged as an important individual difference variable, possibly reflecting differential task engagement and differential reward processing across the sexes. Third, the inclusion of feedback delay differentially affected the fERN and the P300. This finding suggests a hybrid ERP paradigm that can be used to study the complementary functioning of dopamine [Holroyd and Coles, 2002] and norepinephrine systems [Nieuwenhuis, 2005a], both of which are altered with prenatal cocaine exposure [Mayes et al., 2003; Stanwood et al., 2001]. Our future work will explore the differences between males and females in task engagement and capacity for sustained attention, as well as the differences between prenatally cocaine and non-cocaine-exposed adolescents and between adolescents with different profiles of early and current adversity.
Acknowledgments
This research was supported by a National Research Service Award Institutional Post-Doctoral Training Fellowship (M.J.C; T-32 MH18268, James Leckman, PI); NARSAD Young Investigator Award (M.J.C.); NIDA grants: RO1-DA-06025 (L.C.M.), DA-017863 (L.C.M.) and KO5 (L.C.M.); and a grant from the Pfeffer Foundation (L.C.M.).
References
- Aklin WM, Lejuez CW, Zvolensky MJ, Kahler CW, Gwadz M. Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behav Res Ther. 2005;43:215–228. doi: 10.1016/j.brat.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev Rev. 1992;12:339–373. [Google Scholar]
- Bauer CR, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, et al. The Maternal Lifestyle Study: drug exposure during pregnancy and short-term maternal outcomes. Am J Obstet Gynecol. 2002;186:487–495. doi: 10.1067/mob.2002.121073. [DOI] [PubMed] [Google Scholar]
- Bendersky M, Alessandri S, Gilbert P, Lewis M. Characteristics of pregnant substance abusers in two cities in the northeast. Am J Drug Alcohol Abuse. 1996;22:349–362. doi: 10.3109/00952999609001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendersky M, Bennett D, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32 suppl:i–iv. 1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Byrnes JP, Miller DC, Schafer WD. Gender differences in risk-taking: a meta-analysis. Psychol Bull. 1999;125:367–383. [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, et al. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Dev Psychobiol. 1997;30:61–69. [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ, Raymond KM, Mikulich-Gilbertson SK, Thompson LL, Lejuez CW. A risk-taking ‘set’ in a novel task among adolescents with serious conduct and substance problems. J Am Acad Child Adolesc Psychiatry. 2006;45:175–183. doi: 10.1097/01.chi.0000188893.60551.31. [DOI] [PubMed] [Google Scholar]
- Curry MA. The interrelationships between abuse, substance use, and psychosocial stress during pregnancy. J Obstet Gynecol Neonatal Nurs. 1998;27:692–699. doi: 10.1111/j.1552-6909.1998.tb02640.x. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Dev Neuropsychol. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- DiClemente RJ, Hansen WB, Ponton LE. Handbook of Adolescent Health Risk Behavior. New York: Plenum Press; 1996. [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Estelles J, Rodriguez-Arias M, Maldonado C, Aguilar MA, Minarro J. Gestational exposure to cocaine alters cocaine reward. Behav Pharmacol. 2006;17:509–515. doi: 10.1097/00008877-200609000-00017. [DOI] [PubMed] [Google Scholar]
- Falkenstein M. Errors, conflicts, and the brain: a review of the contributions to the error conference, Dortmund 2003. J Psychophysiol. 2004;18:153–163. [Google Scholar]
- Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug Alcohol Depend. 2008;92:141–148. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, D’Lauro C, Curran T. Cross-task individual differences in error processing: neural, electrophysiological, and genetic components. Cogn Affect Behav Neurosci. 2007;7:297–308. doi: 10.3758/cabn.7.4.297. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Woroch BS, Curran T. Error-related negativity predicts reinforcement learning and conflict biases. Neuron. 2005;47:495–501. doi: 10.1016/j.neuron.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Dev Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Medial prefrontal cortex and rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gendle MH, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Impaired sustained attention and altered reactivity to errors in an animal model of prenatal cocaine exposure. Brain Res Dev Brain Res. 2003;147:85–96. doi: 10.1016/j.devbrainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Griffiths MD, Davies MN, Chappell D. Online computer gaming: a comparison of adolescent and adult gamers. J Adolesc. 2004;27:87–96. doi: 10.1016/j.adolescence.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Gullone E, Moore S. Adolescent risk-taking and the five-factor model of personality. J Adolesc. 2000;23:393–407. doi: 10.1006/jado.2000.0327. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biol Psychol. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Res. 2002;110:63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Watson CL, Kesler SR, Bettinger KE, Reiss AL. Gender differences in the mesocorticolimbic system during computer game-play. J Psychiatr Res. 2008;42:253–258. doi: 10.1016/j.jpsychires.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Cohen JD. Errors in reward prediction are reflected in the event-related brain potential. Neuroreport. 2003;14:2481–2484. doi: 10.1097/00001756-200312190-00037. [DOI] [PubMed] [Google Scholar]
- Jessor SL, Jessor R. Problem Behavior and Psychosocial Development: A Longitudinal Study of Youth. New York: Academic Press; 1977. [Google Scholar]
- Johnston VS. Stimuli with biological significance. In: Begleiter H, editor. Evoked Brain Potentials and Behavior. New York: Plenum; 1979. pp. 1–12. [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clin Neurophysiol. 1999;110:1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Kuczkowski KM. The effects of drug abuse on pregnancy. Curr Opin Obstet Gynecol. 2007;19:578–585. doi: 10.1097/GCO.0b013e3282f1bf17. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev Sci. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin W, Daughters S, Zvolensky M, Kahler C, Gwadz M. Reliability and validity of the Youth Version of the Balloon Analogue Risk Task (BART-Y) in the assessment of risk-taking behavior among inner-city adolescents. J Clin Child Adolesc Psychol. 2007;36:106–111. doi: 10.1080/15374410709336573. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J Adolesc. 2003;26:475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk-taking: The Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Bornstein MH, Chawarska K, Haynes O, et al. Impaired regulation of arousal in 3-month-old infants exposed prenatally to cocaine and other drugs. Dev Psychopathol. 1996;8:29–42. [Google Scholar]
- Mayes LC, Cicchetti D, Acharyya S, Zhang H. Developmental trajectories of cocaine-and-other-drug-exposed and non-cocaine-exposed children. J Dev Behav Pediatr. 2003;24:323–335. doi: 10.1097/00004703-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Molfese DL, Key AP, Hunter NC. Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicol Teratol. 2005;27:797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005a;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. Eur J Neurosci. 2005b;21:3161–3168. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: on the functional significance of the Pe vis-à-vis the ERN/Ne. J Psychophysiol. 2005;19:319–329. [Google Scholar]
- Poklis A. Evaluation of TDx cocaine metabolite assay. J Anal Toxicol. 1987;11:228–230. doi: 10.1093/jat/11.5.228. [DOI] [PubMed] [Google Scholar]
- Sato A, Yasuda A, Ohira H, Miyawaki K, Nishikawa M, Kumano H, et al. Effects of value and reward magnitude on feedback negativity and P300. Neuroreport. 2005;16:407–411. doi: 10.1097/00001756-200503150-00020. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–283. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Tzilos GK, Pimentel PJ, Yurgelun-Todd DA. Trajectories of adolescent emotional and cognitive development: effects of sex and risk for drug use. Ann NY Acad Sci. 2004;1021:363–370. doi: 10.1196/annals.1308.046. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Levitt P. Identification of a sensitive period of prenatal cocaine exposure that alters the development of the anterior cingulate cortex. Cereb Cortex. 2001;11:430–440. doi: 10.1093/cercor/11.5.430. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Shumsky JS, Levitt P. Prenatal cocaine exposure produces consistent developmental alterations in dopamine-rich regions of the cerebral cortex. Neuroscience. 2001;106:5–14. doi: 10.1016/s0306-4522(01)00256-1. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Grant TM, Barr HM, Brown ZA, Martin JC, Mayock DE, et al. Cocaine and the use of alcohol and other drugs during pregnancy. Am J Obstet Gynecol. 1991;164(part 1):1239–1243. doi: 10.1016/0002-9378(91)90691-j. [DOI] [PubMed] [Google Scholar]
- Sutton S, Tueting P, Hammer M, Hakerem G. Evoked potentials and feedback. In: Otto D, editor. Multidisciplinary Perspectives in Event-Related Potential Research. Washington: Government Printing Office; 1978. pp. 184–188. [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. J Neurosci. 2004;24:6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]