Abstract
Background and Purpose
Proximal aortic plaques are a risk factor for vascular embolic events. However, this association in the general population is unclear. We sought to assess whether proximal aortic plaques are associated with vascular events in a community-based cohort.
Methods
Stroke-free subjects from the Aortic Plaques and Risk of Ischemic Stroke (APRIS) study were evaluated. Aortic arch and proximal descending aortic plaques were assessed by transesophageal echocardiography (TEE). Vascular events (myocardial infarction, ischemic stroke, vascular death) were prospectively recorded and their association with aortic plaques was assessed.
Results
209 subjects were studied (age 67.0±8.6 years). Aortic arch plaques were present in 130 subjects (62.2%); large plaques (≥4 mm) in 50 (23.9%). Descending aortic plaques were present in 126 subjects (60.9%), large plaques in 41 (19.8%).
During a follow-up of 74.4±26.3 months, 29 events occurred (12 myocardial infarctions, 11 ischemic strokes, 6 vascular deaths). After adjustment for risk factors, large aortic arch plaques were not associated with combined vascular events (hazard ratio [HR] 1.03, 95% confidence intervals [CI] 0.35 to 3.02) or ischemic stroke (HR 0.59, 95% CI 0.10 to 3.39). Large descending aortic plaques were also not independently associated with vascular events (HR 1.99, 95% CI 0.52 to 7.69) or ischemic stroke (HR 1.43, 95% CI 0.27 to 7.48).
Conclusions
In a population-based cohort, the incidental detection of plaques in the aortic arch or proximal descending aorta was not associated with future vascular events. Associated co-factors may affect the previously reported association between proximal aortic plaques and vascular events.
Keywords: Thoracic aorta, atherosclerosis, thromboembolism, prognosis, transesophageal echocardiography
Introduction
Atherosclerotic aortic plaques are associated with cerebrovascular and peripheral embolic events. The evidence supporting this association derives from autopsy studies,1 case-control studies on patients surviving a vascular event (cerebrovascular or peripheral)2-6 and studies on high-risk patients referred for transesophageal echocardiography (TEE).7-9
The prevalence of aortic atherosclerosis increases with age and is affected by comorbidities, with ample variations depending on the age strata considered, the frequency of associated risk factors and the definition of plaque used.3,10,11 Given the high prevalence of aortic arch atherosclerosis in the elderly, it is crucial to understand the vascular risk burden associated with this condition in the general population. In the only population study conducted so far, the prevalence of aortic plaques in the aorta was 51%, but the prevalence of severe plaques in the proximal aorta (ascending/arch), which carry the highest stroke risk,3,4,8,9 was only 2.4%.12 In that study, aortic atherosclerosis was not associated with subsequent cardiovascular events after adjustment for age and other risk factors, but the results regarding ischemic stroke were not conclusive because of the uneven distribution of complex (i.e., large or mobile or ulcerated) aortic plaques (68% of complex plaques were located in the descending aorta, an unlikely location for brain embolism).
In the present study, we investigated the prevalence of aortic plaques in an unselected stroke-free tri-ethnic cohort from Northern Manhattan, and the risk of vascular events and ischemic stroke associated with plaques in the aortic arch or in the proximal portion of the descending aorta.
Methods
Study Population
As part of the NINDS (National Institute of Neurological Disorders and Stroke)-funded Aortic Plaques and Risk of Ischemic Stroke (APRIS) study, 209 stroke-free subjects over the age of 50 years and available to undergo TEE were selected from the participants of the Northern Manhattan Study (NOMAS) on the basis of being matched to APRIS cases by age, sex and race-ethnicity. Detailed recruitment methods for the NOMAS have been previously published.13,14 Informed consent was obtained from all study participants. The study was approved by the Institutional Review Board of Columbia University Medical Center.
Definition of Baseline Subject Characteristics
Cardiovascular risk factors were ascertained through direct examination and interview by trained research assistants. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg (mean of two readings), self-reported history of hypertension, or current antihypertensive medication. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL, self-reported diabetes history, use of insulin or oral hypoglycemic medication. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, self-reported history of hypercholesterolemia or current lipid-lowering treatment. Current smoking was defined by use of tobacco at the time of the interview; smoking history was defined by use of tobacco at any time. Atrial fibrillation was documented by a current or past EKG. Coronary artery disease (CAD) was defined as a history of myocardial infarction, coronary artery bypass grafting or percutaneous coronary intervention, or typical angina and use of anti-ischemic medications. Carotid atherosclerosis was defined as a stenosis ≥ 60% in the internal or in the common carotid artery detected by Doppler examination. Body mass index was calculated as weight (kilograms) divided by height (meters) squared. Race/ethnicity was determined by subject's self-report using a questionnaire modeled after the U.S. Census Bureau questionnaire.
Detection of Aortic Plaques
TEE was performed upon enrollment. The aorta was analyzed in a systematic fashion as previously described.15 The aortic arch was defined as the portion of aorta between the curve at the end of the ascending portion and the takeoff of the left subclavian artery. Descending aorta was defined as the portion of aorta distal to the takeoff of the left subclavian artery.
A plaque was defined as a discrete protrusion of the intimal surface of the vessel at least 2 mm thick, different in appearance and echogenicity from the adjacent intact intimal surface. Plaques were classified as small (< 4 mm) or large (≥4 mm). Plaque ulcerations or mobile components were also recorded. An ulceration was defined as a discrete indentation of the luminal surface of the plaque with base width and maximum depth of at least 2 mm each.15 TEEs were interpreted by a single experienced echocardiographer (MDT) blinded to subjects' characteristics and risk factors.
Follow-up and Outcome Evaluation
All subjects were followed-up annually by telephone interviews. End-points were ischemic stroke, myocardial infarction (MI) and vascular death. Any vascular event or acknowledgment of neurological or cardiac symptoms during the interview triggered an in-person assessment by a neurologist. In addition, active hospital surveillance of admission and discharge ICD-9 codes was performed. All events were reviewed by a trained research assistant, and reported to a study physician for adjudication. Stroke was defined by the occurrence of any type of stroke by TOAST criteria.16 Diagnosis of ischemic stroke was determined by two neurologists independently, disagreements were adjudicated by the NOMAS principal investigator (RLS).
MI was defined by criteria adapted from the Cardiac Arrhythmia Suppression Trial17 and the Lipid Research Clinics Coronary Primary Prevention Trial18, requiring at least 2 of the following: (1) cardiac pain (typical angina); (2) elevation of creatine-phosphokinase MB isoenzyme (CPK-MB) fraction or troponin I values; and/or (3) typical EKG abnormalities.
Deaths were classified as vascular or nonvascular based on information obtained from the family, medical records, and death certificate. Causes of death were validated by a study physician. Vascular causes of death included stroke, MI, heart failure, cardiac arrhythmia (e.g., sudden or unwitnessed death).
Statistical Analysis
Differences between groups were assessed by unpaired Student's t-test for continuous variables and by the chi-square test for proportions. Event rates were calculated in the overall population and in subjects with different definitions of aortic plaque. Kaplan-Meier curves were used to estimate the event-free survival and compared by the log-rank test. Cox proportional hazards regression was used to assess the variables associated with subsequent vascular events. Variables associated with vascular events and ischemic stroke in univariate analysis (p value<0.1) were entered as independent variables in multivariate models testing the risk of events associated with different definitions of aortic plaques. Unadjusted and adjusted hazard ratios (HR) and 95% confidence intervals (CI) were obtained.
For all statistical analyses, a 2-tailed p<0.05 was considered significant.
Results
Study Cohort
Clinical characteristics of the study cohort are shown in Table 1. The aortic arch was visualized in all subjects. Aortic arch plaques of any size were found in 130 subjects (62.2%), large aortic arch plaques in 50 subjects (23.9%). Complex plaque morphology (ulcerations/mobile components) was observed in 14 subjects (6.7%). The proximal descending aorta was visualized in 207 subjects (99.0% of the cohort). Plaques of any size were found in the descending aorta in 126 subjects (60.9%), large plaques in 41 subjects (19.8%).
Table 1.
Baseline characteristics of the study cohort
| Overall Population (n=209) |
|
|---|---|
| Age, years | 67.0±8.6 |
| Women, n (%) | 93 (44.5) |
| Race/Ethnicity | |
| White, n (%) | 30 (14.4) |
| Black, n (%) | 62 (29.7) |
| Hispanic, n (%) | 117 (55.9) |
| Body Mass Index, kg/m2 | 27.6±4.9 |
| Hypertension, n (%) | 145 (69.4) |
| Diabetes, n (%) | 48 (23.0) |
| Smoking History, n (%) | 126 (60.3) |
| Current Smoking, n (%) | 33 (16.1) |
| Hypercholesterolemia, n (%) | 102 (48.8) |
| Coronary Artery Disease, n (%) | 45 (21.5) |
| Atrial Fibrillation, n (%) | 11 (5.3) |
| Carotid Stenosis (≥ 60%)*, n (%) | 1 (0.6) |
| Medications | |
| Aspirin, n (%) | 66 (31.6) |
| Warfarin, n (%) | 5 (2.4) |
| Lipid-lowering Drugs, n (%) | 32 (15.3) |
| Aortic Arch Plaques, n (%) | 130 (62.2) |
| ≥ 4 mm, n (%) | 50 (23.9) |
| Descending Aorta Plaques, n (%) | 126 (60.9) |
| ≥ 4 mm, n (%) | 41 (19.8) |
Data available in 169 subjects (81% of the study cohort)
Table 2 shows the cohort clinical characteristics by aortic plaque presence/thickness and location. Age increased with increasing plaque thickness in both the aortic arch and in the descending aorta (all p <0.01). Subjects with small arch plaques were more frequently smokers and had higher prevalence of CAD (p values <0.05) than those without plaques. Subjects with large arch plaques had higher prevalence of hypercholesterolemia than those without plaque (p<0.01) or those with small plaques (p<0.05). No differences in aspirin treatment was observed between different arch plaque groups, while warfarin treatment was more frequent in subjects with large arch plaques (p<0.01 vs. no plaques; p<0.05 vs. small plaques). Lipid-lowering drugs were more frequent in those with large aortic arch plaques compared to those with no or small plaques (both p<0.05).
Table 2.
Baseline characteristics of the study cohort by aortic plaque presence/thickness and location
| Aortic Arch | Descending Aorta | |||||
|---|---|---|---|---|---|---|
| No Plaque (n=79) |
Small Plaque (n=80) |
Large Plaque (n=50) |
No Plaque (n=81) |
Small Plaque (n=85) |
Large Plaque (n=41) |
|
| Age, years | 63.3±6.8 | 69.1±8.4** | 69.5±9.5** | 63.5±7.4 | 67.7±7.8** | 72.5±9.3**§§ |
| Women, n (%) | 35 (44.3) | 39 (48.8) | 19 (38.0) | 34 (42.0) | 39 (45.9) | 18 (43.9) |
| Race/Ethnicity | ||||||
| White, n (%) | 6 (7.6) | 13 (16.3) | 11 (22.0)* | 5 (6.2) | 15 (17.7)* | 10 (24.4)** |
| Black, n (%) | 27 (34.2) | 21 (26.3) | 14 (28.0) | 23 (28.4) | 25 (29.4) | 14 (34.2) |
| Hispanic, n (%) | 46 (58.2) | 46 (57.5) | 25 (50.0) | 53 (65.4) | 45 (52.9) | 17 (41.5)* |
| Body Mass Index, kg/m2 | 28.0±4.7 | 26.9±4.2 | 28.3±5.9 | 26.8±4.8 | 28.1±4.9 | 27.9±4.6 |
| Hypertension, n (%) | 48 (60.8) | 60 (75.0) | 37 (74.0) | 43 (53.1) | 65 (76.5)** | 35 (85.4)** |
| Diabetes, n (%) | 20 (25.3) | 21 (26.3) | 7 (14.0) | 12 (14.8) | 23 (27.1) | 13 (31.7)* |
| Smoking History, n (%) | 41 (51.9) | 51 (63.8) | 34 (68.0) | 51 (63.0) | 47 (55.3) | 27 (65.9) |
| Current Smoking, n (%) | 7 (9.1) | 16 (20.5)* | 10 (20.0) | 11 (13.8) | 10 (12.2) | 11 (26.8)§ |
| Hypercholesterolemia, n (%) | 33 (41.8) | 37 (46.3) | 32 (64.0)*§ | 34 (42.0) | 42 (49.4) | 25 (61.0)* |
| Coronary Artery Disease, n (%) | 11 (13.9) | 22 (27.5)* | 12 (24.0) | 9 (11.1) | 23 (27.1)** | 12 (29.3)* |
| Atrial Fibrillation, n (%) | 4 (5.1) | 4 (5.1) | 3 (6.0) | 3 (3.7) | 7 (8.2) | 1 (2.5) |
| Carotid Stenosis ≥ 60%, n (%) | 1 (1.3) | 0 | 0 | 1 (1.2) | 0 | 0 |
| Medications | ||||||
| Aspirin, n (%) | 24 (30.4) | 25 (31.3) | 17 (34.0) | 20 (24.7) | 29 (34.1) | 15 (36.6) |
| Warfarin, n (%) | 0 | 1 (1.3) | 4 (8.2)**§ | 0 | 3 (3.5) | 2 (5.0)* |
| Lipid-lowering Drugs, n (%) | 9 (11.4) | 10 (12.5) | 13 (26.0)*§ | 12 (14.8) | 14 (16.5) | 6 (14.6) |
P<0.05,
P<0.01 vs. no plaque;
P <0.05,
P<0.01 vs. small plaque
Subjects with large plaques in the descending aorta had higher prevalence of hypertension (p<0.01), diabetes, hypercholesterolemia and history of CAD than those without plaque (all p<0.05). Hypertension and CAD were more frequent in subjects with small descending aortic plaques compared to those without plaques (both p<0.01).
Risk of Combined Cardiovascular Events
Mean follow-up was 74.4 months (4.2 to 120.5 months). No subject was lost to follow-up. Overall, 29 end-points occurred (11 ischemic strokes, 12 MIs, 6 vascular deaths). Overall incidence of cardiovascular events in the entire study population was 22.4 per 1000 person-years, increasing from 15.5 per 1000 person-years in patients with no aortic arch plaque to 25.2 and 29.5 per 1000 person-years in those with small and large arch plaques. Event rates were 32.5 and 36.0 per 1000 person-years in those with small and large descending aortic plaques, respectively, whereas it was 7.4 per 1000 person-years for subjects without plaques. Kaplan-Meier curves for combined vascular events are showed in Figure 1. There was no significant difference in event-free survival between subjects with no, small and large aortic arch plaques (p=0.387, Figure 1A), while a significantly worse outcome was observed in subjects with descending aortic plaques compared to those without plaque (p<0.01, Figure 1B).
Figure 1.
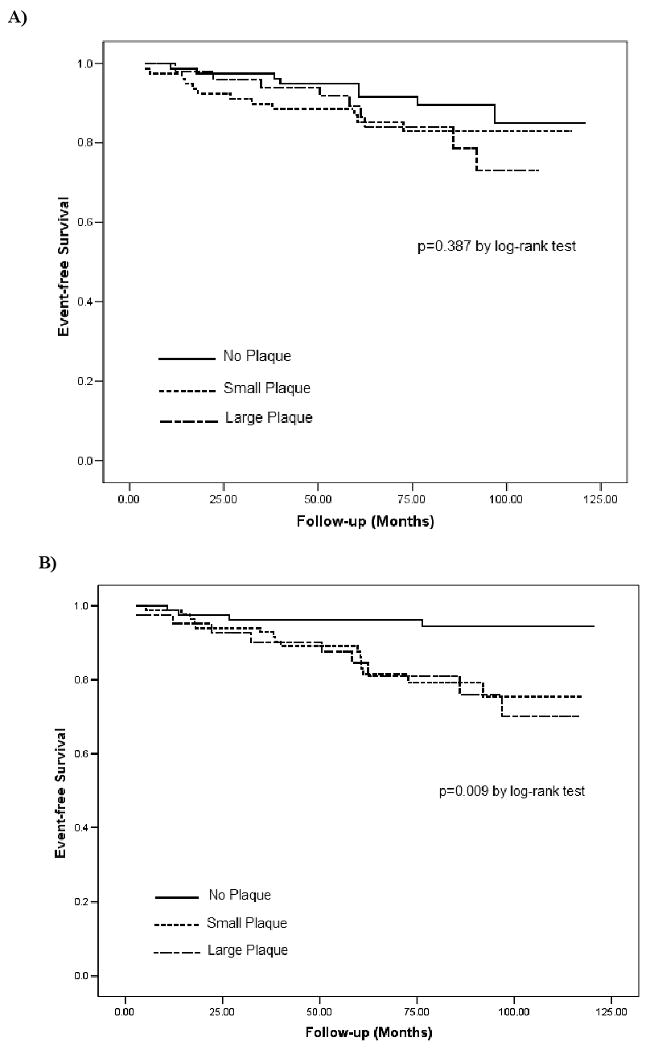
Kaplan-Meier curves for time-to-event by presence/thickness of aortic arch plaques (A) and proximal descending aorta plaques (B).
Table 3 shows the variables associated with vascular events at univariate analysis. Age (p<0.05), body mass index (p=0.01), and history of CAD (p<0.01) were univariate predictors of events. Hypertension and hypercholesterolemia were associated with a borderline increase in risk of events.
Table 3.
Univariate predictors of cardiovascular events
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age | 1.05 | 1.01-1.09 | 0.02 |
| Male Gender | 1.12 | 0.53-2.34 | 0.77 |
| Race-Ethnicity | |||
| Black* | 1.33 | 0.63-2.83 | 0.45 |
| Hispanic* | 0.62 | 0.29-1.29 | 0.20 |
| White* | 1.46 | 0.59-3.59 | 0.41 |
| Body Mass Index | 1.09 | 1.02-1.16 | 0.01 |
| Hypertension | 2.82 | 0.98-8.11 | 0.054 |
| Diabetes | 1.91 | 0.89-4.11 | 0.10 |
| Current Smoking | 0.37 | 0.09-1.57 | 0.18 |
| Smoking History | 1.69 | 0.75-3.81 | 0.21 |
| Coronary Artery Disease | 3.27 | 1.57-6.80 | 0.002 |
| Hypercholesterolemia | 2.11 | 0.98-4.54 | 0.06 |
| Atrial Fibrillation | 2.16 | 0.65-7.14 | 0.21 |
Reference is the combination of the other two groups
Table 4 shows the risk of events associated with the aortic plaques. Small arch plaques were not associated with cardiovascular events in either univariate (HR 1.62, 95% CI 0.66 to 3.97) or multivariate analysis (HR 1.27, 95% CI 0.49 to 3.28). Similar results were observed for large arch plaques, in both univariate (HR 1.88, 95% CI 0.73 to 4.88) and multivariate analysis (HR 1.03, 95% CI 0.35 to 3.02). When severe plaque was defined as either ≥ 4 mm thick or by the presence of ulceration/mobile components as in previous studies,12 HRs were comparable to those for large plaques (data not shown), because all but one of ulcerated/mobile plaques were > 4 mm in thickness.
Table 4.
Risk of vascular events by aortic plaque thickness and location
| Combined Vascular Events | ||
|---|---|---|
| Unadjusted Hazard Ratio (95% CI) |
Adjusted Hazard Ratio* (95% CI) |
|
| Aortic Arch | ||
| No Plaque | Reference | Reference |
| Small Plaque | 1.62 (0.66-3.97) | 1.27 (0.49-3.28) |
| Large Plaque | 1.88 (0.73-4.88) | 1.03 (0.35-3.02) |
| Descending Aorta | ||
| No Plaque | Reference | Reference |
| Small Plaque | 4.35 (1.45-13.03) | 2.50 (0.78-7.97) |
| Large Plaque | 4.83 (1.49-15.70) | 1.99 (0.52-7.69) |
Covariates: age, body mass index, hypertension, diabetes, coronary artery disease, hypercholesterolemia and medical treatment.
Plaques in the descending aorta were associated with an increased risk of events in univariate analysis (HR 4.35, 95% CI 1.45 to 13.03 for small plaques; HR 4.83, 95% CI 1.49 to 15.70 for large plaques) but not in multivariate analysis (HR 2.50, 95% CI 0.78 to 7.97 for small plaques; HR 1.99, 95% CI 0.52 to 7.69 for large plaques).
Risk of Ischemic Stroke
Overall ischemic stroke incidence was 8.3 per 1000 person-years. Event rates were 7.7, 10.2 and 6.3 per 1000 person-years respectively in subjects with no, small and large arch plaques (p=0.813). In subjects with descending aortic plaques, ischemic stroke incidence was 10.4 per 1000 person-years (5.6, 9.8 and 11.6/1000 person-year in subjects with no, small and large descending aortic plaques, p=0.635).
In Cox regression analysis, neither small nor large aortic arch plaques were associated with ischemic stroke occurrence (adjusted HR 1.29, 95% CI 0.34 to 5.00 for small plaques; HR 0.59, 95% CI 0.10 to 3.39 for large plaques). Similar results were observed for descending aortic plaques (HR 1.30, 95% CI 0.30 to 5.57 for small plaques; HR 1.43, 95% CI 0.27 to 7.48 for large plaques).
Discussion
We report on the risk of incident vascular events associated with atherosclerotic plaques in the thoracic aorta in a population-based cohort of stroke-free subjects. This is the study with the longest follow-up among the few that have investigated this topic. We investigated the presence of plaques in the aortic arch, which has been associated with an increased risk of stroke,1-9 and in the thoracic descending aorta, from which retrograde embolization to the brain has also been hypothesized.19 In our cohort, the presence of aortic arch plaques was not an independent predictor of subsequent vascular events, including stroke. This finding is consistent with another population study in the U.S., the Stroke Prevention: Assessment of Risk in a Community (SPARC), which also found no association between aortic atherosclerosis and cardiac events or stroke.12 However, there are substantial differences between the two studies. Our cohort had high prevalence of aortic arch atherosclerosis (62.2%), nearly two-fold greater than in SPARC (32.3% in the ascending aorta/arch). In SPARC, the prevalence of complex plaques (defined as > 4 mm thick and/or with complex morphology) was only 2.4% in the proximal aorta, compared to 24% in our cohort. Such large differences could be at least partially explained by the different composition of the two cohorts and by exposition to different environmental factors. Unlike the exclusively white study group of SPARC, our study cohort was predominantly Hispanic (56%) and black (30%), and had a higher prevalence of cardiovascular risk factors. Diabetes was more than twice as frequent (23.0% vs. 8.9%), hypertension was more prevalent (69.4% vs. 55.2%), and both past and current smokers were more represented in our cohort (60.3% vs. 39.0% and 16.1% vs. 8.2%, respectively). A history of CAD was more frequent in our population (21.5% vs. 13.7%), and body mass index was higher (27.6 vs. 26.9 kg/m2). Despite the significantly higher prevalence of aortic plaques in our cohort, we could not demonstrate an independent association between thoracic aortic plaques and vascular events. For large plaques in the aortic arch, this observation is in apparent conflict with previous data showing their strong independent association with stroke and peripheral embolic events.3,8,9,20 This discordance, however, may stem from the quite different populations studied. The association between large arch plaques and embolic events was established in case-control studies including patients with recent strokes,3-5 and in prospective TEE studies that selected high-risk patients with prior embolic events,6,9 or other miscellaneous indications for TEE.7,8 The results of the present study, as well as those of SPARC, were obtained in subjects drawn from, and representative of, the general population, in whom aortic plaque detection was merely an incidental finding. It is possible that the previous positive results were driven by high-risk subjects, strongly represented in those studies, but who may represent only a small subset of subjects with aortic plaques in the general population. In that high-risk group, coexisting factors may increase the risk of embolic events in patients with arch plaques, but this combination may be infrequent in the general population. Also, cofactors may exist that increase the plaque embolic risk and that are more frequently observed in the presence of an acute disease. In the APRIS study, patients with acute stroke and large aortic arch plaques showed an activation of coagulation that paralleled the plaque thickness, and the combination of large arch plaques and activated coagulation was associated with an increased risk of recurrent stroke and death.21 However, a similar relationship between large plaque and activation of coagulation was not observed in the control subjects of APRIS, who constitute the study group of the present report. Moreover, in patients with acute ischemic stroke, large aortic arch plaques have been found to be associated with the detection of microembolic signals to the brain by transcranial Doppler.22-24 Other yet unidentified cofactors may increase the risk of embolization from arch plaques and may have contributed to the positive results of previous studies. Plasma fibrinogen, homocysteine and neutrophile count have been found associated to aortic plaque severity and progression, which in turn is a strong predictor of incident vascular events in patients with previous stroke/TIA.25-28 Pro-inflammatory and pro-coagulant states might play a role in both plaque growth and event triggering, although the latter possibility needs further investigation.
Retrograde embolization to the brain from the proximal descending aorta is theoretically possible,19 although probably infrequent. We found that plaques in the proximal descending aorta were associated with an increased risk of combined events in unadjusted analyses (Table 4 and Figure 2), but not after adjustment for other risk factors. This observation suggests that plaques in the descending aorta may act more as a marker of atherosclerosis rather than a direct cause for the events. Descending aortic plaques were more strongly associated with atherosclerotic risk factors than arch plaques (Table 2), further suggesting a more prominent role of descending aortic plaques as indicators of diffuse atherosclerosis. However, given the significantly higher event rate associated with large plaques in the descending aorta, these plaques may help identify a high-risk subgroup that should be targeted for aggressive preventive strategies.
Study Limitations
The main limitation of our study is the relatively small sample size, which may have affected the statistical power to detect a significant risk associated with large plaques. Moreover, we could not compare different race/ethnic groups. Given the small number of complex plaques, we could not evaluate the separate effect of complex plaque morphology, which has been linked to increased embolic risk.1,8,20
Summary
Our study shows that, in a tri-ethnic community-based sample of stroke-free subjects, large plaques in the aortic arch are not associated with an increased risk of vascular events over a 6-years follow-up. Plaque characteristics and cofactors infrequently found in the general population may play a role in explaining the increased risk of events observed in previous studies on different patient populations. The possibility that plaques in the proximal descending aorta may independently contribute to an increased risk in the general population needs further investigation.
Acknowledgments
The authors thank Inna Titova, MPH and Gabrielle Gaspard, MPH for their assistance in data collection.
The study was supported by R01 NS36286 from the National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
Authors have no conflicts of interest to disclose.
Reference List
- 1.Amarenco P, Duyckaerts C, Tzourio C, Henin D, Bousser MG, Hauw JJ. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221–5. doi: 10.1056/NEJM199201233260402. [DOI] [PubMed] [Google Scholar]
- 2.Tunick PA, Perez JL, Kronzon I. Protruding atheromas in the thoracic aorta and systemic embolization. Ann Intern Med. 1991;115:423–7. doi: 10.7326/0003-4819-115-6-423. [DOI] [PubMed] [Google Scholar]
- 3.Amarenco P, Cohen A, Tzourio C, Bertrand B, Hommel M, Besson G, Chauvel C, Touboul PJ, Bousser MG. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474–9. doi: 10.1056/NEJM199412013312202. [DOI] [PubMed] [Google Scholar]
- 4.Jones EF, Kalman JM, Calafiore P, Tonkin AM, Donnan GA. Proximal aortic atheroma. An independent risk factor for cerebral ischemia. Stroke. 1995;26:218–24. [PubMed] [Google Scholar]
- 5.Di Tullio MR, Sacco RL, Gersony D, Nayak H, Weslow RG, Kargman DE, Homma S. Aortic atheromas and acute ischemic stroke: a transesophageal echocardiographic study in an ethnically mixed population. Neurology. 1996;46:1560–6. doi: 10.1212/wnl.46.6.1560. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto S, Yasaka M, Otsubo R, Oe H, Nagatsuka K, Minematsu K. Aortic arch atherosclerotic lesions and the recurrence of ischemic stroke. Stroke. 2004;35:1426–9. doi: 10.1161/01.STR.0000127788.32550.d4. [DOI] [PubMed] [Google Scholar]
- 7.Tunick PA, Rosenzweig BP, Katz ES, Freedberg RS, Perez JL, Kronzon I. High risk for vascular events in patients with protruding aortic atheromas: a prospective study. J Am Coll Cardiol. 1994;23:1085–90. doi: 10.1016/0735-1097(94)90595-9. [DOI] [PubMed] [Google Scholar]
- 8.Mitusch R, Doherty C, Wucherpfennig H, Memmesheimer C, Tepe C, Stierle U, Kessler C, Sheikhzadeh A. Vascular events during follow-up in patients with aortic arch atherosclerosis. Stroke. 1997;28:36–9. doi: 10.1161/01.str.28.1.36. [DOI] [PubMed] [Google Scholar]
- 9.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. The French Study of Aortic Plaques in Stroke Group. N Engl J Med. 1996;334:1216–21. doi: 10.1056/NEJM199605093341902. [DOI] [PubMed] [Google Scholar]
- 10.Demopoulos LA, Tunick PA, Bernstein NE, Perez JL, Kronzon I. Protruding atheromas of the aortic arch in symptomatic patients with carotid artery disease. Am Heart J. 1995;129:40–4. doi: 10.1016/0002-8703(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 11.Fazio GP, Redberg RF, Winslow T, Schiller NB. Transesophageal echocardiographically detected atherosclerotic aortic plaque is a marker for coronary artery disease. J Am Coll Cardiol. 1993;21:144–50. doi: 10.1016/0735-1097(93)90729-k. [DOI] [PubMed] [Google Scholar]
- 12.Meissner I, Khandheria BK, Sheps SG, Schwartz GL, Wiebers DO, Whisnant JP, Covalt JL, Petterson TM, Christianson TJ, Agmon Y. Atherosclerosis of the aorta: risk factor, risk marker, or innocent bystander? A prospective population-based transesophageal echocardiography study. J Am Coll Cardiol. 2004;44:1018–24. doi: 10.1016/j.jacc.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 13.Olson SH, Kelsey JL, Pearson TA, Levin B. Evaluation of random digit dialing as a method of control selection in case-control studies. Am J Epidemiol. 1992;135:210–22. doi: 10.1093/oxfordjournals.aje.a116273. [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Roberts JK, Boden-Albala B, Gu Q, Lin IF, Kargman DE, Berglund L, Hauser WA, Shea S, Paik MC. Race-ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan Stroke Study. Stroke. 1997;28:929–35. doi: 10.1161/01.str.28.5.929. [DOI] [PubMed] [Google Scholar]
- 15.Di Tullio MR, Homma S, Sacco RL. Ultrasound Examination of the Aortic Arch in Stroke. In: Welch KMA, Caplan LR, Reis DJ, Siesjo BK, Weir B, editors. Primer on Cerebrovascular Diseases. Academic Press; 1997. pp. 628–34. [Google Scholar]
- 16.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., III Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Greene HL, Richardson DW, Barker AH, Roden DM, Capone RJ, Echt DS, Friedman LM, Gillespie MJ, Hallstrom AP, Verter J. Classification of deaths after myocardial infarction as arrhythmic or nonarrhythmic (the Cardiac Arrhythmia Pilot Study) Am J Cardiol. 1989;63:1–6. doi: 10.1016/0002-9149(89)91065-5. [DOI] [PubMed] [Google Scholar]
- 18.Morris DL, Kritchevsky SB, Davis CE. Serum carotenoids and coronary heart disease. The Lipid Research Clinics Coronary Primary Prevention Trial and Follow-up Study. JAMA. 1994;272:1439–41. doi: 10.1001/jama.272.18.1439. [DOI] [PubMed] [Google Scholar]
- 19.Tenenbaum A, Motro M, Feinberg MS, Schwammenthal E, Stroh CI, Vered Z, Fisman EZ. Retrograde flow in the thoracic aorta in patients with systemic emboli: a transesophageal echocardiographic evaluation of mobile plaque motion. Chest. 2000;118:1703–8. doi: 10.1378/chest.118.6.1703. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari E, Vidal R, Chevallier T, Baudouy M. Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants. J Am Coll Cardiol. 1999;33:1317–22. doi: 10.1016/s0735-1097(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 21.Di Tullio MR, Homma S, Jin Z, Sacco RL. Aortic atherosclerosis, hypercoagulability, and stroke the APRIS (Aortic Plaque and Risk of Ischemic Stroke) study. J Am Coll Cardiol. 2008;52:855–61. doi: 10.1016/j.jacc.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rundek T, Di Tullio MR, Sciacca RR, Titova IV, Mohr JP, Homma S, Sacco RL. Association between large aortic arch atheromas and high-intensity transient signals in elderly stroke patients. Stroke. 1999;30:2683–6. doi: 10.1161/01.str.30.12.2683. [DOI] [PubMed] [Google Scholar]
- 23.Viguier A, Pavy le TA, Massabuau P, Valton L, Larrue V. Asymptomatic cerebral embolic signals in patients with acute cerebral ischaemia and severe aortic arch atherosclerosis. J Neurol. 2001;248:768–71. doi: 10.1007/s004150170092. [DOI] [PubMed] [Google Scholar]
- 24.Castellanos M, Serena J, Segura T, Perez-Ayuso MJ, Silva Y, Davalos A. Atherosclerotic aortic arch plaques in cryptogenic stroke: a microembolic signal monitoring study. Eur Neurol. 2001;45:145–50. doi: 10.1159/000052113. [DOI] [PubMed] [Google Scholar]
- 25.Tribouilloy C, Peltier M, Colas L, Senni M, Ganry O, Rey JL, Lesbre JP. Fibrinogen is an independent marker for thoracic aortic atherosclerosis. Am J Cardiol. 1998;81:321–6. doi: 10.1016/s0002-9149(97)00900-4. [DOI] [PubMed] [Google Scholar]
- 26.Tribouilloy CM, Peltier M, Iannetta Peltier MC, Trojette F, Andrejak M, Lesbre JP. Plasma homocysteine and severity of thoracic aortic atherosclerosis. Chest. 2000;118:1685–9. doi: 10.1378/chest.118.6.1685. [DOI] [PubMed] [Google Scholar]
- 27.Konecky N, Malinow MR, Tunick PA, Freedberg RS, Rosenzweig BP, Katz ES, Hess DL, Upson B, Leung B, Perez J, Kronzon I. Correlation between plasma homocyst(e)ine and aortic atherosclerosis. Am Heart J. 1997;133:534–40. doi: 10.1016/s0002-8703(97)70148-0. [DOI] [PubMed] [Google Scholar]
- 28.Sen S, Hinderliter A, Sen PK, Simmons J, Beck J, Offenbacher S, Ohman EM, Oppenheimer SM. Aortic arch atheroma progression and recurrent vascular events in patients with stroke or transient ischemic attack. Circulation. 2007;116:928–35. doi: 10.1161/CIRCULATIONAHA.106.671727. [DOI] [PubMed] [Google Scholar]


