Abstract
In Temperate zones day length changes markedly across the year, and in many mammals these photoperiodic variations are associated with physiological adaptations. However, the influence of this environmental variable on human behavior and physiology is less clear, and the potential underlying mechanisms are unknown. To address this issue we examined the effect of changing photoperiods on adrenal gland function in ovariectomized female rhesus macaques (Macaca mulatta), both in terms of steroid hormone output and in terms of gene expression. The animals were sequentially exposed to the following lighting regimens, which were designed to simulate photoperiods associated with winter, spring/autumn and summer, respectively: 8L:16D (short days), 12L:12D and 16L:8D (long days). Remote 24-hour serial blood sampling failed to disclose any effect of photoperiod on mean or peak plasma levels of cortisol or dehydroepiandrosterone sulfate (DHEAS). However, there was a marked phase-advancement of both hormonal rhythms in short days, which was reflected as a similar phase-advancement of the daily motor activity rhythm. Gene microarray analysis of the adrenal gland transcriptome revealed photoperiod-induced differences in the expression of genes associated with homeostatic functions, including: development, lipid synthesis and metabolism, and immune function. Taken together the results indicate that in primates, both circadian adrenal physiology and gene expression are influenced by seasonal changes in day length, which may have implications for adrenal-regulated physiology and behavior.
Keywords: adrenal cortex, circadian rhythms, corticosteroids, gene expression, photoperiod
Introduction
In mammals, many aspects of physiology and behavior are temporally regulated, showing circadian as well as circannual rhythms. Whereas circadian rhythms reflect the daily organization of body functions, circannual rhythms represent an adaptation to the seasonal variations that occur in a natural environment throughout the year. It is well established that circadian rhythms are intrinsic to a wide range of body functions, including the sleep-wake cycle, metabolism, immune response and reproduction (Hastings et al. 2007). On the other hand, circannual rhythms have been reported in metabolism, reproduction, and immune function in a number of mammalian species (Bilbo et al. 2002, Nakao et al. 2008). In humans, seasonal variations have been reported for blood pressure, immune response, birth rate and sleep duration, as well as for behavioral traits associated with seasonal affective disorders, bulimia nervosa, anorexia and suicide (Bronson 2004).
Although the underlying mechanism that regulates circannual rhythms is unclear, there is evidence that seasonal neuroendocrine changes are among the main causal agents. In particular, circannual oscillations in one of the major neuroendocrine structures, the hypothalamus-pituitary-adrenal (HPA) axis, has been regarded as a potential mediator of seasonal changes, a view supported by the major role played by adrenal steroids in physiology and behavior. This hypothesis stems from the fact that the HPA axis is timely regulated by the circadian pacemaker located in the suprachiasmatic nuclei (SCN) of the hypothalamus, which drives the rhythmic secretion of two major adrenal steroids, cortisol and dehydroepiandrosterone sulfate (DHEAS) (Hastings 1991, Urbanski et al. 2004, Downs et al. 2008). Furthermore, light itself exerts a remarkable effect on adrenal physiology; in mice, light exposure at night induces both gene expression and the secretion of corticosterone, through a pathway that involves the SCN and the sympathetic nervous system (Ishida et al. 2005).
Although circannual variations have also been reported for adrenal corticoids, the available data are largely inconclusive. In the case of cortisol, some human studies have reported seasonal differences (Van Cauter et al. 1981, Levine et al. 1994, Walker et al. 1997, King et al. 2000, Hansen et al. 2001), whereas others have failed to disclose such variations (Agrimonti et al. 1982, Wehr et al. 1993, Van Dongen et al.1998, Lac & Chamoux 2006). Similarly, some studies have reported seasonal differences in DHEAS levels (Deslypere et al. 1983, Nicolau et al. 1984, Garde et al. 2000), whereas one study found no seasonal difference (Bjornerem et al. 2006).
In the present study we used the rhesus macaque animal model to examine if circannual changes in day length can significantly influence adrenal gland function. Specifically, our first aim was to examine the effect of different photoperiods on plasma corticosteroid rhythms, both in terms of magnitude and in relation to motor activity rhythms. Our second aim was to explore potential photoperiod-induced gene expression changes within the adrenal gland using gene microarray. The results show that day length affects specific parameters of the 24-hour plasma cortisol and DHEAS rhythms as well as the daily activity-rest cycle. Moreover, seasonal-like changes in day length influence the expression of genes involved in development, lipid synthesis and metabolism, and immune response.
Materials and methods
Animals
To avoid the confounding influence of changing sex-steroid concentrations across the menstrual cycle and across different photoperiods, the study used three long-term (>3 months) ovariectomized adult female rhesus macaques (Macaca mulatta; age range: 8.5 - 12 years old). Each animal was fitted with an indwelling sub-clavian vein catheter, as previously described (Downs et al. 2008), which remained implanted for the duration of the study. The animals were caged singly in a temperature-controlled environment, with fixed light cycles that comprised 12 hours of light per day (i.e., 12L:12D), and were cared for by the ONPRC Division of Animal Resources, in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
As part of a longitudinal experimental design, the animals were then sequentially exposed to the following three photoperiods: 8L:16D; 12L:12D and 16L:8D, for 10 weeks in each case. These three photoperiods were selected because they represent the winter, spring equinox, and summer, respectively in Oregon. Note, in order to further facilitate the comparison of phases between the photoperiods, the time of lights on was fixed at 0700 h for each photoperiod. Primate chow (Purina Mills Inc., St. Louis, MO, USA) was made available to the animals twice daily, at 0800 h and again at 1500 h. This diet was supplemented with fresh fruit and vegetables, which were provided at the time of the afternoon meal; drinking water was available at all times.
For the gene microarray analysis, nine ovariectomized adult (age range: 8.5 - 12 years old) female rhesus macaques were used. The animals (three per light regimen) were maintained under the photoperiods 12L:12D, 8L:16D and 16L:8D for 10 weeks. At the end of that period, the animals were anesthetized with ketamine (15-25 mg/kg i.m.) followed by pentobarbital sodium (25-30 mg/kg i.v.) and exsanguinated. This method of euthanasia is consistent with the recommendations of the American Veterinary Medical Association's Panel on Euthanasia. In all cases, necropsies were performed within a narrow window of time (1000 h – 1300 h). Various postmortem tissues were collected and made available to other investigators through the Oregon National Primate research Center (ONPRC) Tissue Distribution Program. This research was approved by the Institutional Animal Care and Use Committee.
Activity recording and analysis
The activity-rest cycles of individual animals were continuously monitored using Actiwatch activity recorders (Mini Mitter Company Inc., Bend, OR, USA). Measure of gross motor activity by the accelerometers was digitally integrated into activity bouts of 5-min duration. Analysis of the activity bouts was performed using Sleepwatch software (Mini Mitter Company Inc.). Total daily activity was estimated by calculating the average number of bouts per day, per animal, over time. Total photophase and scotophase activity were calculated by measuring the average number of bouts per animal, during photophase and scotophase, respectively. Cosine correlation was used to estimate the phase of motor activity rhythm in each photoperiod.
Remote 24-h blood sampling
After ∼10 weeks of exposure to each of the three photoperiods, blood samples were collected remotely from an adjacent room, via the indwelling vascular catheter and a swivel/tether-based sampling system (Downs et al., 2008). Beginning at 0700 h, hourly blood samples were collected from the undisturbed animals for an entire day, including their sleep period. The blood was immediately transferred into EDTA-coated borosilicate glass tubes, centrifuged at 4 °C, and the plasma supernatant was stored at −20 °C until assay.
Hormone assays
Plasma concentrations of cortisol and DHEAS were determined at the ONPRC Endocrine Services Laboratory as previously described (Downs et al. 2008). Briefly, cortisol levels were measured by electrochemiluminescence using an Elecsys 2010 Platform (Roche Diagnostics, Indianapolis, IN, USA). DHEAS levels were determined by radioimmunoassay (RIA), using an antibody raised against DHEAS-17-(O-carboxymethyl)oxime-BSA, and [3H]DHEAS (22 Ci/mmol). Intra-assay and inter-assay coefficients of variation were less than 10% for each assay. Assay detection limits were 3 ng/ml, for both of the steroids.
Terminology and Statistical analysis
The terms photophase, and scotophase refer to the illuminated and the dark segment of a light-dark cycle respectively. Rhythmic parameters, including amplitude and mesor were determined by Cosinor analysis. The acrophase measures the timing of a rhythm relative to a reference time point defined by the investigator, and is used for data which fits a mathematical model; for example, in a cosine curve fit the acrophase represents the rhythm's peak. The mesor represents the adjusted mean of a cosine function fitted to the data. Statistical comparisons for motor activity, as well as hormone measurements, were performed using repeated measures ANOVA followed by the Bonferroni test.
RNA extraction and Gene Microarray
During necropsy, the adrenal glands were quickly frozen in liquid N2 and total RNA was extracted using RNeasy columns (Qiagen, Valencia, CA, USA); the final concentration and purity were determined by spectrophotometry, and the integrity was assessed using an Agilent 2100 Bioanalyzer (Agilent Technology, Palo Alto, CA, USA). Complementary DNA synthesis, cRNA synthesis, hybridization and array scanning were performed by the OHSU Affymetrix Microarray Core, as described in the Affymetrix GeneChip Expression Analysis Technical Manual. As in previous studies (Dillman & Phillips 2005, Wang et al. 2004), the Affymmetrix human HG_U133A gene microarray platform was used. RNA samples from individual adrenal glands were hybridized separately on their own GeneChip arrays.
Raw scanner image files were analyzed using the Affymetrix MAS 5.0 absolute expression analysis comprised in the GeneSifter software (Geospiza Inc, Seattle, WA, USA). The parameters α1 and α2, which set the point at which a probe set is called present (P), marginal (M), or undetectable (A) were set to 0.1 and 0.15 respectively. Comparisons were performed using global scaling, with the target intensity value set to an average intensity of 325; this allowed for the direct comparison of hybridization values from the different arrays. Pair-wise statistical comparisons between photoperiods were made using Student's t-test, with photoperiod 12L:12D as the control group.
Taqman quantitative reverse-transcription PCR
Random-primed reverse transcription was performed to prepare the cDNA samples, using 200 ng of RNA and the Omniscript kit (Qiagen). The reverse transcription reaction was diluted 1:100 for PCR analysis. The PCR reaction mixtures contained 5 μl of Taqman Universal PCR Master Mix, 300 nM specific target gene primers, 50 nM human β-actin primers, 250 nM specific probes and 2 μl cDNA, as previously described (Lemos et al. 2006). Standard curves were used to convert the critical threshold values into relative RNA concentrations for each sample, and the results were expressed relative to β-actin, a housekeeping gene. The primers and probes were designed based on human sequences, using PrimerExpress software (Applied Biosystems, Foster City, CA, USA), and purchased from Invitrogen and Sigma Genosys (St Louis, MO, USA) respectively.
The sequences were: NCKAP1-F, CATTGGCACAAGAAGCACTTAGAG; NCKAP1-R, GGTAATCCG GCAGCTGATGA, NCKAP1 probe, 5′-6FAM-CCACATTCCTTTTCTTGTAAGTTCAA-TAMRA-3′; FADS1-F, CCCCTGGTGCAGTCCTTGT; FADS1-R, GGCAGGGTGGCTGTTGTTAT; FADS1 probe, 5′-6FAM-TCCAAGCCCCTGCTGTCAGC-TAMRA-3′; IL11-F, GGCTGCACCTGACACTTGACT; IL11-R, TGTTTCGCCCCCAGTACTG; IL11- probe, 5′-6FAM-TGCTGCTGAAGACTCGGCTGTGA CC-TAMRA-3′; ACSL1-F, CAAGCCTCCAGCACCTGAAG; ACSL1-R, TGGGCAAGGATTGACTG TATTCT, ACSL1 probe, 5′-6FAM-CAAAAGCTGAACAATCGCTCACT-TAMRA-3′; β-actin-F, CGTGGACATCCGCAAAGAC; β-actin-R, GGGCGGTGATCTCCTTCTG; β-actin probe, 5′-VIC-TGCTGTCTGGCGGCACCACC-TAMRA-3′
Results
Effect of photoperiod on plasma cortisol and DHEAS rhythms in the rhesus macaque
Mean 24-hour plasma cortisol rhythms, obtained under each of the three photoperiods, are depicted in Fig. 1A. No significant differences were detected in either the mesor or amplitude (Table 1). The acrophases under both the 12L:12D and the 16L:8D photoperiods were attained around the time when lights came on (i.e., at 0700 h), but a significant phase advancement was disclosed under 8L:16D (P<0.05 vs. 12L:12D; P<0.01 vs. 16L:8D); notably, plasma cortisol levels reached a maximum while the animals were still in the dark phase of their daily photoperiodic cycle (Fig. 1A, Table 1). Although the ascending phase of the 24-hour plasma cortisol rhythm appears to be shorter in 16L:8D, this change was not statistically significant (Table 1). Mean 24-hour plasma DHEAS rhythms, obtained under each of the three photoperiods, are depicted in Fig. 1B. No significant differences were detected in either the mesor or amplitude, although both parameters showed a tendency to decrease under the 16L:8D photoperiod (Table 2). Under both the 12L:12D and the 16L:8D photoperiods the acrophase of the DHEAS rhythm occurred approximately 3-4 hours after the lights came on in the morning; in contrast, under 8L:16D the acrophase occurred significantly earlier (P<0.05 vs. 12L:12D and 16L:8D), within an hour of the beginning of the light phase (Fig. 1B, Table 2). As with cortisol, the ascending phase of the 24-hour plasma DHEAS rhythm appeared to be shorter in 16L:8D, but this change was not statistically significant (Table 2).
Figure 1.
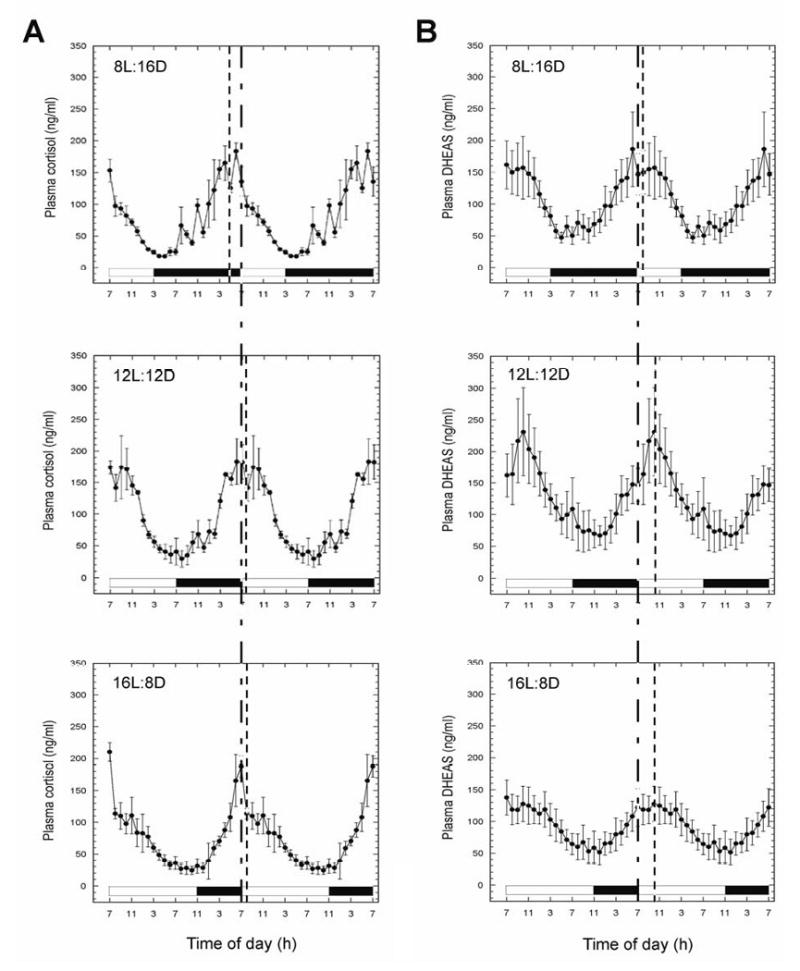
Effect of photoperiod on the 24-hour circulating (A) cortisol and (B) DHEAS rhythms in female rhesus macaques. Plasma samples were collected from the same animals after 10 weeks of exposure to each of the three photoperiods. Values are expressed as means ± SEM (N = 3). Note, the data are double plotted to aid visualization of circadian changes. Vertical dashed lines within each panel indicate the acrophases. The Vertical dashed line across all panels, indicate the beginning of the light phase. The horizontal white and black bars represent day and night, respectively.
TABLE 1.
Effect of photoperiod on plasma Cortisol in rhesus macaques.
| Photoperiod | Mesor (mean ±SEM) | Amplitude (mean ± SEM) | Acrophase (h, min.) | Rising period (h) |
|---|---|---|---|---|
| 8L:16D | 79.4 ± 3.9 | 64.9 ± 10.5 | 04:48 ± 00:13 | 11:00 ± 01:09 |
| 12L:12D | 96.9 ± 8.9 | 73.5 ± 5.1 | 07:24 ± 00:12* | 11:18 ± 01:18 |
| 16L:8D | 76.1 ± 11.9 | 61.0 ± 5.9 | 07:57 ± 00:14** | 09:18 ± 01:50 |
Statistical comparisons were made by repeated measures ANOVA, followed by Bonferroni test
P<0.05, vs. 8L:16D
P<0.01, vs. 8L:16D.
No significant differences were found for mesor, amplitude or rising period (P>0.05).
TABLE 2.
Effect of photoperiod on plasma DHEAS in rhesus macaques.
| Photoperiod | Mesor (mean ± SEM) | Amplitude (mean ± SEM) | Acrophase (h, min.) | Rising period (h) |
|---|---|---|---|---|
| 8L:16D | 105.4 ± 24.0 | 55.8 ± 5.2 | 07:44 ± 00:20 | 10:18 ± 01:12 |
| 12L:12D | 125.6 ± 32.8 | 63.7 ± 12.4 | 10:12 ± 00:12* | 10:00 ± 00:34 |
| 16L:8D | 91.2 ± 21.8 | 36.6 ± 5.0 | 10:11 ± 00:04* | 08:40 ± 01:40 |
Statistical comparisons were made by repeated measures ANOVA, followed by Bonferroni test
P<0.05, vs. 8L:16D.
No significant differences were found for mesor, amplitude or rising period (P>0.05).
Effect of photoperiod on motor activity
Activity-rest profiles from a representative animal, obtained under each of the three photoperiodic regimens, are depicted in Fig. 2. One-way ANOVA analysis of total activity spectra from the three animals showed that there was no significant difference in average total activity, or average light-phase activity between the different photoperiods (Fig. 2B). Interestingly, the hourly activity during the light phase, although not significantly different, tended to be higher under 8L:16D than 12L:12D or 16L:8D (data not shown). On the other hand, average dark-phase activity was clearly higher under 8L:16D than 12L:12D or 16L:8D (P<0.05 vs. 12L:12D; P<0.01 vs. 16L:8D) (Fig. 2B). In addition, exposure to the 8L:16D photoperiod was associated with earlier activity onset (Fig. 2A), which occurred during the dark phase, while the lights were still off, as well as a significant advancement of the activity acrophase, compared to the 16L:8D photoperiod (P<0.05, Fig. 2B).
Figure 2.
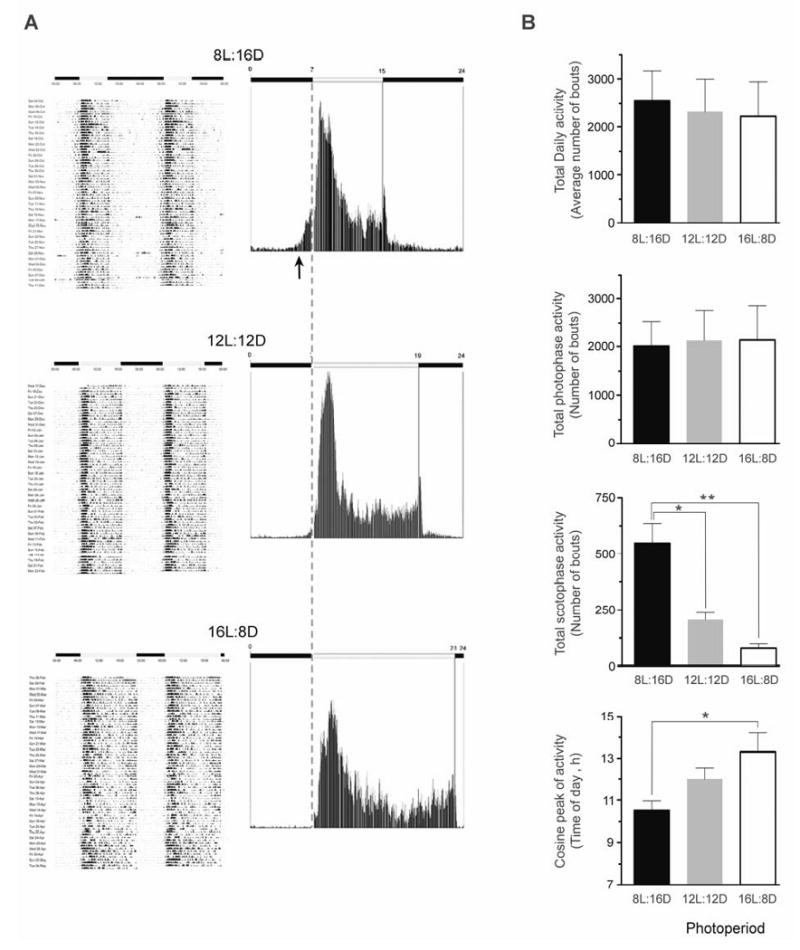
Effect of photoperiod on motor activity in ovariectomized female rhesus macaques. (A) Left panels: Representative actograms from an individual animal that was exposed sequentially for 10 weeks to 8L:16D, 12L:12D and 16L:8D lighting regimens. Note, the activity data are double plotted to aid visualization of circadian changes. Right panels: Representative mean activity during the 10-week periods. The arrow indicates the advancement of activity onset, and the vertical dashed line indicates the beginning of the diurnal phase. The horizontal white and black bars indicate day and night, respectively. (B) Cosinor analysis was used to assess motor activity variables over each 10-week period. Comparisons were made using repeated-measures ANOVA followed by Bonferroni test. Values represent the means ± SEM from all three animals. *P<0.05, **P<0.01.
Effect of photoperiod on adrenal gland gene expression
Rhesus macaque Gene microarrays (Affymetrix, Santa Clara, CA, USA) were used to study the effect of photoperiod on the adrenal transcriptome. Gene expression profiles were obtained from long-term ovariectomized animals that had been maintained for 10 weeks in either 8L:16D, 12L:12D or 16L:8D photoperiods, with three animals per group. Comparisons were made between 12L:12D vs. 8L:16D, and 12L:12D vs. 16L:8D, with fold changes higher than 1.8 or lower than -1.8 being considered significant. A gene annotation tool was used to obtain gene abbreviations and descriptions. The data analysis revealed three main functional clusters: 1) development, 2) lipid synthesis and metabolism, and 3) immune function.
Genes identified as being differentially expressed after exposure to short photoperiods
Genes that were differentially regulated in 8L:16D compared to 12L:12D are depicted in Fig.3. The set of genes associated with development included genes that play functional roles in morphogenesis (HOXD11, ALX3, WNT2), cytoskeletal regulation (NCKAP1), axonal growth (SEMA6B, ROBO1) and neural cell development (NPTX1). The set of genes associated with lipid synthesis and metabolism included genes that play functional roles in activation of long-chain fatty acids (ACSL1) and in the synthesis of glycosylphosphatidylinositol anchor in the endoplasmic reticulum (PIGO). Finally, the set of genes associated with immune response included genes that play functional roles in acute inflammatory response (CSF1), recognition of antigen particles bound to major histocompatibility complex (TRAα), interferon-inducible response to double stranded RNA (PRKR), early lymphoid development (CD7 antigen) and proliferation of hematopoietic stem cells (IL11). To validate the GeneChip analysis, we used quantitative real-time polymerase chain reaction (qRT-PCR) to corroborate differences in the expression of three selected genes (NCKAP1, IL-11 and ACSL1) between the short (8L:16D) and medium (12L:12D) photoperiods (Fig. 5).
Figure 3.
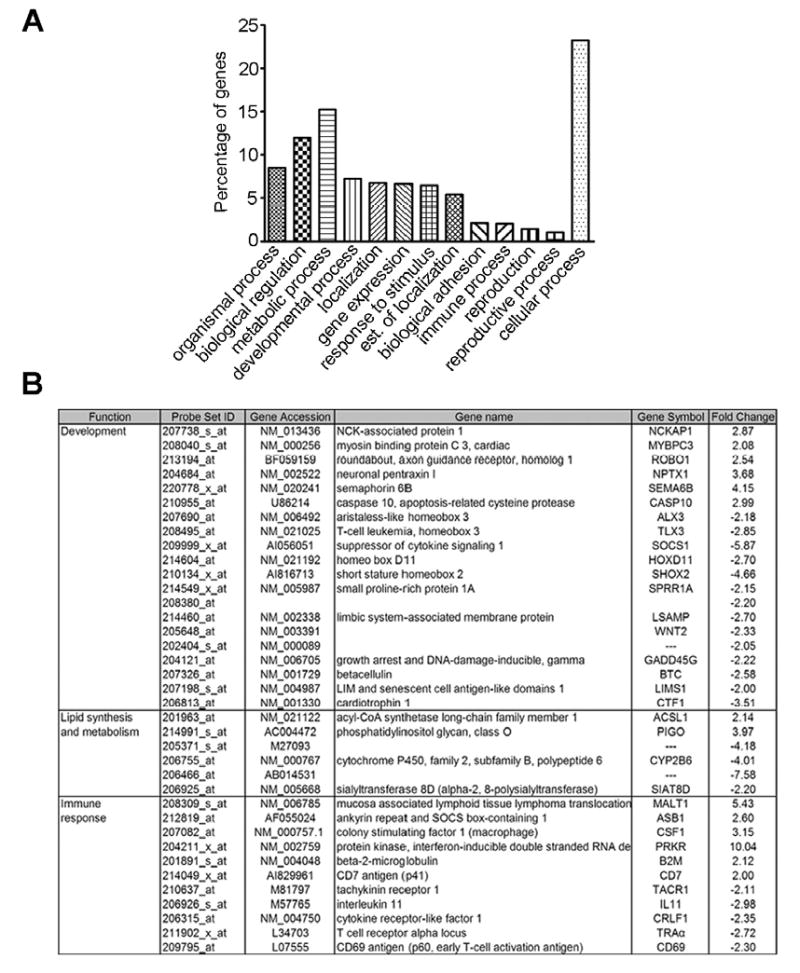
Effect of short days on adrenal gland gene expression. The gene microarrays were analyzed using the algorithm MAS 5.0. (A) Histogram depicting functional clustering of genes differentially regulated after exposure to short photoperiod. (B) Table of genes involved in development, lipid synthesis and metabolism and immune response, differentially expressed in 8L:16D vs. 12L:12D (P<0.05). Statistical comparisons were made by Students t-test.
Figure 5.
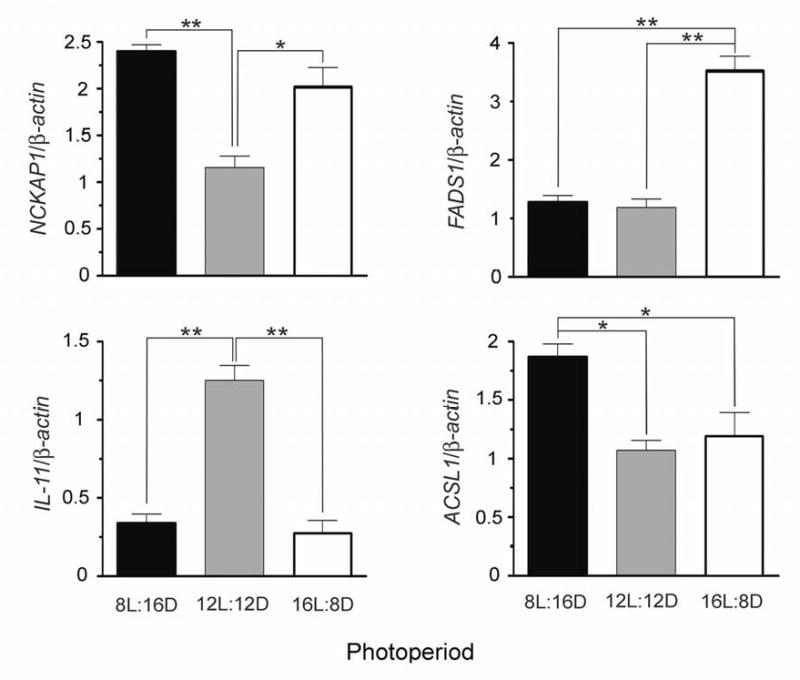
Expression levels for NCKAP1, FADS1, IL-11 and ACSL1, determined by Taqman qRT-PCR Values are expressed as means ± SEM (N = 3). Statistical comparisons were made by one way ANOVA, followed by Bonferroni test *P<0.05, **P<0.01.
Genes identified as being differentially expressed after exposure to long photoperiods
Genes that were differentially regulated in 16L:8D compared to 12L:12D are depicted in Fig. 4. The set of genes associated with development included genes that play functional roles in nervous tissue differentiation (BMP7, DSP, WNT2), cell proliferation (MAC30) and cell-cell contact (PCDHB13). Interestingly, some of the developmental genes that showed differential regulation by short days were also regulated by long days (i.e., NCKAP1, MYBPC3, ROBO1, NPTX1, SEMA6, CASP10, SOCS1, HOXD11, CTF1 and LIMS1). The set of genes associated with lipid synthesis and metabolism included several genes that encode enzymes involved in lipid synthesis, modification and degradation (FADS1, CDS2; UGCG, PLCB3). The set of genes associated with immune response included genes that encode chemokine ligands (XCL2, CXCL14), and genes that are involved in peripheral inflammation (TACR1), virus response (IRF4, IFNA10) and early activation of T cells (CD69), among others. Differences in the expression of NCKAP1, IL-11 and FADS1 between the long (16L:8D) and medium (12L:12D) photoperiods were corroborated using (qRT-PCR) (Fig. 5).
Figure 4.
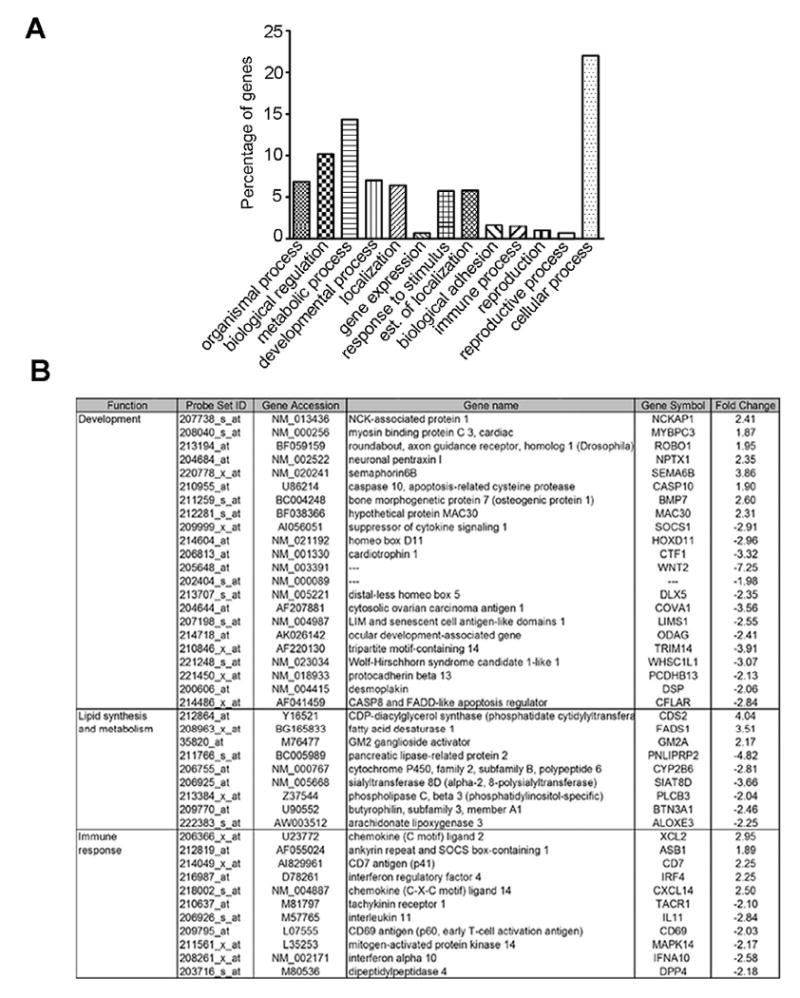
Effect of long days on adrenal gland gene expression. The gene microarrays were analyzed using the algorithm MAS 5.0. (A) Histogram depicting functional clustering of genes differentially regulated after exposure to short photoperiod. (B) Table of genes involved in development, lipid synthesis and metabolism and immune response, differentially expressed in 16L:8D vs. 12L:12D (P<0.05). Statistical comparisons were made by Students t-test.
Discussion
Day/night cycles can exert a profound effect on physiology and behavior, as well as gene expression (Hastings et al. 2003). On the other hand, it is unclear how seasonal changes in environmental conditions can influence these parameters, especially in humans and nonhuman primates. To address this issue, we examined the impact of photoperiodic manipulation on circadian functions and adrenal gene expression in a diurnal nonhuman primate, the rhesus macaque.
In their native habitat, in Northern India and China, these monkeys restrict their breeding activity to the autumn and winter. This biological adaptation ensures that their offspring are born when environmental conditions are more favorable for survival, in the late spring and summer. The well-defined breeding season of rhesus macaques is especially evident at more extreme latitudes (e.g., Oregon at ∼45° North), where the most likely proximate factor (Baker 1938) synchronizing it to the environment is the annual change in photoperiod (Urbanski 1995). Though less obvious, a seasonal reproductive rhythm also occurs in humans, with presumed conception rates peaking around the time of the vernal equinox (Bronson 1995, 2004; Roenneberg & Aschoff 1990a, 1990b). Consequently, both rhesus macaques and humans may show some seasonal fluctuation in circulating sex-steroid concentrations, which would be partially masked by the marked concentrations changes that occur across the menstrual cycle. In female rhesus macaques and women the menstrual cycle is remarkably similar, with a peak of circulating estradiol concentrations occurring during the late follicular phase and a peak of progesterone concentrations occurring during the mid-luteal phase (Urbanski 1995; Downs & Urbanski 2006). It was unclear to us whether seasonal and monthly fluctuating sex-steroid concentrations could affect adrenal gland function (Fonseca et al. 2001; Stavisky et al. 2003), and so to avoid this potentially confounding issue we performed our study using long-term ovariectomized rhesus macaques, exclusively.
The animals were chronically maintained under each of the three photoperiods, which were selected because they resemble the natural photoperiods that occur in Temperate zones during the winter, spring/autumn and summer. Also, the use of a moderately long 16L:8D photoperiod to mimic summer was expected to induce a larger effect on clock-mediated gene expression, than the use of a very long photoperiod (>20 hours of light per day) (Wagner et al. 2007). Although natural photoperiods increase and decrease gradually across the seasons, we chose to use more abrupt photoperiodic transitions. This ensured that the animals had a full 10 weeks to stabilize their physiological rhythms to each new photoperiod. Equally important, by limiting the exposure to only 10 weeks, we reduced the chances of photorefractoriness developing. This condition is characterized by a spontaneous reversion in physiology to that of the previous photoperiodic condition, and has been observed in other species, such sheep, when they are exposed to constant photoperiods for extended periods (Lincoln et al. 2005). At the end of each photoperiodic treatment the serial collection of blood samples, at multiple time points across the day and night, enabled changes in plasma hormone rhythms to be determination more precisely.
The results indicate that exposing the animals longitudinally to the different photoperiods had no obvious impact on either plasma cortisol mean levels or amplitude. On the other hand, these photoperiodic manipulations did affect some circadian aspects of the hormonal rhythm, especially the acrophase and rising kinetics. Also, the nocturnal periods of rising cortisol levels changed in response to duration of the scotoperiod. These findings are in agreement with the clinical results of Wehr et al., which showed adaptive changes in the rhythm of cortisol when human subjects were chronically maintained under controlled experimental conditions (Wehr et al. 1993). They also support the idea that the rhythm-generating component of the cortisol secretion mechanism undergoes adjustments in response to length of day, or duration of scotophase. In the present study, a significant phase-advancement of approximately 2.5 hours was evident for the cortisol rhythm under short photoperiods, reaching the acrophase in the dark phase.
In addition, we observed that the phase of the motor activity rhythm adjusts to photoperiodic changes. An advance in both the onset and phase of the activity rhythm were observed under short days, resulting in higher levels of activity during the scotophase. Since the phase-advancement in cortisol rhythm parallels that of the activity rhythm a causal relationship may exist between the two rhythms. It could be hypothesized that, in view of its physiological functions, an earlier peak of cortisol helps an individual to achieve a state of arousal earlier in the day, thus facilitating an earlier awakening relative to dawn. In natural environments, short photoperiods are generally associated with the onset of unfavorably low temperatures and a scarce food supply. In this context, the advancement of the activity rhythm and other physiological functions in the winter would help to optimize the use of a shorter light phase. Another way of interpreting these data is that the animals centralize their daily activity around the middle of the day, regardless of photoperiod. This means that in short winter photoperiods their daily activity onset occurs several hours before dawn. Note that in our study we kept dawn fixed for each of the photoperiods, at 700 h. Thus, under 8L:16D the animals woke up at ∼500 h, 6 hours before the middle of the day (i.e. at 1100 h). Similarly, under 12L:12D they woke up at ∼700 h, again 6 hours before the middle of the day (i.e., at 1300 h). Under 16L:8D, however, they woke up at ∼700 h, which was 8 hours before the middle of the day (i.e., at 1500 h). When viewed from this alternative perspective (i.e., relative to mid-day rather than dawn), our monkey activity data are in complete agreement with those of Honma et al. (1992), which showed that humans wake-up earlier in summer than in winter.
Also in agreement with previous human studies (Wehr et al. 1993), we did not observe changes in daily average plasma cortisol levels or amplitude, in response to different day lengths. Consequently, if such variations do occur seasonally, they are unlikely to be triggered by changes in the photoperiod per se. A potential effect of other external factors such as temperature, diet or social habits, which show seasonal variations in increased latitudes, cannot be excluded.
Analysis of the DHEAS rhythm did not reveal an effect of day length on either plasma DHEAS mean levels or amplitude, although both values tended to decrease slightly during exposure to a long photoperiod; more conclusive analysis of these parameters would necessitate a larger number of animals, to reduce the impact of high variability between individuals. Nevertheless, the lack of an obvious photoperiodic impact on the magnitude of plasma DHEAS levels contrasts with the marked attenuation of circulating DHEAS concentrations that has been observed during aging (Perrer & Aujard 2005, Downs et al, 2008). Similar to cortisol, a significant phase advancement of approximately 2.5 hours was observed for DHEAS under short photoperiods. Considering the reported effects of DHEAS within the central nervous system, this circadian alteration may have important behavioral consequences. Although no receptor has been reported for DHEAS to date, this steroid acts as an excitatory neuromodulator with proconvulsant activity (Carete & Poulain 1984, Demirgoren et al. 1991). It has been reported that DHEAS acts both as a negative allosteric modulator on GABAA receptors and as a positive modulator on glutamate NMDA receptor activity. In support of this view is the finding that DHEAS increases the excitability of CA3 neurons in the hippocampus (Bergeron et al. 1996), suggesting that DHEAS helps to promote a general arousal state. In agreement with the phase-shifts observed for the plasma cortisol and activity rhythms, the phase-advancement in DHEAS rhythm may be interpreted as another component in the adaptation to short winter days, allowing for an earlier increase in the alert/arousal state.
The gene microarray and real-time PCR data demonstrate that photoperiod can influence gene expression in the primate adrenal gland, upregulating the expression of certain genes while downregulating the expression of others. The microarray analysis focused on changes in gene expression that are likely to occur during the annual cycle, especially after the transition from a winter photoperiod to a spring/autumn photoperiod, and from the latter to a summer photoperiod. The three main sets of genes identified (i.e., development, lipid synthesis and metabolism, and immune response) suggest that the adrenal gland undergoes both structural and functional changes as an adaptive response to long-term exposure to both short and long photoperiods. This hypothesis is supported by our observation that homeobox regulators are differentially expressed in both environmental conditions.
The adrenal gland is a dynamic organ that undergoes structural changes as part of its adaptive role in stress response. Ultrastructural changes in this gland have been reported in response to environmental stressors such as noise (Soldani et al. 1999) and heat (Koko et al. 2004). More importantly, from the perspective of the present study, the effect of light on gene expression in the adrenal gland has previously been examined in rodents. For example, a brief light stimulus (400 lux, 30 min) was shown to affect gene expression in the adrenal glands of mice kept under constant darkness, through a pathway that involves the SCN and the autonomic nervous system (Ishida et al. 2005). As demonstrated by Kalsbeek et al., signals from the SCN are transmitted to peripheral organs, included the adrenal glands, through a pathway that involves vasopressin secretion activating preautonomic neurons of the paraventricular nucleus, and autonomic neurons from the intermediolateral column (Kalsbeek et al. 2007). Furthermore, exposure of male rats to either constant light or constant darkness was found to upregulate the expression of genes that encode catecholamine biosynthetic enzymes in the adrenal gland (Gallara et al. 2004). In keeping with these previous reports, our results demonstrate that some genes can be activated by exposure to extreme photoperiods (i.e., either short days or long days), and suggest that common pathways may be involved. In the natural environment, photoperiodic extremes are separated by the vernal and autumnal equinoxes (i.e., 12L:12D), which could allow resetting of these genes to a more basal level twice per year.
The gene expression data also suggest that the primate adrenal gland may be capable of responding to changes in photoperiod by undergoing tissue remodeling. These structural changes do not seem to affect the endocrine functions of the adrenal cortex; instead, they likely constitute cellular adaptations that allow the tissue to maintain normal function under environmental conditions associated with long and short photoperiods (e.g., high and low temperatures, respectively, and changing food availability). A potential candidate that could underlie the observed changes in gene expression is the pineal hormone melatonin. In mammals, melatonin secretion conveys photoperiodic information to the rest of the body, in part by modulating the secretion of other hormones. In addition, melatonin receptor activation regulates gene expression through a pathway that involves protein kinase C and the extracellular signal-regulated kinases 1 and 2 (Sainz et al. 1999, Roy & Belsham 2002). Expression of MT1 melatonin receptor has been reported in the primate adrenal gland, and it has been suggested that melatonin may play a role in regulating adrenal clock-gene expression (Valenzuela et al. 2008). Therefore, although the autonomic nervous system is known to regulate adrenal gland function, it is plausible that a melatonin-mediated pathway plays a key role in the seasonal modulation of adrenal gland gene expression.
In summary, the present study represents the first multi-level analysis of photoperiod-induced responses in a nonhuman primate, involving 24-hour endocrine profiling, whole animal behavioral observations, as well as gene expression analysis. Although the responses of rhesus macaques to photoperiodic manipulations may be more pronounced than those of humans, the similar organization and endocrine function of the rhesus and human adrenal glands (Conley et al. 2004; Abbott & Bird 2008), emphasize the translational importance of our data. Together they provide a new perspective on the effects of seasonal variations in photoperiod on primate behavior, physiology and gene expression, and may have clinical value in the development of therapies for seasonal human pathophysiology, such as seasonal affective disorders (Wirz-Justice et al. 1984).
Acknowledgments
We wish to thank Dr. Roberto Refinetti for technical advice and for providing the software used for Cosinor analysis (available at http://www.circadian.org/softwar.html). Thanks are also due to Danny Stockdale for editorial assistance with the manuscript.
Funding: This work was supported by a grant from the Collins Medical Trust and the following National Institutes of Health grants: AG023477, AG026472, AG029612, HD029186 and RR00163.
Footnotes
The authors declare that there was no conflict of interest that would prejudice the impartiality of the research reported.
References
- Abbott DH, Bird IM. Nonhuman primates as models for human adrenal androgen production: function and dysfunction. Reviews in Endocrine and Metabolic Disorders. 2008 doi: 10.1007/s11154-008-9099-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrimonti F, Angeli A, Frairia R, Fazzari A, Tamagnone C, Fornaro D, Ceresa F. Circannual rhythmicities of cortisol levels in the peripheral plasma of healthy subjects. Chronobiologia. 1982;9:107–114. [PubMed] [Google Scholar]
- Baker JR. The evolution of breeding season. In: de Beer GR, editor. Evolution (Essays presented to E.S. Goodrich. Oxford University Press; London: 1938. pp. 161–177. [Google Scholar]
- Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. Journal of Neuroscience. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, Nelson RJ. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proceedings of the National Academy of Science. 2002;99:4067–4072. doi: 10.1073/pnas.062001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornerem A, Straume B, Oian P, Berntsen GK. Seasonal variation of estradiol, follicle stimulating hormone, and dehydroepiandrosterone sulfate in women and men. Journal of Clinical Endocrinolology and Metabolism. 2006;91:3798–3802. doi: 10.1210/jc.2006-0866. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Seasonal variation in human reproduction: environmental factors. The Quarterly Review of Biology. 1995;70:141–164. doi: 10.1086/418980. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Are humans seasonally photoperiodic. Journal of Biological Rhythms. 2004;19:180–192. doi: 10.1177/0748730404264658. [DOI] [PubMed] [Google Scholar]
- Carette B, Poulain P. Excitatory effect of dehydroepiandrosterone, its sulphate ester and pregnenolone sulphate, applied by iontophoresis and pressure, on single neurones in the septo-preoptic area of the guinea pig. Neuroscience Letters. 1984;45:205–210. doi: 10.1016/0304-3940(84)90100-9. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Seminars in Reproductive Medicine. 2004;22:311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- Demirgoren S, Majewska MD, Spivak CE, London ED. Receptor binding and electrophysiological effects of dehydroepiandrosterone sulfate, an antagonist of the GABAA receptor. Neuroscience. 1991;45:127–135. doi: 10.1016/0306-4522(91)90109-2. [DOI] [PubMed] [Google Scholar]
- Deslypere JP, de Biscop G, Vermeulen A. Seasonal variation of plasma dehydroepiandrosterone sulphate and urinary androgen excretion in post-menopausal women. Clinical Endocrinology. 1983;18:25–30. doi: 10.1111/j.1365-2265.1983.tb03182.x. [DOI] [PubMed] [Google Scholar]
- Dillman JF, Phillips CS. Comparison of non-human primate and human whole blood tissue gene expression profiles. Toxicological Science. 2005;87:306–314. doi: 10.1093/toxsci/kfi243. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macacaca mulatta) Biology of Reproduction. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiology of Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca E, Basurto L, Velázquez S, Zárate A. Hormone replacement therapy increases ACTH/dehydroepiandrosterone sulfate in menopause. Maturitas. 2001;39:57–62. doi: 10.1016/s0378-5122(01)00192-x. [DOI] [PubMed] [Google Scholar]
- Gallara RV, Bellavia SL, Serova LL, Sabban EL. Environmental light conditions alter gene expression of rat catecholamine biosynthetic enzymes and Neuropeptide Y: differential effect in superior cervical ganglia and adrenal gland. Brain Research Molecular Brain Research. 2004;124:152–158. doi: 10.1016/j.molbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Garde AH, Hansen AM, Skovgaard LT, Christensen JM. Seasonal and biological variation of blood concentrations of total cholesterol, dehydroepiandrosterone sulfate, hemoglobin A(1c), IgA, prolactin, and free testosterone in healthy women. Clinical Chemistry. 2000;47:1877. [PubMed] [Google Scholar]
- Hansen AM, Garde AH, Skovgaard LT, Christensen JM. Seasonal and biological variation of urinary epinephrine, norepinephrine, and cortisol in healthy women. Clinica Chimica Acta. 2001;309:25–35. doi: 10.1016/s0009-8981(01)00493-4. [DOI] [PubMed] [Google Scholar]
- Hastings MH. Neuroendocrine rhythms. Pharmacology and Therapeutics. 1991;50:35–71. doi: 10.1016/0163-7258(91)90072-t. [DOI] [PubMed] [Google Scholar]
- Hastings M, O'Neill JS, Maywood ES. Circadian clocks: regulators of endocrine and metabolic rhythms. Journal of Endocrinology. 2007;195:187–198. doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nature Reviews Neuroscience. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S, Kosaka M, Fukuda N. Seasonal changes of human circadian rhythms in Antarctica. American Journal of Physiology Regulatory Integrative Comparative Phsysiology. 1992;262:885–891. [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metabolism. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Kreier F, Fliers E, Sauerwein HP, Romjin JA, Buijs RM. Minireview: Circadian control of metabolism by the suprachiasmatic nuclei. Endocrinology. 2007;148:5635–5639. doi: 10.1210/en.2007-0776. [DOI] [PubMed] [Google Scholar]
- King JA, Rosal MC, Ma Y, Reed G, Kelly TA, Stanek EJ, 3rd, Ockene IS. Sequence and seasonal effects of salivary cortisol. Behavioral Medicine. 2000;26:67–73. doi: 10.1080/08964280009595753. [DOI] [PubMed] [Google Scholar]
- Koko V, Djordjeviae J, Cvijiae G, Davidoviae V. Effect of acute heat stress on rat adrenal glands: a morphological and stereological study. Journal of Experimental Biology. 2004;207:4225–4230. doi: 10.1242/jeb.01280. [DOI] [PubMed] [Google Scholar]
- Lac G, Chamoux A. Do circannual rhythm of cortisol and testosterone interfere with variations induced by other events. Annals of Endocrinology. 2006;67:60–63. doi: 10.1016/s0003-4266(06)72542-2. [DOI] [PubMed] [Google Scholar]
- Lemos DR, Downs JL, Urbanski HF. Twenty-four-hour rhythmic gene expression in the rhesus macaque adrenal gland. Molecular Endocrinology. 2006;20:1164–1176. doi: 10.1210/me.2005-0361. [DOI] [PubMed] [Google Scholar]
- Levine ME, Milliron AN, Duffy LK. Diurnal and seasonal rhythms of melatonin, cortisol and testosterone in interior Alaska. Arctic Medical Research. 1994;53:25–34. [PubMed] [Google Scholar]
- Lincoln GA, Johnston JD, Andersson H, Wagner G, Hazlerigg DG. Photorefractoriness in mammals: dissociating a seasonal timer from the circadian-based photoperiod response. Endocrinology. 2005;146:3782–3790. doi: 10.1210/en.2005-0132. [DOI] [PubMed] [Google Scholar]
- Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, Kasukawa T, Iigo M, Sharp PJ, Iwasawa A, Suzuki Y, Sugano S, Niimi T, Mizutani M, Namikawa T, Ebihara S. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;20:317–22. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- Nicolau GY, Lakatua D, Sackett-Lundeen L, Haus E. Circadian and circannual rhythms of hormonal variables in elderly men and women. Chronobiology International. 1984;1:301–319. doi: 10.3109/07420528409063911. [DOI] [PubMed] [Google Scholar]
- Perret M, Aujard F. Aging and season affect plasma dehydroepiandrosterone sulfate (DHEA-S) levels in a primate. Experimental Gerontology. 2005;40:582–587. doi: 10.1016/j.exger.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Aschoff J. Annual rhythm of human reproduction: I. biology, sociology, or both. Journal of Biological Rhythms. 1990a;5:195–216. doi: 10.1177/074873049000500303. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Aschoff J. Annual rhythm of human reproduction: II. Environmental correlations. Journal of Biological Rhythms. 1990b;5:217–239. doi: 10.1177/074873049000500304. [DOI] [PubMed] [Google Scholar]
- Roy D, Belsham DD. Melatonin receptor activation regulates GnRH gene expression and secretion in GT1-7 GnRH neurons. Signal transduction mechanisms. Journal of Biological Chemistry. 2002;277:251–258. doi: 10.1074/jbc.M108890200. [DOI] [PubMed] [Google Scholar]
- Sainz RM, Mayo JC, Reiter RJ, Antolin I, Esteban MM, Rodriguez C. Melatonin regulates glucocorticoid receptor: an answer to its antiapoptotic action in thymus. FASEB Journal. 1999;13:1547–1556. doi: 10.1096/fasebj.13.12.1547. [DOI] [PubMed] [Google Scholar]
- Soldani P, Gesi M, Lenzi P, Natale G, Fornai F, Pellegrini A, Ricciardi MP, Paparelli A. Long-term exposure to noise modifies rat adrenal cortex ultrastructure and corticosterone plasma levels. Journal of Submicroscopic Cytology and Pathology. 1999;31:441–448. [PubMed] [Google Scholar]
- Stavisky RC, Watson SL, Anthony MS, Manuck SB, Adams MR, Kaplan JR. Influence of estradiol on cortisol secretion in ovariectomized cynomolgus macaques (Macaca fascicularis) American Journal of Primatology. 2003;60:17–22. doi: 10.1002/ajp.10076. [DOI] [PubMed] [Google Scholar]
- Urbanski HF. Excitatory amino acids and the control of seasonal breeding. In: Brann DW, Mahesh VB, editors. Excitatory Amino Acids: Their Role in Neuroendocrine Function. CRC Press; Boca Raton: 1995. pp. 253–279. Chapter 9. [Google Scholar]
- Urbanski HF, Downs JL, Garyfallou VT, Mattison JA, Lane MA, Roth GS, Ingram DK. Effect of caloric restriction on the 24-hour plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Annals of the New York Academy of Science. 2004;1019:443–447. doi: 10.1196/annals.1297.081. [DOI] [PubMed] [Google Scholar]
- Valenzuela FJ, Torres-Farfan C, Richter HG, Mendez N, Campino C, Torrealba F, Valenzuela GJ, Serón-Ferré M. Clock gene expression in adult primate suprachiasmatic nuclei and adrenal: is the adrenal a peripheral clock responsive to melatonin? Endocrinology. 2008;149:1454–1461. doi: 10.1210/en.2007-1518. [DOI] [PubMed] [Google Scholar]
- Van Cauter EW, Virasoro E, Leclercq R, Copinschi G. Seasonal, circadian and episodic variations of human immunoreactive beta-MSH, ACTH and cortisol. International Journal of Peptide and Protein Research. 1981;17:3–13. doi: 10.1111/j.1399-3011.1981.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Kerkhof GA, Souverijn JH. Absence of seasonal variation in the phase of the endogenous circadian rhythm in humans. Chronobiology International. 1998;15:623–632. doi: 10.3109/07420529808993198. [DOI] [PubMed] [Google Scholar]
- Walker BR, Best R, Noon JP, Watt GC, Webb DJ. Seasonal variation in glucocorticoid activity in healthy men. Journal of Clinical Endocrinology and Metabolism. 1997;82:4015–4019. doi: 10.1210/jcem.82.12.4430. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Johnston JD, Clarke IJ, Lincoln GA, Hazlerigg DG. Redefining the limits of day length responsiveness in a seasonal mammal. Endocrinology. 2007;149:32–39. doi: 10.1210/en.2007-0658. [DOI] [PubMed] [Google Scholar]
- Wang ZN, Lewis MG, Nau ME, Arnold A, Vahey MT. Identification and utilization of inter-species conserved (ISC) probesets on Affymetrix human GeneChip platforms for the optimization of the assessment of expression patterns in nonhuman primate (NHP) samples. BMC Bioinformatics. 2004;5:165. doi: 10.1186/1471-2105-5-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C. Conservation of photoperiod-responsive mechanisms in humans. American Journal of Physiology. 1993;265:846–857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Wever RA, Aschoff J. Seasonality in freerunning circadian rhythms in man. Naturwissenschaften. 1984;71:316–319. doi: 10.1007/BF00396615. [DOI] [PubMed] [Google Scholar]


